Abstract
Aims:
Tumour budding (TB), desmoplastic reaction (DR) and intraepithelial tumour infiltrating lymphocytes (iTILs) are recently recognised prognostic factors in colorectal cancer (CRC). In this study, we evaluated their significance and relationship to each other and their cumulative effect on survival.
Methods and results:
A total of 372 stages I–III CRC cases from 2013 to 2016 were included. Low TB was identified in 302 (81%) cases, immature/myxoid DR in 67 (18%) cases and iTILs in 130 (35.0%) cases. iTILs was associated with low budding (P = 0.0247), non-myxoid DR (P = 0.0004), poorly differentiated histology (P = 0.0015), absence of perineural invasion (P = 0.0367) and loss of mismatch repair proteins (P = 0.0002). Absence of iTILs and presence of immature/myxoid DR were associated with a worse recurrence-free survival (RFS) [hazard ratio (HR) = 2.191, 95% confidence interval (CI) = 1.232–3.895; and HR = 5.706, 95% CI = 3.632–8.964, respectively]. A competing risk analysis showed statistically significant prognostic groups combining iTILs and TB (P < 0.0001). Cases with iTILs and low TB were associated with better RFS compared to cases without iTILs and with intermediate/high TB (HR = 0.214, 95% CI = 0.109–0.421). Similarly, combining iTILs and DR revealed statistically significant prognostic groups (P < 0.0001). Cases with iTILs and a non-myxoid DR had better RFS compared to cases without iTILs and immature/myxoid DR (HR = 0.113, 95% CI = 0.056–0.230). On multivariate cause-specific analysis, patients’ age (P = 0.0045), iTILs (P = 0.0345), DR (P < 0.0001) and pTNM prognostic groups (P < 0.0001) were associated with RFS.
Conclusions:
Our study validates the association of iTILs and DR as independent prognostic finding in CRC, and propose a prognostic model using the combinations of iTILs with TB and stromal reaction in CRC.
Keywords: intraepithelial tumour infiltrating lymphocytes, tumour budding, myxoid stromal reaction, colorectal cancer, recurrence-free survival
Introduction
Despite advances in screening and treatment modalities for colorectal cancer, it remains the third most common cause of cancer-related deaths in the United States.1 Recently, the tumour microenvironment has been recognised to play an important role in patient prognosis. The presence of tumour budding (TB) has been implicated with a worse prognosis in colorectal cancers and in other epithelial neoplasms.2–9 The International Tumour Budding Consensus Conference (ITBCC)4 has proposed including the presence of TB into colorectal cancer staging systems and recommend using a three-tier system based on quantification of tumour buds. Similar to TB, the presence of an immature/myxoid desmoplastic reaction (DR) at the tumour edge has been associated with a worse prognosis in colorectal cancer.10–13 Contrary to TB and an immature/myxoid DR, the presence of intraepithelial tumour infiltrating lymphocytes (iTILs) has been associated with a better prognosis in colorectal cancer14–20 and with a better neoadjuvant treatment response in rectal adenocarcinoma.21–23 However, the relationship between TB, DR and iTILs has not been assessed. Moreover, a recent study by Lang-Schwarz and colleagues24 proposed combining TB and tumour infiltrating lymphocytes to further subdivide colorectal cancer patients into new prognostic groups. Their study, with stages I–IV cases of colorectal cancer, demonstrated that cases with a low TB and tumour infiltrating lymphocytes had the longest survival.24 This proposed model has not yet been validated in other cohorts of patients or evaluated in a cohort of cases excluding stage IV disease.
In this study, we sought to validate the prognostic significance of TB, DR and iTILs in a cohort of stages I–III colorectal cancer patients. We excluded stage IV patients to obtain a meaningful recurrence free survival (RFS) analysis. Additionally, we assessed the relationship between these parameters and their associations to other clinicopathological findings. Also, a validation of the proposed models combining TB and iTILs, and DR and iTILs was made.
Materials and methods
PATIENT POPULATION
A retrospective review of colonic adenocarcinoma resection specimens from 2013 to 2016 in our institution was performed. Exclusion criteria included stage IV disease, neoadjuvant treatment, anorectal location and/or those where the archival haematoxylin and eosin (H&E) slides were not available for review. The clinical and follow-up information was obtained from the electronic medical record. Approval from the institutional review board (IRB) was obtained prior to initiation of the study.
TUMOUR CHARACTERISTICS
All archival H&E slides of the resection specimens were reviewed by two pathologists simultaneously at a multiheader microscope, blinded to the clinical information. All the cases were grossed according to our institutional protocol, which includes submission of at least one section per centimetre of tumour, and entire submission for all tumours 3 cm or less in size, for histological examination. The tumour location was recorded and grouped into right or left colon. Right colon was defined as the region from the caecum to the distal third of the transverse colon, and left as the region between the distal third of the transverse colon to the rectum.
Tumour grade was assigned according to the 5th edition of the World Health Organisation (WHO) Classification of Tumours of the Digestive System.25 The presence of TB was recorded as per the 2016 ITBCC4 as the presence of a single tumour cell or a cell cluster of up to four tumour cells at the invasive front. TB was recorded using the proposed three-tier approach by the ITBCC, which relies upon the quantification of the buds present per 0.785 mm2 and classified into three categories: 0–4 (low), 5–9 (intermediate) or ≥ 10 (high). The presence of iTILs was defined as ≥ 5 lymphocytes per high-power field (Figure 1).26 The DR at the tumour edge was evaluated based on Ueno and colleagues’ method,10–13 which classifies the DR into three distinct groups: immature/myxoid, intermediate and mature. Immature/myxoid DR is defined as the presence of myxoid stroma at the tumour edge involving at least one high power field (×40); cases not meeting this criterion and with keloid-like collagen at the tumour edge are considered as intermediate DR; and those with fine mature collagen fibres, without keloid-like collagen or myxoid stroma, are categorised as showing a mature DR (Figure 1).10–13 All cases were evaluated for the stromal reaction regardless of the pT stage, in contrast to a prior study were the stromal reaction was only assessed in stages II and III (pT3 or pT4 cases).10
Figure 1.
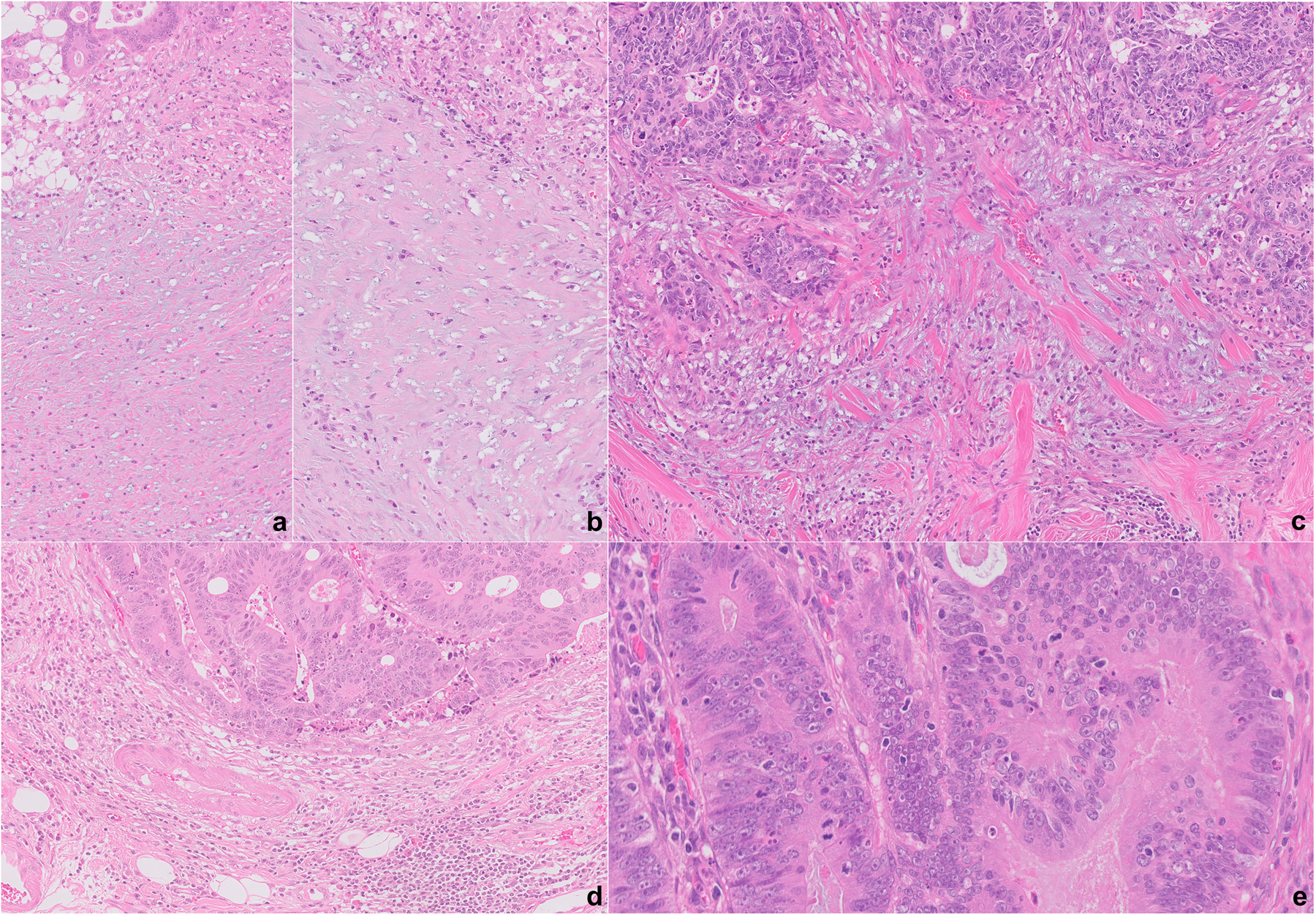
A–D, Examples of desmoplastic reaction (DR) at the tumour edge. An immature/myxoid DR, extending at least a high-power field (×40) (A,B); intermediate DR, with keloid-like collagen (C); and mature DR, without immature/myxoid stroma or keloid-like collagen (D). Intraepithelial tumour infiltrating lymphocytes, defined as ≥ 5 lymphocytes per high-power field (×40) (E).
Other pathological parameters evaluated included lymphovascular invasion (LVI), perineural invasion (PNI), presence of tumour deposits, number of tumour deposits, lymph node involvement, number of lymph nodes involved by carcinoma and number of lymph nodes examined. All the cases were staged according to the American Joint Committee on Cancer, 8th edition for colorectal adenocarcinoma.27 The expression of DNA mismatch repair proteins (MMR) by immunohistochemical analysis was obtained from the pathology reports. Retained MMR proteins was defined as nuclear tumour cell reactivity for MutS homologue (MSH2), MSH6, PMS1 homologue 2 (PMS2) and MutL homologue 1 (MLH1), and MMR loss as complete absence of nuclear reactivity for any protein in all tumour cells. Cases with retained MMR proteins were termed proficient MMR (pMMR), and cases with MMR loss as MMR-deficient (dMMR).
STATISTICAL ANALYSIS
The clinical and pathological characteristics were summarised using descriptive statistics. Continuous and categorical variables were compared by Kruskal–Wallis and χ2 tests, respectively. RFS was defined as the time from the date of resection to recurrence. Alive patients without recurrence were considered as a competing event at the last follow-up. Recurrence-free probabilities were calculated using cumulative incidence curves. Differences between the categories were determined using the Fine and Gray approach. Cause-specific analysis of competing risks were used to evaluate the relationship of the interested variables for RFS analysis. The variables with P < 0.25 from univariate models were considered in the multivariable model. The pathological stages, including T- and N-stages, and tumour–node–metastasis (TNM) prognostic groups had the highest priority. The final multivariable model was built using the backward stepwise selection approach to identify all significant risk factors. Factors significant at a 10% level were kept in the final model. All statistical tests were two-sided using an α = 0.05 level of significance. SAS version 9.4 (Cary, NC, USA) was used to perform all statistical analysis.
Results
PATIENT POPULATION
A total of 372 cases were included in the study with an even distribution in gender, as 50.8% of the cases presented in men. The mean age at resection was 65.7 ± 12.9 years. The majority of the patients were of Caucasian descent (314 patients, 84.4%) followed by African American (49 patients, 13.2%) and of other descent, including Asian and Hispanic (nine patients, 2.4%). In 54 patients (14.5%) a prior history of malignancy was noted, with breast and skin cancer being the most common (28 and 16%, respectively). Additionally, seven patients (1.9%) had a prior diagnosis of Lynch syndrome. In 275 patients the carcinoembryonic antigen (CEA) level was available at the time of resection with a mean value of 6.8 ± 21.2 ng/ml (reference range = 0.0–5.0 ng/ml). None of the cases received neoadjuvant treatment and 105 cases (28.3%) received adjuvant chemotherapy, with FOLFOX being the most common regimen (52 cases, 49.5%). The clinical follow-up range was 0–6.2 years (median = 2.8). The patient population characteristics are summarised in Table 1.
Table 1.
Patient and tumour characteristics (n = 372)
| Gender, n% | |
| Male | 189, 50.8% |
| Female | 183, 49.2% |
| Age, mean ± SD | 65.7 ± 12.9 |
| Race, n% | |
| Caucasian | 314, 84.4% |
| African American | 49, 13.2% |
| Othera | 9, 2.4% |
| History of prior malignancy, n% | |
| Yes | 54, 14.5% |
| No | 318, 85.5% |
| Known Lynch syndrome, n% | |
| Yes | 7, 1.9% |
| No | 365, 98.1% |
| CEA level at resection, n, mean ± SD | 275, 6.8 ± 21.2 |
| Adjuvant chemotherapy, n% | n = 371 |
| Yes | 105, 28.3% |
| No | 266, 71.7% |
| Location, n% | |
| Right | 227, 61.0% |
| Left | 145, 39.0% |
| Tumour buds, n% | |
| Low | 302, 81.2% |
| Intermediate | 31, 8.3% |
| High | 39, 10.5% |
| Desmoplastic reaction, n% | |
| Immature/myxoid | 67, 18.0% |
| Intermediate | 140, 37.6% |
| Mature | 165, 44.4%% |
| Intra-epithelial tumour-infiltrating lymphocytes, n% | |
| Yes | 130, 35.0% |
| No | 242, 65.1% |
| Tumour grade, n% | |
| Well/moderately differentiated | 294, 79.0% |
| Poorly differentiated | 56, 15.1% |
| Mucinous carcinoma | 22, 5.9% |
| Lymphovascular invasion, n% | |
| Yes | 138, 37.1% |
| No | 234, 62.9% |
| Perineural invasion, n% | |
| Yes | 57, 15.3% |
| No | 315, 84.7% |
| Tumour deposits, n% | |
| Yes | 43, 11.6% |
| No | 329, 88.4% |
| Number of tumour deposits, mean ± SD | 0.39 ± 2.07 |
| Number of involved lymph nodes, mean ± SD | 1.29 ± 2.73 |
| Number of lymph nodes evaluated, mean ± SD | 20.1 ± 8.0 |
| MMR expression, n% | n = 324 |
| Deficient | 67, 20.7% |
| Proficient | 257, 79.3% |
| pT stage, n% | |
| pT1 | 26, 7.0% |
| pT2 | 74, 19.9% |
| pT3 | 224, 60.2% |
| pT4 | 48, 12.9% |
| pN stage, n% | |
| pN0 | 233, 62.6% |
| pN1 | 89, 23.9% |
| pN2 | 50, 13.4% |
| pTNM prognostic group, n% | |
| I | 83, 22.3% |
| II | 150, 40.3% |
| III | 139, 37.4% |
SD, standard deviation; CEA, carcinoembryonic antigen; MMR, mismatch repair; pT, pathological T category; pTNM, pathological tumour–node–metastasis.
Asian or Hispanic
Percentages might not total 100% due to rounding
TUMOUR CHARACTERISTICS
The tumour characteristics are summarised in Table 1. The majority of the cases were located in the right colon (227 cases, 61.0%), with the most common specific location being the sigmoid colon (98 cases, 26.3%), the caecum (81 cases, 21.8%) and the ascending colon (76 cases, 20.4%). Most cases were considered well/moderately differentiated (294 cases, 79.0%); 56 cases (15.1%) were poorly differentiated and a total of 22 cases (5.9%) were considered as mucinous carcinoma. The majority of the cases showed low budding (302 cases, 81.2%), followed by high budding in 39 cases (10.5%) and intermediate budding in 31 cases (8.3%). In 165 cases (44.4%) a mature DR was noted, an intermediate reaction in 140 cases (37.6%), and an immature/myxoid reaction in 67 cases (18.0%). iTILs were noted in 130 cases (35.0%). LVI and PNI was identified in 138 (37.1%) and 57 cases (15.3%), respectively. Tumour deposits were present in 43 cases (11.6%), with a mean number of tumour deposits of 0.39 ± 2.07. In 324 cases where MMR protein status was available, 67 cases (20.7%) were dMMR. In cases with dMMR the most common protein losses were MLH1 and PMS2 in 53 cases (79.1%). dMMR cases were more likely to be located in the right colon (57 cases, 85%; P < 0.0001).
The pT-stage was pT1, pT2, pT3 and pT4 in 26 cases (7.0%), 74 cases (19.9%), 224 cases (60.2%) and 48 cases (12.9%), respectively. In 233 cases (62.6%) no lymph node involvement by carcinoma was identified (pN0), and 89 (23.9%) and 50 cases (13.4%) were considered as pN1 and pN2, respectively. The mean number of lymph nodes involved by carcinoma was 1.29 ± 2.73, with a mean number of lymph nodes evaluated per case of 20.1 ± 8.0. The pTNM prognostic stage group was I in 83 (22.3%), II in 150 (40.3%) and III in 139 cases (37.4%).
CLINICOPATHOLOGICAL ASSOCIATIONS WITH INTRAEPITHELIAL TUMOUR INFILTRATING LYMPHOCYTES
The clinicopathological associations with iTILs are summarised in Table 2. iTILs were significantly associated with female gender (P = 0.0045), low budding (P = 0.0247), non-myxoid DR (P = 0.0004), poorly differentiated histology (P = 0.0015), absence of PNI (P = 0.0367) and dMMR (P = 0.0002). Although not significant, iTILs were more frequently detected in tumours arising in the right colon (88 cases, 67.7%) (P = 0.0532). iTILs were not significantly associated with age, race, history of prior malignancy, known Lynch syndrome, CEA level at presentation, adjuvant chemotherapy, LVI, tumour deposits, number of tumour deposits, lymph node involvement, number of lymph nodes examined, pT-stage, pN-stage or pTNM prognostic groups.
Table 2.
Clinicopathological associations with intra-epithelial tumour-infiltrating lymphocytes
| Intra-epithelial lymphocytes | P | ||
|---|---|---|---|
| Yes (n = 130) | No (n = 242) | ||
| Gender, n% | |||
| Male | 53, 40.8% | 136, 56.2% | 0.0045 |
| Female | 77, 59.2% | 106, 43.8% | |
| Age, mean ± SD | 67.4 ± 13.7 | 64.7 ± 12.4 | 0.0470 |
| Race, n% | |||
| Caucasian | 109, 83.9% | 205, 84.7% | 0.4061 |
| African American | 16, 12.3% | 33, 13.6% | |
| Other1 | 5, 3.9% | 4, 1.7% | |
| History of prior malignancy, n% | |||
| Yes | 18, 13.9% | 36, 14.9% | 0.7880 |
| No | 112, 86.2% | 206, 85.1% | |
| Known Lynch syndrome, n% | |||
| Yes | 3, 2.3% | 4, 1.7% | 0.6990 |
| No | 127, 97.7% | 238, 98.4% | |
| CEA level at resection, n, mean ± SD | 93, 5.6 ± 9.9 | 182, 7.5 ± 25.1 | 0.8593 |
| Adjuvant chemotherapy, n% | n =129 | n = 242 | |
| Yes | 33, 25.6% | 72, 29.8% | 0.3957 |
| No | 96, 74.4% | 170, 70.3% | |
| Location, n% | |||
| Right | 88, 67.7% | 139, 57.4% | 0.0532 |
| Left | 42, 32.3% | 103, 42.6% | |
| Tumour buds, n% | |||
| Low | 115, 88.6% | 187, 77.3% | 0.0247 |
| Intermediate | 8, 6.2% | 23, 9.5% | |
| High | 7, 5.4% | 32, 13.2% | |
| Desmoplastic reaction, n% | |||
| Immature/myxoid | 11, 8.5% | 56, 23.1% | 0.0004 |
| Intermediate | 47, 36.2% | 93, 38.4% | |
| Mature | 72, 55.4% | 93, 38.4% | |
| Tumour grade, n% | |||
| Well/moderately differentiated | 90, 69.2% | 204, 84.3% | 0.0015 |
| Poorly differentiated | 31, 23.9% | 25, 10.3% | |
| Mucinous carcinoma | 9, 6.9% | 13, 5.4% | |
| Lymphovascular invasion, n% | |||
| Yes | 40, 30.8% | 98, 40.5% | 0.0641 |
| No | 90, 69.2% | 144, 59.5% | |
| Perineural invasion, n% | |||
| Yes | 13, 10.0% | 44, 18.2% | 0.0367 |
| No | 117, 90.0% | 198, 81.8% | |
| Tumour deposits, n% | |||
| Yes | 13, 10.0% | 30, 12.4% | 0.4906 |
| No | 117, 90.0% | 212, 87.6% | |
| Number of tumour deposits, mean ± SD | 0.6 ± 3.2 | 0.3 ± 1.0 | 0.5392 |
| Number of involved lymph nodes, mean ± SD | 1.3 ± 2.8 | 1.3 ± 2.7 | 0.5139 |
| Number of lymph nodes evaluated, mean ± SD | 20.4 ± 8.9 | 20.0 ± 7.5 | 0.9093 |
| MMR expression, n% | N = 115 | N = 209 | |
| Deficient | 37, 32.2% | 30, 14.4% | 0.0002 |
| Proficient | 78, 67.8% | 179, 85.7% | |
| pT stage, n% | |||
| pT1 | 8, 6.2% | 18, 7.4% | 0.9127 |
| pT2 | 28, 21.5% | 46, 19.0% | |
| pT3 | 78, 60.0% | 146, 60.3% | |
| pT4 | 16, 12.3% | 32, 13.2% | |
| pN stage, n% | |||
| pN0 | 86, 66.2% | 147, 60.7% | 0.5362 |
| pN1 | 27, 20.8% | 62, 25.6% | |
| pN2 | 17, 13.1% | 33, 13.6% | |
| pTNM prognostic group, n% | |||
| I | 29, 22.3% | 54, 22.3% | 0.5279 |
| II | 57, 43.8% | 93, 38.4% | |
| III | 44, 33.8% | 95, 39.3% | |
SD, standard deviation; CEA, carcinoembryonic antigen; MMR, mismatch repair protein; pT, pathological T category; pTNM, pathological tumour–node–metastasis.
Asian or Hispanic percentages might not total 100% due to rounding.
Bold indicates statistical significance.
COMPETING RISK ANALYSIS FOR RECURRENCE-FREE SURVIVAL
The absence of iTILs was significantly associated with a worse RFS [P = 0.0069; hazard ratio (HR) = 2.141, 95% confidence interval (CI) = 1.233–3.717] (Figure 2). Similarly, high budding was significantly associated with a worse RFS (P = 0.0003; HR = 2.601, 95% CI = 1.480–4.570) (Figure 3). A model combining iTILs and TB showed significant association with RFS (P < 0.0001) (Figure 3). A two-tiered scale for TB was used for the prediction model with iTILs. Cases with iTILs and low budding were associated with a better RFS compared to cases with no iTILs and intermediate/high budding (HR = 0.214, 95% CI = 0.109–0.421); among cases with intermediate/high budding, cases with no iTILs were associated with a worse RFS compared to cases with iTILs (HR = 2.754, 95% CI = 0.855–8.873). The presence of intermediate/high budding in cases without iTILs was associated with a worse RFS compared to cases with iTILs and low budding (HR = 4.666, 95% CI = 2.377–9.159) (Figure 3). The combined analyses of iTILs and TB are presented in the Supporting information, Table S1.
Figure 2.
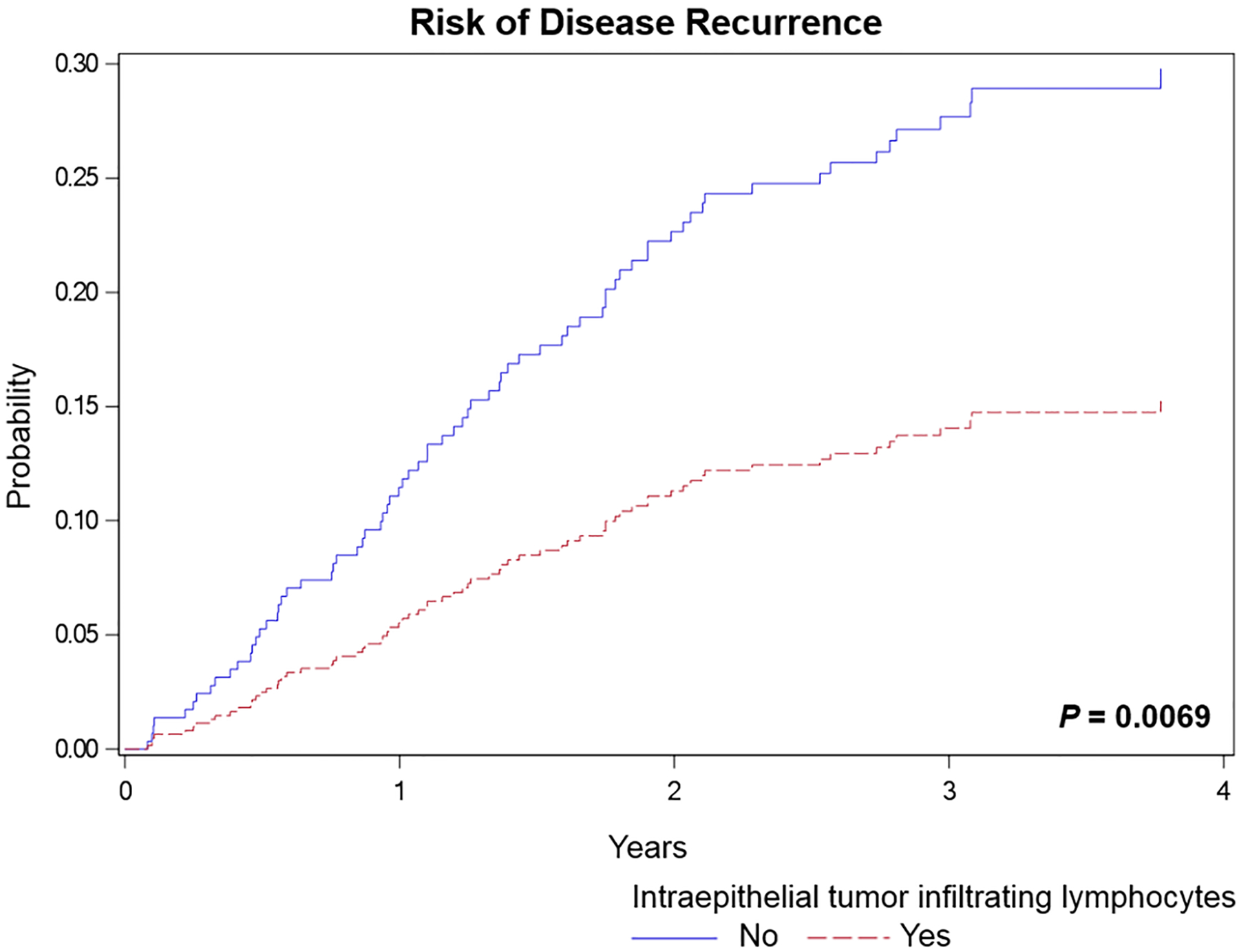
Risk of disease recurrence for cases with no intraepithelial tumour infiltrating lymphocytes (iTILs) compared to cases with iTILs (hazard ratio = 2.141, 95% confidence interval = 1.233–3.717).
Figure 3.
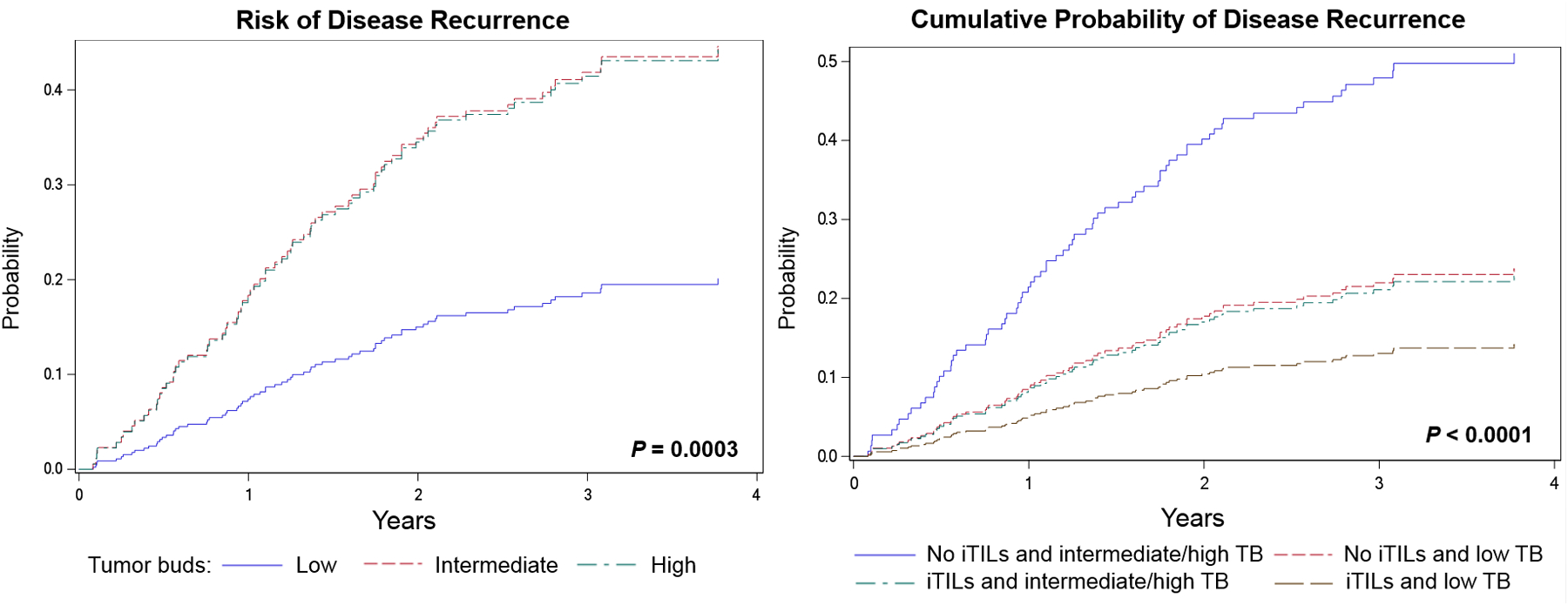
Risk of disease recurrence for cases with low tumour budding (TB) compared to cases with intermediate, and high TB (left). Cumulative probability of disease recurrence using a combination of intraepithelial tumour infiltrating lymphocytes (iTILs) and TB (right) (hazard ratios and 95% confidence intervals presented in Supporting information, Table S1).
Using the three-tier system proposed by Ueno et al. for DR,10–13 a significant difference among the groups is noted with RFS (P < 0.0001) (Figure 4). Cases with an immature/myxoid DR have a worse RFS compared to cases with an intermediate DR (HR = 5.814; 95% CI = 3.282–10.301) and those with a mature DR (HR = 5.615; 95% CI = 3.270–9.639). Given the marked significance of an immature/myxoid DR with a poor RFS, cases were grouped into those with an immature/myxoid DR and those without (non-myxoid), the latter including both intermediate DR as well as mature DR. Cases with an immature/myxoid DR were significantly associated with a worse RFS compared to those with a non-myxoid DR (P < 0.0001; HR = 5.706, 95% CI = 3.632–8.964) (Figure 4).
Figure 4.
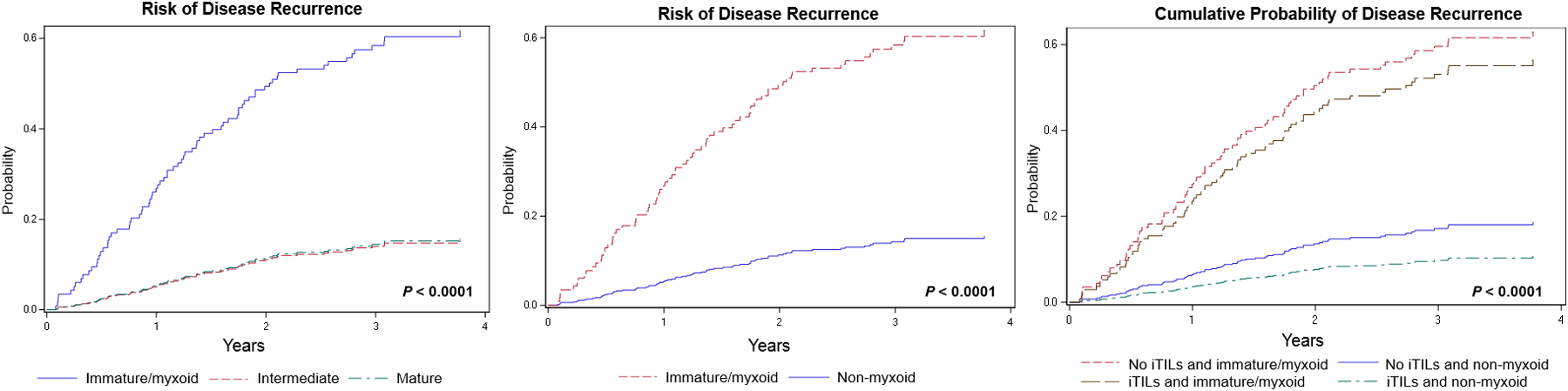
Risk of disease recurrence for cases showing immature/myxoid, intermediate and mature desmoplastic reaction (left). Risk of disease recurrence for cases with an immature/myxoid desmoplastic reaction compared to cases with a non-myxoid desmoplastic reaction [hazard ratio (HR) = 5.706, 95% confidence interval (CI) = 3.632–8.964] (centre). Cumulative probability of disease recurrence using a combination of intraepithelial tumour infiltrating lymphocytes (iTILs) and desmoplastic reaction censored as immature/myxoid and non-myxoid (right) (HR and 95% CI presented in Supporting information, Table S2).
A combined model of DR and iTILs is significantly associated with RFS (P < 0.0001) (Figure 4). The DR for the combined model was considered as immature/myxoid and non-myxoid. Cases with iTILs and a non-myxoid DR were associated with a better RFS compared to cases without iTILs and an immature/myxoid DR (HR = 0.113, 95% CI = 0.056–0.230) and compared to cases with iTILs and an immature/myxoid DR (HR = 0.136, 95% CI = 0.049–0.375). Cases with an immature/myxoid DR and no iTILs were associated with a worse prognosis compared to cases with a non-myxoid DR and with iTILs (HR = 8.816, 95% CI = 4.346–17.883). Cases with both iTILs and an immature/myxoid DR were associated with a worse RFS (HR = 7.371, 95% CI = 2.663–20.400) compared to cases with iTILs and a non-myxoid DR. The combined analysis of iTILs and DR are presented in the Supporting information, Table S2.
UNIVARIATE AND MULTIVARIATE CAUSE-SPECIFIC ANALYSIS FOR RFS
Univariate cause-specific analysis of competing risks models for RFS showed that a prior history of malignancy (P = 0.0365), TB (P = 0.0006), iTILs (P = 0.0068), DR (P < 0.0001), LVI (P < 0.0001), PNI (P = 0.0036), tumour deposits (P < 0.0001), pT-stage (P < 0.0001), pN-stage (P < 0.0001) and pTNM prognostic stage groups (P < 0.0001) were associated with RFS (Table 3). Age, gender, tumour grade, tumour location and MMR expression were not associated with RFS on univariate analysis. Multivariate cause-specific analysis showed that age (P = 0.0045), iTILs (P = 0.0348), DR (P < 0.0001) and pTNM-stage (P < 0.0001) were significantly associated with RFS (Table 3).
Table 3.
Cause-specific analysis of recurrence-free survival
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
| P | HR (95% CI) | P | |
| Age | 0.0979 | 1.029 (1.009 – 1.049) | 0.0045 |
| Gender | 0.2260 | ||
| History of prior malignancy | 0.0365 | ||
| Tumour grade | 0.7892 | ||
| Tumour location | 0.9962 | ||
| Tumour budding | 0.0006 | ||
| No | 1.862 (1.045–3.316) | ||
| Mature | 1 | ||
| Lymphovascular invasion | < 0.0001 | ||
| Perineural invasion | 0.0036 | ||
| Tumour deposits | < 0.0001 | ||
| MMR expression | 0.8230 | ||
| pT stage | < 0.0001 | ||
| pN stage | < 0.0001 | ||
| III | 1 | ||
HR, hazard ratio; CI, confidence interval; pT, pathological T category; pTNM, pathological tumour–node–metastasis; MMR, mismatch repair protein.
Bold indicates statistical significance.
Discussion
The tumour microenvironment plays an important role in the prognosis of colorectal cancers. In our study, cases with iTILs were independently associated with a better RFS (P = 0.0348) and immature/myxoid DR with a worse RFS (P < 0.0001). TB was associated with a worse RFS on univariate analysis (P = 0.0006). Furthermore, the presence of iTILs was significantly associated with low TB (P = 0.0247) and a non-myxoid DR (P = 0.0004). The pathological stages (pT, pN and pTNM) were also significantly associated with RFS (P < 0.0001 each).
The presence of TB has been associated with a worse outcome in several neoplasms, including colorectal carcinoma.2–8,24,28–33 The ITBCC4 recommends using a three-tier system, low buds – 4), intermediate buds (5– 9) and high buds (≥ 10), which has been validated recently by several groups.30,31,34–38 Our results further validate the association of TB with a worse RFS (P = 0.0003) in stages I–III CRC. The intermediate and high TB groups in our cohort did not show a statistically significant difference in RFS (Figure 3). This could be related to inherent challenges in evaluation due to intense tumour-associated inflammation39 or a selection bias in our cohort, with exclusion of stage IV and rectal adenocarcinomas. Nonetheless, there is a marked difference in the intermediate/high budding group compared to the low budding group, as reported in other studies.29–31,33 This reinforces the importance of including TB in pathology reports, which is recommended by National Comprehensive Cancer Network40 and by the US Multi-Society Task Force on Colorectal Cancer.41
DR in colorectal cancer has gained recognition in the last decade as an independent indicator of RFS.10–13 Nonetheless, this feature has not been adopted in pathological reporting for clinical care. One of the largest recent studies analysing the stromal reaction pattern at the tumour edge in colorectal cancer was performed by Ueno et al.10 Their cohort consisted of 821 cases of stages II and III colorectal cancer from four institutions. A total of 214 patients (26%) had an immature/myxoid DR, and on multivariate analysis this feature was significantly associated with a worse RFS (HR = 3.1, 95% CI = 2.1–4.6).10 As previously reported and validated by their group, a type C reaction (immature/myxoid) was an independent indicator of RFS in rectal adenocarcinoma.11 An immature/myxoid DR has also been identified as an independent indicator for extrahepatic recurrence in a cohort of colorectal cancer with synchronous or metachronous resectable liver metastases.12
In this study, DR was classified into three groups: immature/myxoid, intermediate and mature.10–13 An immature/myxoid DR was present in 18%, intermediate in 38% and a mature DR in 44% of the cases. The prevalence of the immature/myxoid DR in our cohort (18%) was lower compared to Ueno et al.’s cohort, at 26.1%; and mature DR in our cohort (44%) was slightly higher compared to their cohort, at 40%.10 The difference can probably be explained by the variability in selection of patient cohort in these two studies. In the study by Ueno et al., only stages II–III (pT3 and pT4) colorectal cancer cases were included.10 In contrast, our cohort consisted of cases involving stages I–III and the DR was evaluated in all cases regardless of pT stage. Interestingly, in our cohort cases with iTILs were significantly associated with a non-myxoid DR (P = 0.0004). Using a combined model with iTILs and DR divided into a two-tier system (immature/myxoid and non-myxoid), cases were further stratified prognostically, with cases showing iTILs and a non-myxoid DR having a better RFS compared to cases without iTILs and an immature/myxoid DR (HR = 0.113, 95% CI = 0.056–0.230).
In recent years, numerous studies have demonstrated the prognostic significance of the colorectal cancer-associated lymphocytic response.42 Different studies have used different methods of assessment, including stromal (intra- and peritumoural) as well as intraepithelial lymphocytic infiltration. Subset analyses of the lymphocytic responses based on immunohistochemical expression and molecular markers are also being studied.43–45 It is clear from these multiple studies that tumour associated lymphocytes are an integral component of the tumour microenvironment, and play an important prognostic role. However, to date, there is no standardisation in the histopathological assessment for this parameter that has been translated into clinical use, and recent efforts are being made to address this.20
iTILs have been associated with an improved RFS regardless of MMR status; however, cases with dMMR and high iTILs had a better prognosis compared to dMMR with intermediate and low iTILs (P = 0.013).17 The presence of iTILs was significantly associated with dMMR in our cohort (P < 0.0002), which is congruent with previous reports.17,18,43,45 Moreover, the presence of iTILs in our cohort has been shown to be an independent prognostic factor. dMMR cases were significantly associated with a right-sided location (P < 0.0001) in our study; similar results were seen by Landau et al. in their cohort of 328 stages I–IV colorectal cancers.33 MLH1 and PMS2 were the most commonly lost MMR proteins in our cohort (79.1%), whereas MSH2 and MSH6 were reported to be most commonly lost in small intestinal adenocarcinomas (P < 0.001).46 Interestingly, in this cohort, MMR status was not associated with RFS on univariate or multivariate analysis. One probable explanation in our cohort could be the exclusion of stage IV adenocarcinomas, which could have created a selection bias for MMR-proficient tumours. Moreover, there could be other molecular factors that could impact prognosis, such as low tumour mutational burden and BRAF and RAS gene mutations, which are shown to be negative prognostic factors among the MSI group of tumours.47 Detailed molecular analysis has not been performed in our cohort to offer a firm explanation.
To our knowledge, this is the first study evaluating the association of iTILs with TB and DR. In our cohort of stages I–III colorectal cancer, the presence of iTILs was significantly associated with low TB (P = 0.0247) and with a non-myxoid DR (P = 0.0004). The underlying mechanism resulting in these associations is unclear. We hypothesise that the high immunological response manifested by iTILs perhaps acts as a barrier for tumour budding formation or for the stroma to show an immature/myxoid DR, thereby trying to prevent the neoplasm from disseminating. In our cohort, the absence of iTILs was significantly associated with a worse RFS on multivariate analysis (P = 0.0348; HR = 1.862, 95% CI = 1.045–3.316), clearly supporting the protective role of iTILs from the damaging effects of the cancer. The negative association of iTILs with the poor histological prognosticators such as TB and immature/myxoid DR may actually reflect the integral tumour microenvironment, rather than these processes being addressed as separate phenomenon.
In conclusion, our study validates TB, DR and iTILs as important prognostic markers for RFS. In our cohort, iTILs and DR are independently associated with RFS. Moreover, our study explores the association of these parameters to each other, and we show that iTILs are significantly associated with a non-myxoid DR and low TB. Our findings support routine reporting of TB, iTILs and DR patterns as additional pathological parameters in clinical practice.
Supplementary Material
Acknowledgements
The authors thank the reviewers for their valuable comments, which led to substantial improvement in the presentation of our findings. The expenses of this project were covered by the Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO. REDCap supported by Clinical and Translational Science Award (CTSA) grant (UL1 TR000448).
Footnotes
Conflicts of interest
None of the authors have any relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.American Cancer Society. Cancer facts and figures 2018. Am. Cancer Soc [internet] 2018; 1–71. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. [cited 5 Febraury 2020]
- 2.Berg KB, Schaeffer DF. Tumor budding as a standardized parameter in gastrointestinal carcinomas: more than just the colon. Mod. Pathol 2018; 31; 862–72. Available at: 10.1038/s41379-018-0028-4. [DOI] [PubMed] [Google Scholar]
- 3.Boxberg M, Kuhn P, Reiser M et al. Tumor budding and cell nest size are highly prognostic in laryngeal and hypopharyngeal squamous cell carcinoma. Further evidence for a unified histopathologic grading system for squamous cell carcinomas of the upper aerodigestive tract. Am. J. Surg. Pathol 2019; 43; 303–311. [DOI] [PubMed] [Google Scholar]
- 4.Lugli A, Kirsch R, Ajioka Y et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol 2017; 30; 1299–1311. Available at: 10.1038/modpathol.2017.46. [DOI] [PubMed] [Google Scholar]
- 5.Jesinghaus M, Boxberg M, Konukiewitz B et al. A Novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am. J. Surg. Pathol 2017; 41; 1112–1120. [DOI] [PubMed] [Google Scholar]
- 6.Ohike N, Coban I, Kim GE et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am. J. Surg. Pathol 2010; 34; 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ, Kauppila JH. Tumor budding and prognosis in gastric adenocarcinoma. Am. J. Surg. Pathol 2019; 43; 229–234. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor K, Li-Chang HH, Kalloger SE et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol 2015; 39; 472–478. [DOI] [PubMed] [Google Scholar]
- 9.Kadota K, Miyai Y, Katsuki N et al. A grading system combining tumor budding and nuclear diameter predicts prognosis in resected lung squamous cell carcinoma. Am. J. Surg. Pathol 2017; 41; 750–760. [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, Kanemitsu Y, Sekine S et al. Desmoplastic pattern at the tumor front defines poor-prognosis subtypes of colorectal cancer. Am. J. Surg. Pathol 2017; 41; 1506–1512. [DOI] [PubMed] [Google Scholar]
- 11.Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut 2004; 53; 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno H, Konishi T, Ishikawa Y et al. Histologic categorization of fibrotic cancer stroma in the primary tumor is an independent prognostic index in resectable colorectal liver metastasis. Am. J. Surg. Pathol 2014; 38; 1380–1386. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Jones A, Jass JR, Talbot IC. Clinicopathological significance of the ‘keloid-like’ collagen and myxoid stroma in advanced rectal cancer. Histopathology 2002; 40; 327–334. [DOI] [PubMed] [Google Scholar]
- 14.Berntsson J, Svensson MC, Leandersson K et al. The clinical impact of tumour infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a cohort study. Int. J. Cancer 2017; 141; 1654–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksen AC, Sørensen FB, Lindebjerg J et al. The prognostic value of tumor-infiltrating lymphocytes in stage II colon cancer. A nationwide population-based study. Transl. Oncol 2018; 11: 979–87. Available at: 10.1016/j.tranon.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emile JF, Julié C, Le Malicot K et al. Prospective validation of a lymphocyte infiltration prognostic test in stage III colon cancer patients treated with adjuvant FOLFOX. Eur. J. Cancer 2017; 82; 16–24. [DOI] [PubMed] [Google Scholar]
- 17.Dahlin AM, Henriksson ML, Van Guelpen B et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol 2011; 24; 671–682. [DOI] [PubMed] [Google Scholar]
- 18.Lee WS, Park S, Lee WY, Yun SH, Chun HK. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 2010; 116; 5188–5199. [DOI] [PubMed] [Google Scholar]
- 19.Pagès F, Berger A, Camus M et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med 2005; 353; 2654–2666. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs TL, Sioson L, Sheen A et al. Assessment of tumor-infiltrating lymphocytes using International TILs Working Group (ITWG) system is a strong predictor of overall survival in colorectal carcinoma: a study of 1034 patients. Am. J. Surg. Pathol 2019; 44; 536–544. [DOI] [PubMed] [Google Scholar]
- 21.Teng F, Mu D, Meng X et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am. J. Cancer Res 2015; 5; 2064–2074. [PMC free article] [PubMed] [Google Scholar]
- 22.Matsutani S, Shibutani M, Maeda K et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018; 109; 966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González I, Bauer PS, Chapman WC, Alipour Z, Rais R. Clinicopathologic determinants of pathologic treatment response in neoadjuvant treated rectal adenocarcinoma. Ann. Diagn. Pathol 2020; 45; 151452 Available at: 10.1016/j.anndiagpath.2019.151452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang-Schwarz C, Melcher B, Haumaier F et al. Budding and tumor-infiltrating lymphocytes – combination of both parameters predict survival in colorectal cancer and leads to new prognostic subgroups. Hum. Pathol 2018; 79; 160–167. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organisation (WHO). WHO classification of tumours: digestive system tumours, 5th edn. Lyon, France: WHO Classification of Tumours; 2019. [Google Scholar]
- 26.Royal College of Pathologists of Australasia. Colorectal cancer structured reporting protocol [internet]. 2016. [cited 2020 Mar 17]. Available at: www.rcpa.%0Aedu.au/Library/Practising-Pathology/Structured-Pathology-Reporting-of-%0ACancer/Cancer-Protocols/Gastrointestinal/Protocol-colorectal-cancer.
- 27.Amin MB, Edge S, Greene F et al. eds. AJCC cancer staging manual, 8th edn. Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 28.Landau MS, Hastings SM, Foxwell TJ, Luketich JD, Nason KS, Davison JM. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod. Pathol 2014; 27; 1578–189. Available at: 10.1038/modpathol.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham RP, Vierkant RA, Tillmans LS et al. Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am. J. Surg. Pathol 2015; 39; 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LM, Kevans D, Mulcahy H et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am. J. Surg. Pathol 2009; 33; 134–141. [DOI] [PubMed] [Google Scholar]
- 31.Ryan É, Khaw YL, Creavin B et al. Tumor budding and PDC grade are stage independent predictors of clinical outcome in mismatch repair deficient colorectal cancer. Am. J. Surg. Pathol 2018; 42; 60–68. [DOI] [PubMed] [Google Scholar]
- 32.Lohneis P, Sinn M, Klein F et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br. J. Cancer 2018; 118; 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landau MA, Zhu B, Akwuole FN, Pai RK. Site-specific differences in colonic adenocarcinoma: KRAS mutations and high tumor budding are more frequent in cecal adenocarcinoma. Am. J. Surg. Pathol 2018; 42; 351–358. [DOI] [PubMed] [Google Scholar]
- 34.Kawachi H, Eishi Y, Ueno H et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod. Pathol 2015; 28; 872–879. [DOI] [PubMed] [Google Scholar]
- 35.Petrelli F, Pezzicam E, Cabiddu M et al. Tumor budding and survival in stage II colorectal cancer: a systematic review and pooled analysis. J. Gastrointest. Cancer 2015; 46; 212–218. [DOI] [PubMed] [Google Scholar]
- 36.Ueno H, Ishiguro M, Nakatani E et al. Prospective multicenter study on the prognostic and predictive impact of tumor budding in stage II colon cancer: results from the SACURA trial. J. Clin. Oncol 2019; 37; 1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi T, Shimada Y, Lee LH et al. Poorly differentiated clusters predict colon cancer recurrence: an in-depth comparative analysis of invasive-front prognostic markers. Am. J. Surg. Pathol 2018; 42; 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Wyk HC, Roseweir A, Alexander P et al. The relationship between tumor budding, tumor microenvironment, and survival in patients with primary operable colorectal cancer. Ann. Surg. Oncol 2019; 26; 4397–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho S-J, Kakar S. Tumor budding in colorectal carcinoma: translating a morphologic score into clinically meaningful results. Arch. Pathol. Lab. Med 2018; 142; 952–957. [DOI] [PubMed] [Google Scholar]
- 40.National Comprehensive Cancer Network and Colon Cancer (version 3.2020) [internet] Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf [cited 20 March 2020]
- 41.Kaltenbach T, Anderson J, Burke C et al. Endoscopic removal of colorectal lesions: recommendations by the US Multi-society Task Force on Colorectal Cancer. Am. J. Gastroenterol 2020; 115; 435–464. [DOI] [PubMed] [Google Scholar]
- 42.Kong JC, Guerra GR, Pham T et al. Prognostic impact of tumor-infiltrating lymphocytes in primary and metastatic colorectal cancer: a systematic review and meta-analysis. Dis. Colon Rectum 2019; 62; 498–508. [DOI] [PubMed] [Google Scholar]
- 43.Turksma AW, Coupé VMH, Shamier MC et al. Extent and location of tumor-infiltrating lymphocytes in microsatellite-stable colon cancer predict outcome to adjuvant active specific immunotherapy. Clin. Cancer Res 2016; 22; 346–356. [DOI] [PubMed] [Google Scholar]
- 44.Berntsson J, Svensson MC, Leandersson K et al. The clinical impact of tumour infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a cohort study. Int. J. Cancer 2017; 141; 1654–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips SM, Banerjea A, Feakins R, Li SR, Baustin SA, Dorudi S. Tumour infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br. J. Surg 2004; 91; 469–475. [DOI] [PubMed] [Google Scholar]
- 46.González I, Goyal B, Xia MD, Pai RK, Ma C. DNA mismatch repair deficiency but not ARID1A loss is associated with prognosis in small intestinal adenocarcinoma. Hum. Pathol 2019; 85; 18–36. [DOI] [PubMed] [Google Scholar]
- 47.Innocenti F, Ou FS, Qu X et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J. Clin. Oncol 2019; 37; 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


