Abstract
Regional alterations in kinetics of catecholamine uptake are due in part to variations in clearance mechanisms. The rate of clearance is a critical determinant of the strength of catecholamine signaling. Catecholamine transmission in the nucleus accumbens core (NAcc) and basolateral amygdala (BLA) is of particular interest due to involvement of these regions in cognition and motivation. Previous work has shown that catecholamine clearance in the NAcc is largely mediated by the dopamine transporter (DAT), but clearance in the BLA is less DAT-dependent. A growing body of literature suggests that organic cation transporter 3 (OCT3) also contributes to catecholamine clearance in both regions. Consistent with different clearance mechanisms between regions, catecholamine clearance is more rapid in the NAcc than in the BLA, though mechanisms underlying this have not been resolved. We compared the expression of DAT and OCT3 and their contributions to catecholamine clearance in the NAcc and BLA. We found DAT protein levels were ~4-fold higher in the NAcc than in the BLA, while OCT3 protein expression was similar between the two regions. Immunofluorescent labeling of the two transporters in brain sections confirmed these findings. Ex vivo voltammetry demonstrated that the magnitude of catecholamine release was greater, and the clearance rate was faster in the NAcc than in the BLA. Additionally, catecholamine clearance in the BLA was more sensitive to the OCT3 inhibitor corticosterone, while clearance in the NAcc was more cocaine sensitive. These distinctions in catecholamine clearance may underlie differential effects of catecholamines on behavioral outputs mediated by these regions.
Keywords: Dopamine, Cocaine, Corticosterone, Uptake, Release, Voltammetry
Introduction
The nucleus accumbens core (NAcc) and basolateral amygdala (BLA) receive dopaminergic projections from the ventral tegmental area (VTA) that have been implicated in regulating aspects of cognition 1,2,3,4, fear 5,6,7, and motivated behaviors8,9,10. Though both NAcc and BLA are implicated in these behaviors, dopamine signaling seems to have opposite effects in the two areas. Dopamine neurotransmission in the NAcc has been historically linked to the psychomotor stimulating and rewarding effects of drugs of abuse 11,12,13, and functional reduction of dopamine transmission in this region has been observed following chronic drug exposure and withdrawal 14,15,16,17,18. The behavioral effects of dopamine in the BLA are often the opposite. For example, augmented dopamine in the NAcc is linked to positive-affective states 11,12,13, while increased dopamine in the BLA is associated with negative affect or anxiogenic states 19,20,21. Further, dopamine is decreased in the NAcc following chronic adolescent social isolation in rats, a model of early life stress, whereas dopamine release is increased in the BLA using the same paradigm 21.
Catecholamine neurotransmission can be influenced by both release and clearance mechanisms, which can differ widely between brain regions. Multiple mechanisms of dopamine clearance have been delineated, including uptake by both high-affinity 22,23,24 and low-affinity transporters 25,26,27,28,29, in addition to enzymatic degradation 30,31 and diffusion 32,33. Dopamine clearance is regulated by distinct mechanisms in the NAcc and BLA. Dopamine transporters (DAT), are high-affinity, low-capacity transport proteins abundantly expressed in the NAcc 22,23,24,21, but are less abundant in the BLA. A growing body of literature suggests that the organic cation transporter 3 (OCT3)26,27,28,29,34,35,36,37,38,39, which is expressed in both the NAcc 39 and BLA 29, can mediate uptake of a variety of monoamine species including serotonin 26,40, norepinephrine28, and dopamine 27,29. Compared to the DAT, OCT3 has a lower affinity for dopamine28, but a higher capacity for dopamine transport41. The relative contributions of OCT3 and the DAT to dopamine clearance in the NAcc and BLA have not been directly investigated. Elucidating this relationship could provide unique pharmacological treatment strategies for ameliorating substance use disorders and anxiety-related disorders by regulating distinct dopamine transmission in the NAcc and BLA.
Exposure to stressors engages the hypothalamic-pituitary-adrenal (HPA) axis42,43 to increase synthesis and release of corticosteroid hormones (predominantly cortisol in humans, and corticosterone (CORT) in rodents)44,45,46,47. In the NAcc, CORT decreases the rate of dopamine clearance39 and thereby increases the duration of neurotransmitter action in this region. CORT and other corticosteroids acutely and directly inhibit OCT3-mediated transport 34,26; in fact, recent work has demonstrated that the pharmacological interaction between CORT and OCT339 is functionally analogous to uptake inhibition by cocaine of the DAT 48. CORT treatment, likely by inhibiting OCT3-mediated clearance, has been shown to augment extracellular concentrations of serotonin26, norepinephrine49 and dopamine39. Taken together, this suggests that OCT3 blockade may augment extracellular catecholamine levels40,26,27,28,29. Notably, OCT3 is densely expressed in the BLA29 where CORT may exert similar inhibitory effects upon OCT3 to decrease catecholamine clearance. By regulating the duration of released catecholamines, clearance mechanisms contribute greatly to the extent to which receptors are activated on target cells, and thus can have powerful effects on behavior modulated by catecholamines.
Previous work has shown that the catecholamine clearance rate in the NAcc is significantly faster than clearance rates in the BLA50. It is plausible that this distinction is due to differences in both the specific transporters expressed, and the density of transporter expression in the two areas. Our lab has previously shown that DAT protein expression is lower in the BLA than in the NAcc 21, and that the apparent affinity of transporters for catecholamines is lower in the BLA compared to the NAcc50. Together, these factors likely contribute to regional differences in clearance rates. In consideration of these data, we examined the localization and expression levels of DAT and OCT3 in the NAcc and BLA using immunofluorescence and western blot, and assessed the functional contributions of DAT and OCT3 to catecholamine clearance in the two regions of drug-naïve rats using ex vivo fast-scan cyclic voltammetry.
Methods:
Experimental Animals
Male Sprague-Dawley rats (n=6–10 per group, 9–10 months old, Harlan Laboratories, Indianapolis, IN) and homozygote DAT knock-out (DAT KO) mice were used for experiments. DAT KO mice were bred in-house via crossing heterozygous DAT+/− 129SvJ and C57BL/6 mice99, and then breeding resultant DAT+/− mice. Tissue samples were obtained from DAT+/− × DAT+/− breeding pair litters and digested prior to phenol:chloroform DNA extraction. Polymerase chain reaction was run with forward and reverse oligonucleotide primers for DAT to verify animals with full DAT KO. All animals were maintained on a 12:12 light cycle and given standard rodent chow and water ad libitum, and all experiments were performed in the dark phase of the animals’ light cycle. Animal care and handling, as well as experimental protocols, were approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee and adhered to all National Institutes of Health Animal Care Guidelines.
Brain slice preparation for fast scan cyclic voltammetry
Animals were deeply anesthetized with isoflurane gas in an induction chamber, then rapidly decapitated. Brains were removed and placed into ice-cold, pre-oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF; in mM: 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.4 CaCl2, 2.4 MgCl2, 25 NaHCO3, 11.0 glucose, 0.4 L-ascorbic acid; pH adjusted to 7.4). A vibrating tissue slicer (Leica Biosystems, Buffalo Grove, IL, USA) was used to prepare coronal brain slices (400μm thick from rats, 300μm thick from mice) containing the NAcc (both rats and mice) and BLA (rats only). NAcc and BLA tissue for protein analysis was excised from slices by hand.
Western Blot Hybridization
DAT and OCT3 protein levels were quantified using Western blot hybridization following methods described previously100, with slight alterations for OCT3 blots described below. Tissue samples from rats were homogenized in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% Triton-X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM Trizma Base, pH 8.0) and centrifuged at 12,000 × g for 30 min. Protein concentrations were determined using a BCA protein assay kit (ThermoScientific, Rockford, IL) and Molecular Devices Spectra Max 384 Plus spectrophotometer (Sunnyvale, CA) running SoftMax Pro software. A MagicMark™ XP molecular weight ladder (LC5602; ThermoFisher Scientific, Grand Island, NY) and 30μg of protein were loaded into 4–12% NuPAGE Bis-Tris gels (NP0321BOX; ThermoFisher Scientific, Grand Island, NY). Proteins from the gel were transferred onto a polyvinylidene difluoride membrane and probed using the following primary antibody dilutions: DAT (1:4000; 2231, EMD Millipore, Billerica, MA), OCT3 (1:1000; (OCT31-A, Alpha Diagnostic Int., San Antonio, TX) and β-actin (1:4000; ab8229, Abcam, Cambridge, MA). A horseradish peroxidase (HRP)-conjugated secondary antibody was used (1:5000 for DAT and β-actin, 1:4000 for OCT3) (65–6120, ThermoFisher Scientific, Grand Island, NY), in combination with Pierce ECL chemiluminescence (32106, ThermoFisher Scientific, Grand Island, NY). Blots were exposed using a Bio-Rad Chemidoc imaging system, and the band signal intensity was assessed using QuantityOne software (Bio-Rad, Hercules, CA).
Perfusion and Histology
Rats were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (100 mg/kg) and were transcardially perfused with ice-cold 0.05 M phosphate-buffered saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB, pH 7.4). Following perfusion, brains were removed and post-fixed in the 4% paraformaldehyde solution for 12 hours at 4°C, and were rinsed twice in 0.1 M PB for 12 hours. The brains were then incubated in 30% sucrose in 0.1 M PB for approximately 72 hours. Brains were then blocked into two pieces with a cut in the coronal plane at the caudal border of the mammillary bodies (approximately –5.30 mm bregma) using a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI, USA). Brains were frozen rapidly in dry-ice-chilled liquid isopentane and stored at –80 °C until sectioning. Forebrain sections (25 μm), including the BLA, were cut across the coronal plane using a cryostat (Leica Biosystems, Buffalo Grove, IL, USA), and stored as six alternate sets of sections in cryoprotectant (30% ethylene glycol (w/w)/20% glycerol (w/w) in 0.05 M PB, pH 7.4) at –20 °C until immunostaining.
Antibodies
For immunodetection of OCT3, an affinity-isolated antibody (RRID AB_1622571, rabbit anti-OCT3, cat # OCT31A, Alpha Diagnostics International, San Antonio, TX, USA) raised against an 18-amino acid sequence in the large intracellular loop of rat OCT3 (amino acids 313–330: HLSSNYSEITVTDEEVSN) was used. This amino acid sequence is 100% conserved between mouse and rat OCT3 and has no significant sequence homology with other organic cation/carnitine transporters. The specificity of this antibody was confirmed previously in immunohistochemical and immunofluorescence applications27,72,25,101. For immunodetection of DAT, an affinity-purified antibody (RRID AB_1586991, rabbit anti-DAT, cat # AB2231, MilliporeSigma, Burlington,MA, USA), was used at a dilution of 1:500.
Immunofluorescence
Separate coronal sections (25μm thick) containing BLA and NAcc were used for detection of OCT3 and DAT. After rinsing in PBS, sections were incubated overnight with anti-OCT3 antibody (1:400) or anti-DAT antibody (1:500) in phosphate buffered saline with 0.1%Tween 20 (PBST). Sections were rinsed the next day and incubated for two hours with fluorophore-conjugated secondary antibodies (AlexaFluor594-conjugated donkey anti-rabbit; 1:2000; Jackson ImmunoResearch, West Grove, PA, USA). Sections were then rinsed briefly in PB, mounted onto SuperFrost microscope slides, dried and coverslipped with EverBrite antifade mounting medium (Biotium, Fremont, CA, USA).
Imaging
Photomicrographs were acquired using a Nikon 80i microscope fitted with an ORCA-Flash 4.0LT digital camera (Hammamatsu,Japan) linked to a computer running NIS Elements-BR software (Nikon Instruments, Melville, NY).
Ex vivo fast scan cyclic voltammetry
Fast scan cyclic voltammetry was used to characterize catecholamine terminal function in the NAcc and BLA. Brain slices were transferred to testing chambers and incubated in oxygenated ACSF, heated to 32oC for one hour prior to experiment start. A glass capillary containing a carbon fiber (house-made; ≈100 μM length, 7 μM radius; Goodfellow Corporation, Berwyn, PA) and a bipolar stimulating electrode (Plastics One, Roanoke, VA) were placed in close proximity (≈100μm) on the surface of the slice. Endogenous catecholamine efflux was induced by a single pulse (350μA, 4msec, NAcc) or 10 pulse stimulation (20Hz, 350μA, 4msec/pulse, BLA), applied every five or seven minutes, respectively. Extracellular catecholamine concentrations were detected by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs. silver/silver chloride, 400 V/sec) every 100 milliseconds to the recording electrode and measuring changes in current at the oxidation potential of dopamine and norepinephrine (~0.6V). Pharmacological agents (cocaine and/or CORT; as described in Results) were applied once a stable baseline was achieved (three consecutive stimulations within 10% of the previous collection). Electrically evoked catecholamine concentrations were assessed by comparing the current at the peak oxidation potential for dopamine and norepinephrine to each electrode’s calibration to known concentrations of catecholamine (3.0μM).
Baseline catecholamine release ([CA] per pulse, [CA]/pulse) and the maximal rate of uptake at catecholamine transporters (Vmax), were analyzed to assess release and uptake (Wightman et al., 1988). Baseline release and uptake was assessed in every slice that was used for recordings, typically 2–4 slices per animal. Standard Michaelis-Menten modeling procedures were used to assess kinetic parameters of baseline stimulation before application of cocaine or CORT. [CA]/pulse was calculated as the amount of catecholamine released per electrical stimulation and catecholamine uptake was determined with Vmax and T80, the time it takes for [CA] to decay to 80% of its peak. Km was set to 160nM for each slice in both regions, based on the known affinity of dopamine for DATs102 while pre-drug Vmax values were allowed to vary. Because the affinity of transporters for their endogenous monoamine neurotransmitter (Km) varies across transporter species, the use of apparent Km (the apparent alteration in affinity of a transporter for its neurotransmitter based on blockade of that transporter, e.g. inhibition of DAT function with cocaine application), was not possible in these experiments, given the examination of multiple transporter species. Therefore, uptake in the NAcc and BLA was assessed using T80, the time it takes for a catecholamine trace to return to 80% of its peak height, a measure of clearance rate. As dopamine and norepinephrine are released in the BLA, “catecholamines” (CA) will be used to describe neurotransmission within this region. All data were collected and analyzed with Demon Voltammetry and Analysis software56.
Statistics
Graph Pad Prism (version 7, La Jolla, CA, USA) was used for all statistical analyses and to prepare all graphs. Student’s t-tests were used to determine regional differences in DAT and OCT3 protein levels, baseline [CA]/pulse and clearance measures, effects of maximal concentrations of cocaine or CORT, as well as the relative contribution of the DAT and OCT3 in uptake. A two-way repeated measures analysis of variance (ANOVA), with region and drug concentration as factors, was used to determine differences in cocaine and CORT potency between regions. In the event of significant main effects, Bonferroni post-hoc analysis was used to determine significant group differences. A one-way ANOVA was used to calculate the effects of CORT on dopamine release in the NAcc of DAT-KO mice. A Tukey’s post-hoc test was applied when a significant finding was revealed. In voltammetry experiments, 2–4 slices from each animal were used to determine basal catecholamine kinetics (release and uptake; N=15–22 slices/group), whereas one slice per animal was used for drug applications (N=5–10 animals/group).
Results
Carbon fiber microelectrodes have equal sensitivity to dopamine and norepinephrine.
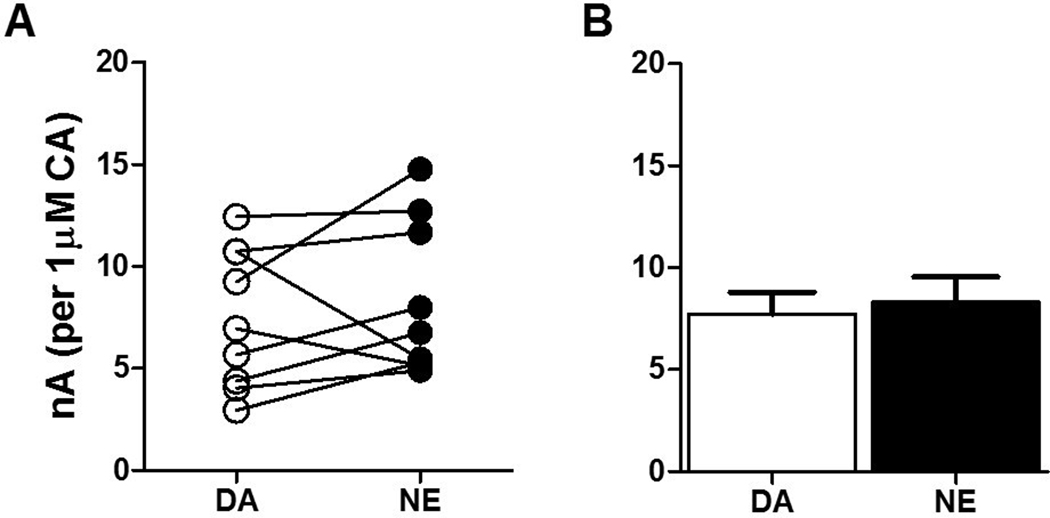
Proper identification of electroactive neurotransmitters using voltammetry relies on neurotransmitter species-specific cyclic voltammograms, which trace the peak oxidation and reduction potentials of the neurotransmitter. Notably, the oxidation and reduction potentials of dopamine and norepinephrine are nearly identical51, preventing the separation of these neurotransmitters using voltammetry without the assistance of additional pharmacology. Electrical stimulation of the NAcc causes dopamine efflux52, while stimulation of the BLA causes release of both dopamine and norepinephrine53,54. Unfortunately, any pharmacological measures to isolate either dopamine or norepinephrine in the BLA would further reduce the low catecholamine levels measured in this region50, and compromise accurate quantification of stimulated neurotransmitter release. Furthermore, exogenous application of neurotransmitter over the slice does not replicate electrically stimulated endogenous release with reasonable temporal fidelity, and therefore physiological uptake kinetics would be difficult to ascertain utilizing this method. We sought to discern the sensitivity of our electrodes to each catecholamine to identify any bias to either neurotransmitter that may influence the interpretation of our experiments. To this end, we flowed a moderately high concentration (3μM) of either dopamine or norepinephrine over the carbon fiber microelectrodes. We chose this concentration in order to more precisely assess sensitivity to the catecholamines through the production of a somewhat larger signal, while by no means reaching the maximum limit of detection. Our data show no difference in electrode sensitivity with respect to dopamine versus norepinephrine (t17=0.3516, p=0.729; Figure 1A, B), indicating that the carbon fiber microelectrodes used in the present voltammetry experiments do not favor one catecholamine over the other at the concentration tested.
Figure 1: Electrodes have equal sensitivity to dopamine and norepinephrine.
(A) Peak oxidation potentials for dopamine (DA) and norepinephrine (NE) showed little variation when detected by the same carbon fiber microelectrode. (B) Within-electrode analysis revealed no group difference in electrode sensitivity with respect to 3μM dopamine and norepinephrine (t17=0.3516, p=0.729). N=9/group.
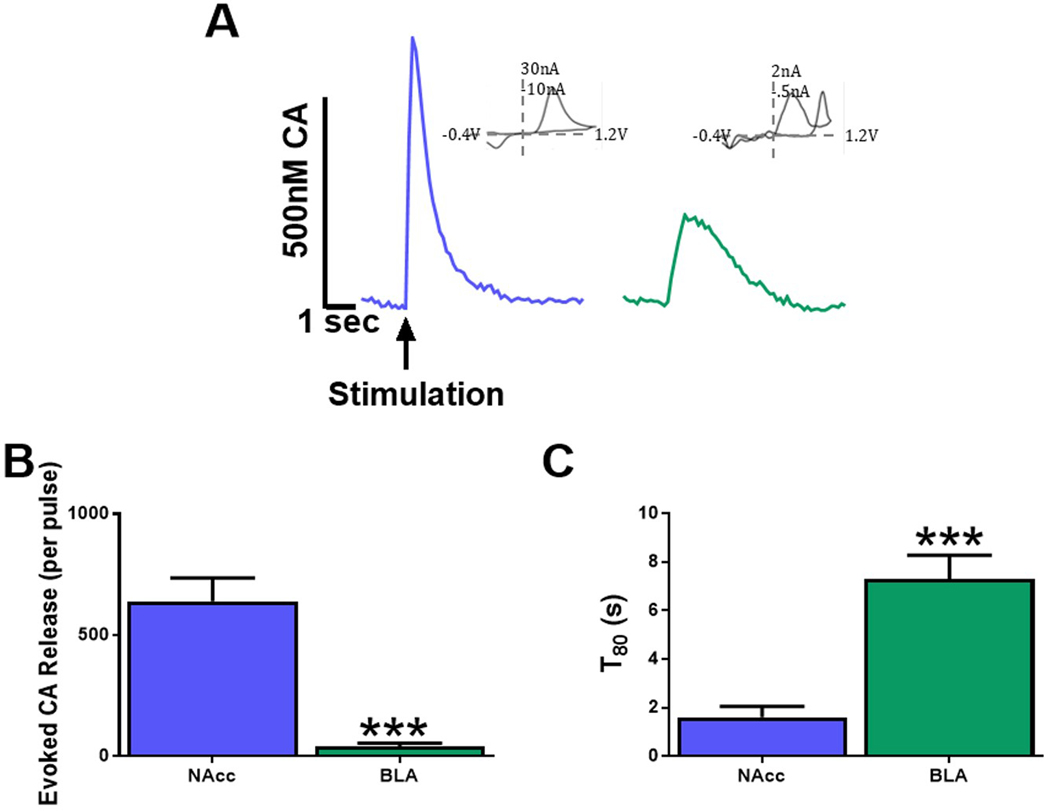
Catecholamine release is greater and uptake is faster in the NAcc compared to the BLA.
To determine regional differences in catecholamine release and uptake kinetics, pre-drug catecholamine release and uptake were analyzed. The BLA has reduced catecholamine release compared to the NAcc50,55, so stronger stimulation parameters were used in the BLA (NAcc, single pulse; BLA, 10 pulse/20hz). For this reason, release data are analyzed as a measure of catecholamine release per stimulation pulse ([CA]/pulse) rather than overall catecholamine release (μM). Representative traces of these stimulation parameters in the NAcc (blue line) and BLA (green line) are depicted in Figure 2A. Two to four slices from each animal were used to assess release and uptake, prior to subsequent drug application. Student’s t-test revealed significantly reduced [CA]/pulse (t19=3.558, p<0.0001, N=22 NAcc, N=17 BLA; Figure 2B) in the BLA compared to the NAcc. Because lower levels of release, particularly within the BLA, may not be great enough to ensure clearance at a saturable level consistently52, we chose to utilize T80, the amount of time it takes for the voltammetric trace to decay to 80% of its peak height, rather than Vmax in order to ascertain reuptake rates. The rate of catecholamine uptake was significantly reduced in the BLA compared to the NAcc as indicated by increased T80 (t34=5.828, p<0.0001; N=22 NAcc, N=15 BLA Figure 2C). Additionally, we find that Vmax is significantly reduced in the BLA compared to the NAcc (data not shown) demonstrating that the effect on Vmax is consistent with T80 data. These data confirm an overall reduction in catecholamine release and uptake dynamics in the BLA.
Figure 2: Catecholamine release is greater and uptake is faster in the NAcc compared to the BLA.
(A) Representative traces of catecholamine (CA) release and uptake from brain slices containing the NAcc and BLA. (B) Evoked CA release was significantly lower in the BLA compared with to the NAcc (p<0.0001). (C) CA uptake, as measured by T80, of the NAcc is significantly faster than BLA slices (p<0.0001). NAcc = nucleus accumbens core; BLA = basolateral amygdala; CA = Catecholamine. N=15–22 slices/group from 6–8 animals.
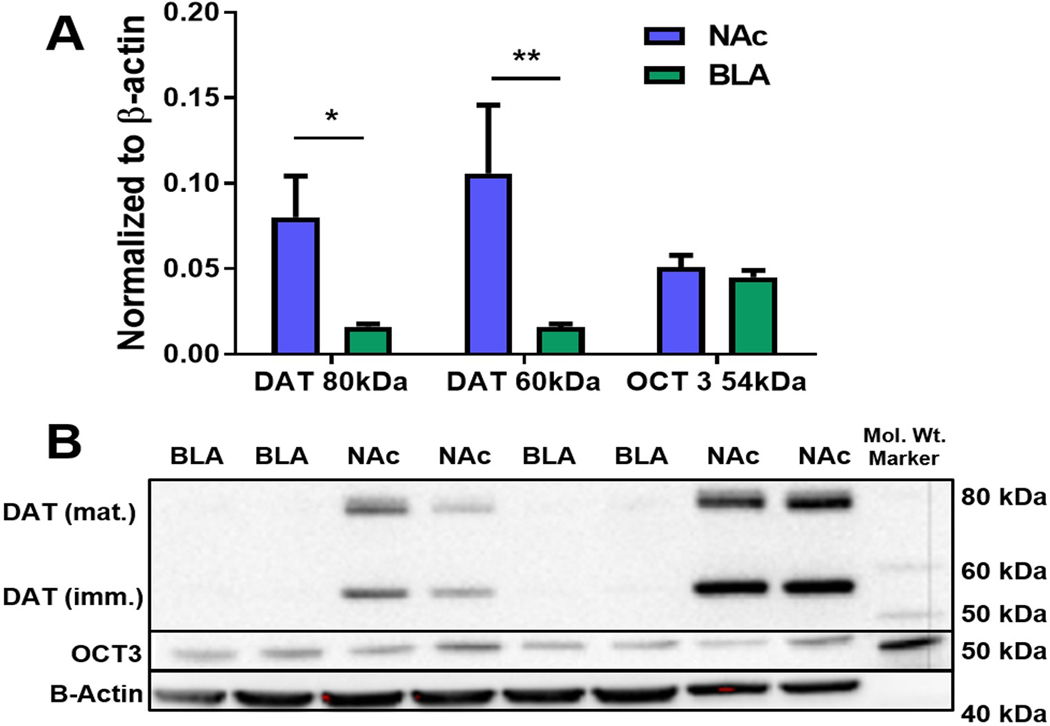
DAT protein levels are higher in the NAcc than in the BLA, but OCT3 protein levels are similar between regions
Western blot hybridization was used to compare protein levels of DAT and OCT3 in the NAcc and BLA (N=8 animals/group). DAT protein content, both 80 kDa (functionally mature; p=0.0247), and 60 kDa (functionally immature; p=0.0011) isoforms, was significantly higher in NAcc tissue than in BLA tissue (Figure 3A, B). OCT3 protein levels were not different between the two regions (p>0.05; Figure 3A). Representative Western blots are shown in Figure 3B.
Figure 3: DAT protein levels are higher in the NAcc compared to the BLA, but OCT3 levels are similar between regions.
Western blot hybridization was used to detect and quantitate the observed variations in DAT and OCT3 densities in the NAcc and BLA. (A) DAT protein levels, normalized to β-actin, were significantly elevated in the NAcc compared to the BLA at both 78 kDa (p=0.0247) and 56 kDa (p=0.0011) molecular weights representing the mature and immature DAT isoforms, respectively. Conversely, the protein levels of OCT3 were similar in the NAcc and BLA (p>0.05). (B) Representative blot showing NAcc and BLA DAT and OCT3 protein levels quantified in the graph above. NAcc = nucleus accumbens core; BLA = basolateral amygdala; *p<0.05. (N=8 animals/group).
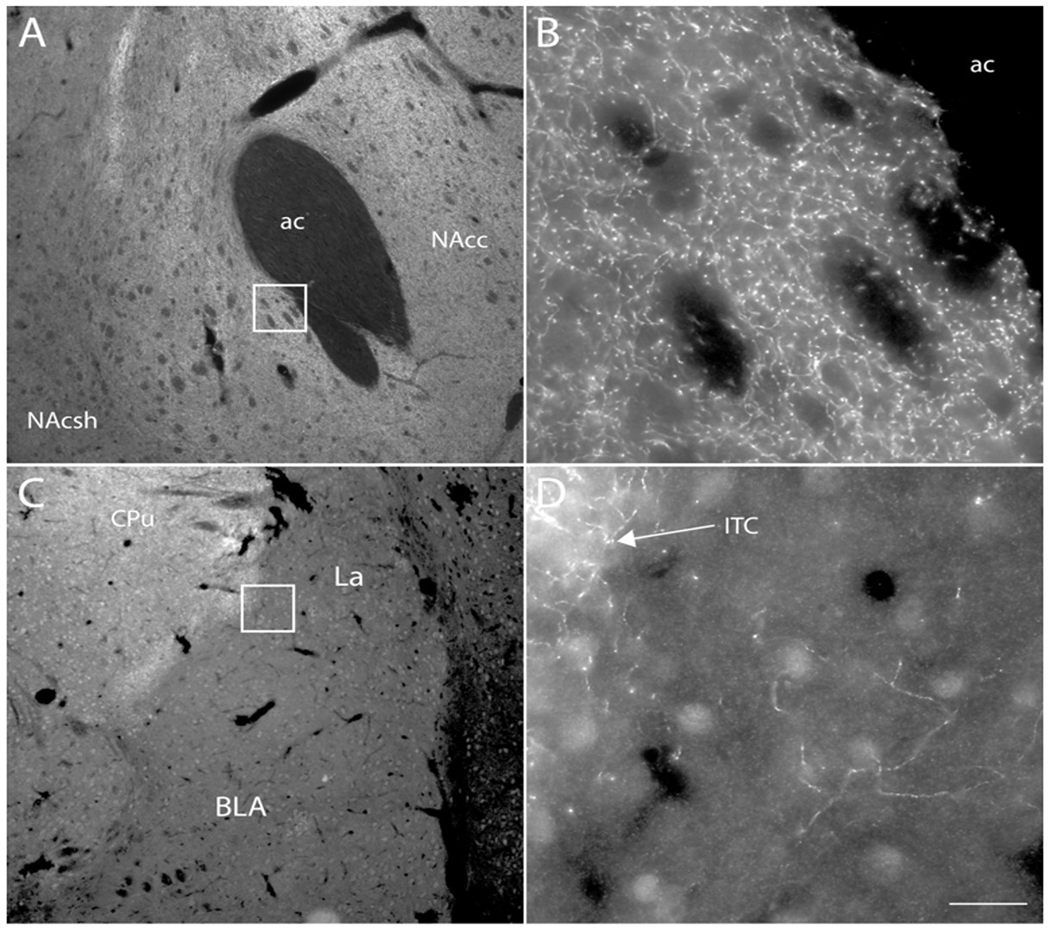
Localization and density of OCT3 and DAT immunoreactivity in the NAcc and BLA.
The relative distributions of DAT and OCT3 protein in the NAcc and BLA were examined using immunofluorescence techniques. DAT-like immunoreactive fibers were observed in both regions; however they occurred at a much higher density in the NAcc than in the BLA (Figure 4). OCT3-like immunoreactive perikarya and processes were observed at similar densities in NAcc and BLA (Figure 5).
Figure 4. Localization of DAT expression in NAcc and BLA.
Fluorescence photomicrographs depicting immunofluorescence localization of dopamine transporter (DAT) immunoreactivity in the nucleus accumbens (A, B) and basolateral amygdala (C, D) of the rat. Boxes in (A) and (C) are shown at higher magnification in (B) and (D), respectively. DAT-immunoreactive fibers were observed at high density in the nucleus accumbens (B) and dorsal striatum (C), and at much lower density in the BLA (D). DAT-immunoreactive fibers were observed at higher density in the intercalated cell groups (ITC) of the amygdala (C, D) than in the rest of the amygdala. Scale bar = 200 μm (A, C); 20 μm (B, D). ac – anterior commissure; BLA – basolateral amygdala; ITC –intercalated cell group; NAcc – nucleus accumbens core; NAcsh – nucleus accumbens shell.
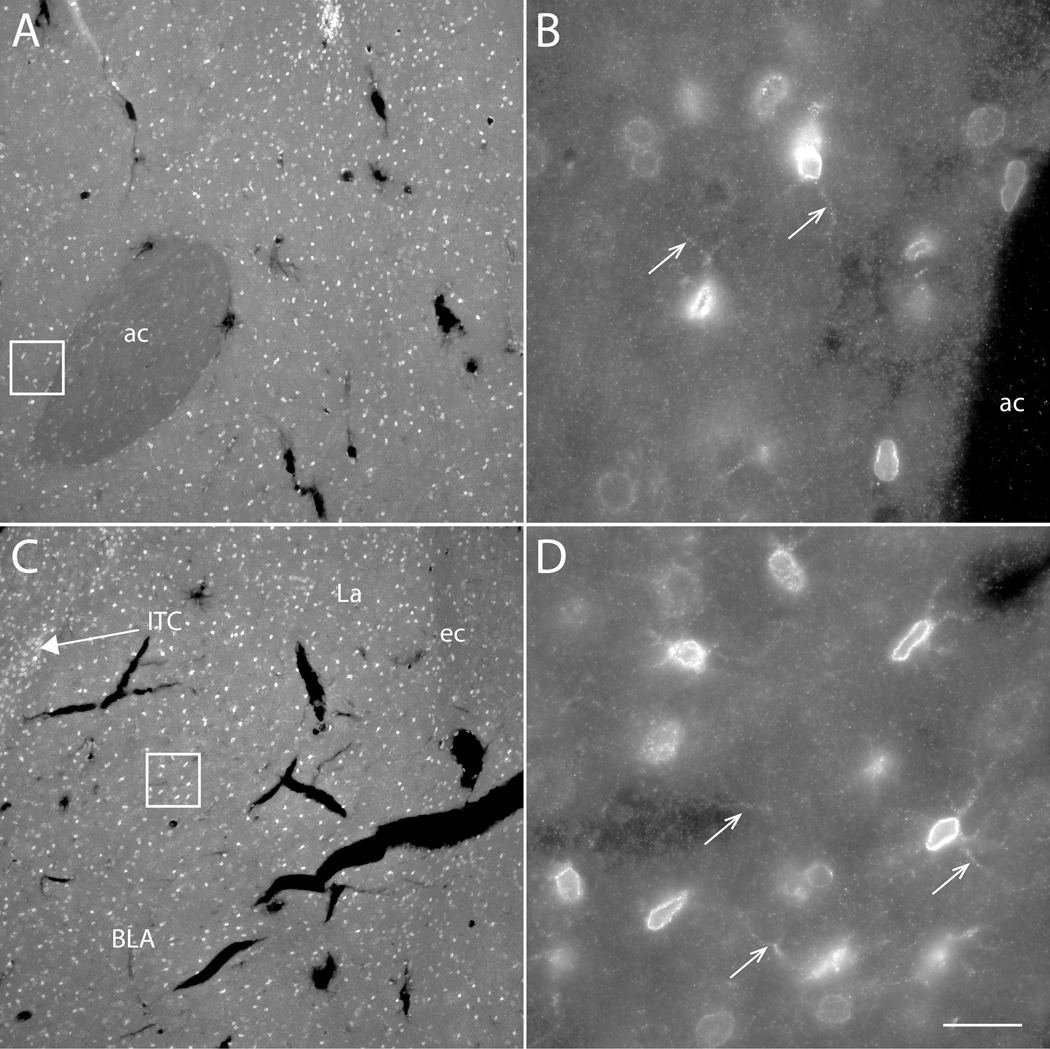
Figure 5. Localization of OCT3 expression in NAcc and BLA.
Fluorescence photomicrographs depicting immunofluorescence localization of OCT3 immunoreactivity in the nucleus accumbens (A, B) and basolateral amygdala (C, D) of the rat. Boxes in (A) and (C) are shown at higher magnification in (B) and (D), respectively. OCT3-immunoreactive punctae, perikaryae and processes (arrows in B, D) were observed at similar densities in both areas. OCT3-perikarya were observed at higher density in the intercalated cell groups (ITC) of the amygdala (C) than in the rest of the amygdala. Arrows in B, D indicate OCT3-immunoreactive processes. Scale bar = 200 μm (A, C); 20 μm (B, D). ac – anterior commissure; BLA – basolateral amygdala; ITC –intercalated cell group.
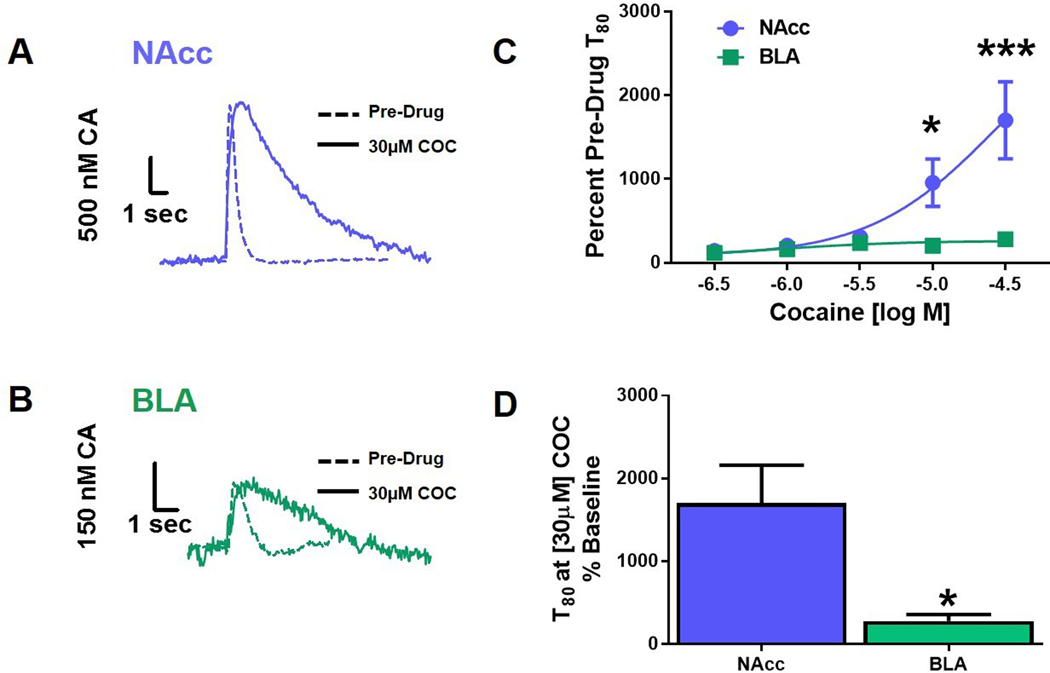
Inhibition of catecholamine uptake by cocaine is greater in the NAcc than the BLA.
In order to assess regional differences in the DAT-mediated catecholamine uptake, we examined the effects of cocaine, a DAT inhibitor, on catecholamine clearance in the NAcc and BLA (Figure 6, N=5 per group). Cocaine inhibits catecholamine uptake through DAT and other monoamine transporters56,50,57,58, an effect that is measured as an increase in T80. Figure 6 depicts representative traces collected from NAcc (Figure 6A) and BLA (Figure 6B) slices before and after stimulation under control conditions and following incubation with 30μM cocaine. Two-way repeated measures ANOVA comparing the effects of a cumulative cocaine concentration response curve on inhibition of dopamine uptake in the NAcc and BLA revealed a main effect of region (F1,33=18.90, p<0.0001; Figure 6C) and drug concentration (F4,32=8.246, p<0.0001; Figure 6C) on T80 over the cocaine concentration response curve. Additionally, an interaction between region and drug dose (F5,35=6.835, p<0.0008; Figure 6C) was detected. Bonferroni post-hoc analysis demonstrated a significant difference in uptake inhibition between regions at the 10μM (p<0.05) and 30μM dose (p<0.001). Comparison of T80 at the highest cocaine concentration as a percentage of pre-drug T80 in the NAcc and BLA using a Student’s t-test revealed a significantly greater potency of cocaine in the NAcc compared to the BLA (t5=2.581, p<0.0494; Figure 6D). Of note is a lack of increased release amplitude in the presence of cocaine, which may seem counterintuitive. However, this is consistent with previous work in our laboratory that demonstrates biphasic effects of dopamine amplitude with increasing concentrations of cocaine. Augmentation of signal amplitude with cocaine administration peaks at approximately the 1–3 μM range; however, we have observed consistently that at greater concentrations, the peak amplitude begins to decline56, 59. At a concentration of 30 μM, cocaine application no longer induces an increase in signal amplitude due primarily to activation of presynaptic autoreceptors60.
Figure 6: Inhibition of catecholamine uptake by cocaine is greater in the NAcc compared to the BLA.
(A) Representative traces of evoked catecholamine release and uptake in brain slices containing the nucleus accumbens (NAcc) at baseline (Pre-Drug) and 30μM cocaine (COC), overlaid. (B) Represented traces from basolateral amygdala (BLA)-containing brain slices at baseline and 30μM COC overlaid. (C) Cocaine (10μM and 30μM) significantly impaired catecholamine clearance in the NAcc compared to the BLA, (p<0.05, and p<0.001, respectively). (D) An increase in NAcc T80 revealed a significantly greater potency of COC in the NAcc, compared to the BLA (p<0.05). NAcc = nucleus accumbens core; BLA = basolateral amygdala; CA = catecholamine; COC = cocaine; *p<0.05; ***p<0.001. N=5 animals/group
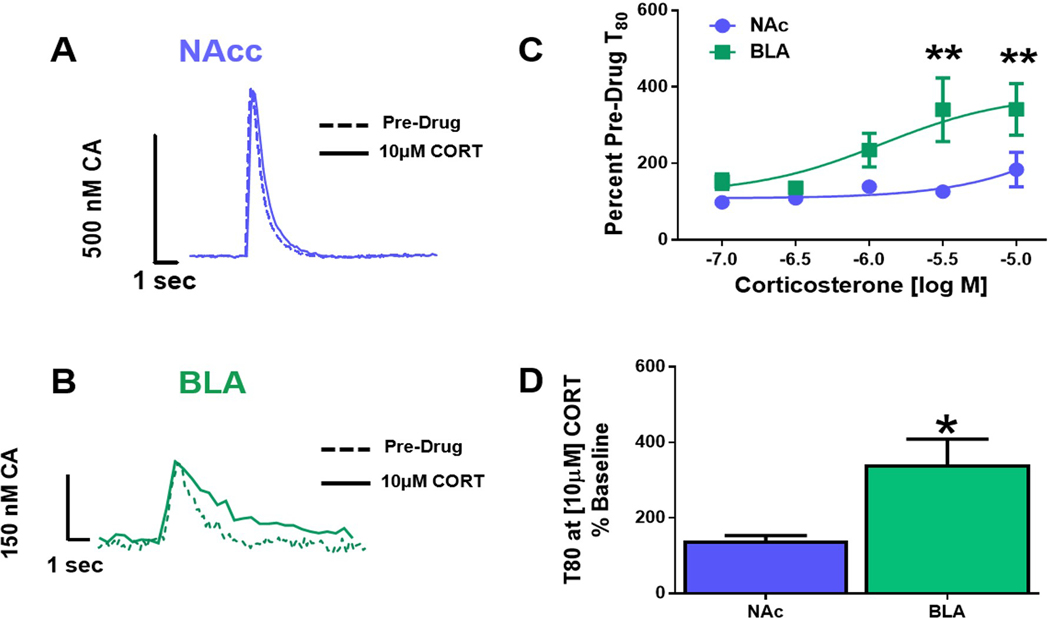
Inhibition of catecholamine uptake by corticosterone is greater in the BLA than the NAcc
To determine the relative contribution of OCT3 to catecholamine clearance in the NAcc and BLA, we examined the effects of CORT, which inhibits OCT3-mediated transport, on catecholamine uptake in the two regions. Figure 7 depicts representative traces collected from the NAcc (Figure 7A) and BLA (Figure 7B) before and after stimulation, under control conditions and following treatment with 10 μM CORT. Two-way repeated measures ANOVA revealed a dose-dependent increase in catecholamine clearance time, T80, in the BLA across the concentration response curve (F4,42= 3.929, p<0.0085; Figure 7C). Bonferroni post hoc analysis revealed a significant difference in T80 between regions at the 3.0μM (p<0.01) and 10μM (p<0.01) concentrations. Student’s t-test of group data at the highest CORT concentration tested (10μM) further underscores the potency CORT to inhibit uptake in the BLA (t8=2.368, p<0.0454; Figure 7D).
Figure 7: Corticosterone inhibits uptake more in the BLA than the NAcc.
(A) Representative traces from the nucleus accumbens (NAcc) at baseline and 10μM corticosterone (CORT) overlaid. (B) Representative basolateral amygdala (BLA) traces at baseline and 10μM CORT overlaid. (C) CORT (3μM and 10μM) significantly inhibited catecholamine uptake in the BLA compared to the NAcc, (p<0.01, and p<0.001, respectively). (D) CORT (10μM) significantly increased T80 in the BLA compared to the NAcc (p<0.05). NAcc = nucleus accumbens core; BLA = basolateral amygdala; CA = catecholamine; CORT = Corticosterone; *p<0.05; **p<0.01. N=5–8 animals/group
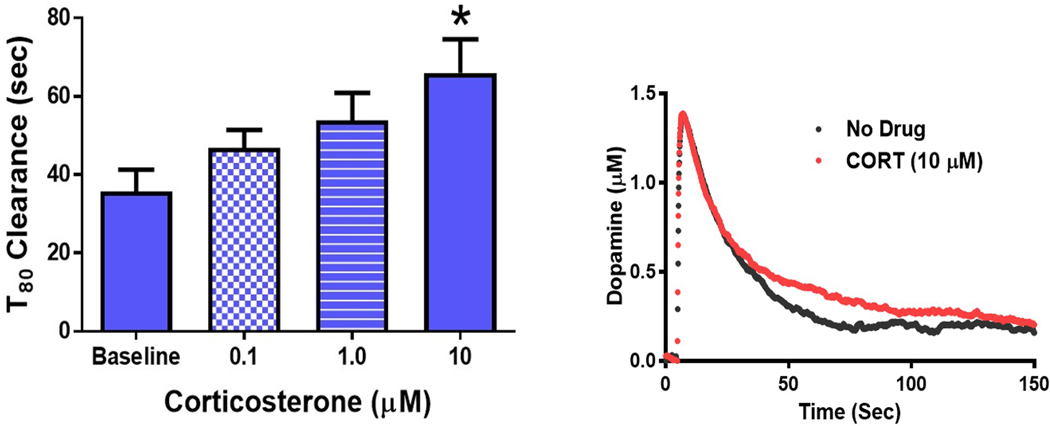
Corticosterone increases dopamine clearance time in the NAcc of DAT KO mice
CORT treatment had a much smaller effect on inhibiting catecholamine uptake in the NAcc than in the BLA. We believed this was likely because the high levels of DAT expression in the NAcc mask the effects of CORT-induced inhibition of OCT3. To examine the contribution of OCT3 function in the NAcc in the absence of DAT, we examined the effects of CORT on catecholamine clearance in NAcc slices obtained from DAT-KO mice. A one-way ANOVA revealed that CORT treatment increased catecholamine clearance time over the concentration response curve (F4,23=3.676; Figure 8) Tukey’s post-hoc analysis revealed a significant increase in T80 at the 10μM concentration versus baseline (p<0.05).
Figure 8: Corticosterone increases dopamine clearance time in the NAcc of DAT KO mice.
Corticosterone (CORT) dose dependently decreased catecholamine clearance in DAT-KO mice, indicated by an increase in T80 time (p<0.05). DAT KO – dopamine transporter knock out mice; *p<0.05. N=6 animals.
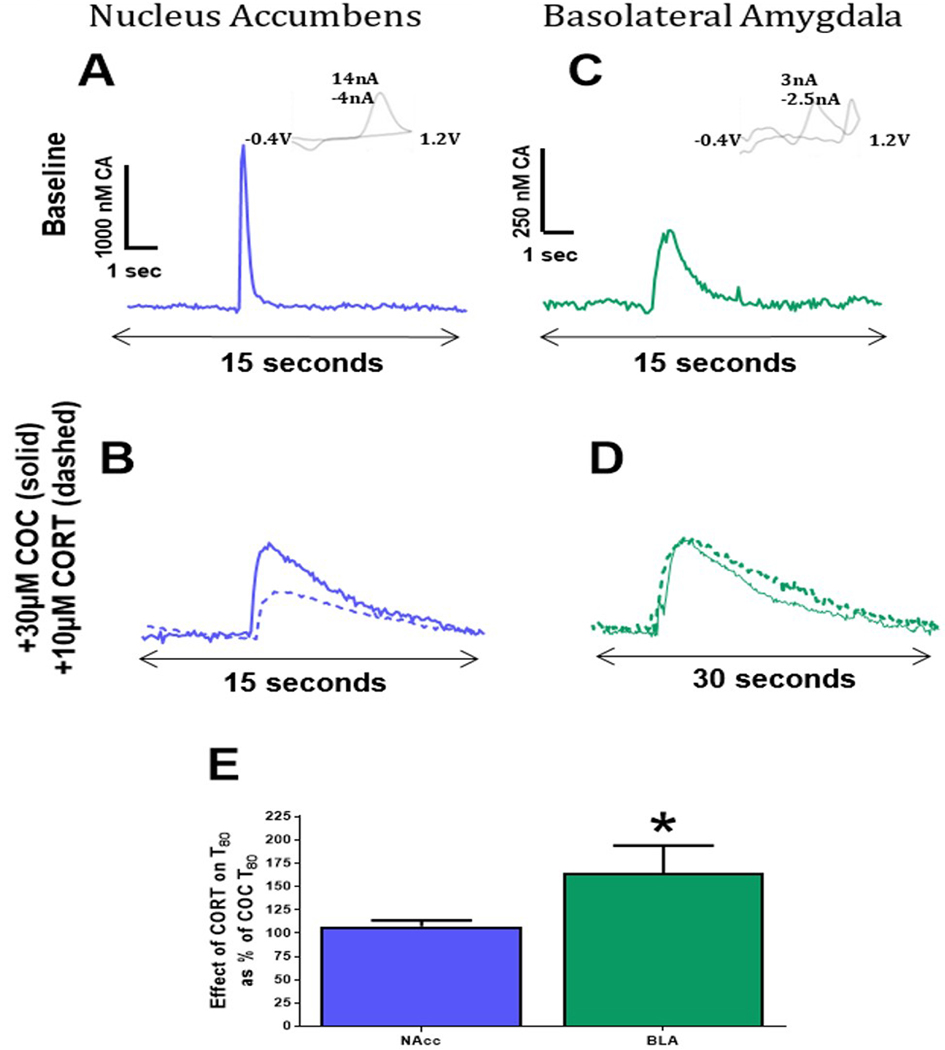
Catecholamine clearance in the NAcc is governed by DATs while clearance in the BLA involves OCT3 activity.
To better compare the relative contribution of DAT and OCT3 to catecholamine uptake in the NAcc and BLA, catecholamine uptake was measured twice in NAcc and BLA slices: first after incubation with the maximum concentration of cocaine studied here (30μM), and second after the addition of the maximum concentration of CORT tested here (10μM), in the presence of cocaine. Representative NAcc and BLA traces can be seen at baseline, following incubation with 30μM cocaine, and after incubation with cocaine + 10 μM CORT (Figure 9A-D). The effect of CORT on clearance was expressed as the CORT-induced increase in T80 as a percentage of the cocaine-induced T80. A Student’s T-test revealed that magnitude of the CORT-induced increase in T80 as a percent of cocaine-induced changes in T80, was greater in the BLA than in the NAcc (t15=2.268, p<0.0386; Figure 9E). Of note, it can be seen that release amplitude is reduced in the NAcc with the combination of cocaine and CORT, but this effect is not present in the BLA.
Figure 9: Catecholamine clearance in the NAcc is governed by DAT function while catecholamine clearance in the BLA favors OCT3 activity.
(A) Representative traces of the nucleus accumbens (NAcc) at baseline, (B) after incubation with 30μM cocaine (COC; solid line) and after the addition of 10μM corticosterone (CORT; dashed line). (C) Traces from BLA slices at baseline, (D) after incubation with 30μM COC (solid line), and after the addition of 10μM CORT (dashed line). (E) Increases in T80 due to CORT application as a percent of post-COC clearance time revealed a greater effect of CORT on uptake inhibition in the BLA compared to the NAcc. NAcc- nucleus accumbens; BLA – basolateral amygdala; COC - cocaine (COC) CORT - corticosterone CORT; *p<0.05. N=7–10 animals/group.
Discussion
Dopaminergic signaling in the NAcc and BLA contribute to the regulation of affect19,61,6,7 and motivated behaviors 8,12,13,9. The magnitude of catecholamine effects in a given region can be regulated by alterations in release magnitude and uptake kinetics. Previous work50 and the present study describe reduced catecholamine release and slower uptake in the BLA compared to the NAcc. The present work aimed to expand our understanding of uptake mechanisms in these regions by examining the expression and activity of two catecholamine transporters that mediate clearance of extracellular catecholamines in the NAcc and BLA. The present studies demonstrated that the expression of DAT protein is greater in the NAcc than in the BLA, and that OCT3 protein expression is similar between regions. Consistent with the transporter expression data, ex vivo voltammetry data demonstrated that the DAT inhibitor cocaine inhibits catecholamine uptake to a greater extent in the NAcc than in the BLA, while the OCT inhibitor CORT exerted a greater inhibitory effect in the BLA than in the NAcc. We suspect that differences in uptake rates are due to regional differences in the density of transporter expression. The distinct uptake mechanisms in the NAcc and BLA may contribute to differential regulation of catecholamine neurotransmission within these regions.
The magnitude of catecholamine release is significantly lower in the BLA than in the NAcc
While the present work mainly focused on differences in catecholamine uptake in the NAcc and the BLA, presynaptic mechanisms influencing catecholamine release also play a major role in shaping catecholamine signaling. The present findings using fast scan cyclic voltammetry corroborate previous work demonstrating that stimulated catecholamine release is significantly greater in the NAcc than in the BLA50. While we did not further explore release mechanisms here, we and others have previously examined many potential modulators of catecholamine release that may differ between the NAcc and the BLA. Previous studies have shown that dopamine D2 and D3 autoreceptors62 and ⍺2-adrenergic autoreceptors reduce catecholamine release63 in subnuclei of the NAcc64,62 and BLA65,5,66,67. It is possible that differences in dopaminergic and noradrenergic autoreceptor expression between the NAcc and BLA may contribute to differential dopamine release in these two regions. Additionally, we have shown that kappa opioid receptor-mediated inhibition of dopamine release in the NAcc is associated with negative affect-like behaviors in rodents16,68. Kappa opioid receptors are densely expressed in both the NAcc and the BLA, where their activation leads to reductions in presynaptic release of neurotransmitters, including dopamine, and differential receptor function or expression could also influence release kinetics from these regions69,70,71. Future work aimed at differentiating release mechanisms between these regions will further aid in refining therapeutic treatment strategies for disorders of negative affect and substance use disorders.
Cocaine and corticosterone were more potent at inhibiting uptake rates in the NAcc and BLA, respectively
Catecholamine clearance is a complex, regionally variable process. Our Western blot and immunofluorescence findings reveal that while OCT3 is expressed at similar levels in NAcc and BLA, DAT expression is significantly greater in the NAcc than in the BLA. These data are consistent with previous studies demonstrating abundant DAT protein in the NAcc65,66, and OCT3 more uniformly distributed throughout the brain 72.
Transporter protein density is not the sole determinant of overall transporter function. Although Western blot data here show higher levels of DAT in the NAcc compared to the BLA, differences in membrane localization and transport modulators, among other factors, can contribute to differences in transporter function between regions73,74. Studies examining the relative subcellular localizations of DAT and OCT3 and their proximity to dopamine receptors would be particularly informative. While colocalization of these transporters has not been examined37, ultrastructural studies have demonstrated that OCT3 is localized to dendritic spines and presynaptic terminals in the BLA 38, suggesting important roles for the transporter in regulating perisynaptic monoamine concentrations. OCT3 expression has also been observed on DAT+ neurons75, suggesting possible co-expression at dopamine terminals. Interestingly, OCT3 also appears to be present on striatal cholinergic neurons76, which strongly regulate terminal dynamics in the nucleus accumbens77,78,79. Therefore, it is possible that there may be additional effects of OCT3 activity on cholinergic neurons, which may alter catecholamine clearance kinetics. While localization studies will certainly be necessary for future delineation of the mechanisms of OCT3 function, we chose to focus on catecholamine clearance as a functional output of DAT and OCT3 activity.
With these studies in mind, we evaluated the functional importance of the DAT and OCT3 in the NAcc and BLA with slice voltammetry using cocaine and CORT to inhibit DAT and OCT3 function, respectively. The concentrations of both cocaine and corticosterone utilized in this study are admittedly somewhat high compared to physiological levels in the presence of systemic cocaine or stress exposure, although they are difficult to directly compare. For instance, repeated administration of 1mg/kg cocaine over 10 injections in anesthetized rats does begin to show the characteristic decline in maximal dopamine amplitude that we observe (and have commented on here) with higher concentrations of cocaine on slice80; however, this effect is not accompanied by uptake inhibition that is as dramatic as is observed with higher concentrations of cocaine on slice. Similarly, corticosterone concentrations used here are around 1–2 orders of magnitude higher than brain levels of corticosterone following administration of stressors in vivo81,82,83, though the concentration response curve for corticosterone in Figure 8 does more closely approximate this range. Here we have chosen somewhat higher concentrations that we and others have published previously in ex vivo studies in order to more clearly observe effects on reuptake56,84.
Cocaine inhibited uptake more in the NAcc, which expresses higher levels of DAT protein, than in the BLA. The fact that CORT was more effective at reducing catecholamine uptake in the BLA than NAcc, despite our evidence that OCT3 protein levels were similar in the two regions, is likely due to the abundant expression of DAT in the NAcc. Thus, the effects of OCT3 inhibition are likely obscured by continued abundant DAT activity in the presence of CORT.
This interpretation was supported by our data examining CORT effects on catecholamine clearance in DAT KO mice, in which CORT is better able to inhibit catecholamine clearance. It is likely that CORT inhibits catecholamine uptake in the NAcc of wild-type animals, but that the effect is not detectable due to the high levels of DAT activity. It is important to note that, as with any constitutive knockout approach, some of the effects observed in the DAT KO mouse line may be mitigated at least in part by compensatory mechanisms that could be driving altered OCT3 function in these animals. However, consistent with our findings, a recent study reported that CORT treatment decreased the clearance of naturally occurring dopamine transients in the NAcc even in the absence of DAT blockade 85. These studies also demonstrated that CORT pre-treatment potentiates the effect of a previously sub-threshold dose of cocaine on NAcc dopamine clearance.
Though the findings outlined here appear at the surface to report relatively straight-forward phenomena, it should be noted that there are many factors modulating uptake kinetics, and should not be viewed as overly simplistic. As noted above, the cyclic voltammograms for dopamine and norepinephrine are virtually identical, making it difficult to identify which neurotransmitter kinetics are being affected by a given treatment through voltammetry assessment alone. This is particularly problematic in the BLA, where dopamine and norepinephrine are estimated to be released at similar concentrations. Blockade of either CA either pharmacologically or using optogenetic stimulation approaches is particularly troublesome in the BLA, a region with limited stimulated catecholamine release. Though one possible solution could involve application of exogenous neurotransmitter, this approach is troublesome in that temporal kinetics observed with electrical stimulation are difficult to faithfully replicate in a slice preparation.
Other monoamine transporters in addition to DAT and OCT3 are also likely to contribute to CA clearance, especially in the BLA. The norepinephrine transporter is capable of clearing catecholamines at equal or greater efficacy than the DAT, and is expressed at similar levels between the accumbens and BLA in humans86.In rodents, NET plays a far larger role in catecholamine reuptake in the nucleus accumbens shell, where greater norepinephrine innervation is observed, than in the NAc core, which is primarily dopaminergic87. However, in regions with high norepinephrine innervation, such as the BLA, NET consistently plays a critical role in clearance of both norepinephrine and dopamine87. Though beyond the scope of this study, it would be interesting to ascertain the role of NET in concert with DAT and OCT3 in differential uptake kinetics, particularly within the BLA. Additionally, other low-affinity, high capacity transporters with Uptake2-like function - OCT1, OCT2, and PMAT - may contribute to CA clearance. These transporters also function to clear monoamines from the extracellular fluid and are inhibited to CORT. Among the Uptake2transporters, OCT3 has the highest sensitivity to CORT37, and is expressed at greater density in the NAcc and BLA than other OCT isoforms88. PMAT is expressed in relatively similar levels between the NAcc and BLA89 and has greater affinity for dopamine than norepinephrine28. Future studies examining the role of this transporter in differential uptake kinetics between the NAcc and BLA will shed further light on Uptake2 transporters in monoamine signaling, particularly with regards to dopaminergic signaling. Interestingly, it has been recently demonstrated that CORT, via inhibition of OCT3, potentiates the effect of a low systemic dose of cocaine on dopamine clearance in the NAc85. This work both differs from and compliments our own in some regards: while this study was performed in awake and behaving animals with a low dose of cocaine, we also observed further inhibition of uptake with a high concentration of cocaine over a deafferented slice when CORT was applied in the bath. It is important to note that OCT3 is canonically cocaine-insensitive at concentrations consistent with our data (Gasser and Lowry, 2018). The ability of CORT to augment cocaine’s effects of uptake inhibition resonates in behavioral studies outlined below.
Of note, we do find that CORT applied following cocaine decreases release amplitude in the NAcc, but not the BLA. While the reason for this difference is not completely clear, it is known that this combination of drugs enhances spontaneous dopamine release transients as well as reducing uptake 39,85, we hypothesize that elevated extracellular levels of dopamine in the slice tonically activate presynaptic D2-type autoreceptors, which then inhibit electrically-evoked release in the NAcc60. The BLA may not exhibit this effect because dopamine terminals in the BLA have fewer presynaptc autoreceptors and are much less sensitive to autoreceptor-mediated inhibition of release than in the NAcc 90. These aspects of NE regulation in the BLA are unknown and require further exploration.
The behavioral implications of elevated corticosterone in the NAcc and BLA
OCT3 is directly and specifically inhibited by CORT, a hormone released in rodents during times of stress34,26. Indeed, augmented levels of CORT have been measured in the NAcc91 and BLA92, following exposure to a stressor. Our findings support previous work that demonstrated CORT-induced inhibition of dopamine clearance in the NAcc in the presence of the DAT inhibitor GBR12909 39. These data specifically implicate OCT3 in increased extracellular catecholamine levels during times of stress. Further, through OCT3, CORT is able to augment the ability of a low dosage of systemic cocaine to inhibit uptake of dopamine in the NAcc of an awake and behaving rat85. A growing body of literature suggests that augmented CORT, via inhibition of OCT3-mediated dopamine clearance, potentiates cocaine-induced reinstatement of drug-seeking behavior39,93. It is possible that increased CORT, and subsequent reduction in catecholamine uptake in the NAcc and BLA, drive complementary aspects of drug abuse-related behaviors.
We hypothesize that in individuals with a substance use disorder, stress-induced increases in glucocorticoids potentiate the effects of sub-threshold stimuli on catecholamine concentrations in the NAcc and BLA, thus “priming” the subject to seek substances of abuse. For example, previous work has shown that increased catecholamine signaling in the BLA is anxiogenic19,20, and CORT-mediated increases in dopamine transmission may underlie stress-induced increases in the motivation for drug self-administration, as discussed above39. In fact, humans94,95,96 and rodents 97,98 exposed to stressors have augmented drug seeking behaviors. Moreover, during long periods of abstinence characterized by augmented CORT levels, rats with a history of cocaine self-administration exhibited increased drug seeking99. Despite strong evidence supporting our hypothesis, additional in vivo work examining the specific contribution of NAcc and BLA OCT3 blockade to stress-induced drug seeking is necessary.
Conclusions
We explored the expression of DAT and OCT3, and their contribution to catecholamine clearance, in the NAcc and BLA. Our findings demonstrate the existence of distinct mechanisms of catecholamine release and clearance in the NAcc and BLA. Specifically, we report that OCT3 blockade significantly reduces catecholamine uptake in the BLA compared to the NAcc, while cocaine is more effective at reducing uptake in the NAcc. We suspect that OCT3 removes excess synaptic catecholamines in the NAcc when transporters are saturated due to high release volumes, or when transporters are pharmacologically inhibited, for example by psychostimulant drugs of abuse. This putative DAT-OCT3 mediated process allows for the relative maintenance of homeostasis within the synapse under normal conditions. However, in times of stress, blockade of OCT3 is hypothesized to augment catecholamine concentrations in the BLA and NAcc and prime the subject for drug-seeking behavior39,93.
Acknowledgements:
Joanne Konstantopolous for her technical expertise.
Abbreviations:
- ACSF
artificial cerebrospinal fluid
- BLA
basolateral amygdala
- CA
catecholamine
- CORT
corticosterone
- DAT
dopamine transporter
- DAT KO
dopamine transporter knockout mice
- NET
norepinephrine transporter
- NAcc
nucleus accumbens core
- OCT3
organic cation transporter 3
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PMAT
plasma membrane monoamine transporter
Footnotes
Competing Interests: The authors declare no conflicts of interest.
Data Accessibility: Data presented here can be accessed online at the following site: https://osf.io/xfqr2/?view_only=33ecfd5e9636443891a450a964d52575
References
- 1.(a) Fuxe K; Borroto-Escuela DO; Romero-Fernandez W; Zhang WB; Agnati LF, Volume transmission and its different forms in the central nervous system. Chin J Integr Med 2013, 19 (5), 323–9;. [DOI] [PubMed] [Google Scholar]; (b) Perez de la Mora M; Jacobsen KX; Crespo-Ramirez M; Flores-Gracia C; Fuxe K, Wiring and volume transmission in rat amygdala. Implications for fear and anxiety. Neurochem Res 2008, 33 (8), 1618–33 [DOI] [PubMed] [Google Scholar]
- 2.Touzani K; Bodnar R; Sclafani A, Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci 2008, 27 (6), 1525–33. [DOI] [PubMed] [Google Scholar]
- 3.Churchwell JC; Morris AM; Heurtelou NM; Kesner RP, Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci 2009, 123 (6), 1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y; Zuo Y; Yu P; Ping X; Cui C, Role of basolateral amygdala dopamine D2 receptors in impulsive choice in acute cocaine-treated rats. Behav Brain Res 2015, 287, 187–95. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson CW; Gratton A, Basolateral amygdala dopamine receptor antagonism modulates initial reactivity to but not habituation of the acoustic startle response. Behav Brain Res 2004, 153 (2), 383–7. [DOI] [PubMed] [Google Scholar]
- 6.Klumpers F; Morgan B; Terburg D; Stein DJ; van Honk J, Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Soc Cogn Affect Neurosci 2015, 10 (9), 1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li SS; McNally GP, A role of nucleus accumbens dopamine receptors in the nucleus accumbens core, but not shell, in fear prediction error. Behav Neurosci 2015, 129 (4), 450–6. [DOI] [PubMed] [Google Scholar]
- 8.Simmons DA; Neill DB, Functional interaction between the basolateral amygdala and the nucleus accumbens underlies incentive motivation for food reward on a fixed ratio schedule. Neuroscience 2009, 159 (4), 1264–73. [DOI] [PubMed] [Google Scholar]
- 9.Shiflett MW; Balleine BW, At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci 2010, 32 (10), 1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballester Gonzalez J; Dvorkin-Gheva A; Silva C; Foster JA; Szechtman H, Nucleus accumbens core and pathogenesis of compulsive checking. Behav Pharmacol 2015, 26 (1–2), 200–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baik JH, Dopamine signaling in reward-related behaviors. Front Neural Circuits 2013, 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T; Sato A; Kitsukawa T; Momiyama T; Yamamori T; Sasaoka T, Distinct motor impairments of dopamine D1 and D2 receptor knockout mice revealed by three types of motor behavior. Front Integr Neurosci 2014, 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fish EW; DiBerto JF; Krouse MC; Robinson JE; Malanga CJ, Different contributions of dopamine D1 and D2 receptor activity to alcohol potentiation of brain stimulation reward in C57BL/6J and DBA/2J mice. J Pharmacol Exp Ther 2014, 350 (2), 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markou A; Koob GF, Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology 1992, 7 (3), 213–24. [PubMed] [Google Scholar]
- 15.Schulteis G; Markou A; Cole M; Koob GF, Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A 1995, 92 (13), 5880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose JH; Karkhanis AN; Chen R; Gioia D; Lopez MF; Becker HC; McCool BA; Jones SR, Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol 2016, 19 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siciliano CA; Calipari ES; Ferris MJ; Jones SR, Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci 2015, 6 (1), 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siciliano CA; Calipari ES; Yorgason JT; Lovinger DM; Mateo Y; Jimenez VA; Helms CM; Grant KA; Jones SR, Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology (Berl) 2016, 233 (8), 1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz MR; Chappell AM; Christian DT; Anderson NJ; McCool BA, Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology 2011, 36 (5), 1090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bananej M; Karimi-Sori A; Zarrindast MR; Ahmadi S, D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J Psychopharmacol 2012, 26 (4), 564–74. [DOI] [PubMed] [Google Scholar]
- 21.Karkhanis AN; Rose JH; Huggins KN; Konstantopoulos JK; Jones SR, Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend 2015, 150, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gainetdinov RR; Jones SR; Fumagalli F; Wightman RM; Caron MG, Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev 1998, 26 (2–3), 148–53. [DOI] [PubMed] [Google Scholar]
- 23.Gainetdinov RR; Jones SR; Caron MG, Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry 1999, 46 (3), 303–11. [DOI] [PubMed] [Google Scholar]
- 24.Budygin EA; John CE; Mateo Y; Jones SR, Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J Neurosci 2002, 22 (10), RC222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lips KS; Volk C; Schmitt BM; Pfeil U; Arndt P; Miska D; Ermert L; Kummer W; Koepsell H, Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol 2005, 33 (1), 79–88. [DOI] [PubMed] [Google Scholar]
- 26.Baganz N; Horton R; Martin K; Holmes A; Daws LC, Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci 2010, 30 (45), 15185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasser PJ; Lowry CA; Orchinik M, Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci 2006, 26 (34), 8758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan H; Wang J, Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 2010, 335 (3), 743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill JE; Gasser PJ, Organic cation transporter 3 is densely expressed in the intercalated cell groups of the amygdala: anatomical evidence for a stress hormone-sensitive dopamine clearance system. J Chem Neuroanat 2013, 52, 36–43. [DOI] [PubMed] [Google Scholar]
- 30.Akil M; Kolachana BS; Rothmond DA; Hyde TM; Weinberger DR; Kleinman JE, Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci 2003, 23 (6), 2008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napolitano A; Bellini G; Borroni E; Zurcher G; Bonuccelli U, Effects of peripheral and central catechol-O-methyltransferase inhibition on striatal extracellular levels of dopamine: a microdialysis study in freely moving rats. Parkinsonism Relat Disord 2003, 9 (3), 145–50. [DOI] [PubMed] [Google Scholar]
- 32.Kehr J; Hoistad M; Fuxe K, Diffusion of radiolabeled dopamine, its metabolites and mannitol in the rat striatum studied by dual-probe microdialysis. Prog Brain Res 2000, 125, 179–90. [DOI] [PubMed] [Google Scholar]
- 33.Trouillon R; Lin Y; Mellander LJ; Keighron JD; Ewing AG, Evaluating the diffusion coefficient of dopamine at the cell surface during amperometric detection: disk vs ring microelectrodes. Anal Chem 2013, 85 (13), 6421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wieland A; Hayer-Zillgen M; Bonisch H; Bruss M, Analysis of the gene structure of the human (SLC22A3) and murine (Slc22a3) extraneuronal monoamine transporter. J Neural Transm (Vienna) 2000, 107 (10), 1149–57. [DOI] [PubMed] [Google Scholar]
- 35.Iversen LL; Salt PJ, Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol 1970, 40 (3), 528–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lightman SL; Iversen LL, The role of uptake2 in the extraneuronal metabolism of catecholamines in the isolated rat heart. Br J Pharmacol 1969, 37 (3), 638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasser PJ, Roles for the uptake2 transporter OCT3 in regulation of dopaminergic neurotransmission and behavior. Neurochem Int 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gasser PJ; Hurley MM; Chan J; Pickel VM, Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Struct Funct 2017, 222 (4), 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graf EN; Wheeler RA; Baker DA; Ebben AL; Hill JE; McReynolds JR; Robble MA; Vranjkovic O; Wheeler DS; Mantsch JR; Gasser PJ, Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 2013, 33 (29), 11800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt A; Mossner R; Gossmann A; Fischer IG; Gorboulev V; Murphy DL; Koepsell H; Lesch KP, Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res 2003, 71 (5), 701–9. [DOI] [PubMed] [Google Scholar]
- 41.Zhu HJ; Appel DI; Grundemann D; Markowitz JS, Interaction of organic cation transporter 3 (SLC22A3) and amphetamine. J Neurochem 2010, 114 (1), 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hek K; Direk N; Newson RS; Hofman A; Hoogendijk WJ; Mulder CL; Tiemeier H, Anxiety disorders and salivary cortisol levels in older adults: a population-based study. Psychoneuroendocrinology 2013, 38 (2), 300–5. [DOI] [PubMed] [Google Scholar]
- 43.Franco AJ; Chen C; Scullen T; Zsombok A; Salahudeen AA; Di S; Herman JP; Tasker JG, Sensitization of the Hypothalamic-Pituitary-Adrenal Axis in a Male Rat Chronic Stress Model. Endocrinology 2016, 157 (6), 2346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung C; Greco S; Nguyen HH; Ho JT; Lewis JG; Torpy DJ; Inder WJ, Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr Disord 2014, 14, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth N; Thorn L; Oskis A; Hucklebridge F; Evans P; Clow A, Anxious attachment style predicts an enhanced cortisol response to group psychosocial stress. Stress 2015, 18 (2), 143–8. [DOI] [PubMed] [Google Scholar]
- 46.Ottenweller JE; Natelson BH; Pitman DL; Drastal SD, Adrenocortical and behavioral responses to repeated stressors: toward an animal model of chronic stress and stress-related mental illness. Biol Psychiatry 1989, 26 (8), 829–41. [DOI] [PubMed] [Google Scholar]
- 47.Jones AB; Gupton R; Curtis KS, Estrogen and voluntary exercise interact to attenuate stress-induced corticosterone release but not anxiety-like behaviors in female rats. Behav Brain Res 2016, 311, 279–286. [DOI] [PubMed] [Google Scholar]
- 48.Grundemann D; Schechinger B; Rappold GA; Schomig E, Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci 1998, 1 (5), 349–51. [DOI] [PubMed] [Google Scholar]
- 49.Ayala-Lopez N; Jackson WF; Burnett R; Wilson JN; Thompson JM; Watts SW, Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol 2015, 309 (11), H1904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones SR; Garris PA; Kilts CD; Wightman RM, Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J Neurochem 1995, 64 (6), 2581–9. [DOI] [PubMed] [Google Scholar]
- 51.Heien ML; Phillips PE; Stuber GD; Seipel AT; Wightman RM, Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 2003, 128 (12), 1413–9. [DOI] [PubMed] [Google Scholar]
- 52.John CE; Jones SR, Fast Scan Cyclic Voltammetry of Dopamine and Serotonin in Mouse Brain Slices In Electrochemical Methods for Neuroscience, Michael AC; Borland LM, Eds. Boca Raton (FL), 2007. [PubMed] [Google Scholar]
- 53.Zhang J; Muller JF; McDonald AJ, Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience 2013, 228, 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinley-Reed M; McDonald AJ, Evidence that dopaminergic axons provide a dense innervation of specific neuronal subpopulations in the rat basolateral amygdala. Brain Res 1999, 850 (1–2), 127–35. [DOI] [PubMed] [Google Scholar]
- 55.Yetnikoff L; Lavezzi HN; Reichard RA; Zahm DS, An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience 2014, 282, 23–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yorgason JT; Espana RA; Jones SR, Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 2011, 202 (2), 158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calligaro DO; Eldefrawi ME, High affinity stereospecific binding of [3H] cocaine in striatum and its relationship to the dopamine transporter. Membr Biochem 1987, 7 (2), 87–106. [DOI] [PubMed] [Google Scholar]
- 58.Uhl GR; Hall FS; Sora I, Cocaine, reward, movement and monoamine transporters. Mol Psychiatry 2002, 7 (1), 21–6. [DOI] [PubMed] [Google Scholar]
- 59.John CE; Jones SR, Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology 2007, 52 (8), 1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wieczorek WJ; Kruk ZL, A quantitative comparison on the effects of benztropine, cocaine and nomifensine on electrically evoked dopamine overflow and rate of re-uptake in the caudate putamen and nucleus accumbens in the rat brain slice. Brain Res 1994, 657 (1–2), 42–50. [DOI] [PubMed] [Google Scholar]
- 61.Chartoff EH; Carlezon WA Jr., Drug withdrawal conceptualized as a stressor. Behav Pharmacol 2014, 25 (5–6), 473–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maina FK; Mathews TA, A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem Neurosci 2010, 1 (6), 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia AS; Barrera G; Burke TF; Ma S; Hensler JG; Morilak DA, Autoreceptor-mediated inhibition of norepinephrine release in rat medial prefrontal cortex is maintained after chronic desipramine treatment. J Neurochem 2004, 91 (3), 683–93. [DOI] [PubMed] [Google Scholar]
- 64.Ihalainen JA; Tanila H, In vivo regulation of dopamine and noradrenaline release by alpha2A-adrenoceptors in the mouse nucleus accumbens. J Neurochem 2004, 91 (1), 49–56. [DOI] [PubMed] [Google Scholar]
- 65.Gurevich EV; Joyce JN, Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 1999, 20 (1), 60–80. [DOI] [PubMed] [Google Scholar]
- 66.Perez de la Mora M; Gallegos-Cari A; Crespo-Ramirez M; Marcellino D; Hansson AC; Fuxe K, Distribution of dopamine D(2)-like receptors in the rat amygdala and their role in the modulation of unconditioned fear and anxiety. Neuroscience 2012, 201, 252–66. [DOI] [PubMed] [Google Scholar]
- 67.Ferry B; Parrot S; Marien M; Lazarus C; Cassel JC; McGaugh JL, Noradrenergic influences in the basolateral amygdala on inhibitory avoidance memory are mediated by an action on alpha2-adrenoceptors. Psychoneuroendocrinology 2015, 51, 68–79. [DOI] [PubMed] [Google Scholar]
- 68.Karkhanis AN; Rose JH; Weiner JL; Jones SR, Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 2016, 41 (9), 2263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Svingos AL; Chavkin C; Colago EE; Pickel VM, Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 2001, 42 (3), 185–92. [DOI] [PubMed] [Google Scholar]
- 70.Thompson AC; Zapata A; Justice JB Jr.; Vaughan RA; Sharpe LG; Shippenberg TS, Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci 2000, 20 (24), 9333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Werling LL; Frattali A; Portoghese PS; Takemori AE; Cox BM, Kappa receptor regulation of dopamine release from striatum and cortex of rats and guinea pigs. J Pharmacol Exp Ther 1988, 246 (1), 282–6. [PubMed] [Google Scholar]
- 72.Gasser PJ; Orchinik M; Raju I; Lowry CA, Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol 2009, 512 (4), 529–55. [DOI] [PubMed] [Google Scholar]
- 73.Kivell B; Uzelac Z; Sundaramurthy S; Rajamanickam J; Ewald A; Chefer V; Jaligam V; Bolan E; Simonson B; Annamalai B; Mannangatti P; Prisinzano TE; Gomes I; Devi LA; Jayanthi LD; Sitte HH; Ramamoorthy S; Shippenberg TS, Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology 2014, 86, 228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao A; Sorkin A; Zahniser NR, Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness. Synapse 2013, 67 (10), 668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayer FP; Schmid D; Owens WA; Gould GG; Apuschkin M; Kudlacek O; Salzer I; Boehm S; Chiba P; Williams PH; Wu HH; Gether U; Koek W; Daws LC; Sitte HH, An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology 2018, 43 (12), 2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vialou V; Balasse L; Dumas S; Giros B; Gautron S, Neurochemical characterization of pathways expressing plasma membrane monoamine transporter in the rat brain. Neuroscience 2007, 144 (2), 616–22. [DOI] [PubMed] [Google Scholar]
- 77.Siciliano CA; McIntosh JM; Jones SR; Ferris MJ, alpha6beta2 subunit containing nicotinic acetylcholine receptors exert opposing actions on rapid dopamine signaling in the nucleus accumbens of rats with high-versus low-response to novelty. Neuropharmacology 2017, 126, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melchior JR; Ferris MJ; Stuber GD; Riddle DR; Jones SR, Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. J Neurochem 2015, 134 (5), 833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yorgason JT; Rose JH; McIntosh JM; Ferris MJ; Jones SR, Greater ethanol inhibition of presynaptic dopamine release in C57BL/6J than DBA/2J mice: Role of nicotinic acetylcholine receptors. Neuroscience 2015, 284, 854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brodnik ZD; Ferris MJ; Jones SR; Espana RA, Reinforcing Doses of Intravenous Cocaine Produce Only Modest Dopamine Uptake Inhibition. ACS Chem Neurosci 2017, 8 (2), 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makino S; Gold PW; Schulkin J, Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res 1994, 640 (1–2), 105–12. [DOI] [PubMed] [Google Scholar]
- 82.Pitman DL; Ottenweller JE; Natelson BH, Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav 1988, 43 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan RM; Gratton A, Relationships between stress-induced increases in medial prefrontal cortical dopamine and plasma corticosterone levels in rats: role of cerebral laterality. Neuroscience 1998, 83 (1), 81–91. [DOI] [PubMed] [Google Scholar]
- 84.Caliskan G; Schulz SB; Gruber D; Behr J; Heinemann U; Gerevich Z, Corticosterone and corticotropin-releasing factor acutely facilitate gamma oscillations in the hippocampus in vitro. Eur J Neurosci 2015, 41 (1), 31–44. [DOI] [PubMed] [Google Scholar]
- 85.Wheeler DS; Ebben AL; Kurtoglu B; Lovell ME; Bohn AT; Jasek IA; Baker DA; Mantsch JR; Gasser PJ; Wheeler RA, Corticosterone regulates both naturally occurring and cocaine-induced dopamine signaling by selectively decreasing dopamine uptake. Eur J Neurosci 2017, 46 (10), 2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith HR; Beveridge TJ; Porrino LJ, Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience 2006, 138 (2), 703–14. [DOI] [PubMed] [Google Scholar]
- 87.Carboni E; Silvagni A; Vacca C; Di Chiara G, Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem 2006, 96 (2), 473–81. [DOI] [PubMed] [Google Scholar]
- 88.Amphoux A; Vialou V; Drescher E; Bruss M; Mannoury La Cour C; Rochat C; Millan MJ; Giros B; Bonisch H; Gautron S, Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 2006, 50 (8), 941–52. [DOI] [PubMed] [Google Scholar]
- 89.Dahlin A; Xia L; Kong W; Hevner R; Wang J, Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience 2007, 146 (3), 1193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garris PA; Wightman RM, Distinct pharmacological regulation of evoked dopamine efflux in the amygdala and striatum of the rat in vivo. Synapse 1995, July;20(3):269–79. [DOI] [PubMed] [Google Scholar]
- 91.Yu P; An S; Tai F; Wang J; Wu R; Wang B, Early social deprivation impairs pair bonding and alters serum corticosterone and the NAcc dopamine system in mandarin voles. Psychoneuroendocrinology 2013, 38 (12), 3128–38. [DOI] [PubMed] [Google Scholar]
- 92.Bouchez G; Millan MJ; Rivet JM; Billiras R; Boulanger R; Gobert A, Quantification of extracellular levels of corticosterone in the basolateral amygdaloid complex of freely-moving rats: a dialysis study of circadian variation and stress-induced modulation. Brain Res 2012, 1452, 47–60. [DOI] [PubMed] [Google Scholar]
- 93.McReynolds JR; Taylor A; Vranjkovic O; Ambrosius T; Derricks O; Nino B; Kurtoglu B; Wheeler RA; Baker DA; Gasser PJ; Mantsch JR, Corticosterone Potentiation of Cocaine-Induced Reinstatement of Conditioned Place Preference in Mice is Mediated by Blockade of the Organic Cation Transporter 3. Neuropsychopharmacology 2017, 42 (3), 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Min M; Farkas K; Minnes S; Singer LT, Impact of childhood abuse and neglect on substance abuse and psychological distress in adulthood. J Trauma Stress 2007, 20 (5), 833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopez-Castro T; Hu MC; Papini S; Ruglass LM; Hien DA, Pathways to change: Use trajectories following trauma-informed treatment of women with co-occurring post-traumatic stress disorder and substance use disorders. Drug Alcohol Rev 2015, 34 (3), 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sullivan TP; Flanagan JC; Dudley DN; Holt LJ; Mazure CM; McKee SA, Correlates of smoking status among women experiencing intimate partner violence: Substance use, posttraumatic stress, and coping. Am J Addict 2015, 24 (6), 546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Funk D; Li Z; Le AD, Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience 2006, 138 (1), 235–43. [DOI] [PubMed] [Google Scholar]
- 98.Briand LA; Blendy JA, Not all stress is equal: CREB is not necessary for restraint stress reinstatement of cocaine-conditioned reward. Behav Brain Res 2013, 246, 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lack CM; Jones SR; Roberts DC, Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology (Berl) 2008, 195 (4), 517–25. [DOI] [PubMed] [Google Scholar]
- 100.Giros B; Jaber M; Jones SR; Wightman RM; Caron MG, Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379 (6566), 606–12. [DOI] [PubMed] [Google Scholar]
- 101.Yorgason JT; Calipari ES; Ferris MJ; Karkhanis AN; Fordahl SC; Weiner JL; Jones SR, Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology 2016, 101, 471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vialou V; Amphoux A; Zwart R; Giros B; Gautron S, Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci 2004, 24 (11), 2846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu Q; Reith ME; Wightman RM; Kawagoe KT; Garris PA, Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods 2001, 112 (2), 119–33. [DOI] [PubMed] [Google Scholar]