Abstract
Introduction:
Obesity and binge eating disorder are associated with high levels of impulsivity, but the causal role of eating and palatable food in these associations is unclear. Studies in rodents show that a high-fat diet can increase one aspect of impulsivity (impulsive action); it is less clear, however, whether a dissociable aspect of impulsivity (impulsive choice) is similarly affected. Hence, the aim of this study was to ascertain whether chronic exposure to a high-fat diet would alter impulsive choice.
Methods:
Male rats were maintained on either a high-fat or control chow diet for two weeks ad libitum. They then underwent equi-caloric food restriction for the duration of the experiment, with each group maintained on their respective diet. To measure impulsive choice, rats were trained on a delay discounting task (DDT) in which they made discrete choices between a lever that delivered a small food reward immediately and a lever that delivered a large food reward accompanied by systematically increasing delays. Upon reaching stable performance on the DDT, rats were given acute systemic injections of amphetamine prior to testing in the DDT to determine whether increased monoamine transmission affected impulsive choice differently in the two diet groups. Lastly, subjects were tested on a progressive ratio schedule of reinforcement to assess motivation for a sucrose reward.
Results:
There was no significant effect of the high-fat diet on impulsive choice. Further, amphetamine decreased choice of the large, delayed reward (increased impulsive choice) to the same extent in both groups. Exposure to the high-fat diet did, however, increase motivation to obtain a sucrose reward.
Conclusions:
These experiments reveal that, under conditions that do not promote weight gain, a chronic high-fat diet does not affect impulsive choice in a delay discounting task. The data are surprising in light of findings showing that this same diet alters impulsive action, and highlight the necessity of further research to elucidate relationships between palatable food consumption and impulsivity.
Keywords: High Fat, Decision Making, Delay Discounting, Impulsive Choice, Amphetamine, Rats
1. Introduction
In a time when over two-thirds of the American population are considered either overweight or obese [1], research examining how palatable food affects behavior and the brain has never been more important. With the notable exception of anorexia nervosa [2, 3], most food-related disorders involve the misuse/overconsumption of palatable food. An abundance of evidence shows that palatable food shares similarities with addictive substances in that food can cause pathological brain changes similar to drugs of abuse [4, 5]. In addition, disordered eating shares genetic vulnerabilities with substance use disorder (SUD), including in genes related to impulse control and dopamine transmission [4]. This relationship is further supported by clinical observations that binge eating disorder (BED) is comorbid with SUD [6].
Among the behaviors overlapping between SUDs and food-related disorders, impulsivity is of particular importance due to its centrality in both psychiatric diseases. Impulsivity is a multifaceted trait that can be separated into impulsive action and impulsive choice. Whereas impulsive action involves the inability to withhold a prepotent motor response, impulsive choice refers to the inability to delay short-term gratification in favor of larger, but later rewards [7]. Although these two aspects of impulsivity are considered to be largely independent of one another, with distinct although overlapping neural substrates [8-12], there is some evidence that in clinical populations, such as those diagnosed with gambling disorder, these two facets of impulsivity not only correlate with the severity of the disorder, but also with one another [13-15]. Further, both facets of impulsivity contribute to the development of and persistence of SUDs, with impulsive action being associated more with development of substance dependence and impulsive choice promoting continued use and relapse [16]. A well-established finding in human subjects is that individuals with SUDs discount delayed rewards at a steeper rate than controls [i.e., display greater impulsive choice; 17, 18-20, but see 21, 22, 23 for more nuanced views of associations between mpulsive choice and adverse life conditions] and tend to show deficits in response inhibition [i.e., display greater impulsive action; 24, 25-27]. Moreover, in rodent models, cocaine exposure (either passively or self-administered) can both cause [28-32] and be predicted by greater impulsive choice [33-37]. Similar to SUDs, impulsive action predicts “food addiction” in obese individuals, as categorized by the Yale Food Addiction Scale 2.0 [38]. In addition, obese patients with BED have higher rates of impulsive action [39] and higher rates of impulsive choice [40] compared to obese patients without BED. Relatively less is known, however, about the relationship between impulsivity and palatable food independent of the effects of binge eating or extreme weight gain. In particular, it is not clear whether heightened impulsivity is a consequence of chronic palatable food intake and/or a marker of vulnerability to food-related disorders.
Findings in rodent models have provided initial insight into understanding this relationship. For example, in one study assessing the relationship between individual differences in impulsive action and the motivation for and consumption of palatable food, rats exhibiting more impulsive action ate more palatable food, displayed greater motivation for palatable food, and showed heightened compulsive eating when compared to less impulsive rats [41]. This suggests that high levels of impulsivity may confer vulnerability to the development of pathological eating behavior. Other studies show that rats selectively bred for high levels of saccharine intake display greater impulsive choice [42] and that chronic exposure to a high-fat diet increases impulsive action [43]. More recently, others have demonstrated that a diet high in either sugar or fat increases choice of smaller rewards delivered after short delays over larger rewards delivered after a long delay [44, 45], providing initial evidence that chronic exposure to palatable food can increase impulsive choice. The increase in impulsive choice in these studies, however, was accompanied by coincident increases in body weight in rats exposed to the high-fat diet; hence, it is not clear whether the chronic exposure to the high-fat diet itself caused the increase in impulsive choice or whether the behavioral changes were a result of body weight gain either in addition to or independent of diet exposure.
The objective of the current study was to therefore determine whether a diet high in fat, independent of weight gain, alters impulsive choice in a delay discounting task in which rats choose between a small, immediate food reward and a large reward that is preceded by systematically increasing delays. We hypothesized that chronic exposure to a high-fat diet would cause an increase in impulsive choice in a manner similar to drugs of abuse such as cocaine [46]. To test this hypothesis, we used the same diet manipulations employed by Adams et al. [43] that caused increases in impulsive action without significant weight gain to determine whether this diet would also increase impulsive choice.
2. Methods
2.1. Subjects
Sixteen adult male Long-Evans rats (270-300 g upon arrival; Charles River Laboratories, Raleigh, NC) were single housed and kept on a 12-h light/dark cycle (lights on at 7:00AM) with ad libitum access to water. One animal was excluded from the experiment due to failure to learn the delay discounting task, leaving final group sizes of n=7 rats in the high-fat diet group and n=8 rats in the control diet group. Animal procedures were conducted in accordance with the University of Florida’s Institutional Animal Care and Use Committee and guidelines set forth by the National Institutes of Health.
2.2. Diets and Food Administration
The diets and feeding procedures followed those used by Adams et al. (2015). The high-fat diet (HFD) was kept refrigerated at all times except when in use to prolong its integrity (Research Diets; D12492; 5.2 kcal/g; % of calories: 20% protein, 20% carbohydrates, 60% fat). The control diet (CON) was kept at room temperature (Research Diets; D12450J; 3.8 kcal/g; % of calories: 20% protein, 70% carbohydrates, 10% fat). The nutritional consistency of the CON diet was equivalent to that of the food reinforcers in the task. Both diets contained a small but equivalent amount of sucrose. For the free-feeding phase, rats were exposed to their respective diets ad libitum in their home cages for 14 days. Every day at 10:00AM, both the rats and the remaining food in the cages were weighed. Food intake was calculated by subtracting the current day’s food weight from the previous day’s food weight. After 14 days, rats were food restricted to 48 kcal/d of their respective diets (i.e., high-fat or control diet) to increase motivation for task performance (note that rats also received additional calories in the form of the reinforcers used in the behavioral tasks). Rats were always fed immediately after each behavioral test session.
2.3. Behavioral Apparatus
The delay discounting task was conducted in 8 standard operant chambers (30.5 cm x 25.4 cm x 30.5 cm; Coulbourn Instruments), which were housed in sound-attenuating cabinets. Each chamber had two retractable levers located 11 cm above the floor. A food trough, through which food reinforcers were delivered, was located between the two levers and 2 cm from the floor. The reinforcers in the task consisted of 45 mg soy-free pellets (Test Diet, AIN-76A, 5TUL) with a nutritional consistency (3.44 kcal/g; % of calories: 20.6% protein, 12.7% fat, 66.7% carbohydrates) distinct from that of the high-fat diet. The flooring consisted of stainless-steel rods with a catch pan underneath. The food trough was illuminated with a 1.12 W lamp and outfitted with a photobeam to detect nosepoke entries. Another 1.12 W house lamp was mounted on the rear of the cabinet, but was only illuminated during specific events that corresponded with food delivery. Locomotion was monitored using a sensor positioned on the ceiling of the operant chamber. This sensor contained an array of infrared (body heat) detectors, which were focused throughout the entire chamber. Movement was defined as a change of infrared energy on the detectors and measured in arbitrary units. Operant chambers were interfaced with computers running Graphic State 3.0 software (Coulbourn Instruments), which controlled task events and collected data. The chambers were cleaned with dilute chlorhexidine solution between successive rats.
The progressive ratio schedule of reinforcement was conducted in a different set of test chambers (Coulbourn Instruments) located in a separate room from that used for the delay discounting task. These chambers were identical in design to those used in the delay discounting task, except that instead of two retractable levers, there were two nosepokes, each equipped with green lights that illuminated their interiors. A liquid dipper was located in a trough between the nosepokes and was used to deliver sucrose rewards. Panels, floors, house lights, and software were otherwise identical to those used in the delay discounting task.
2.4. Behavioral Procedures
2.4.1. Overview of Experimental Design.
The timeline of experiments is presented in Figure 1. The free-feeding phase lasted 14 days, after which rats were food restricted but maintained on their respective high-fat or control diets. Rats were shaped to perform the different components of the delay discounting task (10 days) and were then trained in a delay discounting task with a fixed delay block design [47]. In this task, rats chose between 1 food pellet delivered immediately and 2 food pellets delivered after various delays (hereafter referred to as the 2:1 delay discounting task or DDT). After 35 days, rats reached performance stability (see Data Analyses for definition). Rats were then transitioned to a delay discounting task in which the reward magnitude increased from 2 vs. 1 food pellets to 4 vs. 1 food pellets. Rats were trained in this delay discounting task (hereafter referred to as the 4:1 delay discounting task or DDT) for 18 days, at which point performance was stable. Rats then received acute systemic injections of amphetamine prior to testing in the 4:1 DDT across eight days, including washout test sessions. After the last washout day, rats were shaped and tested in a progressive ratio schedule of reinforcement task for six days.
Figure 1: Experimental Timeline.

Overview of the experiments in chronological order.
2.4.2. Shaping for Delay Discounting.
Detailed shaping procedures for the delay discounting task have been previously described [31, 32, 48, 49]. Briefly, rats underwent one session of magazine training, in which a single food pellet (45 mg) was delivered every 100 ± 40 s over a 64 min session. This session allowed rats to learn the association between the food trough and food delivery and collection. In the next phase of shaping, rats were trained to press one of the levers (left or right, counterbalanced across conditions) for a single food pellet. Once rats reached 50 lever presses in a single 30 min session, they were trained on the opposite lever until they met the same criterion. Finally, rats learned to nosepoke upon food trough illumination to trigger extension of one of the levers. The order of lever extensions was pseudorandomly determined such that the same lever was never presented more than twice in consecutive trials. A lever press resulted in delivery of a single food pellet reward. Criterion for passing this final stage of shaping was 30 presses on each lever in a 60 min session.
2.4.3. Delay Discounting Task.
The delay discounting task (DDT) was conducted as previously described [31, 32, 48, 49]. Testing occurred between 0730 and 1000 (30 to 180 min into the light phase). Sessions were 60 min in duration and consisted of five blocks, each of which contained twelve 60-s trials. At the start of each trial, the house and food trough light were illuminated, prompting the rats to nosepoke. If rats did not nosepoke in the trough within 10 s, the trial was scored as an omission. If rats nosepoked, both the house light and the trough light were extinguished and either one lever (forced choice trials) or both levers (free choice trials) were extended. If rats failed to lever press within 10 s of lever extension, the trial was scored as an omission. The two forced choice trials (one for each lever) always appeared at the start of each block and were used to remind the rats of the delay contingencies in effect for that block. A press on one lever resulted in immediate delivery of a small reward (a single food pellet), whereas a press on the other resulted in delivery of a large reward (2 or 4 pellets depending on the DDT version) after a delay period, the duration of which systematically increased across the five successive blocks (0, 4, 8, 16, and 32 s). The forced choice trials were followed by 10 free choice trials, in which both levers were extended and rats were free to choose between them. Upon a lever press, the lever was retracted, the food trough light was illuminated, and food was delivered (either immediately or after the variable delays). The location (left vs. right) of the small and large reward levers was counterbalanced across groups, but the lever identities remained constant for an individual rat across the entire experiment. During the 2:1 DDT, the large reward consisted of 2 pellets and the small reward consisted of 1 pellet. During the 4:1 DDT, the large reward was increased to 4 pellets while the small reward remained at 1 pellet.
2.4.4. Progressive Ratio Schedule of Reinforcement.
These sessions occurred at the same time of day as the delay discounting sessions described above (0730-1000). Rats were first shaped using a fixed ratio (FR) schedule of reinforcement, in which a nosepoke in the illuminated (active) port resulted in delivery of 40 μl of a 20% sucrose solution. Reward delivery was accompanied by a 10-s tone and illumination of the trough light. To advance to the progressive ratio (PR) reinforcement schedule, rats were required to nosepoke over 100 times in two consecutive 30-min FR1 sessions. In the PR schedule of reinforcement, the number of nosepokes required for reward delivery increased in a geometric progression. This resulted in the sequence 1, 4, 10, 20, 35, 56, 84, 120, 165, etc. (, where N is the number of nosepokes required to earn a reward r and where r is the ordinal number of the reward; for example, for the second reward, r = 2; for the third reward, r = 3). The session ended when 20 min had elapsed since the last reward delivery. The reward, tone, and light presentation were the same as those presented in the FR sessions.
2.5. Amphetamine Administration
D-amphetamine sulfate (0.3, 1.0 and 1.5 mg/kg; National Institute on Drug Abuse Drug Supply Program) was dissolved in 0.9% saline vehicle and administered 15 min before the start of 4:1 DDT sessions. Amphetamine was administered via i.p. injection every other day using a randomized within-subjects Latin square design [50, 51] such that every rat received every dose of amphetamine (and vehicle) in a randomized, counterbalanced fashion.
2.6. Data Analysis
Raw data were compiled using Graphic State 3.0 software and processed with a custom macro for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). After the data were extracted with the macro, they were analyzed in Statistical Package for the Social Sciences (SPSS) 22.0. For every repeated-measures ANOVA presented, Mauchly’s test of sphericity was used to test for violations in sphericity. If sphericity was violated, a Huynh-Feldt correction was used to determine the p-value. All p-values less than or equal to 0.05 were considered statistically significant.
2.6.1. Weights and Food Consumption
Rats’ weights were collected daily during the free-feeding phase and Mondays, Wednesdays and Fridays during behavioral testing. To determine whether weights differed between groups during these time periods, a two-factor repeated-measures ANOVA was used, with day as a within-subjects factor and diet group as a between-subjects factor.
Food consumption was also measured during the free-feeding phase. There were two different dependent variables that were analyzed: grams of food consumed per day and kCals consumed per day. Both dependent variables were analyzed using a two-factor repeated-measures ANOVA, with day as a within-subjects factor and diet group as a between-subjects factor.
2.6.2. Delay Discounting
The two main dependent variables in the delay discounting task were the number of lever presses for the large reward in free choice trials in each delay block and the percentage of free choice trials in each delay block on which the rat chose the large, delayed reward. To determine whether rats reached stability in the DDT, a repeated-measures ANOVA was used to analyze choice of the large, delayed reward across five consecutive test sessions. Stable performance was defined as the absence of both a main effect of day and an interaction between day and delay [52, 53].
A two-factor repeated-measures ANOVA was used to analyze DDT choice performance across training, with day and delay as the within-subjects factors and diet group (HFD vs. CON) as the between-subjects factor. Due to missing data (e.g., data were not properly recorded by the software for several consecutive days), two rats (n = 1, HFD; n = 1, CON) were excluded from the analysis of behavior during training in the 2:1 DDT. Stable performance in the task (averaged across the five days of stable performance) was also analyzed with a two-factor repeated-measures ANOVA. In this analysis, the within-subjects factor was delay and the between-subjects factor was diet group. As an additional index of choice performance, the area under the curve (AUC) was calculated for individual rats’ stable performance in each version of the DDT (2:1 and 4:1). The AUC was calculated using a trapezoidal method similar to that proposed by Myerson et al. (2001) and used in other studies employing a fixed delay block design [55-57]. Ancillary measures, such as latency to press the levers, trial omissions, and locomotor activity, were also analyzed. Latency to press the levers was defined as the time between the nosepoke to initiate a trial and a subsequent lever press (excluding omitted trials). Only latencies from forced choice trials were analyzed so as to minimize confounds of comparative reward values impacting latencies in free choice trials [58]. To analyze latencies, a three-factor repeated-measures ANOVA was used, with lever identity (small, immediate vs. large, delayed) and delay as within-subjects factors and diet group as a between-subjects factor. The percentage of omitted free choice trials was compared between diet groups using an unpaired t-test. Locomotor activity during the intertrial intervals (ITI) was averaged across the five blocks of trials and compared between diet groups using an unpaired t-test.
2.6.3. Amphetamine Administration
A three-factor repeated-measures ANOVA was used to analyze the effects of amphetamine on choice behavior, with delay and dose as within-subjects factors and diet group as a between-subjects factor. The percentage of omitted free choice trials was analyzed using a repeated-measures ANOVA, with dose as a within-subjects factor and diet group as a between-subjects factor. Locomotor activity and latency to press levers were analyzed in the same way as in the DDT (see section 2.6.2), but with dose as an additional within-subjects factor.
2.6.4. Progressive Ratio
A two-factor repeated-measures ANOVA was used to analyze number of nosepokes during the six days of PR testing, with diet group as the between-subjects factor and day as the within-subjects factor. The numbers of nosepokes in the active port were also averaged across the six days of PR testing and compared between diet groups with an unpaired samples t-test. The number of nosepokes was used to determine the ratio at which rats ceased nosepoking (breakpoint), which was then compared between diet groups using an unpaired samples t-test. Finally, an identical analysis was conducted to determine whether there were differences in the number of rewards earned between the diet groups.
3. Results
3.1. Body weight and food consumption
3.1.1. Free-feeding phase
Before behavioral testing, subjects underwent 14 days of ad libitum exposure to their respective diets. Each day, subjects and the remaining food in the cage were weighed in order to calculate food consumption per day. A repeated-measures ANOVA revealed that both diet groups gained weight across the 14-day period [Figure 2A; day, F (13, 169) = 583.04, p < 0.001]. Although there was only a trend toward a main effect of diet group [F (1, 13) = 4.20, p = 0.06], there was a significant day X diet group interaction [F (13, 169) = 6.80, p < 0.001], such that the HFD group gained significantly more weight than the CON group across successive days. Food consumption was assessed using both the amount of food (in grams) consumed per day (Figure 2B) and kCal consumed per day (Figure 2C). For both variables, there was a main effect of day [grams consumed, F (13, 169) = 6.09, p < 0.001; kCal consumed, F (13, 169) = 4.59, p < 0.001], indicating that food consumption fluctuated across the ad libitum period in both diet groups. In addition to a main effect of diet group on the amount of food consumed [F (1, 13) = 7.76, p = 0.015], there was also a significant diet group X day interaction [F (13, 169) = 10.34, p < 0.001], confirming that the HFD group consumed significantly less food by weight during the 14-day period relative to the CON group. With respect to kCal consumed, there was a main effect of diet group [F (1, 13) = 15.14, p < 0.001] such that the HFD group consumed more kCals compared to the CON group. There was also a diet group X day interaction [F (13, 169) = 8.99, p < 0.001], which seemed to be driven by changes in kCal intake in both diet groups: whereas rats in the HFD group decreased their kCal intake across the first half of the ad libitum feeding phase, the CON group increased their kCal intake across this same period. These diet-induced changes in kCals, as well as the overall differences in the effect of the diets on food consumption and kCal intake, are likely due to the different caloric densities of the diets (HFD = 5.2 kcals/g; control = 3.8 kcals/g), with the HFD rats consuming less of the calorically dense food and CON rats consuming more of the calorically sparse food.
Figure 2. Body weight and food consumption during the free-feeding phase.

A. Rats exposed to the high-fat diet (HFD) gained significantly more weight than rats exposed to the control diet (CON). B. Rats exposed to the high-fat diet ate significantly less food than rats exposed to the control diet. C. Rats exposed to the high-fat diet consumed significantly more kCals than rats exposed to the control diet. Data are represented as the mean ± SEM.
3.1.2. Delay discounting
When subjects’ weights were analyzed across training and testing in the 2:1 DDT (Figure 3), there was a trend toward a main effect of diet group [F (1, 13) = 3.72, p = 0.08] and a main effect of day [F (25, 325) = 8.34, p < 0.001] such that weights gradually increased across training in the task, although not differentially between diet groups [diet group X day interaction, F (25, 325) = 0.61, p = 0.93]. In the 4:1 DDT, there was a similar trend toward a main effect of diet group [F (1, 13) = 3.40, p = 0.09] and a main effect of day [F (17, 221) = 132.60, p < 0.01; this gradual increase in weight gain, however, did not differ between diet groups [F (17, 221) = 1.22, p = 0.25]. Food consumption was not recorded or analyzed as it was under experimenter control throughout all behavioral testing.
Figure 3. Body weights during each experimental phase.
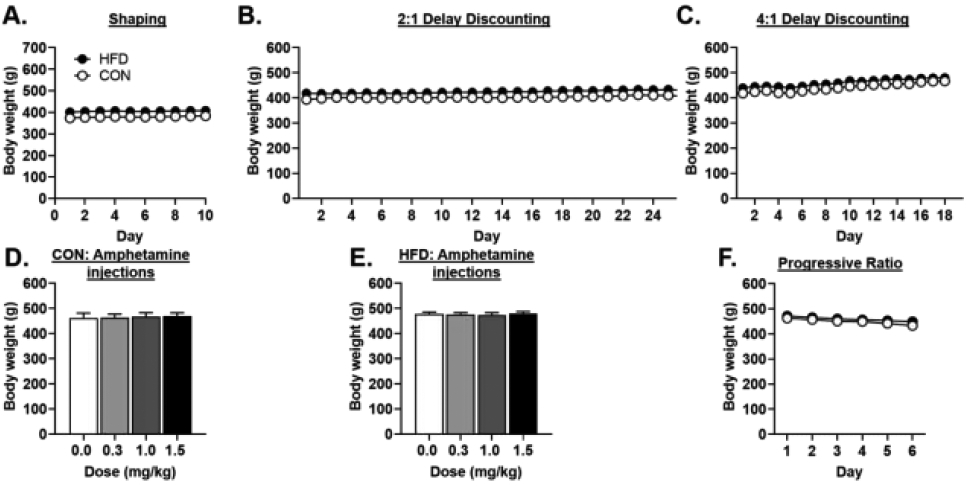
A. There were no differences in body weight between rats that had been exposed to the high-fat diet (HFD) and rats that had been exposed to a control (CON) diet during behavioral shaping. B. There were no significant differences in body weight between HFD and CON rats during testing in the 2:1 delay discounting task. C. There were no significant differences in body weight between HFD and CON rats during testing in the 4:1 delay discounting task. D. There were no differences in body weight across doses of amphetamine in either the CON rats or the E. HFD rats. F. There were no differences in body weight between CON and HFD rats during Progressive Ratio testing. Data are represented as the mean ± SEM.
3.1.3. Amphetamine administration
A two-factor repeated-measures ANOVA was used to analyze subjects’ weights during the regimen of amphetamine administration (Figure 3D, E). In addition to a lack of a main effect of diet group [F (1, 13) = 1.76, p = 0.21], there was neither a main effect of dose [F (3, 39) = 0.87, p = 0.46] nor a dose X diet group interaction [F (3, 39) = 0.69, p = 0.56].
3.1.4. Progressive Ratio Schedule of Reinforcement
Weights were recorded on each day of PR testing (Figure 3F) and subjected to a two-factor repeated-measures ANOVA. This analysis revealed that there was an overall decrease in weight across PR testing [F (5, 60) = 70.12, p < 0.01], but that this reduction in weight did not differ between diet groups [diet group, F (1, 12) = 0.74, p = 0.41; day X diet group, F (5, 60) = 1.74, p= 0.14].
3.2. 2:1 Delay Discounting Task
Rats were trained on a 2:1 delay discounting task in which they chose between a small, immediate reward (1 food pellet) and a large, delayed reward (2 food pellets) until stable behavior emerged (35 days). There were no differences in performance in the task during training [large lever presses: diet group, F (1, 11) = 1.18, p = 0.30; day, F (34, 374) = 1.08, p = 0.36; diet group X day, F (34, 374) = 0.74, p = 0.86; day X delay X diet group, F (136, 1496) = 0.99, p = 0.52; percent choice: diet group, F (1, 11) = 1.12, p = 0.32; day, F (34, 374) = 1.10, p = 0.33; diet group X day, F (34, 374) = 0.74, p = 0.86; day X delay X diet group, F (136, 1496) = 1.00, p = 0.50]. The effects of diet on stable choice behavior (Figure 4A) were analyzed using a two-factor repeated-measures ANOVA, which revealed a robust effect of delay such that rats decreased their choice of the large reward as delay durations increased [large lever presses: F (4, 52) = 59.02, p < 0.01; percent choice: F (4, 52) = 56.58, p < 0.01]. There was, however, neither a main effect of diet group [large lever presses: F (1, 13) = 0.91, p = 0.36; percent choice: F (1, 13) = 0.928, p = 0.35] nor a diet group X delay interaction [large lever presses: F (4, 52) = 0.42, p = 0.80; percent choice: F (4, 52) = 0.55, p = 0.70]. Similarly, there was no main effect of diet group on AUC [t (13) = 1.17, p = 0.26; Figure 4C]. These results indicate that there were no effects of the high-fat diet on choice performance in the 2:1 delay discounting task.
Figure 4: Effects of a high-fat diet on impulsive choice in a delay discounting task.
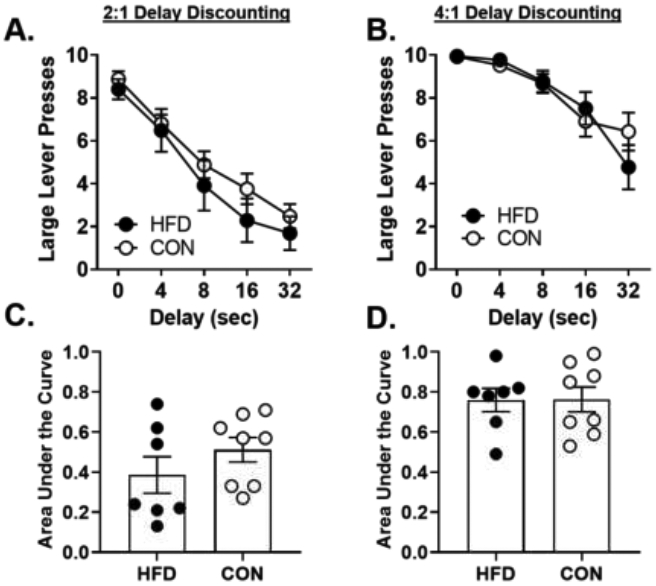
A. There were no differences between diet groups on the number of large lever presses in a delay discounting task in which rats chose between a small, immediate reward (1 pellet) and a large, delayed reward (2 pellets). B. There were no differences between diet groups on the number of large lever presses in a delay discounting task in which rats chose between a small, immediate reward (1 pellet) and a large, delayed reward (4 pellets). Data are represented as the mean ± SEM number of large lever presses during stable performance. C. There was no difference between diet groups in the area under the curve in the 2 vs. 1 delay discounting task. D. There was no difference between diet groups in the area under the curve in the 4 vs. 1 delay discounting task. Each circle represents the area under the curve for an individual subject. HFD, high-fat diet; CON, control diet.
Latency to press the levers during forced choice trials was analyzed using a repeated-measures ANOVA. Although there was no main effect of lever identity [F (1, 13) = 0.62, p = 0.44] or delay [F (4, 52) = 0.75, p = 0.56], there was a significant lever identity X delay interaction [F (4, 52) = 11.07, p < 0.001] such that as the delay increased, the latency to press the small, immediate reward lever decreased, but the latency to press the large, delayed reward lever increased [59, 60]. There were, however, no significant lever identity X diet group [F (1, 13) = 0.19, p = 0.67], delay X diet group [F (4, 52) = 0.67, p = 0.61] or lever identity X delay X diet group [F (4, 52) = 0.15, p = 0.30] interactions, indicating that this pattern of differential latencies on the two levers did not differ between diet groups.
There was no difference between diet groups in mean locomotor activity during ITIs (Table 1; t (13) = −0.96, p = 0.35]. Finally, although there was unequal variance between the diet groups (Levene’s Test of Equality of Variances, p = 0.03) in the percentage of omissions during free choice trials (Table 1), there was no difference between the diet groups [t (13) = 1.02, p = 0.33].
Table 1.
Effects of high-fat diet and amphetamine on trial omissions and locomotor activity in the delay discounting task. Data presented as: mean (± standard error of the mean)
| Experiment | % Omitted Trials | Locomotion (locomotor units/ITI) |
|---|---|---|
| 2:1 Delay Discounting Task | ||
| Control | 1.15 (0.47) | 20.55 (9.19) |
| HFD | 2.17 (0.89) | 16.45 (6.91) |
| 4:1 Delay Discounting Task | ||
| Control | 1.80 (1.41) | 17.46 (7.34) |
| HFD | 1.31 (0.60) | 13.78 (6.00) |
| Amphetamine on the 4:1 Delay Discounting Task | ||
| Vehicle | ||
| Control | 1.50 (0.98) | 13.64 (1.58) |
| HFD | 5.14 (4.49) | 9.81 (1.36) |
| 0.3 mg/kg | ||
| Control | 5.00 (4.44) | 16.43 (1.83) |
| HFD | 6.29 (3.16) | 14.67 (1.08) |
| 1.0 mg/kg | ||
| Control | 24.00 (10.19) | 21.85 (2.01) |
| HFD | 32.57 (14.58) | 20.43 (3.08) |
| 1.5 mg/kg | ||
| Control | 39.25 (14.78) | 18.84 (2.51) |
| HFD | 56.85 (12.64) | 18.22 (2.65) |
3.3. 4:1 Delay Discounting Task
Changes in reward magnitude alter behavior in both humans and rodents, especially behaviors involving risk taking or impulsivity [23, 61-65]. For example, amphetamine is ineffective in altering impulsive choice in a delay discounting task with a 3:1 reinforcer magnitude difference, but does increase impulsive choice when the difference in reward magnitudes is increased to 6:2 [64]. Given these data, it is possible that the reward magnitude difference in the 2:1 DDT was too small to observe effects of a high-fat diet on impulsive choice. Consequently, the same rats were trained in the delay discounting task with the reward magnitude ratio increased to 4:1. Similar to training in the 2:1 DDT, there were no group differences during training in the DDT [large lever presses: F (1, 13) = 0.27, p = 0.61; percent choice: F (1, 13) = 0.33, p = 0.58], although there was a significant increase in choice of the large, delayed reward in both groups across the training period [large lever presses: day, F (17, 221) = 7.76, p < 0.001; diet group X day, F (17, 221) = 0.79, p = 0.70; day X delay X diet group, F (68, 884) = 0.83, p = 0.84; percent choice: day, F (17, 221) = 8.12, p < 0.001; diet group X day, F (17, 221) = 0.70, p = 0.80; day X delay X diet group, F (68, 884) = 0.84, p = 0.82]. After stable behavior emerged (18 days), the effects of diet on choice performance were analyzed using a repeated-measures ANOVA (Figure 4B). This analysis revealed that although there was a robust main effect of delay such that rats decreased their choice of the large reward as the delay to its delivery increased [large lever presses: F (4, 52) = 29.47, p < 0.01; percent choice: F (4, 52) = 31.50, p < 0.01], there was neither a main effect of diet group [large lever presses: F (1, 13) = 0.05, p = 0.82; percent choice: F (1, 13) < 0.01, p = 0.96] nor a diet group X delay interaction [large lever presses: F (4, 52) = 1.73, p = 0.16; percent choice: F (4, 52) = 1.7, p = 0.17]. Finally, there was no main effect of diet group on AUC [t (13) = 0.03, p = 0.98; Figure 4D]. Thus, similar to performance in the 2:1 DDT, there was no effect of the high-fat diet on choice of the large, delayed reward. Finally, to determine whether the increase in large reward magnitude in the DDT (i.e., switching from 2:1 to 4:1) differentially affected the diet groups, a three-factor repeated-measures ANOVA was used to compare choice performance in the 4:1 DDT to choice performance in the 2:1 DDT. Although there was a main effect of reward magnitude [4 vs. 2 pellets; large lever presses: F (1, 13) = 48.38, p < 0.01; percent choice: F (1, 13) = 49.07, p < 0.01], there was no main effect of diet group [large lever presses: F (1, 13) = 0.72, p = 0.41; percent choice: F (1, 13) = 0.53, p = 0.48] nor were there diet group X reward magnitude [large lever presses: F ( 1, 13) = 0.50, p = 0.49; percent choice: F (1, 13) = 0.72, p = 0.41] or diet group X reward magnitude X delay [large lever presses: F (4, 52) = 1.53, p = 0.21; percent choice: F (4, 52) = 1.52, p = 0.21] interactions. Hence, as expected, altering the magnitude of the large reward caused rats to increase their choice of the large, delayed reward; this manipulation did not discriminate between the diet groups, however, as rats in both diet groups exhibited an equivalent shift upwards in their choice of the large, delayed reward.
Analyses of latency to press the levers in the 4:1 DDT revealed a main effect of lever identity [F (1, 13) = 4.76, p = 0.05] such that latencies to press the small, immediate reward lever were significantly shorter than latencies to press the large, delayed reward lever. Despite a main effect of delay [F (4, 52) = 2.70, p = 0.04] and a significant lever identity X delay interaction [F (4, 52) = 9.70, p < 0.001], there was no main effect of diet group [F (1, 13) = 1.48, p = 0.25] and no significant lever identity X diet group [F (1, 13) = 0.74, p = 0.41], delay X diet group [F (4, 52) = 1.10, p = 0.37] or lever identity X delay X diet group [F (4, 52) = 1.55, p = 0.20] interactions. Thus, similar to choice performance, the high-fat diet had no effect on lever press response latencies.
Similar to the 2:1 DDT, there was no difference between diet groups in locomotor activity during the 4:1 DDT [t (13) = −1.05, p = 0.31; Table 1]. Lastly, there were no differences in percentage of omissions between the diet groups [t (13) = 0.30, p = 0.77].
3.4. Effects of Amphetamine on Delay Discounting
Although the high-fat diet alone had no effect on impulsive choice, it may have had more subtle effects on monoamine signaling. Indeed, previous studies showed that rats maintained on a high-fat diet that did not affect baseline impulsive choice were nevertheless more sensitive to the effects on impulsive choice of dopaminergic manipulations, including administration of the D2 dopamine receptor antagonist haloperidol [66, 67]. There is also abundant evidence that maintenance on a high-fat diet decreases ventral striatal D2 dopamine receptor expression and baseline levels of extracellular dopamine [43, 68, 69]. Hence, it is conceivable that, even if exposure to a high-fat diet has no effect on baseline impulsive choice, it may have still altered dopamine signaling such that the sensitivity of impulsive choice to monoaminergic manipulations is augmented. To begin to address this, rats received systemic injections of the indirect dopamine agonist amphetamine (0, 0.3, 1.0, 1.5 mg/kg) prior to testing in the 4:1 DDT. Analysis of the percent choice of the large, delayed reward revealed a trend toward a main effect of dose [F (3, 9) = 3.16, p = 0.08], but no significant interactions [dose X diet group, F (3, 9) = 2.43, p = 0.13; dose X delay, F (12, 36) = 0.82, p = 0.63; dose X diet group X delay, F (12, 36) = 1.59, p = 0.14]. Amphetamine, however, caused a significant increase in the percentage of omissions in the free choice trials to a similar extent in both diet groups [Table 1; dose, F (3, 39) = 12.33, p < 0.01; diet group, F (1, 13) = 0.78, p = 0.39; dose X diet group, F (3, 39) = 0.36, p = 0.78], rendering analyses of percent choice of the large reward difficult to interpret given the reduction in the number of subjects with sufficient data available for analysis (sample sizes for analysis were n = 2, HFD and n = 3, CON). Specifically, when compared to vehicle, the medium [t (14) = −3.39, p < 0.01] and high [t (14) = −4.84, p < 0.01] doses increased the percentage of omissions [low dose; t (14) = −0.73, p = 0.48].
The analyses of the number of large lever presses was more revealing as it allowed all subjects to be included (HFD, n=7; CON, n=8; Figure 5). These analyses revealed that amphetamine decreased the number of lever presses for the large, delayed reward [dose, F (3, 39) = 17.4, p < 0.01; dose X delay, F (12, 156) = 3.0, p < 0.01]. Post-hoc tests revealed that this effect was specific to the medium [dose, F (1, 13) = 10.80, p = 0.01; dose X delay, F (4, 52) = 2.68, p = 0.04] and high [dose, F (1, 13) = 35.80, p < 0.01; dose X delay, F (4, 52) = 21.90, p < 0.01] doses. Although there was a trend toward a main effect of diet group [F (1, 13) = 288.2, p = 0.06] such that the number of large reward lever presses was greater in the CON group than the HFD group across all doses, there were no significant interactions between diet group and delay [F (4, 52) = 7.96, p = 0.42], dose and diet group [F (3, 39) = 0.17, p = 0.92], or dose, diet group and delay [F (12, 56) = 1.10, p = 0.37], indicating that the medium and high doses of amphetamine decreased lever pressing for the large, delayed reward similarly in both diet groups.
Figure 5: Effects of amphetamine in the 4:1 delay discounting task.
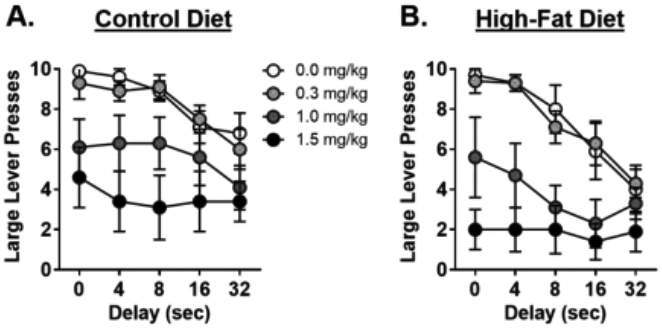
A. Systemic amphetamine decreased the number of lever presses for the large, delayed reward in the rats on the control diet (CON). B. Systemic amphetamine also decreased the number of lever presses for the large, delayed reward in rats on the high-fat diet (HFD). Data are represented as mean ± SEM number of large, delayed lever presses for each dose of amphetamine.
Similar to the analysis of choice data, it was not possible to analyze latencies to press levers on forced choice trials due to the significant increase in omissions in the forced choice trials in both diet groups [dose, F (3, 39) = 16.88, p < 0.01; diet group, F (1, 13) = 0.74, p = 0.40; dose X diet group, F (3, 39) = 0.06, p = 0.97]. Because of the high percentage of omissions, data were missing for 14 of the 15 rats, which decreased the sample size and power needed for this analysis.
Finally, there was a main effect of amphetamine dose on locomotor activity [F (3, 36) = 21.36, p < 0.001], but there was no main effect of diet group [F (1, 12) = 0.32, p = 0.58] nor a diet group X dose interaction [F (3, 36) = 0.94, p = 0.43]. Hence, locomotor activity increased in a dose-dependent manner to the same extent in both diet groups (Table 1).
3.5. Progressive Ratio Schedule of Reinforcement
Although there was no effect of the high-fat diet on choice behavior, it is possible that the high-fat diet could still affect motivation to obtain palatable rewards [70-72]. To evaluate this possibility, rats were tested on a progressive ratio schedule of reinforcement (PR) with a liquid sucrose reward. Initial inspection of the data revealed an outlier (any data point falling above the third quartile or below the first quartile by 1.5 interquartiles) in the HFD group, which was subsequently excluded from the analyses. A repeated-measures ANOVA revealed a main effect of day, indicating that nosepoking increased across the six days of PR testing [F (5, 60) = 3.41, p < 0.01]. This behavioral pattern did not differ between diet groups as there was no main effect of diet group [F (1, 12) = 1.15, p = 0.30] nor a diet group X day interaction [F (5, 60) = 1.38, p = 0.24]. Consistent with this, an unpaired t-test revealed that there was no difference in mean nosepokes between the diet groups [t (12) = 1.09, p = 0.31]. When breakpoints (the ratio at which the rat ceased nosepoking) were compared between the diet groups, however, the HFD group had a significantly greater breakpoint than the CON group [t (12) = 3.52, p < 0.01; Figure 6A). Similarly, the HFD group earned significantly more rewards than the CON group [t (12) = 3.18, p = 0.01; Figure 6B]. Hence, these data demonstrate that, despite an absence of effects on impulsive choice, exposure to a high-fat diet does cause an overall increase in motivation to work for palatable rewards.
Figure 6: Performance in a progressive ratio schedule of reinforcement.
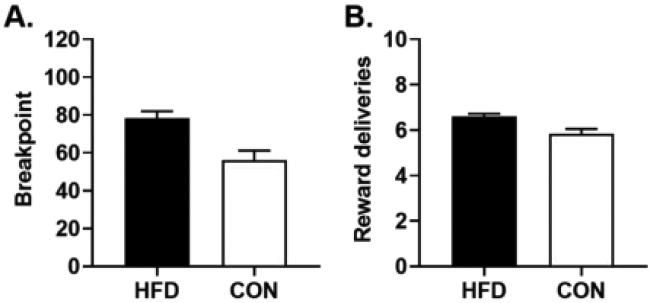
A. On average, rats in the high-fat diet group (HFD) had a significantly higher breakpoint than rats in the control diet group (CON). B. Rats in the HFD group earned significantly more rewards than rats in the CON group during PR testing. Data represent mean breakpoint (A) or reward deliveries (B) ± SEM averaged across 6 days of testing.
4. Discussion
The objective of the current study was to determine whether consumption of a high-fat diet affects impulsive choice. Unlike its effects on impulsive action [43], exposure to a high-fat diet, administered in a way that controlled for excessive weight gain, did not alter impulsive choice. Furthermore, systemic amphetamine did not differentially alter delay discounting in rats consuming the high-fat diet. Exposure to the high-fat diet did, however, increase motivation to work for a sucrose reward. Despite this, the results do not replicate previous reports that a high-fat diet increases impulsive choice [44, 45].
The finding that a high-fat diet had no effect on impulsive choice is surprising in light of evidence that the same diet, administered using identical procedures, increases impulsive action [43]. These discrepant findings suggest that the effects of chronic exposure to a high-fat diet on impulsivity are specific to one aspect of impulsivity. This interpretation is not unreasonable given that impulsive action and impulsive choice are not correlated in healthy humans or rats [8] and have different, although overlapping neurobiological substrates [12, 73]. This explanation is insufficient, however, in addressing why, when it has been demonstrated in other studies (those by Steele et al. [44, 45]), there was no effect of the high-fat diet on impulsive choice. Rather, the incongruency between findings of the current study and those of Steele et al. may be a result of procedural differences. One critical difference is the duration of ad libitum exposure to the high-fat diet. In the current study, rats were exposed to the high-fat diet ad libitum for only two weeks, whereas rats in the Steele et al. studies [44, 45] were exposed to their high-fat diet ad libitum for eight weeks before behavioral testing began. The rats in the current study were also exposed to their respective diets for a long period of time because they were maintained on these diets throughout behavioral testing, albeit at restricted quantities. The critical factor may therefore be the duration of exposure to a diet high in fat under ad libitum conditions rather than the overall duration of exposure itself. Recent work showing a similar absence of effects on impulsive choice after limited exposure (15 d) to a high-sugar diet supports this hypothesis [74]. A second difference is that, in contrast to the current study, maintenance on the high-fat diet continued to promote body weight gain throughout behavioral testing in the Steele et al. studies despite the fact that, similar to the current study, their food intake was restricted during behavioral testing. Hence, in addition to requiring a long-duration exposure to the high-fat diet, increased impulsive choice may only occur under conditions that encourage coincident weight gain. It is clear that future studies are warranted to determine the specific conditions that induce susceptibility to the effects of a high-fat diet on impulsivity.
Additional procedural differences that could explain the lack of effects of a high-fat diet on impulsive choice involve the way in which the food was administered. Compared with studies that have found effects of a high-fat diet on impulsivity [44, 45], there are two important distinctions in the food administration protocol used in the present study. First, the diet used here was specifically formulated to provide rats a diet with balanced nutrient content, and therefore avoided the possibility that any observed effects could be due to malnutrition. This differs from studies in which rats’ diets consisted of purely fatty substances [44, 45]. Second, the administration protocol used in the current study did not promote binge eating. In contrast to ad libitum access to food, short, infrequent access to palatable food, which promotes binge-eating behavior, sensitizes responses to drugs of abuse [71, 75, 76]. For example, rats that binge drink sucrose solution during short-access conditions display symptoms of withdrawal and craving during sugar abstinence; these effects are absent in rats who have access ad libitum [75]. Similarly, brief, infrequent access to fat enhances cocaine seeking and taking compared with an ad libitum diet of fat [77]. Given that binge eating results in behavioral effects similar to those resulting from repeated exposure to drugs of abuse [75] and that exposure to drugs of abuse itself increases impulsive choice [46, 78], it is possible that highly palatable food would more readily increase impulsive choice if it were administered in a manner that promotes binge-like behavior. Indeed, there is clinical evidence that people with binge eating disorder exhibit heightened impulsivity [both impulsive action and choice; 79, 80]. Considered together, these data suggest that relationships between palatable food consumption, impulsive choice, and impulsive action may change depending on the parameters of food access.
Recent work showed that although maintenance on a high-fat diet did not change baseline impulsive choice, it did increase the sensitivity of impulsive choice to the D2 dopamine receptor antagonist haloperidol, in that only rats exposed to a high-fat diet exhibited haloperidol-induced increases in impulsive choice [66, 67]. Relatedly, long-term exposure to a high-fat diet causes reductions in ventral striatal expression of D2 dopamine receptor mRNA [81] and protein [43], as well as decreases in basal levels of extracellular dopamine [82, 83]. Based on this evidence, we hypothesized that, despite no baseline changes in impulsive choice, exposure to the high-fat diet might still have led to changes in dopamine signaling that result in differential sensitivity to dopaminergic manipulations. To evaluate this possibility, rats received acute systemic administration of amphetamine prior to testing in the 4:1 DDT. The results showed that amphetamine dose-dependently increased impulsive choice (reflected in reduced choice of the large, delayed reward), but that this effect did not differ between the two diet groups. Notably, amphetamine administration caused a significant increase in omitted free choice trials. On the surface, this lack of responding, particularly in the first block in which there is no delay to the large reward, may reflect a reduction in overall motivation rather than a change in discounting. This interpretation is less appealing, however, in light of previous work showing that decreased responding for large rewards in the 0 s block is driven by a reduction in sensitivity to reward magnitude differences (as opposed to other factors, including decreases in motivation) [53, 56, 84, 85]. Further, similar doses of amphetamine can actually increase motivation to obtain natural rewards in a PR task [86-88]. Hence, although this alternate explanation cannot be ruled out entirely, a more likely interpretation is that amphetamine reduced the sensitivity to detecting reward magnitude differences, leading to an overall decrease in responding.
The fact that amphetamine increased impulsive choice in the present study contrasts with several previous studies in which amphetamine decreased impulsive choice [64, 89-91]. The reasons for this difference are not clear, although some data suggest that the presence or absence of cues during delays [53] or the order in which delays are presented [55] can alter the effects of amphetamine on delay discounting. Nevertheless, the data show that systemic amphetamine decreased choice of the large, delayed reward irrespective of previous diet exposure. It remains unclear as to why, when previous work shows otherwise [66, 67], such a dopaminergic manipulation did not affect impulsive choice differently between diet groups. One possible explanation is that, similar to studies showing effects of a high-fat diet on impulsive choice [44, 45], the duration of exposure to the high-fat diet was substantially longer (three months) than that used in the current study. As discussed above, such differences across studies underscore the need for additional work to determine the specific conditions that contribute to diet-induced changes in impulsive choice.
Previous work suggests that exposure to a high-fat diet alters motivation for sucrose reward, although the direction of this alteration (increase or decrease) varies across studies [71, 72, 92, 93]. In the current study, the high-fat diet had a significant impact on PR responding for a liquid sucrose reward, increasing the breakpoint and the number of rewards earned. The discrepancy in findings across studies may be explained by differences in both diet content and duration of exposure. For instance, three weeks of ad libitum exposure to a high-fat (40% fat) diet had no effect on motivation for a sucrose reward, whereas six weeks of the same exposure suppressed motivation for this reward [70]. In the current study, although rats only had ad libitum access for two weeks, they were exposed to a 60% high-fat diet, suggesting that the greater fat content of the diet may be a critical factor in determining the impact of such diets on motivation to work for food. Irrespective of inconsistencies across studies, the increase in PR responding served as a positive control in the current study in that it provided evidence that, despite having no effect on choice behavior, exposure to the high-fat diet did have a long-lasting impact on motivated behavior. The greater motivation to obtain sucrose rewards in the HFD group may also explain the lack of effects of the high-fat diet on impulsive choice: had a more palatable reinforcer (relative to the 45 mg pellets used in the current study) been used, it is conceivable that diet-induced differences in impulsive choice could have been detected.
Limitations
It is important to note several limitations of the current study, the first of which is the use of only male subjects. In the last several years, previous studies have provided evidence of sex differences in various forms of decision making, including risk-based decision making [94-96], although the consensus regarding sex differences in impulsive choice is less clear [42, 59, 97-100]. Of even more importance, however, is the fact that the prevalence of eating disorders, including binge eating disorder, is greater in females than males [101-103]. Hence, it is critical that future research in this area includes females, as they may be more sensitive to such diet manipulations than males, resulting in changes in impulsive choice that were otherwise absent in males. Another related limitation is the small sample size of each group, which could have accounted for the lack of effects of the high-fat diet on impulsive choice. Nonetheless, the fact that the high-fat diet had a significant impact on PR breakpoint indicates that the diet manipulation was sufficient to have an effect on behavior, and argues against the group sizes being too small to detect group differences.
The use of food restriction in experiments in the current study could also be considered a limitation. Caloric restriction by itself can enhance the hedonic value of palatable food (e.g., food deprivation enhances positive hedonic reactions, such as tongue protrusion and paw licking, to oral sucrose solutions in rats [104]). Hence, being in a state of chronic food restriction could unintentionally confound food-motivated behavioral tasks such as those used in the current study. This effect of caloric restriction, however, seems to be limited to palatable food rewards, with enhanced hedonic reactions only occurring in response to palatable tastants (as opposed to aversive tastants) [104]. Other work has shown that, in comparison to satiated rats, food-restricted rats prefer sweeter rewards than non-palatable reinforcers (e.g., water) [105]. Because the food reinforcers in the current study were high in neither sugar or fat and were not hedonically palatable, it is unlikely that food restriction caused an exaggerated motivational drive to seek the food reinforcers in the task. Food restriction can also impact body weight and energy expenditure, leading to decreases in weight and a conservation of energy [106, 107]. Conversely, however, free access to standard low-fat rodent chow can lead to excess weight gain, fat deposition and potential metabolic dysfunction. Hence, a food-restricted diet such as that used in the current study (~85% of free feeding weight) is actually recommended for use with laboratory rodents to slow weight gain and fat deposition over time. Although half of the rats were fed a high-fat diet, the restricted conditions under which they were fed still offered those same health benefits.
Conclusion
In summary, the current study shows that exposure to a high-fat diet under conditions that do not promote weight gain does not affect impulsive choice in a delay discounting task, nor does it alter the effects of acute amphetamine on impulsive choice. Future experiments will investigate whether different exposure parameters reveal nuanced effects of a high-fat diet on impulsive choice. Longer durations of high-fat diet exposure, as employed by Steele et al. (2019), or feeding designs that promote binge eating, for example, may be critical factors in revealing effects of palatable food consumption on impulsive choice. Furthermore, under shorter durations of exposure, it is possible that different facets of impulsivity are more vulnerable to the impact of a high-fat diet: as shown by Adams et al. (2015), impulsive action is susceptible to the same high-fat diet regimen used here. Ultimately, identifying such factors will help elucidate the complex relationship between food and impulsivity, which will be necessary to create novel treatments to combat the growing obesity crisis.
Highlights:
Exposure to a high-fat diet does not alter impulsive choice.
Amphetamine increases impulsive choice.
Effects of amphetamine did not differ between diet groups.
High-fat diet exposure increases motivation for sucrose.
Acknowledgments:
We thank the Drug Supply Program at the National Institute on Drug Abuse for kindly providing d-amphetamine, and Shelby Blaes and Shannon Wall for technical assistance. This work was supported by the National Institutes of Health [R01DA036534] awarded to Dr. Barry Setlow and a Thomas H. Maren Fellowship awarded to Dr. Caitlin Orsini.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ogden CL, Carroll MD, Kit BK, Flegal KM Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014,311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Decker JH, Figner B, Steinglass JE On Weight and Waiting: Delay Discounting in Anorexia Nervosa Pretreatment and Posttreatment. Biological psychiatry. 2015,78:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc. 2012,18:773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Volkow ND, Wang GJ, Tomasi D, Baler RD Obesity and addiction: neurobiological overlaps. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2013,14:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Volkow ND, Wise RA How can drug addiction help us understand obesity? Nature Neuroscience. 2005,8:555–60. [DOI] [PubMed] [Google Scholar]
- [6].Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Archives of General Psychiatry. 2011,68:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Evenden JL Varieties of impulsivity. Psychopharmacology. 1999,146:348–61. [DOI] [PubMed] [Google Scholar]
- [8].Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, et al. The relationship between impulsive choice and impulsive action: a cross-species translational study. PloS One. 2012,7:e36781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nautiyal KM, Wall MM, Wang S, Magalong VM, Ahmari SE, Balsam PD, et al. Genetic and Modeling Approaches Reveal Distinct Components of Impulsive Behavior. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2017,42:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biological psychiatry. 2008,63:301–8. [DOI] [PubMed] [Google Scholar]
- [11].Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. The European journal of neuroscience. 2013,37:1779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dalley JW, Everitt BJ, Robbins TW Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011,69:680–94. [DOI] [PubMed] [Google Scholar]
- [13].Mackillop J, Miller JD, Fortune E, Maples J, Lance CE, Campbell WK, et al. Multidimensional examination of impulsivity in relation to disordered gambling. Experimental and clinical psychopharmacology. 2014,22:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Michalczuk R, Bowden-Jones H, Verdejo-Garcia A, Clark L Impulsivity and cognitive distortions in pathological gamblers attending the UK National Problem Gambling Clinic: a preliminary report. Psychol Med. 2011,41:2625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Steward T, Mestre-Bach G, Vintro-Alcaraz C, Aguera Z, Jimenez-Murcia S, Granero R, et al. Delay Discounting of Reward and Impulsivity in Eating Disorders: From Anorexia Nervosa to Binge Eating Disorder. Eur Eat Disord Rev. 2017,25:601–6. [DOI] [PubMed] [Google Scholar]
- [16].Winstanley CA, Olausson P, Taylor JR, Jentsch JD Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010,34:1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011,216:305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mejía-Cruz D, Green L, Myerson J, Morales-Chainé S, Nieto J Delay and probability discounting by drug-dependent cocaine and marijuana users. Psychopharmacology. 2016,233:2705–14. [DOI] [PubMed] [Google Scholar]
- [19].Johnson MW, Bickel WK, Baker F Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Experimental and Clinical Psychopharmacology. 2007,15:187–94. [DOI] [PubMed] [Google Scholar]
- [20].Mitchell JM, Fields HL, D'Esposito M, Boettiger CA Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005,29:2158–69. [DOI] [PubMed] [Google Scholar]
- [21].Hayden BY Time discounting and time preference in animals: A critical review. Psychon Bull Rev. 2016,23:39–53. [DOI] [PubMed] [Google Scholar]
- [22].Namboodiri VM, Mihalas S, Marton TM, Hussain Shuler MG A general theory of intertemporal decision-making and the perception of time. Frontiers in behavioral neuroscience. 2014,8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Green L, Myerson J A discounting framework for choice with delayed and probabilistic rewards. Psychological bulletin. 2004,130:769–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perry JL, Carroll ME The role of impulsive behavior in drug abuse. Psychopharmacology. 2008,200:1–26. [DOI] [PubMed] [Google Scholar]
- [25].Noël X, Linden M. V. d., d’Acremont M, Bechara A, Dan B, Hanak C, et al. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology. 2007,192:291–8. [DOI] [PubMed] [Google Scholar]
- [26].Verdejo-García AJ, Perales JC, Pérez-García M Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive Behaviors. 2007,32:950–66. [DOI] [PubMed] [Google Scholar]
- [27].Monterosso JR, Aron AR, Cordova X, Xu J, London ED Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005,79:273–7. [DOI] [PubMed] [Google Scholar]
- [28].Mitchell MR, Weiss VG, Ouimet DJ, Rita AF, Morgan D, Setlow B Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behavioral Neuroscience. 2014,128:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dandy KL, Gatch MB The effects of chronic cocaine exposure on impulsivity in rats. Behavioural pharmacology. 2009,20:400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S Cocaine decreases self-control in rats: a preliminary report. Psychopharmacology. 1992,109:245–7. [DOI] [PubMed] [Google Scholar]
- [31].Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behavioral neuroscience. 2010,124:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Simon NW, Mendez IA, Setlow B Cocaine exposure causes long-term increases in impulsive choice. Behavioral neuroscience. 2007,121:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anker JJ, Perry JL, Gliddon LA, Carroll ME Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology Biochemistry and Behavior. 2009,93:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008,320:1352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007,315:1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koffarnus MN, Woods JH Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addiction biology. 2013,18:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Perry JL, Larson EB, German JP, Madden GJ, Carroll ME Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005,178:193–201. [DOI] [PubMed] [Google Scholar]
- [38].Meule A, de Zwaan M, Müller A Attentional and motor impulsivity interactively predict 'food addiction' in obese individuals. Comprehensive Psychiatry. 2017,72:83–7. [DOI] [PubMed] [Google Scholar]
- [39].Nasser JA, Gluck ME, Geliebter A Impulsivity and test meal intake in obese binge eating women. Appetite. 2004,43:303–7. [DOI] [PubMed] [Google Scholar]
- [40].Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE Discounting of Various types of rewards by women with and without binge eating Disorder: Evidence for general rather than specific Differences. The Psychological Record. 2011,61:561–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Velázquez-Sánchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014,39:2463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacology, biochemistry, and behavior. 2007,86:822–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Adams WK, Sussman JL, Kaur S, D'Souza AM, Kieffer TJ, Winstanley CA Long-term, calorie-restricted intake of a high-fat diet in rats reduces impulse control and ventral striatal D2 receptor signalling - two markers of addiction vulnerability. The European Journal of Neuroscience. 2015,42:3095–104. [DOI] [PubMed] [Google Scholar]
- [44].Steele CC, Pirkle JRA, Davis IR, Kirkpatrick K Dietary effects on the determinants of food choice: Impulsive choice, discrimination, incentive motivation, preference, and liking in male rats. Appetite. 2019,136:160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Steele CC, Pirkle JRA, Kirkpatrick K Diet-induced impulsivity: Effects of a high-fat and a high-sugar diet on impulsive choice in rats. PloS one. 2017,12:e0180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mitchell MR, Orsini CA, Setlow B Cocaine and intertemporal decision making. In: Preedy V, ed. The Neuroscience of Cocaine: Mechanisms And Treatment: Elsevier; in press. [Google Scholar]
- [47].Evenden JL, Ryan CN The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996,128:161–70. [DOI] [PubMed] [Google Scholar]
- [48].Orsini CA, Mitchell MR, Heshmati SC, Shimp KG, Spurrell MS, Bizon JL, et al. Effects of nucleus accumbens amphetamine administration on performance in a delay discounting task. Behavioural brain research. 2017,321:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B Effects of developmental nicotine exposure in rats on decision-making in adulthood. Behavioural pharmacology. 2012,23:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology. 2011,218:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, et al. Dopaminergic Modulation of Risky Decision-Making. Journal of Neuroscience. 2011,31:17460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Winstanley CA, Theobald DE, Dalley JW, Robbins TW Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005,30:669–82. [DOI] [PubMed] [Google Scholar]
- [53].Cardinal RN, Robbins TW, Everitt BJ The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000,152:362–75. [DOI] [PubMed] [Google Scholar]
- [54].Myerson J, Green L, Warusawitharana M Area under the curve as a measure of discounting. J Exp Anal Behav. 2001,76:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tanno T, Maguire DR, Henson C, France CP Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology. 2014,231:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Maguire DR, Henson C, France CP Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology. 2014,87:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Slezak JM, Anderson KG Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behavioural pharmacology. 2009,20:424–36. [DOI] [PubMed] [Google Scholar]
- [58].Shimp KG, Mitchell MR, Beas BS, Bizon JL, Setlow B Affective and cognitive mechanisms of risky decision making. Neurobiology of learning and memory. 2015,117:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hernandez CM, Orsini CA, Wheeler AR, Ten Eyck TW, Setlow B, Bizon JL Testicular hormones mediate robust sex differences in impulsive choice. eLife. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hernandez CM, Vetere LM, Orsini CA, McQuail JA, Maurer AP, Burke SN, et al. Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344 x brown Norway hybrid rats. Neurobiol Aging. 2017,60:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zoratto F, Laviola G, Adriani W The subjective value of probabilistic outcomes: Impact of reward magnitude on choice with uncertain rewards in rats. Neurosci Lett. 2016,617:225–31. [DOI] [PubMed] [Google Scholar]
- [62].Steele CC, Peterson JR, Marshall AT, Stuebing SL, Kirkpatrick K Nucleus accumbens core lesions induce sub-optimal choice and reduce sensitivity to magnitude and delay in impulsive choice tasks. Behav Brain Res. 2018,339:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kubanek J, Snyder LH, Abrams RA Reward and punishment act as distinct factors in guiding behavior. Cognition. 2015,139:154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Krebs CA, Reilly WJ, Anderson KG Reinforcer magnitude affects delay discounting and influences effects of d-amphetamine in rats. Behavioural processes. 2016,130:39–45. [DOI] [PubMed] [Google Scholar]
- [65].Galtress T, Kirkpatrick K The Role of the Nucleus Accumbens Core in Impulsive Choice, Timing, and Reward Processing. Behav Neurosci. 2010,124:26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Boomhower SR, Rasmussen EB Haloperidol and rimonabant increase delay discounting in rats fed high-fat and standard-chow diets. Behavioural pharmacology. 2014,25:705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Robertson SH, Rasmussen EB Effects of a cafeteria diet on delay discounting in adolescent and adult rats: Alterations on dopaminergic sensitivity. J Psychopharmacol. 2017,31:1419–29. [DOI] [PubMed] [Google Scholar]
- [68].Johnson PM, Kenny PJ Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010,13:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behavioural brain research. 2006,175:415–9. [DOI] [PubMed] [Google Scholar]
- [70].Tracy AL, Wee CJM, Hazeltine GE, Carter RA Characterization of attenuated food motivation in high-fat diet-induced obesity: Critical roles for time on diet and reinforcer familiarity. Physiology & Behavior. 2015,141:69–77. [DOI] [PubMed] [Google Scholar]
- [71].Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behavioral Neuroscience. 2008,122:1257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Finger BC, Dinan TG, Cryan JF Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology. 2012,220:173–81. [DOI] [PubMed] [Google Scholar]
- [73].Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L, et al. Dissociated neural substrates underlying impulsive choice and impulsive action. NeuroImage. 2016,134:540–9. [DOI] [PubMed] [Google Scholar]
- [74].Moore CF, Blasio A, Sabino V, Cottone P Impulsive choice does not predict binge-like eating in rats. Behavioural pharmacology. 2018,29:726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Avena NM, Rada P, Hoebel BG Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Biobehavioral Reviews. 2008,32:20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rada P, Avena NM, Hoebel BG Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005,134:737–44. [DOI] [PubMed] [Google Scholar]
- [77].Puhl MD, Cason AM, Wojnicki FHE, Corwin RL, Grigson PS A history of bingeing on fat enhances cocaine seeking and taking. Behavioral Neuroscience. 2011,125:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behavioral Neuroscience. 2010,124:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schag K, Schönleber J, Teufel M, Zipfel S, Giel KE Food-related impulsivity in obesity and binge eating disorder--a systematic review. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2013,14:477–95. [DOI] [PubMed] [Google Scholar]
- [80].Mole TB, Irvine MA, Worbe Y, Collins P, Mitchell SP, Bolton S, et al. Impulsivity in disorders of food and drug misuse. Psychological medicine. 2015,45:771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Carlin JL, McKee SE, Hill-Smith T, Grissom NM, George R, Lucki I, et al. Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice. Neuroscience. 2016,326:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cone JJ, Robbins HA, Roitman JD, Roitman MF Consumption of a high fat diet affects phasic dopamine release and uptake in the nucleus accumbens. Appetite. 2010,54:640. [Google Scholar]
- [83].Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiology & behavior. 2010,101:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pitts RC, Febbo SM Quantitative analyses of methamphetamine's effects on self-control choices: implications for elucidating behavioral mechanisms of drug action. Behavioural processes. 2004,66:213–33. [DOI] [PubMed] [Google Scholar]
- [85].Pitts RC, McKinney AP Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005,83:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology. 2000,152:47–54. [DOI] [PubMed] [Google Scholar]
- [87].Hailwood JM, Heath CJ, Robbins TW, Saksida LM, Bussey TJ Validation and optimisation of a touchscreen progressive ratio test of motivation in male rats. Psychopharmacology. 2018,235:2739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bensadoun JC, Brooks SP, Dunnett SB Free operant and discrete trial performance of mice in the nine-hole box apparatus: validation using amphetamine and scopolamine. Psychopharmacology. 2004,174:396–405. [DOI] [PubMed] [Google Scholar]
- [89].Slezak JM, Krebs CA, Anderson KG A within-subject analysis of d-amphetamine exposure on delay discounting in rats. Pharmacology, biochemistry, and behavior. 2012,102:502–6. [DOI] [PubMed] [Google Scholar]
- [90].Yates JR, Day HA, Evans KE, Igwe HO, Kappesser JL, Miller AL, et al. Effects of d-amphetamine and MK-801 on impulsive choice: Modulation by schedule of reinforcement and delay length. Behavioural brain research. 2019,376:112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Setlow B, Mendez IA, Mitchell MR, Simon NW Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behavioural pharmacology. 2009,20:380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiology & Behavior. 2006,89:611–6. [DOI] [PubMed] [Google Scholar]
- [93].la Fleur SE, Vanderschuren LJMJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan R. a. H. A reciprocal interaction between food-motivated behavior and diet-induced obesity. International Journal of Obesity. 2007,31:1286–94. [DOI] [PubMed] [Google Scholar]
- [94].Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B Sex differences in a rat model of risky decision making. Behavioral neuroscience. 2016,130:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Orsini CA, Blaes SL, Hernandez CM, Wheeler AR, Ten Eyck TW, Garman TS, et al. Regulation of risky decision making by gonadal hormones in male and female rats. Neuropharmacology. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Islas-Preciado D, Wainwright SR, Sniegocki J, Lieblich SE, Yagi S, Floresco SB, et al. Risk-based decision making in rats: Modulation by sex and amphetamine. Hormones and behavior. 2020,125:104815. [DOI] [PubMed] [Google Scholar]
- [97].Perry JL, Nelson SE, Carroll ME Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Experimental and clinical psychopharmacology. 2008,16:165–77. [DOI] [PubMed] [Google Scholar]
- [98].Eubig PA, Noe TE, Floresco SB, Sable JJ, Schantz SL Sex differences in response to amphetamine in adult Long-Evans rats performing a delay-discounting task. Pharmacology, biochemistry, and behavior. 2014,118:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sackett DA, Moschak TM, Carelli RM Prelimbic Cortical Neurons Track Preferred Reward Value and Reflect Impulsive Choice during Delay Discounting Behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019,39:3108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Panfil K, Bailey C, Davis I, Mains A, Kirkpatrick K A time-based intervention to treat impulsivity in male and female rats. Behavioural brain research. 2020,379:112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Keel PK, Baxter MG, Heatherton TF, Joiner TE Jr. A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. Journal of abnormal psychology. 2007,116:422–32. [DOI] [PubMed] [Google Scholar]
- [102].Rolls BJ, Fedoroff IC, Guthrie JF Gender differences in eating behavior and body weight regulation. Health Psychol. 1991,10:133–42. [DOI] [PubMed] [Google Scholar]
- [103].Liechty JM, Lee MJ Longitudinal predictors of dieting and disordered eating among young adults in the U.S. Int J Eat Disord. 2013,46:790–800. [DOI] [PubMed] [Google Scholar]
- [104].Berridge KC Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991,16:103–20. [DOI] [PubMed] [Google Scholar]
- [105].Yiin YM, Ackroff K, Sclafani A Food deprivation enhances the expression but not acquisition of flavor acceptance conditioning in rats. Appetite. 2005,45:152–60. [DOI] [PubMed] [Google Scholar]
- [106].Rowland NE Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp Med. 2007,57:149–60. [PubMed] [Google Scholar]
- [107].Santos-Pinto FN, Luz J, Griggio MA Energy expenditure of rats subjected to long-term food restriction. Int J Food Sci Nutr. 2001,52:193–200. [PubMed] [Google Scholar]


