Abstract
Neuroblastoma is a solid tumor (a lump or mass), often found in the small glands on top of the kidneys, and most commonly affects infants and young children. Among neuroblastomas, high-risk neuroblastomas are very aggressive and resistant to most kinds of intensive treatment. Immunotherapy, which uses the immune system to fight against cancer, has shown great promise in treating many types of cancer. However, high-risk neuroblastoma is often resistant to this approach as well. Recent studies revealed that small vesicles known as exosomes, which are envelopes, could deliver a cargo of small RNA molecules and provide communication between neuro-neuroblastoma cells and the surrounding cells and trigger metastasis and resistance to immunotherapy. In this chapter, we describe the role of exosomes and small RNA molecules in the metastasis and regression of neuroblastoma and the potential therapeutic approaches to combat this menace.
Keywords: Neuroblastoma, Exosomes, Non-coding RNAs, MYCN, Metastasis, Extracellular vesicles, Multivesicular bodies, Exosome biogenesis, Tumor microenvironment, Tumor-associated monocytes, Pericytes, Disialoganglioside, Antibody-dependent cell cytotoxicity, Chemotherapy, Immunotherapy
5.1. Neuroblastoma
Neuroblastoma is an embryonal tumor of the autonomic nervous system. This means that the origin of a cell is preceded to be developing immaturely in the neural-crest tissues [26, 29]. Neuroblastoma is the most common solid tumor found in infants and children. They account for almost 8–10% of all childhood tumors. The median age of diagnosis with neuroblastoma is 17 months [22]. Almost 15% of all deaths that are related to this cancer are in pediatrics [26, 29]. Almost 500 new cases are reported every year [26, 29]. 90% of cases are usually diagnosed before the age of five, and 30% of those are within the first year of life [11]. Neuroblastoma has been found to be more prevalent in males compared to females [26, 29], and the occurrence of neuroblastoma is unusual in adolescents and adults. 95% of all neuroblastomas occur in children under five years of age [11]. However, cases have been detected pre-birth, during an ultra-sound examination. Many patients diagnosed with neuroblastoma have shown to undergo immense relapse of neuroblastoma. In infants, the prognosis is very good, while it is somewhat at a disadvantage in older children. The patient outcome with the diagnosis of neuroblastoma has improved over the last 30 years. The 5-year survival rates in low- and medium-risk patients vary from 52% to 74% [26]. There is a prediction that around 50–60% of patients diagnosed with high-risk neuroblastoma will relapse [26]. The tumors begin in tissues of the sympathetic nervous system. This may cause a mass in the neck, chest, abdomen, or pelvis. A mass can either cause no symptoms or may progress into a tumor that causes severe illness. The diagnosis of neuroblastoma is 10.2 cases per million children under 15 years of age, and it is the most common cancer diagnosed during the first year of life [21, 26].
5.2. Exosomes
Exosomes are small extracellular vesicles secreted from cells and have lately attracted the attention of researchers worldwide owing to their critical role in intercellular signaling and disease. These are nanoparticles ranging from approximately 30–100 nm in size [41]. Exosomes are released out of the cell into the extracellular surrounding after multivesicular bodies (MVBs) fuse with the cellular membrane. All biological fluids tested have been shown to contain vesicles, including in vitro grown cell lines, which have also been shown to release vesicles to different extents. Canonical exosomes display a particular biconcave or cup-like shape when produced by artificially drying during preparation, while they appear spheroid in solution under a transmission electron microscope [31]. Typically, they have a density range from 1.13 g/mL (B cell-derived exosomes) [31] up to 1.19 g/mL (epithelial cell-derived exosomes) [31] on sucrose gradients.
Exosome biogenesis begins within the endosomal system. The early endosomes grow into multivesicular bodies (MVBs). During this process, the endosomal membrane encloses to generate intraluminal vesicles (ILVs) in the lumen of the organelles [15, 21]. The protein sorting of ILVs is a highly regulated mechanism that is dependent on the endosomal sorting complex required for transport (ESCRT) machinery [24] or ESCRT-independent mechanism [16]. Both pathways are not entirely separated. ESCRT has four different protein complexes: ESCRT-0, ESCRT-1, ESCRT-2, and ESCRT-3 [32].
The ESCRT mechanism is initiated by recognition and sequestration of ubiquitinated proteins to specific domains of the endosomal membrane via ubiquitin binding subunits of ESCRT-0. After interaction with ESCRT-I and -II complexes, the total complex will then combine with ESCRT-III, a protein complex that is involved in promoting the budding processes. Finally, following cleaving the buds to form ILVs, the ESCRT-III complex separates from the MVB membrane with energy supplied by the sorting protein Vps4 [32]. Despite the controversy of whether exosome release is an ESCRT-regulated mechanism, different ESCRT components and ubiquitinated proteins have already been identified in exosomes isolated from various cell types. Additionally, the typical exosomal protein Alix, which is associated with several ESCRT (TSG101 and CHMP4) proteins, has been reported to participate in endosomal membrane budding and abscission, as well as exosomal cargo selection via interaction with syntenin [32]. These observations led to a hypothesis implicating ESCRT function in exosomal biogenesis. ESCRT-independent manner depends on raft-based microdomains for the lateral segregation of cargo within the endosomal membrane. These microdomains are thought to be highly enriched in sphingomyelinases, from which ceramides can be formed by hydrolytic removal of the phosphocholine moiety [32]. Ceramides are known to induce lateral phase separation and coalescence of microdomains in model membranes. Moreover, the cone-shaped structure of ceramide might cause a spontaneous negative curvature of the endosomal membrane, thereby promoting domain-induced budding.
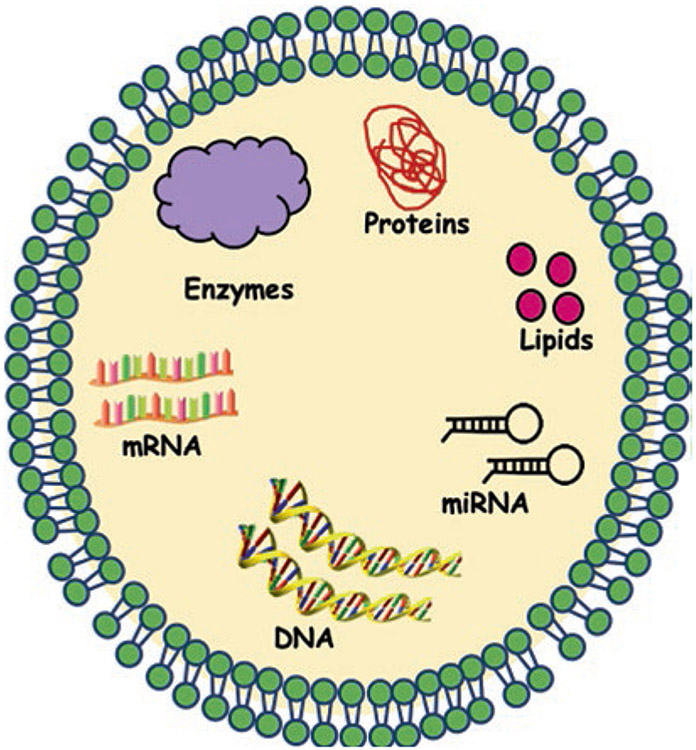
Consequently, this ceramide-dependent mechanism emphasizes the key role of exosomal lipids in exosome biogenesis [32]. Proteins, such as tetraspanins, also participate in exosome biogenesis and protein loading. Tetraspanin-enriched microdomains (TEMs) are ubiquitous specialized membrane platforms for compartmentalization of receptors and signaling proteins in the plasma membrane [32]. It has been shown that TEMs, together with tetraspanin CD81, plays a key role in sorting target receptors and intracellular components toward exosomes [32]. Exosomes play a critical role in physiological and pathological settings, strategies that interfere with the release of exosomes; and the impairment of exosome-mediated cell-to-cell communication could potentially be used in the future [32]. The general structure of the exosome molecule is given in Fig. 5.1.
Fig. 5.1.
General structure of exosome molecule
5.3. Neuroblastoma and Exosomes
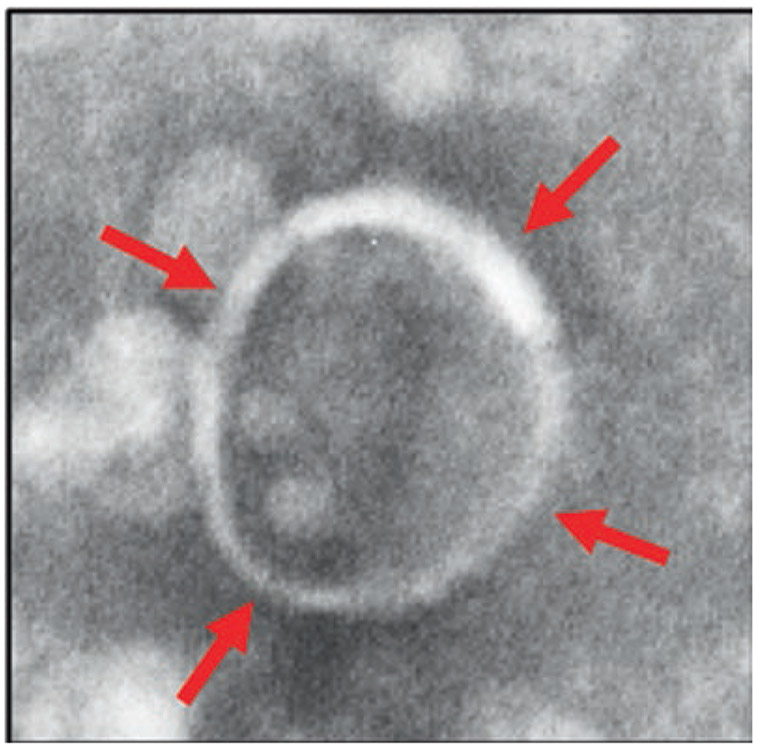
The majority of neuroblastoma deaths occur within two years of diagnosis due to the aggressiveness of the cancer. Exosomes are released by many cell types and transfer their molecular cargo to the target cells, thereby modulating the signaling pathways in the recipient cells. Fibroblasts, endothelial cells, and infiltrating immune cells are the major cell types within a tumor microenvironment that interacts with tumor cells by exosome signaling [7]. The consequences of these interactions depend on the origin of the exosomes determining the exosome cargo. Stressful conditions such as hypoxia, starvation, and acidosis increase exosome release from malignant cells leading to tumor microenvironment alteration and expansion, which subsequently results in tumor progression. Several pieces of evidence show that the major mechanism involved in tumor progression is the role of tumor-derived exosomes in cellular communication. Cancer cell-derived exosomes operate numerous functions. These include angiogenesis, anti-tumor immune responses, and metastatic ability, whereas the non-cancer cell-derived exosomes function by promoting the inhibition of malignant cells [5, 25, 27]. A transmission electron microscopy image of the exosome, showing 95-nm size, enclosed by a lipid bilayer, isolated from neuroblastoma (CHLA-255) cell culture supernatant is given in Fig. 5.2.
Fig. 5.2.
A transmission electron microscopy image of the exosome, showing 95-nm size, enclosed by a lipid bilayer, isolated from neuroblastoma (CHLA-255) cell culture supernatant. Scale: 100 nm
Nearly 20–30% of neuroblastoma cases are associated with the amplification of N-Myc oncogene, which are considered as high risk. Even though it is known that exosomes secreted from N-Myc-amplified neuroblastoma cells contain a tumor-specific signature, it is not known whether exosomes derived from N-Myc-amplified neuroblastoma cells can transfer the aggressive phenotype including chemoresistance between the cells. To test this, exosomes were isolated from derived N-Myc-amplified cancer cells and added to the non-Myc-amplified cell culture, and their properties studied. Addition of exosomes to the non-N-Myc-amplified cells induced migration, colony-forming abilities, and protected the cells against doxorubicin-induced apoptosis. This suggests that exosomes derived from N-Myc-amplified cancer cells can transfer the aggressive phenotype to the neighboring cells, thereby aiding in cancer progression. Proteomic analysis of N-Myc-amplified cancer cell exosomes showed enrichment of TGS101, FlOT1, and VPS35. In addition, exosomes of N-Myc-amplified cells are also enriched in signaling proteins such as NEDD4, β-catenin, and RhoA [10, 14].
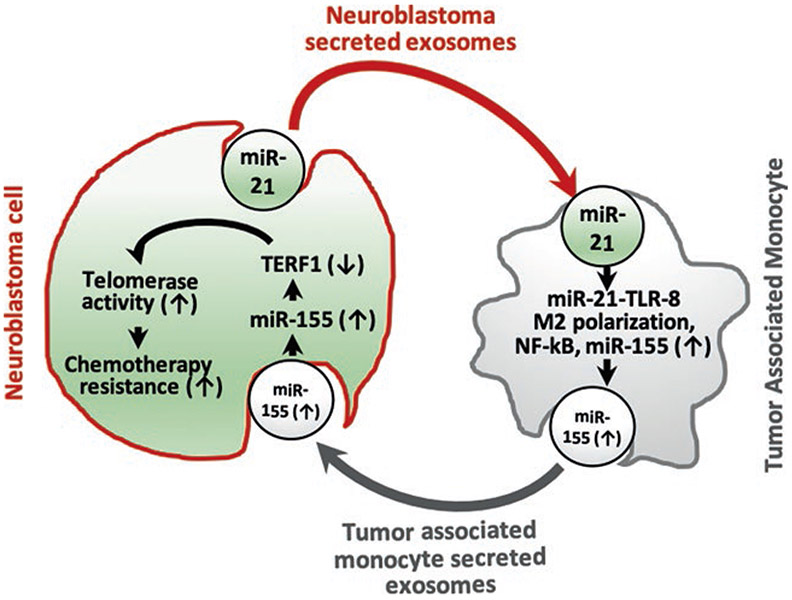
In addition to proteins, exosomes carry various molecules, including mRNAs, DNA, and microRNAs (miRNAs). MiRNAs play a significant role in the regulation of genes. MiRNAs work by inhibiting the translation of messenger-RNAs (mRNAs) or inducing mRNA breakdown by binding to the 3′-untranslated region (UTR) of the mRNAs [6]. The primary function of the miRNAs is the downregulation of gene expression. Recent studies on NB cell line exosomes explored the several miRNAs in them, and functional studies of these miRNAs revealed their profound influence on the target cells. Challagundla et al. purified exosomes from neuroblastoma cell lines (SK-N-B(E)2, CHLA-255, and IMR-32) and quantified the content of miR-21, miR-29a, and miR-155 by quantitative real-time PCR. Among these miRs, miR-21 and miR-29a are implicated in inflammatory reactions in lung cancer; miR-155 is induced during macrophage inflammatory response. In NBL cell line exosomes, miR-21P has been shown to be the top represented miRNA [7]. Monocytes on co-culture with the neuroblastoma cell lines revealed that miR-21 is transferred to human monocytes through exosomes. In monocytes, miR-21 induces upregulation of miR-155 levels in a Toll-like receptor-8 (TLR8)-dependent manner, and miR-155 is transferred from monocytes to neuroblastoma cells through exocytic vesicles. Exosomal targeting of miR-155 in NBL cell lines leads to the downregulation of TERF1 mRNA, an inhibitor of telomerase, thereby leading to increased telomerase activity. Higher telomerase activity is commonly associated with chemotherapy resistance in neuroblastoma patients through a novel exosomic miR-21/TLR8-NF-κB/miR-155/TERF1 signaling pathway [7]. The functional transfer of exosomal miRNAs from neuroblastoma cell to the surrounding monocyte and the development of chemotherapy resistance are given in Fig. 5.3.
Fig. 5.3.
A model depicting the transfer of exosomal miRNAs from neuroblastoma cell to the surrounding monocyte and the development of chemotherapy resistance
Pericytes are a type of fibroblast-like cells, capable of tumor homing and constitute one of the main components of the tumor microenvironment. Pericytes were first named as adventitial cells by Rouget in the nineteenth century and named as pericyte by Zimmermann in 1923. Pericytes are located within the basement membrane of the on-blood vessel walls, thus regulating blood flow, blood vessel permeability, and stabilization of the vascular wall. However, how many types of pericytes present and their role on the development of angiogenesis are not known until Birbrair et al. discovered a mechanistic approach in 2014 using a series of in vitro and in vivo experimentation involving a double transgenic Nestin-GFP/NG2-DsRed mice [4]. The authors identified two pericyte populations: type 1 pericytes expressing Nestin-GFP(−)/NG2-DsRed(+)] and type 2 pericytes expressing Nestin-GFP(+)/NG2-DsRed(+). These pericyte populations were functionally characterized using several in vitro assays and confirmed that type 2 pericytes, but not type 1, exhibit angio-genic potential and were recruited during tumor angiogenesis, raising more questions on the potential role of exosomes secreted from pericytes within the tumor microenvironment [4].
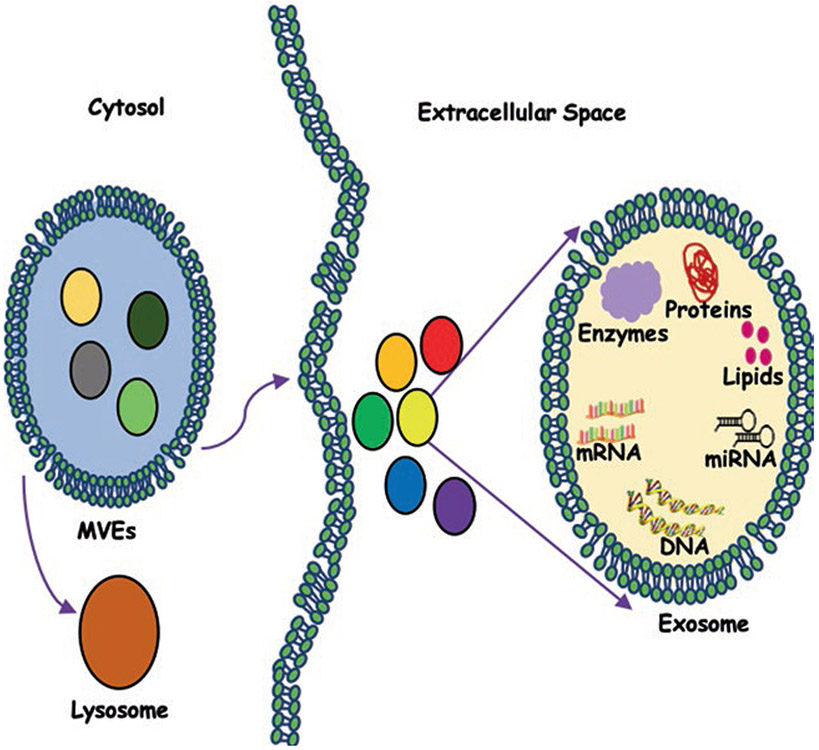
In neuroblastoma, cancer cells modulate the tumor microenvironment and play a role in immune escape mechanisms and drug resistance. Neviani et al. have shown that interleukin-15 (IL-15)-activated natural killer (NK) cells secrete exosomes, which exhibit cytotoxicity against MYCN-amplified neuroblastoma cells. The cytotoxic potential of these exosomes was partly dependent upon the expression of miR-186. Interestingly, authors have shown that miR-186 has been shown to be downregulated in high-risk neuroblastoma patients, and its lower levels are a poor prognostic factor. MiR-186 ectopic expression has been shown to downregulate the expression of neuroblastoma oncogenes – MYCN, AURKA, TGFβR1, and TGFβR2. In addition, ectopic expression of miR-186 in MYCN-amplified neuroblastoma cell lines inhibits its growth and migration. Targeted delivery of miR-186 to MYCN-amplified neuroblastoma or NK cells resulted in inhibition of neuroblastoma tumorigenic potential and prevented the TGFβ1-dependent inhibition of NK cells. Neviani et al. have shown targeted delivery of miR-186 in high-risk neuroblastoma is practicable and may lead to an inhibition of tumor growth and spreading [28]. The discovery that NK exosomes are cytotoxic and have their own killing ability even in an immunosuppressive microenvironment supports the belief of including ex vivo derived NK exosomes as a potential future benefit alongside the NK cell-based iimnunotherapy [28]. A schematic model of releasing exosomes into extracellular space is given in Fig. 5.4.
Fig. 5.4.
A schematic model showing the release of exosomes into extracellular space
Most of the studies on exosomes’ role in neuroblastoma are based on cell culture experiments, but studies on human neuroblastoma patient exosomes were lacking. To investigate the functions of tumor-derived exosomal miRNAs in neuroblastoma patients in progression and migration of neuroblastoma cells, Ma et al. utilized plasma-derived exosomes and carried out differential exosomal miRNA expression profiles [23]. Ma et al. identified that the expression of hsa-miR199a-3p is significantly upregulated, and strongly correlates with the severity in neuroblastoma patients. Exosomal hsa-miR199a-3p promotes tumor proliferation and migration via decreasing neuronal precursor cell-expressed developmentally downregulated 4 (NEDD4) expression in neuroblastoma. Ma et al. have shown that hsa-miR199a-3p may inhibit NEDD4 expression by binding to the 366–373 site of the 3′-UTR of NEDD4 mRNA in neuroblastoma cells, thereby miR-199a-3p promotes proliferation and facilitates migration of NB cells by regulating NEDD4 expression [23]. This work has shown that exosomal hsa-miR-199a-3p can be utilized as a fast, easy, and non-invasive detection biomarker and contribute to the development of novel therapeutic strategies for neuroblastoma in the future. Thus, the content analysis of the exosomes reveals their function in tumor microenvironment progression in malignancies, and this will further lead to developing more efficient micro vesicle-based strategies for cancer prognosis and therapy.
5.4. Treatment Options for Neuroblastoma
Treatment for neuroblastoma depends on the classification of the tumor. There are three broad categories: Low-risk, intermediate-risk, and high-risk [7-9]. Low-risk patients include those with localized tumors and tumors that show characteristics that indicate the tumor is not likely to come back. Low-risk patients are subject to minimal treatment or none at all [9]. Surgery may be the best option for these patients if the tumor is small enough to remove easily. Chemotherapy may be used as a treatment post-surgery, but most often, the patients are monitored for recurrence [9]. Chemotherapy used in low-risk patients includes a mixture of carboplatin, cyclophosphamide, doxorubicin, and etoposide most often [9]. Infants with very small tumors are usually monitored because these tumors are likely to disappear on their own without treatment [9].
Patients are classified as having intermediate risk if the tumor shows different characteristics, if the tumor is too large to fully resect, or if the tumor is causing damage to other organs [3]. These patients are given cycles of chemotherapy to initially shrink the size of the tumor. The amount of cycles depends on the size and severity of the tumor [3]. The typical combination of chemotherapy in this group is similar to low-risk patients. Carboplatin, cyclophosphamide, doxorubicin, and etoposide are generally used [30]. Once the tumor size has been decreased, surgery is the next step. In some cases, surgery is done prior to chemotherapy, to target the remaining tumor not resected in surgery [30]. Radiation is rarely used in the intermediate-risk group [3].
High-risk neuroblastoma patients have characteristics of metastasis or aggressive features of cancer cells [12, 17]. There are many ways to treat this category of neuroblastoma, and the most effective way has yet to be determined [12, 17]. The most common protocols include multiple phases of treatment: induction, surgery, consolidation, and maintenance [12, 17]. The induction phase focuses on reducing the size of the tumor and removing as much of the tumor as it is able in a quick manner [12, 17]. High dosages of chemotherapy are used, and the most common medications include cisplatin, etoposide, vincristine, cyclophosphamide, doxorubicin, and topotecan in a variety of alternating combinations [35]. Surgery is performed next to remove large amounts of the tumor [35]. After the surgery is performed, the next round of treatment begins to eliminate the body of any remaining cancer cells. Consolidation involves both chemotherapy and stem cell transplants. Research has shown that patients who are given high-dose chemotherapy followed by a stem cell transplant, have better results than straight chemotherapy [34, 35]. Stem cell transplant is autologous, so stem cells are harvested during the induction phase of therapy [34]. Also, stem cell transplants given back to back have shown promising results [34]. Once chemotherapy and the stem cell transplant are complete, radiation is done to the primary tumor site as well as any places of previous metastasis [35]. This ensures any left behind remnants are eliminated. The final phase in the treatment is maintenance, which is performed to prevent patients from relapse [35]. Treatments in this phase include medications to stimulate the immune system or to mature tumor cells [35]. Retinoid therapy using 13-cis-retinoic acid (isotretinoin) is most common, along with other immunotherapies [35]. Retinoid therapy causes cancer cells to differentiate into mature cells. Maintenance includes heavy monitoring of tumor relapse. Relapse occurs in approximately 50% of high-risk patients and 5–15% in low- and intermediate-risk patients. Normally, relapse occurs within the first few years after the initial treatment [35].
5.5. Neuroblastoma and Immunotherapy
Several studies have shown that the neuroblastoma microenvironment is immunosuppressive and tumor growth promoting. In recent times, many strategies are devised and tested to overcome this, and they are being developed to promote anti-tumor immunotherapy. The understanding of the biology of immunotherapy of neuroblastoma has increased a lot over the past 40 years [7, 8, 33]. Monoclonal antibody (MAb)-based immunotherapy, along with the discoveries in immune biology, has revolutionized the immunotherapeutic field to design more effective therapies for the treatment of high-risk neuroblastoma. These will be combined with new cytotoxic drugs and radiation therapies to improve survival and quality of life for patients with high-risk neuroblastoma [7, 8, 33].
Current therapy options for neuroblastoma are separated into three sections: induction, consolidation, and post-consolidation or maintenance therapy [33]. Treatment includes chemotherapy, surgical resection, and high-dose chemotherapy. Also included are stem cell rescue, radiation therapy, immunotherapy, and isotretinoin. The current treatment lasts approximately 18 months. The induction phase includes chemotherapy, stem cell collection, and surgery. The consolidation phase includes high dosages of chemotherapy and radiation therapy. The maintenance phase includes immunotherapy and retinoid therapy.
In immunotherapy, the patient’s immune cells are used to recognize and destroy cancer cells. Monoclonal antibodies are used to recognize and attack a very particular neuroblastoma target cell [30]. Anti-GD2 (disialoganglioside) mAbs are a part of standard immunotherapy for high-risk neuroblastoma [38]. Dinutuximab (Unituxin) is a humanized monoclonal antibody that recognizes and binds to GD2 on neuroblastoma membranes; these antibodies in turn bind to Fc-receptors on the surface of granulocytes and NK cells and eliminate neuroblastoma cells through antibody-dependent cell cytotoxicity (ADCC) and cell-mediated toxicity. Dinutuximab is administered along with cytokine, interleukin-2 (IL-2). These will help the child’s immune system seek out and demolish neuroblastoma cells. This is a new and advanced form of immunotherapy for children diagnosed with high-risk neuroblastoma. This antibody is usually given after all treatment options are exhausted, and a stem cell transplant has been done.
When diagnosed with high-risk neuroblastoma, it requires intensive treatment to achieve the current survival rate of slightly less than 50% [7, 8, 33]. With further research and a more robust understanding of the biology of neuroblastoma, there will be a way to identify factors that change the outcomes of patients who are diagnosed with this disease. Current research is focusing on further intensification of therapy to improve outcomes and evaluating the role of precision medicine in this patient population. With ground-breaking clinical trials and intense research into neuroblastoma, all possible options for treating patients who are diagnosed with this cancer are being explored, and the immunotherapy options are allowing for better hope for children diagnosed with this cancer.
5.6. Exosome-Mediated Therapeutics
Mesenchymal stem and stromal cells (MSCs) that have originated from multiple organs such as the bone marrow, umbilical cord (in-utero), adipose tissues, or placentas have shown to carry a therapeutic capacity of exosomes [36]. These were tested in numerous models of diseases. Exosome treatment has been compared to MSC treatment and has shown similar and even substantially better results [2]. When tested, MSC exosomes have exhibited favorable results that promote functional recovery and neurovascular plasticity. Some examples of these are subject, but not limited, to traumatic brain injury [40], the reduction in myocardial infarction size [1, 19], amelioration hypoxia-induced pulmonary hypertension [20], helping with the reparation of an injury to a kidney [13, 39], and the arrangement of neurological protection with the transfer of miRNA [18, 37].
Therapies that are exosome based portray a strong promise for the upcoming future in providing care for patients with various types of diseases. Patients with inflammatory diseases will especially benefit from exosome-based therapies [36]. The test for effectiveness in MSC-exosome treatments has been examined in multiple pre-clinical models. Safety concerns for the effectiveness of MSC-exosome treatment are of primary focus. All in all, though, cell-free exosome-based clinical trials are shown to have a slighter side-effect efficacy compared to live cell MSC trials [36].
5.7. Translational Advances
Exosomes could potentially play a role in the treatment of cancers. Exosomal vesicles can be used in a variety of ways to target different aspects of cancer, including diagnosis and treatment. Specifically, exosomes can be used as biomarkers for cancer diagnosis. Cancer cells are known to secrete more exosomes than healthy cells, which leads researchers to use this as a marker. The process of obtaining exosome samples from patients is fairly non-invasive, so this would be a clinically applicable way to help diagnose cancer. Not only can the exosomes be used as biomarkers, but also the proteins within the exosomes can be used to indicate cancer. Exosomes carry various molecules throughout the body, including proteins, miRNA, mRNA, etc. The overexpression of these molecules could be used as a potential prognostic factor for cancer. As well as a marker for cancer, exosomes could be used for treatment. Exosomes primarily function in cell communication, showing that they can interact with cell membranes to deliver their signals. This leads researchers to determine if exosomes could deliver drug therapy. Exosomes could be used as a method of delivering chemotherapy to malignant cells in the body. Also, it is thought that exosomes could be used to stimulate cytotoxic T cells into a response against cancer cells. The target of exosomes to stop tumor growth is also a consideration. Many studies have shown that exosomes display oncogenic and tumor-promoting effects. Therefore, the targeting of exosomes could be a key element to inhibiting tumor growth. Similarly, inhibiting the ability of cells to receive signals from the exosomes would also inhibit tumor progression. Although there are many possibilities of using exosome in cancer diagnosis and treatment, many mechanisms of exosomes are still undetermined. Further research into this field needs to be conducted.
5.8. Conclusion
Neuroblastoma is the most frequent solid tumor that is diagnosed in children under the age of 5. It develops in the immature nerve cells of the sympathetic nervous system during embryonic development. These tumors are most often found on the adrenal glands. Lumps in the abdomen or neck, bruising around the eyes, pain, fatigue, and weight loss are all common signs and symptoms of neuroblastoma. The current treatments for neuroblastoma involve a mixture of surgery, chemotherapy, radiation, retinoid therapy, and immunotherapy. Exosomes play an important role in the progression of this cancer. Exosomes are small vesicles that are secreted by cells. They regulate cell communication, and transfer molecules between many cells in the body. Exosomes have been shown to progress tumor development, cause resistance to chemotherapy, and serve as a biomarker for tumors. Recent advances made in understanding the function of exosomes hold a promise to develop anti-cancer therapies.
Acknowledgments
Dr. Challagundla’s laboratory is supported in whole or part from the NIH/NCI grant (CA197074); Leukemia Research Foundation (LRF) grant, the Nebraska State DHHS (LB506); UNMC Pediatric Cancer Research Center; Fred and Pamela Buffett Cancer Center’s pilot grant (P30 CA036727) in conjunction with the UNMC Pediatric Cancer Research Center; and the Department of Biochemistry and Molecular Biology start-up. Heather Richard and Arya Pokhrel are thankful to Terri L. Gulick, Jaynie E. Bird, Michele Merrill, and Heidi N. Kaschke and acknowledge the support of the UNMC High School Alliance Health Sciences Enrichment Program.
Abbreviations
- 3′-UTR
Three prime untranslated region
- ADCC
Antibody-dependent cell cytotoxicity
- AURKA
Aurora kinase A
- EFS
Event-free survival
- ESCRT
Endosomal-sorting complex required for transport
- GD2
Disialoganglioside
- IL-15
Interleukin-15
- IL-2
Interleukin-2
- ILV
Intraluminal vesicle
- MAb
Monoclonal antibody
- miRNA
MicroRNA
- mRNA
Messenger RNA
- MSCs
Mesenchymal stem/stromal cells
- MVBs
Multivesicular bodies
- MYCN
v-myc myelocytomatosis viral-related oncogene, neuroblastoma-derived
- NEDD4
Neuronal precursor cell-expressed developmentally downregulated 4
- NF-κB
Nuclear factor-kappa B
- NK
Natural killer
- PCR
Polymerase chain reaction
- PNTs
Peripheral neuroblastic tumors
- RNA
Ribonucleic acid
- TERF1
Telomeric repeat-binding factor 1
- TGFβ 1
Transforming growth factor beta 1
- TGFβR1
Transforming growth factor beta receptor 1
- TGFβR2
Transforming growth factor beta receptor 2
- TLR8
Toll-like receptor 8
Footnotes
Conflict of Interest The authors declare no conflict of interest.
Contributor Information
Heather Richard, High School Alliance Health Sciences Enrichment Program, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE, USA.
Arya Pokhrel, High School Alliance Health Sciences Enrichment Program, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE, USA.
Srinivas Chava, Department of Biochemistry and Molecular Biology & The Fred and Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE, USA.
Anup Pathania, Department of Biochemistry and Molecular Biology & The Fred and Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE, USA.
Santharam S. Katta, REVA University, Rukmini Knowledge Park Kattigenahalli, Yelahanka, Bangalore, Karnataka, India
Kishore B. Challagundla, Department of Biochemistry and Molecular Biology & The Fred and Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE, USA
References
- 1.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN et al. (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10(3):301–312. 10.1016/j.scr.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA et al. (2009) Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180(11):1122–1130. 10.1164/rccm.200902-0242OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A et al. (2010) Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 363(14):1313–1323. 10.1056/NEJMoa1001527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A et al. (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307(1):C25–C38. 10.1152/ajpcell.00084.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobrie A, Colombo M, Raposo G, Thery C (2011) Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12(12):1659–1668. 10.1111/j.1600-0854.2011.01225.x [DOI] [PubMed] [Google Scholar]
- 6.Brummer A, Hausser J (2014) MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. BioEssays 36(6):617–626. 10.1002/bies.201300104 [DOI] [PubMed] [Google Scholar]
- 7.Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T et al. (2015) Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst 107(7). 10.1093/jnci/djv135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW et al. (2020) miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol 14(1):180–196. 10.1002/1878-0261.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM et al. (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27(2):289–297. 10.1200/JCO.2008.16.6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colletti M, Petretto A, Galardi A, Di Paolo V, Tomao L, Lavarello C et al. (2017) Proteomic analysis of neuroblastoma-derived exosomes: new insights into a metastatic signature. Proteomics 17(23–24). 10.1002/pmic.201600430 [DOI] [PubMed] [Google Scholar]
- 11.Colon NC, Chung DH (2011) Neuroblastoma. Adv Pediatr Infect Dis 58(1):297–311. 10.1016/j.yapd.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daudigeos-Dubus E, Led L, Rouffiac V, Bawa O, Leguerney I, Opolon P et al. (2014) Establishment and characterization of new orthotopic and metastatic neuroblastoma models. In Vivo 28(4):425–434 [PubMed] [Google Scholar]
- 13.Dorronsoro A, Robbins PD (2013) Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther 4(2):39 10.1186/scrt187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseka P, Liem M, Ozcitti C, Adda CG, Ang CS, Mathivanan S (2019) Exosomes from N-Myc amplified neuroblastoma cells induce migration and confer chemoresistance to non-N-Myc amplified cells: implications of intra-tumour heterogeneity. J Extracell Vesicles 8(1):1597614 10.1080/20013078.2019.1597614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94(11):3791–3799 [PubMed] [Google Scholar]
- 16.Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75(2):193–208. 10.1007/s00018-017-2595-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Weiss WA (2013) Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 3(10):a014415 10.1101/cshperspect.a014415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalani A, Tyagi N (2015) Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res 10(10):1565–1567. 10.4103/1673-5374.165305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS et al. (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4(3):214–222. 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G et al. (2012) Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126(22):2601–2611. 10.1161/CIRCULATIONAHA.112.114173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS et al. (1999) Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst 91(16):1382–1390. 10.1093/jnci/91.16.1382 [DOI] [PubMed] [Google Scholar]
- 22.London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H et al. (2005) Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol 23(27):6459–6465. 10.1200/JCO.2005.05.571 [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Xu M, Yin M, Hong J, Chen H, Gao Y et al. (2019) Exosomal hsa-miR199a-3p promotes proliferation and migration in neuroblastoma. Front Oncol 9:459 10.3389/fonc.2019.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maas SLN, Breakefield XO, Weaver AM (2017) Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 27(3):172–188. 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B (2018) Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol 6:18 10.3389/fcell.2018.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362(23):2202–2211. 10.1056/NEJMra0804577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakata R, Shimada H, Fernandez GE, Fanter R, Fabbri M, Malvar J et al. (2017) Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J Extracell Vesicles. 6(1):1332941 10.1080/20013078.2017.1332941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY et al. (2019) Natural killer-derived Exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res 79(6):1151–1164. 10.1158/0008-5472.CAN-18-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JR, Eggert A, Caron H (2008) Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin N Am 55(1):97–120, x. 10.1016/j.pcl.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 30.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF et al. (2015) Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 33(27):3008–3017. 10.1200/JCO.2014.59.4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt O, Teis D (2012) The ESCRT machinery. Curr Biol 22(4):R116–R120. 10.1016/j.cub.2012.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeger RC (2011) Immunology and immunotherapy of neuroblastoma. Semin Cancer Biol 21(4):229–237. 10.1016/j.semcancer.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speleman F, Park JR, Henderson TO (2016) Neuroblastoma: a tough nut to crack. Am Soc Clin Oncol Educ Book 35:e548–e557. 10.14694/EDBK_159169. [DOI] [PubMed] [Google Scholar]
- 35.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5(3):219–234. 10.1038/nrd1984 [DOI] [PubMed] [Google Scholar]
- 36.Willis GR, Kourembanas S, Mitsialis SA (2017) Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med 4:63 10.3389/fcvm.2017.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X et al. (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564. 10.1002/stem.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX et al. (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363(14): 1324–1334. 10.1056/NEJMoa0911123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang G, Wang D, Miao S, Zou X, Liu G, Zhu Y (2016) Extracellular vesicles derived from mesenchymal stromal cells may possess increased therapeutic potential for acute kidney injury compared with conditioned medium in rodent models: A meta-analysis. Exp Ther Med 11(4):1519–1525. 10.3892/etm.2016.3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A et al. (2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122(4):856–867. 10.3171/2014.11.JNS14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19 10.1186/s13578-019-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]