Abstract
The regulation of the pleiotropic transcription factor, nuclear factor-κB (NF-κB) by miRNAs and proteins is extensively studied. More recently, the NF-κB signaling was also reported to be regulated by several long non-coding RNAs (lncRNAs) that constitute the major portion of the noncoding component of the human genome. The common NF-κB associated lncRNAs include NKILA, HOTAIR, MALAT1, ANRIL, Lethe, MIR31HG, and PACER. The lncRNA and NF-κB signaling crosstalk during cancer and other diseases such as cardiomyopathy, celiac disease, cerebral infarction, chronic kidney disease, diabetes mellitus, Kawasaki disease, pregnancy loss, and rheumatoid arthritis. Some NF-κB related lncRNAs can affect gene expression without modulating NF-κB signaling. Most of the lncRNAs with a potential to modulate NF-κB signaling are regulated by NF-κB itself suggesting a feedback regulation. The discovery of lncRNAs have provided a new type of regulation for the NF-κB signaling and thus could be explored for therapeutic interventions. The manner in which lncRNA and NF-κB crosstalk affects human pathophysiology is discussed in this review. The challenges associated with the therapeutic interventions of this crosstalk are also discussed.
Keywords: Cancer, Chronic disease, LncRNA, NF-κB, Non-coding RNA
1. Introduction
The pro-inflammatory transcription factor, nuclear factor-kappa B (NF-κB), plays a crucial role in the pathogenesis of several human diseases. It was originally identified as a regulator of immunoglobulin κ light chain in B lymphocytes [1]. Discovered over three decades ago [2], this transcription factor has now been identified in all cell types of the body and conserved all the way from Drosophila to human [3,4]. Being a crucial modulator of inflammatory and immune response, the dysregulation in NF-κB signaling is associated with plethora of diseases [5]. While acute NF-κB is required for the proper functioning of the innate immune system, chronic NF-κB activation facilitate the pathogenesis of human diseases [6].
In humans, NF-κB consists of 5 subunits which include NF-κB1 (p105/p50), NF-κB2 (p100/p52), c-Rel, RelA (p65), and RelB [1]. These subunits regulate the NF-κB dependent target genes expression by forming homodimers or heterodimers [7,8]. Further, these subunits associate with the inhibitory κB (IκB) proteins in the cytoplasm and control NF-κB activation under normal conditions [1,9]. The canonical (classical) and noncanonical (alternative) are two main pathways for the NF-κB activation [10]. While NF-κB1 is involved in the canonical pathway, NF-κB2 is required for the noncanonical pathway [11]. Before the translocation of active NF-κB to the nucleus, NF-κB1 and NF-κB2 undergo cleavage to form the active p50 and p52 subunits, respectively. The IκBα kinase (IKK) complex consisting of IKKα, IKKβ, and NF-κβ essential modulator (NEMO, also called IKKγ) is activated during the classical pathway of NF-κB activation. In the classical pathway, IKK phosphorylates IκBα at two serine residues that trigger its ubiquitination and degradation. The newly released NF-κB heterodimeric complex consisting of p65/RelA and p50 then translocate to the nucleus and regulate the expression of hundreds of NF-κB dependent genes. During the alternative pathway, the p52 subunit processed from p100 is translocated to the nucleus along with RelB as a heterodimer and regulate the gene expression [12].
In addition to the common pathways described above, NF-κB can also be regulated by additional mechanisms. Simply defined as the transcribed but untranslated component of the genome, the non-coding RNAs mainly comprised of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). While miRNAs are 18–22 nucleotides in length, the lncRNAs contain ≥200 nucleotides. Although the regulation of NF-κB signaling by miRNAs is relatively well characterized, only a few studies have demonstrated the association of lncRNAs with NF-κB signaling. The lncRNAs play a crucial role in numerous physiological and pathological processes [13]. Because of their presence in the cells, serum, plasma, urine, and saliva, lncRNAs could be used as a diagnostic and prognostic biomarker in human patients [14,15]. In this review, we discuss the crosstalk between lncRNA and NF-κB signaling. The significance of NF-κB related lncRNAs in the etiology of cancer and other human diseases are also discussed (Table 1).
Table 1.
Functions of NF-κB associated lncRNAs.
| NKILA |
|
| Carlr |
| HOTAIR |
| MALAT1 |
|
| ANRIL |
| Lethe |
| MIR31HG |
|
| PACER |
| lincRNA-Cox2 |
| lincRNA-p21 |
| C2dat1 |
| Arid2-IR |
|
| Gm4419 |
|
| Mirt1 |
2. NF-κB associated lncRNAs
The NF-κB is one of the thoroughly studied pro-inflammatory transcription factors which is regulated by a number of proteins and miRNAs. During the last 5 years, the lncRNAs are reported to regulate NF-κB signaling either in a direct or indirect manner. In some instances, NF-κB signaling also regulate the lncRNA expression. Although the mechanistic association of NF-κB signaling with some lncRNAs is dissected out, it is yet to be elucidated for others (Fig. 1). In the sections to follow, we discuss the lncRNAs that are associated with NF-κB signaling. How the crosstalk between NF-κB signaling and lncRNA contribute to the pathophysiology of human diseases is discussed below and illustrated in Fig. 2. The challenges associated with the targeting of NF-κB associated lncRNAs for therapy are also discussed.
Fig. 1.
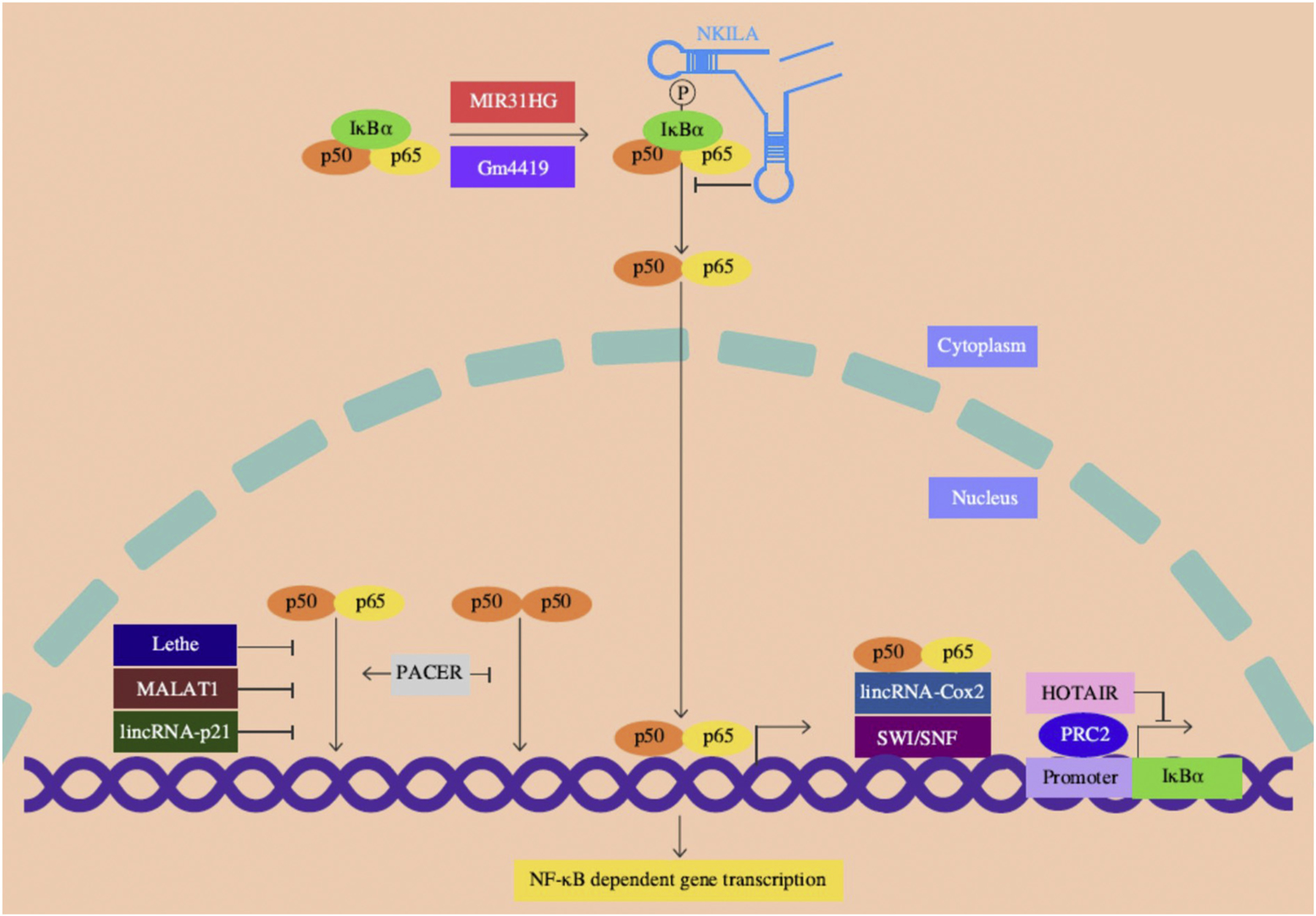
Schematic representation for the role of lncRNAs in NF-κB signaling. NKILA associate with the NF-κB-IκB complex in the cytoplasm masking the IκB phosphorylation thereby suppressing its degradation and NF-κB activation. Lethe, MALAT1 and lincRNA-p21 are largely located in the nucleus, directly associate with p65 and prevent the p65/p50 heterodimer binding to the target promoters. Upon LPS stimulation, PACER occludes the binding of inhibitory p50/p50 homodimers but promotes the active p65/p50 heterodimer formation at the COX2 promoter. HOTAIR acts in trans of the IκBα gene and suppresses its expression partly by recruiting PRC2, which is a chromatin-modifying enzyme complex. The lincRNA-Cox2 facilitate the p65/p50 heterodimer binding to the target site by recruiting the SWI/SNF complex. Under normal conditions, MIR31HG is located in the nucleus. Upon inflammatory stimulation, MIR31HG is exported to the cytoplasm, induces IκBα phosphorylation by direct interaction leading to NF-κB activation. Gm4419 can also physically associate with and induce IκBα phosphorylation and NF-κB activation. Other lncRNAs discussed in the text are not indicated as their mechanistic association with NF-κB signaling is not clearly known. Abbreviations: HOTAIR: HOX transcript antisense RNA; IκBα: inhibitor of kappa B alpha; lincRNA-p21: long intergenic noncoding RNA-p21; MALAT1: metastasis associated lung adenocarcinoma transcript 1; MIR31HG: MIR31 host gene; NKILA: NF-κB interacting lncRNA; PACER: P50 associated cyclooxygenase-2 (COX-2) extragenic RNA; PRC2: polycomb repressive complex 2; SWI/SNF: switch/sucrose nonfermentable.
Fig. 2.
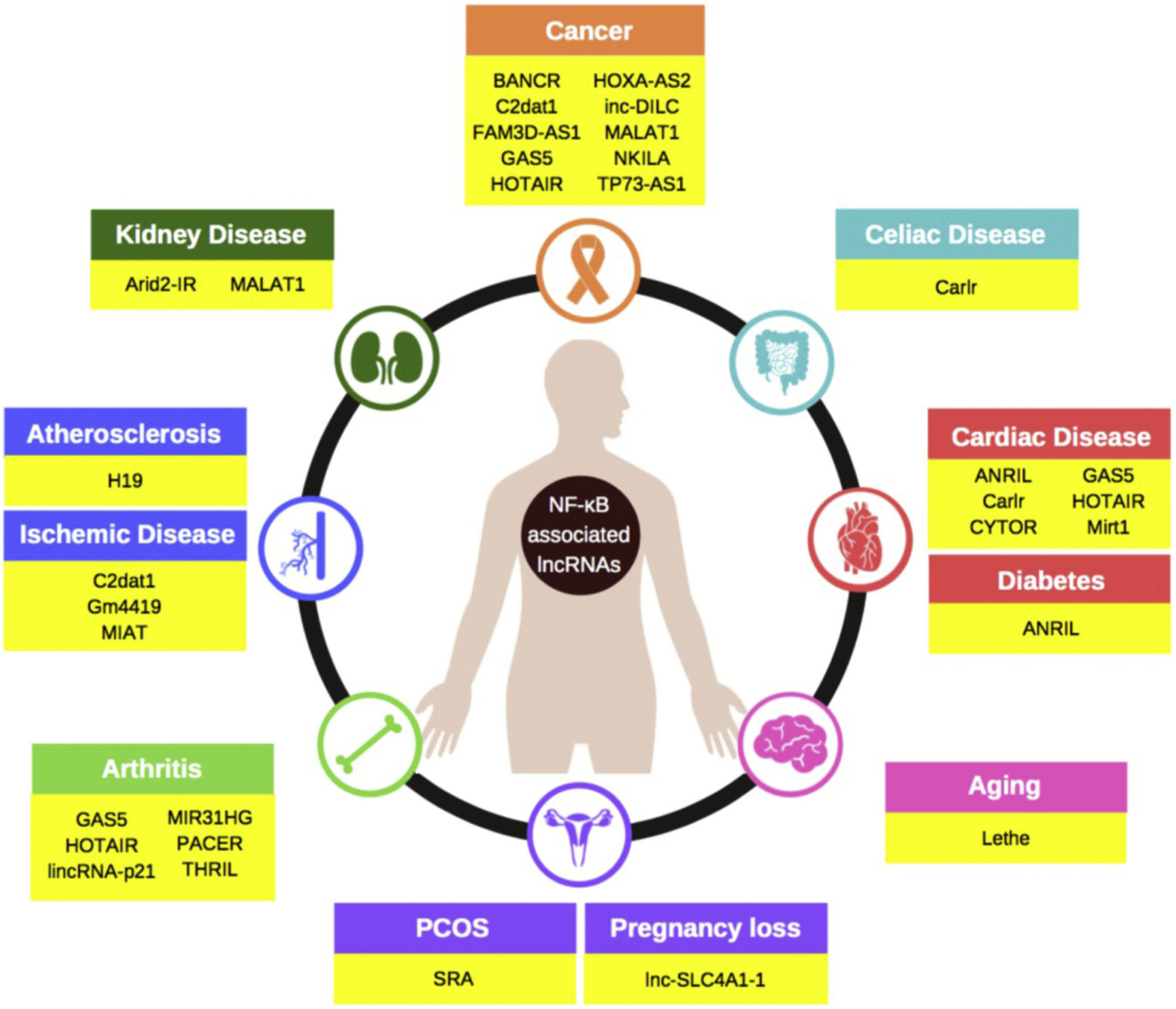
The NF-κB associated lncRNAs involved in human diseases. Abbreviations: ANRIL: antisense non-coding RNA in the INK4 locus; Arid2: AT rich interaction domain 2; BANCR: BRAF activated non-coding RNA; C2dat1: CAMK2D associated transcript 1; Carlr: cardiac and apoptosis related lncRNA; CYTOR: cytoskeleton regulator RNA; FAM3D-AS1: family with sequence similarity 3 member D antisense RNA 1; GAS5: growth arrest specific 5; HOTAIR: HOX antisense intergenic RNA; HOXA-AS2: HOXA cluster antisense RNA 2; Lnc-DILC: lncRNA downregulated in liver cancer stem cells; lincRNA-p21: long intergenic noncoding RNA p21; MALAT1: metastasis associated lung adenocarcinoma transcript 1; MIR31HG: MIR31 host gene; Mirt1: myocardial infarction associated transcript 1; NKILA: nuclear factor κB interacting lncRNA; PACER: P50 associated cyclooxygenase 2 extragenic RNA; THRIL: TNFα and hnRNPL related immunoregulatory lincRNA; SRA: steroid receptor RNA activator; TP73-AS1: tumor protein P73-antisense RNA1.
2.1. NKILA
Originally identified in breast cancer, the NF-κB interacting lncRNA (NKILA) is located in the cytoplasm [16]. The lower level of NKILA is reported in hepatocellular carcinoma [17], melanoma [18], non-small cell lung cancer (NSCLC) [19], and tongue squamous cell carcinoma (TSCC) under normal conditions [20].
When breast cancer cells were challenged with NF-κB inducing stimuli, NKILA was upregulated by > 12-folds suggesting that its expression may be driven by NF-κB [16]. In fact, the promoter region of the NKILA gene harbors NF-κB binding motif. In cytoplasm, the NKILA binds to NF-κB p65-IκB complex to form a stable ternary complex and masks the phosphorylation sites of IκB, leading to inhibition of IκB phosphorylation and NF-κB activation [16]. In breast epithelial cells stimulated with inflammation, NKILA prevents NF-κB over-activation. The lower level of NKILA also associates with metastasis and poor prognosis of breast cancer patients. The lower levels of NKILA associate with aberrant NF-κB activation even in the absence of an inflammatory stimulus. NKILA thus acts as NF-κB “gatekeeper” in unstimulated cells. The NKILA is highly expressed in non-invasive breast cancers and normal breast epithelia. Furthermore, the gene silencing of NKILA results in phosphorylation and degradation of IκB. Most of the currently available NF-κB suppressors act upstream to IKK activation or affect the level of NF-κB or IκB [21,22]. That NKILA masks the phosphorylation motifs of IκB without affecting IKK activity is a new type of negative regulation on NF-κB signaling.
In human melanoma tissues, NF-κB negatively correlates with NKILA [18]. NKILA can suppress the progress of melanoma cells possibly through its negative effects on NF-κB signaling [18]. The low level of NKILA in tumors significantly correlate with lymph node metastasis and advanced TNM stage in NSCLC [19]. Furthermore, the NKILA expression is regulated via classical TGF-β signaling, that in turn interferes with NF-κB/Snail signaling. This suppresses the migration and invasion of NSCLC cells [19]. In TSCC patients, the low level of NKILA was significantly correlated with tumor metastasis and poor prognosis [20]. NKILA can inhibit the IκBα phosphorylation, NF-κB activation, and epithelial-mesenchymal transition (EMT) resulting in reduced migration and invasion of TSCC cells.
In certain cases, NKILA can also enhance the efficacy of anti-cancer agents. For example, the anti-cancer activities of baicalein, which is a flavonoid [23], were enhanced by NKILA in hepatocellular carcinoma cells [17]. Mechanistically, NKILA inhibited IκBα phosphorylation as well as nuclear translocation and activity of NF-κB in HCC cells. Although both baicalein and NKILA inhibited NF-κB signaling, the effect was significantly enhanced by the combination of two approaches. NKILA is antisense to the PMEPA1, a highly conserved gene that encodes protein associated with immune pathways [24]. Although NKILA has been proposed to be evolved as an additional means of PMEPA1 regulation, the mechanistic link between the two is yet to be elucidated [24].
In summary, NKILA negatively regulates NF-κB signaling, acts as a tumor suppressor and thus its upregulation could be potentially therapeutic. Consistent with these observations, other negative regulators of NF-κB such as deubiquitinase [25], A20 [26], ubiquitin ligase SOCS-1 [21], and a group of miRNAs [27] also act as tumor suppressors. Because NF-κB is crucial in several other pathophysiological conditions, the role of NKILA on NF-κB signaling should be elucidated under these conditions.
2.2. Carlr
Cardiac and apoptosis-related lncRNA (Carlr) was originally characterized in mouse cardiomyocytes [28]. Carlr can suppress apoptosis and mitochondrial fission by targeting miRNA-539 and PHB2 in cardiomyocytes. Both mouse and human cells of multiple tissues are known to express Carlr [28]. Upon stimulation with LPS, Carlr expression is increased in mouse and human macrophages in NF-κB dependent manner. Furthermore, Carlr is translocated to the cytoplasm after NF-κB activation in mouse and human macrophages [29]. The knockdown of Carlr impairs the expression of NF-κB related genes. In human celiac disease patient samples, Carlr expression correlates with the expression of NF-κB pathway genes. Moreover, Carlr can interact with activated NF-κB in intestinal biopsies suggesting a possibility for the role of Carlr in the development of inflammation. Thus, Carlr is a novel player of the NF-κB signaling and a clinically relevant lncRNA for immune-mediated diseases. Further studies should delineate the molecular mechanism by which Carlr modulate NF-κB activation.
2.3. HOTAIR
HOX Antisense Intergenic RNA (HOTAIR) is an oncogene that is dysregulated in multiple cancers [30]. Localized both in the nucleus and the cytoplasm, HOTAIR can regulate multi-step of tumor development. The immunoregulatory factors such as TNF-α can modulate HOTAIR expression in cancer cells [31]. The NF-κB can bind to the HOTAIR promoter and induce its expression by 16-fold in ovarian cancer cells leading to the maintenance of DNA damage response (DDR) [32]. The increased HOTAIR expression correlates with a significant reduction in IκBα and enhanced NF-κB activation. It was concluded that HOTAIR-NF-κB-DDR-positive feedback loop leads to persistent DNA damage and reduced genomic integrity in ovarian cancer [32]. The increased HOTAIR expression also leads to an up-regulation in NF-κB target genes and the acquisition of chemotherapy resistance [33]. Consistency with these observations, HOTAIR-NF-κB-DDR loop has also been reported during deregulated immunity in septic cardiomyopathy [34]. Whether the HOTAIR-NF-κB-DDR feedback loop lies at interface between an innate immunity and autoimmunity should also be elucidated.
In addition to its negative effects on the IκBα expression, HOTAIR can also regulate NF-κB activity by modifying p65 subunit at the posttranslational level. For example, in septic mice model and in cardiomyocytes, LPS-induced HOTAIR expression was associated with an elevation in TNF-α levels and p65 phosphorylation [34]. However, how HOTAIR phosphorylates p65 remains to be elucidated. Conversely, a significant reduction in HOTAIR expression was observed in LPS-treated chondrocytes and in a mouse model of rheumatoid arthritis [35]. Furthermore, HOTAIR overexpression also significantly inhibited NF-κB activation leading to down-regulation of IL-1β and TNF-α in response to LPS [35]. Overall, these results suggest that HOTAIR expression and its role on NF-κB signaling is context-dependent.
2.4. MALAT1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a lncRNA that is highly conserved across the mammals. The prognostic significance of this lncRNA is reported for NSCLC [36]. MALAT1 is involved in the pathogenesis of several cancer types including esophageal squamous cell carcinoma [37], glioma [38], renal cell carcinoma [39], colorectal cancer [40], breast cancer [41], gallbladder cancer [42], and prostate cancer [43]. Additionally, MALAT1 is involved during several other pathological and physiological conditions such as vessel growth, synaptogenesis, and myogenesis [44–46]. In spite of all this, the genetic deletion of MALAT1 in murine model produces no phenotype under normal physiological conditions [47]. Therefore, it is crucial to identify the right pathological and environmental context under which MALAT1 is required [47]. In one study, MALAT1 was found to regulate the production of cytokines in macrophages under inflammatory conditions [48]. Upon LPS stimulation, MALAT1 expression was increased in human THP1 monocyte cell line in an NF-κB dependent manner. MALAT1 is also known to physically interact with both p65 and p50 and suppresses NF-κB DNA binding activity and the NF-κB target gene expressions such as TNF-α and IL-6 [48]. In agreement with these observations, MALAT1 knockdown increases TNF-α and IL-6 expression upon LPS stimulation. MALAT1 can modulate EMT through the modulation of NF-κB activity [48,49]. Overall, MALAT1 negatively regulate TNF-α and IL-6 expression in human monocytes. In the mouse model of renal ischemia-reperfusion injury, an increase in the expression of MALAT1 is reported [50]. MALAT1, in turn, could inhibit the inflammatory response induced by hypoxia through the NF-κB pathway.
Conversely, MALAT1 is also required for TNF-α and IL-6 up-regulation in response to glucose in human umbilical vein endothelial cells [51]. It is likely that the MALAT1 exhibits anti-inflammatory and pro-inflammatory effects depending upon cellular context and the cell type. In another study, overexpression of MALAT1 was reported in oral squamous cell carcinoma (OSCC) tissues in comparison to normal oral mucosa [52]. Further, MALAT1 was proposed as a prognostic factor [52]. Gene silencing of MALAT1 revealed that this lncRNA is required for maintaining EMT mediated cell migration and invasion in OSCC cell lines. A significant suppression in the vimentin and N-cadherin expression but induction in the E-cadherin expression was observed by the MALAT1 knockdown. MALAT1 knockdown also attenuated both nuclear and cytoplasmic contents of β-catenin and NF-κB. Consistency with these observations, an elevation in MALAT1 was found to trigger β-catenin and NF-κB. Interestingly, targeting MALAT1 suppressed the TSCCA tumor growth in xenograft mice model. Thus, MALAT1 represent crucial prognostic factor and therapeutic target for OSCC.
In conclusion, MALAT1 appears to be an important oncogenic molecule in multiple cancer types and can regulate several steps of tumor development including EMT, migration, invasion, and metastasis. Mechanistically, MALAT1 modulates key cancer-related pathways such as NF-κB and β-catenin. That MALAT1 modulate NF-κB signaling has been demonstrated by the computational studies as well [53]. Additionally, the knockdown of tumor necrosis factor-α-inducible protein 8 (TNFAIP8) was associated with decreased expression of MALAT1 [54]. However, MALAT1 appears to act as a double-edged sword on the NF-κB signaling. Future studies should investigate the molecular mechanism by which MALAT1 regulates cancer-related genes; this will help in the development of better treatment for cancer therapy.
2.5. ANRIL
The antisense noncoding RNA in the INK4 locus (ANRIL) is transcribed from Chr9p21, which is a high-risk locus for atherosclerosis, coronary artery disease (CAD), cancer, diabetes and periodontitis [55]. ANRIL is a PRC2 ‘scaffold’ lncRNA known to have a role in the epigenetic silencing of genes [56]. This lncRNA is highly induced by LPS, IL-1β, IFN-γ, and TNF-α in human vascular endothelial cells [57]. Furthermore, induction of ANRIL expression by TNF-α is mediated through NF-κB. The elevated ANRIL can modulate the expression of NF-κB dependent inflammatory molecules such as IL-6 and IL-8 [57]. Mechanistically, ANRIL interacts with and facilitates the biding of Ying Yang 1 (YY1) transcription factor with IL-6 and IL-8 promoters [57,58]. The binding of YY1 with IL-6/IL-8 promoter is abrogated by ANRIL silencing. These observations suggest that ANRIL and YY1 cooperatively regulate the expression of inflammatory genes. In the rat model of cerebral infarction and diabetes mellitus, ANRIL can induce VEGF expression and angiogenesis through NF-κB activation [59]. Overall, these results suggest the role of NF-κB mediated ANRIL in the etiology of CAD and diabetes mellitus. However, extensive studies are required before ANRIL can be recommended as a therapeutic target for CAD.
2.6. Lethe
Lethe is a pseudogene lncRNA that can modulate NF-κB signaling [60]. Lethe was selectively induced by TNF-α in an NF-κB-dependent manner in mouse embryonic fibroblast (MEF) cells [60]. The chromatin immunoprecipitation assay in p65−/− cells suggested that Lethe directly regulate p65 transcription [60]. Mostly located in the nucleus, Lethe directly interacts with p65 and inhibit the DNA binding activity of NF-κB. This reduces the production of NF-κB dependent inflammatory molecules [60]. Therefore, Lethe negatively regulates NF-κB and fine tunes the inflammatory response [60]. Furthermore, Lethe was dramatically downregulated in the aged spleen. The association of aging with NF-κB-mediated gene expression programs is well established [61]. It is likely that the age associated loss of Lethe contribute to NF-κB activation during aging. These observations suggest that a pseudogene may function as lncRNA. Although Xist has also been reported to function as pseudogene lncRNA [62], Lethe is the first pseudogene lncRNA known to regulate NF-κB signaling. Additionally, Lethe can also regulate ROS production in macrophages through modulation of NOX-2 expression and NF-κB signaling [63]. Whether other pseudogene lncRNAs influence NF-κB signaling remains to be elucidated.
2.7. MIR31HG
MIR31HG is located on chromosome 9p21.3 that contain putative NF-κB binding sites on its promoter [64]. MIR31HG affects proliferation, differentiation, and motility of tumor cells [65]. In one study the functions of MIR31HG in osteogenesis of human adipose-derived stem cells (hASCs) was evaluated in vitro and in vivo [66]. The silencing of MIR31HG significantly promoted osteogenic differentiation in hASCs. The suppression in MIR31HG also abrogated inflammation-induced suppression of osteogenesis in hASCs. The p65 can bind to the MIR31HG promoter and enhance its expression. The MIR31HG also directly interacted with IκBα and facilitated its phosphorylation and NF-κB activation. Upon inflammatory stimulation, MIR31HG was exported to the cytoplasm from the nucleus. The authors of this study concluded that targeting MIR31HG-NF-κB pathway may improve the osteogenic activity of hASCs in the inflammatory microenvironment. However, MIR31HG regulated several other signaling pathways as well. It is also possible that MIR31HG regulates bone formation by interaction with other signaling pathways.
2.8. PACER
P50-associated COX-2 extragenic RNA (PACER) is expressed upstream to COX-2 and controls its expression [67]. Apart from being involved in the biosynthesis of prostaglandins, COX-2 also plays a crucial role during tumor development [68]. PMA is known to induce COX-2 and PACER expression in human monocytes [67]. Similarly, PACER and COX-2 expression are markedly upregulated when macrophages are exposed to LPS [67]. However, PMA-induced and LPS-stimulated COX-2 expression are drastically reduced in PACER knockdown cells [67]. The implication of these observations suggests that PACER can modulate COX2 expression. Under normal conditions, the inhibitory p50/p50 NF-κB homodimers interact with the COX2 promoter and repress COX2 transcription. In response to LPS, the elevated PACER occludes the binding of inhibitory p50/p50 homodimers but promotes the association of active p65/p50 heterodimers with the COX2 promoter. Eventually, this helps with the assembly of histone acetyltransferase p300 and RNAP II pre-initiation complexes to the promoter leading to gene activation [67]. Thus, PACER functions in cis to promote COX2 transcription. In agreement with these observations, PACER was rapidly induced upon IL-1β stimulation in primary human osteoarthritis chondrocytes [69]. However, whether PACER physically associates with COX2 upon IL-1β stimulation is not known. Similarly, it is not clear if PACER acts in trans to modulate other NF-κB dependent genes expression. An overexpression in PACER expression is also reported in osteosarcoma cell lines and tissues [70]. PACER was found to activate COX-2 in osteosarcoma cells in an NF-κB-dependent manner. Furthermore, PACER knockdown reduced the osteosarcoma cell proliferation and invasion by downregulating the COX-2 expression. Overall, PACER could be a potential therapeutic target for COX-2 associated cancers.
2.9. lincRNA-Cox2
The lincRNA-Cox2 is located at the chromosome 1, proximal to, but downstream of the COX-2 gene and is a critical component of the inflammatory response. This lncRNA is highly induced after stimulation with LPS and during the innate immune response [71]. Upon stimulation with toll-like receptor (TLR) ligand, lincRNA-Cox2 and the adjacent Cox2 display similar expression patterns in dendritic cells and in macrophages [71]. However, lincRNA-Cox2 silencing does not alter Cox2 expression [71]. Upon LPS stimulation, the NF-κB-regulated late-primary inflammatory response genes transcription requires lincRNA-Cox2 [72]. The lincRNA-Cox2 can physically interact with the switch/sucrose nonfermentable (SWI/SNF) chromatin remodeling complex in both microglia and macrophages in response to LPS. The SWI/SNF complex comprising of SWI and SNF encoded proteins, can promote late-primary and secondary response genes transcription upon NF-κB activation [73,74]. The resulting lincRNA-Cox2/SWI/SNF complex is recruited to the late inflammatory genes at its promoter/enhancer region. This, in turn, recruits NF-κB, remodels chromatin and induces gene transcription in response to LPS stimulation. Overall, these results suggest that lincRNA-Cox2 interact with SWI/SNF and NF-κB to activate the transcription of late inflammatory genes in LPS-stimulated murine macrophages. Conversely, lincRNA-Cox2 is also responsible for the suppression of inflammatory genes transcription upon TLR signaling in intestinal epithelial cells and macrophages [71,75].
2.10. lincRNA-p21
The lincRNA-p21 is located 15 kb upstream of the Cdkn1a/p21 gene and can participate in a variety of biological processes [76,77]. The lincRNA-p21 can physically interact with hnRNP-K and play a role in protein stability and p53-regulated processes [76,78]. One study was aimed to determine the interrelationships between the expression of lincRNA-p21, NF-κB activity, and methotrexate (MTX) responses in rheumatoid arthritis (RA) patients and cell culture models [79]. An increase in the basal level of p-p65 was correlated with reduced level of lincRNA-p21 in RA patients. Furthermore, low-dose MTX reduced p-p65 and enhanced lincRNA-p21 in patients. MTX induced lincRNA-p21 via the mediation of DNA-dependent protein kinase catalytic subunit (DNA PKcs) in cell culture model. Furthermore, MTX suppressed TNF-α induced NF-κB activity through DNA PKcs and lincRNA-p21. The reduced levels of TP53 and lincRNA-p21 was associated with increased NF-κB activity in cell lines. The lincRNA-p21 was found to physically interact with p65 mRNA. Notably, the level of p65 mRNA transcript was not altered by a reduced level of lincRNA-p21. These observations suggest that lincRNA-p21 may suppress NF-κB activity by inhibiting p65 mRNA translation. It may be concluded that the lincRNA-p21 negatively regulate NF-κB activity that contribute to the anti-inflammatory activities of MTX in RA patients. However, the possibility of involvement of additional mechanisms cannot be ruled out. Extensive studies are required before lincRNA-p21 can be implied as a therapeutic target for RA patients.
2.11. C2dat1
CAMK2D-associated transcript 1 (C2dat1) overlaps with the introns 13–15 and exon 14 of CaMK2D. Originally identified in the ischemia/reperfusion (I/R) murine model, C2dat1 can promote tumor progression by interacting with Sirt1 and miR-34–5p in osteosarcoma cells [80]. C2dat1 is upregulated in an in vivo I/R murine model as well as in mouse neuronal cells in response to ischemia oxygen-glucose deprivation/reoxygenation (OGD/R) [81]. In response to OGD/R, C2dat1 induced CAMK2D expression and promoted neural survival. The OGD/R also induced IKKα/β phosphorylation, IκB degradation, NF-κB activation, as well as induction of Bcl-xL in neural cells. These observations suggest that NF-κB plays a pro-survival role in the N2a cells. Upon C2dat1 knockdown, suppression in CaMKIID expression, IKK expression, IKK phosphorylation, and IκBα degradation was observed [81]. This resulted in an inhibition of NF-κB activity [81]. Overall, these observations suggest that C2dat1 plays a neuroprotective role and thus can be therapeutically targeted for I/R-induced neuronal injury.
2.12. Arid2-IR
Arid2-IR is one of the highly expressed lncRNAs in the mouse unilateral ureteral obstructive (UUO) chronic kidney disease model [82]. It is situated in the intronic region of the mouse Arid2 gene and is functionally associated with Smad3 in UUO model [82]. Structurally, Arid2-IR promoter contains Smad3 binding site and knockout of Smad3 can abolish Arid2-IR expression in the diseased kidney [82]. Furthermore, genetic manipulation studies suggest that Arid2-IR can enhance NF-κB activity and the pro-inflammatory cytokine genes induced by IL-1β [82]. In conclusion, Smad3-induced lncRNA drives NF-κB-mediated renal inflammation [82].
2.13. DLEU
DLEU1 and DLEU2 maps at the chromosome 13q14.3 which is recurrently deleted in hematopoietic malignancies and solid tumors. Additionally, the 13q14.3 locus contains genes encoding tumor suppressors C13ORF1, KPNA3, miR-15a/16–1 and RFP2 [83]. These tumor suppressors are known to regulate the NF-κB activity [83]. In CML cells, these tumor suppressors are down-regulated, while DLEU1 and DLEU2 are up-regulated [83]. The epigenetic characterization revealed appreciable DNA-demethylation of two specific sequences at the transcriptional start sites (TSS) of DLEU1 and DLEU2. The DNA demethylation of DLEU1 and DLEU2 correlated with transcriptional deregulation of the tumor suppressors at the neighboring sites. It is likely that the two lncRNAs (DLEU1, DLEU2) and the neighboring tumor suppressors within chromosome 13q14.3 are functionally related [83]. In support of this view, KPNA3, RFP2, and C13ORF1 within 13q14.3 were found to positively regulate NF-κB activity. Interestingly, miR-15/16 family was the strongest inducer of NF-κB activity. Although these results suggest the possibility of regulation of NF-κB by DLEU1 and DLEU2, the exact molecular link is not known.
2.14. Gm4419
The lncRNA Gm4419 was recently reported to play a crucial role in the activation of NF-κB signaling and inducing neuroinflammation in the microglial cells stimulated with glucose OGD/R damage [84]. The OGD/R stimulus can abnormally upregulate Gm4419 in microglial cells [84]. Furthermore, Gm4419 can physically associate with and promote IκBα phosphorylation leading to an increase in the NF-κB nuclear content and the activation of IL-1β, IL-6 and TNF-α at the transcription level [84]. Additionally, Gm4419 knockdown functions as NF-κB inhibitor. Thus, Gm4419 down-regulation may protect against OGD/R injury. In conclusion, Gm4419 induced NF-κB activation is essential for OGD/R injury in microglial cells. Thus, Gm4419 represents an attractive therapeutic target in ischemic stroke.
2.15. Mirt1
Originally identified in response to myocardial infarction, myocardial infarction associated transcript 1 (Mirt1) is expressed mostly in cardiac fibroblasts [85]. The knockdown of Mirt1 is associated with an improved cardiac function, and reduced apoptosis and inflammatory cell infiltration in vivo [86]. The apoptosis of cardiomyocytes was suppressed after Mirt1 knockdown in cardiac fibroblasts. Further, the knockdown of Mirt1 suppressed the hypoxia-induced migration of macrophages. Mechanistically, hypoxia-induced NF-κB activation is suppressed in Mirt1 knockdown fibroblasts. In aged diabetic rats, an improvement in the myocardial I/R injury was observed after down-regulation of MIRT1 through suppression of NF-κB activation [87]. Collectively, Mirt1 may induce NF-κB activation and inflammatory response in cardiomyocytes. The apoptosis of cardiomyocytes is crucial for AMI pathogenesis and is a determinant to the fate of the heart [88]. Thus, Mirt1 represent a potential target for AMI. However, the human homolog of murine Mirt1 is yet to be identified. Future studies should also elucidate the molecular mechanism by which Mirt1 regulate NF-κB signaling.
2.16. IL1β-RBT46
In one study, RNA sequencing was used to characterize the lncRNA transcriptome in primary human monocytes [89]. Upon LPS stimulation, a number of antisense lncRNAs, canonical lncRNAs, enhancer RNAs (eRNAs), and regions of bidirectional transcription (RBT) were differentially expressed. The IL1β-RBT46 and IL1β-eRNA that surrounds the IL1β locus are localized in the nucleus. The NF-κB was required for the expression of both IL1β-eRNA and IL1β-RBT46. Furthermore, the knockdown of IL1β-RBT46 and IL1β-eRNA attenuated LPS-induced expression and release of IL1β and CXCL8. Thus, lncRNAs could be crucial modulators of the human innate immune response and help to release inflammatory mediators in human monocytes [89]. However, how the expression of IL1β and CXCL8 is regulated by these lncRNAs remains to be elucidated.
2.17. Other NF-κB associated lncRNAs
In addition to lncRNAs discussed in the above section, a list of lncRNAs has been identified that are either associated with NF-κB signaling or NF-κB regulated gene expression. For example, lnc-IL7R overlaps the 3’untranslated region (3’UTR) of the interleukin-7 receptor α-subunit (IL7R) gene. Upon LPS stimulation, this lncRNA is significantly upregulated [90]. In turn, lnc-IL7R negatively modulates the expression of proinflammatory molecules (IL-6, IL-8, VCAM-1, E-selectin) induced by LPS. lnc-IL7R is also induced by engagement of TLR2 or TLR4. Similarly, TNF-α, which is downstream to NF-κB signaling can be regulated by lncRNA THRIL [91]. In osteoarthritis cell injury model, THRIL exerted pro-inflammatory roles by suppressing miR-125b and activating JAK1/STAT3 and NF-κB pathways [92]. AS-IL1α, which is a natural antisense transcript can regulate IL-1α transcription during the innate immune response [93]. The lncRNA PVT1 can regulate TNF-α and JNK/NF-κB signaling, and promote inflammatory response in LPS-induced septic acute kidney injury (AKI) model [94]. Under glucose starvation, the p53-regulated long noncoding RNA, TRING can protect cancer cells from necrotic cell death partly through modulation of NF-κB signaling [95]. The NF-κB associated lncRNAs have also been reported in women with repeated implantation failure [96]. FIRE, a highly conserved lncRNA is controlled by NF-κB signaling in macrophages and intestinal epithelial cells [97]. The lincRNA-IBIN acts as a link between innate immune response and metabolism and is regulated by NF-κB signaling in Drosophila melanogaster [98]. The lncRNA, CamK-A is aberrantly expressed in multiple cancer types and remodels tumor microenvironment via activation of Ca2+-dependent signaling pathway [99]. Mechanistically, CamK-A-induced Ca2+/calmodulin-dependent kinase activation, IκBα phosphorylation, and calcium-dependent NF-κB activation. Clinically, the activation of CaMK-NF-κB axis was in coordination with CamK-A expression. Further, the high expression of CamK-A correlates with poor patient survival suggesting that this lncRNA could be used as a potential biomarker and therapeutic target.
In triple-negative breast cancers (TNBCs), environmental and chemotherapeutic stresses are known to induce the lncRNA BMP/OP-responsive gene (BORG) expression [100]. BORG, in turn, promotes the survival of TNBC cells and make them resistant to the doxorubicin. The chemoresistant traits of BORG were dependent upon its ability to induce NF-κB activation and to bind and activate RPA1. The BORG-expressing TNBCs were sensitized to doxorubicin after the suppression of NF-κB activation and an interruption in the DNA-binding activity of RPA1. Thus, BORG could be targeted therapeutically to alleviate TNBC recurrence. The lncRNA LOXL1-AS1 contributes to mesenchymal characteristics of glioblastoma through NF-κB signaling [101]. The lincRNA-tumor necrosis factor α-induced protein 3 (lincRNA-Tnfaip3) is an early response gene which is located at mouse chromosome 10 proximal to the Tnfaip3 gene [102]. Upon LPS stimulation, lincRNA-Tnfaip3 modulate the NF-κB-regulated inflammatory genes transactivation by forming NF-κB/Hmgb1/lincRNA-Tnfaip3 complex [102]. DNA damage-sensitive RNA1 (DDSR1) is induced in response to DNA damage. Some other NF-κB associated lncRNAs include Tmevpg1 in T helper type 1 cells [103]; HOXA-AS2 in breast cancer [104]; BANCR in gastric cancer [105]; FAM3D-AS1 in colorectal cancer [106]; TP73-AS1 in hepatocellular carcinoma [107]; GAS5 in colorectal cancer [108], osteosarcoma [109], chondrocytes [110], and in cardiomyocytes [111]; MIAT in ischemia/reperfusion injury model [112]; lnc-SLC4A1–1 in unexplained recurrent pregnancy loss (URPL) patients [113]; CYTOR in cardiac hypertrophy model [114]; XIST in the bovine mammary epithelial cells [115]; SRA in the polycystic ovary syndrome model [116]; H19 in multiple myeloma [117], melanoma [118], osteosarcoma [119], and in atherosclerosis model [120]; IFNG-AS1 in human T cells [121]; lncRNA-POIR during osteogenic differentiation [122]; lnc-DILC in liver cancer stem cells [123]; UCA1 in the hippocampus of rats [124]; and CUDR in hepatocyte-like stem cells [125].
3. Conclusions and future perspective
The lncRNAs constituting a major fraction of the human genome can regulate many aspects of gene expression and are thereby functionally involved in the human pathophysiology. As discussed in this review, lncRNAs are frequently expressed only in a subset of cells that provides an exclusive opportunity for exploring their therapeutic potential in a selective manner. Moreover, the lncRNAs are often expressed in a context-dependent manner. For example, despite being abundant and highly conserved across the taxa, loss of MALAT1 has been associated with a lack of adverse phenotype in mice [47]. On the other hand, loss of NF-κB components such as NEMO, IKK, p65, and IκBα is lethal for the development of embryo and neonates. Thus, targeting MALAT1 in disease and context-specific manner may produce only minimal side effects [47,126].
Considering the involvement of lncRNAs in NF-κB signaling and their association with human diseases, targeting lncRNAs appears promising. The NF-κB associated lncRNAs can be targeted employing small molecule inhibitors, antisense technology, small interfering RNA/short hairpin RNA-mediated RNA interference and masking the binding motifs required for NF-κB-lncRNA interactions. Another potential anti-cancer strategy could be the use of synthetic lncRNA mimics that negatively regulate NF-κB signaling. The phytochemicals are also known to target lncRNAs [127–129]. Being relatively easy and efficient, the CRISPR/Cas9 technology can also be employed to manipulate lncRNA sequences [130].
In summary, lncRNAs have emerged as promising molecules for controlling NF-κB signaling, the aberrant activation of which is linked with several pathological conditions. Like protein and miRNA regulators, lncRNAs can also be induced by NF-κB inducing stimuli and thus are involved in the feedback regulation. Because of redundancy in the cell signaling pathways, modulation in one pathway may be compensated by the alternate pathways. Thus, co-targeting lncRNAs and NF-κB could be an ideal strategy. The field is still in infancy and several avenues remains to be explored. How do lncRNAs and NF-κB work together in time and space to precisely regulate human pathophysiology? Can the diagnosis/prognosis of human diseases be improved by combining lncRNAs and NF-κB based strategy? Can the lncRNA-NF-κB crosstalk be targeted selectively in patients? What is the downside of the combined targeting of lncRNAs and NF-κB signaling? What is the evolutionary significance of lncRNAs and NF-κB? Are they evolved together? These outstanding questions require thorough investigation before lncRNA-NF-κB crosstalk can move to the clinic.
Acknowledgement
This work was supported in part by funds from the Science and Engineering Research Board (ECR/2016/000034), and University Grants Commission [30-112/2015 (BSR)] to SCG. Dr. Challagundla’s laboratory is supported in whole or part from the NIH/NCI grant (CA197074); Leukemia Research Foundation grant, the Nebraska State DHHS (LB506); UNMC Pediatric Cancer Research Center; Fred and Pamela Buffett Cancer Center’s pilot grant (P30 CA036727) in conjunction with the UNMC Pediatric Cancer Research Center; and the Department of Biochemistry and Molecular Biology start-up. The financial assistance from Indian Council of Medical Research, Government of India to NA (5/3/8/40/ITR-F/2019-ITR) and VR (3/2/2/43/2018/Online Onco Fship/NCD-III) is thankfully acknowledged.
Abbreviations:
- ANRIL
antisense noncoding RNA in the INK4 locus
- Arid2
AT-rich interaction domain 2
- C2dat1
CAMK2D-associated transcript 1
- CAMK2D
calcium/calmodulin-dependent protein kinase II delta
- Carlr
cardiac and apoptosis-related lncRNA
- EMT
epithelial-to-mesenchymal transition
- HCC
hepatocellular carcinoma
- HOTAIR
HOX antisense intergenic RNA
- IL
interleukin
- LPS
lipopolysaccharide
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- MIR31HG
MIR31 host gene
- miRNA
microRNA
- Mirt1
myocardial infarction associated transcript 1
- MTX
methotrexate
- NKILA
NF-κB interacting lncRNA
- NOX2
NADPH oxidase 2
- NSCLC
non-small-cell lung carcinoma
- OGD/R
oxygen-glucose deprivation/reoxygenation
- OSCC
oral squamous cell carcinoma
- PACER
P50-associated COX-2 extragenic RNA
- PHB2
prohibitin 2
- RA
rheumatoid arthritis
- ROS
reactive oxygen species
- Sirt1
sirtuin1
- TNF-α
tumor necrosis factor alpha
- TNM
tumor node metastasis
- TSCC
tongue squamous cell carcinoma
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of Competing Interest
None.
References
- [1].Hayden MS, Ghosh S, Signaling to NF-κB, Genes Dev. 18 (2004) 2195–2224. [DOI] [PubMed] [Google Scholar]
- [2].Sen R, Baltimore D, Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism, Cell 47 (1986) 921–928. [DOI] [PubMed] [Google Scholar]
- [3].Yadav AK, Srikrishna S, Gupta SC, Cancer drug development using drosophila as an in vivo tool: from bedside to bench and back, Trends Pharmacol. Sci 37 (2016) 789–806. [DOI] [PubMed] [Google Scholar]
- [4].Ghosh S, May MJ, Kopp EB, NF-kappa B, Rel proteins: evolutionarily conserved mediators of immune responses, Annu. Rev. Immunol 16 (1998) 225–260. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Q, Lenardo MJ, Baltimore D, 30 years of NF-kappaB: a blossoming of relevance to human pathobiology, Cell 168 (2017) 37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aggarwal BB, Sung B, NF-κB in cancer: a matter of life and death, Cancer discovery 1 (2011) 469–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karin M, Greten FR, NF-κB: linking inflammation and immunity to cancer development and progression, Nat. Rev. Immunol 5 (2005) 749. [DOI] [PubMed] [Google Scholar]
- [8].Luo J-L, Kamata H, Karin M, IKK/NF-κB signaling: balancing life and death–a new approach to cancer therapy, J. Clin. Invest 115 (2005) 2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ghosh S, Karin M, Missing pieces in the NF-κB puzzle, Cell 109 (2002) S81–S96. [DOI] [PubMed] [Google Scholar]
- [10].Zhang Q, Lenardo MJ, Baltimore D, 30 years of NF-κB: a blossoming of relevance to human pathobiology, Cell 168 (2017) 37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hayden MS, Ghosh S, Shared principles in NF-κB signaling, Cell 132 (2008) 344–362. [DOI] [PubMed] [Google Scholar]
- [12].Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li Z-W, Karin M, Ware CF, Green DR, The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways, Immunity 17 (2002) 525–535. [DOI] [PubMed] [Google Scholar]
- [13].Wang KC, Chang HY, Molecular mechanisms of long noncoding RNAs, Mol. Cell 43 (2011) 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huarte M, The emerging role of lncRNAs in cancer, Nat. Med 21 (2015) 1253. [DOI] [PubMed] [Google Scholar]
- [15].Chandra Gupta S, Nandan Tripathi Y, Potential of long non-coding RNAs in cancer patients: from biomarkers to therapeutic targets, Int. J. Cancer 140 (2017) 1955–1967. [DOI] [PubMed] [Google Scholar]
- [16].Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D, A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis, Cancer Cell 27 (2015) 370–381. [DOI] [PubMed] [Google Scholar]
- [17].Yu X, Tang W, Yang Y, Tang L, Dai R, Pu B, Feng C, Xia J, Long noncoding RNA NKILA enhances the anti-cancer effects of baicalein in hepatocellular carcinoma via the regulation of NF-κB signaling, Chem. Biol. Interact 285 (2018) 48–58. [DOI] [PubMed] [Google Scholar]
- [18].Bian D, Gao C, Bao K, Song G, The long non-coding RNA NKILA inhibits the invasion-metastasis cascade of malignant melanoma via the regulation of NF-ĸB, Am. J. Cancer Res 7 (2017) 28. [PMC free article] [PubMed] [Google Scholar]
- [19].Lu Z, Li Y, Wang J, Che Y, Sun S, Huang J, Chen Z, He J, Long non-coding RNA NKILA inhibits migration and invasion of non-small cell lung cancer via NF-κB/snail pathway, J. Exp. Clin. Cancer Res 36 (2017) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang W, Cui X, Chen J, Feng Y, Song E, Li J, Liu Y, Long non-coding RNA NKILA inhibits migration and invasion of tongue squamous cell carcinoma cells via suppressing epithelial-mesenchymal transition, Oncotarget 7 (2016) 62520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ruland J, Return to homeostasis: downregulation of NF-κB responses, Nat. Immunol 12 (2011) 709. [DOI] [PubMed] [Google Scholar]
- [22].Awasthee N, Rai V, Chava S, Nallasamy P, Kunnumakkara AB, Bishayee A, Chauhan SC, Challagundla KB, Gupta SC, Targeting IkappaappaB kinases for cancer therapy, Semin. Cancer Biol 56 (2019) 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Awad R, Arnason J, Trudeau V, Bergeron C, Budzinski J, Foster B, Merali Z, Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties, Phytomedicine 10 (2003) 640–649. [DOI] [PubMed] [Google Scholar]
- [24].Dijkstra JM, Alexander DB, The “NF-ĸ B interacting long noncoding RNA”(NKILA) transcript is antisense to cancer-associated gene PMEPA1, F1000Research 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brummelkamp TR, Nijman SM, Dirac AM, Bernards R, Loss of the cylin-dromatosis tumour suppressor inhibits apoptosis by activating NF-κB, Nature 424 (2003) 797. [DOI] [PubMed] [Google Scholar]
- [26].Shembade N, Ma A, Harhaj EW, Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes, Science 327 (2010) 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boldin MP, Baltimore D, MicroRNAs, new effectors and regulators of NF-κB, Immunol. Rev 246 (2012) 205–220. [DOI] [PubMed] [Google Scholar]
- [28].Wang K, Long B, Zhou L-Y, Liu F, Zhou Q-Y, Liu C-Y, Fan Y-Y, Li P-F, CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation, Nat. Commun 5 (2014) 3596. [DOI] [PubMed] [Google Scholar]
- [29].Castellanos-Rubio A, Kratchmarov R, Sebastian M, Garcia-Etxebarria K, Garcia L, Irastorza I, Ghosh S, Cytoplasmic form of Carlr lncRNA facilitates inflammatory gene expression upon NF-κB activation, J. Immunol 199 (2017) 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu X, Li Z, Long non-coding RNA HOTAIR: a novel oncogene (review), Mol. Med. Rep 12 (2015) 5611–5618. [DOI] [PubMed] [Google Scholar]
- [31].Hajjari M, Salavaty A, HOTAIR: an oncogenic long non-coding RNA in different cancers, Cancer biology & medicine 12 (2015) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Özeş AR, Miller DF, Özeş ON, Fang F, Liu Y, Matei D, Huang T, Nephew KP, NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer, Oncogene 35 (2016) 5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Janssens S, Tinel A, Lippens S, Tschopp J, PIDD mediates NF-κB activation in response to DNA damage, Cell 123 (2005) 1079–1092. [DOI] [PubMed] [Google Scholar]
- [34].Wu H, Liu J, Li W, Liu G, Li Z, LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway, Biochem. Biophys. Res. Commun 471 (2016) 240–246. [DOI] [PubMed] [Google Scholar]
- [35].Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang XY, Geng Q, Cui YH, Wang XH, LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-kappaB pathway, Int. Immunopharmacol 50 (2017) 283–290. [DOI] [PubMed] [Google Scholar]
- [36].Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer, Oncogene 22 (2003) 8031. [DOI] [PubMed] [Google Scholar]
- [37].Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y, Yang K, Up-regulation of long noncoding RNA MALAT1 contributes to proliferation and metastasis in esophageal squamous cell carcinoma, J. Exp. Clin. Cancer Res 34 (2015) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma K, Wang H, Li X, Li T, Su G, Yang P, Wu J, Long noncoding RNA MALAT1 associates with the malignant status and poor prognosis in glioma, Tumor Biol. 36 (2015) 3355–3359. [DOI] [PubMed] [Google Scholar]
- [39].Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R, Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205, Cancer Res. 75 (2015) 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhuang M, Zhao S, Jiang Z, Wang S, Sun P, Quan J, Yan D, Wang X, MALAT1 sponges miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility, EBioMedicine 41 (2019) 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhao Z, Chen C, Liu Y, Wu C, 17β-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level, Biochem. Biophys. Res. Commun 445 (2014) 388–393. [DOI] [PubMed] [Google Scholar]
- [42].Wu X-S, Wang X-A, Wu W-G, Hu Y-P, Li M-L, Ding Q, Weng H, Shu Y-J, Liu T-Y, Jiang L, MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway, Cancer biology & therapy 15 (2014) 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang F, Ren S, Chen R, Lu J, Shi X, Zhu Y, Zhang W, Jing T, Zhang C, Shen J, Development and prospective multicenter evaluation of the long non-coding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancer, Oncotarget 5 (2014) 11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Han X, Yang F, Cao H, Liang Z, Malat1 regulates serum response factor through miR-133 as a competing endogenous RNA in myogenesis, FASEB J. 29 (2015) 3054–3064. [DOI] [PubMed] [Google Scholar]
- [45].Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, Zhang MQ, Sedel F, Jourdren L, Coulpier F, A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression, EMBO J. 29 (2010) 3082–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Michalik KM, You X, Manavski Y, Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel GrowthNovelty and Significance, Circ. Res, 114 (2014) 1389–1397. [DOI] [PubMed] [Google Scholar]
- [47].Gutschner T, Hämmerle M, Diederichs S, MALAT1—a paradigm for long non-coding RNA function in cancer, J. Mol. Med 91 (2013) 791–801. [DOI] [PubMed] [Google Scholar]
- [48].Zhao G, Su Z, Song D, Mao Y, Mao X, The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-κB, FEBS Lett. 590 (2016) 2884–2895. [DOI] [PubMed] [Google Scholar]
- [49].Luo F, Wei H, Guo H, Li Y, Feng Y, Bian Q, Wang Y, L.N.A. MALAT1, A lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM2.5 in lung bronchial epithelial cells, American journal of physiology, Lung Cellular and Molecular Physiology, 2019. [DOI] [PubMed] [Google Scholar]
- [50].Tian H, Wu M, Zhou P, Huang C, Ye C, Wang L, The long non-coding RNA MALAT1 is increased in renal ischemia-reperfusion injury and inhibits hypoxia-induced inflammation, Ren. Fail 40 (2018) 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S, Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells, J. Cell. Mol. Med 19 (2015) 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhou X, Liu S, Cai G, Kong L, Zhang T, Ren Y, Wu Y, Mei M, Zhang L, Wang X, Long non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial-Mesenchymal transition in Oral squamous cell carcinoma, Sci. Rep 5 (2015) 15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li X, Zhu M, Brasier AR, Kudlicki AS, Inferring genome-wide functional modulatory network: a case study on NF-κB/RelA transcription factor, J. Comput. Biol 22 (2015) 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Day TF, Mewani RR, Starr J, Li X, Chakravarty D, Ressom H, Zou X, Eidelman O, Pollard HB, Srivastava M, Transcriptome and proteome analyses of TNFAIP8 knockdown cancer cells reveal new insights into molecular determinants of cell survival and tumor progression, Cancer Gene Networks, Springer, 2017, pp. 83–100. [DOI] [PubMed] [Google Scholar]
- [55].Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, Green F, Clarke R, Collins R, Franzosi MG, Tognoni G, Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p, Hum. Mol. Genet 17 (2007) 806–814. [DOI] [PubMed] [Google Scholar]
- [56].Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou M-M, Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a, Mol. Cell 38 (2010) 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhou X, Han X, Wittfeldt A, Sun J, Liu C, Wang X, Gan L-M, Cao H, Liang Z, Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway, RNA Biol. 13 (2016) 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Holdt LM, Hoffmann S, Sass K, Langenberger D, Scholz M, Krohn K, Finstermeier K, Stahringer A, Wilfert W, Beutner F, Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks, PLoS Genet. 9 (2013) e1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang B, Wang D, Ji TF, Shi L, Yu JL, Overexpression of lncRNA ANRIL up-regulates VEGF expression and promotes angiogenesis of diabetes mellitus combined with cerebral infarction by activating NF-kappaB signaling pathway in a rat model, Oncotarget 8 (2017) 17347–17359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [60].Rapicavoli NA, Qu K, Zhang J, Mikhail M, Laberge R-M, Chang HY, A mammalian pseudogene lncRNA at the interface of inflammation and anti-in-flammatory therapeutics, Elife 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY, Motif module map reveals enforcement of aging by continual NF-κB activity, Genes Dev. 21 (2007) 3244–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Duret L, Chureau C, Samain S, Weissenbach J, Avner P, The Xist RNA gene evolved in eutherians by pseudogenization of a protein-coding gene, Science 312 (2006) 1653–1655. [DOI] [PubMed] [Google Scholar]
- [63].Zgheib C, Hodges MM, Hu J, Liechty KW, Xu J, Long non-coding RNA Lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages, PLoS One 12 (2017) e0177453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rajbhandari R, McFarland BC, Patel A, Gerigk M, Gray GK, Fehling SC, Bredel M, Berbari NF, Kim H, Marks MP, Loss of tumor suppressive microRNA-31 enhances TRADD/NF-κB signaling in glioblastoma, Oncotarget 6 (2015) 17805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Montes M, Nielsen MM, Maglieri G, Jacobsen A, Højfeldt J, Agrawal-Singh S, Hansen K, Helin K, Van De Werken HJ, Pedersen JS, The lncRNA MIR31HG regulates p16 INK4A expression to modulate senescence, Nat. Commun 6 (2015) 6967. [DOI] [PubMed] [Google Scholar]
- [66].Jin C, Jia L, Huang Y, Zheng Y, Du N, Liu Y, Zhou Y, Inhibition of lncRNA MIR31HG promotes Osteogenic differentiation of human adipose-derived stem cells, Stem Cells 34 (2016) 2707–2720. [DOI] [PubMed] [Google Scholar]
- [67].Krawczyk M, Emerson BM, p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-κB complexes, Elife 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hoellen F, Kelling K, Dittmer C, Diedrich K, Friedrich M, Thill M, Impact of cyclooxygenase-2 in breast cancer, Anticancer Res. 31 (2011) 4359–4367. [PubMed] [Google Scholar]
- [69].Pearson MJ, Philp AM, Heward JA, Roux BT, Walsh DA, Davis ET, Lindsay MA, Jones SW, Long intergenic noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage, Arthritis & Rheumatology 68 (2016) 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Qian M, Yang X, Li Z, Jiang C, Song D, Yan W, Liu T, Wu Z, Kong J, Wei H, P50-associated COX-2 extragenic RNA (PACER) overexpression promotes proliferation and metastasis of osteosarcoma cells by activating COX-2 gene, Tumor Biol. 37 (2016) 3879–3886. [DOI] [PubMed] [Google Scholar]
- [71].Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, A long noncoding RNA mediates both activation and repression of immune response genes, Science 341 (2013) 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hu G, Gong A-Y, Wang Y, Ma S, Chen X, Chen J, Su C-J, Shibata A, Strauss-Soukup JK, Drescher KM, LincRNA-Cox2 promotes late inflammatory gene transcription in macrophages through modulating SWI/SNF-mediated chromatin remodeling, J. Immunol 196 (2016) 2799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST, Selective and antagonistic functions of SWI/SNF and mi-2β nucleosome remodeling complexes during an inflammatory response, Genes Dev. 20 (2006) 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Euskirchen GM, Auerbach RK, Davidov E, Gianoulis TA, Zhong G, Rozowsky J, Bhardwaj N, Gerstein MB, Snyder M, Diverse roles and interactions of the SWI/SNF chromatin remodeling complex revealed using global approaches, PLoS Genet. 7 (2011) e1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tong Q, Gong A-Y, Zhang X-T, Lin C, Ma S, Chen J, Hu G, Chen X-M, LincRNA-Cox2 modulates TNF-α–induced transcription of Il12b gene in intestinal epithelial cells through regulation of Mi-2/NuRD-mediated epigenetic histone modifications, FASEB J. 30 (2015) 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response, Cell 142 (2010) 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA, LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint, Mol. Cell 54 (2014) 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Barichievy S, Magagula L, Shibayama Y, Mhlanga MM, Microbial manipulation host dark matter, Non-Coding RNAs and Inter-Kingdom Communication, Springer, 2016, pp. 27–52. [Google Scholar]
- [79].Spurlock CF, Tossberg JT, Matlock BK, Olsen NJ, Aune TM, Methotrexate inhibits NF-κB activity via long intergenic (noncoding) RNA–p21 induction, Arthritis & rheumatology 66 (2014) 2947–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jia D, Niu Y, Li D, Liu Z, lncRNA C2dat1 promotes cell proliferation, migration, and invasion by targeting miR-34a-5p in osteosarcoma cells, Oncol. Res 26 (2018) 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [81].Xu Q, Deng F, Xing Z, Wu Z, Cen B, Xu S, Zhao Z, Nepomuceno R, Bhuiyan M, Sun D, Long non-coding RNA C2dat1 regulates CaMKIIδ expression to promote neuronal survival through the NF-κB signaling pathway following cerebral ischemia, Cell Death Dis. 7 (2016) e2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhou Q, Huang XR, Yu J, Yu X, Lan HY, Long noncoding RNA Arid2-IR is a novel therapeutic target for renal inflammation, Mol. Ther 23 (2015) 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Garding A, Bhattacharya N, Claus R, Ruppel M, Tschuch C, Filarsky K, Idler I, Zucknick M, Caudron-Herger M, Oakes C, Epigenetic upregulation of lncRNAs at 13q14. 3 in leukemia is linked to the in Cis downregulation of a gene cluster that targets NF-kB, PLoS Genet. 9 (2013) e1003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wen Y, Yu Y, Fu X, LncRNA Gm4419 contributes to OGD/R injury of cerebral microglial cells via IκB phosphorylation and NF-κB activation, Biochem. Biophys. Res. Commun 487 (2017) 923–929. [DOI] [PubMed] [Google Scholar]
- [85].Zangrando J, Zhang L, Vausort M, Maskali F, Marie P-Y, Wagner DR, Devaux Y, Identification of candidate long non-coding RNAs in response to myocardial infarction, BMC Genomics 15 (2014) 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li X, Zhou J, Huang K, Inhibition of the lncRNA Mirt1 attenuates acute myocardial infarction by suppressing NF-kappaB activation, Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 42 (2017) 1153–1164. [DOI] [PubMed] [Google Scholar]
- [87].Liu Y, Wang T, Zhang M, Chen P, Yu Y, Down-regulation of myocardial infarction associated transcript 1 improves myocardial ischemia-reperfusion injury in aged diabetic rats by inhibition of activation of NF-kappaB signaling pathway, Chem. Biol. Interact 300 (2019) 111–122. [DOI] [PubMed] [Google Scholar]
- [88].Krijnen P, Nijmeijer R, Meijer C, Visser C, Hack C, Niessen H, Apoptosis in myocardial ischaemia and infarction, J. Clin. Pathol 55 (2002) 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ilott NE, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes, Nat. Commun 5 (2014) 3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cui H, Xie N, Tan Z, Banerjee S, Thannickal VJ, Abraham E, Liu G, The human long noncoding RNA lnc-IL7R regulates the inflammatory response, Eur. J. Immunol 44 (2014) 2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li Z, Chao T-C, Chang K-Y, Lin N, Patil VS, Shimizu C, Head SR, Burns JC, Rana TM, The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL, Proc. Natl. Acad. Sci 111 (2014) 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Liu G, Wang Y, Zhang M, Zhang Q, Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells, Int. Immunopharmacol 66 (2019) 354–361. [DOI] [PubMed] [Google Scholar]
- [93].Chan J, Atianand M, Jiang Z, Carpenter S, Aiello D, Elling R, Fitzgerald KA, Caffrey DR, Cutting edge: a natural antisense transcript, AS-IL1α, controls inducible transcription of the proinflammatory cytokine IL-1α, J. Immunol 195 (2015) 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huang W, Lan X, Li X, Wang D, Sun Y, Wang Q, Gao H, Yu K, Long non-coding RNA PVT1 promote LPS-induced septic acute kidney injury by regulating TNFalpha and JNK/NF-kappaB pathways in HK-2 cells, Int. Immunopharmacol 47 (2017) 134–140. [DOI] [PubMed] [Google Scholar]
- [95].Khan MR, Xiang S, Song Z, Wu M, The p53-inducible long noncoding RNA TRINGS protects cancer cells from necrosis under glucose starvation, EMBO J. 36 (2017) 3483–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chen M-Y, Liao G-D, Zhou B, Kang L-N, He Y-M, Li S-W, Genome-wide profiling of Long noncoding RNA expression patterns in women with repeated implantation failure by RNA sequencing, Reprod. Sci, (2018) 1933719118756752. [DOI] [PubMed] [Google Scholar]
- [97].Lu Y, Liu X, Xie M, Liu M, Ye M, Li M, Chen XM, Li X, Zhou R, The NF-kappaB-responsive Long noncoding RNA FIRRE regulates posttranscriptional regulation of inflammatory gene expression through interacting with hnRNPU, J. Immunol 199 (2017) 3571–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Valanne S, Salminen TS, Jarvela-Stolting M, Vesala L, Ramet M, Immune-inducible non-coding RNA molecule lincRNA-IBIN connects immunity and metabolism in Drosophila melanogaster, PLoS Pathog. 15 (2019) e1007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sang LJ, Ju HQ, Liu GP, Tian T, Ma GL, Lu YX, Liu ZX, Pan RL, Li RH, Piao HL, Marks JR, Yang LJ, Yan Q, Wang W, Shao J, Zhou Y, Zhou T, Lin A, LncRNA CamK-A regulates Ca(2+)-Signaling-mediated tumor microenvironment Remodeling, Mol. Cell 72 (2018) 71–83 (e77). [DOI] [PubMed] [Google Scholar]
- [100].Gooding AJ, Zhang B, Gunawardane L, Beard A, Valadkhan S, Schiemann WP, The lncRNA BORG facilitates the survival and chemoresistance of triple-negative breast cancers, Oncogene 38 (2019) 2020–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang H, Li L, Yin L, Silencing LncRNA LOXL1-AS1 attenuates mesenchymal characteristics of glioblastoma via NF-κB pathway, Biochem. Biophys. Res. Commun 500 (2018) 518–524. [DOI] [PubMed] [Google Scholar]
- [102].Ma S, Ming Z, Gong A-Y, Wang Y, Chen X, Hu G, Zhou R, Shibata A, Swanson PC, Chen X-M, A long noncoding RNA, lincRNA-Tnfaip3, acts as a coregulator of NF-κB to modulate inflammatory gene transcription in mouse macrophages, FASEB J. 31 (2016) 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Collier SP, Henderson MA, Tossberg JT, Aune TM, Regulation of the Th1 genomic locus from Ifng through Tmevpg1 by T-bet, J. Immunol 193 (2014) 3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fang Y, Wang J, Wu F, Song Y, Zhao S, Zhang Q, Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge, Oncotarget 8 (2017) 46090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang ZX, Liu ZQ, Jiang B, Lu XY, Ning XF, Yuan CT, Wang AL, BRAF activated non-coding RNA (BANCR) promoting gastric cancer cells proliferation via regulation of NF-kappaB1, Biochem. Biophys. Res. Commun 465 (2015) 225–231. [DOI] [PubMed] [Google Scholar]
- [106].Meng Y, Yu F, Long non coding RNA FAM3D-AS1 inhibits development of colorectal cancer through NF-kB signaling pathway, Biosci. Rep 39 (7) (2019), 10.1042/BSR20190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li S, Huang Y, Huang Y, Fu Y, Tang D, Kang R, Zhou R, Fan X, The long non-coding RNA TP73-AS1 modulates HCC cell proliferation through miR-200a-dependent HMGB1/RAGE regulation, J. Exp. Clin. Cancer Res 36 (2017) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Li Y, Li Y, Huang S, He K, Zhao M, Lin H, Li D, Qian J, Zhou C, Chen Y, Long non-coding RNA growth arrest specific transcript 5 acts as a tumour suppressor in colorectal cancer by inhibiting interleukin-10 and vascular endothelial growth factor expression, Oncotarget 8 (2017) 13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang Y, Kong D, LncRNA GAS5 Represses Osteosarcoma Cells Growth and Metastasis via Sponging MiR-203a, Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 45 (2018) 844–855. [DOI] [PubMed] [Google Scholar]
- [110].Li F, Sun J, Huang S, Su G, Pi G, LncRNA GAS5 overexpression reverses LPS-induced inflammatory injury and apoptosis through up-regulating KLF2 expression in ATDC5 chondrocytes, Cell. Physiol. Biochem 45 (2018) 1241–1251. [DOI] [PubMed] [Google Scholar]
- [111].Yue Q, Zhao C, Wang Y, Zhao L, Zhu Q, Li G, Wu N, Jia D, Ma C, Downregulation of growth arrestspecific transcript 5 alleviates palmitic acid-induced myocardial inflammatory injury through the miR26a/HMGB1/NFkappaB axis, Mol. Med. Rep 18 (2018) 5742–5750. [DOI] [PubMed] [Google Scholar]
- [112].Chen L, Zhang D, Yu L, Dong H, Targeting MIAT reduces apoptosis of cardiomyocytes after ischemia/reperfusion injury, Bioengineered 10 (2019) 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Huang Z, Du G, Huang X, Han L, Han X, Xu B, Zhang Y, Yu M, Qin Y, Xia Y, Wang X, Lu C, The enhancer RNA lnc-SLC4A1–1 epigenetically regulates unexplained recurrent pregnancy loss (URPL) by activating CXCL8 and NF-kB pathway, EBioMedicine 38 (2018) 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yuan Y, Wang J, Chen Q, Wu Q, Deng W, Zhou H, Shen D, Long non-coding RNA cytoskeleton regulator RNA (CYTOR) modulates pathological cardiac hypertrophy through miR-155-mediated IKKi signaling, Biochimica et biophysica acta. Molecular basis of disease 1865 (2019) 1421–1427. [DOI] [PubMed] [Google Scholar]
- [115].Ma M, Pei Y, Wang X, Feng J, Zhang Y, Gao MQ, LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-kappaB/NLRP3 inflammasome pathway, Cell Prolif. 52 (2019) e12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Li Y, Zhao W, Wang H, Chen C, Zhou D, Li S, Zhang X, Zhao H, Zhou D, Chen B, Silencing of LncRNA steroid receptor RNA activator attenuates polycystic ovary syndrome in mice, Biochimie 157 (2019) 48–56. [DOI] [PubMed] [Google Scholar]
- [117].Sun Y, Pan J, Zhang N, Wei W, Yu S, Ai L, Knockdown of long non-coding RNA H19 inhibits multiple myeloma cell growth via NF-kappaB pathway, Sci. Rep 7 (2017) 18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liao Z, Zhao J, Yang Y, Downregulation of lncRNA H19 inhibits the migration and invasion of melanoma cells by inactivating the NFkappaB and PI3K/Akt signaling pathways, Mol. Med. Rep 17 (2018) 7313–7318. [DOI] [PubMed] [Google Scholar]
- [119].Zhao J, Ma ST, Downregulation of lncRNA H19 inhibits migration and invasion of human osteosarcoma through the NF-kappaB pathway, Mol. Med. Rep 17 (2018) 7388–7394. [DOI] [PubMed] [Google Scholar]
- [120].Pan J, LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway, Eur. Rev. Med. Pharmacol. Sci 21 (2017) 322–328. [PubMed] [Google Scholar]
- [121].Spurlock CF 3rd, Shaginurova G, Tossberg JT, Hester JD, Chapman N, Guo Y, Crooke PS 3rd, Aune TM, Profiles of Long noncoding RNAs in human naive and memory T cells, J. Immunol 199 (2017) 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang L, Wu F, Song Y, Li X, Wu Q, Duan Y, Jin Z, Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients, Cell Death Dis. 7 (2016) e2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wang X, Sun W, Shen W, Xia M, Chen C, Xiang D, Ning B, Cui X, Li H, Li X, Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis, J. Hepatol 64 (2016) 1283–1294. [DOI] [PubMed] [Google Scholar]
- [124].Wang HK, Yan H, Wang K, Wang J, Dynamic regulation effect of long non-coding RNA-UCA1 on NF-kB in hippocampus of epilepsy rats, Eur. Rev. Med. Pharmacol. Sci 21 (2017) 3113–3119. [PubMed] [Google Scholar]
- [125].Zheng Q, Lin Z, Li X, Xin X, Wu M, An J, Gui X, Li T, Pu H, Li H, Inflammatory cytokine IL6 cooperates with CUDR to aggravate hepatocyte-like stem cells malignant transformation through NF-κB signaling, Sci. Rep 6 (2016) 36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mendell JT, Targeting a long noncoding RNA in breast cancer, N. Engl. J. Med 374 (2016) 2287–2289. [DOI] [PubMed] [Google Scholar]
- [127].Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM, Kumar AP, Challagundla KB, Sethi G, Gupta SC, Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases, Cellular and molecular life sciences : CMLS 76 (2019) 1947–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Awasthee N, Rai V, Verma SS, Sajin Francis K, Nair MS, Gupta SC, Anti-cancer activities of Bharangin against breast cancer: evidence for the role of NF-kappaB and lncRNAs, Biochimica et Biophysica Acta. General Subjects 1862 (2018) 2738–2749. [DOI] [PubMed] [Google Scholar]
- [129].Yoshida K, Toden S, Ravindranathan P, Han H, Goel A, Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression, Carcinogenesis 38 (2017) 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hilton IB, D’ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA, Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers, Nat. Biotechnol 33 (2015) 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals, Nature 458 (2009) 223. [DOI] [PMC free article] [PubMed] [Google Scholar]


