Abstract
Children growing up on farms have low rates of allergy, but the mechanism for this protective effect has not been fully elucidated. Short chain fatty acids (SCFAs) produced by the gut microbiota may play a role in protection from allergy. We measured fecal SCFA levels in samples collected from 28 farming and 37 control children over the first 3 years of life using gas chromatography. Data on diet and other host factors were recorded and allergy was diagnosed at 8 years of age. Among all children, median propionic and butyric acid concentration increased over the first 3 years, and longer SCFAs typically appeared by 1 year of age. Farm children had higher levels of iso-butyric, iso-valeric and valeric acid at 3 years of age than rural controls. In addition, children with elder siblings had higher levels of valeric acid at 3 years of age, and dietary factors also affected SCFA pattern. High levels of valeric acid at 3 years of age were associated with low rate of eczema at 8 years of age. The fecal SCFA pattern in farm children suggests a more rapid maturation of the gut microbiota. Valeric acid or associated microbes may have protective potential against eczema.
Subject terms: Epidemiology, Paediatric research, Biomarkers
Introduction
Living on a farm is protective against immunoregulatory and inflammatory diseases, including allergic diseases1,2 and inflammatory bowel disease (IBD)3. Farmers also have reduced rates of colorectal and other cancers4. The prevalence of allergy and IBD has increased dramatically over the last century in industrialized countries5,6, while rates in farming families have remained low by comparison1,3. Which exposures in the farming environment that exert the protective effect is unknown, but exposure to a wider range of microbes during infancy7, or typical dietary patterns in farming families8 may contribute.
Short chain fatty acids (SCFAs) are the most abundant bacterial metabolites in the colon and have been proposed as key mediators in microbiota-induced effects on the host9. They contain between two and six carbon atoms, and are produced when dietary fibre and protein, mucous and sloughed epithelial cells are metabolized by gut bacteria. The major SCFAs generated in the colon are short unbranched varieties such as acetic, propionic and butyric acid. As the microbiota becomes more complex, longer SCFAs, such as valeric acid, are produced. Further, the branched SCFAs, iso-butyric and iso-valeric acid, appear. These are generated by the bacterial metabolism of protein10. Iso-caproic acid is a branched SCFA considered a marker of colonization by Clostridium difficile11, an obligate anaerobe that is fairly common in the gut microbiota of young children12.
Many SCFAs exhibit effects that could play a role in immunoregulatory and inflammatory diseases. Within the gut, SCFAs maintain epithelial barrier function by decreasing intestinal tight junction permeability13 and inducing secretion of the epithelium’s protective mucous layer14. Butyric acid is the major source of energy for colonocytes15 and is also a histone deacetylase inhibitor, making it important in the control of gene expression16. While butyrate is almost fully consumed in the colon, acetic and propionic acids can be detected in peripheral blood, where they may elicit physiological effects in various body tissues9,17, e.g. via activation of G-protein coupled receptors, found on the surface of many different cell types9. Very little is known regarding the immunomodulatory effects of longer and branched SCFAs. However, in a birth cohort study, higher levels of iso-butyric, valeric and iso-valeric acid at 1 year of age were inversely related to the development of food allergy by age four18.
The aim of this study was to investigate whether children growing up on small dairy farms had an altered fecal SCFA pattern compared to children living in the same rural area but not on farms, and if so to investigate whether this pattern was related to subsequent allergy outcomes. A secondary aim was to describe the development in fecal SCFA pattern over the first 3 years of life.
Methods
Subjects
The FARMFLORA birth cohort comprised 28 infants raised on small family-owned dairy farms in Skaraborg County in South-West Sweden and 37 infants from the same rural area but not raised on farms who were followed until 8 years of age. Pregnant women were recruited between September 2005 and May 2008. Healthy children born at term were included, and subjects from dairy farms with infrequent animal contact, from non-dairy farms, or from urban areas were excluded. The study conforms to the standards of the Declaration of Helsinki and was approved by the Regional Ethics Committee in Gothenburg (No. 363-05). Written informed consent was obtained from the parents of all participants.
The cohort characteristics are shown in Table 1. There were fewer boys in the farm group than in the rural control group (36% vs. 62%, P = 0.04), and farm children more often had a cat or dog (75% vs. 51%, P = 0.054). Farm children consumed more full-fat milk products and less margarine and oils compared with the rural control children, as reported previously8,19. At 1 year of age, farm children also consumed more oily fish (P = 0.02) and farm milk (P < 0.01), while control children consumed more poultry (P = 0.03)8,19.
Table 1.
General and nutritional characteristics of farm and control children.
| Farm (n = 28) | Control (n = 37) | P | |||
|---|---|---|---|---|---|
| Family | |||||
| Allergic heredity, mothera | 7 (25%) | 11 (30%) | 0.68 | ||
| Allergic heredity, fathera | 1 (4%) | 12 (32%) | 0.01* | ||
| Level of education, motherb | 2 (1–5) | 4 (1–5) | 0.20 | ||
| Level of education, fatherb | 2 (1–5) | 2 (1–5) | 0.02* | ||
| Smoking, motherc | 0 (0%) | 1 (3%) | 1.00 | ||
| Smoking, fatherc | 1 (4%) | 4 (11%) | 0.38 | ||
| Elder sibling(s) | 18 (64%) | 17 (46%) | 0.15 | ||
| Cat or dog in housed | 21 (75%) | 19 (51%) | 0.05 | ||
| Birth and child | |||||
| Mother’s age at delivery (years) | 33 (21–42) | 32 (22–41) | 0.46 | ||
| Caesarian section | 3 (11%) | 7 (19%) | 0.50 | ||
| Male | 10 (36%) | 23 (62%) | 0.04* | ||
| Allergic at 3 yearse | 1 (4%) | 10 (32%) | 0.02* | ||
| Allergic at 8 yearse | 3 (11%) | 7 (19%) | 0.72 | ||
| Breastfeeding and child’s diet at 1 year | |||||
| Exclusive breastfeeding (months) | 4.0 (0.0–6.0) | 3.5 (0.0–6.0) | 0.11 | ||
| Fat (g/day) | 33 (7–78) | 32 (14–62) | 0.70 | ||
| Margarine and oil (g/day) | 3 (0–19) | 5 (0–26) | 0.10 | ||
| Cream (g/day) | 1 (0–28) | 0 (0–14) | 0.02* | ||
| Milk fats (g/day) | 11 (0–35) | 5 (0–29) | 0.04* | ||
| Oily fish (g/day) | 0 (0–63) | 0 (0–15) | 0.02* | ||
| Protein (g/day) | 35 (5–60) | 34 (19–62) | 0.69 | ||
| Poultry (g/day) | 0 (0–5) | 0 (0–75) | 0.03* | ||
| Carbohydrates (g/day) | 130 (23–183) | 135 (65–194) | 0.11 | ||
| Fibre (g/day) | 11 (2–18) | 11 (4–18) | 0.93 | ||
| Fruit and vegetables (g/day) | 150 (77–200) | 180 (90–240) | 0.22 | ||
| Farm milk (g/day)f | 0(0–343) | 0 (0–0) | < 0.01* | ||
| Homemade porridge (g/day) | 0 (0–175) | 0 (0–100) | 0.06 | ||
Data are n (%) or median (range).
*P < 0.05, Chi-squared or Fisher’s exact test for counts, Mann–Whitney U-test for continuous variables.
aDoctor-diagnosed asthma, rhinitis, or atopic eczema.
b1 = Elementary school, 2 = upper secondary school 2–3 years or equivalent, 3 = qualified graduate from upper secondary school, 4 = university < 1 year, 5 = university > 1 year.
cDuring the last month of pregnancy.
dAt the time of recruitment.
eDoctor-diagnosed asthma, atopic eczema, allergic rhino-conjunctivitis or food allergy. At 3 years, n = 27 farm children and 36 rural controls; at 8 years, n = 18 farm children and 30 rural controls.
fPasteurized and unpasteurized. Data selected from a previous study on the FARMFLORA birth cohort8, with intake of milk fats calculated here.
Clinical examination
Children in the FARMFLORA study were examined by study pediatricians to diagnose eczema, asthma, allergic rhino-conjunctivitis and food allergy according to predefined protocols at 18 months, 3 years and 8 years of age. All diagnoses were confirmed by a specialist pediatric allergologist (BH). As described previously, living on a farm was associated with protection from allergy at 3 years of age8, but by 8 years of age there was no such association20. In brief, at 8 years of age, eczema was diagnosed according to William’s criteria, or as having recurring itchy spots on typical locations over at least 6 months, with symptoms during the last 12 months. Asthma was diagnosed based on wheeze or heavy breathing together with response to anti-inflammatory maintenance therapy, bronchial hyperresponsiveness on methacholine challenge (PD20 < 0.6 mg) or bronchial obstruction reversible to β2-agonist (FEV1rev ≥ 12%). Food allergy was defined as symptoms of food allergy supported by either planned or accidental open food challenge. Allergic rhino-conjunctivitis was defined as eye and/or nasal symptoms on exposure to pollen or animals together with a positive allergen-specific IgE test or skin prick test against a corresponding allergen.
Specific IgE was measured in venous blood against food (milk, egg, soy, fish, wheat and peanut; six-mix food test) and inhalant allergens (birch, timothy grass, mugwort, cat, dog, horse and house dust mite; Phadiatop), followed by specific IgE tests by ImmunoCAP (all from Phadia/Pharmacia Diagnostics, Uppsala, Sweden). An allergen-specific IgE level ≥ 0.35 kU/L was considered positive. Skin prick tests were performed in accordance with European guidelines using standard allergen extracts (birch, grass, mugwort, cat, dog, horse, rabbit, Dermatophagoides pteronyssinus, Dermatophagoides farinae and Cladosporium (Soluprick SQ; ALK Abello AS, Hørsholm, Denmark), as previously described20.
SCFA analysis
Fecal samples were collected at regular intervals by parents and transported in gas-tight sachets to the laboratory at Gothenburg University, where they were stored frozen at − 80 °C until further analysis. Samples obtained when the children were 4 weeks, 1 year and 3 years of age were selected for analysis of the concentration of acetic, propionic, iso-butyric, butyric, iso-valeric, valeric, iso-caproic and caproic acids by gas chromatography, using a method modified from Zhao et al.21. In brief, samples were thawed at room temperature, then the feces (200 mg) was mixed with water (1 mL) and homogenized for 20 s. The suspension was adjusted to pH 2–3 using dilute hydrochloric acid, kept at room temperature for 10 min, and centrifuged for 20 min at 5000 rpm. 180 μL of the supernatant was mixed with internal standard solution (20 μL; 7.9 mM 2-ethylbutyric acid in 12% v/v aqueous formic acid). A gas chromatograph with flame ionization detector, fitted with a fused silica 30 m × 0.53 mm capillary column with free fatty acid stationary phase and with glass wool in the injection port liner, was used for analysis. The helium pressure was 30 kPa, injector temperature 200 °C, detector temperature 210 °C and injection volume 1 μL. The oven program was: 1 min at 100 °C, ramp to 170 °C at 10 °C/min, ramp to 220 °C at 20 °C/min, 4 min at 220 °C. Peaks were identified using retention time. The SCFA:internal standard peak area ratio was determined for each SCFA peak, and the corresponding SCFA concentration determined from calibration curves. SCFA levels were not determined for 18 (28%) children at 4 weeks, 1 (2%) at 1 year and 13 (20%) at 3 years of age, due to lack of material. Variables for analysis were the prevalence and concentration of each SCFA.
Dietary assessment
Data regarding the diet of the FARMFLORA children, including full details of the dietary assessment methodology, have been published previously8,19. In brief, parents continuously recorded breastfeeding and formula practices, and introduction of new foods, in diaries up to 18 months of age. At 1 year of age (10–14 months), parents completed an unannounced 24-h dietary recall, followed by a 24-h food diary, of their children’s diet8. The collected dietary information was registered and calculated based on the food composition database of the Swedish National Food Agency using the software Dietist Net Pro (Kost och Näringsdata, version 15.11.02, Stockholm, Sweden).
Statistical methods
The Mann–Whitney U test was used for the evaluation of differences in continuous variables between groups, as SCFA concentration and other variables were typically not normally distributed. For categorical variables the Chi-Squared test was used, or the Fisher’s Exact test for small samples. Friedman tests were used to compare the concentration of fecal SCFAs at different time points.
Multiple logistic regression was used to confirm associations between farm residence and SCFA levels after adjusting for covariates, and to identify explanatory variables. Dichotomous variables for high and low SCFA concentration were created. Variables that were associated with farm residence, and also predictive of SCFA level in univariate logistic regression analysis, (P ≤ 0.2 for both) were included as covariates. In addition, elder siblings and dietary intakes of protein and fibre were also included, as they have been linked theoretically with SCFA levels9,18. Models were built using a backward conditional method.
Statistical analyses were performed with SPSS Statistics version 25 (IBM Corporation, New York, USA). Two-tailed P-values of < 0.05 were considered significant.
Results
Maturation of fecal SCFA pattern during early childhood in cohort children
We first examined how fecal SCFA patterns developed during early childhood, from 4 weeks to 3 years of age, among the cohort as a whole. There was considerable variation in both the prevalence and concentration of the various SCFAs between children at any given time. Nonetheless, a notable transition was observed in SCFA patterns over the first 3 years of life (Fig. 1).
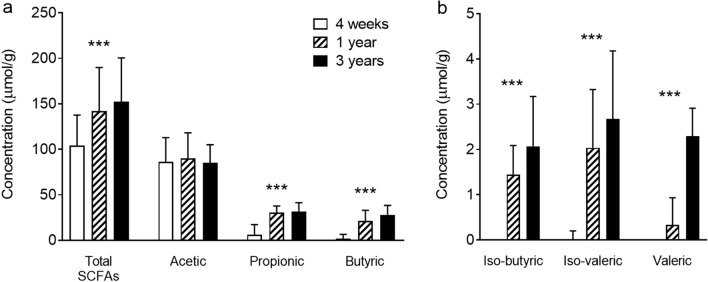
Figure 1.
Development of fecal SCFA levels during early childhood. Median concentration of (a) total SCFAs and acetic, propionic and butyric acid, and (b) iso-butyric, iso-valeric and valeric acid, in children at 4 weeks (n = 47), 1 year (n = 64) and 3 years of age (n = 51). Error bars show the interquartile range. ***P < 0.001, Friedman test.
Short chain fatty acids are made up of between two and six carbon atoms (C2–C6), and may be straight-chain or branched (iso-, or i). At 4 weeks of age the shortest SCFA, acetic acid (C2), was present in all infants with a median concentration of 86 μmol per gram of feces, and represented > 80% of the total SCFA concentration. Propionic (C3) and butyric (C4) acids were each detected in at least 70% of infants, at much smaller median concentrations (6.1 and 1.9 μmol/g respectively, Fig. 1a). Iso-butyric (iC4) and iso-valeric (iC5) acids were also represented in up to 28% of infants, at concentrations up to 4 μmol/g. Finally, valeric (C5), caproic (C6) and/or iso-caproic (iC6) acids were detected in a small minority of infants, at concentrations up to 9.8, 1.8 and 1.7 μmol/g, respectively.
With age, the median total concentration of fecal SCFAs increased, from 104 μmol/g at 4 weeks of age to 152 μmol/g at 3 years of age. By 3 years of age, acetic, propionic and butyric acids were present in all children, at median concentrations of 85, 32 and 28 μmol/g, respectively (Fig. 1a). Iso-butyric, iso-valeric and valeric acids were also detected in almost all children, although at much lower median concentrations (2–3 μmol/g; Fig. 1b: note different scale than in Fig. 1a). Further, caproic acid was detected in a third of children, at concentrations up to 2 μmol/g, while iso-caproic acid was detected in just one child in twenty, at concentrations up to 0.3 μmol/g.
Fecal SCFA patterns in farm and control children
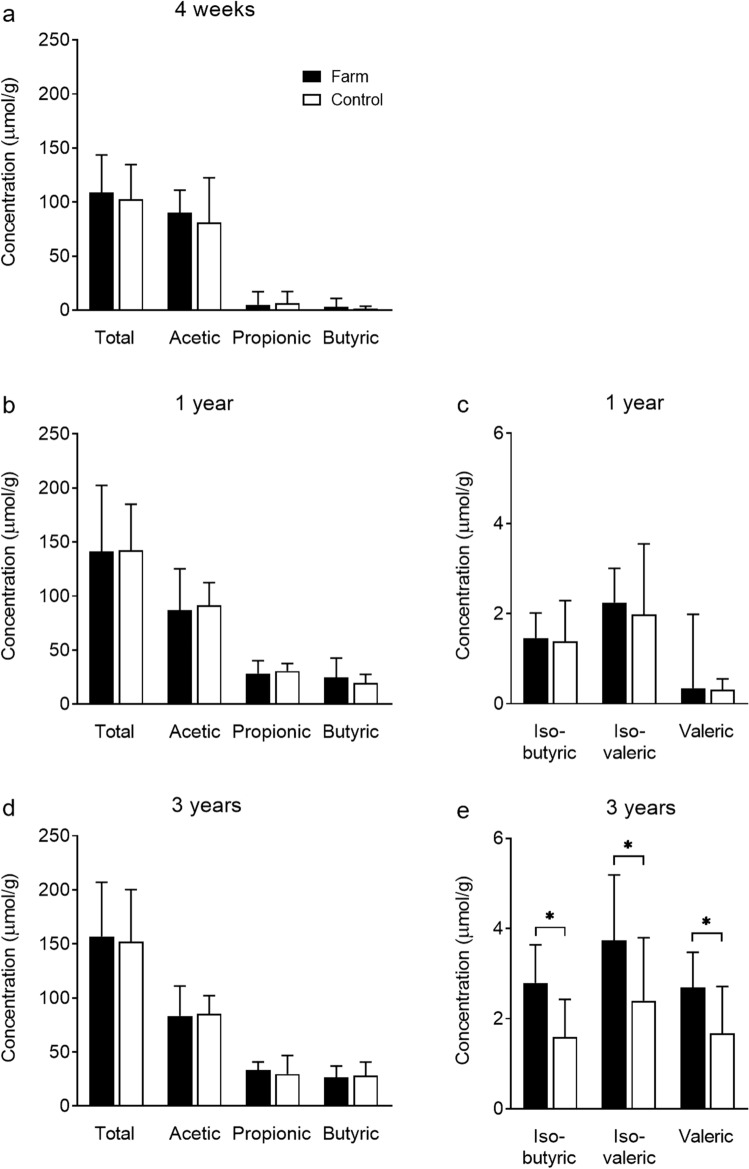
At 4 weeks and 1 year of age, no significant differences were observed in the concentration of fecal SCFAs in children living on farms, compared to children living in the same rural area but not on farms (Table 2, Fig. 2a–c). However, at 3 years of age (Fig. 2d,e), children living on farms had higher median concentrations of several fecal SCFAs including iso-butyric (2.8 vs. 1.6 μmol/g, P = 0.03), iso-valeric (3.7 vs. 2.4 μmol/g, P = 0.03) and valeric acid (2.7 vs. 1.7 μmol/g, P = 0.01) than control children.
Table 2.
Prevalence and concentration of fecal SCFAs in farm and control children.
| Prevalence n (%) |
Concentration, μmol/g Median (range) |
|||||
|---|---|---|---|---|---|---|
| Farm | Control | Farm | Control | |||
| 4 weeksa | ||||||
| Total SCFAs | – | – | 109 (42–220) | 103 (29–269) | ||
| Acetic | 24 (100) | 23 (100) | 90 (34–177) | 81 (20–259) | ||
| Propionic | 17 (71) | 18 (78) | 5.0 (ND–63) | 6.3 (ND–53) | ||
| Butyric | 18 (75) | 15 (65) | 2.9 (ND–37) | 1.1 (ND–30) | ||
| Iso-butyric | 3 (13) | 7 (30) | ND (ND–4.1) | ND (ND–4.0) | ||
| Iso-valeric | 6 (25) | 7 (30) | ND (ND–3.8) | ND (ND–4.4) | ||
| Valeric | 4 (17) | 1 (4) | ND (ND–9.8) | ND (ND–0.4) | ||
| Iso-caproic | 3 (13) | 1 (4) | ND (ND–1.7) | ND (ND–0.3) | ||
| Caproic | 4 (17) | 1 (4) | ND (ND–1.8) | ND (ND) | ||
| 1 yearb | ||||||
| Total SCFAs | – | – | 141 (57–359) | 142 (82–253) | ||
| Acetic | 28 (100) | 36 (100) | 87 (44–221) | 92 (45–154) | ||
| Propionic | 28 (100) | 36 (100) | 28 (3–48) | 31 (8–87) | ||
| Butyric | 28 (100) | 36 (100) | 25 (8–81) | 20 (4–59) | ||
| Iso-butyric | 22 (79) | 29 (81) | 1.5 (ND–4.8) | 1.4 (ND–5.2) | ||
| Iso-valeric | 27 (96) | 35 (97) | 2.2 (ND–7.6) | 2.0 (0.2–6.9) | ||
| Valeric | 24 (86) | 27 (75) | 0.3 (ND–4.8) | 0.3 (ND–3.8) | ||
| Iso-caproic | 5 (18) | 7 (19) | ND (ND–1.2) | ND (ND–0.9) | ||
| Caproic | 5 (18) | 7 (19) | ND (ND–0–3) | ND (ND–0–3) | ||
| 3 yearsc | ||||||
| Total SCFAs | – | – | 157 (71–287) | 152 (62–283) | ||
| Acetic | 19 (100) | 32 (100) | 83 (46–163) | 86 (32–133) | ||
| Propionic | 19 (100) | 32 (100) | 33 (14–49) | 30 (8–66) | ||
| Butyric | 19 (100) | 32 (100) | 26 (6–94) | 28 (7–70) | ||
| Iso-butyric | 19 (100) | 29 (91) | 2.8* (ND–5.0) | 1.6* (ND–5.3) | ||
| Iso-valeric | 19 (100) | 30 (94) | 3.7* (1.3–7.6) | 2.4* (ND–8.8) | ||
| Valeric | 19 (100) | 31 (97) | 2.7* (0.4–6.2) | 1.7* (ND–4.4) | ||
| Iso-caproic | 0 (0) | 3 (10) | ND (ND) | ND (ND–0.3) | ||
| Caproic | 6 (32) | 11 (34) | ND (ND–1.9) | ND (ND–1.3) | ||
*P < 0.05, Chi-squared or Fisher’s exact test for counts, Mann–Whitney U-test for continuous variables.
ND, not detected. Feces analyzed from: a24 (86%) farm and 23 (62%) control children.
b28 (100%) farm and 36 (97%) control children.
c19 (68%) farm and 32 (87%) control children.
Figure 2.
Fecal SCFA levels in farm and control children. Median concentration of total SCFAs and individual species of SCFAs in farm and rural control children at (a) 4 weeks, n = 24 (farm) and 23 (control); (b,c) 1 year, n = 28 (farm) and 36 (control), and (d,e) 3 years of age, n = 19 (farm) and 32 (control). Error bars show the interquartile range. *P < 0.05, Mann–Whitney U-test.
Association between life-style factors and SCFA patterns
There were a number of differences in the demographic, family, birth and dietary characteristics of farm and rural control children (Table 1), many of which could influence gut microbiota composition. Multiple logistic regression models were used to determine whether living on a farm independently predicted the SCFA levels at 3 years of age significant in univariate analysis, after adjusting for covariates (Table 3). The models also enabled us to investigate factors that may help explain, and possibly mediate, the distinct SCFA profile seen in farm children, as well as identify factors that predicted SCFA levels in all children. Variables listed in Table 1 were screened for inclusion in the models. In addition, elder siblings and dietary intakes of protein and fibre were included as they have been linked previously with SCFA levels9,18.
Table 3.
Logistic regression models of demographic, family, birth and dietary characteristics on the associations between farm residence and high concentrations of fecal iso-butyric, iso-valeric and valeric acids at 3 years of age.
| SCFAa | Model type | Predictor variable(s) | OR | (95% CI) | P |
|---|---|---|---|---|---|
| Iso-butyric | Univariate | Living on a farm | 4.7 | (1.3–16) | 0.02* |
| Multivariateb | Living on a farm | 17 | (2.1–131) | 0.008** | |
| Protein (g/day)c,d | 1.3 | (1.1–1.5) | 0.002** | ||
| Fibre (g/day)c,d | 0.5 | (0.3–0.8) | 0.003** | ||
| Homemade porridge (g/day)c,e | 1.0 | (1.0–1.1) | 0.08 | ||
| Iso-valeric | Univariate | Living on a farm | 3.6 | (1.0–12) | 0.04* |
| Multivariateb | Living on a farm | 6.7 | (1.5–30) | 0.01* | |
| Protein (g/day)c,d | 1.1 | (1.0–1.3) | 0.01* | ||
| Fibre (g/day)c,d | 0.7 | (0.5–1.0) | 0.04* | ||
| Valeric | Univariate | Living on a farm | 6.9 | (1.7–28) | 0.008** |
| Multivariateb | Living on a farm | 6.4 | (1.4–31) | 0.02* | |
| Siblings | 6.2 | (1.5–26) | 0.01* | ||
| Cat or doge | 3.3 | (0.8–13) | 0.10 |
*P < 0.05, binary logistic regression, n = 19 (farm) and 32 (control).
aSCFA levels dichotomized as concentration of iso-butyric acid, > or ≤ 2.0 μmol/g; iso-valeric acid, > or ≤ 2.5 μmol/g; valeric acid, > or ≤ 2.0 μmol/g.
bFinal multivariate model. Additional variables related to farm residence and fecal SCFA concentration with P < 0.2, and therefore included in the model-building process, were: (iso-butyric acid) intake of homemade porridge and cream; (iso-valeric acid) cream; (valeric acid) father’s education level. Elder siblings and dietary intakes of protein and fibre were also included, as they have been linked theoretically with SCFA levels.
cAt 1 year of age. We hypothesized that intake at 1 year of age may well be similar to that at 3 years of age and/or may influence the development trajectory of the gut microbiota and short chain fatty acid profile.
d,eThe primary OR (d) increased or (e) decreased by ≥ 10% when the variable was removed and subsequently reintroduced into the model.
After adjustment, living on a farm continued to predict the concentrations of fecal iso-butyric, iso-valeric and valeric acids at 3 years of age (iso-butyric acid, aOR 17, 2.1–131, P = 0.008; iso-valeric acid, aOR 6.7, 1.5–30, P = 0.01; valeric acid, aOR 6.4, 1.4–31, P = 0.02). Further, having elder siblings predicted the concentration of fecal valeric acid independent of the farming effect (aOR 6.2; 95% CI 1.5–26; P = 0.01). Living with a cat or dog partly explained the higher concentration of fecal valeric acid in farm children, as indicated by a decrease in the principal odds ratio of 10% on addition of this variable to the logistic regression model (aOR 3.3; 95% CI 0.8–13; P = 0.10).
Iso-butyric and iso-valeric acids are produced by microbial fermentation of the amino acids valine and leucine10, and valeric acid is likewise generated from the amino acids proline and hydroxyproline22. Using data from dietary registration at 1 year of age, we observed associations between dietary protein intake at 1 year of age and the concentration of fecal iso-butyric and iso-valeric acid at 3 years of age (aOR 1.3; 95% CI 1.1–1.5; P = 0.002 and aOR 1.1; 95% CI 1.0–1.3; P = 0.01, respectively), independent of farm residence. Dietary fibre intake at 1 year of age was negatively associated with the concentration of fecal iso-butyric and iso-valeric acid at 3 years of age (aOR 0.5; 95% CI 0.3–0.8; P = 0.003 and aOR 0.7; 95% CI 0.5–1.0; P = 0.04, respectively), independent of farm residence. No association was seen between protein or fibre intake and fecal valeric acid. Dietary intake of homemade porridge at 1 year of age partly explained the higher concentration of iso-butyric acid in farm children (aOR 1.0; 95% CI 1.0–1.1; P = 0.08).
Links between SCFA levels and allergy development
We investigated whether fecal SCFAs at 3 years of age were associated with eczema, asthma or allergic rhino-conjunctivitis at 8 years of age. No children had food allergy at 8 years of age. Of the 48 children who were clinically evaluated, nine had no fecal samples available. The analysis therefore included 39 children, nine of whom were allergic and 30 of whom had no allergy and were classified as non-allergic.
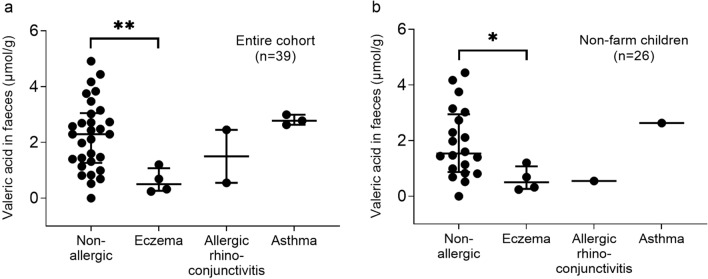
Children with eczema at 8 years of age had a lower median concentration of valeric acid in their feces at 3 years of age than non-allergic children (0.5 vs. 2.3 μmol/g, P = 0.007; Fig. 3a). Other allergic manifestations could not be linked to valeric acid concentration (Fig. 3a), and concentrations of other SCFAs were not related to any allergy diagnosis at 8 years of age.
Figure 3.
Fecal valeric acid levels at 3 years of age in relation to allergic manifestation at 8 years of age. In (a) the whole cohort (n = 39), including both farm and non-farm children, are shown, while (b) shows control children only (n = 26). Non-allergic children were defined as having no allergy at 8 years of age. *P < 0.05, **P < 0.01, Mann–Whitney U-test. Vertical bars show the median and inter-quartile range.
An analysis of only control children, to avoid possible confounding by farm residence, included just six allergic and 20 non-allergic children. Again, children who had eczema at 8 years of age had a lower median concentration of fecal valeric acid at 3 years of age than non-allergic children (0.5 vs. 1.5 μmol/g, P = 0.03; Fig. 3b).
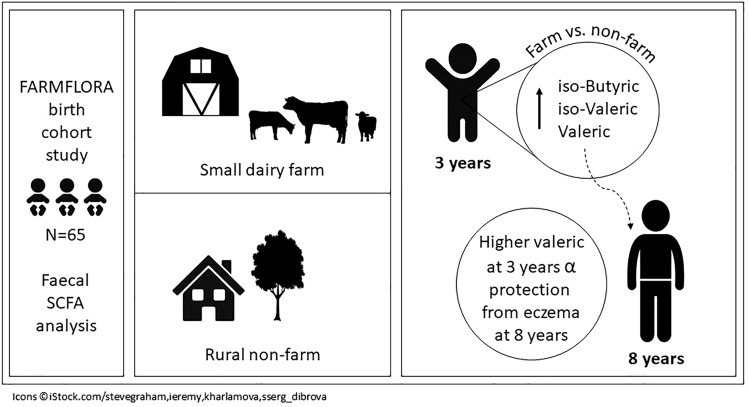
An overview of key findings is presented in Fig. 4.
Figure 4.
Overview of key findings.
Discussion
Short chain fatty acids are end-products of fermentation of carbohydrate and protein by bacteria in the colon, and the pattern of individual SCFAs depends on microbiota composition, and also on the availability of substrates for fermentation. In early childhood, a gradual development of a more and more complex gut microbiota is seen, from a rather simple composition in early infancy, with the successive establishment of more and more strict anaerobes leading to a pronounced dominance of anaerobic bacteria at 2 or 3 years of age23.
In the early months, the major end points of metabolism of the gut microbes are acetic and lactic acid. Lactic acid is non-volatile and not detected by gas chromatography. Thus, acetic acid is by far the most abundant SCFA measured in the colon. The trends we observed in SCFA level from 4 weeks to 3 years of age, namely an early dominance of acetic acid (C2) followed by the progressive increase in levels of the longer (C3–5) and branched (iC4–5) SCFAs, are consistent with findings from a number of previous studies18,24,25. In an adult-pattern microflora containing hundreds of different species26, different groups of bacteria species possess specific enzymes involved in the production of the various SCFAs9. Indeed, the longest (C5–6) and branched SCFAs are produced by anaerobes belonging to the dominant Bacteroidetes and Firmicutes phyla, often via cross-feeding pathways27–29.
Iso-butyric and iso-valeric acids are produced via the microbial fermentation of the amino acids valine and leucine10. Valeric acid is likewise generated from the amino acids proline and hydroxyproline22, as well as by the microbial metabolism of lactate30 and propionate31. Accordingly, dietary protein intake at 1 year of age predicted levels of fecal iso-butyric and iso-valeric acid at 3 years of age. We have earlier reported quite pronounced differences in dietary pattern between farming and non-farming families in our cohort, regarding the maternal diet during pregnancy32, fatty acid pattern in maternal breastmilk32, and the children’s diet at 12 months of age8, with intake of more full-fat dairy products and saturated fat in the farm mothers and children. However, protein intake did not vary between farm and control children in this study. Multiple logistic regression models confirmed that differences in diet and other factors we collected data on did not account for the increased levels of iso-butyric, iso-valeric and valeric acids observed at 3 years of age in farm children compared to controls in this study. Our results accordingly suggest that a more mature, complex gut microbiota in the farm children is the chief explanation for the increased levels of these SCFAs.
We observed substantially lower concentrations of fecal valeric acid at 3 years of age in children who had eczema at 8 years of age, as compared to non-allergic children. This relationship was evident in our entire cohort, and also in a sub-analysis of control children, indicating that high levels of fecal valeric acid derived from non-farm exposures are also associated with protection from eczema, as discussed more fully below. However, levels of fecal valeric acid were higher in farm children than in non-allergic controls (median 2.7 vs. 1.5 μmol/g, P = 0.04), in line with the particularly strong degree of allergy protection conferred by growing up on a farm. We could not link valeric acid levels with allergic rhino-conjunctivitis or asthma, but these categories contained few children. Also, we did not find any association between the concentration of iso-butyric or iso-valeric acid at 3 years of age and any of the allergic conditions we assessed for at 8 years of age. Our findings suggest that farm-related protection from allergy may be mediated in part by fecal valeric acid, or by characteristics of the gut microbiota associated with fecal valeric acid.
To date, few other prospective studies have described fecal SCFA patterns in relation to subsequent allergy. However, consistent with our findings, Sandin et al. observed lower levels of fecal iso-butyric, iso-valeric and valeric acid at 1 year of age in children with reported food allergy at 4 years of age in the BarnAllergiStudie (BAS) birth cohort study18. Further, data from the same birth cohort show lower levels of fecal valeric acid at 1 year of age in children with eczema, and also food allergy, at 13 years of age (Gio-Batta et al., Low levels of fecal valeric acid at 1 year of age are associated with eczema and food allergy at 13 years of age in a prospective birth cohort). Other prospective studies have generally not included valeric acid in the SCFA analysis. However, in another birth cohort, lower levels of fecal propionate and butyrate at 12 months of age were linked with higher risk of sensitization between 3 and 6 years of age33. Further, in a clinical trial of non-digestible oligosaccharides in infant formula in high-risk infants, higher levels of fecal propionate and butyrate at 3 months of age, but lower levels of the same SCFAs at 6 months of age, were associated with eczema at 18 months34.
The pathogenesis of allergy has variously been related to impairment of epithelial barrier function, altered immune responses and dysbiosis of the gut microbiota35–37. Recent studies demonstrate that valeric acid upregulates tight junction proteins and also keratin (KRT1), promoting tissue integrity and barrier function38,39. Conversely, KRT1-deficient mice show impaired skin barrier function and have a gene expression signature similar to that observed in skin of human eczema patients40. Valeric acid also induces IL-10 production, inhibits IL-17 and increases survival of regulatory B cells, thereby promoting the regulatory activity of lymphocytes41,42. In addition, valeric acid is antimicrobial43 and oral administration of valeric acid esters to rodents either fed a high fat diet or subjected to irradiation helps protect against gut dysbiosis39,44. Further, administration of valeric acid or its esters to animal models protects against colitis39 and necrotic enteritis45, while adoptive transfer of valerate-treated Bregs together with naive CD4 + T cells into Rag1-deficient mice protects against colitis and experimental autoimmune encephalitis41. These studies suggest multiple mechanisms by which valeric acid may potentially protect against allergy, and show in-vivo efficacy of valeric acid against other immune-mediated diseases.
Besides farm residence and diet, some other factors also affected SCFA pattern. These included living with a cat or dog, which helped explain the increased fecal valeric acid levels in children raised on farms, as they were more likely to own a pet than control children. Accordingly, data regarding the microbial composition of this cohort show a larger influence of cats and dogs on the gut microbiota than farm residence itself (Ljung et al., Effects of pets and farm residence on gut microbiota establishment and implications for allergy development). We also observed that children with older siblings had higher levels of fecal valeric acid, as was also noted by Sandin et al.18. Interestingly, infants with no older siblings tend to have an impoverished microbiota compared to infants with siblings46. Both elder siblings and early pet exposure have also been associated with reduced risk of allergic disease47,48.
The major limitation of this study was the moderate number of study subjects, yielding a limited number of allergic children. The strengths were the longitudinal design, permitting analysis of SCFA pattern development during early childhood and measurement of a broad range of dietary and other exposure data, as well as allergy being stringently diagnosed by study pediatricians. However, no environmental microbial samples were collected, and therefore the hypothesis that increased microbial exposure on farms may affect the structure and function of the gut microbiota could not be addressed directly.
In conclusion, growing up on a small dairy farm is associated with a fecal SCFA pattern suggestive of a more mature and complex anaerobic gut microbiota at 3 years of age, as compared to living in the surrounding rural area but not on a farm. Specifically, farm children had higher levels of fecal iso-butyric, iso-valeric and valeric acids. Diet could not explain this pattern, but exposure to pets accounted for part of the farming effect. High levels of fecal valeric acid were inversely associated with eczema at 8 years of age, suggesting that valeric acid may explain part of the farm-related protection from allergy. Although this finding needs to be confirmed in larger studies, it implies that valeric acid or microbes producing this metabolite could potentially offer therapeutic potential in preventing or treating eczema.
Acknowledgements
We thank the study nurses Helen Andersson and Anders Nordberg for their skillful work in coordinating sampling and questionnaires. Also, we gratefully acknowledge pediatricians Margareta Ceder, Gunhild Lindhagen, Stefan Stentoft, Carl-Johan Törnhage and Susanne Johansen, who diagnosed the children, and all the families for their participation in this study. This research was supported by the Swedish Research Council for Environmental, Agricultural Sciences and Spatial Planning (Formas) under Grant number 222-2004-1958; the Ekhaga Foundation; the Västra Götaland Region’s Food and Health Concept Centre; the Swedish Research Council (Vetenskapsrådet) under Grant number 521-2013-3154; and the Swedish federal government under the LUA/ALF agreement.
Author contributions
A.W., I.A. and A.S. designed and supervised the FARMFLORA birth cohort study, in which A.S. supervised nutritional aspects. K.J. compiled the dietary data, and K.J., M.B. and A.S. jointly discussed and interpreted it. B.H. was responsible for confirmation of allergy diagnoses. A-C.L. planned and implemented the 8-year follow-up. F.S. and A.W. designed this study. M.G-B. determined SCFA levels, analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Funding
Open Access funding provided by Gothenburg University Library.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
AW holds shares in Flora Innovation, a small researcher-driven company investigating potential preventive treatments against immune-regulated diseases. The other authors have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riedler J, et al. Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 2.Stein MM, et al. Innate immunity and asthma risk in amish and hutterite farm children. N. Engl. J. Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cholapranee A, Ananthakrishnan AN. Environmental hygiene and risk of inflammatory bowel diseases: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2016;22:2191–2199. doi: 10.1097/MIB.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pukkala E, et al. Occupation and cancer—follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- 5.Pawankar R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014;7:12. doi: 10.1186/1939-4551-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018;15:440–452. doi: 10.1038/s41575-018-0003-z. [DOI] [PubMed] [Google Scholar]
- 7.Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson K, et al. Diet in 1-year-old farm and control children and allergy development: Results from the FARMFLORA birth cohort. Food Nutr. Res. 2016;60:32721. doi: 10.3402/fnr.v60.32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan J, et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 10.Zarling EJ, Ruchim MA. Protein origin of the volatile fatty acids isobutyrate and isovalerate in human stool. J. Lab. Clin. Med. 1987;109:566–570. [PubMed] [Google Scholar]
- 11.Böttcher MF, Nordin EK, Sandin A, Midtvedt T, Björkstén B. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy. 2000;30:1590–1596. doi: 10.1046/j.1365-2222.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- 12.Nowrouzian F, et al. Escherichia coli in infants' intestinal microflora: Colonization rate, strain turnover, and virulence gene carriage. Pediatr. Res. 2003;54:8–14. doi: 10.1203/01.PDR.0000069843.20655.EE. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 14.Burger-van Paassen N, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 15.Roediger WEW. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: An emerging perspective in obesity and allergic inflammation. Obes. Rev. 2017;18:18–31. doi: 10.1111/obr.12484. [DOI] [PubMed] [Google Scholar]
- 18.Sandin A, Bråbäck L, Norin E, Björkstén B. Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatr. 2009;98:823–827. doi: 10.1111/j.1651-2227.2008.01215.x. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson K, et al. Late introduction of fish and eggs is associated with increased risk of allergy development—results from the FARMFLORA birth cohort. Food Nutr. Res. 2017;61:1393306. doi: 10.1080/16546628.2017.1393306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strombeck A, et al. Allergic disease in 8-year-old children is preceded by delayed B cell maturation. Clin. Exp. Allergy. 2017;47:918–928. doi: 10.1111/cea.12922. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Nyman M, Jonsson JA. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006;20:674–682. doi: 10.1002/bmc.580. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen HS, Holtug K, Mortensen PB. Degradation of amino acids to short-chain fatty acids in humans. An in vitro study. Scand. J. Gastroenterol. 1988;23:178–182. doi: 10.3109/00365528809103964. [DOI] [PubMed] [Google Scholar]
- 23.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midtvedt AC, Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J. Pediatr. Gastroenterol. Nutr. 1992;15:395–403. doi: 10.1097/00005176-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Norin E, Midtvedt T, Björkstén B. Development of faecal short-chain fatty acid pattern during the first year of life in Estonian and Swedish infants. Microbial. Ecol. Health Dis. 2004;16:8–12. doi: 10.1080/08910600410026364. [DOI] [Google Scholar]
- 26.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rios-Covian D, et al. Different metabolic features of Bacteroides fragilis growing in the presence of glucose and exopolysaccharides of bifidobacteria. Front. Microbiol. 2015;6:825. doi: 10.3389/fmicb.2015.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsden SR, Hilton MG. Volatile acid production from threonine, valine, leucine and isoleucine by clostridia. Arch. Microbiol. 1978;117:165–172. doi: 10.1007/BF00402304. [DOI] [PubMed] [Google Scholar]
- 29.Lanjekar VB, Marathe NP, Ramana VV, Shouche YS, Ranade DR. Megasphaera indica sp. Nov., an obligate anaerobic bacteria isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2014;64:2250–2256. doi: 10.1099/ijs.0.059816-0. [DOI] [PubMed] [Google Scholar]
- 30.Bourriaud C, et al. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005;99:201–212. doi: 10.1111/j.1365-2672.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- 31.Jeon BS, Choi O, Um Y, Sang BI. Production of medium-chain carboxylic acids by Megasphaera sp. MH with supplemental electron acceptors. Biotechnol. Biofuels. 2016;9:129. doi: 10.1186/s13068-016-0549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonsson K, et al. Fat intake and breast milk fatty acid composition in farming and nonfarming women and allergy development in the offspring. Pediatr. Res. 2016;79:114–123. doi: 10.1038/pr.2015.187. [DOI] [PubMed] [Google Scholar]
- 33.Roduit C, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74:799–809. doi: 10.1111/all.13660. [DOI] [PubMed] [Google Scholar]
- 34.Wopereis H, et al. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 2018;141:1334–1342.e1335. doi: 10.1016/j.jaci.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 35.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J. Allergy Clin. Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Mattila P, Joenvaara S, Renkonen J, Toppila-Salmi S, Renkonen R. Allergy as an epithelial barrier disease. Clin. Transl. Allergy. 2011;1:5. doi: 10.1186/2045-7022-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascal M, et al. Microbiome and allergic diseases. Front. Immunol. 2018;9:1584. doi: 10.3389/fimmu.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen TD, Hållenius FF, Lin X, Nyman M, Prykhodko O. Monobutyrin and monovalerin affect brain short-chain fatty acid profiles and tight-junction protein expression in ApoE-knockout rats fed high-fat diets. Nutrients. 2020;12:1202. doi: 10.3390/nu12041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, et al. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes. 2020;11:789–806. doi: 10.1080/19490976.2019.1709387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth W, et al. Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J. Cell Sci. 2012;125:5269–5279. doi: 10.1242/jcs.116574. [DOI] [PubMed] [Google Scholar]
- 41.Luu M, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019;10:760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuille S, Reichardt N, Panda S, Dunbar H, Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE. 2018;13:e0201073. doi: 10.1371/journal.pone.0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovanda L, et al. In vitro antimicrobial activities of organic acids and their derivatives on several species of gram-negative and gram-positive bacteria. Molecules. 2019;24:3770. doi: 10.3390/molecules24203770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen TD, Prykhodko O, Fåk Hållenius F, Nyman M. Monovalerin and trivalerin increase brain acetic acid, decrease liver succinic acid, and alter gut microbiota in rats fed high-fat diets. Eur. J. Nutr. 2019;58:1545–1560. doi: 10.1007/s00394-018-1688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onrust L, et al. Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis. Poult. Sci. 2018;97:2303–2311. doi: 10.3382/ps/pey085. [DOI] [PubMed] [Google Scholar]
- 46.Adlerberth I, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol. 2007;120:343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hesselmar B, Aberg N, Aberg B, Eriksson B, Björkstén B. Does early exposure to cat or dog protect against later allergy development? Clin. Exp. Allergy. 1999;29:611–617. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.