Abstract
Postharvest waste and loss of horticultural crops exacerbates the agricultural problems facing humankind and will continue to do so in the next decade. Fruits and vegetables provide us with a vast spectrum of healthful nutrients, and along with ornamentals, enrich our lives with a wide array of pleasant sensory experiences. These commodities are, however, highly perishable. Approximately 33% of the produce that is harvested is never consumed since these products naturally have a short shelf-life, which leads to postharvest loss and waste. This loss, however, could be reduced by breeding new crops that retain desirable traits and accrue less damage over the course of long supply chains. New gene-editing tools promise the rapid and inexpensive production of new varieties of crops with enhanced traits more easily than was previously possible. Our aim in this review is to critically evaluate gene editing as a tool to modify the biological pathways that determine fruit, vegetable, and ornamental quality, especially after storage. We provide brief and accessible overviews of both the CRISPR–Cas9 method and the produce supply chain. Next, we survey the literature of the last 30 years, to catalog genes that control or regulate quality or senescence traits that are “ripe” for gene editing. Finally, we discuss barriers to implementing gene editing for postharvest, from the limitations of experimental methods to international policy. We conclude that in spite of the hurdles that remain, gene editing of produce and ornamentals will likely have a measurable impact on reducing postharvest loss and waste in the next 5–10 years.
Subject terms: Molecular engineering in plants, Molecular engineering in plants
Introduction
Plant gene editing may be the greatest innovation in plant breeding since the Green Revolution. It has already been used to make discoveries in plant biology and has a profound potential to create new crops with desirable characteristics1. There are already exciting developments, which show that gene editing may be able to live up to expectations and can be used to produce novel plant phenotypes that would improve agricultural production.
Most authorities estimate that food production will have to double in the next 50 years to keep pace with population growth2. The focus on global food security, however, is usually on starch-rich cereals and ignores or underestimates the vital importance of horticultural crops. These perishable commodities are often nutrient-dense with bioactive phytochemicals, the consumption of which is needed for a healthy and thriving population3–6. However, an uncomfortable fact is that in addition to losses that may result from disease, drought, extremes of temperature, and other environmental stresses experienced in the field, an additional 25–40%—an average of 33%—of all fruit and vegetables produced globally are never eaten after harvest7. This estimate still does not illustrate the extreme losses that can occur in some developing countries, which may be as high as 75%8,9. Current worldwide horticultural crop production is insufficient to meet human nutritional requirements, making postharvest loss and waste all the more unsustainable10. Only recently has the need to reduce the loss of horticultural crops after harvest been given the attention it deserves7–9,11–14.
Although the causes of postharvest loss and waste are complicated, we suggest that technology-assisted breeding for new and improved fruit, vegetables, and ornamentals, compatible with supply chain constraints but delivered at peak quality to the consumer, could be an important part of the solution over the long-term. In this review, we examine the potential for gene editing to make a measurable and robust impact on postharvest waste and loss. Rather than a technical or critical assessment of methodologies or research areas, we focus on connecting the bio-physiology of postharvest produce, the needs of the produce industry, and the wealth of existing molecular research, to suggest a holistic yet straightforward approach to crop improvement. The main focus of the review is the discussion of genes that could influence the quality and shelf-life of produce. First, we examine the steps that are taken to extend shelf-life in the produce supply chain, and the impact of supply chain management on consumer-desired quality traits. Then we briefly review the CRISPR–Cas9 method to emphasize the flexibility, ease, and power with which traits can be modified. Finally, we take a critical look at remaining barriers which must be overcome to make gene editing for postharvest traits technically and economically viable. This review serves both as an introduction to postharvest and gene editing and as a resource for researchers attempting to utilize the latter for the former.
Overview of postharvest loss and waste (PLW)
Postharvest waste and postharvest loss are sometimes used interchangeably, but this is incorrect. Postharvest loss is unintentional. It describes the incidental losses that result from events occurring from farm-to-table, such as physical damage, internal bruising, premature spoiling, and insect damage, among others. Produce loss is also described as quantitative because it is measurable. This does not imply that data is easily available, only that it can be assessed8,12.
Postharvest waste, in contrast, is intentional. It describes when produce is discarded because it does not meet buyer expectations, even though it is edible8,12. Produce may be rejected by growers, distributors, processing companies, retailers, and consumers for failing to meet desired or established preferences. Produce waste is described as qualitative because it is difficult to measure and assess8. Still, in the US, it is estimated that 7% of postharvest losses of fruit and vegetables occur on the farm, while more than twice that, i.e., 17% and 18% are wasted in consumer-facing businesses and in homes, respectively14.
Produce postharvest loss and waste (PLW) threatens environmental sustainability, and is especially catastrophic when viewed in the light of the twin challenges of global climate change and increasing population growth. PLW means inefficient use of financial investments in horticulture and more critically, non-renewable natural resources. Technological measures to curb PLW, such as maintaining a cold-chain and use of plastic packaging, additionally have energy and carbon costs. Improving the shelf-life and quality attributes of postharvest crops by genetic modification or smart breeding could be among many solutions to lessen the severity of these problems.
The challenge of the postharvest supply web
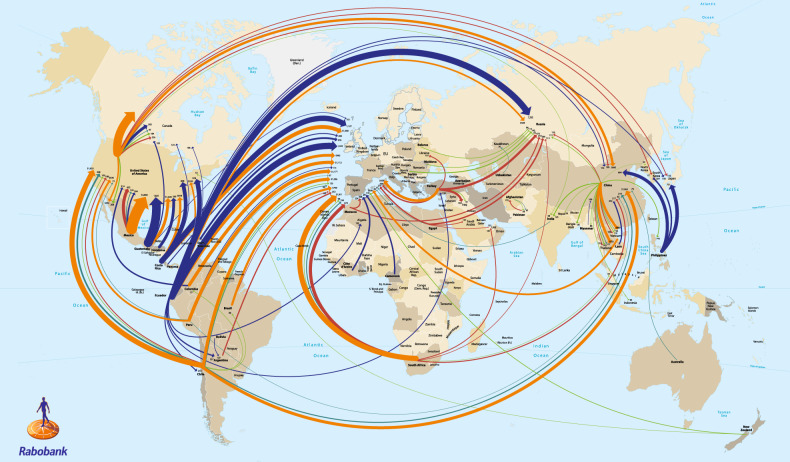
Produce must be kept alive from farm to table; however, the biological nature of horticultural produce is often incongruent with modern commercial supply chain operations15. Produce and ornamentals are high in water content, and often metabolically active, which makes them highly perishable15–17. This becomes a challenge given the number of food miles fruit, vegetables, and ornamentals can travel in the global supply chain (Fig. 1).
Fig. 1. Map of the global trade of fruit in 2016.
An estimated 80% of all fruits grown globally are sold as whole fresh fruit. Key to colored lines: orange—trade movement and monetary value of total fresh fruit, excluding nuts and frozen fruit. Other lines illustrate commodity volume. Blue—bananas and plantains; green—apples; aquamarine—grapes; red—citrus. The minimum requirement for trade values to be shown is USD 500 million, while for commodity volume, it was bananas, plantain, and citrus—100,000 tons and for apples and grapes—50,000 tons. Line thickness proportional to the magnitude of the trade. Source: van Rijswick273 RaboResearch, The Netherlands. Reproduced with the kind permission of RaboResearch
Modern postharvest supply chains may be separated spatially by thousands of miles, and temporally, by several months. Produce trucked and shipped from the field is often treated: cooled, washed, sorted, dipped, sprayed, or held at desirable temperatures and modified atmospheres to preserve “health”. The majority of produce from mid- to large-scale operations may move through a byzantine system of processors, distributors, and trucking and shipping entities. Maintaining an unbroken cold-chain, adequate packing, and shipping are essential to preserving quality and shelf-life. (Zoom in on map to read text).
Produce, even after harvest, respires (taking up oxygen and producing carbon dioxide), transpires water, and, for the “climacteric fruits”, can emit high levels of ethylene, which can be accelerated at high temperatures. Optimizing storage and handling conditions requires managing these biological processes (Fig. 2), which may differ for each produce-type or variety, and from how the preharvest environment influences biology at harvest and thereafter15. Temperature, humidity, ethylene levels, and the storage oxygen-to-carbon dioxide ratio must be controlled to slow down maturation and senescence in order to maintain produce shelf-life and quality15,18,19. Low temperatures are used to reduce respiration, thereby extending shelf-life18, but also have the added benefit of suppressing water loss, shrinkage, and fungal growth, which can occur due to physical injury and physiological disorders18,20. Modifying the atmosphere to change the carbon dioxide-to-oxygen ratio and relative humidity using modified atmosphere packaging or large-scale storage of produce in controlled atmosphere rooms can extend the postharvest life of commodities (Fig. 2).
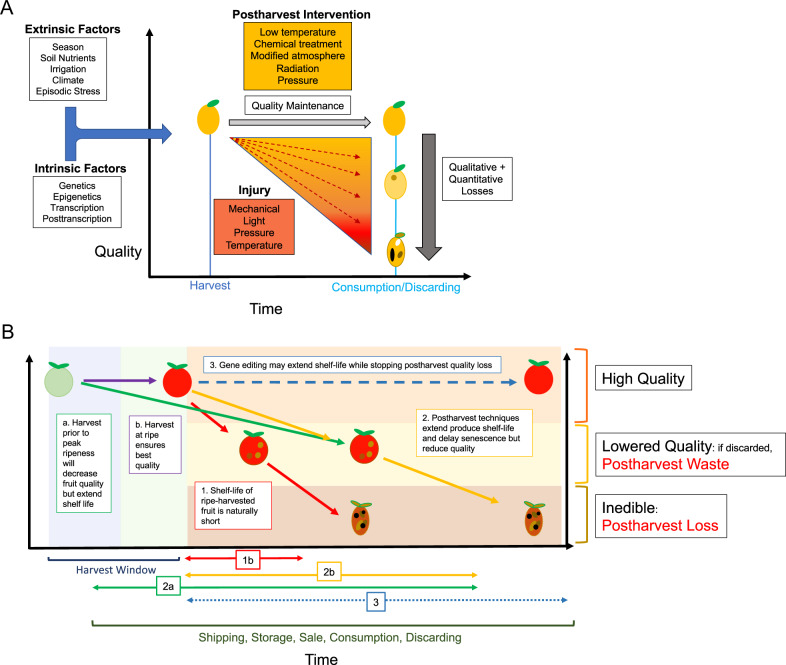
Fig. 2. Determinants of produce quality.
a Extrinsic environmental factors such as season, irrigation, soil nutrition and minerals, climate, stress, pathogens and pests, and agronomic practices as well as physiological genetic factors together determine fruit quality at harvest. Postharvest intervention, including refrigeration, chemical treatment, radiation, and modified atmospheres and pressure aims to maintain that quality through shipping and storage. Minor injury, ranging from mechanical or pathogenic damage to temperature, light, or pressure-induced damage, lowers the quality of fruit. More extensive injury renders produce inedible and contributes to the quantitative loss. b Potential postharvest outcomes for produce. Harvesting fruit prior to full ripeness will increase its shelf-life [a], but compromises quality during and after ripening [2a]. Fruit harvested at ripe [b] has a limited shelf-life before it declines in quality or rots [1b]. Postharvest intervention delays senescence and typically also results in some compromise of quality [2b]. The goal of gene editing is to extend shelf-life without loss of quality [3] and therefore reduce postharvest loss and waste
Ethylene biosynthesis and emission underpin postharvest quality and shelf-life in climacteric fruit21–24 and vegetables24–27. Ethylene accelerates ripening, but also senescence; therefore, ethylene must be managed to optimize shelf-life. This is underscored by the number of ethylene inhibitors, absorbers, and blockers18 on the market (Fig. 4).
Fig. 4. Genetic determinants of ethylene production and their control for extended postharvest shelf-life.
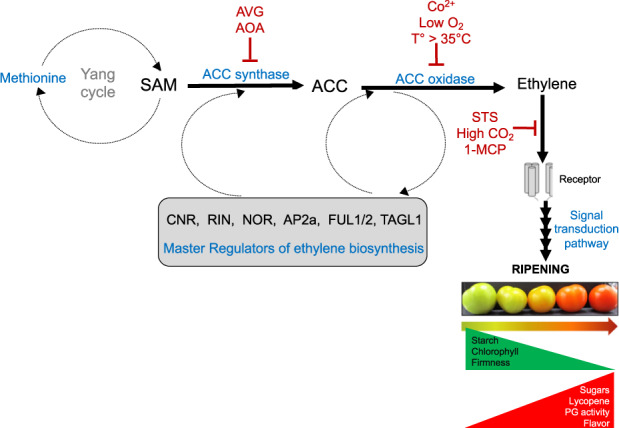
Ethylene biosynthesis occurs in two enzymatic steps, catalyzed by ACC (1-aminocyclopropane-1-carboxylic acid) synthase (ACS) and ACC oxidase (ACO)274. In climacteric fruit, such as tomato, banana, mango, and apple, there is a rapid rate of increase in ethylene at the onset of ripening and continued production leads to ripening and senescence. Enzymatic inhibitors AVG (aminoethoxyvinylglycine) or AOA (aminooxyacetic acid) inhibit ACS275. Cobalt ions (Co2+), high temperatures (T°), and low oxygen concentration inhibit ACO or low oxygen concentration inhibits ACO276. Silver ion, silver thiosulfate (STS), potentially, carbon dioxide, and 1-methylcyclopropene (1-MCP) inhibit ethylene binding to the receptor for activation of ethylene signaling pathway277,278. For example, 1-MCP, a synthetic growth regulator structurally related to ethylene, is commercially used in fruit crops such as apple, kiwifruit, pear, avocado, melons and others, and it has also shown biological benefits in a range of other species16,279. SAM S-adenosyl methionine
The biological reality of ripening is that its natural end is senescence. The goal of postharvest management is therefore to control this progression to senescence, i.e., to pause the ripening process for shipping and storage, and then to restart it with a minimal loss of quality. However, the processes that control the ripening-to-senescence transition dictate quality, creating a dilemma, whereby altering ripening biology via refrigeration, chemicals, or other means to lengthen shelf-life, often unavoidably disrupts ripening outcomes and reduces quality28. This leads to consumer rejection and postharvest waste. The alternative—to maximize consumer preference by harvesting produce close to peak maturity stage, and with no chemical or physical treatment, will invariably increase postharvest losses due to the shortened shelf-life, and increased susceptibility to bruising and pathogenic infection (Fig. 2).
Potential for improving postharvest quality of horticultural crops by gene editing
There is great excitement at the innovation gene editing and the associated technologies potentially bring for improving crop quality, especially for species and traits that have been relatively understudied, such as postharvest traits of horticultural crops. Manipulation of plant genomes in a precise manner has been achieved at a spellbinding pace since the era of genome editing29–31. The current gene-editing tool of choice is CRISPR–Cas9. The researcher is able to generate mutations in narrowly defined regions of the genome, and it has been successfully applied to induce valuable traits in many crop species32,33. Further, CRISPR can bypass other burdens like sterility, self-incompatibility, high heterozygosity, low frequency of recovering desired alleles and traits and long life cycles, which extend or halt entirely conventional breeding efforts34–36.
CRISPR is a prokaryotic system that protects organisms from viral infection37. This naturally occurring mechanism in bacteria has been co-opted by scientists to remove unwanted nucleotides or to insert new or altered ones to promote traits seen as desirable in an organism of interest. For CRISPR editing, a synthetic guide RNA (gRNA) is designed to an identified protospacer adjacent motif (PAM) in the sequence of interest, and this, along with the Cas protein sequence, is inserted into a cell where they are processed using the cell’s gene expression apparatus. The Cas protein synthesized in the plant produces a double-stranded break (DSB) at the bases identified by the gRNA. Repair of the DSB in DNA is usually not faithful to the original sequence, and thus, non-synonymous mutations may be introduced into the genome. The precise changes in nucleotide sequences are difficult to predict, but indels (insertion–deletions) of varying sizes and single-nucleotide polymorphisms are most common, providing diverse genetic variants38. DSB repairs occur naturally in almost all plant tissues, so this is not an inherently foreign process39,40.
Although genomic mutations generated by CRISPR-mediated random repair mechanisms are easily achieved, the ability to specifically express the Cas protein in a controlled spatial-temporal manner, and in conjunction with other enzymes, is often desirable for basic and applied plant research. Precise site-directed editing can be used for single-base substitution of a gene(s) of interest41, which has been achieved in cereals42,43, as well as horticultural crops such as tomato and potato42,44,45. In addition, tissue-specific knockouts using a CRISPR technique, called CRISPR-TSKO, can generate somatic mutations in cells, tissues, and organs by using specific promoters46. Similarly, another gene-editing system uses an inducible chimeric transcription factor (XVE), to control the expression of Cas protein in planta47,48.
Apart from knock-out/in of gene coding regions, transcriptional modulation of gene expression can be achieved by CRISPR targeting of gene regulatory elements49. New alleles generated by CRISPR/Cas in promoters and enhancers where transcription factors (TFs) bind to direct gene expression, can lead to fine-tuned expression1,50,51. Similarly, variants in upstream open reading frame (uORFs) sequences could enhance post-transcriptional modulation of gene expression, influencing phenotype1.
The expression of a gene may also be varied by changing its DNA methylation status. In tomato, orange, and bell pepper52–54, DNA methylation regulates ripening by controlling ripening-related TFs or genes. Binding a methylation modifying protein to a CRISPR complex with a deactivated Cas955 may be a feasible approach to edit regions targeted for de/methylation in ripening-related genes, thus controlling shelf-life.
CRISPR-Cas also enables modulation of traits in species that are difficult to obtain through traditional breeding. Approximately 70% of angiosperms are polyploid, which increases the effort needed for introducing new alleles by crossing and selection56. Transmission of Cas activity in the progeny of CRISPR-expressing lines holds promise for transgenerational gene-editing in polyploid plants. This method was shown to introduce newly mutated alleles, not only in F1 but also in F2 and F3 plants56,57. De novo domestication, a new idea in crop improvement, has been demonstrated in multiple species of the wild Solanum genus by CRISPR targeting58. Novel alleles of selected “domestication genes” are generated in wild species, landraces, or non-commercial genotypes to speed-up their transformation to elite varieties suitable for cultivation and postharvest practices of modern agriculture1,50,51.
In conclusion, various CRISPR techniques and approaches can be used to introduce nuanced changes in the expression of single or multiple genes, however, it also has real value as a tool to dissect the network of biological pathways responsible for ripening, senescence, and quality. It is expected to help identify hitherto unknown genes, that when altered, can promote favorable postharvest phenotypes. These desirable phenotypes are discussed in “Produce postharvest attributes that would minimize PLW” section.
Produce postharvest attributes that would minimize PLW
Recent consumer trends indicate a growing interest in consuming fruit and vegetables for their nutritional value. This is especially notable for middle-class consumers of emerging economies. Gene editing to reduce PLW may improve the overall efficiency of fruit and vegetable production so that costs may be lowered59, thereby bringing fresh produce within the means of more populations60–64 and strengthening the industry as well as worldwide health. There is also a demand for the produce of exceptional quality among discerning consumers65,66, a rising interest in organic and locally sourced produce, and in semi-prepared or “fresh-cut” vegetables and fruit16,17,67,68 that are reasonably priced. Key attributes are outlined below (Fig. 3):
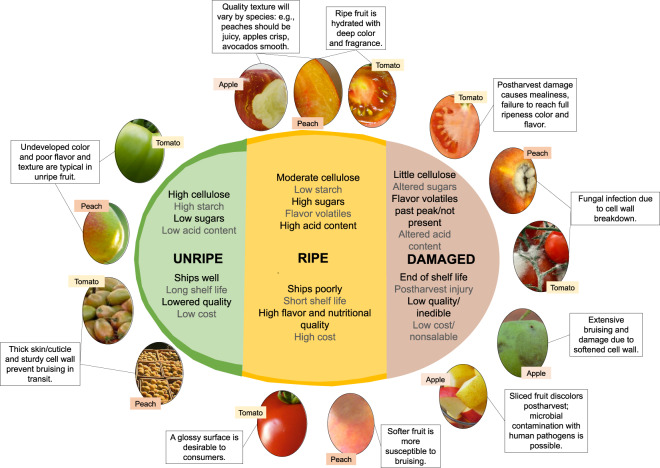
Fig. 3. Linking quality to physio-biological characteristics.
The physiological factors that confer produce quality (flavor, texture, shelf-life, aroma) are determined by the amount and interactions of metabolites, both primary and specialized, present in the various tissues. Texture and shelf-life are tightly connected to the cell wall and cuticle integrity, while flavor and aroma are linked to levels of sugars, acids, and other metabolic products. Biological changes in these factors due to the natural ripening-senescence transition or, due to postharvest handling, determine consumer acceptance
Longer shelf-life with maximal quality retention
Many of the approaches for extending the life of produce often lead to poor taste and flavor, and this link must be broken to increase consumer satisfaction and repeat purchases68,69.
Convenience
The fresh-cut industry has grown over the last 20 years, driven by a demand for convenience. The ability to eat fruit and vegetables directly from the packet has been a boon to the produce industry70. Quality attributes needed to provide safe, long-lasting, visually and texturally appealing fresh-cut products can be challenging to maintain since cut produce often respires faster and is prone to browning and premature senescence70. Microbial contamination, especially of fresh-cut leafy greens and fruit by E. coli, Salmonella, and Listeria is also problematic71.
Better quality
Consumers have shown that fruit and vegetables with desirable appearance, texture, taste, and flavors will have higher salability16,17,65,67. The criteria for a favorable appearance include produce of the right color and color uniformity, correct shape and dimensions, and often a glossy surface area free from defects15,67,70 (Fig. 3). Identifying and manipulating the genes determining these pathways could improve quality. Consumers also have specific notions of what “unacceptable” produce is, and this has consequences for the generation of postharvest waste. This may vary culturally and according to socio-economic status, but general trends are identifiable. Produce with characteristics reminiscent of rotten, infested, or unripe material will be rejected. This is widely accepted as an evolutionary strategy to avoid poisoning or illness from contaminated food72, as well as a learned response based on a previous negative experience. Therefore, lesions or aromas due to age or bruising are associated with “bad” fruit and vegetables and will be rejected not only as “low quality” but as potentially dangerous, despite the produce being largely intact and actually safe. While quality of flavor is widely believed to be a strong predictor of repeat purchase73, visible appearance has a strong role in initial selection or rejection at the point of purchase, and later discarding in the home74,75. These negative traits all interact with the consumer priorities mentioned above and contribute to postharvest waste.
Biological processes “ripe” for editing—shelf-life
Although our knowledge of basic fruit and produce biology is incomplete, there has been extensive work that points to the action of individual genes which, when altered in expression, may deliver useful phenotypes. Manipulating these biological processes by gene editing is a promising new avenue for reducing PLW. Many traits, however, are determined by networks of genes, and although distinct, some networks overlap so that changes in one may have unintended consequences in another. A major challenge is to understand the complicated regulation of these pathways in order to fine-tune them in a beneficial way. Gene editing has the potential to clarify the role of individual constituents in conjunction with the production of novel varieties.
Ethylene production
As mentioned in “The challenge of the postharvest supply web” section, ethylene is a master regulator of ripening; in climacteric fruit, ethylene production must be managed to optimize shelf-life (Fig. 4), but genetic solutions may be more effective. In climacteric fruit, ethylene synthesis, regulation, and perception lead to the transcription of ripening-regulated genes that determine quality attributes desired by consumers. When ACO and ACS (Fig. 4) expression is genetically suppressed or silenced in a range of species, e.g., petunia, tomato, melon, papaya, and kiwifruit, ethylene production is decreased and shelf-life is extended due to slowed ripening processes76–83.
In tomato, the regulation of ethylene biosynthesis is mediated by a complex network centered around the master regulatory proteins: CNR, RIN, and NOR, which are required for normal ripening84. The recent use of CRISPR to induce targeted deletions or substitutions in CNR53 and NOR53,85, and in other transcription factors (Fig. 4), AP2a, FUL1, and FUL286,87 revealed multiple and redundant levels of regulation in the ripening pathway. Using CRISPR to create fruit varying sequentially in one or more of these transcription factors may improve our understanding of the molecular regulation of ethylene response in horticultural crops. This knowledge would allow us to control ethylene production so that ripening proceeds at the rate and with the timing that is optimal for supply chain dynamics while maintaining quality. This would directly mitigate PLW.
Flower vase-life
Global demand for fresh-cut ornamentals has increased in the past years, with an estimated value of $16B in 201588. The top producers, the Netherlands, Ecuador, Columbia, and Kenya, export floral products long distances, primarily to Europe, North America, and East Asia88,89. However, ornamental crops are highly perishable and up to 50% of the farm value may be lost along the cold-chain90, and each extra day in transit leads to a 15% loss of value91. Further, after consumer purchase, ornamental shelf-life, i.e., vase-life, is typically only 10–12 days91, so rapid transport along a cold-chain is essential91,92.
Ethylene has a critical role in accelerating flower senescence in some species, and targeting components of the ethylene signal transduction pathway has been successful in extending vase-life in carnation93–95 and petunia54,96–98 (Table 1). Gene editing was also used to mutate ACO1 in petunia thereby increasing flower longevity99. In species that are not ethylene-responsive, vase-life could also be extended by inhibiting general senescence proteins100.
Table 1.
Targets for improved postharvest quality and enhanced shelf-life
| Species | Trait | Gene | Change* | Method | Phenotype | References |
|---|---|---|---|---|---|---|
| Tomato (Solanum lycopersicum) | Shelf-life | SlFSR | ↓ | RNAi | ↓ Expression of cell wall modification enzymes in fruit | Zhang et al.49 |
| Tomato (Solanum lycopersicum) | Shelf-life | SlACO1 | ↓ | RNAi | ↓ Ethylene, ↓firmness loss associated with ↓ PME and PG activities | Behboodian et al.261 |
| Tomato (Solanum lycopersicum) | Shelf-life | PG | ↓ | Antisense RNA | ↑ Fruit firmness, ↓ postharvest fungal infection | Kramer et al.262 |
| Strawberry (Fragaria × ananassa) | Shelf-life | FaPG1 | ↓ | RNAi | ↑ Soluble solids, firmness and ↓ softening | Quesada et al.263 |
| Tomato (Solanum lycopersicum) | Shelf-life | Del, Ros | ↑ | Ectopic Expression | Double shelf-life and ↓ susceptibility to Botrytis | Zhang et al.143 |
| Tomato (Solanum lycopersicum) | Shelf-life | Aft, atv | – | Breeding | Extended shelf-life ↓ susceptibility to Botrytis | Bassolino et al.145 |
| Tomato (Solanum lycopersicum) | Shelf-life | SlALC | ↓ | CRISPR/Cas9 | Extended shelf life | Yu et al.85 |
| Tomato (Solanum lycopersicum) | Ripening | SlPL | ↓ | CRISPR/Cas9 | ↑ Firmness, no change in color | Wang et al.260 |
| Banana (Musa acuminata) | Ripening | MaMADS1 MaMADS2 | ↓ | RNAi | Delayed ripening | Elitzur et al.264 |
| Eggplant (Solanum aethiopicum) | Color and nutritional content | SmMYB1 | ↑ | Overexpression | ↑ Anthocyanin: flesh and peel | Zhang et al.190 |
| Tomato (Solanum lycopersicum) | Softening | SlPG | ↓ | Antisense RNA | ↓ Pectin depolymerization | Smith et al.127 |
| Tomato (Solanum lycopersicum) | Softening | SlPL | ↓ | RNAi | ↑ Firmness, cellulose, hemicellulose, ↓ soluble pectins | Yang et al.265 |
| Tomato (Solanum lycopersicum) | Softening | PME | ↓ | Antisense RNA | ↓ Soluble pectins and polyuronides, ↑ soluble solids | Tieman et al.266 |
| Tomato (Solanum lycopersicum) | Softening | DkGal1 | ↑ | Heterologous expression | ↓ Cell-to-cell adhesion, ↑ ripening rate, ↑ ethylene production | Ban et al.267 |
| Tomato (Solanum lycopersicum) | Softening | TBG4 | ↓ | Antisense RNA | ↑ Firmness | Smith et al.123 |
| Tomato (Solanum lycopersicum) | Softening | PE1, PE2 (PME) | ↓ | Antisense RNA | ↓ De-esterification of pectins | Wen et al.122 |
| Strawberry (Fragaria × ananassa) | Softening | FaPG1 | ↓ | Antisense RNA | ↑ Firmness, reduced soluble pectin, ↑ cell integrity | Posé et al.115 |
| Strawberry (Fragaria × ananassa) | Softening | FaPL | ↓ | Antisense RNA | ↑ Firmness, ↓ soluble pectins | Jiménez-Bermudez et al.117 |
| Strawberry (Fragaria × ananassa) | Softening | FaPL | ↓ | Antisense RNA | ↓ Soluble pectins, ↑ cell-to-cell adhesion | Santiago-Doménech et al.118 |
| Strawberry (Fragaria × ananassa) | Softening | FaβGal4 | ↓ | Antisense RNA | ↓ Softening, ↓ soluble pectins | Paniagua et al.268 |
| Tomato (Solanum lycopersicum) | Ripening | RIN | ↓ | CRISPR/Cas9 | Slower ripening | Ito et al.87 |
| Tomato (Solanum lycopersicum) | Ripening | LeCTR1, LeEILs LeEIN2 | ↓ | VIGS | Ripening suppression | Fu et al.269 |
| Petunia (Petunia × hybrida cv. “Mitchell diploid”) | Senescence/vase-life | Atetr1-1 | ↑ | Ectopic expression | Doubled vase-life | Wang et al.98 |
| Petunia (Petunia × hybrida cv. Hort. Vilm.-Andr.) | Senescence/vase-life | BoACO1, BoACS1 | ↓ | Antisense | Delayed flower senescence, extended vase-life | Huang et al.270 |
| Petunia (Petunia × hybrida cv. “Primetime Blue” and cv. “Mitchell Diploid”) | Senescence/vase-life | PhHD-Zip | ↓ | VIGS | ↑ Vase-life by 20% | Chang et al.96 |
| Carnation (Dianthus caryophyllus L. cv. “Scania” and “White Sim”) | Senescence/vase-life | ACO | ↓ | Antisense | ↑ Vase-life by 50% | Savin et al.93 |
| Petunia (Petunia hybrida cv. Mirage Rose | Senescence/vase-life | PhACO1 | ↓ | CRISPR | ↑ Vase-life by 50% | Xu et al.99 |
| Potato (Solanum tuberosum) | Shelf-life/quality | StVInv | ↓ | RNAi | ↓ Acrylamide in fried chips | Bhaskar et al.184 |
| Potato (Solanum tuberosum) | Shelf-life/quality | StR1 (GWD), StPhL, StPPO | ↓ | Antisense | ↓ Acrylamide in fried chips, improved fry aroma | Rommens et al.271 |
| Potato (Solanum tuberosum) | Shelf-life/quality | StAs1, StAs2 | ↓ | Antisense | ↓ Acrylamide in fried chips | Rommens et al.272 |
| Potato (Solanum tuberosum) | “High fiber” | StSBEI, II | ↓ | CRISPR | ↑ Resistant starch | Tuncel et al.259 |
| Tomato (Solanum lycopersicum) | Sweetness | SlbZIP1 | ↑ | Overexpression Fruit-promoter | 50% ↑ in fruit sugar, no change in yield | Sagor et al.155 |
| Tomato (Solanum lycopersicum) | Sweetness | SlFgr | ↑ | Wild allele & Overexpression | ↑ Ratio of fructose to glucose, ↑ sweetness | Shammai et al.156 |
| Tomato (Solanum lycopersicum) | Sweetness | SlARF10 | ↑ | Overexpression | ↑ Sucrose, fructose glucose not assayed | Yuan et al.157 |
| Tomato (Solanum lycopersicum) | Sweetness | AGPaseL | ↑ | Allele from S. habrochaites | ↑ Total sugars ↑yield | Petreikov et al.151 |
| Tomato (Solanum lycopersicum) | Varied | fumarase | ↓ | Antisense RNA | ↑ Sugars, ↓ infection by Botrytis, ↓ postharvest water loss ↓ yield | Centeno et al.152 |
| Tomato (Solanum lycopersicum) | Sweetness | Lin5 allele invertase | ↑ | Natural allele | ↑Sugar | Fridman et al.150 |
| Tomato (Solanum lycopersicum) | Sweetness | SlGLK2 | ↑ | Natural allele Transgenic line | ↑ Sugars, ↑ lycopene yield same | Powell et al.153 |
| Cucumber (Cucumis sativus) | Sweetness | CmTST2 | ↑ | Overexpression | ↑ Fructose, glucose, ↑ sucrose, yield unknown | Cheng et al.158 |
| Strawberrry (Fragaria × ananassa Duch) | Sweetness | CmTST2 | ↑ | Overexpression | ↑ Fructose, glucose, ↑ sucrose, yield unknown | Cheng et al.158 |
| Potato (Solanum tuberosum) | Appearance | StPPO2 | ↓ | CRISPR | ↓ Browning | Gonzalez et al.139 |
| Apple (Malus domestica) | Appearance | MdPPO | ↓ | RNAi | ↓ Browning | Okanagan Specialty Fruits Armstrong & Lane199 |
| Potato (Solanum tuberosum) | Appearance health, texture |
StPPO StvINV StAsp StPHO StGWD |
↓ | RNAi |
↓ Browning ↓ Acrylamide ↓ CIS Better texture |
Simplot Company, Richael et al.185 |
*Change in gene expression
Fruit cuticle
The triterpenoids and waxes coating the harvested parts of horticultural crops may have a bigger influence on quality and shelf-life than previously recognized101,102. The plant cuticle is the first layer of defense against water loss and pathogen infestation103. The cuticle is also responsible for multiple traits involved in fruit quality and shelf-life, such as surface brightness104, the characteristic “bloom” of grapes105, blueberries106 and plums107, and potentially modulating texture changes101. Fruit cuticle composition actively changes depending on the environment and organ developmental stage, which affects its protective function during fleshy fruit ripening108.
The interaction between the biomechanical properties of the fruit cuticle and cell wall polysaccharides affects the development of surface cracks in cherries109, apples110, and tomato111. These aesthetically undesirable traits for consumers can also reduce produce shelf-life. Identifying genes key to cuticle compound biosynthesis could improve fruit response to environmental stresses during postharvest storage and reduce pathogen susceptibility.
Fruit softening
The breakdown of the cell wall (CW) during fruit ripening is a crucial process in the development of fruit sensorial quality. Softening the fruit is essential for increasing its appeal to animals and humans for consumption, and thus seed dispersal112. Ripening and senescence, together with fungal attack, accelerate the rate of CW degradation, leading to rotting113. Rotting and ripening are discussed separately, even though they overlap biologically in relation to CW softening and fruit shelf-life. The modern, worldwide food supply chain often necessitates that the breakdown of the cell walls, either by ripening, senescent processes or by fungal rot, be halted or slowed.
CW softening processes are catalyzed by multiple enzymes that respond to developmental and environmental cues and occur over a variety of timelines, depending on the organism and tissue in question. CW degradation is orchestrated by polygalacturonase (PG), pectin methylesterase (PME), pectate lyase (PL), and β-galactosidase (β-Gal)24,114. PG, PME, PL, and β-Gal vary in their biotechnological potential to control firmness/fruit softening (Table 1). PG expression negatively influences firmness and shelf-life in strawberry,115 but only shelf-life in tomato116. In contrast, suppression of PLs reliably increases firmness and shelf-life in the species studied117–119. Suppression of PMEs81,120–122 and β-Gals24 promote fruit softening in many fruits. However, antisense downregulation of β-Gals in tomato caused cracking and other negative phenotypes123–125.
Managing the timing of these CW enzyme activities could support efforts to maintain the physical integrity of fruit and vegetables from farm-to-plate. Because of the cumulative and interactive effects of these enzymes126,127, it may be necessary to intelligently target single or multiple enzymes and their inhibitor proteins128 simultaneously, to create an optimal balance of CW degradation activities. Such efforts may make it possible to surmount, bypass, or control these complex interactions, and produce fruits that retain desirable textures but that show less softening in handling, shipping, and storage.
Fungal rots
Harvested produce is susceptible to pathogenic attack. Invading fungi or bacteria will macerate the fruit components, creating rot: fruits covered in bacteria or spores, and their metabolic by-products. The result: commodities that are unsightly and also inedible due to a combination of the sour, bitter, putrid, or toxic compounds produced129. This may be advantageous in the spreading of seeds by attracting distributing animals or physically destroying CWs130–132 but is inconvenient for postharvest storage of commodities.
Fungal infections typically occur across the physical surface: the cuticle and cell wall. Therefore, all considerations in “Fruit cuticle” and “Fruit softening” sections impact susceptibility to pathogenic attack. One approach for reducing pathogen susceptibility could be to directly target plant CW and ripening-related processes113,133, which would also increase shelf-life by extending the integrity of the CW. Recent work in tomato shows targeting PL with gene editing may protect ripe fruit134.
Gene editing of endogenous plant enzymes that target fungal CW components and linkages could enhance resistance to fungal infection135–137. Further, the accumulation of specialized metabolites conferred pathogen resistance in several citrus species138,139 and could be a target for editing in others140,141.
Composition
Anthocyanins
Many of the red, blue, and purple colors seen in fruit, flowers, and tubers are due to anthocyanins. This class of compounds is not only aesthetically pleasing but has healthful antioxidant properties142. In addition, high-anthocyanin accumulation has been linked to increased shelf-life and reduced Botrytis infection in tomato. This was shown via ectopic expression of anthocyanin biosynthesis genes from snapdragon in tomato under a fruit specific promoter143,144, as well as by introgression of naturally occurring Abg, Aft, and atv high-anthocyanin alleles into tomato145,146. Increasing anthocyanin production in tomato and other fruit, could simultaneously enhance shelf-life for reduced postharvest loss, but also reduce postharvest waste by increasing the attractiveness of the fruit due to healthful properties and novel color.
Carbohydrates
The primary carbohydrates studied are starch and sugars, and their interconversion, content, and relative amounts may influence postharvest quality. Starch breakdown to sugars is undesirable in potato (see “Cold-induced sweetening” section), but during maturation, it is valuable in several species, e.g., apple, banana, and kiwifruit. In others, e.g., sweet peas and sweet corn, the conversion of sugars to starch reduces sweetness and hence quality.
Sugars
Sweetness is an important attribute in fruits. Sweetness is determined by the concentration and relative ratio of the predominant sugars in fruit tissues67,69, although amino acids and other compounds may have an effect147. The biochemical pathways that lead to sugar accumulation have been studied, however, transgenic manipulation of these genes often has negative effects on yield148, indicating that a more fundamental and holistic knowledge of sugar metabolism, especially its regulation, is needed149. However, high sugar Quantitative Trait Loci (QTLs) used in breeding programs150–152 and, the recent discovery of regulatory genes which influence fruit sugar accumulation153–158, are promising targets for improving fruit taste (Table 1). Gene editing for increased sugar may mitigate the loss of tissue sugar content or capacity that occurs due to postharvest handling69 and therefore maintain consumer satisfaction, reducing postharvest waste.
Starch
The starch-rich organs of cassava, yam (Dioscorea spp.), and potato are important staples, but unlike cereal grains, they are highly perishable159. There is interest in changing the digestibility of starch to create varieties with different nutritional attributes160. For example, low-digestible, i.e., “fiber-like” starch would be healthier upon consumption, and could conceivably resist breakdown in storage, reducing postharvest loss. Transgenic alteration of the starch branching enzymes in potato showed that increasing “resistant starch” could be recapitulated in a horticultural crop161–168.
Starch also accumulates in the immature fruit of apples, bananas, tomatoes, and kiwifruit169, and its breakdown to sugars at maturity makes valuable contributions to ripe fruit sugar content151,170. Increasing starch content by manipulating regulatory proteins and transporters (Table 1), has been identified as a viable strategy for increasing the postharvest quality of ripe fruit171,172.
Cold-induced sweetening
Potato tubers are stored at low temperatures (4–8 °C) to extend shelf-life and meet industry demand for round-the-year fresh products. However, sucrose and reducing sugars (glucose and fructose) accumulate during cold storage from starch breakdown, a process referred to as cold-induced sweetening (CIS)173,174. CIS affects the quality of fried potato products: reducing sugars react with amino acids during high temperature cooking to form carcinogenic acrylamide through the Maillard reaction175,176. Several metabolic pathways, including starch biosynthesis and degradation, are involved in CIS177–180. Reducing vacuolar invertase activity decreased reducing sugars and alleviated CIS in transgenic tubers181–185. These genes are therefore ideal targets for manipulation using a gene-editing approach. Reducing CIS would lessen the severity of tuber postharvest starch loss and would also reduce the postharvest waste that results when blackened chips and fries are discarded.
Flavor profiles
There seems to be a consensus among consumers that store-bought fruit and vegetables often lack good flavor; one consequence of this assessment is PW. The flavor is determined by the intricate combination of sugars, acids, and volatiles186. Improving flavor is made even more challenging because “good flavor” is subjective and varies across and among different consumer populations187–189. Postharvest handling and retail systems are major contributors disrupting many of the pathways required for full fruit flavor, especially volatile production190. Painstaking efforts have been made to link the abundance of specific chemicals, especially aroma volatiles, to human likeability using sensory panels191,192. However, because of the complexity of fruit flavor profiles, targeting multiple genes that affect sweetness, acidity, and aroma is likely necessary to truly improve consumer appeal193, a challenge that gene editing may meet more readily than traditional breeding194. For example, in tomato, fructose, citric acid, and six aroma volatiles were associated with a high hedonistic value193. Novel alleles of genes that contribute to enhanced fruit flavor194,195 were related to sugar content, citrate, and volatiles, all of which may be manipulated by gene editing to develop the fruit of optimal flavor196.
Reduced browning
Many horticultural crops exhibit undesirable browning that is a turn-off for consumers who discard these edible but “downgraded” produce197. Browning is common in fresh-cut or bruised produce—lettuce, spinach, apples, and potatoes—or is due to physiological disorders such as heat and chilling injury, or exposure to inappropriate oxygen and carbon dioxide levels197.
There are two types of browning, enzymatic and non-enzymatic. Non-enzymatic browning describes the Maillard reaction, discussed in the “Cold-induced sweetening” section. Enzymatic browning involves the action of three core enzymes: polyphenol oxidase (PPO), peroxidase (POD), and phenylalanine ammonia lyase (PAL)197. Scientists have successfully shown that knocking out PPO genes reduced browning upon wounding. This is a relatively easy target and a proven and effective strategy for reducing postharvest waste198. Non-browning, commercially available produce i.e., Innate potato® and Arctic Apple® (Table 1)185,199,200 are in retail outlets. Non-browning mushrooms have also been produced by CRISPR–Cas9 gene editing201. Manipulation of this trait is expected to make significant inroads in reducing consumer disposal of “browned” but edible produce, especially for those that are “fresh-cut.”
Complex postharvest traits
There are many postharvest phenotypes that influence the quality and shelf-life of crops that are poorly understood at the molecular level. These traits may be influenced by the activity of multiple genes and their alleles, and how their expression is altered by the environment (see “Identifying genes that influence postharvest traits” section). Our understanding of the disorders that result from these combined elements, along with those discussed in “Composition” section, is hampered by the influence of preharvest factors, which are often not taken into account, and study-to-study variability in experimental design and reporting inconsistencies69,202. Postharvest disorders that affect a wide variety of species are discussed below.
Microbial food safety
Salmonella and E. coli contamination of fresh-cut fruit, vegetables, and especially leafy greens can occur at various points in production, causing illness or even death if consumed71,203. E. coli OH157:H7, alone has sickened 72,855 people and led to 173 deaths in the US from 1980–2016 204. These outbreaks have increased in frequency due to (1) intensive farming, (2) the growing complexity of the postharvest supply web, and the (3) popularity of fresh-cut salads, which offer more entry sites for pathogen infestation204–206. The problem is that a localized outbreak of a commodity often temporarily suppresses sales and demand, leading suppliers to dump unaffected product, creating waste.
The development of breeding strategies to reduce bacterial attachment, persistence, and proliferation, have the potential to reduce food contamination203,207. Identifying genomic regions in Salmonella controlling stomatal opening in lettuce leaves208, and screening germplasm for tolerance to microbial pathogens are important steps209. This type of fundamental knowledge could open new avenues for developing genetic strategies for improved food safety210 including gene-editing strategies.
Postharvest chilling injury
Low temperatures typically extend shelf-life, but in some produce, when rewarmed after chilling, the normal maturation program is disrupted, leading to poor quality190. This physiological dysfunction called Postharvest chilling injury (PCI) is manifested in a wide array of symptoms across species, the most severe of which include tissue and seed browning or blackening, pitting, fungal infestation and decay20,211, which contribute to postharvest loss. Mild PCI symptoms include a lack of flavor, and undesirable texture and taste20,211, which leads to postharvest waste.
We estimate that ~56% of the top 50 global commodities are susceptible to PCI. Further, the symptoms are often hard to specifically ascribe to chilling injury: PCI-accelerated decay is often diagnosed as postharvest disease or premature senescence212, and poor flavor induced by PCI is often blamed on variety-type or early-harvest. PCI is insidious because it is difficult to detect, and it is therefore not properly documented28. It also adds constraints to postharvest management strategies for sensitive crops, as preventing PCI requires faster shipping at higher temperatures or shorter storage times.
A new gene discovery holds promise for reducing the occurrence of PCI. The SlGRAS4 gene, when overexpressed in transgenic tomato by RNAi, promoted postharvest chilling tolerance in fruit with no change in yield213. SlGRAS4 overexpression may also be achieved by editing repressor elements in its promoter in the future.
Preharvest factors
The metabolic and physiological state of a commodity before harvest is a key factor determining its postharvest quality. Soil elemental composition, especially nitrogen and calcium, crop exposure to extreme heat, drought, or even wind, and irregularities in irrigation regime can affect a broad array of quality parameters that may make produce unsuitable for sale (Fig. 3)214,215. Visible blemishes such as lettuce tip burn (Fig. 5) are often linked to temperature effects216. Still, overwhelmingly, these physiological traits are some of the most difficult to dissect due to the unpredictable nature of the severity and frequency of their occurrence. Identifying genes that could be modified for improvement may be more problematic than for other traits.
Fig. 5. Postharvest disorders in fruit and vegetables.
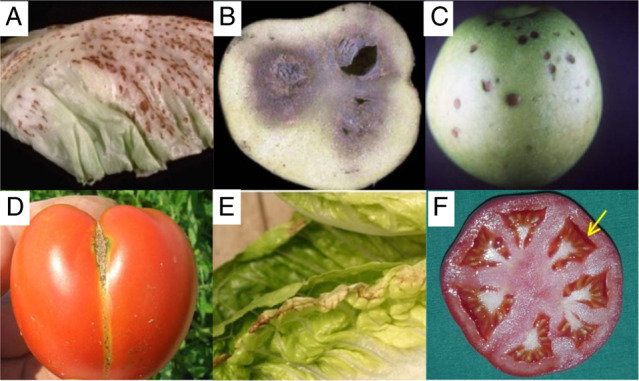
A Russet spotting in lettuce, B blackheart in potato tuber, C bitterpit in apple, D zippering in tomato, E tipburn in lettuce, F puffiness in tomato fruit. Pictures reproduced with kind permission from UC Davis Postharvest Technology Center (A, C), Marita Cantwell (B), Gerald Brust (D), Richard Smith (E), and Elizabeth Maynard (F)
Postharvest storage disorders
Postharvest treatments are used to prolong produce shelf-life (Fig. 2), but incorrect exposure or treatment can disrupt metabolism, leading to physiological disorders occurring in the harvested product217,218. These injuries may be initiated at the cellular level due to the overproduction of reactive oxygen species, membrane damage, and energy imbalances caused by interferences in ATP production219.
Over time, secondary reactions occur and result in visible changes, e.g., water soaking, tissue browning, blackening, microbial growth and decay, off-flavors, and odors214,217,219. Common postharvest disorders include russet spotting in lettuce and blackheart in potato (Fig. 5). The genes underlying these phenotypes are very poorly understood, and studies are complicated because there are multiple interacting factors that result in the trait. The use of -omics technology and the mitigative effects of some hormonal or hormetic physical treatments have led to a better understanding of the signal transduction pathways affected219, but more work needs to be done to determine candidates for gene-editing solutions because these traits lead to PLW.
Roadblocks impeding the broader adoption of gene editing for reducing PLW
Commercial and public implementation of gene editing has been occasional and non-systemic. The reasons for this are manifold and interconnected; however, most stem from two primary issues.
First, many horticultural crops have been traditionally understudied because their lifecycle, genomic structure, or inability to regenerate via tissue culture, are not amenable to methods used in functional genomics. This leads directly to the second reason: many of the genes that contribute to the problems of PLW have not yet been identified. These two identified stumbling blocks will require new and significant financial investments to minimize their effect and accelerate the use of gene editing for reducing PLW (Fig. 6).
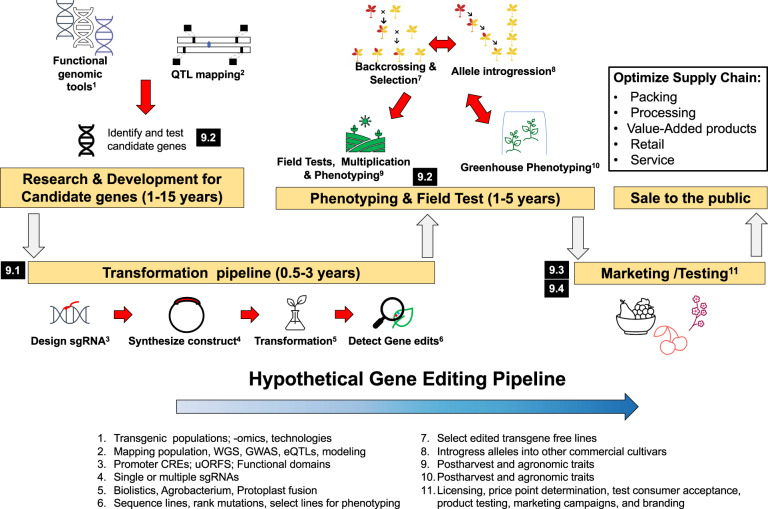
Fig. 6. A flowchart with time estimates for developing gene-edited plants.
Numbers indicate sections referred to in the text. The more unknowns and undeveloped steps in a potential pipeline for a gene-edited crop, the less appealing beginning that process may be to invested parties, especially given the uncertain and changing regulation
Plant transformation and regeneration
Although transgenic approaches for modifying plants have been advanced for three decades, transformation and regeneration (Fig. 6) are bottlenecks in using gene-editing tools to address crop improvements220,221. The efficiency of Agrobacterium-mediated approach varies by Agrobacterium strain, and the plant species and tissue to be transformed183. Almost 95% of woody fruit and nut crops are still recalcitrant to transgenic approaches because of poor transformation efficiencies using Agrobacterium222. Transformation may be achieved using biolistic and electroporation approaches, which are non-tissue specific223 but lead to multiple insertions which can create additional non-intended genetic changes.
Regeneration through tissue culture is even more challenging than the integration of foreign genes, and the process is time-consuming (Fig. 6). Regeneration takes 6–9 months for papaya224, 5–8 months for kiwifruit225, and 4–5 months for potato220,226. Novel delivery approaches to bypass labor-intensive plant regeneration procedures are being developed. “Spray-on” gene editing involves coating nanosized carbon dots with plasmids containing gRNA and Cas9 cDNA, which are delivered directly to the cell227. Inducing meristem formation in tissues transformed with the CRISPR gene construct would produce edited plantlets without a callus explant step, thus saving time228. Seeds produced from such plants could be propagated228. Recent innovations promise to open up the number of crops that can be efficiently modified by gene editing229,230. Specifically, transforming calli with a GRF4-GIF growth factor chimera has been used to accelerate regeneration efficiency more than 5-fold among some of the most recalcitrant species229, and may be a major advance in crop improvement.
Identifying genes that influence postharvest traits
Many postharvest traits are composite, with phenotypes that are the result of multiple environmental factors as well as genetics (Eq. 1). As mentioned, common disorders such as PCI, blossom end rot, and superficial scald, are challenging to study at the molecular level. These traits are likely to be multigenic and multiallelic, with gene expression controlled in networks involving epistatic interactions and epigenetic mechanisms, that are influenced by environmental factors231–237.
Eq. 1. Factors contributing to postharvest phenotype.
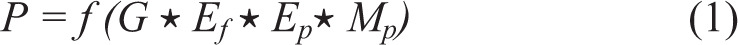
Where P = postharvest phenotype; f = function; G = genotypic factors; Ef = field preharvest environment; Ep = postharvest environment; Mp = postharvest management. Factors are interactive and presumed to be non-additively, nonlinearly compounding: we use the star operator to suggest the dynamism of a cross-correlation function; though this concept is not typically applied to biological systems, it is evocative here.
For many years, environmental control, i.e., refrigeration and modified and controlled atmospheres, was the primary way of maintaining shelf-life and quality15. Forward genetic approaches such as QTL and Association mapping, mutagenesis, and gene expression analysis, have been used to identify candidate genes that exert a significant degree of control over complex agricultural traits238,239. However, introgressing favorable genes into a new cultivar may not always lead to the strong expression of a trait, because of environmental effects240.
Patent landscape
There is much uncertainty about the right to market edited germplasm. In the United States, the Broad Institute holds the patent for CRISPR editing of eukaryotic cells241. However, the University of California continues to challenge the 2018 ruling on multiple grounds242–244. In most of Europe and the Pacific (China, Australia, Singapore), the University of California holds the rights to CRISPR241. As shown in Fig. 1, the perishable fruit, vegetable, and ornamental market is global. Those interested in growing “CRISPR’ed” produce in, e.g., California, and selling it in the US, Canada, China, and Australia, might need to invest millions to acquire permissions from both Broad Institute and the University of California241. This may increase the entry cost to commercialize postharvest gene-edited products, and limit the traits targeted to those with the highest profit-margin and simultaneously, push out smaller, “boutique” biotechnology firms241.
The public sector has not hesitated to use CRISPR245–247. The Broad Institute allows unrestricted use of the Intellectual Property covered in its CRISPR patents for non-profit and academic research uses, but commercial planting of the fruits of that research is open to legal challenge248,249. Currently, in the US, commercial growers frequently invest in public breeding programs by providing land for field trials to state-level institutions. Material support from for-profit entities may become legally tenuous if a laboratory or facility is using CRISPR or any other patented technology.
As CRISPR techniques become more refined246,247 and additional patents are submitted, these legal and financial contingencies may become more labyrinthine. However, the explosion in CRISPR research in plants, shows that the agricultural and horticultural world is eagerly embracing gene editing. The promised profit improvements currently outweigh the potential legal ramifications of patent infringements.
Regulatory issues
Another roadblock to the commercialization of gene-edited horticultural crops is their differing classification across the globe. The United States and China, which produce and consume a majority of the world’s fruit and vegetables (Fig. 1), have readily embraced gene editing240, and regulation in India is based largely on precedent250. Modifications produced by gene editing vs. traditional breeding can be functionally identical, and distinguishing said modifications is near impossible251–253. As a result, in June 2020 the United States announced the SECURE rule, stating that from April 2021, novel crops with DNA changes that could be introduced by traditional breeding can be fast-tracked for marketing254. The European Union, however, has ruled that gene-edited crops are in the same classification as “traditional” GMOs252,255,256. This places additional burdens on companies wishing to market edited produce in the EU and UK255. European produce markets have high quality standards for flavor and texture257,258, and new gene-edited crops could conceivably meet these criteria. The benefits of PLW reductions from gene editing may not be realized as quickly as in Europe.
Conclusion
Gene manipulation alone cannot solve the problem of horticultural loss and waste, as the overall issue remains heterogeneous and multi-faceted, requiring transdisciplinary advances, and the integration of biological, engineering, and socio-cultural solutions. Consumer awareness campaigns about saving produce are notoriously difficult to develop and implement, and success is variable because human behavior is often intractable. Realizing engineering solutions requires massive long-term investments in infrastructure, equipment, and energy. It is against this backdrop that we explored the potential of gene editing for improving produce to be hardier in the supply chain as well as meet consumer expectations.
Manipulating biological processes by gene editing is both a promising new avenue for reducing PLW and a major challenge that relies on understanding the baroque regulation of these pathways in order to “tweak” them in a beneficial way (Figs. 2,3, and 4). Because the technique is relatively cheap and easy, with minimal impact on the genome (Fig. 6), the cost barrier is such that for the first time, breeders can feasibly engineer postharvest traits with the expectation that the new germplasm could be commercially viable. This means that in spite of the challenges we have outlined (Figs. 5 and 6), there is reason to believe widespread gene editing for PLW reduction is possible and imminent.
As shown in Fig. 7, several genes have been proven to provide reliable phenotypes for reducing PLW, and there are others that are very promising. Many projects are underway to recapitulate these findings using gene-editing approaches, for extended shelf-life or better quality in major commercial species85,99,198,201,259,260. Stacking edited alleles of these genes in crops may also lead to additive or valuable transgressive effects. It is our opinion that gene-edited crops will eventually be in broad use across the globe, because of the clear evidence of their potential to minimize postharvest waste and loss in the context of multiple threats to the stability of the world produce supply chain.
Fig. 7. Gene targets for commercialization of novel gene-edited crops.
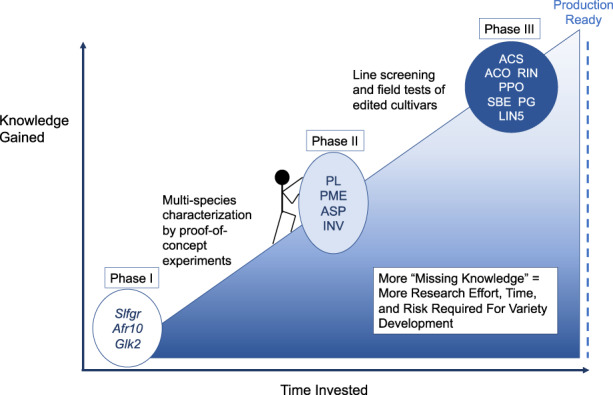
Genes listed in the dark blue oval to the right have been well studied and are likely to have commercially relevant phenotypes, while those in white still require additional testing because the action of these genes have only been demonstrated in a single species. Phase I: Afr10—higher fruit sugars; Glk2—higher tomato sugars and flavonoids; Slfgr—sweeter tomato fruit. Phase II: INV, ASP reduced cold-induced sweetening and acrylamide produced during processing in potato; PME; PL—reduced fruit softening, better storability and consumer acceptance. Phase III: ACS, ACO, RIN—reduced rate of ripening; PG—reduced fruit softening would cause less bruising during shipment, PPO—non-browning, better consumer appeal if bruised postharvest; LIN5—higher accumulation of sugars in fruit; SBE—higher fiber potato for health
Acknowledgements
E.N.S.’s Ph.D. is supported by a National Science Foundation Graduate Research Fellowship; J.Y.’s Master’s degree is supported by the Paulden F. & Dorathea Knowles Scholarship, and a UC Davis Horticulture & Agronomy Graduate Group Scholarship; J.Z. thanks the China Scholarship Council, and the UC Davis Horticulture & Agronomy Graduate Group Scholarship for her Master’s funding. E.N.S., J.Y., and J.Z. thank the Henry A. Jastro Graduate Research Award for research support. K.A. thanks the Departamento de Producción Vegetal at the Universidad de Concepción for grant support; funding for gene-editing research in DB’s lab is provided by USDA Hatch Project CA-D-PLS-2404-H. We thank the UC Davis Postharvest Technology Center, Drs. Elizabeth Maynard, Gerald Brust, Richard Smith, and Marita Cantwell for permission to reuse images. We apologize to those authors whose work we did not cite because of space constraints.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Emma N. Shipman, Email: enshipman@ucdavis.edu
Jingwei Yu, Email: jwyyu@ucdavis.edu.
Jiaqi Zhou, Email: jiqzhou@ucdavis.edu.
Karin Albornoz, Email: karinalbornoz@udec.cl.
Diane M. Beckles, Email: dmbeckles@ucdavis.edu
References
- 1.Wolter, F., Schindele, P. & Puchta, H. Plant breeding at the speed of light: the power of CRISPR/Cas to generate directed genetic diversity at multiple sites. BMC Plant Biol.19, 176 (2019). [DOI] [PMC free article] [PubMed]
- 2.Pradhan, P., Fischer, G., van Velthuizen, H., Reusser, D. E. & Kropp, J. P. Closing yield gaps: how sustainable can we be? PLoS ONE10, e0129487 (2015). [DOI] [PMC free article] [PubMed]
- 3.Alfa HH, Arroo RRJ. Over 3 decades of research on dietary flavonoid antioxidants and cancer prevention: What have we achieved? Phytochem Rev. 2019;18:989–1004. doi: 10.1007/s11101-019-09632-0. [DOI] [Google Scholar]
- 4.Fraga CG, Croft KD, Kennedy DO, Tomas-Barberan FA. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514–528. doi: 10.1039/C8FO01997E. [DOI] [PubMed] [Google Scholar]
- 5.Liskova, A. et al. Dietary phytochemicals targeting cancer stem cells. Molecules24, 899 (2019). [DOI] [PMC free article] [PubMed]
- 6.Saiwal N, Dahiya M, Dureja H. Nutraceutical insight into Vegetables and their Potential for Nutrition Mediated Healthcare. Curr. Nutr. Food Sci. 2019;15:441–453. doi: 10.2174/1573401314666180115151107. [DOI] [Google Scholar]
- 7.FAO. Global Food Losses and Food Waste – Extent, Causes and Prevention. http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (FAO, 2011).
- 8.Porat R, Lichter A, Terry LA, Harker R, Buzby J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: quantifications, causes, and means of prevention. Postharvest Biol. Tec. 2018;139:135–149. doi: 10.1016/j.postharvbio.2017.11.019. [DOI] [Google Scholar]
- 9.Kitinoja L, Tokala VV, Brondy A. A review of global postharvest loss assessments in plant-based food crops: recent findings and measurement gaps. J. Postharvest Technol. 2018;6:1–15. [Google Scholar]
- 10.Bahadur, K. C. K. et al. When too much isn’t enough: does current food production meet global nutritional needs? PLoS ONE13, e0205683 (2018). [DOI] [PMC free article] [PubMed]
- 11.HPLE. FoodLosses and Waste in the Context of Sustainable Food Systems: A Report. http://www.fao.org/3/a-i3901e.pdf (2014).
- 12.Kader, A. A. Increasing food availability by reducing postharvest losses of fresh produce. In Proc. 5th International Postharvest Symposium, Vols 1–3 2169–2175 (2005).
- 13.NASEM (ed). in Reducing Impacts of Food Loss and Waste: Proceedings of a Workshop (Committee on Reducing Food Loss and Waste: A Workshop on Impacts; Science and Technology for Sustainability Program; Policy and Global Affairs. National Academies of Sciences, Engineering, and Medicine, 2019). [PubMed]
- 14.ReFED. A Roadmap to Reduce U.S. Food Waste by 20 Percent (ReFED, 2016).
- 15.Kader, A. A. in Postharvest Technology of Horticultural Crops Vol. Publication 3529 (ed Kader, A. A.) 39–48 (University of California Agriculture and Natural, 2011).
- 16.Hewett, E. W. Postharvest Innovation: Current Trends and Future Challenges in the Global Market. In Southeast Asia Symposium on Quality Management in Postharvest Systems and Asia Pacific Symposium on Postharvest Quality Management of Root and Tuber Crops Vol. 989, 25–37 (2013).
- 17.Hewett EW. Postharvest research for quality horticultural products. Middle East Hortic. Summit. 2014;1051:63–70. [Google Scholar]
- 18.Kader, A. A. & Saltveit, M. E. Postharvest Physiology and Pathology of Vegetables (eds Bartz, J. A. & Brecht, J. K.) (CRC Press, 2002).
- 19.Kays SJ. Quality maintenance of fresh produce. Acta Hortic. 2010;875:27–31. doi: 10.17660/ActaHortic.2010.875.1. [DOI] [Google Scholar]
- 20.Saltveit, M. E. & Morris, L. L. Chilling Injury of Horticultural Crops (ed Wang, C. Y.) 3–15 (CRC Press, 1990).
- 21.Bower JH, Blasi WV, Mitcham EJ. Effect of ethylene in the storage environment on quality of ‘Bartlett pears’. Postharvest Biol. Tec. 2003;28:371–379. doi: 10.1016/S0925-5214(02)00210-7. [DOI] [Google Scholar]
- 22.El-Kereamy A, et al. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003;119:175–182. doi: 10.1034/j.1399-3054.2003.00165.x. [DOI] [Google Scholar]
- 23.Gong YP, Fan XT, Mattheis JP. Responses of ‘Bing’ and ‘Rainier’ sweet cherries to ethylene and 1-methylcyclopropene. J. Am. Soc. Hortic. Sci. 2002;127:831–835. doi: 10.21273/JASHS.127.5.831. [DOI] [Google Scholar]
- 24.Friedman, H. in Plant Breeding Reviews (ed Goldman, I.) Ch 3, 61–94 (Wiley, 2020).
- 25.Liu ZY, Jiang WB. Lignin deposition and effect of postharvest treatment on lignification of green asparagus (Asparagus officinalis L.) Plant Growth Regul. 2006;48:187–193. doi: 10.1007/s10725-005-6112-z. [DOI] [Google Scholar]
- 26.Ritenour MA, Ahrens MJ, Saltveit ME. Effects of temperature on ethylene-induced phenylalanine ammonia-lyase activity and russet spotting in harvested iceberg lettuce. J. Am. Soc. Hortic. Sci. 1995;120:84–87. doi: 10.21273/JASHS.120.1.84. [DOI] [Google Scholar]
- 27.Chalutz E, Devay JE, Maxie EC. Ethylene-induced isocoumarin formation in carrot root tissue. Plant Physiol. 1969;44:235. doi: 10.1104/pp.44.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albornoz K, Cantwell MI, Zhang L, Beckles DM. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019;9:2795. doi: 10.1038/s41598-019-38877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jasin M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 1996;12:224–228. doi: 10.1016/0168-9525(96)10019-6. [DOI] [PubMed] [Google Scholar]
- 30.Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188:773–782. doi: 10.1534/genetics.111.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satheesh V, Zhang H, Wang XT, Lei MG. Precise editing of plant genomes - prospects and challenges. Semin. Cell Dev. Biol. 2019;96:115–123. doi: 10.1016/j.semcdb.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Pandey PK, et al. Versatile and multifaceted CRISPR/Cas gene editing tool for plant research. Semin. Cell Dev. Biol. 2019;96:107–114. doi: 10.1016/j.semcdb.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Enciso-Rodriguez, F. et al. Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front. Plant Sci. 10, 376 (2019). [DOI] [PMC free article] [PubMed]
- 35.Nadakuduti, S. S., Buell, C. R., Voytas, D. F., Starker, C. G. & Douches, D. S. Genome editing for crop improvement - applications in clonally propagated polyploids with a focus on potato (Solanum tuberosum L.). Front. Plant Sci. 9, 1607 (2018). [DOI] [PMC free article] [PubMed]
- 36.Sharma S, Kaur R, Singh A. Recent advances in CRISPR/Cas mediated genome editing for crop improvement. Plant Biotechnol. Rep. 2017;11:193–207. doi: 10.1007/s11816-017-0446-7. [DOI] [Google Scholar]
- 37.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen KL, Wang YP, Zhang R, Zhang HW, Gao CX. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019;70:667–697. doi: 10.1146/annurev-arplant-050718-100049. [DOI] [PubMed] [Google Scholar]
- 39.Metje-Sprink, J., Menz, J., Modrzejewski, D. & Sprink, T. DNA-free genome editing: past, present and future. Front. Plant Sci. 9, 1957 (2019). [DOI] [PMC free article] [PubMed]
- 40.Puchta H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J. Exp. Bot. 2005;56:1–14. doi: 10.1093/jxb/eri123. [DOI] [PubMed] [Google Scholar]
- 41.Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells (vol 19, pg 770, 2018) Nat. Rev. Genet. 2018;19:801–801. doi: 10.1038/s41576-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 42.Shimatani Z, et al. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017;35:441. doi: 10.1038/nbt.3833. [DOI] [PubMed] [Google Scholar]
- 43.Zong Y, et al. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017;35:438. doi: 10.1038/nbt.3811. [DOI] [PubMed] [Google Scholar]
- 44.Veillet F, et al. The Solanum tuberosum GBSSI gene: a target for assessing gene and base editing in tetraploid potato. Plant Cell Rep. 2019;38:1065–1080. doi: 10.1007/s00299-019-02426-w. [DOI] [PubMed] [Google Scholar]
- 45.Veillet, F. et al. Transgene-free genome editing in tomato and potato plants using Agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 20, 402 (2019). [DOI] [PMC free article] [PubMed]
- 46.Decaestecker W, et al. CRISPR-TSKO: a technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. Plant Cell. 2019;31:2868–2887. doi: 10.1105/tpc.19.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brand L, et al. A versatile and reliable two-component system for tissue-specific gene induction in Arabidopsis. Plant Physiol. 2006;141:1194–1204. doi: 10.1104/pp.106.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, X. et al. An inducible genome editing system for plants. Nat. Plants6, 766–772 (2020). [DOI] [PMC free article] [PubMed]
- 49.Zhang HW, et al. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018;36:894. doi: 10.1038/nbt.4202. [DOI] [PubMed] [Google Scholar]
- 50.Li TD, et al. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018;36:1160. doi: 10.1038/nbt.4273. [DOI] [PubMed] [Google Scholar]
- 51.Zsogon A, et al. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018;36:1211. doi: 10.1038/nbt.4272. [DOI] [PubMed] [Google Scholar]
- 52.Cheng, J. F. et al. Downregulation of RdDM during strawberry fruit ripening. Genome Biol. 19, 212 (2018). [DOI] [PMC free article] [PubMed]
- 53.Gao, Y. et al. Diversity and redundancy of the ripening regulatory networks revealed by the fruitENCODE and the new CRISPR/Cas9 CNR and NOR mutants. Hortic. Res.6, 39 (2019). [DOI] [PMC free article] [PubMed]
- 54.Huang H, et al. Global increase in DNA methylation during orange fruit development and ripening. Proc. Natl Acad. Sci. USA. 2019;116:1430–1436. doi: 10.1073/pnas.1815441116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papikian, A., Liu, W. L., Gallego-Bartolome, J. & Jacobsen, S. E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 10, 729 (2019). [DOI] [PMC free article] [PubMed]
- 56.Wang W, et al. Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. Crispr J. 2018;1:65–74. doi: 10.1089/crispr.2017.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaeffer SM, Nakata PA. CRISPR/Cas9-mediated genome editing and gene replacement in plants: transitioning from lab to field. Plant Sci. 2015;240:130–142. doi: 10.1016/j.plantsci.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Eshed Y, Lippman ZB. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science. 2019;366:705. doi: 10.1126/science.aax0025. [DOI] [PubMed] [Google Scholar]
- 59.Hamant O. Plant scientists can’t ignore Jevons paradox anymore. Nat. Plants. 2020;6:720–722. doi: 10.1038/s41477-020-0722-3. [DOI] [PubMed] [Google Scholar]
- 60.Haynes-Maslow, L., Parsons, S. E., Wheeler, S. B. & Leone, L. A. A qualitative study of perceived barriers to fruit and vegetable consumption among low-income populations, North Carolina, 2011. Prev Chronic Dis. 10, E34 (2013). [DOI] [PMC free article] [PubMed]
- 61.Lucan SC, Barg FK, Long JA. Promoters and barriers to fruit, vegetable, and fast-food consumption among urban, low-income african americans—a qualitative approach. Am. J. Public Health. 2010;100:631–635. doi: 10.2105/AJPH.2009.172692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller V, et al. Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Glob. Health. 2016;4:E695–E703. doi: 10.1016/S2214-109X(16)30186-3. [DOI] [PubMed] [Google Scholar]
- 63.Mook, K., Laraia, B. A., Oddo, V. M. & Jones-Smith, J. C. Food security status and barriers to fruit and vegetable consumption in two economically deprived communities of Oakland, California, 2013-2014. Prev. Chronic Dis. 13, 150402 (2016). [DOI] [PMC free article] [PubMed]
- 64.Tallant A, Rettig M, Tennyson S. Barriers and facilitators for fruit and vegetable consumption among adults in rural counties. Fam. Consum. Sci. Res. J. 2018;47:87–100. doi: 10.1111/fcsr.12275. [DOI] [Google Scholar]
- 65.Possingham JV. Fruit and vegetable quality in 21st century—the influence of Japan. J. Jpn Soc. Hortic. Sci. 1998;67:1250–1254. doi: 10.2503/jjshs.67.1250. [DOI] [Google Scholar]
- 66.Florkowski, W. J. et al. in Postharvest Handling 3rd edn (eds Florkowski, W. J., Shewfelt, R. L., Brueckner, B. & Prussia, S. E.) 147–166 (Academic Press, 2014).
- 67.Kader AA. Flavor quality of fruits and vegetables. J. Sci. Food Agr. 2008;88:1863–1868. doi: 10.1002/jsfa.3293. [DOI] [Google Scholar]
- 68.Florkowski, W., Shewfelt, R. & Prussia, S. E. in Postharvest Handling- A Systems Approach (eds Florkowski, W. J., Shewfelt, R. L. & Prussia, S. E.) 592 (Academic Press, 2014).
- 69.Beckles DM. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Tec. 2012;63:129–140. doi: 10.1016/j.postharvbio.2011.05.016. [DOI] [Google Scholar]
- 70.Barrett DM, Beaulieu JC, Shewfelt R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. 2010;50:369–389. doi: 10.1080/10408391003626322. [DOI] [PubMed] [Google Scholar]
- 71.Mogren, L. et al. The hurdle approach-a holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy green vegetables. A review. Front. Microbiol. 9, 1965 (2018). [DOI] [PMC free article] [PubMed]
- 72.Breslin PaulAS. An evolutionary perspective on food and human taste. Curr. Biol. 2013;23:R409–R418. doi: 10.1016/j.cub.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diehl DC, et al. Exploring produce industry attitudes: relationships between postharvest handling, fruit flavor, and consumer purchasing. Horttechnology. 2013;23:642–650. doi: 10.21273/HORTTECH.23.5.642. [DOI] [Google Scholar]
- 74.Hebrok M, Boks C. Household food waste: drivers and potential intervention points for design. An extensive review. J. Clean. Prod. 2017;151:380–392. doi: 10.1016/j.jclepro.2017.03.069. [DOI] [Google Scholar]
- 75.Jaeger SR, et al. Consumers’ visual attention to fruit defects and disorders: a case study with apple images. Postharvest Biol. Tec. 2016;116:36–44. doi: 10.1016/j.postharvbio.2015.12.015. [DOI] [Google Scholar]
- 76.Hamilton, A. J., Lycett, G. W. & Grierson, D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature346, 10.1038/346284a0 (1990).
- 77.López-Gómez R, et al. Ripening in papaya fruit is altered by ACC oxidase cosuppression. Transgenic Res. 2009;18:89–97. doi: 10.1007/s11248-008-9197-0. [DOI] [PubMed] [Google Scholar]
- 78.Nagata M, et al. Modification of tomato fruit ripening by transformation with sense or antisense chimeric 1-aminocyclopropane-1-carboxylate synthase genes. Acta Hortic. 1995;394:213–218. doi: 10.17660/ActaHortic.1995.394.22. [DOI] [Google Scholar]
- 79.Oeller, P. W., Min-Wong, L., Taylor, L. P., Pike, D. A. & Theologis, A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science254, 10.1126/science.1925603 (1991). [DOI] [PubMed]
- 80.Xiong A-S, et al. Different effects on ACC oxidase gene silencing triggered by RNA interference in transgenic tomato. Plant Cell Rep. 2005;23:639–646. doi: 10.1007/s00299-004-0887-7. [DOI] [PubMed] [Google Scholar]
- 81.Xue, C. et al. Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening. BMC Plant Biol.20, 13 (2020). [DOI] [PMC free article] [PubMed]
- 82.Atkinson RG, et al. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J. Exp. Bot. 2011;62:3821–3835. doi: 10.1093/jxb/err063. [DOI] [PubMed] [Google Scholar]
- 83.Ayub R, et al. Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nat. Biotechnol. 1996;14:862–866. doi: 10.1038/nbt0796-862. [DOI] [PubMed] [Google Scholar]
- 84.Lu PT, et al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants. 2018;4:784–791. doi: 10.1038/s41477-018-0249-z. [DOI] [PubMed] [Google Scholar]
- 85.Yu Q-h, et al. CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Sci. Rep. 2017;7:11874. doi: 10.1038/s41598-017-12262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, R. F. et al. Re-evaluation of transcription factor function in tomato fruit development and ripening with CRISPR/Cas9-mutagenesis. Sci. Rep.9, 1696 (2019). [DOI] [PMC free article] [PubMed]
- 87.Ito, Y., Nishizawa-Yokoi, A., Endo, M., Mikami, M. & Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruitripening. Biochem. Biophys. Res. Commun. 467, 76–82 (2015). [DOI] [PubMed]
- 88.MarketWatch. Floriculture Market 2019 Global Industry Size, Share, Forecasts Analysis, Company Profiles, Competitive Landscape and Key Regions 2024. Available at 360 Research Reports. https://www.marketwatch.com/press-release/floriculture-market-2019-global-industry-size-share-forecasts-analysis-company-profiles-competitive-landscape-and-key-regions-2024-available-at-360-research-reports-2019-09-26 (2019).
- 89.Xia, Y., Deng, X., Zhou, P., Shima, K. & Teixeira da Silva, J. A. in Floriculture, Ornamental and Plant Biotechnology - Advances and Topical Issues Vol. 1–4 (ed Teixeira da Silva, J. A.) (Global Science Books, Ltd., 2006).
- 90.Pranuthi P, Suseela T, Swami DV, Salomi Suneetha DR, Sudha Vani V. Effect of packing and storage conditions on physiological loss in weight, diameter of the flower, electrolyte leakage in extending the vase life of Carnation cv. Kiro. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:1278–1287. doi: 10.20546/ijcmas.2018.712.158. [DOI] [Google Scholar]
- 91.Mamias, S. The Floriculture Supply Chain: Characteristics And Prospects (Union Fleurs-International Flower Trade Association, The Netherlands, 2018).
- 92.Fredenburgh, F. The 4,000 mile flower delivery. http://www.bbc.com/future/bespoke/made-on-earth/the-new-roots-of-the-flower-trade/ (2019).
- 93.Savin KW, et al. Antisense Acc oxidase RNA delays carnation petal senescence. Hortscience. 1995;30:970–972. doi: 10.21273/HORTSCI.30.5.970. [DOI] [Google Scholar]
- 94.Woodson WR, Park KY, Drory A, Larsen PB, Wang H. Expression of ethylene biosynthetic-pathway transcripts in senescing carnation flowers. Plant Physiol. 1992;99:526–532. doi: 10.1104/pp.99.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu MJ, Vandoorn WG, Reid MS. Variation in the senescence of carnation (Dianthus-Caryophyllus L) Cultivars .1. Comparison of flower life, respiration and ethylene biosynthesis. Sci. Hortic. 1991;48:99–107. doi: 10.1016/0304-4238(91)90156-S. [DOI] [Google Scholar]
- 96.Chang, X. X. et al. A petunia homeodomain-leucine zipper protein, PhHD-Zip, plays an important role in flower senescence. PLoS ONE9, e88320 (2014). [DOI] [PMC free article] [PubMed]
- 97.Tan YY, et al. PhGRL2 protein, interacting with PhACO1, is involved in flower senescence in the petunia. Mol. Plant. 2014;7:1384–1387. doi: 10.1093/mp/ssu024. [DOI] [PubMed] [Google Scholar]
- 98.Wang, H. et al. Transcriptome changes associated with delayed flower senescence on transgenic petunia by inducing expression of etr1-1, a mutant ethylene receptor. PLoS ONE8, e65800 (2013). [DOI] [PMC free article] [PubMed]
- 99.Xu J, et al. CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol. J. 2020;18:287–297. doi: 10.1111/pbi.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reid, M. S. & Jiang, C.-Z. in Horticultural Reviews Vol. 40 (ed. Janick, J.) 1–44 (John Wiley & Sons, Inc., 2012).
- 101.Lara I, Belge B, Goulao LF. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014;87:103–112. doi: 10.1016/j.postharvbio.2013.08.012. [DOI] [Google Scholar]
- 102.Lara, I., Heredia, A. & Dominguez, E. Shelf life potential and the fruit cuticle: the unexpected player. Front. Plant Sci. 10, 770 (2019). [DOI] [PMC free article] [PubMed]
- 103.Benichou, M. et al. in Postharvest Biology and Technology of Temperate Fruits (eds Mir, S. A., Shah, M. A. & Mir, M. M.) 77–100 (Spring International Publishing, 2018).
- 104.Petit J, Bres C, Mauxion JP, Bakan B, Rothan C. Breeding for cuticle-associated traits in crop species: traits, targets, and strategies. J. Exp. Bot. 2017;68:5369–5387. doi: 10.1093/jxb/erx341. [DOI] [PubMed] [Google Scholar]
- 105.Rid M, Markheiser A, Hoffmann C, Gross J. Waxy bloom on grape berry surface is one important factor for oviposition of European grapevine moths. J. Pest Sci. 2018;91:1225–1239. doi: 10.1007/s10340-018-0988-7. [DOI] [Google Scholar]
- 106.Loypimai P, Paewboonsom S, Damerow L, Blanke MM. The wax bloom on blueberry: application of luster sensor technology to assess glossiness and the effect of polishing as a fruit quality parameter. J. Appl Bot. Food Qual. 2017;90:154–158. [Google Scholar]
- 107.Storey R, Price WE. Microstructure of the skin of d’Agen plums. Sci. Hortic. 1999;81:279–286. doi: 10.1016/S0304-4238(99)00024-2. [DOI] [Google Scholar]
- 108.Trivedi, P. et al. Developmental and environmental regulation of cuticular wax biosynthesis in fleshy fruits. Front. Plant Sci10, 431 (2019). [DOI] [PMC free article] [PubMed]
- 109.Bruggenwirth M, Knoche M. Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta. 2017;245:765–777. doi: 10.1007/s00425-016-2639-7. [DOI] [PubMed] [Google Scholar]
- 110.Knoche M, Grimm E. Surface moisture induces microcracks in the cuticle of ‘Golden Delicious’ apple. Hortscience. 2008;43:1929–1931. doi: 10.21273/HORTSCI.43.6.1929. [DOI] [Google Scholar]
- 111.Dominguez E, et al. Tomato fruit continues growing while ripening, affecting cuticle properties and cracking. Physiol. Plant. 2012;146:473–486. doi: 10.1111/j.1399-3054.2012.01647.x. [DOI] [PubMed] [Google Scholar]
- 112.Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev. Plant Phys. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 113.Cantu D, et al. Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiol. 2009;150:1434–1449. doi: 10.1104/pp.109.138701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Payasi A, Mishra NN, Chaves ALS, Singh R. Biochemistry of fruit softening: an overview. Physiol. Mol. Biol. Plants. 2009;15:103–113. doi: 10.1007/s12298-009-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Posé S, et al. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013;64:3803–3815. doi: 10.1093/jxb/ert210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001;47:311–340. doi: 10.1023/A:1010656104304. [DOI] [PubMed] [Google Scholar]
- 117.Jiménez-Bermudez S, et al. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 2002;128:751–759. doi: 10.1104/pp.010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santiago-Domenech N, et al. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J. Exp. Bot. 2008;59:2769–2779. doi: 10.1093/jxb/ern142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang L, et al. Silencing of SlPL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 2017;15:1544–1555. doi: 10.1111/pbi.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tieman DM, Harriman RW, Ramamohan G, Handa AK. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell. 1992;4:667–679. doi: 10.2307/3869525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang, S. Y., Zhou, Q., Zhou, X., Zhang, F. & Ji, S. J. Ethylene plays an important role in the softening and sucrose metabolism of blueberries postharvest. Food Chem. 310, 125965 (2020). [DOI] [PubMed]
- 122.Wen B, Strom A, Tasker A, West G, Tucker GA. Effect of silencing the two major tomato fruit pectin methylesterase isoforms on cell wall pectin metabolism. Plant Biol. 2013;15:1025–1032. doi: 10.1111/j.1438-8677.2012.00714.x. [DOI] [PubMed] [Google Scholar]
- 123.Smith DL, Abbott JA, Gross KC. Down-regulation of tomato beta-galactosidase 4 results in decreased fruit softening. Plant Physiol. 2002;129:1755–1762. doi: 10.1104/pp.011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moctezuma E, Smith DL, Gross KC. Antisense suppression of a beta-galactosidase gene (TBG6) in tomato increases fruit cracking. J. Exp. Bot. 2003;54:2025–2033. doi: 10.1093/jxb/erg214. [DOI] [PubMed] [Google Scholar]
- 125.Carey AT, et al. Down-regulation of a ripening-related beta-galactosidase gene (TBG1) in transgenic tomato fruits. J. Exp. Bot. 2001;52:663–668. doi: 10.1093/jexbot/52.357.663. [DOI] [PubMed] [Google Scholar]
- 126.Uluisik S, et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016;34:950. doi: 10.1038/nbt.3602. [DOI] [PubMed] [Google Scholar]
- 127.Smith CJS, et al. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol. Biol. 1990;14:369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- 128.Wormit, A. & Usadel, B. The multifaceted role of pectin methylesterase inhibitors (PMEIs). Int. J. Mol. Sci.19, 2878 (2018). [DOI] [PMC free article] [PubMed]
- 129.Encinas-Basurto D, et al. Alterations in volatile metabolites profile of fresh tomatoes in response to Alternaria alternata (Fr.) Keissl. 1912 infection. Chil. J. Agr. Res. 2017;77:194–201. doi: 10.4067/S0718-58392017000300194. [DOI] [Google Scholar]
- 130.Peris, J. E., Rodriguez, A., Pena, L. & Fedriani, J. M. Fungal infestation boosts fruit aroma and fruit removal by mammals and birds. Sci. Rep.7, 5646 (2017). [DOI] [PMC free article] [PubMed]
- 131.Rodrigo JEP, Laffitte JMF, García LP. Los mamíferos frugívoros prefieren frutos de cítricos infectados por Penicillium:¿ se equivocaba Janzen? Rev. Ecosistemas. 2015;24:5–13. doi: 10.7818/ECOS.2015.24-3.02. [DOI] [Google Scholar]
- 132.Rodriguez A, Alquezar B, Pena L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. N. Phytol. 2013;197:36–48. doi: 10.1111/j.1469-8137.2012.04382.x. [DOI] [PubMed] [Google Scholar]
- 133.Vilanova L, et al. Differential contribution of the two major polygalacturonases from Penicillium digitatum to virulence towards citrus fruit. Int J. Food Microbiol. 2018;282:16–23. doi: 10.1016/j.ijfoodmicro.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 134.Silva, C. J. et al. Tomato fruit susceptibility to fungal disease can be uncoupled from ripening by suppressing susceptibility factors. Preprint at https://www.biorxiv.org/content/10.1101/2020.06.03.132829v1.full (2020).
- 135.Porat R, McCollum TG, Vinokur V, Droby S. Effects of various elicitors on the transcription of a beta-1,3-endoglucanase gene in citrus fruit. J. Phytopathol. 2002;150:70–75. doi: 10.1046/j.1439-0434.2002.00719.x. [DOI] [Google Scholar]
- 136.Distefano G, et al. Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Transgenic Res. 2008;17:873–879. doi: 10.1007/s11248-008-9172-9. [DOI] [PubMed] [Google Scholar]
- 137.Muccilli V, et al. Substantial equivalence of a transgenic lemon fruit showing postharvest fungal pathogens resistance. J. Agr. Food Chem. 2020;68:3806–3816. doi: 10.1021/acs.jafc.9b07925. [DOI] [PubMed] [Google Scholar]
- 138.Ballester AR, et al. Transcriptomic profiling of citrus fruit peel tissues reveals fundamental effects of phenylpropanoids and ethylene on induced resistance. Mol. Plant Pathol. 2011;12:879–897. doi: 10.1111/j.1364-3703.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gonzalez-Candelas, L., Alamar, S., Sanchez-Torres, P., Zacarias, L. & Marcos, J. F. A transcriptomic approach highlights induction of secondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biol.10, 194 (2010). [DOI] [PMC free article] [PubMed]
- 140.Chen, J. Y., Shen, Y. T., Chen, C. Y. & Wan, C. P. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: a review. Plants8, 26 (2019). [DOI] [PMC free article] [PubMed]
- 141.Sanzani SM, Schena L, Ippolito A. Effectiveness of phenolic compounds against citrus green mould. Molecules. 2014;19:12500–12508. doi: 10.3390/molecules190812500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khoo, H. E., Azlan, A., Kong, K. & Ismail, A. Phytochemicals and medicinal properties of indigenous tropical fruits with potential for commercial development. Evid. Based Compl. Alt. 2016, 7591951 (2016). [DOI] [PMC free article] [PubMed]
- 143.Zhang Y, et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013;23:1094–1100. doi: 10.1016/j.cub.2013.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, et al. Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol. 2015;169:1568–1583. doi: 10.1104/pp.15.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bassolino L, et al. Accumulation of anthocyanins in tomato skin extends shelf life. N. Phytol. 2013;200:650–655. doi: 10.1111/nph.12524. [DOI] [PubMed] [Google Scholar]
- 146.Mes PJ, Boches P, Myers JR, Durst R. Characterization of tomatoes expressing anthocyanin in the fruit. J. Am. Soc. Hortic. Sci. 2008;133:262–269. doi: 10.21273/JASHS.133.2.262. [DOI] [Google Scholar]
- 147.Nookaraju A, et al. Molecular approaches for enhancing sweetness in fruits and vegetables. Sci. Hortic. 2010;127:1–15. doi: 10.1016/j.scienta.2010.09.014. [DOI] [Google Scholar]
- 148.Beckles DM, Hong N, Stamova L, Luengwilai K. Biochemical factors contributing to tomato fruit sugar content: a review. Fruits. 2012;67:49–64. doi: 10.1051/fruits/2011066. [DOI] [Google Scholar]
- 149.Beauvoit B, et al. Putting primary metabolism into perspective to obtain better fruits. Ann. Bot. 2018;122:1–21. doi: 10.1093/aob/mcy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science. 2004;305:1786–1789. doi: 10.1126/science.1101666. [DOI] [PubMed] [Google Scholar]
- 151.Petreikov, M. et al. Carbohydrate balance and accumulation during development of near-isogenic tomato lines differing in the AGPase-L1 allele. J. Am. Soc. Hortic. Sci.134, 134–140 (2009).
- 152.Centeno DC, et al. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell. 2011;23:162–184. doi: 10.1105/tpc.109.072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Powell ALT, et al. Uniform ripening encodes a golden 2-like transcription factor regulating tomato fruit chloroplast development. Science. 2012;336:1711–1715. doi: 10.1126/science.1222218. [DOI] [PubMed] [Google Scholar]
- 154.Sagar M, et al. SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 2013;161:1362–1374. doi: 10.1104/pp.113.213843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sagor GHM, et al. A novel strategy to produce sweeter tomato fruits with high sugar contents by fruit-specific expression of a single bZIP transcription factor gene. Plant Biotechnol. J. 2016;14:1116–1126. doi: 10.1111/pbi.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shammai A, et al. Natural genetic variation for expression of a SWEET transporter among wild species of Solanum lycopersicum (tomato) determines the hexose composition of ripening tomato fruit. Plant J. 2018;96:343–357. doi: 10.1111/tpj.14035. [DOI] [PubMed] [Google Scholar]
- 157.Yuan, Y. et al. (2018). SlARF10, an auxin response factor, is involved in chlorophyll and sugaraccumulation during tomato fruit development. J. Exp. Bot. 69, 5507–5518 (2018). [DOI] [PMC free article] [PubMed]
- 158.Cheng JT, et al. Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation. J. Exp. Bot. 2018;69:511–523. doi: 10.1093/jxb/erx440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith AM, Denyer K, Martin CR. What controls the amount and structure of starch in storage organs. Plant Physiol. 1995;107:673–677. doi: 10.1104/pp.107.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Andersson M, et al. Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep. 2017;36:117–128. doi: 10.1007/s00299-016-2062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beckles, D. M. & Thitisaksakul, M. in Encyclopedia of Biotechnology in Agriculture and Food (eds Heldman, D., Hoover, D. & Wheeler, M.) 8 (CRC Press, Boca Raton, 2014).
- 162.Wang, H. X. et al. CRISPR/Cas9-based mutagenesis of starch biosynthetic genes in sweet potato (Ipomoea Batatas) for the improvement of starch quality. Int. J. Mol. Sci. 20, 4702 (2019). [DOI] [PMC free article] [PubMed]
- 163.Li HT, Gidley MJ, Dhital S. High-amylose starches to bridge the “fiber gap”: development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019;18:362–379. doi: 10.1111/1541-4337.12416. [DOI] [PubMed] [Google Scholar]
- 164.Jobling S. Improving starch for food and industrial applications. Curr. Opin. Plant Biol. 2004;7:210–218. doi: 10.1016/j.pbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 165.Hofvander P, Andersson M, Larsson CT, Larsson H. Field performance and starch characteristics of high-amylose potatoes obtained by antisense gene targeting of two branching enzymes. Plant Biotechnol. J. 2004;2:311–320. doi: 10.1111/j.1467-7652.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 166.Jobling SA, Westcott RJ, Tayal A, Jeffcoat R, Schwall GP. Production of a freeze-thaw-stable potato starch by antisense inhibition of three starch synthase genes. Nat. Biotechnol. 2002;20:295–299. doi: 10.1038/nbt0302-295. [DOI] [PubMed] [Google Scholar]
- 167.Schwall GP, et al. Production of very-high-amylose potato starch by inhibition of SBE A and B. Nat. Biotechnol. 2000;18:551–554. doi: 10.1038/75427. [DOI] [PubMed] [Google Scholar]
- 168.Visser RGF, et al. Inhibition of the expression of the gene for granule-bound starch synthase in potato by antisense constructs. Mol. Gen. Genet. 1991;225:289–296. doi: 10.1007/BF00269861. [DOI] [PubMed] [Google Scholar]
- 169.Dong SY, Beckles DM. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019;234:80–93. doi: 10.1016/j.jplph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 170.Luengwilai K, Beckles DM. Starch granules in tomato fruit show a complex pattern of degradation. J. Agric. Food Chem. 2009;57:8480–8487. doi: 10.1021/jf901593m. [DOI] [PubMed] [Google Scholar]
- 171.McKibbin RS, et al. Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnol. J. 2006;4:409–418. doi: 10.1111/j.1467-7652.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 172.Regierer B, et al. Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat. Biotechnol. 2002;20:1256–1260. doi: 10.1038/nbt760. [DOI] [PubMed] [Google Scholar]
- 173.Burton WG. The sugar balance in some British potato varieties during storage. II. The effects of tuber age, previous storage temperature, and intermittent refrigeration upon low-temperature sweetening. Eur. Potato J. 1969;12:81–95. doi: 10.1007/BF02364726. [DOI] [Google Scholar]
- 174.Amir J, Kahn V, Unterman M. Respiration, Atp level, and sugar accumulation in potato-tubers during storage at 4-degrees. Phytochemistry. 1977;16:1495–1498. doi: 10.1016/0031-9422(77)84008-9. [DOI] [Google Scholar]
- 175.Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 176.Gokmen V, Palazoglu TK. Acrylamide formation in foods during thermal processing with a focus on frying. Food Bioprocess Technol. 2008;1:35–42. doi: 10.1007/s11947-007-0005-2. [DOI] [Google Scholar]
- 177.Morrell S, Aprees T. Control of the hexose content of potato-tubers. Phytochemistry. 1986;25:1073–1076. doi: 10.1016/S0031-9422(00)81556-3. [DOI] [Google Scholar]
- 178.Lorberth R, Ritte G, Willmitzer L, Kossmann J. Inhibition of a starch-granule-bound protein leads to modified starch and repression of cold sweetening. Nat. Biotechnol. 1998;16:473–477. doi: 10.1038/nbt0598-473. [DOI] [PubMed] [Google Scholar]
- 179.Cochrane MP, Duffus CM, Allison MJ, Mackay GR. Amylolytic activity in stored potato-tubers .2. The effect of low-temperature storage on the activities of alpha-amylase and beta-amylase and alpha-glucosidase in potato-tubers. Potato Res. 1991;34:333–341. doi: 10.1007/BF02360507. [DOI] [Google Scholar]
- 180.Claassen PAM, Budde MAW, Vancalker MH. Increase in phosphorylase-activity during cold-induced sugar accumulation in potato-tubers. Potato Res. 1993;36:205–217. doi: 10.1007/BF02360529. [DOI] [Google Scholar]
- 181.Zrenner R, Schuler K, Sonnewald U. Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta. 1996;198:246–252. doi: 10.1007/BF00206250. [DOI] [PubMed] [Google Scholar]
- 182.Yu XY, et al. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. J. Exp. Bot. 2008;59:2969–2977. doi: 10.1093/jxb/ern158. [DOI] [PubMed] [Google Scholar]
- 183.Cheng M, Lowe BA, Spencer TM, Ye XD, Armstrong CL. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. Vitr. Cell Dev. 2004;40:31–45. doi: 10.1079/IVP2003501. [DOI] [Google Scholar]
- 184.Bhaskar PB, et al. Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol. 2010;154:939–948. doi: 10.1104/pp.110.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Richael, C. et al. POTATO CULTIVAR W8. USA patent (2015).
- 186.Klein D, Gkisakis V, Krumbein A, Livieratos I, Kopke U. Old and endangered tomato cultivars under organic greenhouse production: effect of harvest time on flavour profile and consumer acceptance. Int J. Food Sci. Technol. 2010;45:2250–2257. doi: 10.1111/j.1365-2621.2010.02398.x. [DOI] [Google Scholar]
- 187.Causse M, Buret M, Robini K, Verschave P. Inheritance of nutritional and sensory quality traits in fresh market tomato and relation to consumer preferences. J. Food Sci. 2003;68:2342–2350. doi: 10.1111/j.1365-2621.2003.tb05770.x. [DOI] [Google Scholar]
- 188.Causse M, et al. Consumer preferences for fresh tomato at the european scale: a common segmentation on taste and firmness. J. Food Sci. 2010;75:S531–S541. doi: 10.1111/j.1750-3841.2010.01841.x. [DOI] [PubMed] [Google Scholar]
- 189.Sinesio F, et al. Sensory quality of fresh french and dutch market tomatoes: a preference mapping study with italian consumers. J. Food Sci. 2010;75:S55–S67. doi: 10.1111/j.1750-3841.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 190.Zhang B, et al. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl Acad. Sci. USA. 2016;113:12580–12585. doi: 10.1073/pnas.1613910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klee HJ, Tieman DM. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018;19:347–356. doi: 10.1038/s41576-018-0002-5. [DOI] [PubMed] [Google Scholar]
- 192.Ferrão LFV, et al. Genome-wide association of volatiles reveals candidate loci for blueberry flavor. N. Phytol. 2020;226:1725–1737. doi: 10.1111/nph.16459. [DOI] [PubMed] [Google Scholar]
- 193.Tieman D, et al. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012;22:1035–1039. doi: 10.1016/j.cub.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 194.Zhao, J. T. et al. Meta-analysis of genome-wide association studies provides insights into genetic control of tomato flavor. Nat. Commun. 10, 1534 (2019). [DOI] [PMC free article] [PubMed]
- 195.Mathieu S, et al. Flavour compounds in tomato fruits: identification of loci and potential pathways affecting volatile composition. J. Exp. Bot. 2009;60:325–337. doi: 10.1093/jxb/ern294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Galili G, Galili S, Lewinsohn E, Tadmor Y. Genetic, molecular, and genomic approaches to improve the value of plant foods and feeds. Crit. Rev. Plant Sci. 2002;21:167–204. doi: 10.1080/0735-260291044232. [DOI] [Google Scholar]
- 197.He, Q. & Luo, Y. Enzymatic browning and its control in fresh-cut produce Stewart Postharvest Rev.6, 1–7 (2007).
- 198.González MN, et al. Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front Plant Sci. 2020;10:1649–1649. doi: 10.3389/fpls.2019.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Armstrong, J. & Lane, W. D. Genetically modified reduced-browning fruit producing plant and produce fruit thereof and method of obtaining such. USA patent (2009).
- 200.Brooks, J. Consumer feedback: The world is ready for Arctic® apples. Availabe online: https://www.okspecialtyfruits.com/consumer-feedback-theworld-is-ready-for-arctic-apples/ (accessed on May 30th), (Okanagan Specialty Fruits Inc. Summerland, British Columbia, 2012).
- 201.Waltz E. Gene-edited CRISPR mushroom escapes US regulation. Nature. 2016;532:293. doi: 10.1038/nature.2016.19754. [DOI] [PubMed] [Google Scholar]
- 202.Beckles DM, Thitisaksakul M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch. 2014;66:58–71. doi: 10.1002/star.201300212. [DOI] [Google Scholar]
- 203.Brandi MT, Cox CE, Teplitski M. Salmonella interactions with plants and their associated microbiota. Phytopathology. 2013;103:316–325. doi: 10.1094/PHYTO-11-12-0295-RVW. [DOI] [PubMed] [Google Scholar]
- 204.Machado-Moreira B, Richards K, Brennan F, Abram F, Burgess CM. Microbial contamination of fresh produce: what, where, and how? Compr. Rev. Food Sci. Food Saf. 2019;18:1727–1750. doi: 10.1111/1541-4337.12487. [DOI] [PubMed] [Google Scholar]
- 205.Haymaker J, et al. Prevalence of Shiga-toxigenic and atypical enteropathogenic Escherichia coli in untreated surface water and reclaimed water in the Mid-Atlantic U.S. Environ. Res. 2019;172:630–636. doi: 10.1016/j.envres.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 206.Olaimat AN, Holley RA. Factors influencing the microbial safety of fresh produce: a review. Food Microbiol. 2012;32:1–19. doi: 10.1016/j.fm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 207.Teplitski M, Barak JD, Schneider KR. Human enteric pathogens in produce: un-answered ecological questions with direct implications for food safety. Curr. Opin. Biotechnol. 2009;20:166–171. doi: 10.1016/j.copbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 208.Montano, J. et al. Salmonella enterica serovar typhimurium 14028s genomic regions required for colonization of lettuce leaves. Front. Microbiol. 11, 6 (2020). [DOI] [PMC free article] [PubMed]
- 209.Jacob, C. & Melotto, M. Human pathogen colonization of lettuce dependent upon plant genotype and defense response activation. Front. Plant Sci. 10, 1769 (2020). [DOI] [PMC free article] [PubMed]
- 210.Melotto, M. et al. Breeding crops for enhanced food safety. Front. Plant Sci. 11, 10.3389/fpls.2020.00428 (2020). [DOI] [PMC free article] [PubMed]
- 211.Albornoz, K. et al. Investigating postharvest chilling injury in tomato (Solanum lycopersicum L.) fruit using magnetic resonance imaging and 5-azacytidine, a hypomethylation agent. Acta Hortic.1278, 10.17660/ActaHortic.12020.11278.17635 (2020).
- 212.Biswas, P., East, A. R., Hewett, E. W. & Heyes, J. A. in Horticultural Reviews Vol. 44 (ed Jules Janick) (John Wiley & Sons, 2017).
- 213.Liu, Y. D. et al. SlGRAS4 mediates a novel regulatory pathway promoting chilling tolerance in tomato. Plant Biotechnol. J.10.1111/pbi.13328 (2020). [DOI] [PMC free article] [PubMed]
- 214.Ferguson I, Volz R, Woolf A. Preharvest factors affecting physiological disorders of fruit. Postharvest Biol. Tec. 1999;15:255–262. doi: 10.1016/S0925-5214(98)00089-1. [DOI] [Google Scholar]
- 215.de Freitas ST, Jiang CZ, Mitcham EJ. Mechanisms involved in calcium deficiency development in tomato fruit in response to gibberellins. J. Plant Growth Regul. 2012;31:221–234. doi: 10.1007/s00344-011-9233-9. [DOI] [Google Scholar]
- 216.Kader, A. A. in Postharvest Techology of Horticulture Crops (ed Kader, A. A.) 279–285 (University of California ANR Natural Resources, 2002).
- 217.Kader, A. A. in Postharvest Technology of Horticultural Crops (ed Kader, A. A.) 39–48 (University of California, Agriculture and Natural Resources, 2002).
- 218.Thompson, A. K. Fruit and Vegetable Storage: Hybobaric, Hyperbaric and Controlled Atmosphere. 10.1007/978-3-319-23591-2 (Springer, 2016).
- 219.Pedreschi R, Lurie S. Advances and current challenges in understanding postharvest abiotic stresses in perishables. Postharvest Biol. Technol. 2015;107:77–89. doi: 10.1016/j.postharvbio.2015.05.004. [DOI] [Google Scholar]
- 220.Altpeter F, et al. Advancing crop transformation in the era of genome editing. Plant Cell. 2016;28:1510–1520. doi: 10.1105/tpc.16.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cohen SN, Chang ACY, Boyer HW, Helling RB. Construction of biologically functional bacterial plasmids in-vitro. Proc. Natl Acad. Sci. USA. 1973;70:3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Song, G. Q., Prieto, H. & Orbovic, V. Agrobacterium-mediated transformation of tree fruit crops: methods, progress, and challenges. Front. Plant Sci. 10, 26 (2019). [DOI] [PMC free article] [PubMed]
- 223.Sanford JC. Biolistic Plant Transformation. Physiol. Plant. 1990;79:206–209. doi: 10.1111/j.1399-3054.1990.tb05888.x. [DOI] [Google Scholar]
- 224.Fitch, M. M. M. in Biotechnology of Fruit and Nut Crops (ed Litz, R. E.) 173–208 (CABI Publishing, Cambridge, MA, 2005).
- 225.Oliveira, M. & Fraser, L. in Biotechnology of Fruit and Nut Crops (ed Litz, R. E.) 2–28 (CABI Publishing, Cambridge, MA, 2005).
- 226.Fernandez-Pinan, S. et al. Agrobacterium tumefaciens and Agrobacterium rhizogenes-mediated transformation of potato and the promoter activity of a suberin gene by GUS staining. J. Vis. Exp. https://doi.org/10.3791/59119 (2019). [DOI] [PubMed]
- 227.Doyle, C. et al. A simple method for spray-on gene editing in planta. Preprint at 10.1101/805036 (2019).
- 228.Maher, M. F. et al. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 10.1038/s41587-019-0337-2 (2019). [DOI] [PMC free article] [PubMed]
- 229.Debernardi, J. M. et al. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274–1279 (2020). [DOI] [PMC free article] [PubMed]
- 230.Kong, J. et al. Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front. Plant Sci. 11, 1389 (2020). [DOI] [PMC free article] [PubMed]
- 231.Aurand R, et al. Anatomical and biochemical trait network underlying genetic variations in tomato fruit texture. Euphytica. 2012;187:99–116. doi: 10.1007/s10681-012-0760-7. [DOI] [Google Scholar]
- 232.Nunez-Lillo, G. et al. High-density genetic map and QTL analysis of soluble solid content, maturity date, and mealiness in peach using genotyping by sequencing. Sci. Hortic.257, 108734 (2019).
- 233.Hayes RJ, Galeano CH, Luo YG, Antonise R, Simko I. Inheritance of decay of fresh-cut lettuce in a recombinant inbred line population from ‘Salinas 88’ x ‘La Brillante’. J. Am. Soc. Hortic. Sci. 2014;139:388–398. doi: 10.21273/JASHS.139.4.388. [DOI] [Google Scholar]
- 234.Ogundiwin, E. A., Peace, C. P. & Gradziel, T. M. Molecular genetic dissection of chilling injury in peach fruit. Acta Hortic.78, 633–638 (2007).
- 235.Soltys-Kalina, D. et al. Novel candidate genes AuxRP and Hsp90 influence the chip color of potato tubers. Mol. Breed.35, 224 (2015). [DOI] [PMC free article] [PubMed]
- 236.da Costa JHP, Rodriguez GR, Pratta GR, Picardi LA, Zorzoli R. QTL detection for fruit shelf life and quality traits across segregating populations of tomato. Sci. Hortic. 2013;156:47–53. doi: 10.1016/j.scienta.2013.03.015. [DOI] [Google Scholar]
- 237.Howard, N. P. et al. Two QTL characterized for soft scald and soggy breakdown in apple (Malus x domestica) through pedigree-based analysis of a large population of interconnected families. Tree Genet. Genomes14, 2 (2018).
- 238.Ding YD, et al. Network analysis of postharvest senescence process in citrus fruits revealed by transcriptomic and metabolomic profiling. Plant Physiol. 2015;168:357–U642. doi: 10.1104/pp.114.255711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tang N, Deng W, Hu N, Chen N, Li ZG. Metabolite and transcriptomic analysis reveals metabolic and regulatory features associated with Powell orange pulp deterioration during room temperature and cold storage. Postharvest Biol. Technol. 2016;112:75–86. doi: 10.1016/j.postharvbio.2015.10.008. [DOI] [Google Scholar]
- 240.Metje-Sprink J, Sprink T, Hartung F. Genome-edited plants in the field. Curr. Opin. Biotechnol. 2020;61:1–6. doi: 10.1016/j.copbio.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 241.Mika, A. Flux and Uncertainty in the CRISPR Patent Landscape (The Scientist, 2017).
- 242.Terry, M. UC-Berkely renews U.S. patent dispute with the broad institute over CRISPR. https://www.biospace.com/article/crispr-patent-battle-isn-t-quite-over-yet/ (2019).
- 243.Rizk, C. CRISPR patent fight turns ugly as UC accuses broad researchers of lying about claims. https://www.genomeweb.com/business-news/crispr-patent-fight-turns-ugly-uc-accuses-broad-researchers-lying-about-claims (2019).
- 244.Akst, J. USPTO restarts CRISPR patent dispute between broad and UC. https://www.the-scientist.com/news-opinion/uspto-restarts-crispr-patent-dispute-between-broad-and-uc-66050 (2019).
- 245.Elsevier. CRISPR. https://www.elsevier.com/research-intelligence/campaigns/crispr (2016).
- 246.Kocak DD, et al. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat. Biotechnol. 2019;37:657. doi: 10.1038/s41587-019-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tan YY, et al. Rationally engineered Staphylococcus aureus Cas9 nucleases with high genome-wide specificity. Proc. Natl Acad. Sci. USA. 2019;116:20969–20976. doi: 10.1073/pnas.1906843116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Institute, B. For journalists: statement and background on the CRISPR patent process. https://www.broadinstitute.org/crispr/journalists-statement-and-background-crispr-patent-process (2017).
- 249.Rozen, I. Removing a major CRISPR licensing roadblock in agriculture. The Broad Institute. https://www.broadinstitute.org/news/removing-major-crispr-licensing-roadblock-agriculture (2017).
- 250.Ahuja V. Regulation of emerging gene technologies in India. BMC Proc. 2018;12:14. doi: 10.1186/s12919-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Consortium, E. Regulating genome edited organisms as GMOs has negative consequences for agriculture, society and economy. https://www.mpg.de/13748566/position-paper-crispr.pdf (2019).
- 252.Ledford, H. CRISPR conundrum: strict European court ruling leaves food-testing labs without a plan. Nature572, 10.1038/d41586-019-02162-x (2019). [DOI] [PubMed]
- 253.Weigel, D., Bock, R. & Coupland, G. Scientists call for modernization of EU gene-editing legislation. https://www.mpg.de/13761643/scientists-call-for-modernization-of-the-european-genetic-engineering-law (2019).
- 254.Stokstad, E. United States relaxes rules for biotech crops. (Science Magazine, Plants & Animals Science and Policy, 2020).
- 255.Callaway, E. CRISPR plants now subject to tough GM laws in European Union. Nature560, 10.1038/d41586-018-05814-6 (2018). [DOI] [PubMed]
- 256.European Parliament. 32001L0018 (Official Journal of the European Communities, 2001).
- 257.Priefer C, Jörissen J, Bräutigam K-R. Food waste prevention in Europe – A cause-driven approach to identify the most relevant leverage points for action. Resour. Conserv. Recycling. 2016;109:155–165. doi: 10.1016/j.resconrec.2016.03.004. [DOI] [Google Scholar]
- 258.De Laurentiis V, Corrado S, Sala S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018;77:238–251. doi: 10.1016/j.wasman.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 259.Tuncel A, et al. Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol. J. 2019;17:2259–2271. doi: 10.1111/pbi.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang DD, et al. Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 2019;179:544–557. doi: 10.1104/pp.18.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Behboodian, B., Mohd Ali, Z., Ismail, I. & Zainal, Z. Postharvest analysis of lowland transgenic tomato fruits harboring hpRNAi-ACO1construct. Sci. World J. 439870. 10.1100/2012/439870 (2012). [DOI] [PMC free article] [PubMed]
- 262.Kramer M, Sanders R, Bolkan H, Waters C, Sheeny RE, Hiatt WR. Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biol. Technol. 1992;1:241–255. doi: 10.1016/0925-5214(92)90007-C. [DOI] [Google Scholar]
- 263.Quesada, M. A. et al. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 150, 1022–1032 (2009). [DOI] [PMC free article] [PubMed]
- 264.Elitzur, T. et al. Banana MaMADS transcription factors are necessary for fruit ripening and molecular tools to promote shelf-life and food security. Plant Physiol. 171, 380–391 (2016). [DOI] [PMC free article] [PubMed]
- 265.Yang, L. et al. Silencing of Sl PL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf‐life and reduced susceptibility to grey mould. Plant Biotechnol. J.15, 1544–1555 (2017). [DOI] [PMC free article] [PubMed]
- 266.Tieman, D. M., Harriman, R. W., Ramamohan, G. & Handa, A. K. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. The Plant Cell4, 667–679 (1992). [DOI] [PMC free article] [PubMed]
- 267.Ban, Q. et al. Functional characterization of persimmon β-galactosidase gene DkGAL1 in tomato reveals cell wall modification related to fruit ripening and radicle elongation. Plant Science274, 109–120 (2018). [DOI] [PubMed]
- 268.Paniagua, C. et al. Antisense downregulation of the strawberry β-galactosidase gene FaβGal4 increases cell wall galactose levels and reduces fruit softening. J. Exp. Bot.67, 619–631 (2016). [DOI] [PMC free article] [PubMed]
- 269.Fu, D. Q., Zhu, B. Z., Zhu, H. L., Jiang, W. B., & Luo, Y. B. Virus‐induced gene silencing in tomato fruit. Plant J. 43, 299–308 (2005). [DOI] [PubMed]
- 270.Huang, L. C. et al. Delayed flower senescence of Petunia hybrida plants transformed with antisense broccoli ACC synthase and ACC oxidase genes. Postharvest Biol. Technol. 46, 47–53 (2007).
- 271.Rommens, C. M., Ye, J., Richael, C. & Swords, K. Improving potato storage and processing characteristics through all-native DNA transformation. J. Agric. Food Chem. 54, 9882–9887 (2006). [DOI] [PubMed]
- 272.Rommens, C. M., Yan, H., Swords, K., Richael, C., & Ye, J. (2008). Low‐acrylamide French fries and potato chips. Plant Biotechnol. J. 6, 843–853 (2008). [DOI] [PMC free article] [PubMed]
- 273.van Rijswick, C. World Fruit Map 2018: Global Trade Still Fruitful. RaboResearch Food & Agribusiness (2018).
- 274.Houben, M. & Van de Poel, B. 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): the enzyme that makes the plant hormone ethylene. Front. Plant Sci. 10, 695 (2019). [DOI] [PMC free article] [PubMed]
- 275.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher-plants. Annu Rev. Plant Phys. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 276.Martinez-Romero D, et al. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Crit. Rev. Food Sci. 2007;47:543–560. doi: 10.1080/10408390600846390. [DOI] [PubMed] [Google Scholar]
- 277.Hyde PT, Guan X, Abreu V, Setter TL. The anti-ethylene growth regulator silver thiosulfate (STS) increases flower production and longevity in cassava (Manihot esculenta Crantz) Plant Growth Regul. 2020;90:441–453. doi: 10.1007/s10725-019-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.de Wild HPJ, Otma EC, Peppelenbos HW. Carbon dioxide action on ethylene biosynthesis of preclimacteric and climacteric pear fruit. J. Exp. Bot. 2003;54:1537–1544. doi: 10.1093/jxb/erg159. [DOI] [PubMed] [Google Scholar]
- 279.Watkins CB. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006;24:389–409. doi: 10.1016/j.biotechadv.2006.01.005. [DOI] [PubMed] [Google Scholar]