Abstract
Background:
Anterior cruciate ligament (ACL) injury and reconstruction (ACLR) promotes quadriceps muscle atrophy and weakness that persists for years, suggesting the need for more effective rehabilitation paradigms. Whether neuromuscular electrical stimulation (NMES) could be used to prevent maladaptations in skeletal muscle size and function is unclear.
Purpose:
To examine whether early NMES use, started soon after injury and maintained through 3 weeks post surgery, can preserve quadriceps muscle size and contractile function at the cellular (ie, fiber) level in the injured versus the non-injured legs of ACLR patients.
Study Design:
Prospective, randomized, sham-controlled, blinded trial.
Methods:
Patients (n=25; 12 men/13 women) with acute, first-time ACL rupture were randomized to NMES (5 days/week) or sham (simulated microelectrical neural stimulation; 5 days/week) treatments to the quadriceps of their injured leg. Bilateral biopsies of the vastus lateralis were performed 3 weeks post surgery to measure skeletal muscle fiber size and contractility. Quadriceps muscle size and strength were assessed 6 months post surgery.
Results:
Twenty-one patients (9 men/12 women) completed the trial. ACLR reduced single muscle fiber size and contractility across all fiber types (P<0.01 to 0.001) in the injured compared to the non-injured leg at 3 weeks post surgery. NMES reduced muscle fiber atrophy (P<0.01) through effects on fast-twitch, myosin heavy chain (MHC) II fibers (P<0.01 to P<0.001). NMES preserved contractility in slow-twitch, MHC I fibers (P<0.01 to P<0.001), increasing maximal contractile velocity (P<0.01) and preserving power output (P<0.01), but not in MHC II fibers. Differences in whole muscle strength were not discerned 6 months post surgery.
Conclusion:
Early NMES use reduces skeletal muscle fiber atrophy in MHC II fibers, and preserves contractility in MHC I fibers. These results provide seminal, cellular level data demonstrating the utility of early use of NMES to beneficially modify skeletal muscle maladaptations to ACLR.
Clinical relevance:
Our results provide the first comprehensive, cellular level evidences to show that early use of NMES mitigates early skeletal muscle maladaptations to ACLR.
Keywords: knee, ACL, rehabilitation, weakness
Brief summary:
This is the first randomized, sham-controlled, blinded trial to comprehensively examine the effects of neuromuscular electrical stimulation to preserve skeletal muscle size and function using assessments at the cellular (ie, single muscle fiber) level. Our results provide seminal evidence that early use of NMES following the index injury and post surgery mitigates skeletal muscle atrophy and contractile dysfunction.
INTRODUCTION
Anterior cruciate ligament (ACL) injury and reconstruction (ACLR) promote muscle weakness that can persist for years despite rehabilitation.25, 31 Persistent muscle weakness contributes to dissatisfaction with ACLR16 and may hasten the progression of osteoarthritis,31 suggesting the need for improvements in current rehabilitation programs.
Neuromuscular electrical stimulation (NMES), which initiates muscle contraction by passing current through electrodes placed over muscles, is often used as an adjunct to rehabilitation programs to circumvent neural activation deficits following injury and surgery.20 However, more extensive use of NMES in a more proactive manner following the index injury and surgical repair may derive greater benefits, as NMES prevents muscle atrophy in orthopedic injury/surgical populations.10, 14, 21 Despite these benefits, clinicians may be reluctant to prescribe NMES, as its ability to prevent atrophy and improve strength remains controversial. For instance, when NMES is added as an adjunct to standard rehabilitation beginning several weeks post-surgery, no additive benefit to improve strength has been noted.26 A recent meta-analysis revealed that NMES is more effective at improving muscle strength and function when instituted earlier post-surgery.15 In this context, benefits of NMES to prevent muscle atrophy and weakness may be realized when it is instituted closer to periods of muscle disuse and trauma related to the ACL injury and surgical reconstruction.
Testing the efficacy of rehabilitation interventions instituted early, post-injury and post-surgery on muscle size and strength is complicated by clinical and practical limitations. Maximal or near maximal voluntary contractions are contraindicated due to the potential for further injury2 and are unreliable because of neural activation deficits.20 To circumvent these problems, we can study muscle at the cellular (ie, fiber) level. Fundamentally, whole muscle strength is a function of the size and contractility of its constituent fibers. Muscle fiber size and contractility, in turn, are determined by the quantity and type of myofilament proteins expressed (eg, myosin and actin) and their contractility. As myofilament proteins are the end effectors of contraction,11 any change in their quantity and/or contractility modifies the base functionality of the muscle.11 Accordingly, muscle fiber size and contractility set absolute functional limits on muscle, making them useful outcome measures to test the efficacy of early rehabilitation interventions.
Our primary objective was to evaluate whether NMES, instituted soon after injury and maintained through 3 weeks post ACLR, can preserve skeletal muscle fiber size and contractility. We assessed muscle fiber size and contractility becasuse, as detailed above, they are important determinants of whole muscle function, and methodological and practical limitations in assessing whole skeletal muscle size and functionality at this early post-surgery time point using more conventional tissue imaging and whole muscle functional assays. We hypothesized that early use of NMES post-injury and post-surgery would mitigate muscle fiber atrophy and prevent contractile dysfunction secondary to disuse and trauma related to both the index injury and surgery. A secondary objective was to explore whether early NMES use enhances whole muscle strength at later time points.
MATERIALS AND METHODS
This study was designed as a prospective, randomized, sham-controlled, blinded trial (NCT02945553), where data were available in each patient on both their injured, surgical leg and their contralateral, non-injured limb. For a priori sample size calculations, using published21 and preliminary data in other orthopedic surgical patients, we expected reductions in the injured leg in muscle fiber cross-sectional area (CSA) of −30% and −40% in myosin heavy chain (MHC) I and MHC II fibers, respectively, and maximal isometric tension (−50%) and contractile velocity (−30%) in MHC II fibers. From data in prior publications8, 10, 14 and our preliminary work, we hypothesized that NMES would prevent atrophy in MHC I (0%) and MHC II (−10%) fibers and reduce losses in isometric tension (−40%) and increase contractile velocity (+20%) in MHC II fibers. Detection of group differences in muscle fiber CSA drove the final cohort size, requiring n=20 with a power of 87% at an alpha of 0.05. We planned to enroll 24 patients, assuming ~20% loss to follow-up.
Patient recruitment, enrollment and randomization
Enrollment started in November 2016 and ended in December 2018, with last patient, last follow-up visit in July 2019. A total of 79 patients with an acute, first-time ACL rupture were screened (Figure 1). Inclusion criteria included: 1) 18–50 years of age; 2) body mass index <35 kg/m2 ; 3) acute (<3 weeks from injury), first-time, ACL rupture with or without meniscus injury and 4) scheduled to undergo surgical reconstruction with any type of graft material. Exclusion criteria included: 1) history of knee/lower extremity surgery; 2) abnormal laxity of any knee ligament at surgery other than the injured ACL; 3) signs or symptoms of arthritis, autoimmune/inflammatory disease or diabetes; 4) grade IIIb or greater articular cartilage lesions (ICRS criteria) to the tibiofemoral joint and/or 5) women who are/plan on becoming pregnant.
Figure 1.
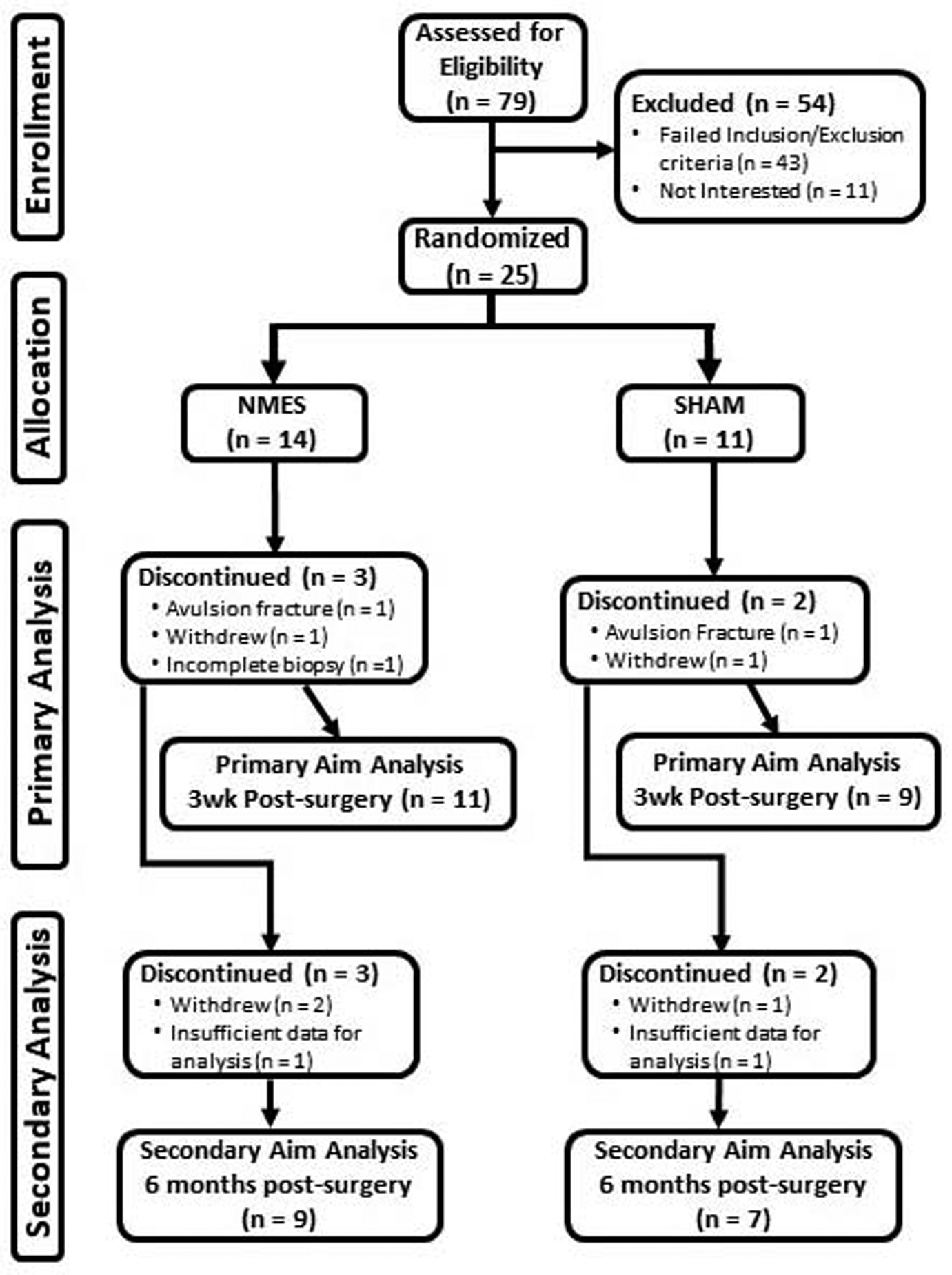
CONSORT diagram.
Patients were randomized (1:1) using a covariate adaptive approach to receive NMES or sham, simulated microelectrical neural stimulation (MENS) interventions, with stratification for age, sex and graft material. Two unblinded study personnel enrolled volunteers, assigned them to intervention arms and trained them to use NMES or sham MENS. All personnel performing assessments on patients/tissue or responsible for analysis of data were blinded to group assignment. Of the 25 (12 men/13 women) patients enrolled and randomized (14 NMES/11 sham MENS), 20 (11 NMES/9 sham MENS) completed 3-week, post-surgery testing and were included in primary analyses (single fiber size and contractility), and 16 (9 NMES/7 sham MENS) completed 6-month, post-surgery testing with data for secondary analyses (whole muscle strength). All protocols were approved by our institutional ethics committee and written informed consent obtained from each volunteer prior to participation.
Protocol
Patients were evaluated twice pre surgery, at the time of enrollment and within 1 week pre surgery, and 3 weeks and 6 months post surgery. Pre-surgery testing at enrollment consisted of muscle strength on the non-injured leg and clinical and patient-oriented assessments. Testing one week pre surgery was identical, but included bilateral muscle strength testing. Weight-bearing physical activity was measured by accelerometry for three 5-day periods both between enrollment and surgery and during the first 3 weeks post surgery. At 3 weeks post surgery, bilateral, percutaneous biopsies were taken from the vastus lateralis muscles and computed tomography performed. For one 5-day period during post-surgery months 2 through 5, weight-bearing activity was assessed by accelerometry. At 6 months post-surgery, whole muscle strength and size were measured bilaterally, whole leg function by single leg hop and clinical and patient-oriented assessments performed.
Treatment interventions
Patients randomized to NMES on the quadriceps of their injured/operative leg (Empi Continuum; EMPI Inc., Clear Lake, SD) began the intervention within 3 weeks of injury and continued until 3 weeks post surgery. NMES was discontinued just prior to surgery and resumed within 72 hours post-surgery. Patients performed NMES at home 5 days/week, 60 minutes/day (5-minute warm-up, 50-minute stimulation session and 5-minute cool down). The device administered symmetrical, biphasic pulses (400 μs at 50 Hz), with a duty cycle of 25% (10 seconds on, 30 seconds off), with parameters based on our prior work.30 Electrode pads (7.5 × 13.5 cm) were placed horizontally on the proximal and distal aspects of the quadriceps. A bolster was placed under the knee joint and an ankle weight used to immobilize the leg at ~40° relative to full knee extension,2 with the goal of obtaining tetanic isometric contractions within pain tolerance.
To remove bias of patients anticipating a benefit from an active treatment, patients randomized to sham MENS (EMPI 300PV; Supplemental Figure 1) performed sham stimulation (ie, device administered no electrical current) and were told that the device administered imperceptible, MENS for pain mitigation. Patients in the NMES group were told that, in addition to causing contraction, NMES had pain mitigating effects to distribute any placebo effect equally across groups. Volunteers performed sham MENS at home 5 days/week, 60 minutes/day.
Each volunteer demonstrated proficiency using their device while supervised by unblinded study personnel and was contacted within 72 hours of training to ensure proficiency, with regular weekly contacts thereafter to encourage adherence and assess problems. Adherence to NMES/sham MENS prescriptions was tracked with the compliance monitoring feature of the device software.
All volunteers underwent ACLR rehabilitation with similar goals and benchmarks, as described.3 Restrictions post-surgery were weight bearing to tolerance, except for patients with meniscal repairs, where it was 50% weight bearing with crutches for 4 weeks post-surgery, progressing to partial weight bearing with one crutch through week 5 and full weight bearing by week 6. One patient with a meniscus root avulsion was non-weight bearing for 6–8 weeks following surgical repair.
Knee extensor muscle function
Isometric (70°) and isokinetic (60 and 180 °/sec) peak torque about the knee were evaluated using a HUMAC/NORM 770 dynamometer (CSMi, Stoughton, MA), as described previously31 and in detail in the on-line supplement.
Physical activity
Weight-bearing activity was measured with a uniaxial accelerometer (Actigraph GT1M; ActiGraph Co., Pensacola, FL) using raw activity counts, with details described in the on-line supplement.
Thigh muscle size
Thigh muscle tissue cross-sectional areas (CSA) were measured by computerized tomography (CT), as described previously4 and in detail in the on-line supplement.
Muscle biopsy
Percutaneous biopsies of the vastus lateralis (VL) muscles were performed bilaterally, as described12 and detailed in the on-line supplement. A portion of muscle was frozen in embedding medium (OCT; Sakura, Torrance, CA) and stored at −80°C until analysis. Another portion of the sample was placed immediately into cold (4°C) dissecting solution, dissected into small fiber bundles, chemically-skinned and then stored for single fiber contractile assessments, with procedures and solutions as described.5
Muscle fiber size
Single muscle fiber size was assessed using immunohistochemistry, as described previously22 and detailed in the on-line supplement. We chose to assess skeletal muscle cross-sectional area because it is the base determinant of overall muscle size, which is an essential determinant of whole muscle functionality. More specifically, single muscle fiber cross-sectoinal area provides an index of the content of myofilaments, which are the end effectors of contraction and, as such, a fundamental determinant of muscle functionality.
Muscle fiber contractility
Segments (~2–3 mm) of chemically-skinned fibers were isolated and cellular level contractility measured (25°C) within 3 weeks of the biopsy, as described previously5 and in detail in the on-line supplement. Measurements were conducted under maximal Ca2+-activated conditions and included maximal isometric force production (Fmax), maximal isometric tension (Tmax; force production per fiber CSA), maximum shortening velocity (Vmax) and maximum power production (Pmax) using isotonic load clamps. These indices represent the basic mechanical/contractility indices that describe the functional patency of skeletal muscle and assessment of these indices in the chemically-skinned single fiber preparation provides a clear indication of the functionality of myofilament proteins that have historically been used in the muscle physiology field and shown to determine whole muscle functionality.18 All fibers were analyzed for determination of MHC isoform composition by gel electrophoresis, as described previously23 and in detail in the on-line supplement.
Lower extremity function and patient- and clinician-reported outcomes
Single leg hop tests were conducted, as described31 and detailed in the on-line supplement. Patient-reported assessments of their knee were performed using the Knee Injury and Osteoarthritis Outcome Score (KOOS)28 and International Knee Documentation Criteria (IKDC) 2000 subjective score.17 Knee range of motion was measured by goniometry with the subject supine and a knee exam performed according to the IKDC 2000 knee examination form.17
Statistics
For variables with multiple observations within an individual (eg, fiber size and contractility), repeated measures mixed model analyses of variance were used with leg (injured and non-injured) and treatment group (sham and NMES) as factors. Analyses were limited to fibers expressing MHC I, MHC IIA and MHC IIA/IIX because other MHC isotype fibers (I/IIA, I/IIA/IIX and IIX) were too few to permit analyses. Whole muscle CSA and strength data were expressed as injured relative to non-injured leg, with the former evaluated using unpaired t-tests and the latter using analyses of covariance, with quadriceps CSA as a covariate. Strength in the non-injured leg over time was evaluated using a repeated measures analyses of variance, with time and group as factors. Data were analyzed using SAS (v9.4; SAS Institute, Cary, NC) or SPSS (v23; IBM, Armonk, NY) and presented as mean ± SE.
RESULTS
Patient characteristics and interventions
Treatment groups were similar for sex distribution, age, height, weight and did not differ for time on study or time between milestones (Table 1). Data are not shown for two subjects who withdrew after enrollment and two who were excluded (3 men/1 woman). For primary aim analysis, two patients in the NMES group had insufficient muscle tissue to allow fiber contractility assessments in one leg. For secondary aim analysis, 3 participants withdrew prior to 6-month testing and 2 lacked whole muscle size data because of technical or scheduling problems. Additionally, 1 patient was not medically cleared to, and 2 others chose not to, complete single leg hop testing. No adverse events or unanticipated problems occurred with NMES or sham interventions. Adherence in the NMES group was 85 ± 5% (range: 42–100%) and 63 ± 8% (range: 19–98%) in the sham group. The intervention period was similar in NMES (58 ±7 days) and sham (57 ± 7 days) groups. Of note, we did not restrict individual therapists treatment of volunteers to exclude electrotherapy (eg, NMES, TENS), but asked volunteers to report any use of these modalities. No patient reported receiving electrotherapy outside of that provided by study interventions.
Table 1.
Physical and clinical characteristics.
| SHAM | NMES | P-value | |
|---|---|---|---|
| N (M/F) | 4/5 | 6/6 | |
| Age (yr) | 24 ± 3 | 25 ± 2 | 0.894 |
| Weight (kg) | 69 ± 4 | 74 ± 3 | 0.366 |
| Height (cm) | 171 ± 3 | 175 ± 3 | 0.469 |
| Injury type | |||
| ACL rupture only (n) | 3 | 3 | NA |
| ACL rupture and meniscus injury (n) | 1 | 3 | NA |
| ACL rupture and cartilage injury (n) | 4 | 2 | NA |
| ACL rupture and cartilage and meniscus injury (n) | 1 | 4 | NA |
| Meniscus repair (n) | 1 | 4 | NA |
| Graft type | |||
| BPTB autograft (n) | 7 | 10 | NA |
| BPTB allograft (n) | 1 | 2 | NA |
| Hamstring autograft (n) | 1 | 0 | NA |
| Tourniquet time (min) | 54 ± 7 | 56 ± 8 | 0.843 |
| Anesthesia | |||
| General (n) | 7 | 11 | NA |
| General + Femoral nerve block (n) | 1 | 1 | NA |
| Spinal (n) | 1 | 0 | NA |
| Injury to enrollment (d) | 12 ± 1 | 13 ± 2 | 0.700 |
| Injury to surgery (d) | 47 ± 6 | 52 ± 6 | 0.519 |
| Surgery to biopsy (d) | 23 ± 1 | 23 ± 1 | 0.980 |
| Return to sport/activity 6-months post-surgery (Yes/No) | 5/2 | 3/6 | NA |
Data are mean ± SE or n, as indicated. Note that for return to sport/activity data 6-months post-surgery, n=7 and 9 for sham and NMES groups to reflect those patients who completed strength and/or functional testing at that time. BPTB, bone patellar tendon bone. NA, these variables were not compared statistically.
Physical activity
No group differences were found in physical activity level pre-surgery or during the first 3 weeks post surgery, or the slope of the activity/time relationship from 1 to 5 months post surgery (Table 2).
Table 2.
Physical activity measured by accelerometry.
| Variable | SHAM | NMES | P-value (n/n) |
|---|---|---|---|
| Pre-surgery (counts) | 195,099 ± 29,299 | 205,704 ± 37,786 | 0.830 (9/10) |
| 3 weeks post-surgery (counts) | 124,027 ± 21,454 | 86,727 ± 14,578 | 0.155 (7/11) |
| Slope of physical activity counts from month 1 through month 5 post surgery (counts/month) | 904 ± 221 | 987 ± 286 | 0.775 (7/9) |
Data are mean ± SE. Units for accelerometry are raw activity counts Sample sizes are variable because of missing data, as detailed in the Results.
Single muscle fiber size 3 weeks post ACLR
We found Leg effects (ie, comparison between injured and non-injured legs; all P<0.001) for lower muscle fiber CSA in the injured leg for all fibers types (Figure 2). Additionally, we observed Leg × Group interaction effects for single fiber CSA for all fiber types pooled (P<0.01) and in MHC IIA (P<0.01) and IIA/IIX (P<0.001) fibers. The interaction effect was driven by reduced atrophy in the NMES group, although atrophy remained apparent in both groups (P<0.001 to P<0.01).
Figure 2.
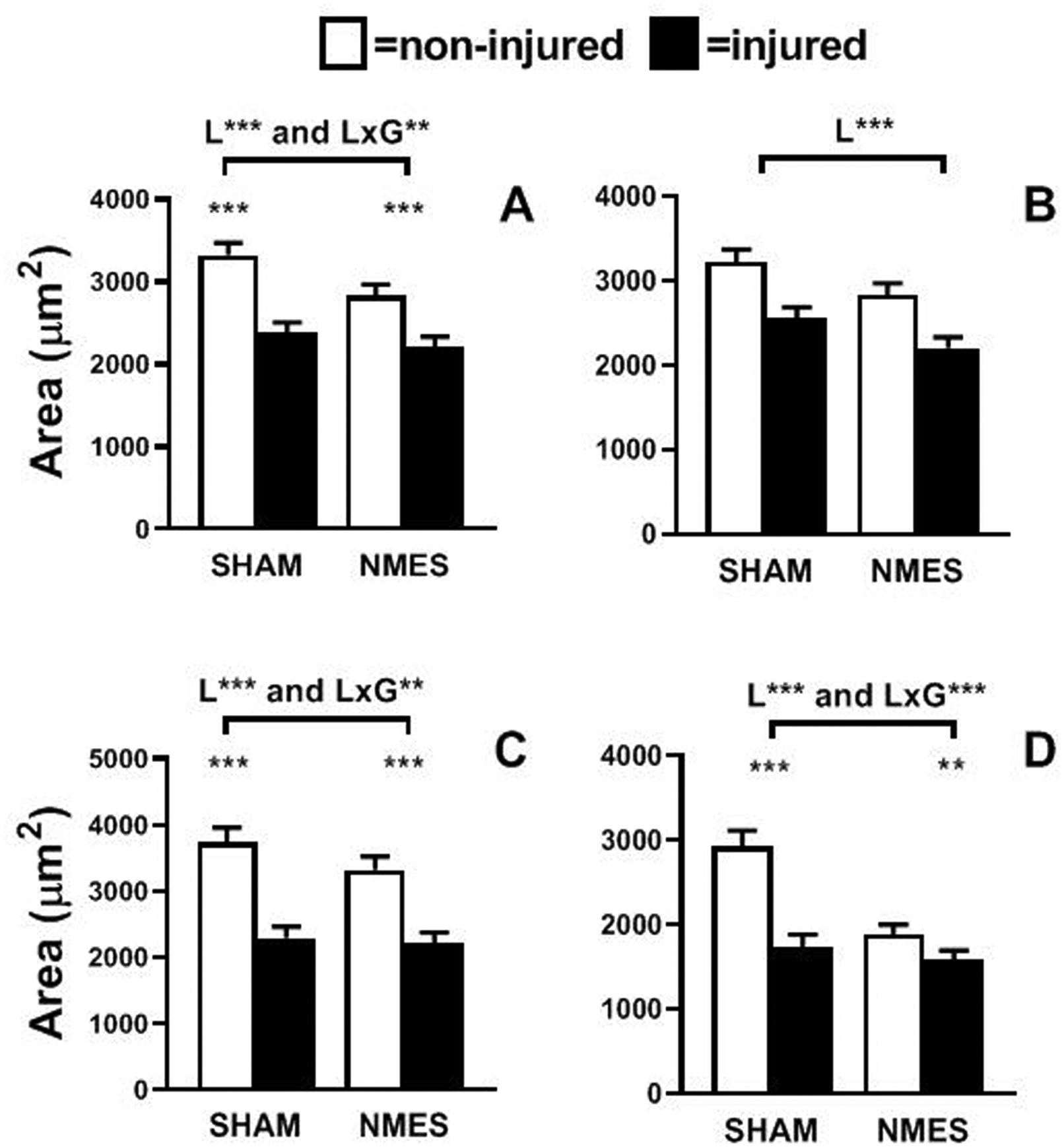
Skeletal muscle fiber size in pooled (A), MHC I (B), MHC IIA (C) and MHC IIA/IIX (D) fibers in non-injured (clear) and injured (filled) legs of volunteers randomized to simulated microelectrical neural stimulation (SHAM) and neuromuscular electrical stimulation (NMES) interventions. Leg (L) and leg by group (L × G) interaction effects are indicated above the brackets. Pairwise analysis of differences between legs, where L × G interaction effects were apparent, are indicated by symbols directly above bars. Data represent mean ± SE. **, P<0.01 and ***, P<0.001.
Single muscle fiber contractility 3 weeks post ACLR
Leg effects were found for lower muscle fiber Fmax in the injured leg for all major fiber types (MHC I: P<0.01; IIA: P<0.001; and IIA/IIX: P<0.001; Table 3). Given fiber atrophy (Figure 2), we evaluated whether deficits in Fmax were explained by atrophy by normalization to CSA (ie, Tmax). Leg effects were found for lower muscle fiber Tmax (P<0.001) in the injured leg across fiber types, with no leg × group interaction effects for Fmax (MHC I: P=0.14; MHC IIA: P=0.50; MHC IIA/IIX: P=0.49) or Tmax (MHC I: P=0.37; MHC IIA: P=0.63; MHC IIA/IIX: P=0.66).
Table 3.
Skeletal muscle fiber function at 3 weeks post surgery.
| Variable | SHAM control (n=9) | NMES (n=10) | Effect | ||
|---|---|---|---|---|---|
| Non-Injured | Injured | Non-Injured | Injured | ||
| MHC I | |||||
| Fmax (mN) | 0.600 ± 0.098 | 0.363 ± 0.089 | 0.877 ± 0.120 | 0.328 ± 0.106 | Leg ** |
| Tmax (mN/mm2) | 127 ± 5 | 106 ± 5 | 126 ± 5 | 109 ± 5 | Leg *** |
| V max (ML/s) | 1.29 ± 0.10 | 1.13 ± 0.09** | 1.04 ± 0.10 | 1.20 ± 0.10** | Leg × Group*** |
| Pmax (mN/mm2 ML/s) | 0.107 ± 0.005 | 0.100 ± 0.005** | 0.096 ± 0.005 | 0.100 ± 0.005 | Leg × Group ** |
| MHC IIA | |||||
| Fmax (mN) | 1.000 ± 0.058 | 0.464 ± 0.061 | 0.985 ± 0.056 | 0.478 ± 0.057 | Leg *** |
| T max (mN/mm2) | 175 ± 7 | 137 ± 8 | 169 ± 7 | 127 ± 7 | Leg *** |
| Vmax (ML/s) | 1.95 ± 0.09 | 1.74 ± 0.10 | 1.92 ± 0.09 | 1.67 ± 0.09 | Leg *** |
| Pmax (mN/mm2 ML/s) | 0.209 ± 0.007 | 0.194 ± 0.008 | 0.205 ± 0.007 | 0.183 ± 0.007 | Leg *** |
| MHC IIAX | |||||
| Fmax (mN) | 0.799 ± 0.087 | 0.372 ± 0.097 | 0.738 ± 0.079 | 0.368 ± 0.082 | Leg*** |
| Tmax (mN/mm2) | 160 ± 10 | 127 ± 12 | 154 ± 9 | 114 ± 9 | Leg *** |
| Vmax (ML/s) | 1.87 ± 0.14 | 1.67 ± 0.16 | 1.83 ± 0.13 | 1.50 ± 0.13 | Leg ** |
| Pmax (mN/mm2 ML/s) | 0.233 ± 0.012 | 0.193 ± 0.014 | 0.230 ± 0.011 | 0.193 ± 0.011 | Leg *** |
Data are mean ± SE.
P<0.01;
P<0.001.
For Leg × Group interaction effects, symbols indicating pairwise differences between legs are placed on the value for the injured leg. n=10 in the NMES group because of inadequate tissue.
Leg effects for Vmax were observed for MHC IIA (P<0.001) and MHC IIA/IIX (P<0.01) fibers, reflecting reductions in both fiber types in the injured leg (Table 3). Leg × group effects were found for Vmax in MHC I fibers. Pairwise comparisons showed that this interaction effect reflected a reduction in sham (P<0.001) and an increase in NMES (P<0.001).
Finally, leg effects for Pmax were found for MHC IIA and IIA/IIX fibers (both P<0.001), reflecting reduced Pmax in both fiber types of the injured leg (Table 3). Leg × group effects were found for Pmax in MHC I fibers, with pairwise comparisons showing that this interaction effect was due to a reduction in sham (P<0.01).
Whole muscle size and function 6 months following ACLR
We observed no group by time interaction effects for any knee extensor muscle strength parameter throughout the study in the non-injured leg, (Supplemental Figure 2). We found no group differences in the ratio of injured to non-injured leg quadriceps muscle CSA at 3 weeks, post surgery (Supplemental Table 1), but the ratio was lower (P<0.05) in the NMES group 6 months post surgery. There were no group differences in knee flexor muscle CSA at 3 weeks or 6 months post surgery.
No group effects were found for isometric or isokinetic strength measurements 6 months post surgery when adjusting for quadriceps muscle CSA (Supplemental Table 2).
We found no group differences in patient- or clinician-reported outcomes or single leg hop performance (Supplemental Table 3) 6 months post surgery.
DISCUSSION
Our study is novel in starting NMES near the time of injury and continuing it into the early post-surgical period. This contrasts with the more common clinical use of NMES, as an adjunct to standard rehabilitation to address neural activation deficits. To our knowledge, this is the first study to provide mechanistic cellular data in the setting of a randomized, sham controlled, blinded trial that demonstrates the efficacy of NMES to beneficially modify both muscle fiber size and contractility.
Skeletal muscle fiber size
ACL injury and reconstruction caused marked muscle fiber atrophy (−26%), with more prominent atrophy in fast-twitch, MHC II fiber types (−29 to −35%). Considering this and reports that MHC II fibers do not regain size by the time patients return to activity,24 the effect of NMES to mitigate atrophy specifically in MHC II fiber types is clinically significant, as atrophy prevention is a goal of orthopedic rehabilitation. Of note, although baseline fiber size was higher in the non-injured leg in the sham group for some fiber types, this likely did not impact differences between legs, as baseline muscle fiber size in the non-injured leg did not correlate to the ratio of injured-to-non-injured muscle fiber size across fiber types (P-values: 0.838 to 0.345). To our knowledge, only one other study evaluated the effects of NMES on muscle fiber size in ACL-injured patients.1 In that study, NMES combined with isometric contractions over 6 weeks post surgery reduced muscle atrophy in women, but not men, compared to isometric contractions alone.1 However, the surgical leg was cast immobilized during the intervention, which is an outdated clinical practice that could possibly influence responsiveness to NMES. Similar to our results, NMES mitigates atrophy in patients undergoing total knee arthroplasty21 or in those with lower extremity fracture10 when started soon after surgery/casting. Thus, using NMES closer to the index injury and surgery may derive benefits in preventing muscle atrophy in patients undergoing ACLR.
Many studies show that NMES completely prevents muscle atrophy,8, 10, 21 although our data suggest a more modest anti-atrophic effect. One key difference is that most prior work evaluated whole muscle size, which could suffer bias from fluid infiltration into muscle, which would register as muscle tissue.27 The amount of fluid infiltration into muscle could vary among patients by surgical trauma/tissue edema, degree of muscle disuse or other factors. Indeed, one could speculate that NMES might modulate fluid content of muscle in patients, with effects in either direction: reducing fluid content via rhythmic contractions or increasing tissue fluid through contraction-induced muscle damage.9 Examples of this potential bias are shown in disagreements between whole muscle and cellular muscle size estimates in studies evaluating the effects of NMES in orthopedic and non-orthopedic populations,8, 10, 24 including the current study. Because of this potential bias, we urge caution in the interpretation of whole muscle size data and suggest single muscle fiber assessments as a more rigorous approach to evaluate the effects of NMES, particularly during the early, post-surgical period.
Skeletal muscle fiber function
ACL rupture and reconstruction reduced all single fiber contractile indices across all major fiber types, sometimes markedly. These results agree with recent studies that that show reduced single muscle fiber force production and tension in patients undergoin ACL reconstruction compared to non-injured control patients.13 Importantly, this study showed that these variables were reduced from the time of surgery until 6-months post-surgery, with a nadir at 1-month post-surgery. Together, these results suggest that the function of myofilament proteins (eg, myosin, actin, etc), the end effectors of muscle contraction, are severely impaired following injury and surgery. Accordingly, intrinsic muscle fiber contractility is a suitable a target for clinical rehabilitation. To this point, NMES prevented reductions in single muscle fiber Pmax and increased Vmax in MHC I fibers. We originally hypothesized that NMES would affect MHC II fibers, as they are thought to be more important determinants of whole muscle strength,18 and, in turn, gains in strength with NMES.15 MHC I fibers, however, may become a more prominent modulator of whole muscle function in patients with orthopedic conditions, as we have shown correlations between whole muscle function and single MHC I fiber function (Vmax) in older adults with knee osteoarthritis,6 and between MHC I fiber contractility 3 weeks post surgery and whole muscle isometric and isokinetic strength 6 months following surgery in a separate cohort of patients undergoing ACL reconstruction.(blinded for review, unpublished data) Indeed, MHC I fibers may limit whole muscle power output.18 Collectively, these results uncover a novel role for MHC I fiber contractility in whole muscle strength/performance in orthopedic populations, as well as NMES to benefit slow-twitch, MHC I fiber contractility in ACLR patients.
Whole muscle strength
We found no effect of NMES to alter isometic or isokinetic knee extensor torque 6 months post surgery. However, we urge caution in interpretation of these results, as loss of patients to follow-up meant we did not have adequate statistical power to detect differences in strength between groups. Additonally, because functional recovery, as reflected in the numbers of patients returning to sport/activity in both groups (Table 1), may have differed between the two groups. To this latter point, this may be due to the fact that extent of meniscal injury was not perfectly balanced between groups. Another reason for the failure to realize improvements in whole muscle strength is that our NMES regimen was only maintained till 3 weeks post ACLR. Recent recommendations29 suggest testing for neural activation deficits 3 weeks post surgery and continuing NMES in patients with persistent neural activation deficits.29 Accordingly, use of NMES longer than 3 weeks post surgery, or more frequently throughout our intervention, may have lead to improvements in whole muscle strength.15
Our study has important strengths, including: 1) carefully selected eligibility criteria to avoid confounding from prior knee injuries; 2) a sham, control intervention; 3) assessments of physical activity throughout the study and 4) measurements of quadriceps muscle at the cellular level to evaluate NMES effects in the early post-surgical period. However, there are limitations that must be acknowledged. First, the extent of concomitant meniscal injury was not perfectly balanced by randomization. More meniscal injuries and repairs in the NMES group could affect post-surgical weight bearing. While no group differences in physical activity were noted, mean activity was 30% lower in the NMES than the sham group during the first 3 weeks post surgery. If anything, this would bias against finding an effect of NMES on primary outcomes. That we still observed effects of NMES underscores the robustness of these effects. Group differences in meniscal pathology/repair and, in turn, weight bearing, could impact whole muscle strength measures at 6 months, albeit the effects of the degree of trauma on whole muscle function is controversial.19 Second, our study was not adequately powered to assess group differences in whole leg function (ie, single leg hop) or patient-reported outcomes/satisfaction because of patients lost from the 3 week to the 6 month post-surgery assessments. Third, effects of NMES to alter contractility of the uninjured limb7 could affect detection of group differences in whole muscle strength, although we found no group difference in knee extensor function in the non-injured leg soon after injury up to 6 months post-surgery. Finally, the generalizability of our results are limited to the confines of our cohort; namely, active patients with no history of significant knee trauma that suffered their first severe, acute ACL disruption that undergo ACLR with a bone-patellar tendon-bone graft (both auto- and allo-graft).
In conclusion, our study shows that both muscle fiber size and contractility were markedly reduced 3 weeks post surgery. These results are clinically relevant, as they suggest that both should be targeted by rehabilitation programs. Additionally, we found that early NMES following injury and surgery beneficially modifies adaptations in both muscle size and contractility at the cellular (ie, muscle fiber) level following ACL injury and reconstruction.While anti-atrophic effects align with clinical indications for NMES use, we provide novel evidence for effects of NMES on single fiber contractility. The functional implications of these beneficial effects of NMES in single muscle fiber size and function are unclear, as differences in whole muscle strength were not discerned 6 months following ACLR. Future trials are needed to determine whether these beneficial effects of NMES on intrinsic muscle fiber size and function early after ACLR, when subjects are rapidly advancing their rehabilitation, translate into improved whole muscle function, patient-reported outcomes or prevention of reinjury or progression towards post-traumatic knee osteoarthritis. Use of NMES over a longer time interval post-surgery, or more frequent daily/weekly use, may better maintain muscle size and strength and limit limb asymmetries when patients begin to return to sport and pre-injury activity levels and, in turn, possibly protect against future joint pathology.31
Supplementary Material
What is known about the subject:
Following ACL injury and reconstruction, most patients suffer muscle weakness that persists for years after surgery despite rehabilitation. Persistent muscle weakness contributes to patient dissatisfaction with surgery and hastens the onset and progression of post-traumatic osteoarthritis, suggesting the need for improvements in current rehabilitation programs. Prior trials of rehabilitation with NMES after ACL injury and reconstruction have had mixed results, possibly because treatment began too late following surgery and/or because whole muscle size and function assessments used to assess efficacy are hindereded by methodological and practical limitations. Whether early NMES can prevent atrophy and preserve muscle function following ACL trauma and reconstruction remains unclear.
What this study adds to existing knowledge:
This translational study provides novel insight regarding the effects of early use of NMES to address quadriceps muscle adaptations following ACL injury and reconstruction. While NMES is conventionally used clinically to address deficits in neural activation of quadriceps muscle post-surgery, results from this randomized, sham-controlled, blinded trial provide seminal, comprehensive evidence at the cellular level (ie, muscle fiber) that early use of NMES following the index injury and post surgery reduces muscle fiber atrophy and preserves muscle fiber contractility. These findings provide a strong mechanistic foundation that can serve to inform future trials designed to evaluate the effects of NMES to preserve neuromuscular function and forestall development of muscle weakness and lower extremity dysfunction that contribute to dissatisfaction with surgical treatment and future joint pathology.
Acknowledgements:
We thank all of the volunteers who dedicated their valuable time to these studies. This study was funded by a grant to MJT and BDB (AR069199).
Footnotes
Site where trial was conducted: University of Vermont College of Medicine
References
- 1.Arvidsson I, Arvidsson H, Eriksson E, Jansson E. Prevention of quadriceps wasting after immobilization: an evaluation of the effect of electrical stimulation. Orthopedics. 1986;9:1519–1528. [DOI] [PubMed] [Google Scholar]
- 2.Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renström PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23(1):24–34. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Johnson RJ, Naud S, et al. Accelerated versus nonaccelerated rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. Am J Sports Med. 2011;39(12):2536–2548. [DOI] [PubMed] [Google Scholar]
- 4.Callahan DM, Bedrin NG, Subramanian M, et al. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex-specific: relationship to single fiber function. J Appl Physiol. 2014;116(12):1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan DM, Miller MS, Sweeny AP, et al. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol. 2014;592(20):4555–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan DM, Tourville TW, Slauterbeck JR, et al. Reduced rate of knee extensor torque development in older adults with knee osteoarthritis is associated with intrinsic muscle contractile deficits. Exp Gerontol. 2015;72:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattagni T, Lepers R, Maffiuletti NA. Effects of neuromuscular electrical stimulation on contralateral quadriceps function. J Electromyogr Kinesiol. 2018;38:111–118. [DOI] [PubMed] [Google Scholar]
- 8.Dirks ML, Wall BT, Snijders T, Ottenbros CLP, Verdijk LB, van Loon LJC. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. 2013;210(3):628–641. [DOI] [PubMed] [Google Scholar]
- 9.Fouré A, Le Troter A, Ogier AC, Guye M, Gondin J, Bendahan D. Spatial difference can occur between activated and damaged muscle areas following electrically-induced isometric contractions. J Physiol. 2019;597(16):4227–4236. [DOI] [PubMed] [Google Scholar]
- 10.Gibson JNA, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet. 1988;332(8614):767–770. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80(2):853–924. [DOI] [PubMed] [Google Scholar]
- 12.Guigni BA, Callahan DM, Tourville TW, et al. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am J Physiol Cell Physiol. 2018;315(5):C744–C756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumucio JP, Sugg KB, Enselman ERS, et al. Anterior cruciate ligament tear induces a sustained loss of muscle fiber force production. Muscle Nerve. 2018;58(1):145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasegawa S, Kobayashi M, Arai R, Tamaki A, Nakamura T, Moritani T. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction. J Electromyogr Kinesiol. 2011;21(4):622–630. [DOI] [PubMed] [Google Scholar]
- 15.Hauger AV, Reiman MP, Bjordal JM, Sheets C, Ledbetter L, Goode AP. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg Sports Trauma Arthro. 2018;26(2):399–410. [DOI] [PubMed] [Google Scholar]
- 16.Ingelsrud LH, Granan L-P, Terwee CB, Engebretsen L, Roos EM. Proportion of patients reporting acceptable symptoms or treatment failure and their associated KOOS values at 6 to 24 months after anterior cruciate ligament reconstruction: a study from the Norwegian Knee Ligament Registry. Am J Sports Med. 2015;43(8):1902–1907. [DOI] [PubMed] [Google Scholar]
- 17.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 18.Ivy JL, Withers RT, Brose G, Maxwell BD, Costill DL. Isokinetic contractile properties of the quadriceps with relation to fiber type. Eur J Appl Physiol Occup Physiol. 1981;47(3):247–255. [DOI] [PubMed] [Google Scholar]
- 19.Lepley LK, Wojtys EM, Palmieri-Smith RM. Does concomitant meniscectomy or meniscal repair affect the recovery of quadriceps function post-ACL reconstruction? Knee Surg Sports Trauma Arthro. 2015;23(9):2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisee C, Lepley AS, Birchmeier T, O’Hagan K, Kuenze C. Quadriceps strength and volitional activation after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Sports Health. 2019;11(2):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin TP, Gundersen LA, Blevins FT, Coutts RD. The influence of functional electrical stimulation on the properties of the vastus lateralis fibers following total knee arthroplasty. Scand J Rehabil Med. 1991;23(4):207–210. [PubMed] [Google Scholar]
- 22.Miller MS, Callahan DM, Tourville TW, et al. Moderate-intensity resistance exercise alters skeletal muscle molecular and cellular structure and function in inactive, older adults with knee osteoarthritis. J Appl Physiol. 2017;122(4):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MS, VanBuren P, LeWinter MM, et al. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2(6):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noehren B, Andersen A, Hardy P, et al. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. J Bone Joint Surg. 2016;98(18):1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sport Med. 2008;27(3):405–424. [DOI] [PubMed] [Google Scholar]
- 26.Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: A randomized clinical trial with an imbedded prospective cohort. Arthritis Care Res. 2009;61(2):174–183. [DOI] [PubMed] [Google Scholar]
- 27.Pieterobelli A, Formica C, Wang Z, Heymsfield SB. Dual energy x-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271(6 Pt 1):E941–E951. [DOI] [PubMed] [Google Scholar]
- 28.Roos EM, Roos HP, Lohmander LS, Ekdahl CE, Beynnon BD. Knee injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 29.Spector P, Laufer Y, Elboim Gabyzon M, Kittelson A, Stevens-Lapsley JE, Maffiuletti NA. Neuromuscular electrical stimulation therapy to restore quadriceps muscle function in patients after orthopaedic surgery: a novel structured approach. J Bone Joint Surg AM. 2016;98(23):2017–2024. [DOI] [PubMed] [Google Scholar]
- 30.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther. 2012;92(2):210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tourville TW, Naud S, Slauterbeck JS, Johnson RJ, Beynnon BD. Relationship between isokinetic strength and tibiofemoral joint space width changes following ACL reconstruction. Am J Sports Med. 2014;42(2):302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


