Abstract
STUDY QUESTION
Which transcriptomic alterations in mid-luteal endometrial scratch biopsies, taken prior to the assisted reproductive treatment (ART) treatment cycle are associated with unsuccessful pregnancy?
SUMMARY ANSWER
Dysregulated interleukin-17 (IL-17) pathway components are demonstrated in women who fail to become pregnant after ART.
WHAT IS KNOWN ALREADY
Implantation failure is now recognised as a critical factor in unexplained infertility and may be an important component of failed ART.
STUDY DESIGN, SIZE, DURATION
Using a prospective longitudinal study design, 29 nulliparous women with unexplained infertility undergoing ART were recruited between October 2016 and February 2018. Mid-luteal stage endometrium and matched serum samples were collected, and patients underwent a single embryo transfer in the subsequent cycle. RNA-seq analysis of endometrial biopsies was performed on the discovery cohort (n = 20).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Gene set enrichment analysis of the differentially expressed genes (DEGs) was performed. Endometrium and serum were then prepared for IL-17A analysis by ELISA.
MAIN RESULTS AND THE ROLE OF CHANCE
There were 204 differentially expressed protein-coding genes identified in tissue from women who became pregnant (n = 9) compared with tissue from women who failed to become pregnant (n = 11) (false discovery rate; P < 0.05). Of the 204 DEGs, 166 were decreased while 38 were increased in the pregnant compared to the non-pregnant groups. Gene set enrichment analysis of the DEGs identified an over-representation of IL-17 and Pl3K-Akt signalling pathways. All the DEGs within the IL-17 signalling pathway (MMP3, MMP1, IL1β, LCN2, S100A9 and FOSL1) demonstrated decreased expression in the pregnant group. Serum IL-17 protein levels were increased in the non-pregnant discovery cohort (n = 11) and these findings were confirmed a validation cohort (n = 9).
LIMITATIONS, REASONS FOR CAUTION
Limitations of our study include the cohort size and the lack of aneuploidy data for the embryos; however, all embryos transferred were single good or top-quality blastocysts.
WIDER IMPLICATIONS OF THE FINDINGS
These findings demonstrate dysregulated IL-17 pathway components in women who fail to become pregnant after ART. Elevated serum levels of the pro-inflammatory cytokine IL-17 may predict failure of ART in women with unexplained infertility. Future trials of anti-IL-17 therapies in this cohort warrant further investigation.
STUDY FUNDING/COMPETING INTEREST(S)
Funding from the UCD Wellcome Institutional Strategic Support Fund, which was financed jointly by University College Dublin and the SFI-HRB-Wellcome Biomedical Research Partnership (ref 204844/Z/16/Z), is acknowledged. The authors have no competing interests.
TRIAL REGISTRATION NUMBER
NA.
Keywords: assisted reproductive treatment, endometrium, interleukin-17, unexplained infertility, RNA-seq
Introduction
Unexplained infertility is defined as failure to establish a clinical pregnancy after 12 months, in couples with apparently normal ovarian function, fallopian tubes, uterus, cervix and pelvis and with adequate coital frequency, as well as apparently normal testicular function, genito-urinary anatomy and a normal ejaculate (Zegers-Hochschild et al., 2017). However, the diagnosis is dependent upon the methodologies used and some pathologies may not be apparent, such as mild endometriosis in cases where laparoscopy is not performed. Nevertheless, it is estimated that a standard fertility evaluation will fail to identify an abnormality in approximately15–30% of infertile couples (Gelbaya et al., 2014).
Embryo implantation is a critical step in the reproductive process and requires a receptive endometrium, a normal functional embryo and a synchronised dialogue between maternal and embryonic tissues (Simon et al., 2000). Implantation failure is now recognised as a critical factor in infertility and early miscarriage, particularly in couples with idiopathic or unexplained infertility and it may also be an important component of failed assisted reproductive treatment (ART) (Macklon, 2017). Improved embryo selection techniques, including developmental morphokinetics and preimplantation genetic testing (Sigalos et al., 2016), mean that an improved understanding of the endometrial factors that mediate receptivity and embryo implantation are now required to improve ART success rates.
Endometrial receptivity is defined as a temporary unique state that makes the endometrium receptive to embryonic implantation (Bergh and Navot, 1992). It is mediated by complex and coordinated hormonal, cytokine and chemokine signalling pathways. These pathways facilitate embryo adhesion during the window of implantation and subsequent decidualisation in preparation for pregnancy (Gellersen et al., 2007). Embryo implantation is suggested to have evolved from an ancestral inflammatory attachment reaction (Griffith et al., 2017). This complex, dynamic inflammatory process is likely regulated by several genes and networks. Embryo-derived trophoblast cells invade the endometrium, initiating a pro-inflammatory response (Van Sinderen et al., 2013). Maintenance of an optimal, balanced inflammatory state is likely necessary for successful implantation to occur (Maybin et al., 2011). Uterine dendritic cells and macrophages release both pro- and anti-inflammatory cytokines that co-ordinate endometrial remodelling during implantation. Pro-inflammatory cytokines, such as interleukin-6 (IL-6), are involved in the implantation process by attracting human trophoblast cells (Dekel et al., 2014). Likewise, anti-inflammatory cytokines such as transforming growth factor beta (TGFβ1) are required to regulate this process, in order to drive and maintain the optimal inflammatory balance (Liang et al., 2015).
Women with active autoimmune disorders such as rheumatoid arthritis have an increased ‘time to pregnancy’ interval, which has been attributed to dysregulation of inflammatory mediators (Brouwer et al., 2015). It is therefore suggested that preconception treatment strategies should aim for maximum suppression of disease activity to improve fertility in rheumatoid arthritis and other autoimmune conditions, such as inflammatory bowel disease (Brouwer et al., 2015; Glover et al., 2016). Similarly, dysregulated inflammation in the eutopic endometrium associated with endometriosis is known to alter endometrial receptivity, which may result in infertility (Lessey and Kim, 2017).
Previous studies of endometrial dysfunction in infertility have identified specific transcriptomic signatures associated with recurrent implantation failure (RIF) and pregnancy loss (Huang et al., 2017). RIF is associated with dysregulated expression of genes involved in cell division, cytoskeleton, cilia formation (Ruiz-Alonso et al., 2013), circadian rhythm, complement and coagulation cascade and inflammation (Bastu et al., 2019) in endometrial tissue from women who fail to become pregnant. Endometrial injury or ‘scratching’ has been hypothesised to support implantation and successful pregnancy due to stimulation of inflammatory and vascular reactions similar to those occurring in decidualisation and trophoblast invasion (Zhou et al., 2008; Almog et al., 2010). However, recent evidence suggests that endometrial scratching is not associated with any improvement in live birth when performed prior to ART (Lensen et al., 2019). However, the findings indicate a lack of clarity regarding the role of inflammation in successful implantation and further support the possibility of a constitutive defect in endometrial function as an important determinant of implantation failure. The impact of dysregulated endometrial gene expression on pregnancy outcomes in unexplained infertility remains unknown. In this study, we therefore investigated endometrial transcriptomic changes in women presenting with unexplained infertility, and sought to identify biomarkers to predict pregnancy outcome following ART.
Materials and methods
Patient recruitment
This was a prospective cohort study. Women with unexplained infertility (ICMART 2017; Zegers-Hochschild et al., 2017) were recruited at Merrion Fertility Clinic, Dublin, Ireland between October 2016 and February 2018. Those scheduled for an endometrial scratch were asked to allow use of the scratch sample for research. The study includes two serial cohorts of patients who underwent ART following endometrial scratch: discovery (n = 20) and validation (n = 9) cohorts. Inclusion criteria included women with unexplained infertility, age <38 years, no previous pregnancy (including no miscarriage), regular menstrual cycles (25–35 days), no steroid hormone use within the preceding 3 months, a normal transvaginal ultrasound scan, normal test of tubal patency and normal semen analysis in the partner. The clinical policy is to recommend laparoscopy if there are symptoms or ultrasound evidence of endometriosis or other pelvic pathology, otherwise a hysterosalpingogram is performed. Of the entire cohort, two women (6.9%) had a laparoscopy preceding the study and both were normal. The remaining cohort (n = 27) had no clinical features suggestive of endometriosis. Exclusion criteria included smoking, systemic disease, known cases of endometriosis, regular medication use and body mass index (BMI) ≥30 kg/m2.
Sample collection
Endometrial tissue was sampled using a pipelle de Cornier endometrial sampler (Pipelle®). Samples were taken at a defined stage of the menstrual cycle: mid-luteal, LH+7. Urinary luteinising hormone (LH) (One Step®, Home Health UK, Herts, UK) was assessed twice daily by the patient from Day 9 of the cycle. Endometrial biopsies were divided into three. The first sample was placed in formalin and menstrual cycle stage was confirmed by histological and morphological criteria by two experienced gynaecologic pathologists (E.E.M. and P.D.). The other two samples were placed in ‘RNA Later’ (Qiagen, Germany) or snap frozen for protein analysis. Both samples were stored in a −80°C freezer for long-term storage. Matched peripheral blood was collected at the same time as endometrial biopsy and centrifuged within 30 min at 3000 rpm at 4°C for 10 minutes, then serum was separated and stored in 0.5 ml aliquots at −80°C.
Patient follow up and ART outcome
Patients were followed up prospectively and underwent a fresh or frozen cycle of ART in the menstrual cycle following biopsy. A single blastocyst of top or good quality was transferred on Day 5. Blastocysts were graded according to a modified Garner scale (Alpha Scientists in Reproductive and Embryology, 2011). ART cycle outcomes were defined using rate of clinical pregnancy (intrauterine pregnancy with ultrasound evidence) (Zegers-Hochschild et al., 2017) or no pregnancy (defined as a negative serum βhCG) per embryo transfer. Pregnant patients were followed up until delivery to compare results from women who had a livebirth to those who did not.
RNA extraction, library preparation and sequencing
RNA was isolated from endometrial tissue samples (10 mg) in the ‘discovery set’ (n = 20) using an EZ-RNA Total RNA Isolation Kit (Qiagen, Germany) as per the manufacturer’s instructions. RNA quantity and quality were determined using a Nanodrop spectrophotometer ND-1000 (Nanodrop Technologies, DE, USA) and Agilent Bioanalyzer 2100 (Agilent Technologies, UK), respectively. Next-generation sequencing libraries were prepared using the NeoPrep Automated Library Prep System (Illumina, San Diego, CA, USA). cDNA was synthesised from the RNA fragments using reverse transcriptase (Superscript II) and random primers and final normalised cDNA libraries were validated on the Agilent Bioanalyser 2100 using the DNA 1000 Nano Lab Chip kit (Agilent Technologies, UK). After quality control procedures, individual RNA-seq libraries were pooled based and sequenced at 2 × 75 bp paired end read length using an Illumina NextSeq 500 sequencer (Illumina, San Diego, CA, USA).
Differential expression analysis
RNA-seq data analysis has been described previously by (Keogh et al., 2015) ). Briefly, raw sequence reads were first checked for quality using FASTQC software (version 0.10.0) and then trimmed of low-quality reads using Trim Galore (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Genes with greater than five read counts per million in at least five samples were included. All genes below this expression level were removed to ease the multiple testing correction burden on the dataset. Trimmed reads were then aligned to the human reference genome (hg19) using TopHat (v2.0.9) (Kim and Salzberg, 2011). HTSeq (v0.5.4p5) (http://pypi.python.org/pypi/HTSeq) was used to calculate the number of sequence reads aligned to all protein-coding genes from the ENSEMBL v74 annotation of the human genome. EdgeR (v3.4.1) (Robinson et al., 2010), which uses a negative binomial distribution model to account for both biological and technical variation, was then applied to identify statistically significant (corrected P-value < 0.05) differentially expressed genes (DEGs), through a generalised linear model likelihood ratio test.
Pathway and functional enrichment analysis
Over-represented biological functions from the DEG sets were identified by functional enrichment analysis using Ingenuity Pathway Analysis (IPA; v. 8.8, Ingenuity Systems, Mountain View, CA, USA; http://www.ingenuity.com). Enrichment analysis was applied to statistically significant genes with increased and decreased expression between the pregnant and not pregnant groups. Gene ontology (GO) terms and Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways were adopted as functional terms. A Benjamini-Hochberg false discovery rate (FDR) of <0.05 was used to identify significantly over-represented biological functions in the DEG sets.
Quantitative RT-PCR validation of RNA-seq data
The RNA sequencing results were validated against gene expression values obtained from the endometrial biopsies for the discovery cohort (n = 20) and from the independent validation cohort meeting the inclusion criteria (n = 9). Total RNA was extracted, the quality was checked as outlined above and 1 µg of total RNA was used for reverse transcription to cDNA. Gene expression was examined using quantitative PCR (qPCR). Expression levels of candidate genes were determined by using TaqMan reagents (Life Technologies, CA, USA). Relative quantification of gene expression was calculated using 2−ΔCt values normalised to 18S (DataAssist, Thermo Fisher Scientific, USA). Our selection of specific genes for qPCR was based on involvement in KEGG pathway analysis, fold-change and FDR significance. The Mann–Whitney U test was used to examine statistically significant between pregnant and non-pregnant groups following (P < 0.05).
Preparation of endometrium and serum samples for IL-17A analysis by ELISA
Endometrial samples were disrupted after thawing by placing the endometrial tissue in a 2 ml eppendorf tube with 500 µl of RIPA buffer (with protease inhibitors leupeptin (2 μg/ml), aproptonin (2 μg/ml), sodium orthovanadate (4 μg/ml) and phenylmethylsulphonyl fluoride (100 μg/ml)) and a 3 mm stainless steel bead and kept on ice. The tubes were loaded into the Tissue Lyser II which was run at full speed for 1.5–2.5 min. All tubes were centrifuged at 12 000g for 2 min at 4°C to precipitate cell debris. The supernatant was transferred to a clean eppendorf tube and samples were stored at −20°C. Protein was quantified using Pierce Bicinchoninic Acid (BCA) Protein Assay Reagents (Thermo Fisher Scientific, IL, USA) after thawing.
ELISA for serum and protein IL-17A
An interleukin-17A (IL-17A) ELISA (Peprotech, NJ, USA) was performed according to the manufacturer’s instructions. Plates were read using the VersaMax microplate reader (Molecular Devices, CA, USA) and analysed using Softmax pro 4.8 (MDS Analytical Technologies, USA). Protein concentrations were determined by standard curve analysis. Cytokine levels for each sample were determined by standard curve analysis. The IL-17A ELISA kit was sensitive to 16 pg/ml.
Analysis of publicly available single-cell RNA sequencing to identify IL-17A origin
To determine the origin of IL-17A and the receptor IL-17RA, we used previously reported single-cell RNA sequencing (scRNA-seq) analysis of human first-trimester decidual cells of pregnant women (Array Express Accession Code: E-MTAB-6678) (Vento-Tormo et al., 2018). Additionally, we sought to determine the source of two further gene products associated with IL-17 molecular cascade that were up-regulated in the non-pregnant group (IL1β and S100A9), using a gene product that was up-regulated in the pregnant group (insulin-like growth factor 1 (IGF1)) as a control.
Statistical analysis
Statistical analysis was performed using SPSS version 23.0 (SPCC Inc., Chicago, IL, USA) and GraphPad Prism version 7.04 for Windows (GraphPad Software, La Jolla, CA, USA). Results are presented as median ± interquartile range (IQR). Differences in continuous variables between groups were analysed using Student’s t-test or Mann–Whitney U test according to distribution of data. The differences for categorical variables were analysed using the Chi-squared test or Fisher’s exact test where applicable. Receiver operator curves (ROCs) were prepared and analysed using GraphPad Prism version 7.04 for Windows (GraphPad Software, La Jolla, CA, USA). P-values <0.05 were considered statistically significant.
Ethical approval
Ethical approval was obtained from the National Maternity Hospital, Dublin, Ethics Committee (EC 27.2016) in October 2016 and informed consent was obtained from all study participants.
Results
Patient characteristics
This study included 29 women with unexplained infertility. Following endometrial scratching, patients underwent ART (in vitro fertilisation (IVF), intracytoplasmic sperm injection (ICSI) or frozen embryo transfer (FET)) in a subsequent menstrual cycle and had a day five single embryo transfer (SET) of a top or good quality blastocyst.
RNA-seq was performed on endometrial samples from the ‘discovery cohort’ (n = 20), in which nine women (45.0%) were pregnant following ART and 11 (55.0%) were not pregnant. Findings from RNA-seq experiments were validated in an independent validation set (n = 9), in which three women (33.3%) were pregnant following ART and six (66.7%) were not. Of the 12 pregnant women, 11 had a livebirth and one miscarried at 7.5 weeks of gestation. Baseline characteristics of these two groups are outlined in Table I. No significant differences in age, BMI, serum anti-Müllerian hormone (AMH) level, duration of infertility, cycle length, ethnicity, number of previous unsuccessful cycles, endometrial thickness or morphology, number of fresh or frozen cycles, fresh cycle protocol or serum progesterone were identified between the pregnant and non-pregnant groups.
Table I.
General characteristics of the study participants.
| Discovery set (n = 20) |
Validation set (n = 9) |
|||||
|---|---|---|---|---|---|---|
| Pregnant (n = 9) | Non-pregnant (n = 11) | P-value | Pregnant (n = 3) | Non-pregnant (n = 6) | P-value | |
| Age (years) | 35.3 (1.3) | 35.2 (1.7) | 0.89 | 34.8 (1.5) | 35.5 (0.9) | 0.40 |
| Body mass index (kg/m²) | 23.7 (3.4) | 22.8 (2.6) | 0.51 | 22.7 (3.3) | 22.9 (1.7) | 0.90 |
| Median serum AMH (pmol/l) | 26.0 (13.4) | 24.2 (16.0) | 0.79 | 21.2 (11.4) | 19.7 (9.9) | 0.84 |
| Cycle length (days) | 29.8 (3.4) | 28.8 (0.9) | 0.36 | 28.0 (0.0) | 29.8 (2.9) | 0.33 |
| Duration of Infertility (months) | 26.0 (12.6) | 30.3 (10.6) | 0.39 | 24.0 (7.5) | 28.8 (13.1) | 0.58 |
| Median Serum Progesterone (nmol/l) | 43.8 (20.5) | 40.4 (15.6)) | 0.68 | 42.0 (14.0) | 38.6 (16.4) | 0.77 |
| Endometrial thickness (mm) | 8.8 (1.5) | 10.1 (2.7) | 0.21 | 10.4 (1.8) | 10.2 (2.2) | 0.90 |
| Previous ART | ||||||
| No previous embryo transfer | 7 (77.8%) | 9 (81.8%) | 1.00 | 2 (66.7%) | 4 (66.7%) | 1.00 |
| One previous embryo transfer | 1 (11.1%) | 2 (18.2%) | 1.00 | 0 (0%) | 2 (33.3%) | 0.50 |
| Two previous embryo transfers | 1 (11.1%) | 0 (0%) | 0.45 | 1 (33.3%) | 0 (0%) | 1.00 |
| Fresh IVF cycle | 3 (33.3%) | 4 (36.4%) | 1.00 | 1 (33.3%) | 4 (66.7%) | 0.52 |
| Fresh ICSI cycle | 3 (33.3%) | 3 (27.3%) | 1.00 | 1 (33.3%) | 1 (16.7%) | 1.00 |
| Antagonist if fresh cycle | 3 (50.0%) | 4 (57.1%) | 1.00 | 0 (0%) | 0 (0%) | 1.00 |
| FET cycle | 3 (33.3%) | 4 (36.4%) | 1.00 | 1 (33.3%) | 1 (16.7%) | 1.00 |
| Good quality embryo | 6 (66.7%) | 8 (72.7%) | 1.00 | 3 (100%) | 4 (66.7%) | 0.50 |
| Top-quality embryo | 3 (33.3%) | 3 (27.3%) | 1.00 | 0 (0%) | 2 (33.3%) | 0.50 |
Values are reported in mean with standard deviation in parentheses, except AMH and serum progesterone which are presented as median and IQR. Number of previous ART cycles, type of ART cycle and embryo quality transferred are reported as absolute values in percentages in parentheses.
Pregnant is defined using clinical pregnancy (intrauterine pregnancy with ultrasound evidence). Non-pregnant is defined as a negative serum βhCG.
FET, frozen embryo transfer; AMH, anti-Müllerian hormone.
Differential gene expression and pathway enrichment
RNA-seq was performed on 20 mid-luteal phase endometrial biopsies. The mean (SD) number of raw reads across all samples was 43.5 × 106 (SD 16.4 × 106). Approximately 94% of reads aligned to the human genome (hg19) with high-quality scores (≥Q30). After normalisation and scaling, unsupervised hierarchical clustering was performed on all samples.
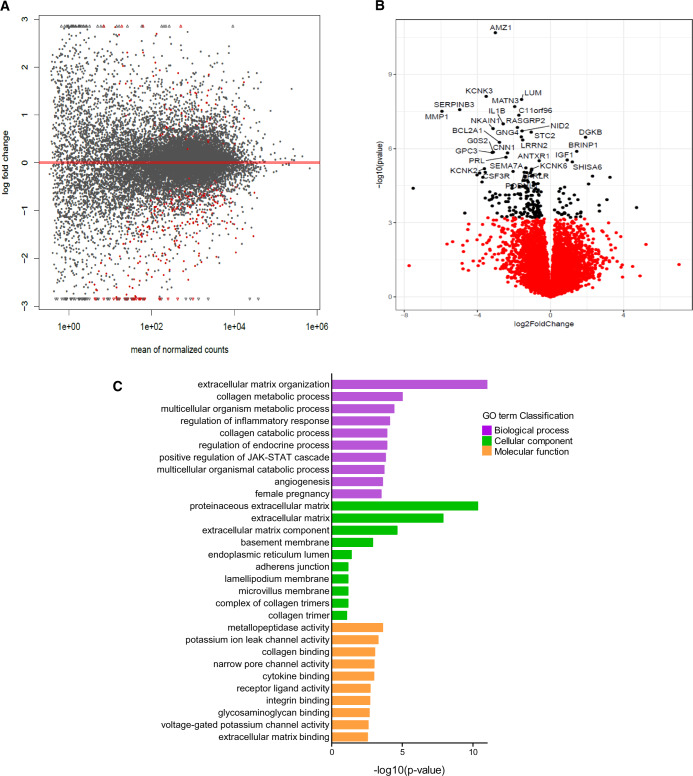
A total of 204 protein-coding genes were found to be differentially expressed between the pregnant (n = 9) and non-pregnant (n = 11) groups (generalised linear model likelihood ratio test EdgeR (v3.4.1; Robinson et al., 2010; P < 0.05) (Fig. 1A)). These manifested as 166 genes with decreased expression and 38 genes with increased expression in the pregnant compared to the non-pregnant group (Supplementary Table SI). The 30 most highly DEGs are illustrated in Fig. 1B. Pathway enrichment analysis was applied to the 204 DEGs.
Figure 1.
RNA-seq analysis. (A) Volcano plot illustrating the 204 genes reaching statistical significance (P < 0.05) illustrated in red between pregnant and not pregnant. (B) Volcano plot illustrating the 30 most DEGs between pregnant and not pregnant. Statistical analyses were performed using generalised linear model likelihood ratio test (EdgeR). (C) ClusterProfiler functional annotation showing GO enrichment analysis of DEGs; groups reflect main categories of GO terms: BP, biological process; CC, cellular component; MF, molecular function. Vertical and horizontal axes represent GO term and −log10 (P-value) of the corresponding GO term, respectively.
Of the total, 202 genes were successfully mapped to a molecular or biological pathway and/or category in the IPA database (IPA; v. 8.8, Ingenuity Systems, Mountain View, CA, USA; http://www.ingenuity.com). Pathway analysis of the 202 DEGs using KEGG, GO biological process and GO cellular components revealed several enriched pathways (Benjamini-Hochberg FDR < 0.05) (Fig. 1C). Enriched GO biological pathways included extracellular matrix organisation (29/202 genes; P = 2.37E-09), regulation of inflammatory response (13/202 genes; P = 0.03), regulation of JAK-STAT cascade (6/202 genes; P = 0.03) and angiogenesis (15/202 genes; P = 0.049). Enriched GO molecular pathways included receptor ligand activity (11/202 genes; P = 0.002), metallopeptidase activity (9/202 genes; P = 0.0002) and cytokine binding (6/202 genes; P = 0.001).
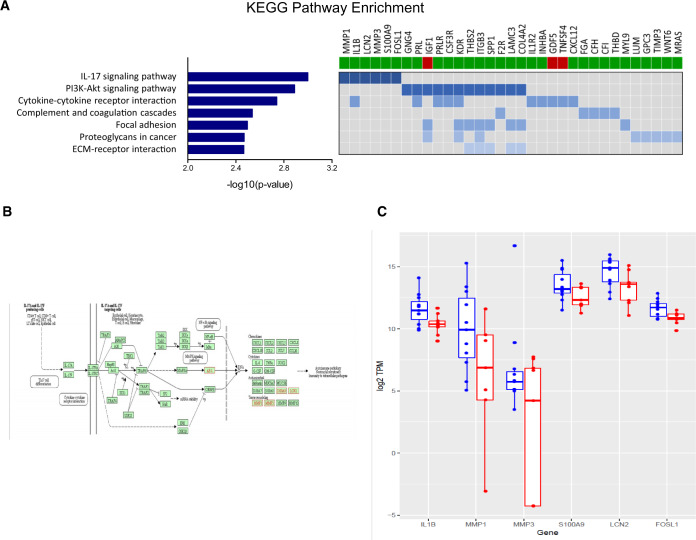
Seven over-represented KEGG enriched pathways identified in the pregnant group reached statistical significance (Benjamini-Hochberg FDR < 0.05) (Fig. 2A). Enriched pathways included those involved in the ‘IL-17 signalling pathway’ (6/202 genes; P = 0.001, Fig. 2A), the ‘PI3K-Akt signalling pathway’ (12/202 genes, P = 0.001) and cytokine–cytokine receptor interaction (10/202 genes, P = 0.002) (Fig. 2A).
Figure 2.
Identification of interleukin-17 signalling pathway using RNA-seq analysis. (A) KEGG pathway enrichment analysis of DEGs between pregnant and not pregnant. Left panel: horizontal axis indicates −log10 (P-value) of the corresponding KEGG classification (left). Right panel shows 33 genes involved in 7 KEGG pathways outlined on the left; bars below the gene names indicates whether a gene is up-regulated (green) or down-regulated (red) in the not pregnant group. (B) IL-17A signalling pathway with DEGs outlined in red. More than one gene may be included in one node. Pathway components were extracted from the KEGG database (http://www.genome.jp/kegg/kegg1.html). (C) Box plots of the six DEGs associated with the IL-17 pathway from RNA-seq analysis. The not pregnant group is illustrated in blue and the pregnant group is illustrated in red. These six genes are up-regulated in the not pregnant group. KEGG, Kyoto Encyclopaedia of Genes and Genomes.
Our analyses identified the pro-inflammatory IL-17 signalling pathway (Fig. 2B) as being prominently up-regulated in the endometrium of women who subsequently had a failed ART cycle. Six genes associated with IL-17 molecular cascades that were up-regulated in the non-pregnant group, specifically MMP3, MMP1, IL1β, S100A9, LCN2 and FOSL1, are illustrated in Fig. 2C, and quantitative PCR analysis of these six genes confirmed these findings (Supplementary Fig. S1).
IL-17 protein levels in endometrial biopsies
Based on this observation, we sought to analyse IL-17 protein expression in the mid-luteal phase endometrial scratch biopsies. We elected to focus specifically on IL-17A, given published evidence of IL-17A expression in human endometrium and its suggested role in the inflammatory pathology underlying endometriosis (Ahn et al., 2015). IL-17A was detected by ELISA in all endometrial samples (n = 29).
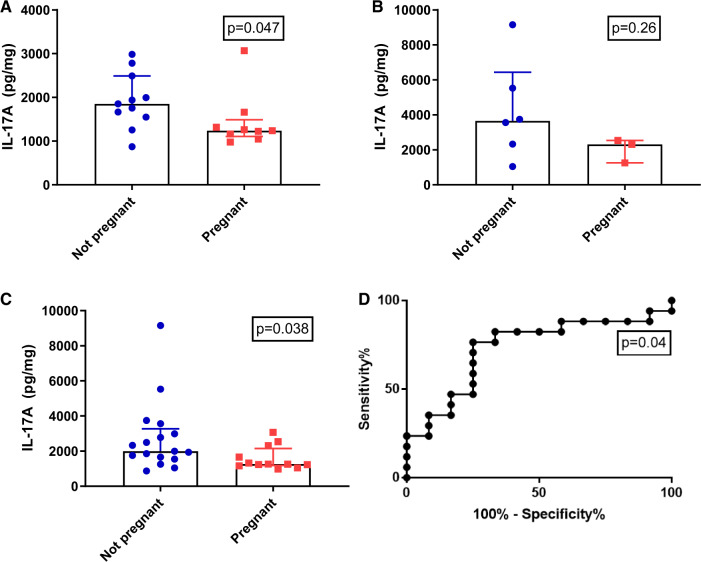
In the discovery cohort (n = 20), the median endometrial IL-17A concentration was significantly elevated in the non-pregnant (n = 11) compared to the pregnant group (n = 9) (1851 pg/mg protein vs. 1237 pg/mg protein; P = 0.047). In the validation cohort, although IL-17A levels were increased in the non-pregnant group (3651 pg/mg protein; n = 6) compared with the pregnant group (2309 pg/mg protein; n = 3), this difference was not significant; P = 0.26). Combining data from both cohorts (n = 29) demonstrated that median endometrial IL-17A levels were higher in the non-pregnant compared to the pregnant group (1993 pg/mg protein vs. 1259 pg/mg protein; P = 0.038, Fig. 3A–C). A post hoc power calculation was performed for our results. To detect the same difference in picogram per milligram protein between two balanced groups with 80% confidence using a Mann–Whitney U test and a type I error rate of 0.05, 70 participants would be required per group, suggesting that tissue analysis of IL-17A protein expression was underpowered in this cohort.
Figure 3.
Identification of interleukin-17 signalling pathway in endometrial samples. (A) ELISA of endometrial tissue IL-17A in (A) Discovery set (n = 20), (B) Validation set (n = 9) and (C) Entire cohort (n = 29). The not pregnant group is illustrated in blue and the pregnant group is illustrated in red. (D) AUC analysis of entire cohort (n = 29) for endometrial tissue IL-17A (AUC = 0.73 (95% CI 0.54–0.92)). AUC, area under the curve.
Predictive potential of raised endometrial IL-17 levels for pregnancy failure
Although underpowered, we assessed mid-luteal phase endometrial IL-17A protein expression levels as a predictor of pregnancy outcome following ART using a ROC. Area under the curve (AUC) analysis of mid-luteal phase endometrial IL-17A of the entire cohort (n = 29) demonstrated an AUC of 0.73 (95% CI 0.54–0.92; P = 0.04, Fig. 3D). Endometrial IL-17A levels of >1664 pg/mg of protein were associated with a sensitivity of 76% and specificity of 75% in those who were not pregnant, giving a positive predictive value (PPV) of 80% and negative predictive value (NPV) of 69%.
Serum IL-17 levels
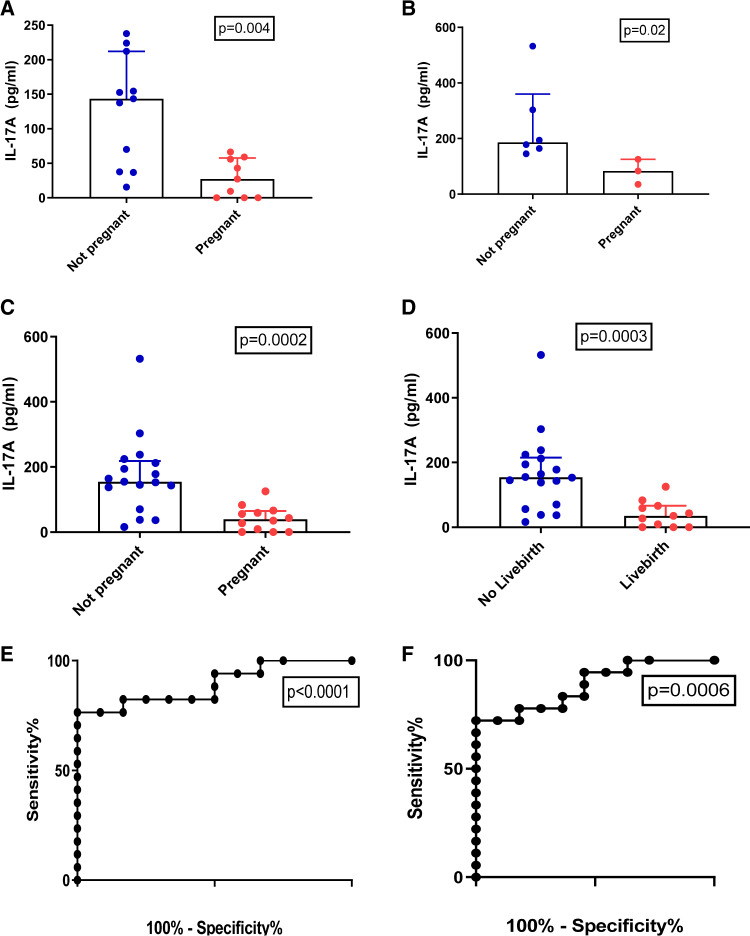
Having identified that increased mid-luteal phase endometrial IL-17A levels were associated with failed ART in unexplained infertility, we sought to determine whether serum IL-17A levels correlated with pregnancy outcome. Serum IL-17A levels were significantly elevated in the non-pregnant group compared with the pregnant group in both the discovery (143.4 pg/ml vs. 27.2 pg/ml; P = 0.004, Fig. 4A) and validation cohorts (186.4 pg/ml vs. 83.6 pg/ml; P = 0.02, Fig. 4B). Three women in the discovery cohort who had undetectable levels of IL-17A in their serum at the time of ART, all became pregnant. Analysis of the entire cohort (n = 29) demonstrated that median mid-luteal phase serum IL-17A levels were higher in non-pregnant group (n = 17) compared to the pregnant group (n = 12) (154.5 pg/ml vs. 39.0 pg/ml; P = 0.0002: Fig. 4C. A post hoc power calculation was performed on our data. To detect the same difference in picogram per millilitre in serum between two balanced groups with 80% confidence using a Mann–Whitney U test and a type I error rate of 0.05, 11 participants would be required per group. When the pregnant group (n = 12) were analysed by livebirth only (n = 11), the median mid-luteal phase serum IL-17A concentration was still significantly higher in the women who did not have a livebirth (n = 18) compared to women who had a livebirth (n = 11) (154.0 pg/ml vs 35.0 pg/ml; P = 0.0003: Fig. 4D).
Figure 4.
Serum IL-17 as a predictor of ART outcome. ELISA of serum IL-17A for pregnant in (A) Discovery set (n = 20), (B) Validation set (n = 9) and (C) Entire cohort (n = 29). The not pregnant group is illustrated in blue and the pregnant group is illustrated in red. (D) ELISA of serum IL-17A for livebirth in entire cohort (n = 29). (E) AUC analysis entire cohort (n = 29) for pregnant group (clinical pregnancy) of serum IL-17A (AUC = 0.89 (95% CI 0.77–1.00)). (F) AUC analysis entire cohort (n = 29) for livebirth of serum IL-17A (AUC = 0.88 (95% CI 0.77–1.00)).
Predictive potential of raised serum IL-17 levels for pregnancy failure
The sensitivity and specificity of mid-luteal phase serum IL-17A as a predictor of pregnancy outcome following ART was also assessed using a ROC. AUC analysis of mid-luteal phase serum IL-17A as a predictor of no clinical pregnancy in this cohort was 0.89 (95% CI 0.78–1.00; P < 0.001, Fig. 4E). A serum IL-17A level of >30 pg/ml was associated with a higher sensitivity of 94%, a lower specificity of 42%, a PPV of 70% and an NPV of 83%, in the prediction of no clinical pregnancy with an odds ratio (OR) of 11.4 (95% CI 1.1–116.7; P = 0.04; Table II).
Table II.
Thresholds of mid-luteal phase serum IL-17A in the prediction of negative outcomes (no clinical pregnancy and no livebirth) following ART (n = 29).
| >20 pg/ml |
>30 pg/ml |
>40 pg/ml |
||||
|---|---|---|---|---|---|---|
| No clinical pregnancy | No livebirth | No clinical pregnancy | No livebirth | No clinical pregnancy | No livebirth | |
| Sensitivity | 94.1% (71.3–99.9) | 94.4% (72.7–99.9) | 94.1% (71.3–99.9) | 94.4% (72.7–99.9) | 82.4% (56.6–96.2) | 83.3% (58.6–96.4) |
| Specificity | 33.3% (13.7–78.8) | 36.4% (10.9–69.2) | 41.7% (15.2–72.3) | 45.5% (16.8–76.6) | 50.0% (21.1–78.9) | 54.6% (23.4–82.3) |
| PPV | 66.7% (56.9–75.2) | 70.8% (60.5–79.4) | 69.6% (58.3–78.9) | 73.9% (62.0–83.1) | 70.0% (56.0–81.1) | 75.0% (60.3–85.6) |
| NPV | 80.0% (33.7–96.9) | 80.0% (33.8–96.9) | 83.3% (40.0–97.4) | 83.3% (40.1–97.4) | 66.7% (38.2–86.6) | 66.7% (38.4–86.5) |
| Accuracy | 69.0% (49.2–84.7) | 72.4% (52.8–87.3) | 72.4% (52.8–87.3) | 75.9% (56.4–89.7) | 69.0% (49.2–84.7) | 72.4% (52.8–87.2) |
| Odds ratio of negative outcome | 8.0 | 9.7 | 11.4 | 14.2 | 4.7 | 6.0 |
| (0.8–83.9) | (0.9–103.0) | (1.1–116.7) | (1.4–147.1) | (0.9–25.1 ) | (1.1–33.4) | |
| P = 0.08 | P = 0.059 | P = 0.04 | P = 0.03 | P = 0.07 | P = 0.04 | |
Values in parentheses are 95% confidence intervals (%).
NPV, negative predictive value; PPV, positive predictive value.
To predict no livebirth following ART cycle, ROC analysis was performed and was associated with an AUC of 0.88 (0.77–1.00; P = 0.0006; Fig. 4F). A serum IL-17A level of >30 pg/ml was associated with a sensitivity of 94%, specificity of 46%, PPV of 74% and NPV of 76% in the prediction of no livebirth with an OR of 14.2 (95% CI 1.4–147.1; P = 0.03).
Source of endometrial IL-17
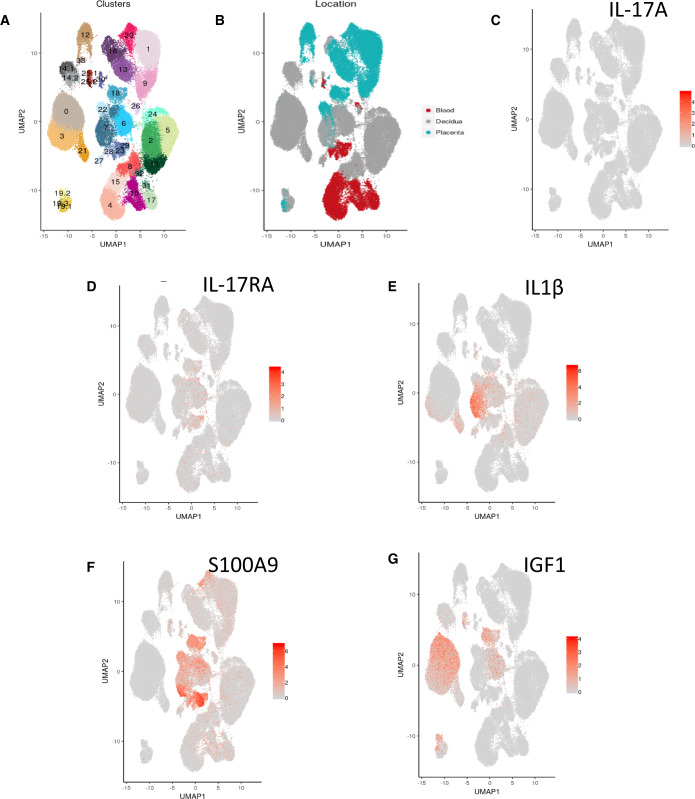
To determine whether decidual cells might be the site of altered IL-17A signalling, we analysed previously reported single-cell RNA-seq (scRNA-seq) analysis of human first-trimester decidual cells of pregnant women (Fig. 5A and B) (Vento-Tormo et al., 2018). This demonstrated minimal expression of IL-17A transcripts in the decidua, placenta and blood of healthy first-trimester pregnancies. Likewise, its receptor (IL-17RA) is expressed at low levels in pregnancy (Fig. 5C and D). IL1β and S100A9 expression, associated with the not pregnant group in our analysis, appeared to be limited to the pro-inflammatory type 1 maternal decidual macrophages as demonstrated in Fig. 5E and F. IGF1 expression, associated with the pregnant group in our analysis, was used as a control. IGF1 expression was limited to decidual stromal cells and type 2 anti-inflammatory maternal decidual macrophages (Fig. 5G). IGF1 is a growth factor that is present at the maternal–embryo interface in a number of mammalian species (Green et al., 2015) and high concentrations of IGF1 are detectable in human maternal circulation during early pregnancy and thought to be involved in regulation of placental and foetal growth (Hills et al., 1996).
Figure 5.
Single-cell RNA-seq (scRNA-seq) analysis of human first-trimester decidual cells of pregnant women. (A) Placental and decidual cell clusters from 10× Genomics and Smart-seq2 (SS2) scRNA-seq analysis (Vento-Tormo et al., 2018). (B) Origin of droplet cells in C by tissue (Vento-Tormo et al., 2018). (C) IL-17 and (D) IL17RA confirming our findings of minimal expression in blood, decidua or placenta in women with successful pregnancy. (E) IL1β and (F) S100A9 expression is limited to type 1 pro-inflammatory maternal decidual macrophages. (G) IGF1 as a control expression is limited to decidual stromal cells and type 2 anti-inflammatory maternal decidual macrophages.
Discussion
This prospective cohort study has identified distinct transcriptomic alterations in women with unexplained primary infertility who become pregnant following ART compared with those who do not become pregnant. In this study, we used RNA-seq performed on carefully timed mid-luteal phase endometrial biopsies to identify IL-17 and PI3K-Akt as dysregulated pathways in women with unexplained infertility who did not become pregnant following ART. We also demonstrated that elevated serum IL-17A predicted an increased risk of ART failure in a subsequent cycle. The association between mid-luteal phase IL-17A serum levels and ART failure was validated in an independent cohort of women with unexplained infertility. To enable further assessment of serum IL-17A levels in independent studies, we suggest that a mid-luteal phase IL-17A level greater than 30 pg/ml prior to treatment may be an appropriate threshold to predict ART failure in a future cycle, with a sensitivity of 94%. As expected, we also demonstrated that women with low serum IL-17A levels have a higher live birth rate. Using previously published scRNA-seq data (Vento-Tormo et al., 2018), we confirmed our findings of minimal expression of IL-17A in the decidua, placenta or blood of healthy first-trimester pregnancies. Furthermore, the expression of genes associated with IL-17 molecular cascades, namely IL1β and S100A9, appears to be limited to the pro-inflammatory type 1 maternal decidual macrophages.
The IL-17 family of pro-inflammatory cytokines (IL-17A–F) have been shown to play a multifaceted role in inflammation, autoimmunity and host defense (Miossec et al., 2009). IL-17A, the best characterised of these, was initially discovered in 1993 as the product of the CTLA8 gene identified in mice and humans (Rouvier et al., 1993). Cells of both the adaptive and innate immune system can produce IL-17. T helper 17 (Th17) cells, gamma delta T (δT) cells and natural killer (NK) cells are the major source of IL-17A in many types of adaptive immunity (Bettelli et al., 2007).
It is possible that higher levels of tissue and serum IL-17A are indicative of aberrant, dysregulated local and systemic inflammatory processes, resulting in implantation failure, potentially resulting in extracellular matrix destruction and tissue damage within the endometrium. Recent work in eutherian mammals suggested that endometrial IL-17 at the fetal–maternal interface was repressed by the ancestral emergence of decidual stromal cells and placentation, thus enabling the evolution of embryo implantation and a sustainable fetal–maternal interface (Chavan, 2018). Consistent with these findings, increased IL-17 levels have been described in the serum and plasma of women with recurrent pregnancy loss, and pre-eclampsia (Zhang et al., 2005; Cornelius and Lamarca, 2014; Ozkan et al. 2014a,b, 2015).
There is evidence that IL-17 is implicated in the pathophysiology of endometriosis (Ozkan et al., 2014a). IL-17 induces or acts synergistically with other cytokines, such as IL-8 to activate cyclooxygenase-2 (COX-2) activity (Lessey and Kim, 2017), prostaglandins and matrix metalloproteinases (MMPs) (Balkowiec et al., 2018), which are central to the eutopic endometrial changes associated with endometriosis. Furthermore, IL-17-induced IL-8 targets the PI3K-Akt signalling pathway (Lee and Kim, 2014), known to be aberrantly activated in endometriosis (Li et al., 2012), which results in overexpression of this pathway. Progesterone resistance, a feature of endometriosis, induces these factors, resulting in inflammation, angiogenesis and cell proliferation (Lessey and Kim, 2017). Serum IL-17 and a number of other inflammatory cytokines including IL-1A and IL-6 are elevated in women with endometriosis (Yoo et al., 2017). It is well recognised that the most common finding in women with unexplained infertility at laparoscopy is endometriosis (Bonneau et al., 2012; Evans-Hoeker et al., 2016; Yu et al. 2019; Xu et al., 2020), and therefore it is plausible that the women in this study may have had undiagnosed, asymptomatic endometriosis.
Few studies have described the effect of IL-17 on infertility. Ozkan et al. compared plasma levels of IL-17 in a heterogenous cohort of 80 infertile women undergoing ovarian stimulation and ICSI for unexplained primary and secondary infertility to a control group of 40 fertile women. Binary logistic regression analysis revealed that IL-17 had a negative influence on the ICSI outcome (RR = 2, 95% CI: 1.531–2.661) (Ozkan et al., 2014a). Unlike our study, these samples were not accurately timed using LH testing and may have been taken outside the window of implantation.
Dysregulation of the IL-17 family of cytokines has been associated with autoimmune diseases, including psoriasis (Martin et al., 2013), inflammatory arthritis (Miossec, 2017), ankylosing spondylitis (Baeten et al., 2015), multiple sclerosis (Park et al., 2005) and inflammatory bowel disease (Jin and Dong, 2013). IL-17 is now a recognised target in chronic inflammation and the first inhibitor was registered in 2015 (Miossec and Kolls, 2012). Secukinumab (Novartis, Switzerland), a chimeric IL-17A-specific monoclonal antibody, has been used successfully in patients with plaque psoriasis, with impressive results in phase three trials (Langley et al., 2014), leading to European Medicines Agency (EMA) and the Food and Drug Administration (FDA) approval for the treatment of psoriatic arthritis and ankylosing spondylitis in 2015 and 2016, respectively (Baeten et al., 2015). Secukinumab has not been associated with an increased rate of fetal abnormalities or adverse pregnancy outcome to date (Gerosa et al., 2018). However, given the limited exposure reported to date, the continuous use of Secukinumab throughout pregnancy requires further research (Warren et al., 2018).
Our study is, to our knowledge, the first to identify differences in IL-17 signalling in the endometrium of women with unexplained infertility who became pregnant following ART compared to those who did not. Although many key gene signatures of endometrial receptivity have been identified (Ruiz-Alonso et al., 2013; Huang et al., 2017; Bastu et al., 2019), there are inconsistencies among clinical studies due to the differences in experimental designs, inclusion criteria and day of the menstrual cycle when the biopsy was taken.
Limitations of our study include the cohort size and lack of aneuploidy data for the embryos; however, all embryos transferred were single good or top-quality blastocysts. Furthermore, most women (93.1%, n = 27) did not undergo laparoscopy prior to ART, therefore there may have been cases of undiagnosed endometriosis in our cohort. Strengths of our study include its prospective design. Endometrial scratching was performed once in the mid-luteal phase by a single operator. Unlike previously published work, the menstrual cycle stage was accurately timed, and samples were precisely collected at LH+7 and the cycle stage was confirmed using serum progesterone and by histological assessment. The strict inclusion criteria adopted for this study, particularly the inclusion of nulliparous women only, allow comparisons to be drawn clearly, minimising the significant clinical heterogeneity seen in previous studies. Another strength of this study is the use of RNA-seq rather than a micro-array, as previously used in other transcriptome studies (Ruiz-Alonso et al., 2013; Koot et al., 2016).
The significant discrete differences in mid-luteal endometrial gene expression seen in this study may predict pregnancy outcome following ART treatment. Our work highlights IL-17 signalling as an important candidate pathway that may contribute to dysregulated immune function in the endometrium of women with unexplained infertility after ART who fail to become pregnant. Importantly, our work demonstrates that changes in IL-17A levels in the local endometrial microenvironment are reflected in the systemic circulation, corroborating serum IL-17A as a potential robust and minimally invasive predictor of endometrial function. These novel findings have the potential to lead to the development of new therapeutic strategies to treat underlying dysfunction and improve pregnancy outcomes in women with unexplained infertility.
Data availability
RNA-seq data are available from the Gene Expression Omnibus (GEO) under accession GSE144895.
Supplementary Material
Acknowledgements
The authors acknowledge the UCD Conway Genomics Core, University College Dublin, Ireland.
Authors’ role
D.A.C., L.E.G., E.P.B., P.D., E.E.M., B.J.L., F.M.A., M.W., C.O.F. and D.J.B. contributed to the study design. All co-authors contributed to the execution and analysis, the manuscript drafting and the critical discussion of this manuscript.
Funding
Funding from the University College Dublin Wellcome Institutional Strategic Support Fund, which was financed jointly by University College Dublin and the SFI-HRB-Wellcome Biomedical Research Partnership (ref 204844/Z/16/Z), is acknowledged.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol 2015;195:2591–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog B, Shalom-Paz E, Dufort D, Tulandi T. Promoting implantation by local injury to the endometrium. Fertil Steril 2010;94:2026–2029. [DOI] [PubMed] [Google Scholar]
- Alpha Scientists in Reproductive and Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270–1283. [DOI] [PubMed] [Google Scholar]
- Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M et al. ; MEASURE 1 Study Group; MEASURE 2 Study Group. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–2548. [DOI] [PubMed] [Google Scholar]
- Balkowiec M, Maksym RB, Wlodarski PK. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (review). Mol Med Rep 2018;18:3123–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastu E, Demiral I, Gunel T, Ulgen E, Gumusoglu E, Hosseini MK, Sezerman U, Buyru F, Yeh J. Potential marker pathways in the endometrium that may cause recurrent implantation failure. Reprod Sci 2019;26:879–890. [DOI] [PubMed] [Google Scholar]
- Bergh PA, Navot D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril 1992;58:537–542. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol 2007;19:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau C, Chanelles O, Sifer C,, Poncelet C. Use of laparoscopy in unexplained infertility. Eur J Obstet Gynecol Reprod Biol 2012;163:57–61. [DOI] [PubMed] [Google Scholar]
- Brouwer J, Hazes JM, Laven JS, Dolhain RJ. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann Rheum Dis 2015;74:1836–1841. [DOI] [PubMed] [Google Scholar]
- Chavan AR, Griffith OW, Stadtmauer D, Maziarz J, Pavlicev M, Fishman R, Koren L, Romero R, Wagner GP. Evolution of embryo implantation was enabled by the origin of decidual cells in eutherian mammals. bioRxiv 2018;doi: 10.1101/429571. [DOI] [PMC free article] [PubMed]
- Cornelius DC,, Lamarca B. TH17- and IL-17-mediated autoantibodies and placental oxidative stress play a role in the pathophysiology of pre-eclampsia. Minerva Ginecol 2014;66:243–249. [PMC free article] [PubMed] [Google Scholar]
- Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol 2014;72:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, Schammel DP, Young SL. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci 2016;23:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv 2014;69:109–115. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA,, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 2007;25:445–453. [DOI] [PubMed] [Google Scholar]
- Gerosa M, Argolini LM, Artusi C, Chighizola CB. The use of biologics and small molecules in pregnant patients with rheumatic diseases. Expert Rev Clin Pharmacol 2018;11:987–998. [DOI] [PubMed] [Google Scholar]
- Glover LE, Fennimore B,, Wingfield M. Inflammatory bowel disease: influence and implications in reproduction. Inflamm Bowel Dis 2016;22:2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CJ, Fraser ST, Day ML. Insulin-like growth factor 1 increases apical fibronectin in blastocysts to increase blastocyst attachment to endometrial epithelial cells in vitro. Hum Reprod 2015;30:284–298. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R,, Wagner GP. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci USA 2017;114:E6566–E6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills FA, English J, Chard T. Circulating levels of IGF-I and IGF-binding protein-1 throughout pregnancy: relation to birthweight and maternal weight. J Endocrinol 1996;148:303–309. [DOI] [PubMed] [Google Scholar]
- Huang J, Qin H, Yang Y, Chen X, Zhang J, Laird S, Wang CC, Chan TF,, Li TC. A comparison of transcriptomic profiles in endometrium during window of implantation between women with unexplained recurrent implantation failure and recurrent miscarriage. Reproduction 2017;153:749–758. [DOI] [PubMed] [Google Scholar]
- Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect 2013;2:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh K. Waters SM. Kelly AK. Wylie ARG. Kenny DA. Effect of feed restriction and subsequent re-alimentation on hormones and genes of the somatotropic axis in cattle. Physiol Genomics. 2015;47:264-273. [DOI] [PubMed] [Google Scholar]
- Kim D, Salzberg SL. TopHat-Fusion: an algorithm for discovery of novel fusion transcripts. Genome Biol 2011;12:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot YE, van Hooff SR, Boomsma CM, van Leenen D, Groot Koerkamp MJ, Goddijn M, Eijkemans MJ, Fauser BC, Holstege FC, Macklon NS. An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep 2016;6:19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B et al. ; ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014;371:326–338. [DOI] [PubMed] [Google Scholar]
- Lee II, Kim JJ. Influence of AKT on progesterone action in endometrial diseases. Biol Reprod 2014;91:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, Wilkinson J, Sadler L, Gupta D, Strandell A et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med 2019;380:325–334. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril 2017;108:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, Li DJ. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod 2012;27:2107–2116. [DOI] [PubMed] [Google Scholar]
- Liang PY, Diao LH, Huang CY, Lian RC, Chen X, Li GG, Zhao J, Li YY, He XB,, Zeng Y. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod Biomed Online 2015;31:823–826. [DOI] [PubMed] [Google Scholar]
- Macklon N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil Steril 2017;108:9–14. [DOI] [PubMed] [Google Scholar]
- Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, Russell CB. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013;133:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybin JA, Critchley HO,, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol 2011;335:42–51. [DOI] [PubMed] [Google Scholar]
- Miossec P. Update on interleukin-17: a role in the pathogenesis of inflammatory arthritis and implication for clinical practice. RMD Open 2017;3:e000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012;11:763–776. [DOI] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009;361:888–898. [DOI] [PubMed] [Google Scholar]
- Ozkan ZS, Deveci D, Kumbak B, Simsek M, Ilhan F, Sekercioglu S,, Sapmaz E. What is the impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility? J Reprod Immunol 2014. a;103:53–58. [DOI] [PubMed] [Google Scholar]
- Ozkan ZS, Deveci D, Simsek M, Ilhan F, Risvanli A, Sapmaz E. What is the impact of SOCS3, IL-35 and IL17 in immune pathogenesis of recurrent pregnancy loss? J Matern Fetal Neonatal Med 2015;28:324–328. [DOI] [PubMed] [Google Scholar]
- Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A,, Sapmaz E. Plasma IL-17, IL-35, interferon-gamma, SOCS3 and TGF-beta levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med 2014. b;27:1513–1517. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 1993;150:5445–5456. [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013;100:818–824. [DOI] [PubMed] [Google Scholar]
- Sigalos G, Triantafyllidou O, Vlahos NF. Novel embryo selection techniques to increase embryo implantation in IVF attempts. Arch Gynecol Obstet 2016;294:1117–1124. [DOI] [PubMed] [Google Scholar]
- Simon C, Martin JC,, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:815–826. [DOI] [PubMed] [Google Scholar]
- Van Sinderen M, Menkhorst E, Winship A, Cuman C,, Dimitriadis E. Preimplantation human blastocyst-endometrial interactions: the role of inflammatory mediators. Am J Reprod Immunol 2013;69:427–440. [DOI] [PubMed] [Google Scholar]
- Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polanski K, Goncalves A.L. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018;563:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RB, Reich K, Langley RG, Strober B, Gladman D, Deodhar A, Bachhuber T, Bao W, Altemeyer E, Hussain S et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol 2018;179:1205–1207. [DOI] [PubMed] [Google Scholar]
- Xu XX, Yu Q, Sun AJ, Tian QJ, Chen R. Clinical study of hysteroscopy combined with laparoscopy in the diagnosis and treatment of unexplained infertility. Zhonghua Fu Chan Ke Za Zhi 2020;55:15–20. [DOI] [PubMed] [Google Scholar]
- Yoo JY, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C, Schammel DP, Young SL, Jeong JW, Lessey BA. KRAS activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep 2017;7:6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Cai H, Guan J, Zheng X, Han H. Laparoscopic surgery: any role in patients with unexplained infertility and failed in vitro fertilization cycles? Medicine (Baltimore ) 2019;98:e14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID et al. The international glossary on infertility and fertility care, 2017. Fertil Steril 2017;108:393–406. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu H, Lin J, Qian Y, Deng L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG 2005;112:1153–1155. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li R, Wang R, Huang HX,, Zhong K. Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertil Steril 2008;89:1166–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data are available from the Gene Expression Omnibus (GEO) under accession GSE144895.