Abstract
Sphingolipids, which function as plasma membrane lipids and signaling molecules, are highly enriched in neuronal and myelin membranes in the nervous system. They are degraded in lysosomes by a defined sequence of enzymatic steps. In the related group of disorders, the sphingolipidoses, mutations in the genes that encode the individual degradative enzymes cause lysosomal accumulation of sphingolipids and often result in severe neurodegenerative disease. Here we review the information indicating that microglia, which actively clear sphingolipid-rich membranes in the brain during development and homeostasis, are directly affected by these mutations and promote neurodegeneration in the sphingolipidoses. We also identify parallels between the sphingolipidoses and more common forms of neurodegeneration, which both exhibit evidence of defective sphingolipid clearance in the nervous system.
Keywords: Aging, Alzheimer’s disease, Microglia, Parkinson’s disease, Sphingolipidoses, Sphingolipids
1. Introduction
Sphingolipids are a diverse family of lipids that are characterized by a sphingosine backbone. They are one of the three major categories of plasma membrane lipids, with the other two being cholesterol and glycerol backbone-based phospholipids [1]. Sphingolipids also have important functions as signaling molecules [2]. The biosynthesis of sphingolipids begins with the condensation of an amino acid, usually serine, with a fatty acid, usually 16 carbons in length, to produce an 18-carbon “sphingoid” base. Derivatization with another fatty acid produces ceramide, a membrane anchor that can be further derivatized with hydrophilic head groups to give rise to plasma membrane sphingolipids, which include sphingomyelin and the glycosphingolipids. Signaling lipids, ceramide, sphingosine, and sphingosine-1-phosphate are produced by the degradation of plasma membrane sphingolipids [2].
Sphingolipids are highly expressed in the brain. Gangliosides–glycosphingolipids containing one or more sialic acid residues–are prominent in the gray matter and are major components of neuronal membranes, where they make up to 10–12% of the total lipid content of neurons [3–5]. Glycosphingolipids in the form of cerebrosides (glucosylceramide and galactosylceramide) and sulfatides (sulfated galactosylceramide) are highly enriched in the white matter [6]. These sphingolipids represent up to 80% of the non-sterol lipid of the highly specialized myelin membranes, which are produced by oligodendrocytes and Schwann cells, and wrap axons to facilitate the neuronal transmission of electrical impulses [6].
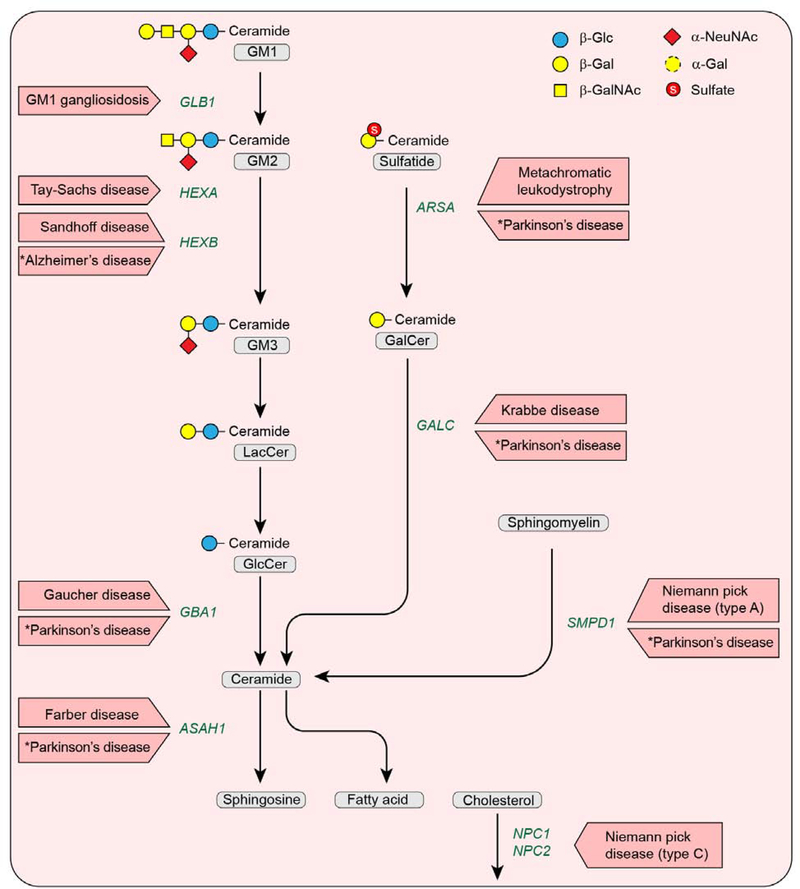
The major sphingolipid degradation pathway occurs in lysosomes and utilizes an orchestrated series of enzymatic reactions (Fig. 1). After the sphingolipids are internalized into lysosomes, hydrolases and activator proteins coordinate the removal of the hydrophilic head groups. For sphingomyelin, the phosphorylcholine head group is removed by acid sphingomyelinase to generate ceramide. For the glycosphingolipids, each sugar residue is removed by an individual reaction in a defined sequence until ceramide is produced. The ceramide backbone is then split by acid ceramidase into sphingosine and a fatty acid, both of which are transferred into the cytoplasm and recycled to serve as substrates in other biosynthetic pathways. If a single sphingolipid degradative step fails to occur due to gene mutation, the lipid substrate that is normally degraded by the missing hydrolase accumulates, giving rise to a lysosomal storage disease. These generally result in severe neurodegenerative diseases. Because the lipids that accumulate are sphingolipids, these disorders are termed sphingolipidoses. In these disorders, an enzyme-deficient cell type will “store” sphingolipid whether it is synthesized endogenously or comes from an exogenous source. The storage process can contribute to the pathogenic pathways of the sphingolipidoses in a number of ways (e.g., formation of toxic lipids, aberrant activation of signaling pathways, blockage in the endosomal-lysosomal pathway [which precludes autophagic function], and an increase in synaptic spines and formation of meganeurites) [7–12]. Inflammatory processes also contribute to disease progression [7, 8].
Fig. 1.
Disorders resulting from mutational defects in the lysosomal sphingolipid degradation pathway. Oligosaccharide structures are illustrated by colored symbols. Substrate names are presented in gray rounded boxes, genes are presented in green text, and disorders are presented in boxed red arrows. An asterisk (*) identifies a disease predisposition associated with a gene variant.
Microglia are the resident macrophages of the brain and have critical functions during development and homeostasis [13, 14]. They mediate injury responses and pathogen defense, and are a primary source of pro-inflammatory cytokines [14]. They also have important roles in the pathogenesis of neurodegenerative diseases [14].
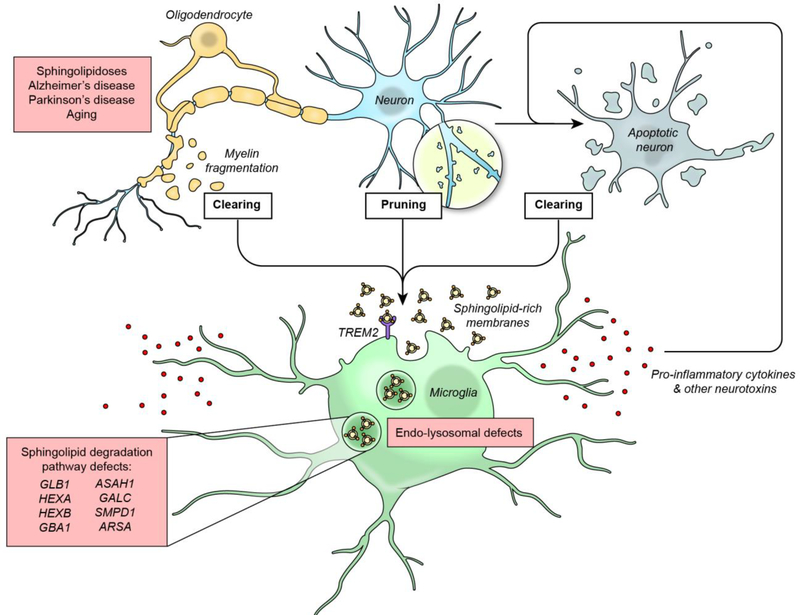
Normally microglia constantly surveil the brain parenchyma and eliminate dying neurons, remove neuronal synapses, and clean up myelin debris [13, 15–17] (Fig. 2). Sphingolipid-rich neuronal and myelin membranes captured through these processes undergo lysosomal degradation within microglia. This degradative process is facilitated by a lipid-sensing receptor, TREM2, that is activated by various lipids (including sphingolipids, sphingomyelin, and sulfatide) (Fig. 2). TREM2 signals via two receptor tyrosine kinases, DNAX-activation protein 10 (DAP10) and DAP12, to modulate several important microglial functions, including lipid uptake, transport, and processing [18, 19].
Fig. 2.
The sphingolipid degradation pathway and microglia in neurodegenerative disease. Microglia function during both development and homeostasis to eliminate dying neurons, prune neuronal synapses, and clear myelin debris. To accomplish these functions, sphingolipid-rich membranes are acquired from exogenous sources through the lipid-sensing receptor TREM2 and then undergo lysosomal degradation within microglia. During aging and the diseases indicated, these microglial processes are accelerated. Gene mutations in the sphingolipid degradation pathway genes reduce catabolic capacity and lead to lysosomal storage of sphingolipids, causing intrinsic microglial defects (such as a dysfunctional endosome-lysosome system), as well as compromising homeostatic functions and inducing a pro-inflammatory phenotype that promotes neurodegeneration.
To provide an understanding of how the sphingolipid degradation pathway influences microglial function, we review the relevant findings for the sphingolipid storage diseases in which the individual steps of the sphingolipid degradation pathway are disrupted. Microglia are directly affected by these mutations and promote neurodegeneration in the sphingolipidoses by impairment of their normal homeostatic functions and the acquisition of disease promoting characteristics. We also present evidence indicating that sphingolipid degradation pathway defects are a risk factor in common forms of neurodegeneration
2. Farber disease
2.1. Disease description
Farber disease is a rare autosomal recessive, progressive neurovisceral disorder that is characterized by macrophages ladened with lipid storage and inflammation throughout the body. Accumulation of ceramide in the visceral organs and cerebral white matter of severely affected Farber disease patients was first reported by Mosher and colleagues [20, 21]. The severity and symptoms vary among patients depending on the degree and location of the lipid build-up. The most common symptoms are subcutaneous nodules, joint deformities, and laryngeal hoarseness associated with neurological deficit. Patients with significant neurological involvement usually die early in infancy. Farber disease is a lysosomal storage disorder caused by mutations in the ASAH1 gene, which encodes acid ceramidase. Acid ceramidase catalyzes the degradation of ceramides to sphingosine and fatty acids (Fig. 1). Therefore, mutations of this gene cause ceramide accumulation in the lysosome [22].
2.2. Microglia in Farber disease
In a limited study of a Farber disease patient’s brain, the neurons were distended with storage materials and neuronal loss was accompanied by marked proliferation and activation of microglia and gliosis [21]. The ASAH1P361R/ P361R mouse model exhibits features of human neurovisceral Farber disease [23]. In ASAH1P361R/ P361R mouse brain, ceramide, hydroxyceramides, dihydroceramides, sphingosine, dihexosylceramides, and GM3 ganglioside were elevated [24]. Numerous microglial and/or macrophage abnormalities were noted as pathological hallmarks [24]. At 3 weeks of age, expanded foamy cells with varying levels of lipid storage appeared in the white matter. These cells formed fused multi-nucleated granuloma-like structures and frequently organized into perivascular cuffs at later ages.
Abnormal neuronophagic clusters appeared later only in specific gray matter regions, such as the hippocampal CA1 region, some thalamic nuclei, and layer II of the primary somatosensory cortex. At later ages, granuloma-like clustering was observed in the cerebellum. The morphologic alteration of neurons was not as pronounced as in the microglia. These findings indicate that microglial and/or macrophage abnormalities are a dominant neuropathological feature in Farber disease.
3. Gaucher disease
3.1. Disease description
Gaucher disease is an autosomal recessive lysosomal storage disorder characterized by the deficiency of lysosomal acid β-glucosylceramidase, which is caused by mutations in the GBA1 gene [7]. Specific GBA1 mutations correlate in part with disease severity and residual enzyme activity. Gaucher disease occurs as a neuropathic form with an early (type 2) or late onset (type 3), or as a systemic form (type 1) without central nervous system (CNS) involvement [25]. Neuropathic Gaucher disease is characterized by progressive neurodegeneration and brain inflammation [26].
β-glucosylceramidase is responsible for the removal of β-linked glucose from glucosylceramide to yield ceramide (Fig. 1) [27]. β-glucosylceramidase activity is dependent on the activator protein saposin C, which facilitates accessibility of the glycosphingolipid substrate [28]. Reduced β-glucosylceramidase activity due to mutations in GBA1 leads to the accumulation of glucosylceramide. Some accumulated glucosylceramide is converted by the action of acid ceramidase to its lyso-form, glucosylsphingosine, which also accumulates in Gaucher disease [29]. Glucosylceramide and glucosylsphingosine storage are particularly predominant in blood, liver, and spleen macrophages, which are enlarged with glycosphingolipid-loaded lysosomes and referred to as Gaucher cells [7]. Neuropathic forms of Gaucher disease are characterized by perivascular and parenchymal accumulation of Gaucher cells, accompanied by severe neuronal loss in several areas of the brain and brain stem, with extensive astrogliosis and microglial activation [30, 31].
3.2. Microglia in Gaucher disease
Accumulation of glucosylceramide and glucosylsphingosine in neuronopathic Gaucher disease leads to massive neuronal loss and inflammation in the brain [30, 32, 33]. Elevated levels of pro-inflammatory cytokines, nitric oxide, and reactive oxygen species were detected in fetal Gaucher disease brain [34]. An increase in inflammatory markers correlated with disease progression and severity in Gaucher disease mice [32]. In addition, a gene expression profile of a Gaucher disease type 2 patient brain revealed a pattern associated with activated astrocytes, a cell type that mediates injury responses and inflammation in the CNS [35].
Activated microglia are a common finding in the brain of Gaucher disease patients [35], and are the only myeloid population detected in significant numbers within the brain parenchyma in Gaucher disease mice [32, 34, 36]. Studies in mouse models indicate that while the neuronal deficiency of GBA1 is a primary determinant of CNS pathogenesis, GBA1-deficient microglia may also contribute by influencing the onset and progression of the disease [32, 37, 38]. Microglial activation together with astrogliosis are spatially and temporally correlated with neuronal loss, but it is not known if these inflammatory events precede or are concomitant with neuronal cell death [33, 37]. However, in a Gaucher disease zebrafish model, glycosphingolipid accumulation induced activated microglia and marked inflammation prior to neuronal loss [39]. Together, these findings support the idea that the activation of microglia in Gaucher disease stemming from the defective sphingolipid degradation pathway contributes to the process of neuronal cell death.
4. Krabbe disease
4.1. Disease description
Krabbe disease, also known as globoid cell leukodystrophy, is a rare autosomal recessive lysosomal storage disorder [40]. Krabbe disease is caused by mutations in the GALC gene resulting in a deficiency of the lysosomal enzyme β-galactocerebrosidase (Fig. 1). Functioning with activator saposin A, β-galactocerebrosidase removes β-linked galactose mainly from galactosylceramide and galactosylsphingosine (also known as psychosine) [41]. Psychosine, a bioactive lipid, accumulates at several hundred-fold levels above normal in Krabbe disease, leading to progressive demyelination and neurodegeneration in both the CNS and the peripheral nervous system (PNS) [42].
More than 90% of Krabbe disease cases have an onset before 6 months. Initial symptoms typically include hyperirritability, hypersensitivity, unknown episodic fever, and limb stiffness. The neurological dysfunction soon progresses into hypertonicity, as well as loss of vision and hearing. The affected patients have difficulty moving, eating, and breathing, which leads to death by the age of 2 [43].
4.2. Microglia in Krabbe disease
Multi-nucleated giant phagocytic globoid cell formation is the pathological hallmark for Krabbe disease [42]. In the brain, globoid cells may originate from residential microglia or infiltrating macrophages [44]. In vivo studies in twitcher mice, a naturally occurring mouse model of Krabbe disease, found that neither inflammation nor the globoid cell population was altered in the brain when macrophage infiltration is blocked by deficiency of the chemokine receptor CXCR2, suggesting that microglia, not infiltrating macrophages, are the major contributor to globoid cell formation in the CNS in Krabbe disease [45]. Moreover, in vitro, primary-cultured microglia formed multi-nucleated globoid cells after psychosine treatment [46], and became cytotoxic for oligodendrocytes [47].
In Krabbe disease, microglia play important roles in disease progression. They may induce astrogliosis via the inflammation mediator prostaglandin D2 and promote myelin loss and neurodegeneration [48]. Microglia are also needed for maintaining myelin homeostasis, and compromised microglia lead to increases in myelin debris and more deleterious demyelination in twitcher mice [49].
Activation of microglia and globoid cell formation are early events in Krabbe disease. Evidence of globoid cell formation was found in 21-week-old Krabbe disease human fetal spinal cord [50]. Activated microglia were observed in 2-week-old twitcher mouse brain before demyelination onset [51]. In mice carrying the E130K missense Krabbe disease mutation (which have a shorter lifespan than twitcher mice), gliosis, globoid cells, and psychosine accumulation were present without significant demyelination in the CNS [52], suggesting that neuroinflammation plays a critical role in disease progression independent of myelin loss.
Galc-deficient microglia in twitcher mice may also have intrinsic defects that contribute to Krabbe disease progression. Scott-Hewitt et al. [53] showed that Galc+/− mouse microglia had reduced upregulation of the TREM2 lipid-sensing receptor (Fig. 2), as well as decreased phagocytic clearance of myelin debris. In addition, PNS macrophages (microglia are not present in the PNS) require β-galactocerebrosidase for phagocytosis and myelin turnover [54]. In this paradigm, hematopoietic stem cell transplantation for Krabbe disease is beneficial by providing β-galactocerebrosidase-competent macrophages/microglia to restore the myelin phagocytosis function, thereby reducing the highly inflammatory globoid cell reaction [54].
5. GM1 gangliosidosis
5.1. Disease description
GM1 gangliosidosis is an autosomal recessive lysosomal storage disorder characterized by the deficiency of lysosomal acid β-galactosidase encoded by GLB1 [55]. β-Galactosidase cleaves β-linked terminal galactosyl residues from GM1 ganglioside (Fig. 1), as well as from glycoproteins and glycosaminoglycans [7]. GM1 ganglioside degradation does not require an activator protein, although it is enhanced by saposin B and GM2 activator protein [56].
GM1 gangliosidosis presents as a fatal infantile form, characterized by developmental arrest and rapid, progressive neurodegeneration. Less severe juvenile and chronic, adult forms of GM1 gangliosidosis also exist. In GM1 gangliosidosis, GM1 ganglioside accumulation occurs in the CNS and other organs, and neurons become filled with membranous cytoplasmic bodies and vacuoles as a result of the GM1 ganglioside storage [7, 57].
5.2. Microglia in GM1 gangliosidosis
Neuroinflammation plays an important role in disease progression for GM1 gangliosidosis, which has been most extensively studied in Glb1-deficient mice, a model that recapitulates features of the infantile form of GM1 gangliosidosis [58]. Brains of GM1 gangliosidosis mice exhibited elevated levels of inflammatory markers and cytokines [59, 60]. Microglial activation was noted in pre-symptomatic GM1 gangliosidosis mouse brains, starting in areas with the highest GM1 ganglioside accumulation and increasing gradually with disease progression, suggesting that microglial activation may contribute to neuronal death in this disease [59]. In addition, infiltration of monocytes and macrophages contributed to the inflammatory response, which was suppressed by bone marrow transplantation [60]. These results are consistent with a block in GM1 ganglioside degradation causing microglial activation and macrophage infiltration that is damaging to the nervous system.
6. Metachromatic leukodystrophy
6.1. Disease description
Metachromatic leukodystrophy (MLD) is a rare autosomal recessive lysosomal storage disease. MLD is caused by deficiency of lysosomal enzyme arylsulfatase A, which is encoded by the ARSA gene [61]. It cleaves 3-sulfate from the galactosyl moiety of sulfatide (Fig. 1), and requires an activator protein, saposin B [62]. In MLD, sulfatide storage was found in neurons, astrocytes, and activated macrophages/microglia, as well as in Schwann cells and oligodendrocytes [63], and leads to progressive demyelination and neurodegenerative manifestations [64].
Based on the disease onset, MLD is classified into three types: late-infantile (age of onset before 30 months); juvenile (2.5–16 years); and adult (after 16 years) [65]. Late-infantile onset accounts for nearly 50% of all cases worldwide, and is generally characterized by rapidly progressive psychomotor regression, including muscle weakness, areflexia, and ataxia. In the late stage of late-infantile onset disease, patients suffer from dysphagia, neuropathic pain, and severe foot deformities. Death usually occurs within a few years after disease onset.
6.2. Microglia in MLD
In human MLD, intense gliosis is marked in demyelinating areas [66, 67]. In an autopsy study, Bergner et al. [68] reported that in human MLD brains, activated amoeboid microglia could be found clustered in “prelesional” areas with normal white matter morphology. Microglia in prelesional areas showed evidence of apoptosis. Closer to the early gliotic scar areas, the percentage of amoeboid microglial cells increased and microglial decay/death became apparent, accompanied by lysosomal breakdown and cell membrane lysis. In contrast, in advanced gliotic scarring centers with complete demyelination, only myelin and oligodendrocyte remnants were observed. In mouse models, introduction of ARSA-competent macrophages/microglia into the MLD nervous system by transplantation with genetically engineered hematopoietic stem cells has been shown to be effective in reducing the severity of the disease [69].
Activation of microglia is an early event in MLD mouse models. ARSA knockout (KO) mice developed activated microglia with no obvious demyelination. In a demyelinating ARSA KO mouse model engineered to exhibit excess sulfatide synthesis, robust microglial activation and inflammatory cytokine (Ccl-2, Ccl-3, Ccl-4, and interleukins) elevation occurred before obvious demyelination appeared [70]. Correspondingly, several inflammatory cytokines (Ccl-2, Ccl-4, IL-1Ra, IL-8, and VEGF) were found to be significantly elevated in the cerebrospinal fluid of MLD patients [71]. In vitro, exposure of cultured primary microglia to sulfatide led to activation and production of inflammatory molecules [72].
These results point to a central pathophysiologic role of activated, inflammatory microglia in MLD stemming from their intrinsic inability to degrade the sphingolipid sulfatide within myelin membrane fragments.
7. Niemann-Pick disease type A
7.1. Disease description
Niemann-Pick disease type A (NPA) is a rare autosomal recessive, neurodegenerative disorder characterized by brain atrophy, hypomyelination, and Purkinje cell degeneration [73–76]. These defects lead to tremors and ataxia, and ultimately result in death in early childhood [7]. NPA is a lysosomal storage disorder caused by mutations in the SMPD1 gene, which encodes acid sphingomyelinase (ASM). ASM catalyzes the degradation of sphingomyelin to ceramide and phosphorylcholine (Fig. 1) [77, 78]. Mutations of this gene therefore cause build-up of sphingomyelin within lysosomes. In the nervous system, extensive sphingomyelin accumulation occurs in macrophages, vascular endothelial cells, and neurons. Schwann cells and, to a minor extent, oligodendrocytes also store sphingomyelin in NPA [79–82].
7.2. Microglia in NPA
Activated and expanded microglia were found in regions of the brain–cerebellum, cortex, and hippocampus–associated with neuronal death in NPA [83, 84]. Further, both inflammatory and anti-inflammatory activated microglia were detected in a mouse model of NPA. The anti-inflammatory microglia initially provided a beneficial function by clearing myelin debris, but became neurotoxic because of lipid overload caused by their intrinsic inability to degrade sphingomyelin. The sphingomyelin accumulation in microglia induced lysosomal damage, resulting in cathepsin B extracellular release, which was toxic to neurons [84]. The findings directly implicate the ASM-deficient microglia in the neurodegenerative process in NPA.
8. Niemann-Pick disease type C
8.1. Disease description
Niemann-Pick disease type C (NPC) is an autosomal recessive, neurodegenerative sphingolipid storage disorder that results in ataxia, dysarthria, dementia, dysphagia, and vertical supranuclear gaze palsy [85]. Disease presentation and symptom onset are variable, but most patients are diagnosed in late childhood and live until 10 to 25 years of age [86]. NPC is predominantly caused by mutations in the NPC1 gene (95% of cases), and very infrequently by mutations in the NPC2 gene [85]. NPC1 and NPC2 both reside in the late endosome/lysosome (LE/L), where NPC1 is an integral membrane protein and NPC2 is a soluble lumenal protein [87]. Both proteins have cholesterol-binding pockets (CBPs) and play a crucial role in regulating intracellular cholesterol levels [88].
In lysosomes, NPC2 binds to free cholesterol and transfers it to the NPC1 N-terminal CBP [87–89]. CBP-bound cholesterol is then moved to the NPC1 transmembrane sterol-sensing domain, from where the lipid is subsequently exported from the LE/L (Fig. 1) [89, 90]. Mutations in either NPC gene cause faulty lipid trafficking, resulting in cholesterol build-up in the LE/L [91]. Although cholesterol accumulation is the hallmark NPC cellular phenotype, mutations in NPC1 and NPC2 also result in sphingolipid accumulation in the LE/L [91, 92].
8.2. Microglia in NPC
Accumulation of cholesterol and sphingolipids were found in brain and other organs in NPC [91]. Because multiple lipids are stored, it is difficult to know the contribution of specific lipids to NPC disease progression. However, it is known that this sphingolipid accumulation occurs in axons of neurons in motor and sensory pathways, with pronounced effect on Purkinje cells in the cerebellum [93]. In fact, Lloyd-Evans et al. [94] found that sphingosine is the first lipid to build up in NPC-mutant cells, while the other lipids, including cholesterol, take longer to accumulate. The accumulation of sphingosine in NPC has been proposed to induce an imbalance in calcium homeostasis and affect lysosomal trafficking [94].
Microglial activation is a crucial CNS event in NPC that occurs before any neurodegeneration is evident [95]. Microglia are profoundly affected in NPC, with enhanced phagocytic uptake and impaired lipid processing that compromises their functions during development [95]. Changes in microglial morphology, gene expression, inflammatory markers, and phagocytic function provide evidence for a highly activated microglial state in NPC [95–97].
In an NPC mouse model, activated microglia showed significant reduction in expression of microglial lineage markers (CX3CR1 and CD11b) and increased expression of chemokines and innate immune receptors [96]. Cologna et al. [97] found an increase in inflammatory markers in cerebrospinal fluid of NPC patients, providing further evidence that neuroinflammation is associated with microglial activation and disease progression.
Loss of NPC1 enhanced phagocytic uptake and impaired lipid trafficking in microglia [95]. This resulted in accumulation of phagocytosed myelin in LE/L and multi-vesicular bodies, possibly resulting from impaired myelin degradation [95] due to impaired vesicular fusion. The increase in phagocytic activity, in addition to impaired lipid turnover, creates a severely dysfunctional microglial phenotype in NPC. Cougnoux et al. [96] found that deletion of IRF8, a transcription factor that enables microglial activation, slowed disease progression and prolonged lifespan in NPC1 KO mice. These findings support the notion that intrinsic changes in microglial function, caused by lipid storage, drive NPC disease progression in the CNS.
9. Sandhoff disease and Tay-Sachs disease
9.1. Disease description
Sandhoff and Tay-Sachs diseases are rare autosomal recessive, lysosomal storage disorders caused by the deficiency of the lysosomal hydrolase β-hexosaminidase. β-Hexosaminidases A and B are dimers composed of two subunits, α and β, encoded by the HEXA gene and HEXB gene, respectively. In Tay-Sachs disease, mutations in the HEXA gene result in a deficiency of β-hexosaminidase A (αβ); in Sandhoff disease, mutations in the HEXB gene result in a deficiency of both β-hexosaminidase A (αβ) and B (ββ) isozymes [98].
β-hexosaminidase A is the only isozyme that removes terminal β-linked N-acetyl-D-galactosamine residues from GM2 ganglioside to form GM3 ganglioside (Fig. 1). The enzyme requires the GM2 activator protein for its ganglioside-degrading activity. Deficiency of β-hexosaminidase A activity results in the accumulation of GM2 ganglioside, as well as GA2 glycolipid, in the lysosomes, forming lamellar membranous inclusions in neurons and glial cells in the CNS in both Sandhoff and Tay-Sachs diseases [7, 98, 99].
Sandhoff and Tay-Sachs diseases present as severe forms in childhood and adolescence, or as chronic, late-onset forms, characterized by progressive neurodegeneration. Symptoms include progressive motor deterioration, hypotonia, blindness, seizures, and macrocephaly in the infantile forms, with slower progression and milder symptoms in later onset forms [98].
9.2. Microglia in Sandhoff and Tay-Sachs diseases
Neuroinflammation has been shown to have a crucial role in the pathogenesis of Sandhoff and Tay-Sachs diseases, and the mechanisms involved have been most extensively studied in the Sandhoff disease (Hexb KO) mouse model, which recapitulates central aspects of both of these human diseases. In this model, increased pro-inflammatory cytokine expression in the brain correlates with disease manifestations [59, 100]. Moreover, deletion of the cytokine tumor necrosis factor-α decreased levels of astrogliosis and reduced neuronal cell death, with no alterations in neuronal storage of gangliosides, and slightly improved lifespan in Sandhoff disease mice [101].
Microglial activation plays a significant role in disease progression in Sandhoff and Tay-Sachs diseases. Evidence for intense inflammatory responses in the brain attributed to macrophage/microglial activation was found in Sandhoff and Tay-Sachs disease patients, as well as in Sandhoff disease mice [59, 100, 102, 103]. Activated microglial expansion and other inflammatory processes attributed to reactive microglia were found to precede the substantial neuronal cell death observed in Sandhoff disease mouse brain, suggesting that microglia drive the inflammatory response and contribute to disease progression, including neuronal cell death [59, 101, 102]. When β-hexosaminidase expression was induced specifically in neurons of Sandhoff disease mice, their lifespan was partially extended, with a substantial improvement in neuropathology [104, 105]. This incomplete rescue may point to an additional requirement for β-hexosaminidase expression in other CNS cell types, such as microglia. Interestingly, HEXB is highly expressed by microglia compared with other cell types in the CNS and is considered a microglial signature gene in mice [106]. Notably, deletion of hexb in zebrafish caused abnormalities in radial glia, which are neuronal and glial progenitors, and within microglia themselves [107].
In addition to microglia functioning as potentially damaging inflammatory mediators, peripheral monocytes and macrophages can infiltrate the brain in Sandhoff disease mice and contribute to inflammation [59, 100, 108, 109]. Supplying normal monocytes and macrophages through the transplantation of normal bone marrow into Sandhoff disease mice prolonged lifespan by suppression of both the explosive expansion of activated microglia and neuronal cell death, without detectable decreases in GM2 ganglioside storage [102, 110]. This effect was thought to be due to enzyme-competent monocytes/macrophages that had infiltrated the CNS. The suppression of enzyme-deficient monocyte infiltration from the peripheral circulation by deletion of monocyte chemoattractant protein-1α in Sandhoff disease mice retarded neurodegeneration [100, 109]. These studies support an important role for inflammation responses mediated by microglia and infiltrating monocytes/macrophages in the neurodegeneration that characterizes Sandhoff and Tay-Sachs diseases.
10. The sphingolipid degradation pathway and microglia in common forms of neurodegeneration
10.1. Aging
Aging is considered the primary risk factor for neurodegenerative disease [111]. During aging, microglia develop defective homeostatic functions and elevated reactive oxygen species and secrete pro-inflammatory cytokines–processes that may contribute to age-related neurodegeneration. Aged microglia have a defective lysosomal degradative pathway and accumulate inclusions and lipofucin (lipid-containing pigment) in their lysosomes [112, 113]. In mouse brain, debris from myelin fragmentation was found to be increased with age. These myelin fragments were cleared by microglia, but led to lysosomal accumulation of myelin fragments and lipofucin-like material. The accumulation of large amounts of myelin debris caused subsequent activation of microglia [16]. Thus, age-related myelin fragmentation is substantial, leading to lysosomal myelin storage and contributing to microglial senescence and immune dysfunction in aging. These results suggest that the microglial lysosomal degradative pathway for sphingolipid-rich myelin debris generated during aging is sensitive to overloading [16].
10.2. Alzheimer’s disease
Alzheimer’s disease, the most prevalent neurodegenerative disorder, is also the most common cause of age-related dementia [114]. Its pathological features include brain atrophy, neurodegeneration, extracellular amyloid plaque accumulation (amyloid β peptide aggregates), and tau protein neurofibrillary tangles. The accumulation of lipids in microglial cells of patients with Alzheimer’s disease was first observed by Alois Alzheimer in 1907 when he described glial cells with numerous fibers and adipose saccules [115]. Microglia are crucial for the preliminary defense against the progression of Alzheimer’s disease, in part because they surround initial fibrillar amyloid depositions and act as a stable barrier inhibiting polymerization [116]. They are also responsible for phagocytotic clearance of amyloid β [117]. Importantly, the majority of risk genes for Alzheimer’s disease are expressed in microglia, with many being microglia-specific [118].
Lipid sensing by microglia appears to reduce the risk for Alzheimer’s disease. A loss of function mutation in the TREM2 gene is associated with Alzheimer’s disease [119]. TREM2 is a transmembrane glycoprotein expressed on microglia that associates with transmembrane adapter DAP12 for downstream signaling to promote microglial proliferation and survival. TREM2 binds to and is activated by various membrane lipids, including the sphingolipids, sphingomyelin, and sulfatide. In the absence of TREM2, microglia accumulate sphingolipids after myelin fragmentation, suggesting that activation of TREM2 promotes the clearance of myelin fragments via the enhancement of the microglial lysosomal degradation pathway for sphingolipids [18, 19, 120].
HEXB (see Section 9) has been identified as a potential Alzheimer’s disease risk gene (Fig. 1) that is specifically expressed in microglia and responsive to amyloid β [121]. In the mouse model of Sandhoff disease (Hexb KO), aggregated proteins associated with Alzheimer’s disease, amyloid β peptide-like, and phospho-tau-like all accumulated in the brain [122]. These findings indicate that impaired degradation of GM2 ganglioside, which accumulates in Sandhoff disease, hinders the clearance of proteins connected with Alzheimer’s disease, possibly by impairment of lysosomal function [123, 124].
10.3. Parkinson’s disease
Parkinson’s disease is the second-most common neurodegenerative disorder. The clinical symptoms of this disease center around motor dysfunction, resulting in a resting tremor, rigid face muscles, and bradykinesia [125]. These symptoms are driven by the loss of dopaminergic neurons within the substantia nigra pars compacta. A hallmark of Parkinson’s disease is that the remaining neurons contain α-synuclein-positive inclusions, referred to as Lewy bodies [125]. Neuroinflammatory processes, including activated microglia, have been reported to play an important role in the pathophysiology of Parkinson’s disease [126].
Whereas biallelic mutations in GBA1 cause Gaucher disease (see Section 3), heterozygous mutations in GBA1 are the most important risk factor for developing Parkinson’s disease [127, 128]. Other damaging sphingolipid storage disease gene variants have also been identified in association with Parkinson’s disease risk. Variants of the SMPD1 gene, whose deficiency is responsible for NPA, the ASAH1 gene, whose deficiency causes Farber disease, GALC, whose deficiency causes Krabbe disease, and ARSA, whose deficiency is responsible for MLD, have been identified as candidate Parkinson’s disease susceptibility genes (Fig. 1) [129–131]. In addition, reduced activities of lysosomal sphingolipid hydrolases have been observed in Parkinson’s disease [132]. These findings link defects in the sphingolipid degradation pathway with increased risk for Parkinson’s disease. However, whether impaired sphingolipid degradation in microglia is a factor in Parkinson’s disease onset or progression remains to be determined.
11. Conclusions
Sphingolipids are widely expressed, and are relatively highly expressed in the brain. As a consequence, genetic disruption of the sphingolipid degradation pathway often causes catastrophic neurodegeneration. While the sphingolipid accumulation that occurs in the sphingolipidoses likely has a direct impact on most nervous system cell types, microglia are often affected. Evidence has emerged that sphingolipidosis microglia have intrinsic defects that can contribute to the disease process by the corruption of their normal homeostatic functions and by the acquisition of damaging pro-inflammatory phenotypes (Fig. 2).
The sphingolipid degradation pathway has been linked to more common forms of neurodegeneration. During aging, a major risk factor for neurodegeneration, sphingolipid-degrading enzyme activities are reduced [132], and microglial lipid storage is increased. In the more common neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, increased disease risk has been associated with genetic variants in the pathway of sphingolipid uptake and degradation (Fig. 1).
Normally, cells have essentially no lysosomal sphingolipid accumulation because the maximum turnover rate of the sphingolipid degradation pathway is much higher than the influx rate of the substrate entering into the lysosomal compartment [133]. Microglia must continually take up and degrade cell membranes containing high levels of sphingolipids during their developmental and homeostatic activities of synaptic pruning, removal of dying neurons, and clearance of myelin debris [13]. During aging and neurodegenerative disease, these processes are accelerated, increasing the sphingolipid substrate influx into the lysosomal compartment, which would be predicted to raise the critical threshold value for catabolic activity required to prevent sphingolipid storage [133]. Reductions in the catabolic activity of the pathway below this critical threshold value would therefore result in sphingolipid accumulation. When sphingolipid degradation pathway genes are mutated, cells with excessive sphingolipid burden, such as microglia and other phagocytic cells, may be affected early in the pathogenic process and contribute to neurodegeneration. Although this appears to be the case for some of the sphingolipidoses, further studies are needed to define the link between microglial sphingolipid degradation and the more common forms of neurodegeneration.
Highlights.
Sphingolipids are highly expressed in the brain
Sphingolipids are degraded in lysosomes by a sequence of enzymatic steps
Sphingolipidoses are caused by defects in sphingolipid degradation pathway genes
Sphingolipid degradation pathway genetic defects affect microglial functions
Altered microglial functions promote neurodegeneration in the sphingolipidoses
Gene variants in the sphingolipid degradation pathway may increase risk for more common neurodegenerative diseases
Acknowledgements:
We thank Linda Raab for expert editing of the manuscript.
Funding sources
This work was supported by the Intramural Research Programs of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health.
This review is dedicated to the memory of Lina Obeid
Abbreviations:
- ASM
acid sphingomyelinase
- CBP
cholesterol-binding pocket
- CNS
central nervous system
- DAP
DNAX-activation protein
- KO
knockout
- LE/L
late endosome/lysosome
- MLD
Metachromatic leukodystrophy
- NPA
Niemann-Pick disease type A
- NPC
Niemann-Pick disease type C
- PNS
peripheral nervous system
Footnotes
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Merrill AH Jr., Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics, Chem Rev 111(10) (2011) 6387–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hannun YA, Obeid LM, Principles of bioactive lipid signalling: lessons from sphingolipids, Nat Rev Mol Cell Biol 9(2) (2008) 139–50. [DOI] [PubMed] [Google Scholar]
- [3].Sandhoff R, Sandhoff K, Emerging concepts of ganglioside metabolism, FEBS Lett 592(23) (2018) 3835–3864. [DOI] [PubMed] [Google Scholar]
- [4].Ledeen RW, Yu RK, Gangliosides-structure, isolation, and analysis., Methods Enzymol. 83 (1982) 139–191. [DOI] [PubMed] [Google Scholar]
- [5].Tettamanti G, Ganglioside/glycosphingolipid turnover: new concepts, Glycoconj J 20(5) (2004) 301–17. [DOI] [PubMed] [Google Scholar]
- [6].Wattenberg BW, Intra- and intercellular trafficking in sphingolipid metabolism in myelination, Adv Biol Regul 71 (2019) 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Platt FM, d’Azzo A, Davidson BL, Neufeld EF, Tifft CJ, Lysosomal storage diseases, Nat Rev Dis Primers 4(1) (2018) 27. [DOI] [PubMed] [Google Scholar]
- [8].Ballabio A, Gieselmann V, Lysosomal disorders: from storage to cellular damage, Biochim Biophys Acta 1793(4) (2009) 684–96. [DOI] [PubMed] [Google Scholar]
- [9].Purpura DP, Suzuki K, Distortion of neuronal geometry and formation of aberrant synapses in neuronal storage disease, Brain Res 116(1) (1976) 1–21. [DOI] [PubMed] [Google Scholar]
- [10].Platt FM, Sphingolipid lysosomal storage disorders, Nature 510(7503) (2014) 68–75. [DOI] [PubMed] [Google Scholar]
- [11].Platt FM, Boland B, van der Spoel AC, The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction, J Cell Biol 199(5) (2012) 723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schulze H, Sandhoff K, Lysosomal lipid storage diseases, Cold Spring Harb Perspect Biol 3(6) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hammond TR, Robinton D, Stevens B, Microglia and the Brain: Complementary Partners in Development and Disease, Annu Rev Cell Dev Biol 34 (2018) 523–544. [DOI] [PubMed] [Google Scholar]
- [14].Colonna M, Butovsky O, Microglia Function in the Central Nervous System During Health and Neurodegeneration, Annu Rev Immunol 35 (2017) 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sakai J, Core Concept: How synaptic pruning shapes neural wiring during development and, possibly, in disease, Proc Natl Acad Sci U S A 117(28) (2020) 16096–16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M, Age-related myelin degradation burdens the clearance function of microglia during aging, Nat Neurosci 19(8) (2016) 995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takahashi K, Rochford CD, Neumann H, Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2, J Exp Med 201(4) (2005) 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, Lucas A, Baskaran S, Haddick PCG, Lenser M, Earr TK, Shi J, Dugas JC, Andreone BJ, Logan T, Solanoy HO, Chen H, Srivastava A, Poda SB, Sanchez PE, Watts RJ, Sandmann T, Astarita G, Lewcock JW, Monroe KM, Di Paolo G, TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge, Neuron 105(5) (2020) 837–854 e9. [DOI] [PubMed] [Google Scholar]
- [19].Poliani PL, Wang Y, Fontana E, Robinette ML, Yamanishi Y, Gilfillan S, Colonna M, TREM2 sustains microglial expansion during aging and response to demyelination, J Clin Invest 125(5) (2015) 2161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Prensky AL, Ferreira G, Carr S, Moser HW, Ceramide and Ganglioside Accumulation in Farber’s Lipogranulomatosis, Proceedings of the Society for Experimental Biology and Medicine 126(3) (1967) 725–728. [Google Scholar]
- [21].Moser HW, Prensky AL, Wolfe HJ, Rosman NP, Farber’s lipogranulomatosis. Report of a case and demonstration of an excess of free ceramide and ganglioside, Am J Med 47(6) (1969) 869–90. [DOI] [PubMed] [Google Scholar]
- [22].Ehlert K, Frosch M, Fehse N, Zander A, Roth J, Vormoor J, Farber disease: clinical presentation, pathogenesis and a new approach to treatment, Pediatr Rheumatol Online J 5 (2007) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alayoubi AM, Wang JC, Au BC, Carpentier S, Garcia V, Dworski S, El-Ghamrasni S, Kirouac KN, Exertier MJ, Xiong ZJ, Prive GG, Simonaro CM, Casas J, Fabrias G, Schuchman EH, Turner PV, Hakem R, Levade T, Medin JA, Systemic ceramide accumulation leads to severe and varied pathological consequences, EMBO Mol Med 5(6) (2013) 827–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sikora J, Dworski S, Jones EE, Kamani MA, Micsenyi MC, Sawada T, Le Faouder P, Bertrand-Michel J, Dupuy A, Dunn CK, Xuan ICY, Casas J, Fabrias G, Hampson DR, Levade T, Drake RR, Medin JA, Walkley SU, Acid Ceramidase Deficiency in Mice Results in a Broad Range of Central Nervous System Abnormalities, Am J Pathol 187(4) (2017) 864–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beutler E, Grabowski GA, Gaucher Disease, Scriver CR, Beaudet AL, Sly WS, Valle D (Eds). McGraw-Hill, NY, USA, In: The Metabolic and Molecular Bases of Inherited Disease, 2001, pp. 3635–3668 [Google Scholar]
- [26].Vitner EB, Futerman AH, Neuronal forms of Gaucher disease, In: Gulbins E, Petrache I (eds) Sphingolipids in Disease. Handbook of Experimental Pharmacology, Springer; 2013, pp. 405–419. [DOI] [PubMed] [Google Scholar]
- [27].Futerman AH, Platt FM, The metabolism of glucocerebrosides - From 1965 to the present, Mol Genet Metab 120(1–2) (2017) 22–26. [DOI] [PubMed] [Google Scholar]
- [28].Sun Y, Qi X, Grabowski GA, Saposin C is required for normal resistance of acid beta-glucosidase to proteolytic degradation, J Biol Chem 278(34) (2003) 31918–23. [DOI] [PubMed] [Google Scholar]
- [29].Ferraz MJ, Marques AR, Appelman MD, Verhoek M, Strijland A, Mirzaian M, Scheij S, Ouairy CM, Lahav D, Wisse P, Overkleeft HS, Boot RG, Aerts JM, Lysosomal glycosphingolipid catabolism by acid ceramidase: formation of glycosphingoid bases during deficiency of glycosidases, FEBS Lett 590(6) (2016) 716–25. [DOI] [PubMed] [Google Scholar]
- [30].Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, Morrison A, Lwin A, Colegial C, Allman JM, Schiffmann R, Neuropathology provides clues to the pathophysiology of Gaucher disease, Mol Genet Metab 82(3) (2004) 192–207. [DOI] [PubMed] [Google Scholar]
- [31].Bosch ME, Kielian T, Neuroinflammatory paradigms in lysosomal storage diseases, Front Neurosci 9 (2015) 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vitner EB, Farfel-Becker T, Eilam R, Biton I, Futerman AH, Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease, Brain 135(Pt 6) (2012) 1724–35. [DOI] [PubMed] [Google Scholar]
- [33].Farfel-Becker T, Vitner EB, Pressey SN, Eilam R, Cooper JD, Futerman AH, Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease, Hum Mol Genet 20(7) (2011) 1375–86. [DOI] [PubMed] [Google Scholar]
- [34].Hong YB, Kim EY, Jung SC, Upregulation of proinflammatory cytokines in the fetal brain of the Gaucher mouse, J Korean Med Sci 21(4) (2006) 733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Myerowitz R, Mizukami H, Richardson KL, Finn LS, Tifft CJ, Proia RL, Global gene expression in a type 2 Gaucher disease brain, Mol Genet Metab 83(4) (2004) 288–96. [DOI] [PubMed] [Google Scholar]
- [36].Cho SM, Vardi A, Platt N, Futerman AH, Absence of infiltrating peripheral myeloid cells in the brains of mouse models of lysosomal storage disorders, J Neurochem 148(5) (2019) 625–638. [DOI] [PubMed] [Google Scholar]
- [37].Enquist IB, Lo Bianco C, Ooka A, Nilsson E, Mansson JE, Ehinger M, Richter J, Brady RO, Kirik D, Karlsson S, Murine models of acute neuronopathic Gaucher disease, Proc Natl Acad Sci U S A 104(44) (2007) 17483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Massaro G, Hughes MP, Whaler SM, Wallom KL, Priestman DA, Platt FM, Waddington SN, Rahim AA, Systemic AAV9 gene therapy using the synapsin I promoter rescues a mouse model of neuronopathic Gaucher disease but with limited cross-correction potential to astrocytes, Hum Mol Genet (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Keatinge M, Bui H, Menke A, Chen YC, Sokol AM, Bai Q, Ellett F, Da Costa M, Burke D, Gegg M, Trollope L, Payne T, McTighe A, Mortiboys H, de Jager S, Nuthall H, Kuo MS, Fleming A, Schapira AH, Renshaw SA, Highley JR, Chacinska A, Panula P, Burton EA, O’Neill MJ, Bandmann O, Glucocerebrosidase 1 deficient Danio rerio mirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death, Hum Mol Genet 24(23) (2015) 6640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bascou N, DeRenzo A, Poe MD, Escolar ML, A prospective natural history study of Krabbe disease in a patient cohort with onset between 6 months and 3 years of life, Orphanet J Rare Dis 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hill CH, Cook GM, Spratley SJ, Fawke S, Graham SC, Deane JE, The mechanism of glycosphingolipid degradation revealed by a GALC-SapA complex structure, Nat Commun 9(1) (2018) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suzuki K, Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status, Neurochem Res 23(3) (1998) 251–9. [DOI] [PubMed] [Google Scholar]
- [43].Wenger DA, Rafi MA, Luzi P, Datto J, Costantino-Ceccarini E, Krabbe disease: genetic aspects and progress toward therapy, Mol Genet Metab 70(1) (2000) 1–9. [DOI] [PubMed] [Google Scholar]
- [44].Borda JT, Alvarez X, Mohan M, Ratterree MS, Phillippi-Falkenstein K, Lackner AA, Bunnell BA, Clinical and immunopathologic alterations in rhesus macaques affected with globoid cell leukodystrophy, Am J Pathol 172(1) (2008) 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reddy AS, Patel JR, Vogler C, Klein RS, Sands MS, Central nervous system pathology progresses independently of KC and CXCR2 in globoid-cell leukodystrophy, PLoS One 8(6) (2014) e64647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ijichi K, Brown GD, Moore CS, Lee JP, Winokur PN, Pagarigan R, Snyder EY, Bongarzone ER, Crocker SJ, MMP-3 mediates psychosine-induced globoid cell formation: implications for leukodystrophy pathology, Glia 61(5) (2013) 765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Claycomb KI, Winokur PN, Johnson KM, Nicaise AM, Giampetruzzi AW, Sacino AV, Snyder EY, Barbarese E, Bongarzone ER, Crocker SJ, Aberrant production of tenascin-C in globoid cell leukodystrophy alters psychosine-induced microglial functions, J Neuropathol Exp Neurol 73(10) (2014) 964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, Fukumoto N, Eguchi N, Kushi A, Sasai H, Kanaoka Y, Ozono K, Narumiya S, Suzuki K, Urade Y, Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher, J Neurosci 26(16) (2006) 4383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kondo Y, Adams JM, Vanier MT, Duncan ID, Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy, J Neurosci 31(10) (2011) 3610–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ida H, Rennert OM, Watabe K, Eto Y, Maekawa K, Pathological and biochemical studies of fetal Krabbe disease, Brain Dev 16(6) (1994) 480–4. [DOI] [PubMed] [Google Scholar]
- [51].Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, MacLean AG, Peterson KE, Lackner AA, Bunnell BA, Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy, Am J Pathol 184(2) (2014) 382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Potter GB, Santos M, Davisson MT, Rowitch DH, Marks DL, Bongarzone ER, Petryniak MA, Missense mutation in mouse GALC mimics human gene defect and offers new insights into Krabbe disease, Human Molecular Genetics 22(17) (2013) 3397–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Scott-Hewitt NJ, Folts CJ, Hogestyn JM, Piester G, Mayer-Proschel M, Noble MD, Heterozygote galactocerebrosidase (GALC) mutants have reduced remyelination and impaired myelin debris clearance following demyelinating injury, Human Molecular Genetics 26(15) (2017) 2825–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Weinstock NI, Shin D, Dhimal N, Hong X, Irons EE, Silvestri NJ, Reed CB, Nguyen D, Sampson O, Cheng YC, Lau JTY, Bongarzone ER, Kofler J, Escolar ML, Gelb MH, Wrabetz L, Feltri ML, Macrophages Expressing GALC Improve Peripheral Krabbe Disease by a Mechanism Independent of Cross-Correction, Neuron (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Okada S, O’Brien JS, Generalized gangliosidosis: beta-galactosidase deficiency, Science 160(3831) (1968) 1002–4. [DOI] [PubMed] [Google Scholar]
- [56].Wilkening G, Linke T, Uhlhorn-Dierks G, Sandhoff K, Degradation of membrane-bound ganglioside GM1. Stimulation by bis(monoacylglycero)phosphate and the activator proteins SAP-B and GM2-AP, J Biol Chem 275(46) (2000) 35814–9. [DOI] [PubMed] [Google Scholar]
- [57].Suzuki Y, Oshima A, Nanba E, Beta-galactosidase deficiency (beta-galactosidosis): GM1 gangliosidosis and Morquio B disease, Scriver CR, Beaudet AL, Sly WS, Valle D (Eds). McGraw-Hill, NY, USA, In: The Metabolic and Molecular Bases of Inherited Disease., 2001, pp. 3775–3809. [Google Scholar]
- [58].Hahn CN, del Pilar Martin M, Schroder M, Vanier MT, Hara Y, Suzuki K, Suzuki K, d’Azzo A, Generalized CNS disease and massive GM1-ganglioside accumulation in mice defective in lysosomal acid beta-galactosidase, Hum Mol Genet 6(2) (1997) 205–11. [DOI] [PubMed] [Google Scholar]
- [59].Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d’Azzo A, Perry VH, Butters TD, Dwek RA, Platt FM, Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis, Brain 126(Pt 4) (2003) 974–87. [DOI] [PubMed] [Google Scholar]
- [60].Sano R, Tessitore A, Ingrassia A, d’Azzo A, Chemokine-induced recruitment of genetically modified bone marrow cells into the CNS of GM1-gangliosidosis mice corrects neuronal pathology, Blood 106(7) (2005) 2259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Austin JH, Balasubramanian AS, Pattabiraman TN, Saraswathi S, Basu DK, Bachhawat BK, A Controlled Study of Enzymic Activities in Three Human Disorders of Glycolipid Metabolism, J Neurochem 10 (1963) 805–16. [DOI] [PubMed] [Google Scholar]
- [62].Fischer G, Jatzkewitz H, The activator of cerebroside sulphatase. Purification from human liver and identification as a protein, Hoppe Seylers Z Physiol Chem 356(5) (1975) 605–13. [DOI] [PubMed] [Google Scholar]
- [63].Eckhardt M, The role and metabolism of sulfatide in the nervous system, Mol Neurobiol 37(2–3) (2008) 93–103. [DOI] [PubMed] [Google Scholar]
- [64].van Rappard DF, Boelens JJ, Wolf NI, Metachromatic leukodystrophy: Disease spectrum and approaches for treatment, Best Pract Res Clin Endocrinol Metab 29(2) (2015) 261–73. [DOI] [PubMed] [Google Scholar]
- [65].Beerepoot S, Nierkens S, Boelens JJ, Lindemans C, Bugiani M, Wolf NI, Peripheral neuropathy in metachromatic leukodystrophy: current status and future perspective, Orphanet J Rare Dis 14(1) (2019) 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Eichler F, Van Haren K, Immune response in leukodystrophies, Pediatr Neurol 37(4) (2007) 235–44. [DOI] [PubMed] [Google Scholar]
- [67].Takashima S, Matsui A, Fujii Y, Nakamura H, Clinicopathological differences between juvenile and late infantile metachromatic leukodystrophy, Brain Dev 3(4) (1981) 365–74. [DOI] [PubMed] [Google Scholar]
- [68].Bergner CG, van der Meer F, Winkler A, Wrzos C, Turkmen M, Valizada E, Fitzner D, Hametner S, Hartmann C, Pfeifenbring S, Stoltenburg-Didinger G, Bruck W, Nessler S, Stadelmann C, Microglia damage precedes major myelin breakdown in X-linked adrenoleukodystrophy and metachromatic leukodystrophy, Glia 67(6) (2019) 1196–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, Sessa M, Fasano S, Brambilla R, Marchesini S, Bordignon C, Naldini L, Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells, J Clin Invest 113(8) (2004) 1118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stein A, Stroobants S, Gieselmann V, D’Hooge R, Matzner U, Anti-inflammatory Therapy With Simvastatin Improves Neuroinflammation and CNS Function in a Mouse Model of Metachromatic Leukodystrophy, Mol Ther 23(7) (2015) 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thibert KA, Raymond GV, Tolar J, Miller WP, Orchard PJ, Lund TC, Cerebral Spinal Fluid levels of Cytokines are elevated in Patients with Metachromatic Leukodystrophy, Sci Rep 6 (2016) 24579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jeon SB, Yoon HJ, Park SH, Kim IH, Park EJ, Sulfatide A Major Lipid Component of Myelin Sheath, Activates Inflammatory Responses as an Endogenous Stimulator in Brain-Resident Immune Cells, Journal of Immunology 181(11) (2008) 8077–8087. [DOI] [PubMed] [Google Scholar]
- [73].Di Rocco M, Rossi A, Parenti G, Allegri AE, Filocamo M, Pessagno A, Tortori-Donati P, Minetti C, Biancheri R, Different molecular mechanisms leading to white matter hypomyelination in infantile onset lysosomal disorders, Neuropediatrics 36(4) (2005) 265–9. [DOI] [PubMed] [Google Scholar]
- [74].Buccinna B, Piccinini M, Prinetti A, Scandroglio F, Prioni S, Valsecchi M, Votta B, Grifoni S, Lupino E, Ramondetti C, Schuchman EH, Giordana MT, Sonnino S, Rinaudo MT, Alterations of myelin-specific proteins and sphingolipids characterize the brains of acid sphingomyelinase-deficient mice, an animal model of Niemann-Pick disease type A, J Neurochem 109(1) (2009) 105–15. [DOI] [PubMed] [Google Scholar]
- [75].Perez-Canamas A, Benvegnu S, Rueda CB, Rabano A, Satrustegui J, Ledesma MD, Sphingomyelin-induced inhibition of the plasma membrane calcium ATPase causes neurodegeneration in type A Niemann-Pick disease, Mol Psychiatry 22(5) (2017) 711–723. [DOI] [PubMed] [Google Scholar]
- [76].Schuchman EH, Desnick RJ, Types A and B Niemann-Pick disease, Mol Genet Metab 120(1–2) (2017) 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kornhuber J, Rhein C, Muller CP, Muhle C, Secretory sphingomyelinase in health and disease, Biol Chem 396(6–7) (2015) 707–36. [DOI] [PubMed] [Google Scholar]
- [78].Shanbhogue P, Hannun YA, Exploring the Therapeutic Landscape of Sphingomyelinases, Handb Exp Pharmacol 259 (2020) 19–47. [DOI] [PubMed] [Google Scholar]
- [79].Kuemmel TA, Schroeder R, Stoffel W, Light and electron microscopic analysis of the central and peripheral nervous systems of acid sphingomyelinase-deficient mice resulting from gene targeting, J Neuropathol Exp Neurol 56(2) (1997) 171–9. [DOI] [PubMed] [Google Scholar]
- [80].Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH, Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease, Nat Genet 10(3) (1995) 288–93. [DOI] [PubMed] [Google Scholar]
- [81].Otterbach B, Stoffel W, Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann-Pick disease), Cell 81(7) (1995) 1053–61. [DOI] [PubMed] [Google Scholar]
- [82].Macauley SL, Sidman RL, Schuchman EH, Taksir T, Stewart GR, Neuropathology of the acid sphingomyelinase knockout mouse model of Niemann-Pick A disease including structure-function studies associated with cerebellar Purkinje cell degeneration, Exp Neurol 214(2) (2008) 181–92. [DOI] [PubMed] [Google Scholar]
- [83].Sarna J, Miranda SR, Schuchman EH, Hawkes R, Patterned cerebellar Purkinje cell death in a transgenic mouse model of Niemann Pick type A/B disease, Eur J Neurosci 13(10) (2001) 1873–80. [DOI] [PubMed] [Google Scholar]
- [84].Gabande-Rodriguez E, Perez-Canamas A, Soto-Huelin B, Mitroi DN, Sanchez-Redondo S, Martinez-Saez E, Venero C, Peinado H, Ledesma MD, Lipid-induced lysosomal damage after demyelination corrupts microglia protective function in lysosomal storage disorders, EMBO J 38(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vanier MT, Niemann-Pick diseases, Handb Clin Neurol 113 (2013) 1717–21. [DOI] [PubMed] [Google Scholar]
- [86].Vanier MT, Wenger DA, Comly ME, Rousson R, Brady RO, Pentchev PG, Niemann-Pick disease group C: clinical variability and diagnosis based on defective cholesterol esterification: A collaborative study on 70 patients, Clinical Genetics 33(5) (1988) 331–348. [DOI] [PubMed] [Google Scholar]
- [87].Li X, Saha P, Li J, Blobel G, Pfeffer SR, Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2, Proc Natl Acad Sci U S A 113(36) (2016) 10079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Subramanian K, Balch WE, NPC1/NPC2 function as a tag team duo to mobilize cholesterol, Proceedings of the National Academy of Sciences 105(40) (2008) 15223–15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Saha P, Shumate JL, Caldwell JG, Elghobashi-Meinhardt N, Lu A, Zhang L, Olsson NE, Elias JE, Pfeffer SR, Inter-domain dynamics drive cholesterol transport by NPC1 and NPC1L1 proteins, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE, Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol, Cell 137(7) (2009) 1213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Newton J, Milstien S, Spiegel S, Niemann-Pick type C disease: The atypical sphingolipidosis, Adv Biol Regul 70 (2018) 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Höglinger D, Nadler A, Haberkant P, Kirkpatrick J, Schifferer M, Stein F, Hauke S, Porter FD, Schultz C, Trifunctional lipid probes for comprehensive studies of single lipid species in living cells, Proceedings of the National Academy of Sciences 114(7) (2017) 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Arenas F, Garcia-Ruiz C, Fernandez-Checa JC, Intracellular Cholesterol Trafficking and Impact in Neurodegeneration, Front Mol Neurosci 10 (2017) 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM, Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium, Nat Med 14(11) (2008) 1247–55. [DOI] [PubMed] [Google Scholar]
- [95].Colombo A, Dinkel L, Müller SA, Monasor LS, Schifferer M, Cantuti-Castelvetri L, König J, Vidatic L, Bremova-Ertl T, Hecimovic S, Simons M, Lichtenthaler SF, Strupp M, Schneider SA, Tahirovic S, Loss of NPC1 enhances phagocytic uptake and impairs lipid trafficking in microglia, bioRxiv (2019) 789511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cougnoux A, Drummond RA, Collar AL, Iben JR, Salman A, Westgarth H, Wassif CA, Cawley NX, Farhat NY, Ozato K, Lionakis MS, Porter FD, Microglia activation in Niemann-Pick disease, type C1 is amendable to therapeutic intervention, Hum Mol Genet 27(12) (2018) 2076–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cologna SM, Cluzeau CV, Yanjanin NM, Blank PS, Dail MK, Siebel S, Toth CL, Wassif CA, Lieberman AP, Porter FD, Human and mouse neuroinflammation markers in Niemann-Pick disease, type C1, J Inherit Metab Dis 37(1) (2014) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gravel RA, Kaback MM, Proia RL, Sandhoff K, Suzuki K, Suzuki K, The GM2 gangliosidoses, Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, Kinzler KW and Vogelstein B (eds). McGraw-Hill, NY, USA, In: The Metabolic and Molecular Basis of Inherited Disease., 2001, pp. 3827–3876. [Google Scholar]
- [99].Breiden B, Sandhoff K, Lysosomal Glycosphingolipid Storage Diseases, Annu Rev Biochem 88 (2019) 461–485. [DOI] [PubMed] [Google Scholar]
- [100].Kyrkanides S, Miller AW, Miller JN, Tallents RH, Brouxhon SM, Olschowka ME, O’Banion MK, Olschowka JA, Peripheral blood mononuclear cell infiltration and neuroinflammation in the HexB−/− mouse model of neurodegeneration, J Neuroimmunol 203(1) (2008) 50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Abo-Ouf H, Hooper AW, White EJ, Janse van Rensburg HJ, Trigatti BL, Igdoura SA, Deletion of tumor necrosis factor-alpha ameliorates neurodegeneration in Sandhoff disease mice, Hum Mol Genet 22(19) (2013) 3960–75. [DOI] [PubMed] [Google Scholar]
- [102].Wada R, Tifft CJ, Proia RL, Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation, Proc Natl Acad Sci U S A 97(20) (2000) 10954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Myerowitz R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL, Molecular pathophysiology in Tay-Sachs and Sandhoff diseases as revealed by gene expression profiling, Hum Mol Genet 11(11) (2002) 1343–50. [DOI] [PubMed] [Google Scholar]
- [104].Kyrkanides S, Brouxhon SM, Tallents RH, Miller JN, Olschowka JA, O’Banion MK, Conditional expression of human beta-hexosaminidase in the neurons of Sandhoff disease rescues mice from neurodegeneration but not neuroinflammation, J Neuroinflammation 9 (2012) 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sargeant TJ, Drage DJ, Wang S, Apostolakis AA, Cox TM, Cachon-Gonzalez MB, Characterization of inducible models of Tay-Sachs and related disease, PLoS Genet 8(9) (2012) e1002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Masuda T, Amann L, Sankowski R, Staszewski O, Lenz M, d Errico P, Snaidero N, Costa Jordao MJ, Bottcher C, Kierdorf K, Jung S, Priller J, Misgeld T, Vlachos A, Luehmann MM, Knobeloch KP, Prinz M, Novel Hexb-based tools for studying microglia in the CNS, Nat Immunol 21(7) (2020) 802–815. [DOI] [PubMed] [Google Scholar]
- [107].Kuil LE, Lopez Marti A, Carreras Mascaro A, van den Bosch JC, van den Berg P, van der Linde HC, Schoonderwoerd K, Ruijter GJG, van Ham TJ, Hexb enzyme deficiency leads to lysosomal abnormalities in radial glia and microglia in zebrafish brain development, Glia 67(9) (2019) 1705–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL, Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease, Hum Mol Genet 17(15) (2008) 2257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wu YP, Proia RL, Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice, Proc Natl Acad Sci U S A 101(22) (2004) 8425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Norflus F, Tifft CJ, McDonald MP, Goldstein G, Crawley JN, Hoffmann A, Sandhoff K, Suzuki K, Proia RL, Bone marrow transplantation prolongs life span and ameliorates neurologic manifestations in Sandhoff disease mice, J Clin Invest 101(9) (1998) 1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA, Ageing as a risk factor for neurodegenerative disease, Nat Rev Neurol 15(10) (2019) 565–581. [DOI] [PubMed] [Google Scholar]
- [112].Mosher KI, Wyss-Coray T, Microglial dysfunction in brain aging and Alzheimer’s disease, Biochem Pharmacol 88(4) (2014) 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T, Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain, Nat Neurosci 23(2) (2020) 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL, Alzheimer’s disease, Nat Rev Dis Primers 1 (2015) 15056. [DOI] [PubMed] [Google Scholar]
- [115].Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR, An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”, Clin Anat 8(6) (1995) 429–31. [DOI] [PubMed] [Google Scholar]
- [116].Condello C, Yuan P, Schain A, Grutzendler J, Microglia constitute a barrier that prevents neurotoxic protofibrillar Abeta42 hotspots around plaques, Nat Commun 6 (2015) 6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sarlus H, Heneka MT, Microglia in Alzheimer’s disease, J Clin Invest 127(9) (2017) 3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hansen DV, Hanson JE, Sheng M, Microglia in Alzheimer’s disease, J Cell Biol 217(2) (2018) 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K, Variant of TREM2 associated with the risk of Alzheimer’s disease, N Engl J Med 368(2) (2013) 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M, TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model, Cell 160(6) (2015) 1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sierksma A, Lu A, Mancuso R, Fattorelli N, Thrupp N, Salta E, Zoco J, Blum D, Buee L, De Strooper B, Fiers M, Novel Alzheimer risk genes determine the microglia response to amyloid-beta but not to TAU pathology, EMBO Mol Med 12(3) (2020) e10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Keilani S, Lun Y, Stevens AC, Williams HN, Sjoberg ER, Khanna R, Valenzano KJ, Checler F, Buxbaum JD, Yanagisawa K, Lockhart DJ, Wustman BA, Gandy S, Lysosomal dysfunction in a mouse model of Sandhoff disease leads to accumulation of ganglioside-bound amyloid-beta peptide, J Neurosci 32(15) (2012) 5223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Walter J, van Echten-Deckert G, Cross-talk of membrane lipids and Alzheimer-related proteins, Mol Neurodegener 8 (2013) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].van Echten-Deckert G, Walter J, Sphingolipids: critical players in Alzheimer’s disease, Prog Lipid Res 51(4) (2012) 378–93. [DOI] [PubMed] [Google Scholar]
- [125].Corti O, Lesage S, Brice A, What genetics tells us about the causes and mechanisms of Parkinson’s disease, Physiol Rev 91(4) (2011) 1161–218. [DOI] [PubMed] [Google Scholar]
- [126].Wang Q, Liu Y, Zhou J, Neuroinflammation in Parkinson’s disease and its potential as therapeutic target, Transl Neurodegener 4 (2015) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG, Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease, N Engl J Med 361(17) (2009) 1651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sidransky E, Samaddar T, Tayebi N, Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset, Neurology 73(17) (2009) 1424–5, author reply 1425–6. [DOI] [PubMed] [Google Scholar]
- [129].Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, C. International Parkinson’s Disease Genomics, T. andMe Research, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR, A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci, Nat Genet 49(10) (2017) 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lee JS, Kanai K, Suzuki M, Kim WS, Yoo HS, Fu Y, Kim DK, Jung BC, Choi M, Oh KW, Li Y, Nakatani M, Nakazato T, Sekimoto S, Funayama M, Yoshino H, Kubo SI, Nishioka K, Sakai R, Ueyama M, Mochizuki H, Lee HJ, Sardi SP, Halliday GM, Nagai Y, Lee PH, Hattori N, Lee SJ, Arylsulfatase A, a genetic modifier of Parkinson’s disease, is an alpha-synuclein chaperone, Brain 142(9) (2019) 2845–2859. [DOI] [PubMed] [Google Scholar]
- [131].Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, C. International Parkinson’s Disease Genomics, Heutink P, Shulman JM, Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease, Brain 140(12) (2017) 3191–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Huebecker M, Moloney EB, van der Spoel AC, Priestman DA, Isacson O, Hallett PJ, Platt FM, Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease, Mol Neurodegener 14(1) (2019) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Conzelmann E, Sandhoff K, Partial enzyme deficiencies: residual activities and the development of neurological disorders, Dev Neurosci 6(1) (1983) 58–71. [DOI] [PubMed] [Google Scholar]