Abstract
Background
Around 20% of patients hospitalized for COVID-19 need mechanical ventilation (MV). MV may be prolonged, thus warranting tracheostomy.
Methods
Observational cohort study enrolling patients admitted due to COVID-19. Demographic and clinical data at hospital and ICU admission were collected. The primary endpoint was to identify parameters associated with a need for tracheostomy; secondary endpoints were to analyze the clinical course of patients who needed tracheostomy.
Results
118 patients were enrolled; 37 patients (31.5%) were transferred to ICU, of which 11 (29.72%) needed a tracheostomy due to prolonged MV. Sequential Organ Failure Assessment (SOFA) score at ICU admission (OR 0.65, 95% CI 0.47–0.92, p 0.015) was the only variable found to be associated with increased risk of the need for tracheostomy, with a cut-off point of 4.5 (sensitivity 0.72, specificity 0.73, positive predictive value 0.57 and negative predictive value 0.85). The main complications were nosocomial infection (100%), supraventricular cardiac arrhythmia (45.5%), agitation (54.5%), pulmonary thromboembolism (9.1%) and depression (9.1%). All patients presented with hypoalbuminemia and significant critical illness polyneuropathy.
Conclusion
SOFA at ICU admission is associated with an increased risk of tracheostomy in patients with COVID-19. Moreover, they present clinical features similar to those with chronic critical illness and suffer SARS-CoV-2-related complications.
Keywords: COVID-19, Tracheostomy, Prolonged mechanical ventilation, Respiratory failure
Introduction
On March 11 2020, coronavirus disease 2019 (COVID-19), caused by novel coronavirus SARS-CoV-2, was declared a global health emergency and pandemic by the World Health Organization [1]. The clinical spectrum of COVID-19 appears broad and can present as asymptomatic infection, mild upper respiratory tract illness or severe bilateral pneumonia 1 or 2 weeks after primary infection, which may progress to acute respiratory distress syndrome (ARDS) [2]. Under these circumstances, around 20% of hospitalized patients may need respiratory support and admittance to an intensive care unit (ICU) [2].
Published data on the need for invasive mechanical ventilation (MV) in COVID-19 indicate that it is required in 3–17% of hospitalized patients [2–4]. Time from disease onset to MV has been established at 14.5 days [4]. Duration of MV is prolonged in some cases, sometimes exceeding 20 days [5]. Tracheostomy is recommended in patients with prolonged mechanical ventilation (PMV) [6] as it facilitates weaning from ventilation and potentially increases ICU bed availability if these patients are transferred to specialized areas such as Respiratory Care Units (RCU). Turri-Manzone et al. recently reported that around 32% of patients with COVID-19-related MV underwent elective tracheostomy due to PMV [5]. PMV is one of the principal factors determining the transition from acute critical process to chronic critical illness (CCI). A CCI patient is defined as having survived acute illness or injury but not yet recovered to the point of liberation from life-sustaining therapies [7].
The aim of our study is to identify which factors may predict the need for tracheostomy in patients with PMV due to COVID-19 and describe their clinical course and prognosis.
Methods
Type of study
Observational cohort study with patients admitted to both the Respiratory Medicine Department and ICU of a tertiary university hospital with a diagnosis of SARS-CoV-2.
Population
All patients admitted to our hospital for COVID-19 from February 27 to May 20 were included in a general registry. COVID-19 diagnosis was made according to WHO interim guidance [8] by real-time reverse-transcription polymerase chain reaction assay for nasal and pharyngeal swab specimens. Exclusion criteria were age under 18 years and refusal to participate in the study. All patients were managed according to the protocol set by Spanish health authorities (Ministerio de Sanidad, Consumo y Bienestar Social) [9]. Treatment of any disease produced by SARS-CoV-2, related complications and criteria for ICU admission followed the same guidelines [9]. ARDS was diagnosed according to the Berlin definition [10].
Ethical issues
This study was approved by the Ethics Research Committee of our institution (HCUV-INCLIVA, project 2020/115). Written informed consent and use of de-identified retrospective data were waived owing to the severity of the situation. However, verbal authorization from the patient or caregiver was required.
Data collection
Clinical, demographic and outcome data of hospitalized patients were extracted from electronic medical records in our center.
A Covid-19 chest X-ray severity score as adapted by Wong et al. [11] here termed Covid19-CXRScore, was used to radiologically determine severity. This radiological scale of lung involvement has a score varying between 0 and 8, with 8 indicating the highest degree of involvement. The Sequential Organ Failure Assessment score (SOFA) was used to assess the severity of SARS-CoV-2 infection [12].
Tracheostomy patient management
Patients on MV for more than 14 days underwent a percutaneous dilatational tracheostomy, as per recommendations. Criteria used for tracheostomy were: PaO2/FiO2 > 200 mmHg and signs of clinical improvement to avoid a futile procedure. After tracheostomy, sedation was reduced progressively and a weaning protocol was implemented based on progressive reduction in pressure support [13]. Once the weaning process was completed and the patient was able to maintain spontaneous ventilation for 48 h, the tracheostomy tube was replaced by an uncuffed, fenestrated one, placing a heat and moisture exchange filter with supplementary oxygen therapy at the top of the tracheostomy tube. During subsequent days a cap was placed at the top of the tracheostomy tube during progressively longer periods of time. The tube was removed and the tracheostomy was closed when the patient was able to maintain adequate ventilation by himself with the capped tracheostomy tube and was able to expel respiratory secretions by coughing. Tracheostomy care was performed daily; chlorhexidine was applied frequently in the skin around the stoma and respiratory secretions were suctioned when required with a closed suction system; no saline instillation through tracheostomy tubes was made.
Patients were continuously cardio-respiratory monitored during the entire process, first at ICU then, when the patient was in a more stable condition (FiO2 < 40%, no need for vasopressor drugs to maintain hemodynamic stability, renal replacement therapy or sedation, combined with improvement in clinical and laboratory COVID-19 features) at the RCU of the Respiratory Medicine Department which have negative pressure rooms. All patients were included in a rehabilitation program to recover from critical illness polyneuropathy and myopathy (CIPM). To assess CIPM severity we used a score adapted from the Medical Research Council scale (MRC-muscle scale), ranging from 0 (paralysis) to 60 (normal strength) [14]. To ensure minimal exposure and risk, the healthcare team managing tracheostomized patients used full personal protective equipment for aerosol-generating procedures, including FFP3 mask, eye protection, fluid-repellent gown, and gloves.
Endpoints
The primary endpoint was to establish parameters at the hospital or at ICU admission associated with a need for tracheostomy in COVID-19 patients.
Secondary endpoints were to analyze demographics, clinical course, and prognosis of patients needing a tracheostomy due to COVID-19. Parameters collected were demographic variables, complications (pneumonia, sepsis, hemoptysis, embolic events), need for MV, duration of MV, length of UCI stay, time to decannulation, length of hospitalization, and all-cause mortality.
Statistical analysis
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies [15]. Binary and categorical variables were summarized using frequency counts and percentages. Continuously distributed variables were expressed as mean ± SD or median and interquartile ranges (IQR). Data comparisons were performed using Student’s t-test and paired Wilcoxon test. Dichotomous variables were compared using the chi-square test. Time from intubation to tracheostomy and survival was assessed with Kaplan–Meier charts. Forward stepwise logistic regression analysis was used to determine variables at hospital admission and ICU admission that were independently associated with need for tracheostomy in COVID-19 patients. The multivariate analysis model included variables that exhibited a significant association in the univariate model. Receiver operating characteristics (ROC) curves were used to identify a cut-off point in the variables that best predicted the need for tracheostomy in logistic regression. Statistical significance was set at p < 0.05.
Results
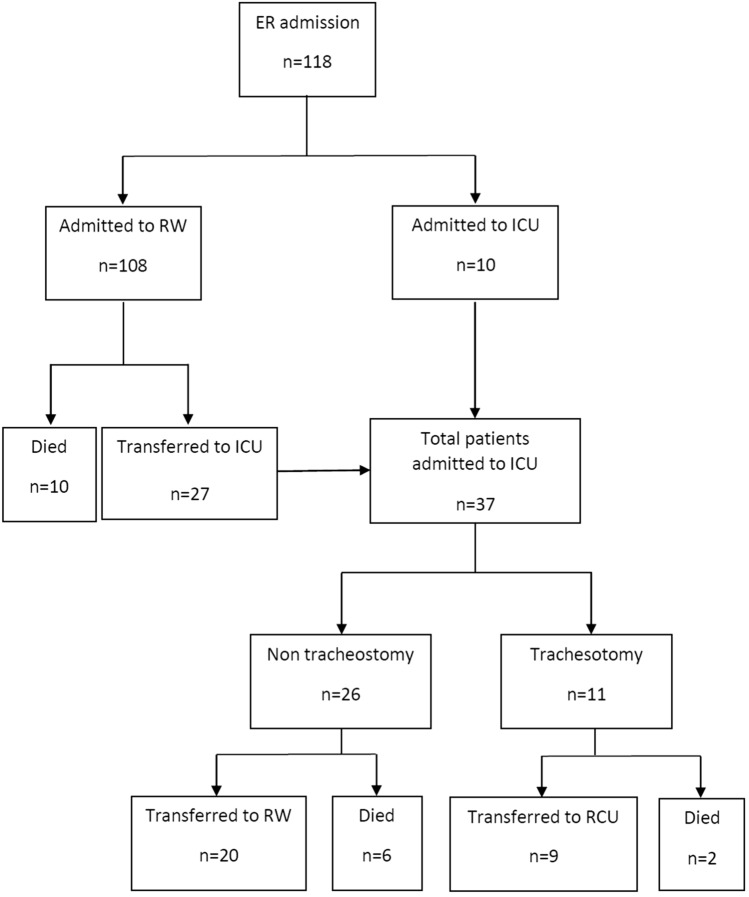
During the study, 118 patients with a confirmed diagnosis of COVID-19 were hospitalized (108 admitted to the Respiratory Ward and 10 directly admitted to ICU from the Emergency Department). Of patients who entered the Respiratory Medicine Department 27 (25%) were transferred to ICU due to worsening clinical condition (mean time from hospital admission to ICU admission 3.87 ± 2.89 days) (Fig. 1). Time from symptoms onset to hospital admission was 8.20 ± 4.78 days. The most common symptoms of COVID-19 were fever (85.6%), cough (78.0%), dyspnea (50.8%) and general discomfort (46.6%). Patients’ demographic and clinical characteristics at hospital admission are shown in Table 1. Thirty-seven patients (31.4%) were admitted to ICU, and 30 (81.1%) of these were intubated and mechanically ventilated, with a median UCI stay of 8.50 days (IQR, 3.75–21.50 days). Eighteen patients (15.3%) died during hospitalization, 10 patients in the Respiratory ward (12.3%) and 8 patients in ICU (mortality at ICU 21.6%); 62.5% of ICU mortality occurred during the first 14 days of ICU stay (Fig. 2).
Fig. 1.
Study flowchart. ER Emergency Department, ICU intensive care unit, RCU respiratory care unit, RW respiratory ward
Table 1.
Demographic and clinical characteristics at hospital admission
| Total population | No tracheostomy | Tracheostomy | p | |
|---|---|---|---|---|
| Sample size (n) | 118 | 107 | 11 | |
| Sex (male/female) | 78/40 | 69/38 | 9/2 | 0.248 |
| Comorbidity (n, %) | ||||
| Smoker | 11 (9.32) | 11 (10.28) | 0 (0) | 0.171 |
| HTA | 47 (39.83) | 40 (37.38) | 7 (63.63) | 0.090 |
| DM | 24 (20.33) | 21 (19.62) | 3 (27.27) | 0.549 |
| Cardiopathy | 11 (9.32) | 10 (9.34) | 1 (9.09) | 0.978 |
| COPD | 5 (4.23) | 4 (3.73) | 1 (9.09) | 0.401 |
| Asthma | 6 (5.08) | 6 (5.60) | 0 (0) | 0.420 |
| Cancer | 15 (12.71) | 13 (12.14) | 2 (18.18) | 0.567 |
| BMI > 30 | 20 (16.94) | 16 (14.95) | 4 (36.36) | 0.078 |
| Age (Years) | 60.01 ± 13.97 | 59.27 ± 14.33 | 67.18 ± 6.63 | 0.004 |
| Lymphocytes (× 109/L) | 1.80 ± 6.16 | 1.89 ± 6.44 | 0.81 ± 0.36 | 0.600 |
| Platelets (× 109/L) | 207.50 ± 105.96 | 204.97 ± 10,504 | 105.04 ± 118.54 | 0.404 |
| Neutrophils/Lymphocytes | 6.39 ± 5.37 | 5.57 ± 4.10 | 16.00 ± 8.72 | 0.007 |
| AST (U/L) | 76.61 ± 222.40 | 77.68 ± 232.75 | 65.55 ± 36.47 | 0.877 |
| ALT (U/L) | 60.41 ± 85.47 | 62.01 ± 89.22 | 45.40 ± 32.71 | 0.562 |
| Urea (mg/dL) | 35.58 ± 19.62 | 33.87 ± 17.79 | 52.90 ± 28.66 | 0.003 |
| Creatinine (mg/dL) | 1.00 ± 0.50 | 0.98 ± 0.50 | 1.22 ± 0.49 | 0.146 |
| LDH (U/L) | 674.00 ± 373.08 | 647.93 ± 371.04 | 957.88 ± 275.76 | 0.016 |
| CRP (mg/L) | 104.17 ± 101.98 | 87.17 ± 77.32 | 284.31 ± 153.89 | 0.003 |
| IL-6 (pg/mL) | 217.93 ± 408.78 | 111.90 ± 136.95 | 562.51 ± 919.40 | 0.209 |
| D-dimer (mg/mL) | 1174.61 ± 2864.70 | 1170.31 ± 2988.02 | 1217.00 ± 1121.17 | 0.961 |
| PaO2/FiO2 | 292.44 ± 106.39 | 297.29 ± 87.66 | 247.91 ± 215.37 | 0.468 |
| SOFA | 1.83 ± 2.05 | 1.53 ± 1.55 | 4.90 ± 3.66 | 0.018 |
| Covid19-CXRScore | 3.11 ± 1.96 | 2.94 ± 1.83 | 4.90 ± 2.55 | 0.002 |
| Mortality (%) | 15.30 | 14.95 | 18.18 | 0.777 |
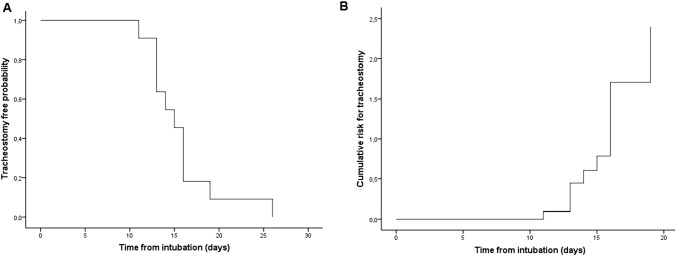
Fig. 2.
a Probability of being tracheostomy-free after intubation. b Risk of tracheostomy after intubation
Tracheostomy due to PMV was performed in 11 patients (36.7% of those on MV), and the time from orotracheal intubation to tracheostomy was 15.55 ± 4.84 days (Fig. 3). All patients presented COVID-19-related ARDS, thus protective lung maneuvers such as prone position were applied during MV. In tracheostomy patients length of time on MV was 27.70 ± 9.79 days and the mean time from tracheostomy to decannulation was 20.50 ± 7.76 days. Duration of hospitalization was 51.40 ± 10.96 days (30.91 ± 10.24 days at ICU). Compared with patients with no tracheostomy, the former were older and presented more criteria of severity at hospital admission (Table 1). Table 2 shows differences in clinical parameters at ICU admission between patients who needed a tracheostomy and those who did not. Univariate analysis revealed the variables at hospital admission (Table 3) and at ICU admission (Table 4) that were independently associated with the need for tracheostomy in COVID-19 patients, while a multivariable logistic regression model found no variable at hospital admission correlated with the need for tracheostomy. This same model found SOFA (OR 0.65, 95% CI 0.47–0.92, p 0.015) at ICU admission to be predictive of tracheostomy. In ROC curve analysis SOFA at ICU admission presented AUC = 0.779, 95% CI 0.59–0.96, p = 0.009, with a cut-off point of 4.5 (sensitivity 0.72, specificity 0.73, positive predictive value 0.57 and negative predictive value 0.85) (Fig. 4).
Fig. 3.
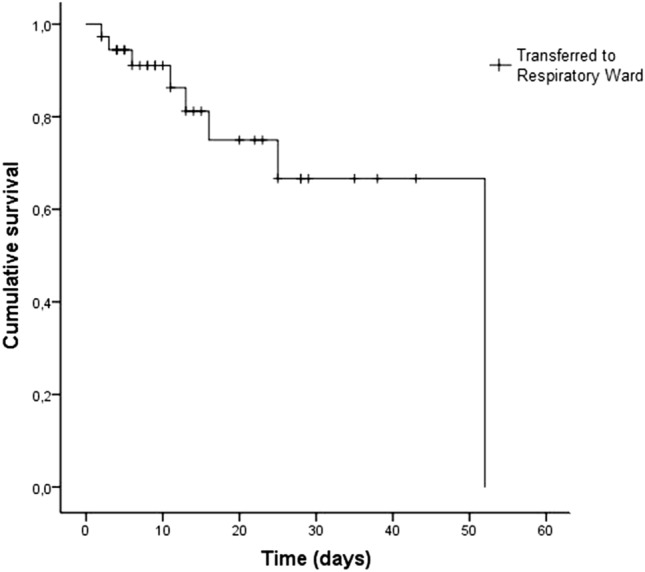
Mortality in ICU patients admitted due to COVID-19
Table 2.
Demographic and clinical characteristics at ICU admission
| No tracheostomy | Tracheostomy | p | |
|---|---|---|---|
| Sample size (n) | 26 | 11 | |
| Sex (male/female) | 20/6 | 9/2 | 0.741 |
| Comorbidity (n, %) | |||
| Smoker | 4 (15.38) | 0 (0) | 0.111 |
| HTA | 12 (46.15) | 7 (63.63) | 0.331 |
| DM | 6 (23.07) | 3 (27.27) | 0.786 |
| Cardiopathy | 5 (19.23) | 1 (9.09) | 0.444 |
| COPD | 1 (3.84) | 1 (9.09) | 0.519 |
| Asthma | 1 (3.84) | 0 (0) | 0.510 |
| Cancer | 6 (23.07) | 2 (18.18) | 0.741 |
| BMI > 30 | 6 (23.07) | 4 (36.36) | 0.446 |
| Age (Years) | 66.38 ± 8.51 | 67.18 ± 6.63 | 0.874 |
| Lymphocytes (× 109/L) | 3.80 ± 13.46 | 0.81 ± 0.30 | 0.492 |
| Platelets (× 109/L) | 200.53 ± 86.97 | 235.88 ± 118.54 | 0.354 |
| Neutrophils/Lymphocytes | 7.16 ± 5.06 | 16.00 ± 8.72 | 0.001 |
| AST (U/L) | 71.20 ± 5.06 | 65.55 ± 36.47 | 0.796 |
| ALT (U/L) | 52.85 ± 41.00 | 45.40 ± 32.71 | 0.622 |
| Urea (mg/dL) | 38.87 ± 22.45 | 45.40 ± 32.71 | 0.136 |
| Creatinine (mg/dL) | 1.09 ± 0.48 | 1.22 ± 0.49 | 0.502 |
| LDH (U/L) | 843.26 ± 301.56 | 957.88 ± 275.76 | 0.331 |
| CRP (mg/L) | 122.77 ± 81.21 | 284.31 ± 153.89 | 0.009 |
| IL-6 (pg/mL) | 176.51 ± 194.92 | 562.51 ± 919.40 | 0.298 |
| D-dimer (mg/mL) | 1429.95 ± 1344.62 | 1217 ± 1121.17 | 0.665 |
| PaO2/FiO2 | 24,825 ± 83.16 | 247.91 ± 215.37 | 0.995 |
| SOFA | 2.59 ± 1.65 | 4.90 ± 3.66 | 0.041 |
| Covid19-CXRScore | 3.96 ± 1.64 | 4.90 ± 2.55 | 0.303 |
| Mortality (%) | 23.07 | 18.18 | 0.741 |
Table 3.
Univariate analysis of factors at hospital admission associated with the need for tracheostomy
| OR | 95% CI | p | |
|---|---|---|---|
| Gender | 0.40 | 0.08–1.96 | 0.261 |
| Comorbidity | |||
| Smoker | 2.47 | 0.70–8.75 | 0.159 |
| HTA | 0.34 | 0.09–1.23 | 0.102 |
| DM | 0.65 | 0.15–2.66 | 0.551 |
| Cardiopathy | 1.03 | 0.11–8.90 | 0.978 |
| COPD | 0.38 | 0.04–3.81 | 0.417 |
| Asthma | – | – | – |
| Cancer | 0.62 | 0.12–3.20 | 0.570 |
| BMI > 30 | 0.31 | 0.082–1.20 | 0.090 |
| Age | 0.94 | 0.893–1.00 | 0.080 |
| Lymphocytes | 1.00 | 1.00–1.00 | 0.280 |
| Platelets | 1.00 | 1.00–1.00 | 0.404 |
| Neutrophils/Lymphocytes | 0.76 | 0.66–0.88 | 0.000 |
| AST | 1.00 | 0.99–1.00 | 0.887 |
| ALT | 1.00 | 0.99–1.01 | 0.566 |
| Urea | 0.96 | 0.94–0.99 | 0.010 |
| Creatinine | 0.54 | 0.22–1.33 | 0.18 |
| LDH | 0.99 | 0.99–1.00 | 0.059 |
| CRP | 0.98 | 0.97–0.99 | 0.000 |
| IL-6 | 0.99 | 0.99–1.00 | 0.126 |
| D-dimer | 1.000 | 1.00–1.00 | 0.960 |
| PaO2/FiO2 | 1.00 | 0.99–1.01 | 0.133 |
| SOFA | 0.59 | 0.44–0.79 | 0.000 |
| Covid19-CXRScore | 0.63 | 0.46–0.87 | 0.005 |
Table 4.
Univariate analysis of factors at ICU admission associated with the need for tracheostomy
| OR | 95% CI | p | |
|---|---|---|---|
| Sex | 0.74 | 0.12–4.40 | 0.742 |
| Comorbidity | |||
| Smoker | 3.20 | 0.70–14.52 | 0.132 |
| HTA | 0.49 | 0.11–2.08 | 0.355 |
| DM | 0.80 | 0.16–4.00 | 0.786 |
| Cardiopathy | 2.38 | 0.24–23.16 | 0.455 |
| COPD | 0.40 | 0.02–7.03 | 0.531 |
| Asthma | – | – | – |
| Cancer | 1.35 | 0.27–8.03 | 0.742 |
| BMI > 30 | 0.55 | 0.11–2.56 | 0.448 |
| Age | 0.98 | 0.90–1.08 | 0.777 |
| Lymphocytes | 1.00 | 0.99–1.00 | 0.547 |
| Platelets | 1.00 | 1.00–1.00 | 0.759 |
| Neutrophils/Lymphocytes | 0.94 | 0.88–1.01 | 0.108 |
| AST | 1.00 | 0.99–1.02 | 0.340 |
| ALT | 1.01 | 0.99–1.03 | 0.204 |
| Urea | 0.98 | 0.96–1.01 | 0.369 |
| Creatinine | 0.57 | 0.17–1.87 | 0.361 |
| LDH | 0.99 | 0.99–1.00 | 0.275 |
| CRP | 0.99 | 0.98–1.00 | 0.036 |
| IL-6 | 1.00 | 0.99–1.00 | 0.946 |
| D-dimer | 1.00 | 1.00–1.00 | 0.861 |
| PaO2/FiO2 | 0.99 | 0.99–1.00 | 0.353 |
| SOFA | 0.65 | 0.46–0.91 | 0.013 |
| Covid19-CXRScore | 0.66 | 0.39–1.11 | 0.123 |
Fig. 4.
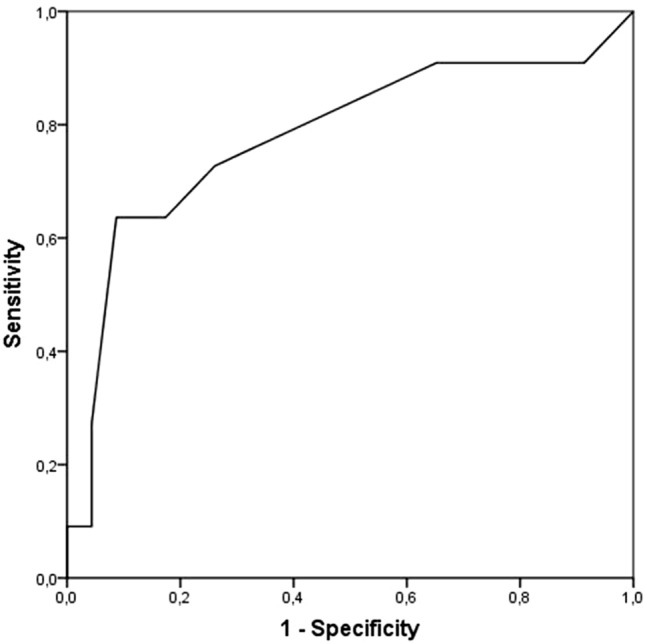
Receiver operating characteristics curves. Sequential Organ Failure Assessment score (SOFA) at ICU admission (area under curve 0.779, 95% confidence interval 0.59–0.96, p = 0.009)
Regarding COVID-19-associated complications and long-term hospitalization, five patients (45.5%) presented supraventricular cardiac arrhythmias, six patients (54.5%) suffered agitation and confusion, one (9.1%) pulmonary thromboembolism, and another one (9.1%) depression. Nosocomial infection was detected in all patients (72.7% respiratory, 18.2% urinary, catheter 9.1%) with no predominant germ.
In patients admitted to RCU after ICU stay all patients presented hypoalbuminemia (2.90 ± 0.45 g/dL), increased D-dimmer (1074.11 ± 608 mg/mL), IL-6 (161.14 ± 126.64 pg/mL) and LDH (601.66 ± 152.10 U/L) and major CIPM (MRC-sumscore 34.22 ± 14.08); two patients (18.2%) had pressure ulcers and another two (18.2%) showed swallowing dysfunction. All patients with tracheostomy were liberated from MV and tracheostomy was successfully closed, except for two who died during ICU stay due to severe ARDS (mortality in tracheostomized patients 18.18%), in this way successfully weaning was achieved in 81.8% and decannulation in 81.8% patients. At hospital discharge swallowing dysfunction was resolved and CIPM improved after daily rehabilitation sessions (MRC-muscle score 51.50 ± 4.62, p = 0.011). None of the healthcare team responsible for patients with tracheostomy were infected with SARS-CoV-2.
Discussion
The findings of our observational study show that SOFA at ICU admission is associated with an increased risk of tracheostomy in patients with COVID-19 and MV. Moreover, these patients present clinical features similar to those with CCI and suffer complications related to SARS-CoV-2.
In patients with critical illness secondary to SARS-CoV-2 requiring MV, Hur et al. [16] reported that around 64% remained intubated for more than 14 days. In those requiring MV the percentage of patients in whom tracheostomy was performed ranges from 11 to 38%. [5, 16–19] Our findings showed that tracheostomy was performed in 36.7% of patients with MV. Tracheostomy confers advantages in patients with PMV that could also be applicable to COVID-19 patients, such as less need for deeper sedation, shorter weaning time, and shorter ICU and hospital stay [20]. Median weaning time after tracheostomy in our patients was 9.00 days (IQR 4.00–14.00) with a mean duration of MV of 27.70 days that is in range with previously published data [19, 21–23]. 81.8% of our patients achieved successfully weaning. This data contrast with the results of the study [23] with more patients included (n = 1890) which found that at 30 days of follow-up, liberation from MV was achieved only in 52% of the patients. However, Long et al. report similar rates of weaning success to our results [24].
The key factors in managing COVID-19 patients under PMV are correctly pinpointing patients who might benefit from a tracheostomy and optimal timing of the procedure. It has been reported that around 85% of deaths in patients with COVID-19-related MV occur within 2 weeks of intubation [16]. Our study reflects similar results, with 62.5% of deaths in patients with MV produced during the first 14 days after intubation. Safety for healthcare workers from viral exposure during aerosol-generating procedures such as tracheostomy is another important issue to take into consideration regarding timing of tracheostomy in COVID-19 patients, in which regard decreasing infectivity has been found beyond 10 days after symptoms onset [25]. In light of this, different international guidelines [6, 26–28] recommend that tracheostomy in COVID-19 patients should be delayed until 14 days of intubation and MV should be considered only in patients with stable pulmonary status, appropriate ventilator requirements, showing signs of clinical improvement and with a clear prognosis.
Recently, Hur et al. [16] found that advanced age (> 65 years) and obesity (BMI > 30 kg/m2) were strongly associated with time to extubation and therefore higher risk for prolonged intubation in intubating COVID-19 patients. However, the results of our study show that SOFA at ICU admission, with a cut-off point of 4.5, was the only variable associated with the need for tracheostomy due to PMV. Tornari et al. [29] found that higher FiO2 (≥ 0.4) at tracheostomy and peak cough flow prior to tracheostomy were associated with delayed decannulation in COVID-19 patients. Different studies conducted in non-COVID-19 patients have been published assessing predictors of PMV, [30] showing that variables that evaluate the severity of critical illness, most of them included in the SOFA score, could predict the need for PMV and tracheostomy.
This is the first study to address the clinical course of tracheostomy in COVID-19 patients. All patients but the two who died (mortality 18.18%) could be liberated from MV and later decannulated and tracheostomy closed. They experienced prolonged hospital stays and suffered SARS-CoV-2-related complications. The agitation has been previously described as a primary neurologic feature in severe COVID-19, present in up to 67% of patients, and was observed in 54% of our tracheostomized patients [31]. 45% of our patients showed cardiac arrhythmias, in line with previously reported COVID-19-associated cardiac complications, in which arrhythmias occurred in 16.7% of patients hospitalized with SARS-CoV-2 pneumonia [32]. Evidence shows that severe COVID-19 can be complicated by coagulopathy that can manifest as pulmonary thromboembolism. It has been reported to occur in 21% of patients; [33] in our study 9.1% of patients presented pulmonary emboli. In addition to SARS-CoV-2 complications, our patients presented a collection of symptoms characteristic of CCI, such as nosocomial infections, hypoalbuminemia, skin breakdown, CIPM, depression, and increased inflammatory markers (mainly IL-6). CCI physiopathology is secondary to persistent inflammation, immunosuppression, and catabolism precipitated by dysregulated host immune responses to an initial insult [34]. The immune system clearly plays a key role in the host defense against SARS-CoV-2. The viruses induce inflammatory responses, mainly in the lungs, resulting in pneumonia. In susceptible patients, SARS-CoV-2 can cause a massive inflammatory response known as a cytokine storm, which in around 20% of patients results in severe disease with ARDS due to diffuse alveolar damage and can lead to multi-organ failure [35]. Some patients who survive this acute critical phase may develop a chronic state known as CCI. This is defined as organ dysfunction that persists more than 14 days in ICU patients and is linked to persistent immune dysregulation [36]. This persistent inflammation is typically associated with significantly elevated levels of inflammatory mediators such as IL-6 (30). In fact, in COVID-19 IL-6 is a significant predictor of mortality [37]. Moreover, this state is also associated with T cell depletion and decreased activation leading to immune suppression [36].
The study has some limitations which must be taken into account when interpreting our findings. It was conducted at a single-center hospital, implying a small sample size; also, due to the exploratory nature of the study, not driven by formal hypotheses, the sample size was not calculated. International guidelines recommend that patients with tracheostomy secondary to COVID-19 need to be managed by experienced staff trained in tracheostomy care and management; [6] (6) in this regard, our Respiratory Care Unit has extensive experience in the management of patients with tracheostomy and patients with CCI [38].
In conclusion, our results suggest that the SOFA score measured at ICU admission, with a cut-off point of 4.5, is an independent risk factor for prolonged mechanical ventilation and the need for tracheostomy in patients with COVID-19. Moreover, COVID-19 patients with tracheostomy present clinical features similar to patients with chronic critical illness in addition to SARS-CoV-2-related complications.
Abbreviations
- 95% CI
95% Confidence interval
- ADRS
Acute respiratory distress syndrome
- AUC
Area under curve
- CCI
Chronic critical illness
- CIPM
Critical illness polyneuropathy and myopathy
- COVID-19
Coronavirus disease 2019
- Covid19-CXRScore
Covid-19 chest X-ray severity score
- ER
Emergency department
- ICU
Intensive care unit
- IQR
Interquartile ranges
- MRC
Medical research council
- MV
Mechanical ventilation
- OR
Odds ratio
- PMV
Prolonged mechanical ventilation
- RCU
Respiratory care unit
- ROC
Receiver operating characteristics
- RW
Respiratory ward
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- SOFA
Sequential organ failure assessment
Author contributions
JS, SF, and JS-C conception and design of the study, acquisition of data, analysis, and interpretation of data, drafting the article and final approval of the submitted version. TP, EB, PB, LF-P, PR, MLB acquisition of data, drafting the article, and final approval of the submitted version. JS is responsible for the overall content of the manuscript as guarantor.
Compliance with ethical standards
Conflict of interest
All the authors have no financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2020) Coronavirus disease (COVID-19) outbreak. (https://www.who.int). Accessed 11 Mar 2020
- 2.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turri-Zanoni M, Battaglia P, Czackes C, Pelosi P, Castelnuovo P, Cabrini L. Elective tracheostomy during mechanical ventilation in patients affected by COVID-19: preliminary case series from Lombardy, Italy. Otolaryngol Head Neck Surg. 2020;163:135–137. doi: 10.1177/0194599820928963. [DOI] [PubMed] [Google Scholar]
- 6.McGrath BA, Brenner MJ, Warrillow SJ, Pandian V, Arora A, Cameron TS, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darson SS. Definitions and epidemiology of the chronic critically ill. Respir Care. 2012;57:848–856. doi: 10.4187/respcare.01736. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation (2020) Clinical management of severe acute respiratory infection when novel coronavirus (nCov) is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 01 Mar 2020
- 9.Gobierno de España (2020) Ministerio de Sanidad. Manejo Clínico del COVID-19: Atención hospitalaria. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf. Accessed 01 Mar 2020
- 10.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019;27:201160. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 13.Jubran A, Gant BJB, Duffner LA, Colins EG, Lanuza DM, Hoffman LA, Tobin MJ. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation. A randomized trial. JAMA. 2013;309:671–677. doi: 10.1001/jama.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleyweg RP, Van der Meche F, Schmitz P. Interobserver agreement in the assessment muscle strength and functional abilities in Guillain-Barre Syndrome. Muscle Nerve. 1991;14:1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Hur K, Price CPE, Gray EL, Gulati RK, Maksimoski M, Racette SD, et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrasa H, Rello J, Tejada S, Martin A, Balziskueta G, Vinuesa C, et al. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Victoria. AnaesthCrit Care Pain Med. 2020;39:553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angel L, Kon ZN, Chang SH, Rafeq S, Shekar SP, Mitzman B, et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann ThoracSurg. 2020;110:1006–1011. doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuazua-Gonzalez A, Collazo-Lorduy T, Coello-Casariego G, Collazo-Louruy A, Leon-Soriano E, Torralba-Moron A, et al. Surgical tracheostomies in COVID-19 patients: indications, technique and results in a second-level Spanish hospital. OTO Open. 2020 doi: 10.1177/2473974X20957636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durbin CG. Tracheostomy: why, when and how? Respir Care. 2010;55:1056–1068. [PubMed] [Google Scholar]
- 21.Bottic D, Lusetti F, Peroni T, Castellucci A, Salsi P, et al. The role of tracheostomy and timing of weaning and decannulation in patients affected by severe COVID-19. Ear Nose Throat J. 2020 doi: 10.1177/145561320965196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picetti E, Fornaciari A, Taccone FS, Malchiodi L, Grossi S, Di Lella F, et al. Safety of bedside surgical tracheostomy during COVID-19 pandemic: a retrospective observational study. PLoS ONE. 2020;15(9):e0240014. doi: 10.1371/journal.pone.0240014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Villares C, Perez C, Bartolome-Benito M, Bernal-Sprekelsen M, COVID ORL ESP Collaborative Group Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long SM, Chern A, Feit NZ, Chung S, Ramaswamy AT, Li C, et al. Percutaneous and open tracheostomy in patients with COVID-19: comparison and outcomes of an institutional series in New York City. Ann Surg. 2020 doi: 10.1097/SLA0000000000004428. [DOI] [PubMed] [Google Scholar]
- 25.Wolfel R, Corman VM, Cuggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-19. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 26.Takhar A, Walker A, Tricklebank S, Wyncoll D, Hart N, Jacob T, et al. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2173–8427. doi: 10.1007/s00405-020-05993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao TN, Bralslow BM, Martin ND, Chalian AA, Atkins J, Haas AR, et al. Tracheotomy in ventilated patients with COVID-19. Ann Surg. 2020;272:e30–e32. doi: 10.1097/SLA.0000000000003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb CR, Desai NR, Chaddha U, Sachdeva A, Sethi S, Bencheqroun H, et al. Use of tracheostomy during the COVID-19 pandemic: CHEST/AABIP/AIPPD: expert panel report. Chest. 2020;158:1499–1514. doi: 10.1016/j.chest.2020.05.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tornary C, Surda P, Takhar A, Amin N, Dinham A, Harding R, et al. Tracheostomy, ventilator wean and decannulation in COVID-19 patients. Eur Arch Otorhinolaryngol. 2020;1:1–10. doi: 10.1007/s00405-020-06187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghauri SK, Javaeed A, Mustafa KJ, Khan AS. Predictors of prolonged mechanical ventilation admitted to intensive care units: a systematic review. Int J Health Sci. 2019;13:31–38. [PMC free article] [PubMed] [Google Scholar]
- 31.Helms J, Kremmer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurological features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Wang Y, Li X, Ren L, ZhaoHu JY. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;2020(395):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hippensteel JA, Burnham EL, Jolley SE. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol. 2020;190:e134–e137. doi: 10.1111/bjh.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson JE, Cox CE, Hope AA, Carson S. Chronic critical Illness. Am J RespirCrit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu N, Zang D, Wng W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins RB, Raymond SL, Storz JA, Horiguchi H, Brakenridge SC, Gardner A, et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front Inmunol. 2018;9:1511. doi: 10.3389/finmu.2018.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sancho J, Servera E, Jara-Palomares L, Barror E, Sanchez-Oro-Gomez R, Gomez de Terreros FJ, et al. Noninvasive ventilation during the weaning process in chronically critically ill patients. ERJ Open Res. 2016;2:00061–2016. doi: 10.1183/23120541.00061-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]