Summary:
In this issue Bayik et al. demonstrated sexual dimorphism in accumulation of different populations of myeloid derived suppressor cells in glioblastoma and showed that they could be targeted by different agents.
Glioblastoma (GBM) is the most common primary malignant brain tumor with very poor survival. It is characterized by immune suppressive tumor microenvironment (TME). Myeloid-derived suppressor cells (MDSC) are one of the major contributors to immune suppressive TME in GBM (1). These are pathologically activated neutrophils and monocytes with potent immune suppressive activity (2). Based on their origin, two large populations of MDSC are currently recognized: pathologically activated neutrophils referred as PMN-MDSC or, as in this report, granulocytic (gMDSC) and pathologically activated monocytes – monocytic (mMDSC). Although MDSC and their classical counterpart neutrophils and monocytes share many phenotypic and morphological characteristics, they have distinct transcriptomic and proteomic profiles, metabolism, biochemical features as well as functions. MDSC accumulation is strongly associated with negative clinical outcome in cancer and failure of cancer immunotherapy. Because of that, therapeutic targeting of MDSC is actively pursued. In recent years, it became evident that therapeutic approaches to targeting gMDSC and mMDSC should be different due to the differences in their biology. However, the specifics of therapeutic approaches need to be elucidated.
Epidemiologic evidence supports male-dominant sexual dimorphism in GBM. Male patients have a worse prognosis than females, underscoring the clinical relevance of biological sex in GBM (3). In general, sex differences in host immunity are well described. Females have a more active immune response, which is mediated by increased type I interferon signaling, pro-inflammatory cytokine production, and T cell activation (4). In the context of tumor immunity, this could translate to more robust immunosurveillance. However, studies in patients treated with immune checkpoint inhibitors suggested that males might benefit more from this treatment compared to females. Thus, the hypothesis of possible role of immune systems in sexual dimorphism in GBM seems appealing.
In this issue Bayik et al. (5) tried to determine sex-dependent immunological changes in two orthotopic models of GBM: GL261 and SB28. They found that mMDSCs accumulated in male tumors leading to a ~5.5–6.5-fold increase in the mMDSC/gMDSC ratio as compared to brain tissues in control animals. In contrast, no significant change was observed in females. Instead, in female mice, there was a 2-fold increase in the peripheral gMDSC frequency, while mMDSCs remained unchanged. Surprisingly, the presence of other myeloid cells (macrophages, dendritic cells) as well as natural killer cells, and T cells did not differ between male and female tumor-bearing mice. The observed sexual dimorphism in MDSC was associated with survival differences. Female mice experienced longer survival compared to male mice. Female hosts reconstituted with male donor bone marrow had decreased survival compared to female-to-female transplant controls, demonstrating that the male immune system may have more tumor-promoting role.
Bayik et al. then sought to determine the functional contribution of MDSC subsets to tumor progression by depleting MDSC using antibodies. Bulk MDSC depletion with anti-Gr-1 antibody resulted in a survival benefit, but it limited only to female mice. Depletion of gMDSC with anti-Ly6G antibody provided survival benefit only to female mice. Targeting mMDSC with Ly6C antibody did not affect mMDSC presence and survival of either male or female mice. Authors hypothesized that the lack of systemic mMDSC reduction was due to mMDSCs proliferation and rapid replacement. Proliferative activity of mMDSC was described previously (6). In current study, mMDSCs highly expressed Ki-67 proliferation marker regardless of sex of the host.
To gain mechanistic insight into the differential roles of MDSC subsets, authors generated mMDSCs and gMDSCs from the bone marrow. Both male and female MDSCs were functionally suppressive. To identify putative drug targets for these subsets, authors used a network medicine approach that takes advantage of reported drug-target interactions using the differentially expressed gene profiles of mMDSCs and gMDSCs. This strategy identified fludarabine, a purine analog, as a potential drug candidate to target mMDSCs. For gMDSC targeting, IL-1β pathway inhibitors were enriched among the top targets. To test the therapeutic utility of these predicted drugs, they assessed their efficacy in pre-clinical models of GL261 and SB28 and observed that fludarabine significantly extended survival in male mice, with no significant benefit for female mice. In contrast, anti-IL-1β antibody treatment significantly prolonged the survival of female mice. In SB28-bearing male mice, one cycle of fludarabine was sufficient to decrease mMDSC presence in tumors by 4-fold. Tumor proliferation rate was reduced, suggesting that inhibition of mMDSC infiltration into the tumors at an early stage can alter the growth dynamics. Interestingly, in female GL261-bearing animals the frequency of the proliferating tumor cells or tumor mMDSCs were unaffected by the drug. Treatment with anti-IL-1β antibody reduced systemic gMDSCs specifically in GL261-bearing females. Anti-IL-1β antibody also delayed the tumor growth in female mice without direct impact on the proliferation of GL261 and SB28 cell in vitro. (Figure)
Figure.
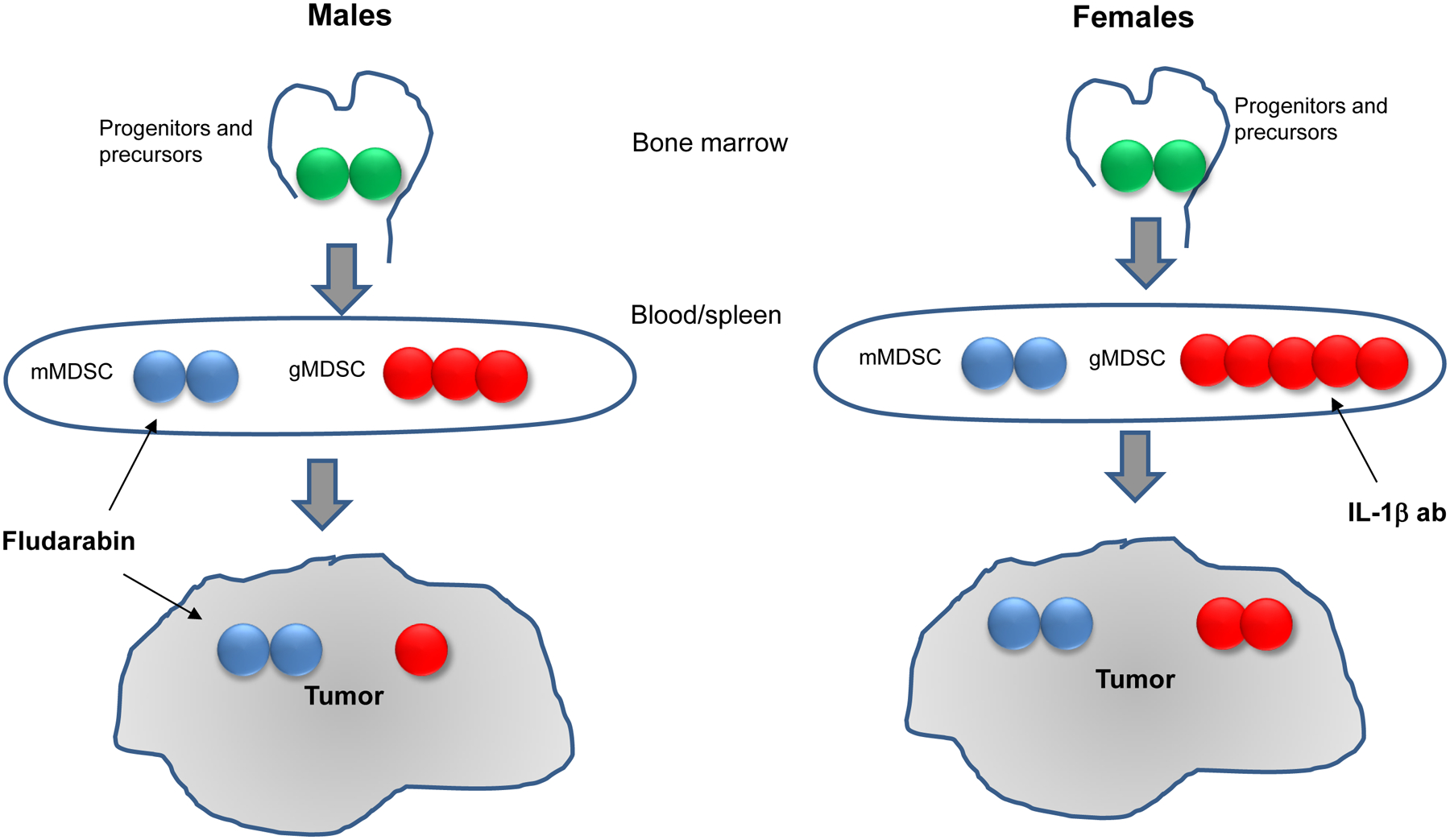
Sexual dimorphism of MDSC response in GBM
To validate preclinical observations in patients, Bayik et al. analyzed fresh tumor tissues with flow cytometry from a cohort of male patients with isocitrate dehydrogenase (IDH)-wild type GBM. They found that mMDSCs were more abundant then gMDSC. Moreover, these tumor-infiltrating mMDSCs were positive for Ki-67, confirming that they were proliferating cells. To evaluate the prognostic value of gMDSC, they analyzed The Cancer Genome Atlas (TCGA) GBM Database for mRNA levels of OLR1 (LOX-1), a gMDSC-specific marker (7), and IL1B and found that there were no differences in the expression these genes between male and female patients. Further investigation of IL-1β protein levels by immunohistochemistry of tumor tissue from patients with IDH-wild type GBM established that this cytokine was abundant in the tumor microenvironment regardless of patient sex. Notably, the expression levels of OLR1 and IL1B were associated with patient survival only when stratified based on sex. In both TCGA and the Chinese Glioma Genome Atlas (CGGA), high OLR1 expression inversely correlated with the survival of females but had no effect in male patients with GBM. A similar trend was observed with IL1B levels. OLR1 expression positively correlated with IL1B expression, suggesting that these genes are part of the same signaling network.
Collectively, these studies indicated that in GBM male hosts have enhanced accumulation of mMDSCs, while a gMDSC-IL-1β axis associates with females and represents a therapeutic target for female patients. This interesting report describes, for the first time, sexual dimorphism of MDSC and suggested specific targets for different populations of these cells. However, as with any novel study it raises number of questions. First, whether observed sexual dimorphism is associated with other types of cancer or only specific to GBM? If the latter, what can then explain such tumor specificity?
Investigators observed sexual dimorphism only in MDSC, but not in macrophages and dendritic cells. Considering that monocyte/mMDSC are the main source of tumor associated macrophages (TAM), it is peculiar that similar dimorphism was not observed in accumulation of those cells. The reason for mMDSC accumulation in tumors of male mice is not clear. Because authors did not observe systemic expansion of these cells, mMDSC accumulation would be the result of increased migration or possibly blocked differentiation to macrophages. In the latter case, it could explain lack of sexual dimorphism in TAM. The other possible explanation is loss of gMDSC in tumor tissues. Granulocytic cells are more sensitive than monocytic cells to tumor microenvironment. It is possible that if mMDSC and gMDSC equally recruited to tumors, mMDSC survive better and thus would be a predominant population. Indirectly, it can explain lack of shift in mMDSC/gMDSC in tumors of female mice. Since these mice have much higher expansion of gMDSC in periphery, more gMDSC would come to tumors and despite their loss the shift of balance towards mMDSC would not be detectable.
Authors presented strong correlation between expression of OLR1, gene encoding gMDSC specific LOX-1, and clinical outcome in female patients. Although the data are suggestive, there is a caveat that require further clarification. OLR1 is expressed on different cells, including macrophages and endothelial cells, present in TEM. Therefore, in whole tumor tissues this may affect interpretation of the results.
There is now clear evidence that because of their biological differences gMDSC and mMDSC are sensitive to different therapeutic targeting. Examples include targeting of their migration (8), epigenetic (9), or metabolism (10). Moreover, it became clear that successful therapy would require combination of targeting of mMDSC and gMDSC (11). Current study provides evidence that different approaches may be beneficial for different MDSC populations. Previous studies demonstrated effect of chemotherapeutics on MDSC including nucleoside analogs, 5-fluorouracil, gemcitabine and others. In this study authors demonstrate the potent effect of fludarabine but only on mMDSC, whereas IL-1β neutralization worked against gMDSC. Since these populations differentially accumulated in male and female mice, it opens an opportunity to select therapy based on patients’ gender. However, such potentially impactful conclusion would require strong confirmation in future studies.
Acknowledgments
Commercial Research Grants from Merck, M1NA Therapeutics, Cour Therapeutics, Syndax, Syntrix, GI Innovation.
Footnotes
Conflict of interest disclosure:
Minor paid consultant for T-Rx Pharmaceutical, Quentis, Third Rock Venture, EMD Serono, Shattuck, Merck, Verseau Theraputic, Takeda, Riley FBR, Compass Theraputic, Elstar.
References
- 1.Raychaudhuri B, Rayman P, Huang P, Grabowski M, Hambardzumyan D, Finke JH, et al. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J Neurooncol 2015;122:293–301 doi 10.1007/s11060-015-1720-6. [DOI] [PubMed] [Google Scholar]
- 2.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19(2):108–19 doi 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol 2018;20(4):576–7 doi 10.1093/neuonc/noy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16(10):626–38 doi 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 5.Bayik D, Zhou Y, Park C, Hong C, Vail D, Silver DJ, et al. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discovery 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 2013;14(3):211–20 doi 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 2016;1(2) doi 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020. doi 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang H, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature 2020;579(7798):284–90 doi 10.1038/s41586-020-2054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019;569(7754):73–8 doi 10.1038/s41586-019-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017;32(5):654–68 e5 doi 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


