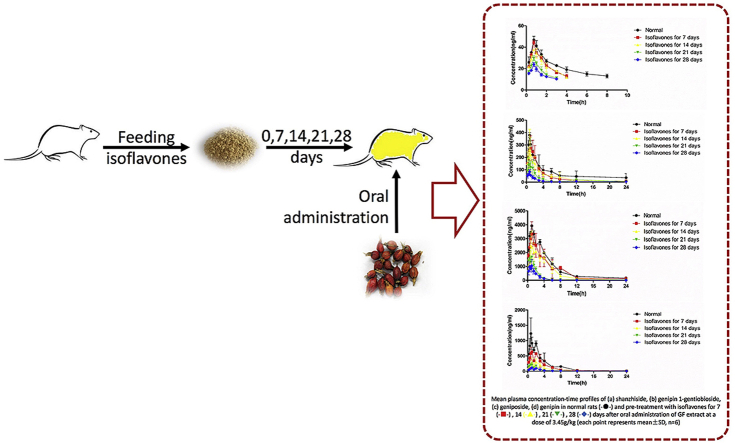
Abstract
Gardeniae Fructus (GF) and Semen Sojae Praeparatum (SSP) are both medicine food homologies and widely used in Chinese clinical prescriptions together. The research investigated the pharmacokinetics of four iridoids in normal rats and isolfavones-fed rats, which were administered with isolfavones from SSP for 7, 14, 21 and 28 consecutive days. A validated LC-MS/MS method was developed for determining shanzhiside, genipin-1-gentiobioside, geniposide and their metabolite genipin in rat plasma. Plasma samples were pretreated by solid-phase extraction using paeoniflorin as the internal standard. The chromatographic separation was performed on a Waters Atlantis T3 (4.6 mm × 150 mm, 3 μm) column using a gradient mobile phase consisting of acetonitril and water (containing 0.06% acetic acid). The mass detection was under the multiple reaction monitoring (MRM) mode via polarity switching between negative and positive ionization modes. The calibration curves exhibited good linearity (r > 0.997) for all components. The lower limit of quantitation was in the range of 1–10 ng/mL. The intra-day and inter-day precisions (RSD) at three different levels were both less than 12.2% and the accuracies (RE) ranged from −10.1% to 16.4%. The extraction recovery of them ranged from 53.8% to 99.7%. Pharmacokinetic results indicated the bioavailability of three iridoid glycosides and the metabolite, genipin in normal rats was higher than that in rats exposed to isoflavones. With the longer time of administration of isoflavones, plasma concentrations of iridoids decreased, while genipin sulfate, the phase Ⅱ metabolite of genposide and genipin-1-gentiobioside, appeared the rising exposure. The pharmacokinetic profiles of main iridoids from GF were altered by isoflavones.
Keywords: Iridoids, Gardeniae fructus, Isoflavones, Rat, Pharmacokinetics, LC-MS/MS
Graphical abstract
Highlights
-
•
A LC-MS/MS method for determination of four iridoids in rat plasma was developed and applied.
-
•
The bioavailability of four iridoids decreased in rats with their increasing isoflavones exposure time.
-
•
Isoflavones could alter the fate of iridoids in vivo when GF and SSP were prescribed together to obtain toxicity-reducing.
1. Introduction
Gardeniae Fructus (GF), commonly called Zhizi in Chinese, is the dried and ripe fruit of Gardenia jasminoides Ellis. As a kind of traditional Chinese medicine (TCM), GF is used to treat diabetes, depression [1], hyperlipidemia, inflammatory condition [2] and postmenopausal syndrome [3]. In particular, iridoids including shanzhiside, genipin-1-gentiobioside and geniposide have been regarded as the main active ingredients in GF [4,5]. It was reported that iridoids not only have a neuroprotective effect on rats after traumatic brain injury, but also exert an anti-inflammatory effect by decreasing the production of proinflammatory mediators [6,7]. Furthermore, geniposide could protect against acute alcohol-induced liver injury in mice via up-regulating the expression of the main antioxidant enzymes [8].
Semen Sojae Praeparatum (SSP) as a medicine food homology by the Ministry of Public Health of China is obtained by fermentation from Soybean, Sweet Wormwood Herb and Mulberry Leaf, officially being listed in the Chinese Pharmacopoeia. The major active constituents of SSP are known as isoflavones which are bioactive compounds structurally similar to estrogen, being also called phytoestrogens. Isoflavones can bind to estrogen receptors and has an estrogenic or anti-estrogenic effect depending on their concentrations [9].
The compatibility of GF and SSP is the main ingredient in traditional Chinese formula, such as Zhi-Zi-Da-Huang decoction and Zhi-Zi-Hou-Pu decoction [[10], [11], [12], [13]]. These formulas, regarded as an indispensable part of the oldest East Asian medical systems, have been used in clinical therapy of various diseases for thousands of years [14,15]. Traditional Chinese medicines are always administered orally. Therefore, the components in the herbs are inevitably exposed to the microbiota in the whole gastrointestinal tract, resulting in reshaped structure of gut microbiota [16]. The gut microbiota exerts an influence on the drug metabolism, which would change the pharmacokinetic profiles of orally administered compounds from traditional Chinese medicines [[17], [18], [19]]. These complicated drug-drug interactions in vivo are considered as an essential feature of traditional Chinese medicine. In our previous studies, it was found that dietary isoflavones could alter the structure of gut microbiota in rats and the disposition of orally administered isoflavones from SSP as well, through changing the activity of β-d-glucosidase [20,21]. Thus, the alternation of gut microbiota might lead to making different pharmacokinetic profiles of iridoids from GF when both of SSP and GF were given to rats.
Few have explored the changes of pharmacokinetic parameters of iridoids, when GF was administered with or without SSP. Currently, most studies focus on the pharmacokinetics of the compounds including the one or more iridoids in rats after their oral administration of traditional Chinese medicine formulas based on GF and SSP [[22], [23], [24], [25], [26]]. For GF and SSP, as the main part in the regular and traditional Chinese formula, there might be the interaction of different compounds in vivo. Therefore, it is necessary to compare the pharmacokinetic profiles of iridoids between rats with normal feeding and isoflavones from SSP and to know about the fate of iridoids, which would contribute to clinical applications and understanding the compatibility of GF and SSP. This study established a sensitive and reliable LC-MS/MS method for simultaneous determination of shanzhiside, genipin-1-gentiobioside, geniposide and its metabolite, genipin, in rat plasma. And then, rats with normal feeding and rats exposed to isoflavones primarily in 7, 14, 21 and 28 consecutive days were administered GF extract orally to explore the pharmacokinetics alteration of the above iridoids, emphasizing the influence of isoflavones from SSP on fates of iridoids from GF in rats.
2. Materials and methods
2.1. Material and reagents
A reference standard of shanzhiside (purity 98%) was purchased from Shanghai Tauto Bio-tech Co., Ltd. (Shanghai, China). A reference standard of geniposide (purity 98%) was purchased from Shanghai ANPEL Laboratory Technologies Inc. (Shanghai, China). Reference standards of genipin-1-gentiobioside and genipin (purity 98%) were purchased from Dalian Meilun Biotechnology Co., Ltd. (Dalian, Liaoning, China). Paeoniflorin (purity 98%) was purchased from Shanghai Yuanye Bio-technology Co., Ltd. (Shanghai, China) and used as an internal standard (IS). GF extract was prepared in our lab and the chemical analysis was performed as our previous research (The proportions of shanzhiside, genipin-1-gentiobioside and geniposide were 0.06%, 3.75% and 12.45%, respectively) [27]. The herbal materials of SSP were purchased from Chengdu and authenticated by Dr. Luping Qin from the Second Military Medical University. Acetonitrile, methanol (analytical grade) and acetic acid (HPLC grade) were purchased from Merck Company (Darmstadt, Germany) and Tedia (Fairfield, USA), respectively. Deionized water (18.2 MΩ/cm) was generated in-house using a Milli-Q System from Millipore (Bedford, MA, USA).
2.2. LC-MS/MS conditions
Sample separation and determination were achieved by a Shimadzu LC-MS/MS system equipped with two LC-30A pumps, a SIL-30AC autosampler, a CTO-30A column oven and LCMS-8045 triple-quadruple mass spectrometry (Shimaszu, Kyoto, Japan). The mass spectrometry detector was equipped with an electrospray ionization (ESI) source. The data were collected and processed using Shimadzu Version 5.86 software.
The chromatographic separation was performed on a Waters Atlantis T3 (3 μm, 4.6 mm × 150 mm) column with a flow rate of 0.6 mL/min at 35 °C. The mobile phase was composed of A (water containing 0.06% acetic acid) and B (acetonitrile containing 0.06% acetic acid) with a liner gradient elution of 10% (V/V) B at 0–3 min, 10%–12% B at 3–4 min, 12%–22% B at 4–5.5 min, 22%–25% B at 5.5–9.5 min, 25%–29% B at 9.5–11 min, 29%–42% B at 11–13 min, 42%−10% B at 13–14 min and 10% B at 14–19 min. The detection of the analytes and IS was carried out on a triple quadrupole mass spectrometer. Quantitation was performed by positive (for genipin-1-gentiobioside) and negative (for shanzhiside, geniposide, genipin and IS) MRM modes to monitor the precursor ion→product ion (m/z) of shanzhiside (391.10 → 149.30), genipin-1-gentiobioside (573.40 → 365.05), geniposide (447.30 → 225.15), genipin (225.20 → 101.10) and paeoniflorin (479.00 → 121.15). The optimal ESI source parameters were set as follows: source temperature 300 °C, the interface voltage 4.5 kV for positive mode and −3.5 kV for negative mode; nebulizer gas, 2.9 L/h; drying gas, 10 L/h; desolvation line (DL) temperature and heat block temperature were maintained at 250 °C and 400 °C, respectively. For collision-induced dissociation (CID), argon was used as the collision gas at 230 kPa.
2.3. Preparation of isoflavones
The SSP (100 g) was chopped into powder and decocted twice (2 h each time) with 70% (V/V) ethanol. All the extraction was combined and condensed to a small volume. The resin D101 was equilibrated in 95% (V/V) ethanol for 24 h and then washed thoroughly with water several times. 300 g of the pretreated resin was put into a flask and then 1000 mL of the crude extract solution was added. After adsorption equilibrium for 2 h, the resin was washed with 500 mL water and 3000 mL 20% (V/V) ethanol. And then the isoflavones fraction was desorbed with 3000 mL of 60% (V/V) ethanol in the resin to produce the isoflavones powder through rotary evaporating to dryness at 60 °C under reduced pressure. The powder was stored at 4 °C in the dark for further oral administration to rats. There were 11 major isofalvones components in SSP [28], and the isoflavones extract powder contained approximately 20% total isoflavones.
2.4. Preparation of the stock, standard and the quality control (QC) solutions
The stock solution of shanzhiside, genipin-1-gentiobioside, geniposide, genipin and paeoniflorin were prepared by dissolving the accurately weighed reference compounds in 50% acetonitrile solution at a concentration of 50 mg/mL. A mixed working solutions containing shanzhiside, genipin-1-gentiobioside, geniposide and genipin were obtained by serial dilution. The IS working solution was diluted with 50% acetonitrile to a final concentration of 10 μg/mL. All the stock solutions and working solutions were stored at −20 °C. For both the calibration standards and QC solutions, 10 μL of standard working solution was spiked into 100 μL of blank rat plasma. The final six concentrations of calibration standards were 10–200 ng/mL for shazhiside, 1–1000 ng/mL for genipin-1-gentiobioside, 1–4000 ng/mL for geniposide and 1–2000 ng/mL for genipin. The three concentrations of QC solutions were 20, 100 and 160 ng/mL for shanzhiside, 2, 500 and 800 ng/mL for genipin-1-gentiobioside, 2, 2000 and 3200 ng/mL for geniposide and 2, 1000 and 1600 ng/mL for genipin.
2.5. Sample preparation
Plasma samples were performed on CNWBOND C18 cartridges (1 mL, 100 mg) (Anpel Laboratory Technologies, Shanghai, China), each of which was pretreated with 1 mL methanol followed by 1 mL water. 100 μL rat plasma sample was mixed with 10 μL of a solution containing IS (10 μg/mL) in 50% acetonitrile and 10 μL of 50% acetonitrile and applied to the extraction column. Then, the cartridge was washed with 130 μL of water and eluted with 500 μL methanol. The eluate was concentrated to dryness by an automated concentration evaporation system at 30 °C under a gentle stream of nitrogen (Turbo Vap 96, Biotage AB, Uppsala, Sweden). After drying, the residue was reconstituted with 50 μL of 10% acetonitrile, vortexed for 3 min and centrifuged for 10 min at 12000 rpm. The supernatant was transferred to a vial with glass insert and 10 μL of aliquots was injected into the LC-MS/MS system.
2.6. Method validation
The method validation was based on the Food and Drug Administration (FDA) [29] guidelines in terms of selectivity, linearity, lower limit of quantitation (LLOQ), precision, accuracy, extraction recovery, matrix effect and stability.
2.6.1. Selectivity
To assess the method selectivity meant the four iridoids and the IS in the rat plasma were not interfered with the endogenous substance as well as the components that had been absorbed in the rats fed with isoflavones. Therefore, the chromatograms of blank plasma samples, those of blank plasma samples spiked the analytes and the IS, those of normal rat obtained at 0.5 h after oral administration of the GF extract, those of 28 days isoflavones-fed rats plasma samples were compared to evaluate the selectivity of the method.
2.6.2. Linearity and LLOQ
Calibration curves consisting of at least six concentrations were established from the peak area ratio of analytes to IS versus the nominal concentration of analytes with weighted (1/X2) least square linear regression. The lowest analytical concentration on the calibration curve was LLOQ, which could be quantified with accuracy within 20% relative error (RE) and a precision below 20% relative standard deviation (RSD).
2.6.3. Precision and accuracy
The intra-day precision and accuracy were determined by QC samples at three concentrations in five replicates during the same day. The inter-day precision and accuracy were determined by QC samples at three concentrations in five replicates during three consecutive days. Sample precision and accuracy at each QC concentration were evaluated by RSD and RE, respectively.
2.6.4. Extraction recovery and the matrix effect
The extraction recovery was determined by comparing the peak areas of the four analytes from the QC samples at the same concentrations with those obtained from blank plasma samples with four analytes spiked into the post-extraction supernatant. The matrix effect was assessed by comparing the peak areas of four analytes spiked into the post-extraction supernatant with those of the neat solution at the same concentration.
2.6.5. Stability
The stability was evaluated by QC samples of four analytes at two concentrations in three replicates. Ambient temperature stability was assessed by leaving the processed samples at room temperature for 24 h. Long-term stability was investigated after samples were stored at −80 °C for 15 days. Autosampler stability was analyzed using post-extraction samples stored in the autosampler room for 24 h. And freeze-thaw cycle stability was analyzed after QC samples undergoing three freeze (−80 °C) and thaw (room temperature) cycles. The measured concentrations were compared to those of freshly prepared QC samples and the percentage concentration deviation was calculated to evaluate stability.
2.7. Animals experiment
Male Sprague-Dawley (SD) rats (200 ± 20 g) were supplied by Shanghai Slac Laboratory Animal and acclimatized for a week with abundant food and water before the experiment. The experimental procedures were approved by the Animal Ethics Committee of the Second Military Medical University. Rats were housed in the controlled environment consisting of temperature and humidity; a 12 h cycle of light and darkness was established.
The male SD rats were divided into five groups with six rats per group. Group A was orally administered GF extract at 3.45 g/kg. Group B, group C, group D and group E were all given SSP isoflavones extract at 0.21 g/kg (42.9 mg/kg total isoflavones) once every day for 7, 14, 21 and 28 days, respectively, because of the alteration of rat intestinal microbiota after their 7-day isoflavones diet with triggering changes in enzyme activity [20]. And then on the next day of the last provision of isoflavones, rats in each group were orally administered GF extract at 3.45 g/kg. The blood (0.3 mL) was collected from the orbital venous plexus at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 h in the clean heparinized EP tubes. All of the collected blood samples were immediately centrifuged at 8000 rpm for 10 min to obtain plasma, which was labeled and frozen at −80 °C until analysis. The main pharmacokinetic parameters including t1/2, AUC0→t, AUC0→∞, MRT, Cmax and Tmax were calculated by the BAPP software (version 2.0, Center of Drug Metabolism and Pharmacokinetics, China Pharmaceutical University, Nanjing, China) using the non-compartmental model analysis.
3. Results and discussion
3.1. Optimization of the LC-MS/MS analysis
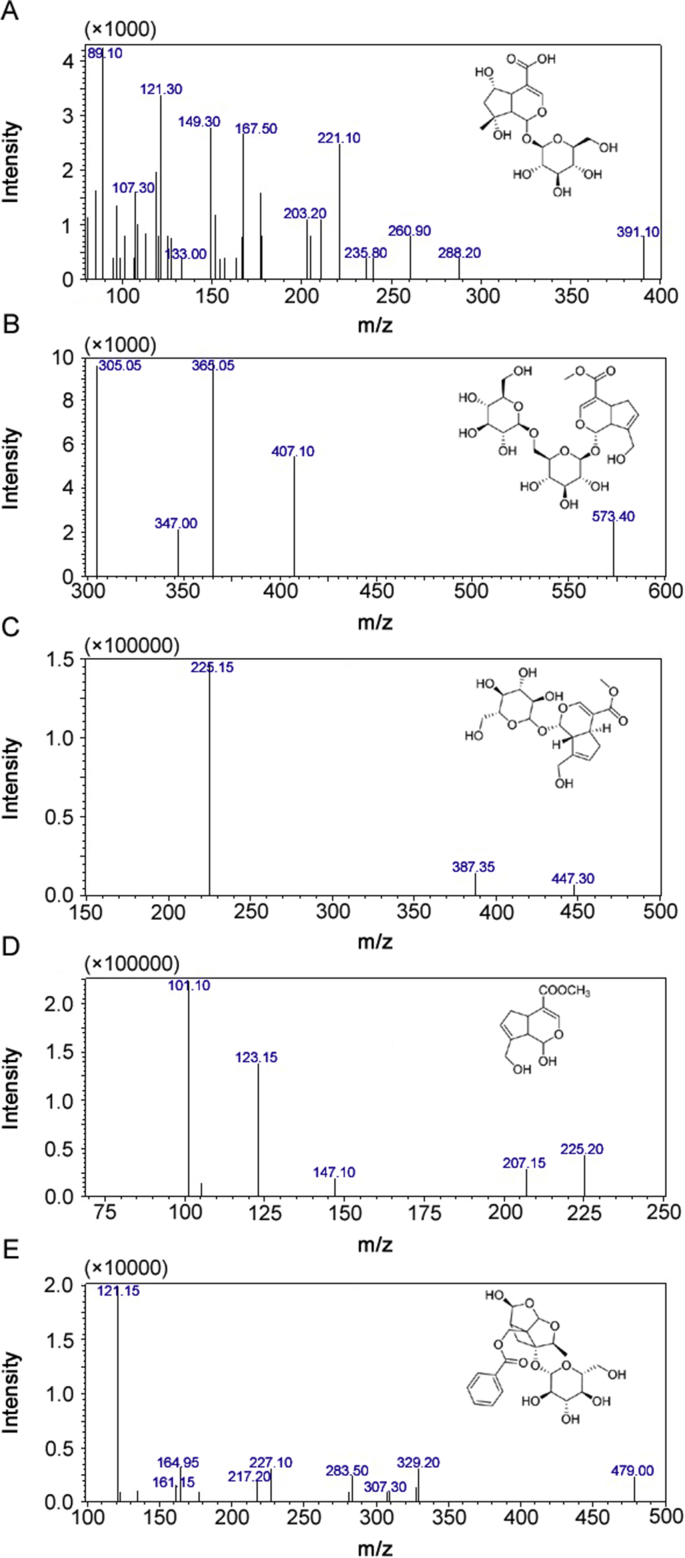
In order to obtain high response signals, the first step was to select a suitable ionization, precursor ion and product ion of the analytes and the IS. The ionization of four reference compounds was investigated in ESI source in both positive and negative ion modes. The MS/MS ion transitions were taken in MRM mode, which could improve the specificity and sensitivity of the detection. Shanzhiside, genipin-1-gentiobioside, and geniposide are iridoid glycosides composed of glucosides and aglycones. Genipin is an aglycone metabolized by geniposide and genipin-1-gentiobioside in vivo. The precursor ion and the product ion of shanzhiside were at m/z 391.00 Da ([M − H]-) and m/z 149.30 Da, respectively, which were attributable to the loss of Glu and –OCOOH in the negative ion mode. The precursor ion of genipin-1-gentiobioside was at m/z 573.40 Da ([M+Na]+), and the loss of Glu and –CO in positive ion mode could produce the product ion peak at m/z 365.05 Da. The precursor ion of geniposide was at m/z 447.3 Da ([M + CH3COO]-), and the product ion peak at m/z 225.15 Da was attributable to the loss of Glu and –C2H3O in the negative ion mode. The precursor ion of genipin was at m/z 225.20 Da ([M − H]-), and the product ion peak at m/z 101.1 Da was attributable to the loss of –C7H8O2 in the negative ion mode. The precursor ion of paeoniflonin (IS) was at m/z 479.00 Da ([M − H]-), and the product ion peak was at m/z 121.15 Da in the negative ion mode. To get the richest relative abundance of precursor and product ions, the parameters for collision energy were optimized. The results are shown in Table 1 and Fig. 1.
Table 1.
Parameters of MRM mode used in quantification.
| Compounds | ESI mode | Precursor ion (m/z) | Product ion (m/z) | Collision energy (eV) |
|---|---|---|---|---|
| Shanzhiside | (−) | 391.10 | 149.30 | 30 |
| Genipin-1-gentiobioside | (+) | 573.40 | 365.05 | −34 |
| Geniposide | (−) | 447.30 | 225.15 | 14 |
| Genipin | (−) | 225.20 | 101.10 | 11 |
| Paeoniflorin (IS) | (−) | 479.00 | 121.15 | 22 |
Fig. 1.
Representative product ion mass spectra of (A) Shanzhiside, (B) Genipin-1-gentiobioside, (C) Geniposide, (D) Genipin and (E) Paeoniflorin (IS).
Chromatographic condition was investigated to obtain the appropriate retention time, sensitive and ideal peak shapes. Because of the polarity of iridoid glycosides and the hydrophobicity of the aglycones, HILIC column was tested first. Although it could give a better analytical run time, it failed because of the bad shape of peaks, which had a negative effect on the quantitation of analytes at lower concentrations. Finally, an Atlantis T3 reversed-phase C18 column (4.6 mm × 150 mm, 3 μm) was chosen to separate these polar and non-polar compounds. The different combinations of methanol, acetonitrile, water and other additives such as formic acid, and acetic acid were tested to select the suitable mobile phase. When using acetonitrile (containing 0.06% acetic acid) and water (containing 0.06% acetic acid) as mobile phase, the responses of four analytes were obviously higher than those with methanol (containing 0.06% acetic acid) and water (containing 0.06% acetic acid). And a gradient elution with acetonitrile (containing 0.06% acetic acid) and water (containing 0.06% acetic acid) could produce narrower chromatographic peak to meet the sensitivity requirement. The endogenous substances in the plasma could not interfere the quantitation under the conditions listed in Section 2.2. An improved separation of the three iridoid glycosides, one aglycones and the IS was achieved within 19 min.
3.2. Sample preparation
The sample pretreatment methods including protein precipitation (PPT), SPE and ultrafiltration were tested in the experiment for improving the recoveries of the analytes and reducing the matrix effects. Ultrafiltration was abandoned primarily due to its low recovery. And then PPT was carried out using methanol (0.1% formic acid) and acetonitrile (0.1% formic acid) to precipitate the protein, while the results showed the low extraction recovery too. The reason might be that iridoids formed intermolecular hydrogen bonds with proteins for their multiple hydroxyls structure and co-precipitated with proteins easily [30]. Finally, SPE was used for the plasma treatment. Firstly, 100 μL of plasma mixed with 10 μL IS and 10 μL 50% acetonitrile was loaded on a preconditioned SPE cartridge. Owning to the hydrophilic of iridoids and the instability of genipin, different washing solution volumes (70 μL, 130 μL, 200 μL, and 260 μL), elution solution volumes (500 μL, 1 mL, 1.5 mL, and 2 mL) and various ratios of elution solution (10%, 30%, 50%, 80%, and 100% methanol) were tested. In the end, the elution procedure was optimized as follows: after sample loaded, the cartridge was washed with 130 μL deionized water and 500 μL elution solution (100% methanol). However, the recovery of shanzhiside was still low because of its strong hydrophile. For the better detection sensitivity, the eluate was needed to be concentrated after SPE followed by reconstitution in smaller volume solvents.
3.3. Method validation
3.3.1. Selectivity
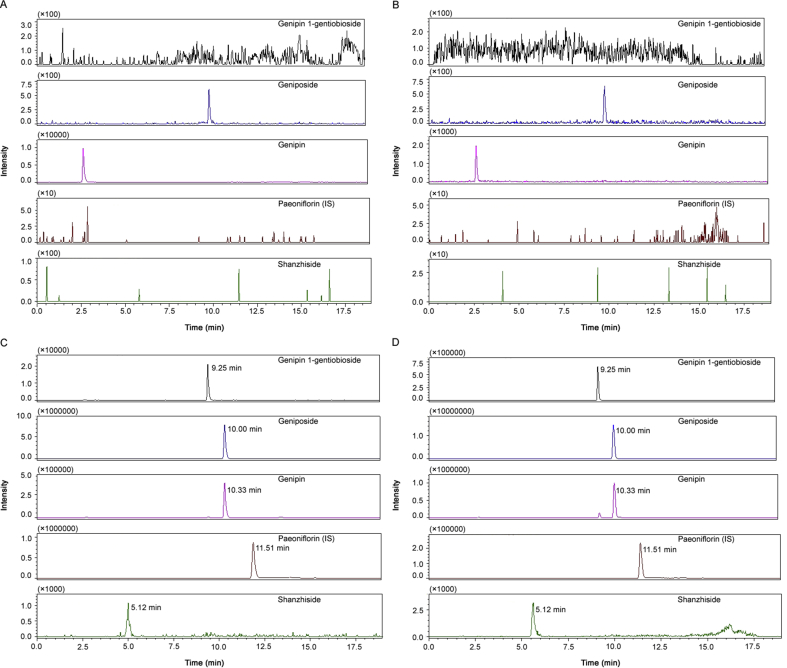
The selectivity of the method towards plasma matrix was evaluated with plasma from six rats. Fig. 2 shows the typical chromatograms. The retention time of shanzhiside, genipin-1-gentiobioside, geniposide, genipin and IS was 5.1, 9.2, 10.0, 10.3 and 11.5 min, respectively. There were no significant interference signals from endogenous substances in normal rats and isoflavones-fed rats.
Fig. 2.
Representative MRM chromatograms of genipin-1-gentiobioside, geniposide, genipin, shanzhiside and IS in rat plasma samples. (A) Blank plasma sample from a normal rat; (B) blank plasma sample from a rat exposed to isoflavones for 28 day; (C) blank plasma sample from a normal rat spiked with four analytes at LLOQ and IS; (D) plasma sample from a normal rat obtained at 0.5 h after oral administration of GF extract at a dose of 3.45 g/kg (37.85 ng/mL, 280.26 ng/mL, 676.35 ng/mL and 64.62 ng/mL for shanzhiside, genipin-1-gentiobioside, geniposide and genipin, respectively).
3.3.2. Linearity and LLOQ
The calibration curve was constructed by plotting the peak area ratio of the analyte to IS (Y) versus the plasma concentration of the analyte at ng/mL (X). All the correlation coefficients (r) for each calibration curve exceeded 0.997. The calibration curve, linear range, correlation coefficient (R), and LLOQ of four compounds are shown in Table 2. The LLOQ was used to evaluate the sensitivity of the analytical method with required precision (RSD, %) ≤ 20%, and the accuracy (RE, %) ranged from −20% to 20% at five replicates on the same day.
Table 2.
The regression equations, linear ranges and LLOQs for the determination of the analytes in rat plasma.
| Compounds | Regression equation | R | Linear range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| Shanzhiside | Y = 0.42X−0.0055 | 0.997 | 10–200 | 10 |
| Genipin-1-gentiobiosode | Y = 0.78X+0.0051 | 0.999 | 1–1000 | 1 |
| Geniposide | Y = 9.31X+0.69 | 0.998 | 1–4000 | 1 |
| Genipin | Y = 1.38X+0.024 | 0.999 | 1–2000 | 1 |
3.3.3. Precision and accuracy
The precision and accuracy experiments were evaluated by QC samples at three concentration levels (low, medium and high) on the same day and on three consecutive validation days. The results are listed in Table 3. The intra- and inter-day precision values (RSD%) were less than 12.2% and the accuracy values ranged from −10.1% to 16.4% for intra-day and from −2.5% to 7.8% for inter-day, which demonstrated that the method was precise and accurate.
Table 3.
Precision, accuracy, matrix effect and extraction recovery for the four analytes in rat plasma.
| Compounds spiked concentration (ng/mL) | Intra-day (n = 5) |
Inter-day (n = 15, 5 replicates per day for 3 days) |
Matrix effect (n = 6) |
Extraction recovery (n = 6) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measured concentration (ng/mL) | Precision (RSD,%) | Accuracy (RE,%) | Measured concentration (ng/mL) | Precision (RSD,%) | Accuracy (RE,%) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | |
| Shanzhiside | ||||||||||
| 10 | 10.50 ± 0.44 | 4.2 | 4.1 | |||||||
| 20 | 19.74 ± 0.46 | 2.3 | −2.3 | 20.82 ± 1.19 | 5.7 | 4.7 | 83.8 | 7.9 | 53.8 | 8.6 |
| 100 | 103.31 ± 6.83 | 6.6 | 0.6 | 101.05 ± 6.39 | 6.3 | 7.3 | 83.2 | 8.5 | 54.6 | 4.6 |
| 160 | 158.62 ± 5.83 | 3.7 | −0.6 | 161.34 ± 8.64 | 5.4 | −2.5 | 85.7 | 7.4 | 60.8 | 4.7 |
| Genipin-1-gentiobioside | ||||||||||
| 1 | 1.15 ± 0.03 | 2.5 | 13.7 | |||||||
| 2 | 2.04 ± 0.10 | 5.0 | 2.9 | 1.99 ± 0.10 | 3.2 | 1.1 | 81.2 | 8.6 | 98.8 | 8.0 |
| 500 | 540.63 ± 17.18 | 3.2 | 7.4 | 533.35 ± 24.73 | 4.6 | −0.3 | 87.7 | 6.5 | 99.7 | 5.3 |
| 800 | 810.89 ± 32.72 | 4.0 | 0.2 | 820.64 ± 26.02 | 5.0 | 4.1 | 83.3 | 3.4 | 84.9 | 9.0 |
| Geniposide | ||||||||||
| 1 | 1.11 ± 0.04 | 4.0 | 8.8 | |||||||
| 2 | 1.96 ± 0.24 | 12.2 | −10.1 | 2.02 ± 0.20 | 9.8 | 7.0 | 82.0 | 3.7 | 89.9 | 6.0 |
| 2000 | 2025.19 ± 164.14 | 8.1 | −0.1 | 2036.98 ± 140.32 | 6.9 | −0.1 | 89.2 | 5.1 | 87.7 | 8.5 |
| 3200 | 3172.78 ± 116.84 | 3.7 | −1.6 | 3271.67 ± 155.95 | 4.8 | 2.3 | 82.0 | 5.0 | 92.5 | 5.1 |
| Genipin | ||||||||||
| 1 | 1.15 ± 0.04 | 3.5 | 16.4 | |||||||
| 2 | 2.03 ± 0.06 | 2.8 | 3.1 | 2.06 ± 0.11 | 3.9 | 7.8 | 88.1 | 5.5 | 71.0 | 9.4 |
| 1000 | 1012.98 ± 23.61 | 1.8 | 2.8 | 1042.44 ± 75.04 | 7.2 | 3.4 | 88.4 | 8.6 | 70.0 | 3.2 |
| 1600 | 1602.72 ± 23.61 | 1.5 | 1.3 | 1603.35 ± 61.85 | 5.5 | 1.4 | 82.5 | 4.4 | 67.6 | 7.9 |
3.3.4. Extraction recovery and matrix effect
The extraction recoveries and matrix effects of four analytes were investigated by analyzing low, medium and high QC samples. The results are shown in Table 3. The extraction recoveries of the analytes ranged from 53.8% to 99.7%, and the matrix effects ranged from 81.2% to 89.2% with a little ion suppression, which suggested that the values were all in the acceptable ranges. The extraction recovery and matrix effect of IS was 86.3% and 81.0%, respectively.
3.3.5. Stability
The stability studies were determined by QC samples at low and high concentration levels under four storage conditions. The results of stability are shown in Table 4 illustrating the enough stable analytes in rat plasma.
Table 4.
Stability of four analytes in rat plasma (n = 3).
| Compounds spiked concentration (ng/mL) | Short-term stability (room temperature for 24 h) |
Long-term stability (−80 °C for 15 days) |
Free-thaw stability (3 free-thaw cycles) |
Post-preparation stability (4 °C for 24 h) |
||||
|---|---|---|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | |
| Shanzhiside | ||||||||
| 20 | 5.6 | 7.9 | 0.2 | 10.7 | 12.7 | −1.2 | 6.4 | 5.2 |
| 160 | 1.9 | 3.2 | 2.4 | −1.0 | 0.1 | −3.1 | 5.2 | 2.9 |
| Genipin-1-gentiobioside | ||||||||
| 2 | 7.0 | 6.3 | 4.0 | −9.1 | 15.0 | −4.1 | 6.1 | 9.8 |
| 800 | 0.8 | 0.9 | 0.9 | 0.8 | 1.0 | 1.2 | 0.9 | 1.7 |
| Geniposide | ||||||||
| 2 | 7.1 | 5.8 | 12.3 | 3.7 | 5.5 | −4.2 | 3.0 | −5.8 |
| 3200 | 0.8 | 0.7 | 1.1 | 0.5 | 2.0 | 1.7 | 0.4 | 1.2 |
| Genipin | ||||||||
| 2 | 10.5 | −0.9 | 1.1 | 10.8 | 5.9 | −8.4 | 12.0 | 9.7 |
| 1600 | 0.3 | 1.7 | 0.8 | −1.3 | 0.6 | −1.4 | 2.1 | −0.8 |
3.4. Pharmacokinetic study
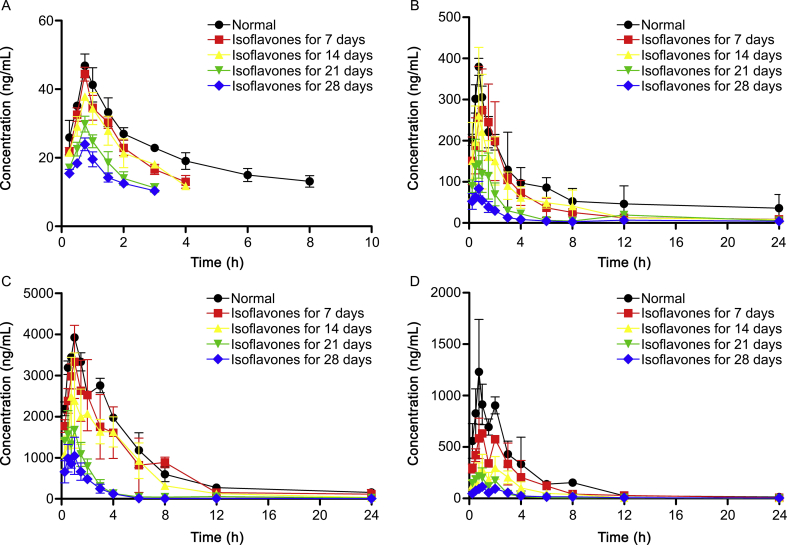
The LC-MS/MS method for the quantitative analysis of four iridoids in rat plasma was successfully established. After oral administration of 3.45 g extract/kg (10 g crude material/kg, shanzhiside 6.43 mg/kg, genipin-1-gentiobioside 376.38 mg/kg, geniposide 1245.36 mg/kg) to rats, the pharmacokinetic parameters and mean plasma concentration-time profiles are presented in Table 5 and Fig. 3.
Table 5.
Pharmacokinetic parameters of the four analytes in rats pre-treated with isoflavones for 0, 7, 14, 21, and 28 days after oral administration of GF extract (mean ± SD, n = 6).
| Group | Compounds | Cmax (ng/mL) | tmax (h) | t1/2 (h) | MRT (h) | AUC0→t (ng·h/mL) | AUC0→∞ (ng·h/mL) |
|---|---|---|---|---|---|---|---|
| A: Isoflavones for 0 day | Shanzhiside | 45.65 ± 4.20 | 0.75 | 5.46 ± 1.06 | 3.31 ± 0.06 | 167.63 ± 9.83 | 260.88 ± 41.14 |
| Genipin 1-gentiobioside | 366.79 ± 53.34 | 0.75 | 8.90 ± 8.58 | 6.03 ± 1.37 | 1441.73 ± 511.48 | 1846.80 ± 1165.18 | |
| Geniposide | 3893.94 ± 84.72 | 1.00 | 5.64 ± 3.94 | 4.98 ± 0.51 | 18192.97 ± 2600.10 | 19281.55 ± 3343.57 | |
| Genipin | 1446.40 ± 281.83 | 1.10 ± 0.52 | 5.90 ± 7.17 | 3.64 ± 0.97 | 4296.24 ± 1871.57 | 4496.07 ± 2014.02 | |
| B: Isoflavones for 7 days | Shanzhiside | 43.90 ± 2.70 | 0.79 ± 0.10 | 2.20 ± 0.61 | 1.97 ± 0.35 | 101.96 ± 17.13 | 136.94 ± 19.99 |
| Genipin 1-gentiobioside | 291.99 ± 98.31 | 0.96 ± 0.29 | 6.23 ± 3.68 | 4.96 ± 0.85 | 1031.64 ± 259.00 | 1081.93 ± 271.64 | |
| Geniposide | 3172.45 ± 609.89 | 1.04 ± 0.49 | 5.20 ± 2.35 | 5.00 ± 0.52 | 14993.84 ± 5073.46 | 15797.05 ± 5627.01 | |
| Genipin | 709.26 ± 123.42 | 1.15 ± 0.49 | 3.75 ± 1.21 | 4.29 ± 0.44 | 3100.08 ± 859.70 | 3130.41 ± 843.56 | |
| C: Isoflavones for 14 days | Shanzhiside | 37.62 ± 1.66 | 0.75 | 1.83 ± 0.54 | 1.73 ± 0.06 | 79.16 ± 7.15 | 105.14 ± 17.07 |
| Genipin 1-gentiobioside | 276.65 ± 94.53 | 0.92 ± 0.54 | 5.50 ± 2.73 | 5.34 ± 0.49 | 968.14 ± 294.04 | 1007.46 ± 278.88 | |
| Geniposide | 2272.13 ± 624.74 | 1.29 ± 0.56 | 3.65 ± 1.29 | 4.93 ± 0.54 | 11380.85 ± 2452.48 | 11561.73 ± 2444.79 | |
| Genipin | 286.19 ± 70.88 | 1.35 ± 0.60 | 2.52 ± 1.14 | 3.82 ± 0.30 | 1140.00 ± 301.24 | 1144.55 ± 297.58 | |
| D: Isoflavones for 21 days | Shanzhiside | 30.80 ± 2.31 | 0.75 | 1.36 ± 0.50 | 1.39 ± 0.03 | 51.02 ± 3.32 | 68.30 ± 10.60 |
| Genipin 1-gentiobioside | 154.70 ± 53.99 | 1.00 ± 0.47 | 7.01 ± 2.74 | 6.07 ± 1.47 | 496.82 ± 139.39 | 746.96 ± 184.26 | |
| Geniposide | 2053.87 ± 194.63 | 0.88 ± 0.38 | 13.23 ± 6.80 | 3.44 ± 6.80 | 5123.81 ± 1115.61 | 5555.66 ± 906.72 | |
| Genipin | 236.78 ± 21.85 | 1.05 ± 0.27 | 4.28 ± 3.62 | 2.65 ± 0.30 | 648.32 ± 83.87 | 654.57 ± 76.64 | |
| E: Isoflavones for 28 days | Shanzhiside | 24.89 ± 4.34 | 0.75 | 2.25 ± 1.33 | 1.45 ± 0.02 | 44.04 ± 6.15 | 77.59 ± 27.68 |
| Genipin 1-gentiobioside | 84.46 ± 22.51 | 0.71 ± 0.10 | 14.62 ± 12.67 | 6.81 ± 1.37 | 224.49 ± 35.04 | 365.19 ± 152.19 | |
| Geniposide | 1150.46 ± 222.73 | 1.00 ± 0.52 | 8.75 ± 8.57 | 2.83 ± 0.82 | 2852.44 ± 711.99 | 2928.61 ± 653.48 | |
| Genipin | 113.70 ± 11.01 | 1.00 | 7.18 ± 3.53 | 4.24 ± 1.06 | 390.01 ± 81.56 | 415.66 ± 102.69 |
Fig. 3.
Mean plasma concentration-time profiles of (A) shanzhiside, (B) genipin-1-gentiobioside, (C) geniposide, and (D) genipin in normal rats (-●-) and rats pre-treated with isoflavones for 7 (-■-), 14 (-▲-), 21 (-▼-), 28 (-◆-) days after oral administration of GF extract at a dose of 3.45 g/kg (each point represents mean ± SD, n = 6).
3.4.1. Pharmacokinetic parameters in normal rats
It could be seen from Table 5 that in normal group, the tmax of four analytes were about 0.75 – 1 h. Both of the Cmax and the AUC0→t of geniposide were higher than those of genipin. Genipin is the metabolite of geniposide and genipin-1-gentiobioside, which are partly biotransformed into their aglycone form by the intestinal microbial β-glucosidase [31], and is absorbed from the gastrointestinal tract. Besides, genipin might be further transformed to some conjugated metabolites such as sulfate conjugation, glucuronidation conjugation of genipin by sulfotransferase in the gut [32]. These contributed to the lower Cmax and the AUC0→t of genipin. In addition, there were distinct double-peaks in both individual and mean plasma concentration-time curves of geniposide and genipin. This phenomenon might be caused by enterohepatic recycling, indicating that geniposide and genipin would be reabsorbed in the gut after being excreted into bile.
3.4.2. The influence of isoflavones on pharmacokinetic profiles of iridoids
Compared with normal rats, the rats exposed to isoflavones had remarkably lower Cmax and decreased AUC0→t of the four analytes. The significant differences in Cmax and AUC0→t among the B, C, D and E groups indicated that the exposure of the iridoids in rats would decrease accordingly with the increased time of rats fed with isoflavones.
Since gut microbiota could biotransform iridoid glycosides into the aglycones, and isoflavones could modify total numbers and/or their relative proportion of specific bacterial communities in the gut [33], resulting in the alteration of the pharmacokinetic profiles of iridoids in isoflavones-fed rats. Our previous work [20] had shown that isoflavones could decrease the abundances of 8 genera (Blautia, Roseburia, Desulfovibrio, Phascolarctobacterium, Butyricimonas, Collinsella, Paraprevotella, and Gemella) and increase the abundances of 13 genera (Lactobacillus, Prevotella, Alloprevotella, Saccharibacteria genera incertae sedis, Bifidobacterium, Odoribacter, Acetatifactor, Intestinimonas, Barnesiella, Streptophyta, Adlercreutzia, Anaeroplasma and Veillonella) in gut microbiota. Some gut microbiota could influence the activity of metabolic enzymes, which played a vital role in drug biotransformation.
Lactobacillus is found to produce extracellular β-d-glucosidase which contributes to biotransform glycosides into aglycones [34,35]. Thus, the decreased bioavailability of glucosides might result from the increased activity of β-d-glucosidase, which improves the biotransformation of glucosides into aglycones largely. That might be the reason why the bioavailability of three iridoid glucosides decreased with the increased isoflavones exposure time.
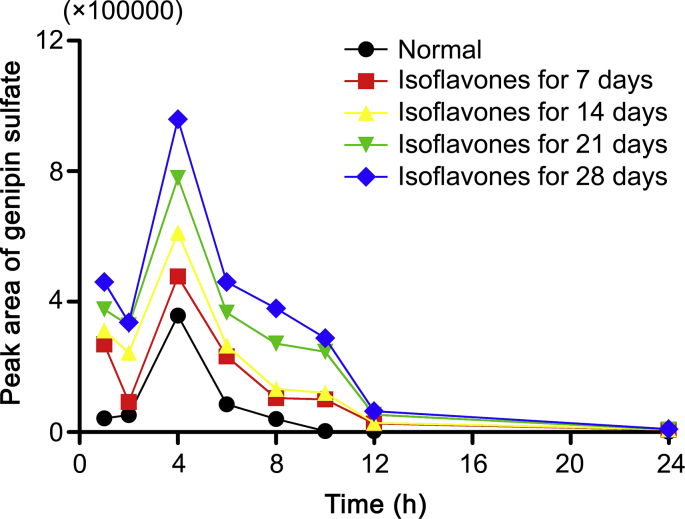
However, with the Cmax and AUC0→t of three glucosides decreasing, those of the aglycone were reduced as well. In the meantime it was found that one conjugated metabolite, genipin sulfate, increased. The time and peak areas plot of genipin sulfate is shown in Fig. 4 due to the lack of the reference standard of it. The plot illustrates genipin sulfate in the rat plasma increased further when the rat group was exposed to isoflavones for longer days. It has been reported that oral Lactobacillus could modulate phase II metabolic enzymes, such as sulfotransferase, and UDP-glucuronosyltransferases [36,37]. Thus, it was indicated that feeding isoflavones generated high abundance of Lactobacillus in rats, producing more biotransformation of genipin into genipin sulfate, and the decreased bioavailability of the aglycone appeared. Meanwhile, Desulfovibrio could reduce sulfate to sulfide. The reduction of Desulfovibrio could inhibit the consumption of genipin sulfate, which might result from the decreased activity of dissimilatory adenosine 50-phosphosulfate reductase (AprAB) and sulfite reductase [38], the key enzymes in this process. Overall, isoflavones might have an impact on pharmacokinetic profiles of iridoids mediated by gut microbiota.
Fig. 4.
The peak area-time curve of genipin sulfate in normal rats (-●-) and rats pre-treated with isoflavones for 7 (-■-), 14 (-▲-), 21 (-▼-), 28 (-◆-) days after oral administration of GF extract at a dose of 3.45 g/kg (each point represents the value from a pooled plasma sample with three rats).
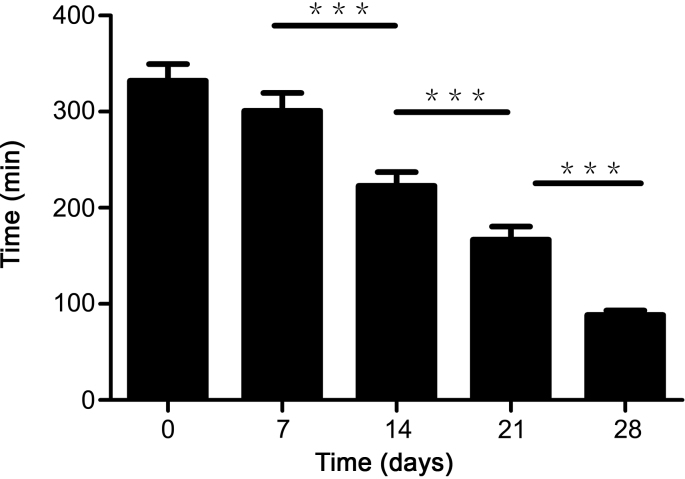
In addition, rats administered with GF extract discharge urine and faeces in blue-green. Owing to the fact that genipin could be transformed to blue conjugates with amino acids [39,40], the dying urine and faeces could be explained by this formation in gut. The blue conjugates had high hydrophilcity and could be excreted from the body through urine and faeces, making urine and faeces blue-green. In this study, we found that the first time of discharging blue-green urine from rats after their oral administration of GF extract became earlier with the longer isoflavones exposure time (Fig. 5), indicating the more accelerated excretion rate of gardenia blue in urine. It has been reported that geniposide would require metabolic activation for genipin to exert its toxicity in HepG2 cells [41,42]. Altogether, isoflavones not only raised the biotransformation of genipin into genipin sulfate, but also improved the discharge of amino acid conjugation of genipin, which demonstrated that the isoflavones might play an indispensable role in detoxification of genipin.
Fig. 5.
The first urination time of blue-green urine from different groups of rats after their oral administration of GF extract. Data were presented as the mean ± SEM. ***P < 0.001.
4. Conclusion
This LC-MS/MS method was successfully established and validated to determine shanzhiside, genipin-1-gentiobioside, geniposide and genipin in rat plasma simultaneously after oral administration of GF extract. This study indicated there were significant differences in pharmacokinetic profiles of iridoids between normal rats and isoflavones exposed rats after oral administration of GF extract. It was revealed that the isoflavones could alter the species and/or their relative proportion of gut microbiota in rats, resulting in the change of the activity of some enzymes, which made the different fates of iridoids in the rats, especially the decreased iridoid glycosides and genipin, increased genipin sulfate in plasma and accelerated excretion of genipin-amino acid conjugation. Thus, the result provided that isoflavones could alter the disposition of iridoids in vivo when GF and SSP were prescribed together in the traditional Chinese medicine to obtain toxicity-reducing. This research gave us a new insight into the compatibility of traditional Chinese formula by analysis of the PK alteration of chemical components via gut microbiota. More attention should be paid to the interaction among different traditional Chinese herbs, the chemical components and gut microbiota in the clinical therapy in the future.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81573584, 81773862).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Jun Wen, Email: wenjunapple@163.com.
Weidong Chen, Email: wdchen@ahtcm.edu.cn.
Tingting Zhou, Email: tingting_zoo@163.com.
References
- 1.Chen Y.I., Cheng Y.W., Tzeng C.Y. Peroxisome proliferator-activated receptor activating hypoglycemic effect of Gardenia jasminoides Ellis aqueous extract and improvement of insulin sensitivity in steroid induced insulin resistant rats. BMC Complement Altern. Med. 2014;14:30. doi: 10.1186/1472-6882-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H., Chen Y.F., Li F. Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2013;15:94–110. doi: 10.1080/10286020.2012.723203. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Wang G.C., Rong J. Identification of steroidogenic components derived from Gardenia jasminoides Ellis potentially useful for treating postmenopausal syndrome. Front. Pharmacol. 2018;9:390. doi: 10.3389/fphar.2018.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y., Wen J., Zhou T. Chemical fingerprinting of Gardenia jasminoides Ellis by HPLC-DAD-ESI-MS combined with chemometrics methods. Food Chem. 2015;188:648–657. doi: 10.1016/j.foodchem.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Coran S.A., Mulas S., Vasconi A. Profiling of components and validated determination of iridoids in Gardenia Jasminoides Ellis fruit by a high-performance-thin-layer- chromatography/mass spectrometry approach. J. Chromatogr. A. 2014;1325:221–226. doi: 10.1016/j.chroma.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Ma D., Wang N., Fan X. Protective effects of cornel iridoid glycoside in rats after traumatic brain injury. Neurochem. Res. 2018;43:959–971. doi: 10.1007/s11064-018-2501-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen J.Y., Wu H., Li H. Anti-inflammatory effects and pharmacokinetics study of geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2015;24:102–109. doi: 10.1016/j.intimp.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Zhang Y., Liu R. Geniposide protects against acute alcohol-induced liver injury in mice via up-regulating the expression of the main antioxidant enzymes. Can. J. Physiol. Pharmacol. 2015;93:261–267. doi: 10.1139/cjpp-2014-0536. [DOI] [PubMed] [Google Scholar]
- 9.Carbonel A.A.F., Simões R.S., Girão J.H.C. Isoflavones in gynecology. Rev. Assoc. Med. Bras. 2018;64:560–564. doi: 10.1590/1806-9282.64.06.560. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Wang D., Yang G. Comparative pharmacokinetics and brain distribution of magnolol and honokiol after oral administration of Magnolia officinalis cortex extract and its compatibility with other herbal medicines in Zhi-Zi-Hou-Po Decoction to rats. Biomed. Chromatogr. 2016;30:369–375. doi: 10.1002/bmc.3557. [DOI] [PubMed] [Google Scholar]
- 11.Luo K., Feng F. Identification of absorbed components and metabolites of Zhi-Zi-Hou-Po decoction in rat plasma after oral administration by an untargeted metabolomics-driven strategy based on LC-MS. Anal. Bioanal. Chem. 2016;408:5723–5735. doi: 10.1007/s00216-016-9674-x. [DOI] [PubMed] [Google Scholar]
- 12.Hu Q., Li X., Shi Q. Deciphering the absorption profile and interaction of multi-components of Zhi-Zi-Da-Huang decoction based on in vitro–in silico–in vivo integrated strategy. Xenobiotica. 2019;49:762–777. doi: 10.1080/00498254.2018.1497220. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Shi Q., Wu C. Dynamic metabolic profile of Zhi-Zi-Da-Huang decoction in rat urine based on hybrid liquid chromatography–mass spectrometry coupled with solid phase extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1036:100–113. doi: 10.1016/j.jchromb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Long Z., Zhang R., Zhao X. Determination and pharmacokinetics of geniposidic acid in rat plasma after oral administration of Gardenia jasminoides fruit crude extract and Zhi-zi-chi decoction. Biomed. Chromatogr. 2013;27:812–816. doi: 10.1002/bmc.2871. [DOI] [PubMed] [Google Scholar]
- 15.Qu K., Dai J., Zhao L. A sensitive liquid chromatographic-mass spectrometric method for simultaneous quantification of six iridoid glycosides from Zhi-zi-chi Decoction in rat plasma and its application to a pharmacokinetic study, J. Pharm. Biomed. Anal. 2013;78:83–91. doi: 10.1016/j.jpba.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Kim D.-H. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab. Dispos. 2015;43:1581–1589. doi: 10.1124/dmd.115.063867. [DOI] [PubMed] [Google Scholar]
- 17.Kong M., Liu H.H., Wu J. Effects of sulfur-fumigation on the pharmacokinetics, metabolites and analgesic activity of Radix Paeoniae Alba. J. Ethnopharmacol. 2018;212:95–105. doi: 10.1016/j.jep.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Xiao B., Sun Z., Sun S. Effect of cortex mori on pharmacokinetic profiles of main isoflavonoids from pueraria lobata in rat plasma. J. Ethnopharmacol. 2017;209:140–146. doi: 10.1016/j.jep.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Balap A., Lohidasan S., Sinnathambi A. Herb-drug interaction of Andrographis paniculata (Nees) extract and andrographolide on pharmacokinetic and pharmacodynamic of naproxen in rats. J. Ethnopharmacol. 2017;195:214–221. doi: 10.1016/j.jep.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Chang R., Zhang X. Non-isoflavones diet incurred metabolic modifications induced by constipation in rats via targeting gut microbiota. Front. Microbiol. 2018;9:3002. doi: 10.3389/fmicb.2018.03002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Y., Ma X., Li T. Quantification of isoflavone glycosides and aglycones in rat plasma by LC–MS/MS: Troubleshooting of interference from food and its application to pharmacokinetic study of Semen Sojae Praeparatum extract. J. Pharm. Biomed. Anal. 2018;161:444–454. doi: 10.1016/j.jpba.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Wu D., Chen X., Hu S. Study on major antitumor components in Yinchenhao decoction in vitro and in vivo based on hollow fiber cell fishing coupled with high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017;1060:118–125. doi: 10.1016/j.jchromb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Li C., Dong J., Tian J. LC/MS/MS determination and pharmacokinetic study of iridoid glycosides monotropein and deacetylasperulosidic acid isomers in rat plasma after oral administration of Morinda officinalis extract. Biomed. Chromatogr. 2016;30:163–168. doi: 10.1002/bmc.3532. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y.X., Liu E.W., Wang L. LC/MS/MS determination and pharmacokinetic studies of six compounds in rat plasma following oral administration of the single and combined extracts of Eucommia ulmoides and Dipsacus asperoides. Chin. J. Nat. Med. 2014;12:469–476. doi: 10.1016/S1875-5364(14)60073-X. [DOI] [PubMed] [Google Scholar]
- 25.Lu C.M., Lin L.C., Tsai T.H. Determination and pharmacokinetic study of gentiopicroside, geniposide, baicalin, and swertiamarin in Chinese herbal formulae after oral administration in rats by LC-MS/MS. Molecules. 2014;19:21560–21578. doi: 10.3390/molecules191221560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi F., Pan H., Li Y. A sensitive LC–MS/MS method for simultaneous quantification of geniposide and its active metabolite genipin in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2018;32 doi: 10.1002/bmc.4126. [DOI] [PubMed] [Google Scholar]
- 27.Chang R., Chen W., Zhou T. A sensitive LC-MS/MS method for simultaneous quantification of three iridiods in Gardenia jasminoides Ellis, J. Pharm. Pract. 2019;37:19–22. [Google Scholar]
- 28.Guo H., Zhang Z., Yao Y. A new strategy for statistical analysis-based fingerprint establishment: application to quality assessment of Semen sojae praeparatum. Food Chem. 2018;258:189–198. doi: 10.1016/j.foodchem.2018.03.067. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration . 2018. Bioanalytical Method Validation Guidance for Industry, U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) pp. 4–10.https://www.fda.gov/drugs/guidances-drugs/all-guidances-drugs [Google Scholar]
- 30.Jiang J., Zhao X., Li X. High-throughput determination of sodium danshensu in beagle dogs by the LCMS/MS method, employing liquid-liquid extraction based on 96-well format plates. Molecules. 2017;22:667. doi: 10.3390/molecules22050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akao T., Kobashi K., Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of Geniposide. Biol. Pharm. Bull. 1994;17:1573–1576. doi: 10.1248/bpb.17.1573. [DOI] [PubMed] [Google Scholar]
- 32.Jiang P., Ma Y., Gao Y. Comprehensive evaluation of the metabolism of genipin-1-β- d -gentiobioside in vitro and in vivo by using HPLC-Q-TOF. J. Agric. Food Chem. 2016;64:5490–5498. doi: 10.1021/acs.jafc.6b01835. [DOI] [PubMed] [Google Scholar]
- 33.Tomás-Barberán F.A., Selma M.V., Espín J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:471–476. doi: 10.1097/MCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 34.Yeo S.K., Liong M.T. Effect of ultrasound on the growth of probiotics and bioconversion of isoflavones in prebiotic-supplemented soymilk. J. Agric. Food Chem. 2011;59:885–897. doi: 10.1021/jf103974d. [DOI] [PubMed] [Google Scholar]
- 35.Rekha C.R., Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010;109:1198–1208. doi: 10.1111/j.1365-2672.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamaladevi A., Ganguli A., Balamurugan K. Lactobacillus casei stimulates phase-II detoxification system and rescues malathion-induced physiological impairments in Caenorhabditis elegans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016;179:19–28. doi: 10.1016/j.cbpc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Ren C., Dokter-Fokkens J., Figueroa Lozano S. Lactic acid bacteria may impact intestinal barrier function by modulating goblet cells. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duarte A.G., Santos A.A., Pereira I.A.C. Electron transfer between the QmoABC membrane complex and adenosine 5′-phosphosulfate reductase. Biochim. Biophys. Acta Bioenerg. 2016;1857:380–386. doi: 10.1016/j.bbabio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Park J.E., Lee J.Y., Kim H.G. Isolation and characterization of water-soluble intermediates of blue pigments transformed from geniposide of Gardenia jasminoides. J. Agric. Food Chem. 2002;50:6511–6514. doi: 10.1021/jf020499b. [DOI] [PubMed] [Google Scholar]
- 40.Hou Y.C., Tsai S.Y., Lai P.Y. Metabolism and pharmacokinetics of genipin and geniposide in rats. Food Chem. Toxicol. 2008;46:2764–2769. doi: 10.1016/j.fct.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Kang M.J., Khanal T., Kim H.G. Role of metabolism by human intestinal microflora in geniposideinduced toxicity in HepG2 cells. Arch Pharm. Res. (Seoul) 2012;35:733–738. doi: 10.1007/s12272-012-0418-y. [DOI] [PubMed] [Google Scholar]
- 42.Khanal T., Kim H.G., Choi J.H. Biotransformation of geniposide by human intestinal microflora on cytotoxicity against HepG2 cells. Toxicol. Lett. 2012;209:246–254. doi: 10.1016/j.toxlet.2011.12.017. [DOI] [PubMed] [Google Scholar]