Abstract
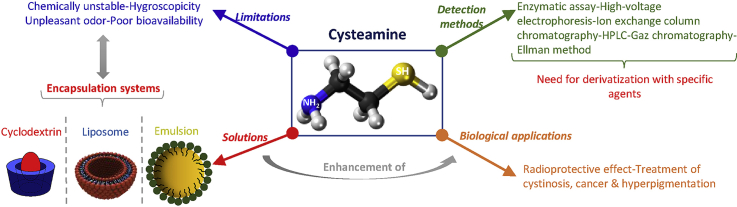
The aminothiol cysteamine, derived from coenzyme A degradation in mammalian cells, presents several biological applications. However, the bitter taste and sickening odor, chemical instability, hygroscopicity, and poor pharmacokinetic profile of cysteamine limit its efficacy. The use of encapsulation systems is a good methodology to overcome these undesirable properties and improve the pharmacokinetic behavior of cysteamine. Besides, the conjugation of cysteamine to the surface of nanoparticles is generally proposed to improve the intra-oral delivery of cyclodextrin-drug inclusion complexes, as well as to enhance the colorimetric detection of compounds by a gold nanoparticle aggregation method. On the other hand, the detection and quantification of cysteamine is a challenging mission due to the lack of a chromophore in its structure and its susceptibility to oxidation before or during the analysis. Derivatization agents are therefore applied for the quantification of this molecule. To our knowledge, the derivatization techniques and the encapsulation systems used for cysteamine delivery were not reviewed previously. Thus, this review aims to compile all the data on these methods as well as to provide an overview of the various biological applications of cysteamine focusing on its skin application.
Keywords: Cysteamine, Detection, Encapsulation, Skin, Stability
Graphical abstract
Highlights
-
•
Cysteamine derivatization is required for quantification purposes.
-
•
Cysteamine applications are reviewed including hyperpigmentation treatment.
-
•
Drug delivery systems loading cysteamine are highlighted.
1. Introduction
Cysteamine (cys) or 2-mercaptoethylamine is an aminothiol endogenously synthesized by human body cells during the co-enzyme A metabolism cycle (Fig. 1).
Fig. 1.
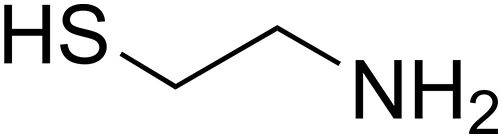
Structure of cys.
In 1953, cys was found to be one of the most potent thiol derivatives in the protection against ionizing radiation [1]. Numerous studies have tested the radioprotective effect and the mechanism of action of this compound using human cell lines [2,3], bacteria [4], and mice models [5].
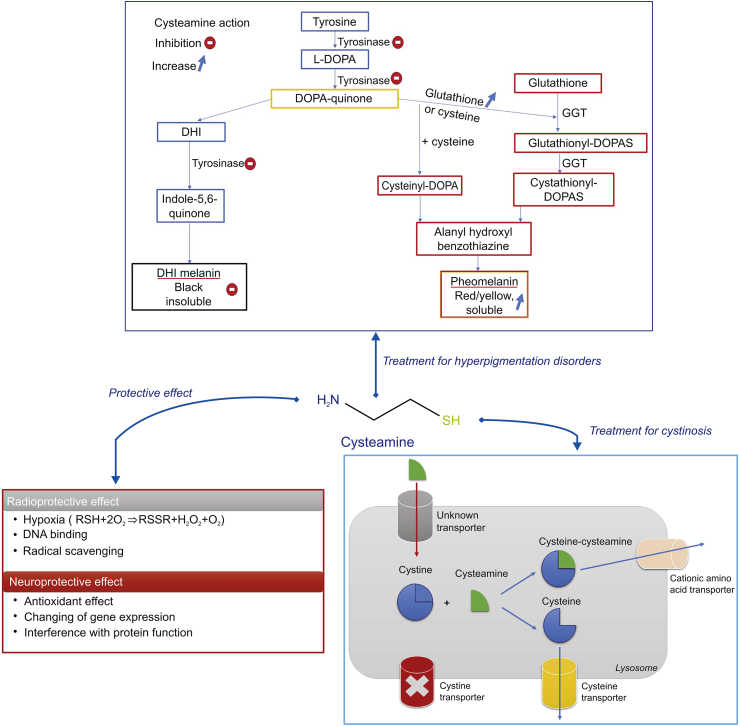
In 1976, cys was first used for the treatment of cystinosis, approved by the US Food and Drug Administration (FDA), and commercialized in the 1990s [6]. Till now, cys is the only specific targeted therapy available for patients with cystinosis. The latter is a rare autosomal recessive metabolic disease, characterized by the accumulation and crystallization of cystine within the lysosome, which eventually results in apoptosis and tissue damage in all organ systems including the cornea [7]. Following oral administration, cys enters the lysosome by an unknown transporter and breaks down cystine into cysteine and cysteine-cys disulfide, which are removed by specific transporters. Consequently, cys rapidly depletes cells and tissues of lysosomal cystine.
Another interesting application was recently discovered for cys, as it was shown to be clinically efficient in treating hyperpigmentation disorders [8,9]. Despite the presence of many potent depigmenting agents, namely hydroquinone and derivatives and kojic acid, they were found to possess local side effects (irritation, permanent depigmentation, etc.) and presented mutagenic and carcinogenic potentials [10]. Cys has shown to be a well-tolerated compound, demonstrating its non-mutagenicity and non-carcinogenicity criteria [11]. Interestingly, it may inhibit the mutagenic effect of some potent mutagens. Besides, it may exert an anti-cancer effect in several cancers, such as melanoma, in in vivo studies [12].
Regardless of its numerous and remarkable biological applications, the efficacy of cys may be limited by its unpleasant organoleptic properties, strong hygroscopicity, chemical instability, and its poor pharmacokinetic profile (T1/2 = 1.75 h) [13]. Moreover, cys is often susceptible to degradation through its rapid oxidation in air or aqueous solution to its disulfide form cystamine (Fig. 2).
Fig. 2.
Oxidation of cys to cystamine.
Consequently, the encapsulation of this molecule into delivery systems has been commonly used as a good approach to overcoming these problems [[14], [15], [16]]. Cys was mainly incorporated into three different types of encapsulating agents: liposomes, cyclodextrin (CDs), and emulsions. For example, its encapsulation in liposomes was used to selectively release cys in lysosomes [17] and to enhance its gastrointestinal absorption [15]. Besides, CDs and emulsions were successfully shown to remove cys odor [13] and to improve cys stability [18], respectively. On the other hand, cys was employed to modify the surface of encapsulation systems for many purposes. For example, cys was linked to the surface of CDs to improve intra-oral drug delivery [19,20], and to the surface of gold nanoparticles to enhance the colorimetric detection of compounds by these particles [21,22].
This review highlights the biosynthesis, the physicochemical properties, and the stability of cys. The quantification of this compound using different analytical methods, as well as the challenges encountered in these techniques, will be for the first time analyzed. Then, we will discuss the biological activities of this compound emphasizing its topical effects. Finally, the encapsulation of cys in different encapsulation systems and the use of cys to modify the surface of these carriers will be presented.
2. Metabolism of cys
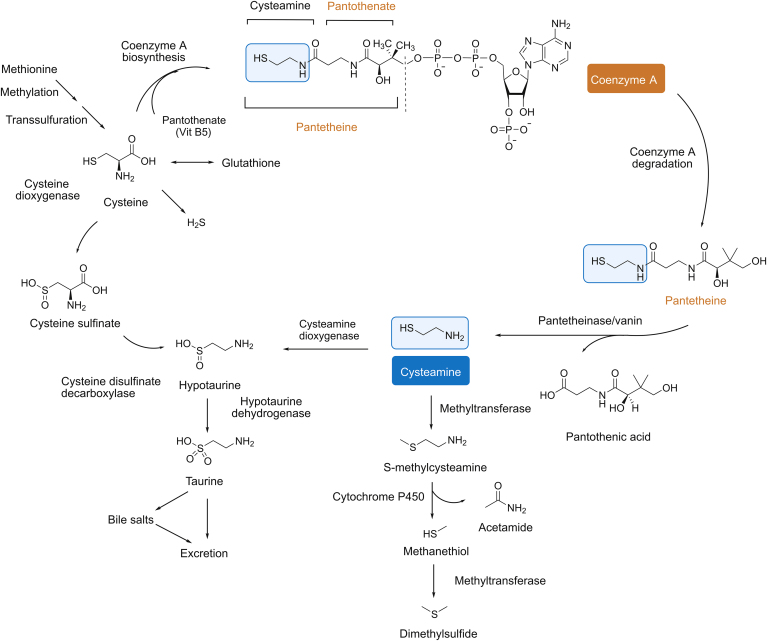
Cys is an aminothiol derived from coenzyme A (CoA) degradation. The degradation of CoA leads to the formation of pantetheine, which is hydrolyzed by pantetheinase to obtain cys and pantothenic acid (Fig. 3) [23,24]. Then, the oxidation of cys by cys dioxygenase produces hypotaurine. Later, cys is eliminated by the taurine pathway in the form of bile salts [25]. After the administration of a high dose of cys, an alternative catabolic route is manifested, involving its conversion to S-methylcys by a thiol-methyltransferase, which is consequently metabolized into methanethiol and acetamide by cytochrome P450. Then, methanethiol is converted to dimethylsulfide by another thiol-methyltransferase [26,27].
Fig. 3.
Metabolism of cys [24] (with permission from John Wiley and Sons).
3. Physicochemical properties and stability of cys
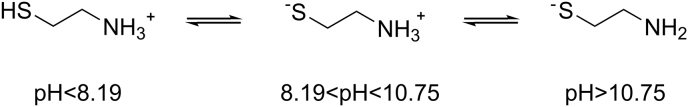
Cys possesses high aqueous solubility (23.5 g/L), a pKa1(SH) = 8.19 and pKa2(NH2) = 10.75 with a melting point of 67.3 ℃ (Table 1 [16,[28], [29], [30], [31]]). This molecule exists in three ionic forms: the positively charged form (cys+), the zwitterionic form (cys-ZW), and the negatively charged form (cys−) (Fig. 4) [32].
Table 1.
Physicochemical properties of cys.
| Physicochemical properties | Detailed information | Refs. |
|---|---|---|
| Melting point | 67.3 °C | [16] |
| pKa values | pKa1 = 8.19; pKa2 = 10.75 | [28] |
| Molecular weight | 77.15 g/mol | [29] |
| Physical description | Solid | [29] |
| Color | Crystal | [29] |
| Odor | Disagreeable odor | [29] |
| Chemical formula | HSCH2CH2NH2 | |
| Boiling point | 133.6 ± 23.0 °C at 760 mmHg (predicted) | |
| Vapor pressure | 0.03167 bar at 25 ℃ | [30] |
| Density | 1.0 ± 0.1 g/mL (predicted) | |
| a LogP | 0.1 | [31] |
| a Water solubility | 23.5 g/L | [31] |
Soluble in methanol, ethanol and freely soluble in alkaline media [31]
Fig. 4.
Chemical structures of cys forms obtained at different pHs.
Besides, cys, as a thiol compound, has a very offensive odor that makes it difficult to be used as a depigmenting agent [9]. Different forms of cys have been used: cys hydrochloride (HCl), phosphocys, and cys bitartrate. Cys hydrochloride forms a eutectic equilibrium with water with a low eutectic temperature of -33.15 ℃. A eutectic mixture is composed of two or three components with a melting point significantly lower than that of its one component [33]. This low temperature causes the rapid dissolution of cys in the presence of water. The presence of a minor quantity of water vapor does not affect the stability state of cys hydrochloride, and cys mass remains stable until a 35% relative humidity. In fact, above this water vapor pressure, cys hydrochloride melts immediately to form a very concentrated saturated solution of 0.85 mass fraction or 0.47 mol fraction of cys hydrochloride [31].
Cys is unstable in aqueous solution; a rapid conversion to cystamine occurs due to the rapid oxidation of the sulfhydryl group. The reactions of oxygen with thiols in aqueous solutions are presented in Eqs. (1) and (2), according to which the reactions give disulfides and hydrogen peroxide or water.
Alkaline pH stimulates the oxidation of cys because the thiolate anion (cys−) is more reactive. Moreover, this reaction is catalyzed by metal ions such as Cu2+, Fe3+, and Zn2+. In fact, the reduction reaction of cys by metal ions produces the reduced ion (Eq. (3)); the latter reacts with oxygen and peroxide. The following series of reactions would explain this fact.
The reaction between Cu+ and oxygen produces superoxide (Eq. (4)), then superoxide reacts with a thiol to generate thiol radical (Eq. (5)) that reacts with itself to produce the disulfide (Eq. (6)).
On the other hand, oxygen consumption is stimulated by a reaction of a reduced metal ion with peroxide, producing, the potent hydroxyl radical (Eq. (7)); the latter reacts with a thiol group, leading to the formation of a water molecule (Eq. (8)). The thiol radical reacts with itself to produce the disulfide (Eq. (6)) as described previously. Therefore, the use of a chelating agent, such as diethyldithiocarbamate, inhibits this reaction [34].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
The degradation of cys is a zero-order reaction, indicating that the concentration of cys decreases linearly with time [35]. The removal of oxygen from a solution of cys by packing under nitrogen and the presence of ascorbic acid (antioxidant) increase the stability of the molecule, but show a lower efficacy in comparison with chelating agents such as disodium edetate [36].
Pescina et al. [16] examined the stability of cys after being dissolved in 0.9% NaCl at different pH, temperature, and in the presence of α-CD, ethylenediaminetetraacetic acid (EDTA), and sodium phosphate. They showed that cys oxidation is a pH-dependent reaction. It is very fast at pH 7.4 because of the presence of the ionized thiol groups while the oxidation decreases at an acidic pH of 4.2. As for the influence of the temperature (−20, 4, and 25 ℃), they found that the stability decreases when the temperature increases. While EDTA plays a crucial role in preventing cys oxidation, the presence of α-CD or sodium phosphate does not affect its stability.
The degradation of 0.1 mg/mL cys has been reached within 18 h in phosphate buffer saline (PBS) with a rate of degradation of 126 μg/h. A similar rate (132 μg/h) was also observed but with a higher concentration (4.4 mg/mL) [18]. In order to improve the stability of cys, the addition of different types of antioxidant to 0.1 mg/mL cys has been evaluated to out-compete cys for oxygen consumption and compared to the degradation rate of free cys (126 μg/h). Nevertheless, the presence of the hydrophilic antioxidant vitamin C increased the degradation rate of cys (523 μg/h). This rate was not also influenced by the presence of the hydrophobic antioxidants, vitamin E, and soybean oil since they are scarcely soluble in aqueous solution. Tween 80 surfactant induced a reduction in degradation rate to 112 μg/h, suggesting some interactions with either dissolved cys or oxygen molecules. While, an emulsion of vitamin E, soybean oil, and Tween 80 surfactant resulted in a greater decrease in cys degradation (101 μg/h). However, these results are not significant because the degradation rate of cys was not well decreased. The use of catalase enzyme, which can revert peroxide species to diatomic oxygen and potentially scavenging the system of radicals essential to oxidize cys, reduced the degradation rate to 58 μg/h. On the other hand, a decline in the cys degradation rate to 20%–30% was obtained after the addition of hydrophobic film (a soybean oil layer) to slow oxygen diffusion [18].
4. Analytical methods for cys detection
Different analytical methods have been suggested to detect and quantify cys in biological samples (i.e., plasma and urine) in the literature, including enzymatic assay, high-voltage electrophoresis, ion-exchange column chromatography, high-performance liquid chromatography (HPLC) with fluorescence and ultraviolet (UV) detection, and gas chromatography with flame ionization and photometric detection. These methods will be for the first time presented in this review.
4.1. Cys derivatization
Due to the lack of a chromophore in cys structure, the quantification of this compound using conventional analytical methods with UV absorbance or fluorescence detection is a challenging mission. Therefore, the derivatization of cys is used for cys separation or quantification. For this purpose, numerous derivatization agents were utilized and optimized according to the analytical method used. In Table 2, the structures of all these agents, as well as the derivatization reactions with cys, are presented [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]].
Table 2.
Structure and reaction of derivatization agents with cys.
| Derivatization agents | Structures | Reaction products | Refs. |
|---|---|---|---|
| Pivaldehyde | 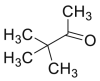 |
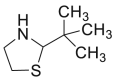 |
[37] |
| BSTFA | 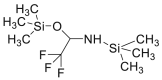 |
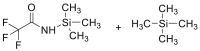 |
[38] |
| mBBr or QBBr | 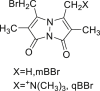 |
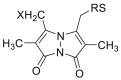 |
[[39], [40], [41], [42]] |
| ABD-F | 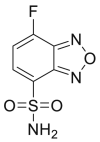 |
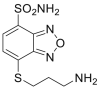 |
[43,44] |
| o-Phthalaldehyde | 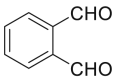 |
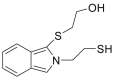 |
[45] |
| isoBCF | 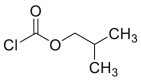 |
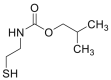 |
[46,47] |
| CMQT | 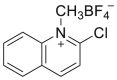 |
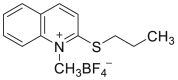 |
[48] |
| NPM | 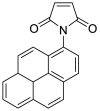 |
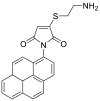 |
[49] |
| DAABD-Cl | 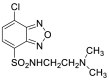 |
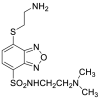 |
[50,51] |
| SBD-F | 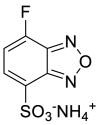 |
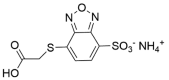 |
[52] |
| ACQ | 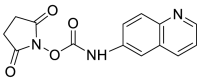 |
[53] |
BSTFA: bis (trimethylsilyl) trifluoroacetamide, ABD-F: 4-fluoro-7-sulfamoyl benzofurazan, isoBCF: isobutyl chloroformate, CMQT: 2-chloro-1-methylquinolinium tetrafluoroborate, NPM: N-(1-pyrenyl) maleimide, DAABD-Cl: 7-chloro-N-[2-(dimethylamino)ethyl]-2,1,3-benzoxadiazole-4-sulfonamide, SBD-F: ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate, ACQ: 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate.
4.1.1. Pivaldehyde
Cys is derivatized with pivaldehyde before its analysis with gas chromatography [37].
4.1.2. Bis (trimethylsilyl) trifluoroacetamide (BSTFA)
The sample containing cys is placed in a screw-capped reaction vial, followed by the addition of dimethylformamide and BSTFA. The mixture is allowed to stand overnight before the analysis [38].
4.1.3. Monobromobimane (mBBr) or monobromotrimethylammoniobimane (qBBr)
mBBr or qBBr were dissolved in methanol, stored at 4 ℃, and protected from light for up to 4 months. This agent was used to quantify cys in plasma, red blood cells, and urine. A certain volume of this agent was added to cys solution and incubated for 15 min in the dark. For biological samples, perchloric acid was added to allow protein precipitation [[39], [40], [41], [42]].
4.1.4. Ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBD-F)
SBD-F was prepared in borate buffer. Borate buffer (125 mM; pH 9.5) containing 4 mM EDTA was added to cys sample solution. After vortex-mixing for 30 s, the reaction mixture was heated at 60°C for 60 min and then cooled at 4°C for 15 min to stop the labeling reaction [43,44].
4.1.5. o-Phthalaldehyde
Cys solution was mixed with sodium hypochlorite followed by the addition of o-phthalaldehyde in the presence of 2-mercaptoethanol. The latter was used as an antioxidant. Sodium hypochlorite increased several times the fluorescence intensity of cys coupled to o-phthalaldehyde [45].
4.1.6. Isobutyl chloroformate (isoBCF)
IsoBCF and NaOH were added to cys sample, and the mixture was shaken at 300 rpm for 5 min at room temperature. The mixture was extracted with n-pentane, and the pentane extract was evaporated to dryness at 80 ℃. The residue was dissolved in ethyl acetate [46,47].
4.1.7. 2-Chloro-1-methylquinolinium tetrafluoroborate (CMQT)
CMQT was synthetized as follows: 2-chloroquinoline, nitromethane, and trimethyloxonium tetrafluoroborate were mixed. Then, diethyl ether was added to the reaction mixture. The white precipitate was filtered off, washed with diethyl ether, and dried over phosphorus pentoxide under vacuum. Unfortunately, the synthesis reaction of CMQT was not presented.
Cys was derivatized by an excess of CMQT in tris buffer solution (pH 8.2), and hydrochloric acid was added to the mixture [48].
4.1.8. N-(1-pyrenyl) maleimide (NPM)
NPM solution was prepared in acetonitrile. Cys solutions were derivatized with 1.0 mM NPM solution and left to stand for 5 min at room temperature. HCl solution (2 M) was added to stop the reaction and stabilize the adducts at the end of the reaction time. The final pH of the solution was kept at about 2, which is necessary for the stability of the NPM-cys adduct [49].
4.1.9. 7-Chloro-N-[2-(dimethylamino)ethyl]-2,1,3-benzoxadiazole-4-sulfonamide (DAABD-Cl)
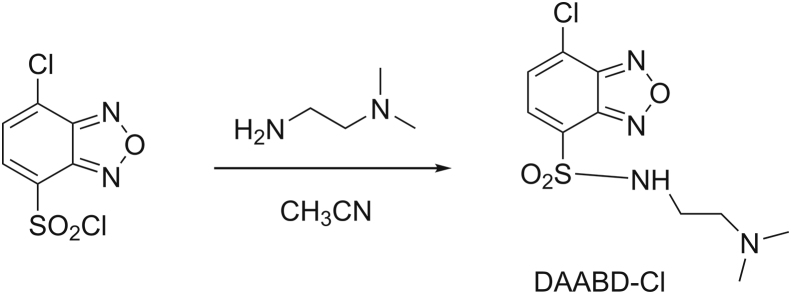
DAABD-Cl was synthesized as follows (Fig. 5): 4-chlorosulfonyl-7-chloro-2,1,3- benzoxadiazole was dissolved in CH3CN. After the addition of N,N-dimethylethylenediamine and triethylamine, the mixture was stirred at room temperature for 10 min. The reaction mixture was evaporated to dryness under reduced pressure to form DAABD-Cl [50].
Fig. 5.
Synthetic route for DAABD-Cl (7-chloro-N-[2-(dimethylamino)ethyl]-2,1,3-benzoxadiazole-4-sulfonamide).
DAABD-Cl was prepared in acetonitrile. A mixture of tris (2-carboxyethyl) phosphine hydrochloride, EDTA, and 3-[(3-cholamidoproryl) dimethylammonio] propanesulfonic acid was prepared in borate buffer. This mixture was added to cys solution, borate buffer, and DAABD-Cl. The reaction mixture was heated at 40 ℃, and aliquots of the reaction mixture were taken out at intervals of 5–20 min, followed by the addition of 20% trifluoroacetic acid to stop the derivatization reaction [51].
4.1.10. 4-Fluoro-7-sulfamoyl benzofurazan (ABD-F)
Cys solutions were directly derivatized with ABD-F reagent. The alkylation reaction was completed at 55 ℃ for 15 min and stopped with HCl 12 N [52].
4.1.11. 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate (ACQ)
Cys solutions were derivatized with ACQ in the presence of borate buffer and heated at 55 ℃ for 10 min. The mixture was allowed to cool at room temperature before HPLC analysis [53].
4.2. Analytical methods
4.2.1. Enzymatic assay
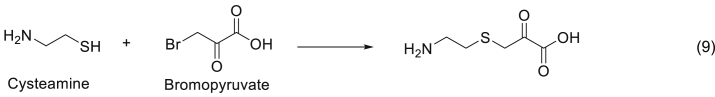
This assay consists of the inhibition of d-amino acid oxidase enzyme activity by the product of the reaction occurring between cys and bromopyruvate (Eq. (9)). The enzyme activity is proportional to the amount of cys [54].
Another method described by Duffel et al. [55] involved the oxidation of cys to hypotaurine by cys dioxygenase (Eq. (10)). Therefore, the quantification of oxygen uptake is proportional to cys concentration with standard solutions between 10 and 100 nmol.
4.2.2. Ion-exchange column chromatography
Hsiung et al. [56] worked on the detection of cys by ion-exchange column chromatography using an amino acid analyzer with a short column of Beckman analyzer. Cys was detected after 268 min, a long time that may lead to the oxidation of the product during the analysis and therefore, an underestimation of the product concentration.
Derivatization of cys with qBBr and mBBr has been realized by Fahey et al. [39], followed by separation of the derivatives by cation-exchange chromatography and detection by fluorometry. The principal limitation of this method was the time required for sample analysis, 3–4 h, for each of the bromobimane derivatives.
Later, Ida et al. [45] used o-phthalaldehyde as the derivatization agent in the presence of 2-mercaptoethanol and sodium hypochlorite. Cation-exchange chromatography, using cation exchange resin (ISC-05/S0504), sodium borate buffer (pH 11.10) as mobile phase, 70 ℃ as temperature and with a linearity range of 2–200 pmol, was used to separate the derivatives, and the elution time was decreased to attain 7.5 min based on a fluorometric detection. For the application of this assay method to biological materials, the pretreatment with a cation exchange column (Dowex 5OWX8) was essential for removing interfering o-phthalaldehyde-reactive substances. This method was found to be suitable for evaluating the cys plus cystamine content in various organs and tissues because cys was quantitatively converted to cystamine in biological materials during these sampling procedures.
4.2.3. HPLC
The detection of cys by HPLC was performed using fluorescence, UV, and electrochemical detections. Electrochemical detection does not require any derivatization, while cys needs to be derivatized when using fluorescence and UV detections (Table 3 [[40], [41], [42], [43], [44],48,49,[51], [52], [53]]). Herein, we will present two methods of HPLC that use derivatization agents.
Table 3.
Detection of cys by HPLC.
| Derivatization agent | Flow rate (mL/min) | Stationary phase | Mobile phase | T (°C) | Elution time (min) | Limit of detection | Refs. |
|---|---|---|---|---|---|---|---|
| CMQTa | 1 | C18 (5μm; 4.6 mm × 150 mm) | Gradient elution or isocratic elution (trichloro acetic acid and acetonitrile) | 25 | 9 | 0.1 μM | [48] |
| mBBrb | 1.5 | C18 (5 μm; 4.6 mm × 150 mm) | Gradient elution (methanol, acetic acid and water) | RT | 12.5 | Not defined | [40] |
| 1.5 | C18 (3 μm; 4.6 mm × 150 mm) | Acetonitrile | RT | 4.3 | 50 nM | [41] | |
| 0.3 | C18 (5 μm; 2.1 mm × 100 mm) | Water: methanol (65:35) | – | 11 | 2 nM | [42] | |
| SBD-Fb | 1 | C18 (8–10 μm; 3.9 mm × 300 mm) | Gradient elution (methanol and sodium acetate) | RT | 10 | 0.07 pmol | [43] |
| 0.3 | C18 (5μm; 2.0 mm × 250 mm) | Phosphate buffer: CH3CN (96:4) | 30 | 5 | 0.47 μM | [44] | |
| NPMb | 1 | C18 (5μm; 4.6 mm × 250 mm) | Acetonitrile: water (70:30) | RT | 10 | 0.01 nM | [49] |
| DAABD-Clb | 0.6 | C18 (2 nm, 4.6 mm × 150 mm) | Gradient elution (water, acetonitrile and trifluoroacetic acid) | 50 | 6.4 | 154 fmol | [51] |
| ABD-Fb | 1 | C18 (3μm; 3.9 mm × 150 mm) | 2.5% methanol and ammonium acetate | – | – | – | [52] |
| ACQb | 0.3 | C18 (5μm; 2.1 mm × 150 mm) | Gradient elution (sodium acetate and trimethyl- amine, acetonitrile and water) | RT | 29 | 0.77 pmol | [53] |
CMQT: 2-chloro-1-methylquinolinium tetrafluoroborate, mBBr: monobromobimane, SBD-F: ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate, NPM: N-(1-pyrenyl) maleimide, DAABD-Cl: 7-Chloro-N-[2-(dimethylamino)ethyl]-2,1,3-benzoxadiazole-4-sulfonamide, ABD-F: 4-Fluoro-7-sulfamoyl benzofurazan, ACQ: 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, RT: room temperature, T: temperature. a HPLC coupled with UV detection; b HPLC coupled with fluorescence detection.
4.2.3.1. Fluorescence detection
HPLC using fluorescence detection is widely used to detect cys. Many derivatization agents are used inducing different analysis conditions and eluting time. For example, mBBr [[40], [41], [42]], qBBr [43,44], NPM [49], DAABD-Cl [51], ABD-F [52], and ACQ [53] were all selected to detect cys. Table 3 resumes the analytical conditions and elution time for each derivatization agent.
4.2.3.2. UV detection
CMQT was synthesized and used as a derivatization agent for UV detection of cys. The elution time was 25 min, the limit of detection was 0.1 and 0.2 μmol/L, and the detection wavelength was 355 nm. This method is highly sensitive and specific. But, it is labor-intensive, as the derivatization reagent has to be synthesized initially for the preparation of stable derivatives [48].
4.2.4. Gas chromatography
Gas chromatography with flame ionization and photometric detection were used to quantify cys (Table 4 [37,38,46,47]).
Table 4.
Gas chromatography detection of cys.
| Derivatization agent | Carrier gas | Column used | T (°C) | Internal standard | Limit of detection | Refs. |
|---|---|---|---|---|---|---|
| Pivaldehydea | Helium (35 mL/min) | 5′ × 1/8″ column | From 80 to 250 ℃ at 10 ℃/min | – | 8 pmole | [37] |
| BSTFAa | Helium (80 mL/min) | 6 ft. ¼ column | From 75 to 230 ℃ at 8 ℃/min | – | Sub nanomole | [38] |
| isoBCFb | Nitrogen (10 mL/min) | 15 m × 0.53 mm column | From 170 to 250 ℃ at 5 ℃/min | p-Toluene sulphonyl anilide | 2 pmole | [46] |
| Nitrogen (8 mL/min) | 15 m × 0.53 mm column | From 170 to 250 ℃ at 5 ℃/min | Thianthrene | 2 pmole | [47] |
BSTFA: bis (trimethylsilyl) trifluoroacetamide, isoBCF: isobutyl chloroformate, T: temperature.
Flame ionization.
Flame photometric detection.
4.2.4.1. Flame ionization
Pivaldehyde (2,2-dimethylpropanal) [37] and (trimethylsilyl) trifluoracetamide [38] were used as derivative agents of cys. The conditions used in the analysis are presented in Table 4.
4.2.4.2. Flame photometric detection
The detection of cys in mouse tissues [46], in urine, and plasma samples [47] using isoBCF as a derivatization agent was studied. It is a sensitive and selective method but requires the preparation of stable derivatives, which is a time-consuming procedure.
4.2.5. Ultra-high performance liquid chromatography-electrospray ionization tandem mass spectrometry (UHPLC-ESI-MS/MS)
5-Aminoisoquinolyl-N-hydroxysuccinimidyl carbamate (5-AIQC) and N-(acridin-9-yl)-2-bromoacetamide (AYBA) were used as derivatization agents for cys quantification using UHPLC-ESI-MS/MS. The different conditions of the analysis are described in Table 5 [57,58].
Table 5.
UHPLC-ESI-MS/MS detection of cys.
| Derivatization agent | Mobile phase | Stationary phase | Flow rate (mL/min) | T (°C) | Gas flow (L/min) | Gas T (°C) | LOD (fmol) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 5-AIQC | Gradient elution (ultra-pure water and methanol containing 0.1% formic acid) | C18 (1.8 μm; 2.1 mm × 100 mm) | 0.6 | 50 °C | 10 | 315 | 4 | [57] |
| AYBA | Gradient elution (0.1% (V/V 1:999) HCOOH and MeOH) | C18 (1.7 μm; 2.1 mm × 100 mm) | 0.4 | N.D. | 3 | 250 | 0.0120 | [58] |
5-AIQC: 5-Aminoisoquinolyl-N-hydroxysuccinimidyl carbamate, AYBA: N-(acridin-9-yl)-2-bromoacetamide, N.D.: not determined, T: temperature.
4.2.6. Colorimetric method using Ellman’s reagent
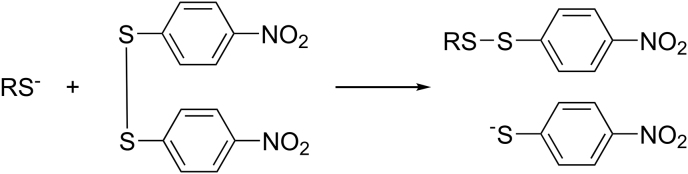
In 1958, George Ellman [59] described a method for the determination of mercaptan based on interchange of bis(p-nitrophenyl) disulfide and mercaptan anions (S−) at pH 8.0 (Fig. 6). The kinetics of the reaction between cys and bis(p-nitrophenyl) disulfide showed that the full absorbance was settled after 60–90 min.
Fig. 6.
Reaction between bis(p-nitrophenyl) disulfide and mercaptan anion [59].
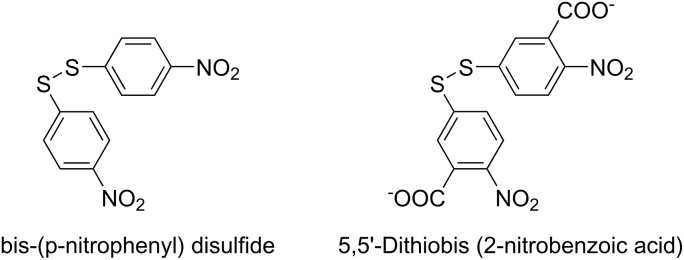
In 1959, bis(p-nitrophenyl) disulfide was replaced by its carboxylated derivative DTNB (5,5′-dithiobis (2-nitrobenzoique acid)) because of its poor solubility in water (Fig. 7) [60].
Fig. 7.
Structure difference between bis(p-nitrophenyl) disulfide and DTNB.
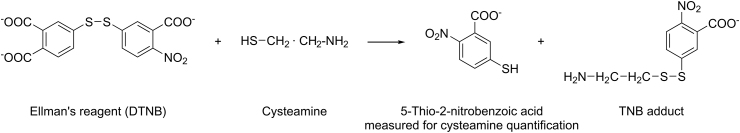
An easy and rapid method was developed by Ellman [60] to quantify sulfhydryl groups based on colorimetric detection after a reaction between sulfhydryl groups and DTNB, resulting in a yellow-colored product (2-nitro-5-thiobenzoic acid). The absorbance of the latter was measured spectrophotometrically at a wavelength of 412 nm, reflecting the concentration of cys.
The detection and quantification of cys using Ellman’s reagent can be achieved using many techniques such as UV–visible spectroscopy [15], HPLC [61], ultra-performance liquid chromatography-tandem mass spectrometer [62], and using microtiter plate [63]. This method is easy to be applied since it does not need any synthesis of the reactive agent, can be used with different analytical techniques from the simplest to the most complicated ones, and the reaction between the reactive and the sulfhydryl group is rapid without the requirement of heat or enzyme (Fig. 8).
Fig. 8.
Reaction between cys and Ellman’s reagent. DTNB: 5,5′-dithiobis (2-nitrobenzoique acid), TNB: 5’-thio-2-nitrobenzoic acid.
Some difficulties could be encountered in the detection of cys by this method in some samples, for example in cloudy solutions such as liposomes loading cys. This issue is not well elaborated in the literature. However, Butler et al. [17] tried to overcome this problem by adding 2% deoxycholate solution in the assay buffer to solubilize liposomes and/or by using matching amounts of liposomes suspension in the reference cuvette. This method is not selective since many compounds possessing a sulfhydryl group may react with DTNB.
4.2.7. Ion pair and micellar chromatography
The quantification of cys was also assessed with the addition of a surfactant into the mobile phase which interacts with cys, affecting its retention through a reversed-phase column. The addition of sodium dodecyl phosphate (40 mM) using micellar chromatography and sodium 1-heptanesulfonate (4 mM) using the ion pair chromatography to the mobile phase were able to separate and quantify cys and cystamine (Table 6 [16,64]).
Table 6.
Cys detection using ion pair and micellar chromatography.
| Method | Surfactant | Mobile phase | Stationary phase | Flow rate (mL/min) | Temperature (℃) | LOD (μM) | Refs. |
|---|---|---|---|---|---|---|---|
| Micellar chromatography | Sodium dodecyl phosphate (40 mM) | Water: acetonitrile: methanol (38:30:32) with phosphoric acid, and sodium dodecyl sulfate | C18 (5 μm; 4.6 mm × 250 mm) | 1.6 | 50 | 4.15 | [16] |
| Ion pair chromatography | Sodium 1-heptanesulfonate (4 mM) | Acetonitrile: water (0.1% phosphoric acid + Sodium 1-heptanesulfonate) (85:15) | C18 (5 μm; 4.6 mm × 250 mm) | 1 | 25 | 12.9 | [64] |
4.2.8. Electrochemical detection of cys
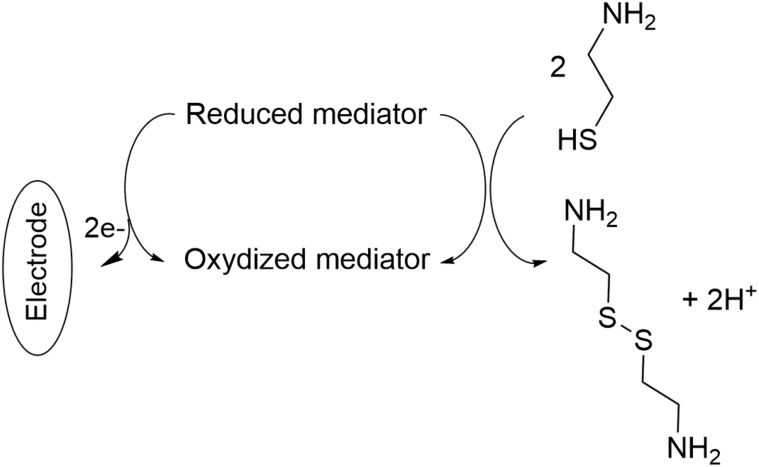
Electrochemical reactions involve the loss or gain of electrons followed by subsequent rearrangements or reactions. If these reactions occur on physically separated metals in a conducting medium, a difference in electrical potential is generated; the electrical signal depends on the analyte concentration [65]. Cys is known for its oxidation to cystamine and therefore can be detected electrochemically. This was realized in literature using different types of electrodes. Cys was first analyzed by HPLC with an electrochemical detector using platinum electrode [66] or a single gold/mercury electrode [67,68]. However, the use of unmodified electrodes has proved a high overpotential and low electrical signal. Therefore, the electrochemical determination of cys was assessed using modified electrodes such as single-wall carbon nanotube modified glassy carbon electrode [69], carbon paste electrode [[70], [71], [72], [73], [74]], multiwall carbon nanotubes paste electrode [[75], [76], [77], [78]], and screen-printed electrode [79]. The electrooxidation of cys was catalyzed using different types of mediators cited in Table 7 [[69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]]. The electrocatalytic mechanism for cys determination at the surface of an electrode in the presence of the mediator is illustrated in Fig. 9. Each method was applied in a certain range of cys concentration where the catalytic oxidation peak current showed a linear relationship with the concentration of cys. The limit of detection was determined for each method and the quantification was evaluated in different biological samples such as urine, tablet, capsules and serum.
Table 7.
The different electrochemical detection methods.
| Electrode | Mediator | Concentrations range (μM) | LOD (μM) | Samples | Refs. |
|---|---|---|---|---|---|
| Single-wall carbon nanotube modified glassy carbon electrode | 1,2-Naphthoquinone-4-sulfonic acid sodium | 5.0–270 | 3.0 | – | [69] |
| Carbon paste electrode | N,N-dimethylaniline/ferrocyanide | 80–1140 | 79.7 | Capsules | [70] |
| (9, 10-dihydro-9, 10-ethanoanthracene-11, 12-dicarboximido)-4-ethylbenzene-1, 2-diol and nickel-oxide-carbon nanotube | 0.01–250 | 0.007 | Tablet and urine | [71] | |
| Ferrocene carboxaldehyde and nickel-oxide nanoparticle | 0.09–300 | 0.06 | Urine and capsule | [72] | |
| N-(4-hydroxyphenyl)-3,5-dinitrobenzamide and magnesium oxide nanoparticles | 0.03–600 | 0.009 | Capsule and pharmaceutical serum | [73] | |
| Acetylferrocene and nickel-oxide-carbon nanotube | 0.1–600 | 0.07 | Drug and pharmaceutical serum | [74] | |
| Multiwall carbon nanotubes paste electrode | Ferrocene | 0.7–200 | 0.3 | Pharmaceutical, serum, and urine samples | [75] |
| 3,4-Dihydroxycinnamic acid | 0.25–400 | 0.09 | – | [76] | |
| Isoproterenol | 0.3–450.0 | 0.09 | Urine and drug | [77] | |
| Promazine hydrochloride | Two dynamic ranges of 2.0–346.5 μM and 346.5–1912.5 μM | 0.8 | Urine and drug | [78] | |
| Screen printed electrode | La2O3/Co3O4 | 1.0–700.0 | 0.3 | Urine and capsule | [79] |
Fig. 9.
Electrocatalytic mechanism for cys determination at the surface of an electrode in the presence of a mediator.
Herein, we present the different methods of cys quantification. The choice of an adequate method is hard, and it is based on various parameters. First, the type of sample used should be widely considered. For example, the Ellman method cannot be applied to plasma samples because of the interference of other thiol compounds during cys detection. Moreover, some derivatization agents (CMQT and cystine thiosulfonate) are not commercialized, and thus they should be synthesized. Additionally, the time required to detect cys seems to be crucial. For instance, the use of ACQ as a derivatization agent elutes cys after 29 min while SBD-F elutes cys after 5 min using HPLC with fluorescence detection.
5. Pharmacokinetics of cys
Limited information is available on the pharmacokinetics of cys. The bioavailability of cys is less than 10%. After the ingestion of cys (15 mg/kg) by children with nephropathic cystinosis, a peak concentration (0.03–0.07 mM) in plasma is reached around 1 h later [80]. The absorption of this molecule in the small intestine is much better than in the stomach or colon [81]. In addition, this bioavailability can be affected by the type of food administered as this drug can potentially bind to food such as fats and high-protein meals. A study showed that taking cys with foods may reduce its absorption by 30%, particularly with a high-protein diet [82]. Cys absorption is enhanced by iron in the proximal duodenum, iron loading accelerates, and iron depletion slows [14C] cys uptake in intestinal epithelial cells [83]. Armas et al. [84] showed that the pharmacokinetics of cys bitartrate delayed-release capsules are not affected by co-administration with orange juice, water only, or omeprazole (with water).
Cys, after oral administration in rats, is primarily distributed in the kidney, the gastrointestinal tract (mainly the duodenum), and the liver. Regardless of the route of administration, cys uptake reaches a maximum in the duodenum after 6 h, which is maintained for up to 12 h, and the efflux is observed only after 24 h. At a concentration higher than 20 mM, cys uptake is blocked, suggesting that the uptake system is saturable. Two studies suggested a carrier-mediated system for cys uptake. Based on an in vitro system, the first study suggested the presence of an unknown cys carrier for the human fibroblast lysosomes [85]. The second one confirmed that cys uptake by intestinal epithelial cells is mediated by an organic cation transport (OCT) system. The latter is inhibited by cys analogs and modulated by inhibitors of the OCTs and by suppression of OCT gene expression [83]. Moreover, these studies showed that cellular uptake of cys is more favorable at alkaline pH, compared to pH 5, due to the reason of pKa of NH2; the protonated form is presumably less well transported into the lysosome and intestinal epithelial cells, compared to its thiolate form [83,85].
After being metabolized to taurine and bile salts, cys is eliminated from the body after 6 h of administration, and this is the main reason of cys administration every 6 h for patients with cystinosis. The problem of repeated administration of cys was resolved in 2013, where Cystagon®, an immediate release cys approved by FDA in 1994, was replaced by PROCYSBI® (Horizon Pharma), an enteric-coated delayed-release cys bitartrate formulation. This formulation bypasses absorption in the stomach, resulting in sustained absorption in the small intestine and thus improving gastro-intestinal tolerability [82]. Consequently, the drug is eliminated after 12 h, reducing the drug administration and thus the treatment side effects [86].
Table 8 shows the pharmacokinetic parameters after the oral and gastrointestinal administration of cys in human body cells. Tmax is the time corresponding to Cmax, half-life (t1/2), the area under the curve between time 0 and the last sample (AUC0-∞), and clearance (CL) are represented in this table [87,88]. The best pharmacokinetic values were obtained for gastro-intestinal administration in humans, more specifically in the small intestine.
Table 8.
Pharmacokinetic parameters of cys after different routes of administration.
| Route of administration of cys | Cmax (mg/L) | Tmax (min) | t1/2 (min) | AUC0-∞ (mg・min/L) | CL (L/min) | Refs. | |
|---|---|---|---|---|---|---|---|
| Oral (450 mg cy) | 2.86 | 72 | 222 | 9.62 | 1.5 | [87] | |
| Gastro-intestinal (500 mg cy) | Stomach | 8.8 | 50 | 94.5 | 880 | N.D. | [88] |
| Small intestine | 11 | 21 | 112 | 983 | N.D. | ||
| Caecum | 5.2 | 64 | 98 | 713 | N.D. | ||
| Mid-ileum | 11 | 30 | 124 | 1034 | N.D. | ||
cys: cysteamine, Cmax: maximum concentration, Tmax: the time to reach Cmax, t1/2: half-life, AUC0-∞: the area under the curve between time 0 and the last sample, CL: clearance, N.D.: not determined.
6. Biological applications of cys
A low concentration of cys induces the transport of cysteine into cells. The latter is a precursor of glutathione (GSH) synthesis, an important antioxidant, thus influencing the oxidative state of a cell [89]. The oxidative state regulates several signaling pathways involved in cell proliferation and influences the gene expression of several redox-sensitive genes [90]. Moreover, the thiol group of cys can react with free thiol or the disulfide bonds of peptides and proteins, ending by interference with their function [91]. The alteration in gene expression and the interference with the protein function are the main causes behind the ability of cys to treat Huntington and Parkinson diseases. At high concentration, the oxidation of cys in the presence of transition metals produces hydrogen peroxide (H2O2) molecules, responsible for oxidative stress. Additionally, it induces the inhibition of GSH peroxidase responsible for cys toxicity at high concentrations (10−4 to 10−3 M) [92]. Therefore, the dose selection is very important to avoid any complications in the treatment of any diseases by cys.
Cys has shown several biological applications: treatment of cystinosis, Huntington and Parkinson diseases, malaria, neuropsychiatric disorders, cancer, and non-alcoholic fatty liver disease, and is used as a radioprotective agent (Fig. 10). Many reviews have profoundly discussed these applications [7,23,24]. We will focus on the main applications of cys as a radioprotective agent, in the treatment of cystinosis, and for anti-tumor proliferation. However, the topical application of cys, for the treatment of hyperpigmentation, has not yet been well elaborated and reviewed. Consequently, all the data in the literature concerning this topic are collected and described below.
Fig. 10.
Cys pharmacodynamics.
6.1. Radioprotective effect
Cys was first used in 1954 as a radioprotective agent [1]. This radioprotective effect is attributed to its sulfhydryl group. It is a great scavenger of hydroxyl radical (.OH). It also reacts slowly with hydrogen peroxide (H2O2), but this reaction can lead to significant rates of H2O2 removal if high concentrations of cys are present [93]. Cys enters the cells rapidly and provides the maximum level of protection within 10 min [94]. Cys radioprotection in vitro is based on three different mechanisms [4]. First, cys can undergo an oxidation reaction with molecular oxygen in the cells, leading to hypoxia. Besides, cys donates hydrogen atoms to hydroxyl radicals (·OH), decreasing the indirect effect of radiation. Moreover, cys activates specific repressor molecules that interrupt the DNA templates activity necessary for DNA replication. A metabolically active DNA molecule is more sensitive to ionizing radiation; thus cys leads to the diminishment of radiation injury to the DNA molecule [5,95].
6.2. Treatment of cystinosis
Cystinosis is a rare autosomal recessive metabolic disorder characterized by a defect in lysosomal cystine transport, leading to the intralysosomal accumulation of cystine crystals in many tissues (kidneys, bone marrow, intestine, etc.) including the eye (retina, conjunctiva, iris, and cornea) [96], affecting muscles and the central nervous system [86]. This disease can cause a generalized proximal tubular damage (called renal Fanconi syndrome), resulting in polyuria, polydipsia, and a development failure within the first year of life [97]. If left untreated, cystinosis can cause end-stage renal disease around the age of ten.
In 1976, cys in the form of cys bitartrate was introduced as a treatment of cystinosis [98]. Cys is a weak base that enters the lysosome and reacts with cystine to form a mixed disulfide of half-cystine and cys. The mixed disulfide has a stearic resemblance to the amino acid lysine; consequently, it rapidly leaves the lysosomes via lysine transporter [99]. However, this treatment presents many side effects such as gastrointestinal complaints, disagreeable breath, sweat odor, development of lupus nephritis, proliferative vascular lesions on their elbows, skin striae, and bone and muscular pain [23]. These side effects are mainly caused by the metabolism of 3% of cys to dimethyl sulfide (Fig. 3) [100].
6.3. Treatment of cancer
Cys has been shown to inhibit gastric [101] and mammary [12] tumors formation. Besides, a study conducted by Wan et al. [102] demonstrated that cys caused autophagosome accumulation in cancer cells and sensitized doxorubicin-elicited chemotherapeutic killing in HeLa, B16 melanoma, doxorubicin-resistant MCF-7 cells, and in a mouse melanoma model. Besides, cys inhibits matrix metalloproteinases conducting to the suppression of invasion, metastasis, and prolonging survival in a mouse model of human pancreatic cancer [103] and human ovarian cancer [104].
6.4. Treatment of hyperpigmentation
There are three types of skin color alteration: darkening, lightening, and the occurrence of unusual skin color [105]. Eumelanin and pheomelanin are the two forms of melanin. Eumelanin is responsible for the brown pigmentation of the skin while pheomelanin produces yellow and red colorations [106]. Pigmentary disorders can occur after an increase or decrease in melanocyte activity. They are divided into two different categories: hyperpigmentation and hypopigmentation [107]. Hyperpigmentation is divided into three main types: melasma, post-inflammatory hyperpigmentation, and sun damage or sunspots. Melasma is a psychologically distressing skin disorder divided into three types: epidermal, dermal, and mixed melasma. Epidermal and dermal melasma is the accumulation of melanin in the epidermis and the dermis, respectively, and mixed melasma is a combination of epidermal and dermal melasma [108]. Post-inflammatory hyperpigmentation is an acquired hypermelanosis occurring after cutaneous inflammation or injury that can arise in all skin types [109], and sunspots are usually light brown (generally called freckles) and appear mostly on the face, neck, chest, and hands, which are primarily exposed to UV rays [110].
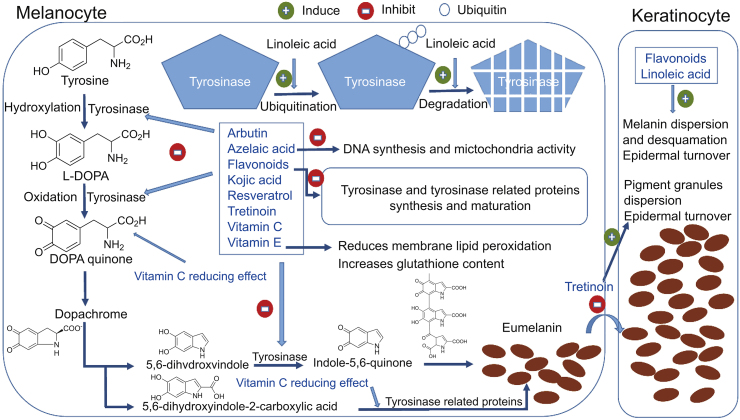
Several natural and synthetic skin depigmenting agents have been developed. Their mechanisms of action may occur before, during or after the melanin synthesis. Moreover, depigmentation by any exogenous agent is induced by the destruction or the loss of melanocytes, alteration of the melanin present in melanosomes, and the interference with (i) the biosynthesis of premelanosomes and melanosomes, (ii) the conversion of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) to melanin, (iii) the biosynthesis of tyrosinase or the active center of the enzyme, and (iv) the transfer of melanosomes to keratinocytes (Fig. 11) [111].
Fig. 11.
Schematic illustration of the mechanism of action of skin whitening agents [111] (with permission from Elsevier).
Cys hydrochloride has been known to be a potent depigmenting molecule for over 5 decades. Chavin et al. [112] examined, for the first time, the ability of several compounds (quinhydrone, cys, N-(2 mercaptoethyl)-dimethylamine HCI, sodium cys-S-phosphate, cystamine, 3-methylcatechol, fluphenazine, hydroquinone, etc.), to induce a specific destructive effect in the melanin synthesizing cells (melanocytes and melanophores). These compounds were injected by subcutaneous injection in black goldfish. They found that all the molecules induced lysis of melanophores and melanocytes locally and systemically; however, hydroquinone was the most potent anti-pigmentary compound. Another study demonstrated that cys was an effective depigmenting agent when applied to the skin of black guinea pigs, and conversely to the previous result, was shown to be more potent than hydroquinone [113]. In fact, melanocytes are the specific target of cys since a noticeable decrease of the epidermal melanocyte was observed after the topical application of this compound to the skin of black guinea pigs. The treatment with cys did not affect the keratinocytes cells adjacent to melanocytes cells [114]. The minimum dose required for depigmentation is 100 μM, and this concentration decreases the percentage of melanin by 21% [11].
The mechanism of cys as a depigmenting agent is not yet deeply studied. It may act through the inhibition of tyrosinase and peroxidase and the increase of intracellular GSH levels.
6.4.1. Inhibition of tyrosinase and peroxidase activity
Tyrosinase is responsible for the conversion of tyrosine to DOPA and of DOPA to DOPA quinone. As for peroxidase, it catalyzes the final step of the oxidative polymerization reaction of the formed indoles to eumelanin pigments [115]. Thiolic depigmenting agents such as cys and GSH [116] are known to be inhibitors of tyrosinase and peroxidase, the two key enzymes involved in melanin biosynthesis [117]. The inhibition of tyrosinase will produce the scavenging of DOPA quinone affecting the production of melanin. Arbutin, azelaic acid, flavonoids, kojic acid resveratrol, tretinoin, and vitamins C and E act by the same mechanism [111]. Qiu et al. [11] reported that the depigmenting action of cys is due to its interaction with the products of the reactions catalyzed by tyrosinase activity (DOPA oxidation products are isolated) inhibiting pigment synthesis. They confirmed that the cys mechanism of action is a melanogenesis inhibition, but not melanocytotoxicity, in contrast to hydroquinone.
6.4.2. Increase in the level of intracellular GSH
The interaction of DOPA-quinone with the thiol groups of GSH or cysteine shifts the melanogenesis from eumelanin to phaeomelanin synthesis (Fig. 10) [118]. Cys enhances the intracellular levels of GSH, thus delaying the melanogenesis procedure. Among the different depigmenting agents, only vitamin E exhibited this action as well [111]. Djurhuus et al. [119] demonstrated the enhancement of GSH content in C3H/10T1/2 cells after the addition of cys. De Matos and Furnus [89] proved that the addition of cys to the culture medium during in vitro maturation of bovine oocytes increased the GSH levels in the mature oocytes, while Wilmer et al. [120] showed that cys increased total GSH and restored GSH redox status in a renal cystinosis cell mode [[89], [119], [121]]. Cys stimulates GSH synthesis by increasing the rate of cellular cysteine uptake through the formation of mixed disulfides with cysteine. Mixed disulfides of cysteine and cys enter cells via transport system L and are reduced intracellularly to release both thiol compounds. The cysteine is then used in GSH synthesis [122].
Several skin whitening agents such as retinoic acid, kojic acid or hydroquinone proved high efficacy. However, they present numerous side effects like hypersensitivity to the sun, skin irritation, inhibition of new melanin formation, and they are responsible for itching, peeling, dryness, and redness of the skin [123]. Cysteamine is a natural skin whitening agent as powerful as hydroquinone, does not present any risk, and is compatible with light exposure. The main obstacle in the use of cys as a depigmenting agent is its fast oxidation once in contact with air as well as the strongly unpleasant organoleptic features of this molecule [13], which can not be covered by perfumes [124]. Recently, Scientis Pharma© developed a new technology that stabilizes cys molecules and significantly reduces its odor. Cys Cream® is eventually the first and only depigmenting agent commercially available based on cys molecule. Hsu et al. [124] applied cys cream® to the ear of black female guinea pigs. Evaluation with dermatoscopic, chromametric, and histologic instruments was performed, and the cys cream® showed a potent depigmenting effect in guinea pig skin. Then, Mansouri et al. [9] evaluated the efficacy and safety of cys cream® for the treatment of epidermal melasma in a randomized, double-blind vehicle-controlled clinical trial. After an evaluation of melanin content and erythema levels, they concluded that the treatment with cys cream® decreased the content of melanin and consequently it was a good treatment for epidermal melasma [8,9].
7. Cys and encapsulation systems
Cys encapsulation into liposomes, CDs, and emulsions was conducted to enhance its effects; studies dealing with encapsulation are addressed in this section. Moreover, the conjugation of cys to the surface of CD induces the formation of disulfide bonds with cysteine-rich substructures of the ocular and glycoproteins mucus, providing a prolonged residence time of the incorporated drugs at the site of action. On the other hand, the conjugation of cys to the surface of gold nanoparticles improves the colorimetric detection of compounds using the gold nanoparticles aggregation method by decreasing the electrostatic repulsion force between the nanoparticles.
7.1. Liposomes
Liposomes are enclosed spherical vesicles organized in one or several concentric phospholipidic bilayers with an internal aqueous phase. Liposomes can entrap lipophilic drugs within the lipid membrane, hydrophilic agents in their internal aqueous compartment, or amphiphilic ones at the water-lipid interface [125]. These carriers are biodegradable, biocompatible, and non-immunogenic [126]. Since liposomes mimic natural membranes, their use in topical applications is generally favorable [127]. Liposomes can be prepared by classical and large-scale techniques [125,128]. In fact, cys was first encapsulated in liposomes by Butler et al. [17], to reduce cystine accumulation in cells. Since cys is a water-soluble compound, it can be incorporated in the internal aqueous cavity of the liposomes, thus facilitating the selective uptake of cys by endocytic target cells. The authors compared different types of liposomes formed from saturated and unsaturated or positively and negatively charged lipids. The best type of liposomes selected for subsequent experiments was those containing saturated dipalmitoyl phosphatidylcholine and negative charge by the inclusion of phosphatidic acid. This is explained as follows: saturated fatty acids are not subject to autoxidation as are unsaturated ones; the use of dipalmitoylphosphatidylcholine liposomes avoids some of the toxic effects of peroxides or epoxides on cells in tissue culture and negatively charged liposomes made with phosphatidic do not present any aggregation contrary to those with positive charge. The encapsulation of cys in liposomes provides a better efficacy to reduce cystine in cystinotic cells in tissue culture [17]. In addition, liposomes serve as a targeting agent to the lysosome since cys incorporated into liposomes will be mainly taken up by phagocytic cells and concentrated in lysosomes. They confirmed that the liposomes were disrupted by lysosomal enzymes releasing cys intralysosomally, followed by the diffusion of mixed disulfide from lysosome [17].
Roman et al. [129] reported the encapsulation of cys in liposomes. It was delivered orally in mice with an evaluation of its radioprotective effect. They found that the liposome encapsulating cys protected the drug up to 3 h after administration in contrast to free cys. In order to investigate the effect of liposome-encapsulation on cys absorption through the intestinal wall, the distribution of the molecule after in vivo administration was studied by Jaskierowicz et al. [15]. They used mixed egg yolk lecithin and cholesterol (4:1, mol/mol) and reported that radioactivity was higher and more persistent in blood, plasma, liver, and spleen in encapsulated cys than that in the free form. The digestive absorption of cys was more important when entrapped, and the drug was protected from digestive degradation.
On the other hand, the encapsulation of cys in liposomes formed from egg yolk phospholipid and cholesterol (4:1) extended the presence and duration of action of cys in pituitary glands, after oral administration. It led to reduce tumor proliferation through the modification of hormone status where cys could reduce somatostatin and/or prolactin levels near the tumor. 10.8% of the initial cys was encapsulated in liposomes. Cys loaded liposomes were stable during storage for six days [130]. Table 9 shows the different studies conducted to encapsulate cys in liposomes [15,17,130].
Table 9.
Liposomes prepared by thin lipid film hydration method encapsulating cys.
| Liposomes composition | Model used | Administration routes | Biological effects | Refs. |
|---|---|---|---|---|
| Mixed egg yolk lecithin and cholesterol (4:1) | In vivo | Intragastric | Enhancement of cys absorption through the intestinal wall | [15] |
| Negatively charged, saturated phosphatidyl choline cholesterol-phosphatidic acid (7:2:1) | Cystinotic cells in tissue culture In vivo |
Intravenously | Reducing cystine contents and improvement of uptake into target tissues | [17] |
| Mixed egg yolk and cholesterol (4:1) | In vivo | Orally | Enhancement of prolactin depletion action period | [130] |
cys: cysteamine.
Unfortunately, liposome’s characteristics (size, shape, homogeneity, encapsulation efficiency, and loading rate) were not investigated in these studies.
7.2. Cyclodextrins
CDs are a family of cyclic oligosaccharides composed of α-(1,4) linked glucopyranose subunits [131]. Three native CDs are known as follows: α-CD, β-CD, and γ-CD, composed of six, seven, and eight α-units, respectively [132]. CDs possess a lipophilic inner cavity and a hydrophilic outer surface that allow the formation of non-covalent inclusion complexes with numerous types of guests [133].
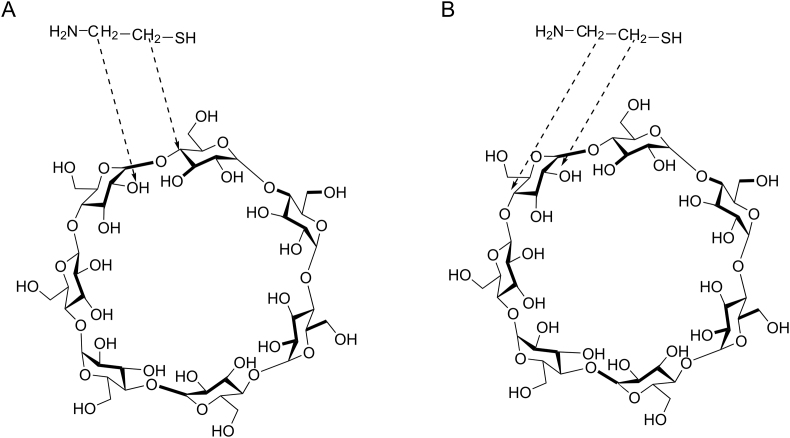
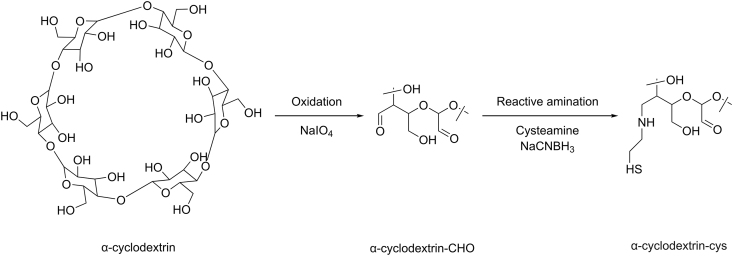
Table 10 shows the methods of preparation and the effects of different molar ratios of cys to CD on cys properties. In fact, the presence of hydroxyl groups (OH) outside the molecule prevents the inclusion of hydrophilic drugs in CDs. To evaluate if the complexation between cys and CDs can be realized, Lahiani-Skiba et al. [13] studied interactions between cys hydrochloride and α-CD in lyophilized inclusion complexes. Inclusion complexes were prepared from solutions obtained by the dissolution of cys hydrochloride in α-CD solution. Lyophilized products were obtained with molar ratios of 1:1, 2:1, 3:1, and 4:1 (cys hydrochloride:α-CD). After the analysis of the lyophilized products by differential scanning calorimetry, mass spectrometry analysis, 1H nuclear magnetic resonance (NMR), and Fourier transform infrared spectroscopy, they confirmed the complex formation. The nuclear over Hauser effect spectroscopy technique showed the proximity of the methylene groups of cys protons with protons H2 and H4, located at the outside of α-CD (Fig. 12). They obtained odorless powder of cys with moderate flavor, storable at room temperature [13].
Table 10.
Interaction of cys with CDs.
| Interaction of cys | CD type | Molar ratio (cys: CD) | Preparation method | Freeze dried | Effect | Refs. |
|---|---|---|---|---|---|---|
| Encapsulation | α-CD | 1:1, 2:1, 3:1, 4:1 (best ratio) | Direct dissolution of cys hydrochloride in α-CD solution | Freeze-dried on a shelf at 50°C for 3 h at least. | An odorless powder and moderate flavor of cys is obtained, storable at room temperature | [13] |
| 1:7, 1:10, 1:11, 1:12.5 (best ratio) | Dissolution of cys in α-CD solution | _ | Increase in the permeation of the trans corneal diffusion of cys (ex vivo model) | [16] | ||
| Modification | β-CD | 1:2 | The oxidation of CD prior to the covalent coupling of cys via reductive amination | _ | Improved water solubility and retention time of miconazole nitrate on porcine intestinal and buccal mucosa | [19] |
| α-CD | 10:3 | Prolong drug residence time of cetirizine on the ocular mucosal surface | [20] |
cys: cysteamine, CD: cyclodextrins.
Fig. 12.
Representation proposed for the interaction between cys and α-CD according to the results of NMR technique (A) interactions of CH2 of cys with H2 and H4 of two different glucopyranose (B) interactions of CH2 of cys with H2 and H4 of a same glucopyranose [13] (with permission from Springer).
Pescina et al. [16] reported the encapsulation of cys in α-CD to improve the trans-corneal permeation of cys. An increase of permeation was observed when CD concentration was added to the range between 3% and 5.5%. The increase of permeation was CD concentration-dependent; for example, a 5.5% concentration of CD increased the amount permeated up to 20 times compared to free cys [16].
7.3. Emulsions
Emulsions are metastable colloids made out of two immiscible fluids, one being dispersed in the other, in the presence of surface-active agents [134].
Gresham et al. [14] reported the use of sustained-release multiple emulsion to extend the period of cys radioprotection. They compared the radioprotective effect in irradiated mice protected by cys with those unprotected. They found that emulsions prolonged the radioprotective effect of cys from 15 min obtained using free cys to 1.5 h when cys was administered in an emulsion [14].
Recently, Dixon et al. [18] tried to enhance cys stability using an emulsion of vitamin E, soybean oil, and Tween 80 surfactants, since emulsions have been shown to decrease the transport of oxygen and increase the stability of other hydrophilic antioxidants [135]. Vitamin E and/or soybean oil were prepared at the solubility limit by adding an excess of the hydrophobic component(s) to PBS and stirring at 300 rpm for 24 h, followed by the addition of cys (0.1 mg/mL). The emulsion formulation was prepared by first mixing vitamin E (0.45 mg/mL) and soybean oil (0.45 mg/mL), followed by the addition of the surfactant solution Tween 80 (0.1 mg/mL) with sonication for 30 min. The degradation rate of cys decreased from 126 μg/h for free cys (0.1 mg/mL) to 111 μg/h for cys, oil, and vitamin E at the solubility limit in PBS. This rate was decreased to 101 μg/h for cys in the emulsion. The solubilization of the antioxidants increased their concentrations in the formulation and consequently allowed the stabilization of cys [18].
7.4. Modification of encapsulation systems by cys
7.4.1. Cyclodextrins
The synthesis and characterization of thiolated β- and α- CD [19,20] as a novel mucoadhesive excipient for intra-oral drug delivery was studied. The synthesis of the thiolated CD was achieved in two steps: the oxidation of CD and the covalent coupling of cys via reductive amination (Fig. 13). α-CD-cys and β-CD-cys conjugates displayed an increase in the retention time of cetirizine on the ocular mucosal surface and miconazole nitrate on porcine intestinal and buccal mucosa. This could be due to the improvement of CD drug encapsulation properties after being thiolated, where the solubility of miconazole nitrate was enhanced. In addition, local mucosal irritating effects of cetirizine were significantly reduced after being complexed with α-CD-cys and applied on the rabbit’s ocular mucosa (Table 10). These findings could be a promising tool for the delivery of poorly water-soluble therapeutic agents.
Fig. 13.
Synthetic pathway for the generation of thiolated α-CD. cys: cysteamine, CD: cyclodextrins.
7.4.2. Gold nanoparticles
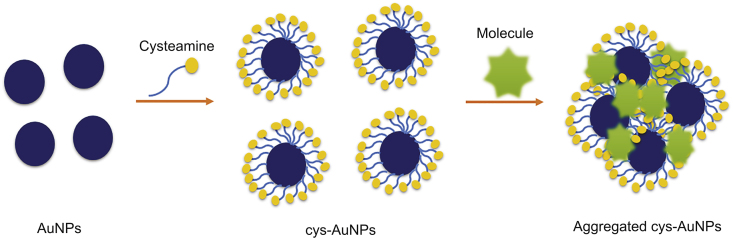
7.4.2.1. Synthesis
Gold nanoparticles (AuNPs) are a diverse group of nanomaterials ranging in size from 5 to 110 nm with different forms, including spheres, cubes, nanorods, and nanoribbons [136]. There are a variety of methods to synthesize AuNPs. We can find chemical methods of synthesis based on the chemical reduction of gold salt in aqueous or/and organic phase. The physical methods involve the γ-irradiation technique, the technique of microwave irradiation, and heat or photochemical reduction. Finally, the biological method was also reported using citrus fruit juice extracts or edible mushrooms [137]. Cys modified AuNPs were prepared by the attachment of cys mercapto group to the surface of the AuNPs by the formation of Au–S bonds with the –NH2 groups exposed on the outer surface of the citrate-capped AuNPs [138].
7.4.2.2. Colorimetric detection of compounds via cys-AuNPs
The use of AuNPs as a colorimetric reporter to detect several compounds of large numbers of samples, such as milk products, eggs, and feeds, has received great attention in the last few years, to substitute the classical techniques like HPLC and gas chromatography which are expensive and need dedicated instruments. This method was developed since AuNPs possess a surface plasmon resonance changing from red to blue corresponding to their dispersion or aggregation state. The development of a more sensitive assay involves the modification of the AuNPs surface by cys to decrease the electrostatic repulsion force between AuNPs. The cys-AuNPs solution is wine-red and displays an absorption peak at 524 nm. An electrostatic repulsion occurs because of the positive charge of cys-AuNPs inhibiting the aggregation of the latter. When the compound is added to the cys-AuNPs solution, the absorption spectrum exhibits an obvious decrease at 524 nm and a strong increase at 650 nm. The color of the conjugates changes from wine-red to purple within several minutes, indicating the aggregation of cys-AuNPs (Fig. 14) [21,22]. The colorimetric detection of gentamycin melamine [21,139], heparin [139], lipopolysaccharides [140], mercury (II) [21], glyphosate [22], trinitrotoluene [141], clenbuterol [142], and sulfate [143] using cys modified AuNPs has been reported. This method presents a lot of advantages like simplicity of preparation and manipulation and high sensitivity. It is more robust and less expensive than the conventional methods.
Fig. 14.
Colorimetric detection strategy of molecules based on cys modified AuNPs. cys: cysteamine, AuNPs: gold nanoparticles.
8. Conclusion and perspectives
Due to the important role of cys in medical and cosmetic fields, it has been extensively studied in literature. However, this molecule suffers from different drawbacks mainly related to instability, organoleptic and pharmacokinetic properties. The quantification of this agent is also challenging because of its low absorptivity. This review presents a broad overview of cys characteristics; it can serve as a reference for novel works focusing on the improvement of cys properties through encapsulation in delivery systems which may enlarge cys application.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Authors thank the Research Funding Program at the Lebanese University and the “Agence Universitaire de la Francophonie, projet PCSI” for supporting the project (2018–2020).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Bacq Z.M., Dechamps G., Fischer P. Protection against x-rays and therapy of radiation sickness with beta-mercaptoethylamine. Science. 1953;117:633–636. doi: 10.1126/science.117.3049.633. [DOI] [PubMed] [Google Scholar]
- 2.Eker P., Pihl A. Studies on the growth-inhibiting and radioprotective effect of cystamine, cysteamine, and AET on mammalian cells in tissue culture. Radiat. Res. 1964;21:165–179. [PubMed] [Google Scholar]
- 3.Takagi Y., Shikita M., Terasima T. Specificity of radioprotective and cytotoxic effects of cysteamine in HeLa S3 cells: generation of peroxide as the mechanism of paradoxical toxicity. Radiat. Res. 1974;60:292–301. [PubMed] [Google Scholar]
- 4.Korystov Y.N., Vexler F.B. Mechanisms of the radioprotective effect of cysteamine in Escherichia coli. Radiat. Res. 1988;114:550–555. [PubMed] [Google Scholar]
- 5.Mitznegg P., Säbel M. On the mechanism of radioprotection by cysteamine. I. Relationship between cysteamine-induced mitotic inhibition and radioprotective effects in the livers of young and senile white mice. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1973;24:329–337. doi: 10.1080/09553007314551181. [DOI] [PubMed] [Google Scholar]
- 6.Cherqui S. Cysteamine therapy: a treatment for cystinosis, not a cure. Kidney Int. 2012;81:127–129. doi: 10.1038/ki.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gahl W.A. Early oral cysteamine therapy for nephropathic cystinosis. Eur. J. Pediatr. 2003;162:S38–S41. doi: 10.1007/s00431-003-1349-x. [DOI] [PubMed] [Google Scholar]
- 8.Farshi S., Mansouri P., Kasraee B. Efficacy of cysteamine cream in the treatment of epidermal melasma, evaluating by Dermacatch as a new measurement method: a randomized double blind placebo controlled study. J. Dermatol. Treat. 2017:1–8. doi: 10.1080/09546634.2017.1351608. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri P., Farshi S., Hashemi Z. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br. J. Dermatol. 2015;173:209–217. doi: 10.1111/bjd.13424. [DOI] [PubMed] [Google Scholar]
- 10.McGregor D. Hydroquinone: an evaluation of the human risks from its carcinogenic and mutagenic properties. Crit. Rev. Toxicol. 2007;37:887–914. doi: 10.1080/10408440701638970. [DOI] [PubMed] [Google Scholar]
- 11.Qiu L., Zhang M., Sturm R.A. Inhibition of melanin synthesis by cystamine in human melanoma cells. J. Invest. Dermatol. 2000;114:21–27. doi: 10.1046/j.1523-1747.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- 12.Tatsuta M., Iishi H., Yamamura H. Inhibitory effect of prolonged administration of cysteamine on experimental carcinogenesis in rat stomach induced by N-methyl-N’-nitro-N-nitrosoguanidine. Int. J. Canc. 1988;41:423–426. doi: 10.1002/ijc.2910410318. [DOI] [PubMed] [Google Scholar]
- 13.Lahiani-Skiba M., Boulet Y., Youm I. Interaction between hydrophilic drug and α-cyclodextrins: physico-chemical aspects. J. Inclusion Phenom. Macrocycl. Chem. 2007;57:211–217. [Google Scholar]
- 14.Gresham P.A., Barnett M., Smith S.V. Use of a sustained-release multiple emulsion to extend the period of radio protection conferred by cysteamine. Nature. 1971;234:149–150. doi: 10.1038/234149a0. [DOI] [PubMed] [Google Scholar]
- 15.Jaskierowicz D., Genissel F., Roman V. Oral administration of liposome-entrapped Cysteamine and the distribution pattern in blood, liver and spleen. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985;47:615–619. doi: 10.1080/09553008514550851. [DOI] [PubMed] [Google Scholar]
- 16.Pescina S., Carra F., Padula C. Effect of pH and penetration enhancers on cysteamine stability and trans-corneal transport. Eur. J. Pharm. Biopharm. 2016;107:171–179. doi: 10.1016/j.ejpb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Butler J.D., Tietze F., Pellefigue F. Depletion of cystine in cystinotic fibroblasts by drugs enclosed in liposomes. Pediatr. Res. 1978;12:46–51. doi: 10.1203/00006450-197801000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Dixon P., Powell K., Chauhan A. Novel approaches for improving stability of cysteamine formulations. Int. J. Pharm. 2018;549:466–475. doi: 10.1016/j.ijpharm.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Ijaz M., Matuszczak B., Rahmat D. Synthesis and characterization of thiolated β-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery. Carbohydr. Polym. 2015;132:187–195. doi: 10.1016/j.carbpol.2015.06.073. [DOI] [PubMed] [Google Scholar]
- 20.Ijaz M., Ahmad M., Akhtar N. Thiolated α-cyclodextrin: the invisible choice to prolong ocular drug residence time. J. Pharmacol. Sci. 2016;105:2848–2854. doi: 10.1016/j.xphs.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y., Jiang L., Mei Y. Colorimetric sensing strategy for mercury(ii) and melamine utilizing cysteamine-modified gold nanoparticles. Analyst. 2013;138:5338–5343. doi: 10.1039/c3an00690e. [DOI] [PubMed] [Google Scholar]
- 22.Zheng J., Zhang H., Qu J. Visual detection of glyphosate in environmental water samples using cysteamine-stabilized gold nanoparticles as colorimetric probe. Anal Methods. 2013;5:917–924. [Google Scholar]
- 23.Besouw M., Masereeuw R., van den Heuvel L. Cysteamine: an old drug with new potential. Drug Discov. Today. 2013;18:785–792. doi: 10.1016/j.drudis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Gallego-Villar L., Hannibal L., Häberle J. Cysteamine revisited: repair of arginine to cysteine mutations. J. Inherit. Metab. Dis. 2017;40:555–567. doi: 10.1007/s10545-017-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripps H., Shen W. Review: taurine: a “very essential” amino acid. Mol. Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- 26.Besouw M., Blom H., Tangerman A. The origin of halitosis in cystinotic patients due to cysteamine treatment. Mol. Genet. Metabol. 2007;91:228–233. doi: 10.1016/j.ymgme.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Gahl W.A., Ingelfinger J., Mohan P. Intravenous cysteamine therapy for nephropathic cystinosis. Pediatr. Res. 1995;38:579–584. doi: 10.1203/00006450-199510000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Serjeant E.P., Dempsey B. Pergamon Press; Oxford; New York: 1979. Ionisation Constants of Organic Acids in Aqueous Solution. [Google Scholar]
- 29.O’Neil M.J. thirteenth ed. Whitehouse Station, N.J.: Merck; 2001. The Merck Index : an Encyclopedia of Chemicals, Drugs, and Biologicals.https://trove.nla.gov.au/version/13531769 [Google Scholar]
- 30.Gana I., Barrio M., Ghaddar C. An integrated view of the influence of temperature, pressure, and humidity on the stability of trimorphic cysteamine hydrochloride. Mol. Pharm. 2015;12:2276–2288. doi: 10.1021/mp500830n. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Biotechnology Information. PubChem Compound Summary for CID 6058, Cysteamine. https://pubchem.ncbi.nlm.nih.gov/compound/Cysteamine
- 32.Riauba L., Niaura G., Eicher-Lorka O. A study of cysteamine ionization in solution by Raman spectroscopy and theoretical modeling. J. Phys. Chem. 2006;110:13394–13404. doi: 10.1021/jp063816g. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q., De Oliveira Vigier K., Royer S. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012;41:7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- 34.Biaglow J.E., Issels R.W., Gerweck L.E. Factors influencing the oxidation of cysteamine and other thiols: implications for hyperthermic sensitization and radiation protection. Radiat. Res. 1984;100:298–312. [PubMed] [Google Scholar]
- 35.Brodrick A., Broughton H.M., Oakley R.M. The stability of an oral liquid formulation of cysteamine. J. Clin. Pharm. Therapeut. 1981;6:67–70. doi: 10.1111/j.1365-2710.1981.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 36.Purkiss R. Stability of cysteamine hydrochloride in solution. J. Clin. Pharm. Therapeut. 1977;2:199–203. [Google Scholar]
- 37.Jellum E., Bacon V.A., Patton W. Quantitative determination of biologically important thiols and disulfides by gas-liquid chromatography. Anal. Biochem. 1969;31:339–347. doi: 10.1016/0003-2697(69)90274-7. [DOI] [PubMed] [Google Scholar]
- 38.Lofberg R.T. Gas chromatographic analysis of aminothiol radioprotective compounds. Anal. Lett. 1971;4:77–86. [Google Scholar]
- 39.Fahey R.C., Newton G.L., Dorian R. Analysis of biological thiols: quantitative determination of thiols at the picomole level based upon derivatization with monobromobimanes and separation by cation-exchange chromatography. Anal. Biochem. 1981;111:357–365. doi: 10.1016/0003-2697(81)90573-x. [DOI] [PubMed] [Google Scholar]
- 40.Newton G.L., Dorian R., Fahey R.C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal. Biochem. 1981;114:383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- 41.Pastore A., Massoud R., Motti C. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin. Chem. 1998;44:825–832. [PubMed] [Google Scholar]
- 42.Stachowicz M., Lehmann B., Tibi A. Determination of total cysteamine in human serum by a high-performance liquid chromatography with fluorescence detection. J. Pharmaceut. Biomed. Anal. 1998;17:767–773. doi: 10.1016/s0731-7085(97)00248-3. [DOI] [PubMed] [Google Scholar]
- 43.Toyo’oka T., Imai K. High-performance liquid chromatography and fluorometric detection of biologically important thiols, derivatized with ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBD-F) J. Chromatogr. 1983;282:495–500. doi: 10.1016/s0021-9673(00)91626-1. [DOI] [PubMed] [Google Scholar]
- 44.Ichinose S., Nakamura M., Maeda M. A validated HPLC-fluorescence method with a semi-micro column for routine determination of homocysteine, cysteine and cysteamine, and the relation between the thiol derivatives in normal human plasma. Biomed. Chromatogr. 2009;23:935–939. doi: 10.1002/bmc.1205. [DOI] [PubMed] [Google Scholar]
- 45.Ida S., Tanaka Y., Ohkuma S. Determination of cystamine by high-performance liquid chromatography. Anal. Biochem. 1984;136:352–356. doi: 10.1016/0003-2697(84)90229-x. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka H., Imamura Y., Tanaka H. Determination of cysteamine and cystamine by gas chromatography with flame photometric detection. J. Pharmaceut. Biomed. Anal. 1993;11:963–969. doi: 10.1016/0731-7085(93)80056-7. [DOI] [PubMed] [Google Scholar]
- 47.Kataoka H., Tanaka H., Makita M. Determination of total cysteamine in urine and plasma samples by gas chromatography with flame photometric detection. J. Chromatogr. B Biomed. Appl. 1994;657:9–13. doi: 10.1016/0378-4347(94)80063-4. [DOI] [PubMed] [Google Scholar]
- 48.Kuśmierek K., Głowacki R., Bald E. Determination of total cysteamine in human plasma in the form of its 2-S-quinolinium derivative by high performance liquid chromatography. Anal. Bioanal. Chem. 2005;382:231–233. doi: 10.1007/s00216-005-3166-8. [DOI] [PubMed] [Google Scholar]
- 49.Ogony J., Mare S., Wu W. High performance liquid chromatography analysis of 2-mercaptoethylamine (cysteamine) in biological samples by derivatization with N-(1-pyrenyl) maleimide (NPM) using fluorescence detection. J. Chromatogr. B. 2006;843:57–62. doi: 10.1016/j.jchromb.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 50.Masuda M., Toriumi C., Santa T. Fluorogenic derivatization reagents suitable for isolation and identification of cysteine-containing proteins utilizing high-performance liquid chromatography−tandem mass spectrometry. Anal. Chem. 2004;76:728–735. doi: 10.1021/ac034840i. [DOI] [PubMed] [Google Scholar]
- 51.Asamoto H., Ichibangase T., Saimaru H. Existence of low-molecular-weight thiols in Caenorhabditis elegans demonstrated by HPLC-fluorescene detection utilizing 7-chloro-N-[2-(dimethylamino)ethyl]-2,1,3-benzoxadiazole-4-sulfonamide. Biomed. Chromatogr. 2007;21:999–1004. doi: 10.1002/bmc.814. [DOI] [PubMed] [Google Scholar]
- 52.Bousquet M., Gibrat C., Ouellet M. Cystamine metabolism and brain transport properties: clinical implications for neurodegenerative diseases: cystamine in neurodegenerative diseases. J. Neurochem. 2010;114:1651–1658. doi: 10.1111/j.1471-4159.2010.06874.x. [DOI] [PubMed] [Google Scholar]
- 53.Soriano B.D., Tam L.-T.T., Lu H.S. A fluorescent-based HPLC assay for quantification of cysteine and cysteamine adducts in Escherichia coli-derived proteins. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2012;880:27–33. doi: 10.1016/j.jchromb.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Ricci G., Nardini M., Chiaraluce R. Detection and determination of cysteamine at the nanomole level. J. Appl. Biochem. 1983;5:320–329. [PubMed] [Google Scholar]
- 55.Duffel M.W., Logan D.J., Ziegler D.M. in: Methods Enzymology, Vol. 143, Academic Press; 1987. Cysteamine and cystamine; pp. 149–154. [DOI] [PubMed] [Google Scholar]
- 56.Hsiung M., Yeo Y.Y., Itiaba K. Cysteamine, penicillamine, glutathione, and their derivatives analyzed by automated ion exchange column chromatography. Biochem. Med. 1978;19:305–317. doi: 10.1016/0006-2944(78)90032-7. [DOI] [PubMed] [Google Scholar]
- 57.Wang J., Zhou L., Lei H. Simultaneous quantification of amino metabolites in multiple metabolic pathways using ultra-high performance liquid chromatography with tandem-mass spectrometry. Sci. Rep. 2017;7:1423. doi: 10.1038/s41598-017-01435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao H.-M., Wang X., Liao Q.-L. Sensitive analysis of multiple low-molecular-weight thiols in a single human cervical cancer cell by chemical derivatization-liquid chromatography-mass spectrometry. Analyst. 2019;144:6578–6585. doi: 10.1039/c9an01566c. [DOI] [PubMed] [Google Scholar]
- 59.Ellman G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958;74:443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- 60.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 61.Belldina E.B., Huang M.Y., Schneider J.A. Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients: pharmacokinetics of cysteamine. Br. J. Clin. Pharmacol. 2003;56:520–525. doi: 10.1046/j.1365-2125.2003.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luaces-Rodríguez A., Díaz-Tomé V., González-Barcia M. Cysteamine polysaccharide hydrogels: study of extended ocular delivery and biopermanence time by PET imaging. Int. J. Pharm. 2017;528:714–722. doi: 10.1016/j.ijpharm.2017.06.060. [DOI] [PubMed] [Google Scholar]
- 63.Coulomb B., Robert-Peillard F., Palacio E. Fast microplate assay for simultaneous determination of thiols and dissolved sulfides in wastewater. Microchem. J. 2017;132:205–210. [Google Scholar]
- 64.Kim Y., Na D.H. Simultaneous determination of cysteamine and cystamine in cosmetics by ion-pairing reversed-phase high-performance liquid chromatography. Toxicol. Res. 2019;35:161–165. doi: 10.5487/TR.2019.35.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S., Ge Y., Piletsky S., editors. Molecularly Imprinted Sensors: Overview and Applications. first ed. Elsevier; Amsterdam; Boston: 2012. [Google Scholar]
- 66.Kelly M.J., Perrett D., Rudge S.R. The determination of cysteamine in physiological fluids by HPLC with electrochemical detection. Biomed. Chromatogr. BMC. 1987;2:216–220. doi: 10.1002/bmc.1130020509. [DOI] [PubMed] [Google Scholar]
- 67.Smolin L.A., Schneider J.A. Measurement of total plasma cysteamine using high-performance liquid chromatography with electrochemical detection. Anal. Biochem. 1988;168:374–379. doi: 10.1016/0003-2697(88)90332-6. [DOI] [PubMed] [Google Scholar]
- 68.Garcia R.A., Hirschberger L.L., Stipanuk M.H. Measurement of cyst(e)amine in physiological samples by high performance liquid chromatography. Anal. Biochem. 1988;170:432–440. doi: 10.1016/0003-2697(88)90655-0. [DOI] [PubMed] [Google Scholar]
- 69.Raoof J.B., Ojani R., Chekin F. Fabrication of functionalized carbon nanotube modified glassy carbon electrode and its application for selective oxidation and voltammetric determination of cysteamine. J. Electroanal. Chem. 2009;633:187–192. [Google Scholar]
- 70.Ojani R., Raoof J.B., Zarei E. Electrocatalytic oxidation and determination of Cysteamine by poly- N,N -dimethylaniline/ferrocyanide film modified carbon paste electrode. Electroanalysis. 2009;21:1189–1193. [Google Scholar]
- 71.Karimi-Maleh H., Biparva P., Hatami M. A novel modified carbon paste electrode based on NiO/CNTs nanocomposite and (9, 10-dihydro-9, 10-ethanoanthracene-11, 12-dicarboximido)-4-ethylbenzene-1, 2-diol as a mediator for simultaneous determination of cysteamine, nicotinamide adenine dinucleotide and folic acid. Biosens. Bioelectron. 2013;48:270–275. doi: 10.1016/j.bios.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 72.Karimi-Maleh H., Salimi-Amiri M., Karimi F. A voltammetric sensor based on NiO nanoparticle-modified carbon-paste electrode for determination of cysteamine in the presence of high concentration of tryptophan. J. Chem. 2013;2013:1–7. [Google Scholar]
- 73.Arabali V., Karimi-Maleh H. Electrochemical determination of cysteamine in the presence of guanine and adenine using a carbon paste electrode modified with N-(4-hydroxyphenyl)-3,5-dinitrobenzamide and magnesium oxide nanoparticles. Anal. Methods. 2016;8:5604–5610. [Google Scholar]
- 74.Salmanpour S., Abbasghorbani M., Karimi F. Electrocatalytic determination of cysteamine uses a nanostructure based electrochemical sensor in pharmaceutical samples. Curr. Anal. Chem. 2016;13:40–45. [Google Scholar]
- 75.Taherkhani A., Karimi-Maleh H., Ensafi A.A. Simultaneous determination of cysteamine and folic acid in pharmaceutical and biological samples using modified multiwall carbon nanotube paste electrode. Chin. Chem. Lett. 2012;23:237–240. [Google Scholar]
- 76.Keyvanfard M., Sami S., Karimi-Maleh H. Electrocatalytic determination of cysteamine using multiwall carbon nanotube paste electrode in the presence of 3,4-dihydroxycinnamic acid as a homogeneous mediator. J. Braz. Chem. Soc. 2013;24:32–39. [Google Scholar]
- 77.Keyvanfard M., Ahmadi M., Karimi F. Voltammetric determination of cysteamine at multiwalled carbon nanotubes paste electrode in the presence of isoproterenol as a mediator. Chin. Chem. Lett. 2014;25:1244–1246. [Google Scholar]
- 78.Rezaei B., Khosropour H., Ensafi A.A. Sensitive voltammetric determination of cysteamine using promazine hydrochloride as a mediator and modified multi-wall carbon nanotubes carbon paste electrodes. Ionics. 2014;20:1335–1342. [Google Scholar]
- 79.Mohammadi S.Z., Tajik S., Beitollahi H. Sensitive cysteamine determination using disposable electrochemical sensor based on modified screen printed electrode. Iran J. Anal. Chem. 2019;6:57–64. [Google Scholar]
- 80.Smolin L.A., Clark K.F., Thoene J.G. A comparison of the effectiveness of cysteamine and phosphocysteamine in elevating plasma cysteamine concentration and decreasing leukocyte free cystine in nephropathic cystinosis. Pediatr. Res. 1988;23:616–620. doi: 10.1203/00006450-198806000-00018. [DOI] [PubMed] [Google Scholar]
- 81.Dohil R., Fidler M., Barshop B.A. Understanding intestinal cysteamine bitartrate absorption. J. Pediatr. 2006;148:764–769. doi: 10.1016/j.jpeds.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 82.Dohil R., Cabrera B.L., Gangoiti J. The effect of food on cysteamine bitartrate absorption in healthy participants. Clin. Pharmacol. Drug Dev. 2012;1:170–174. doi: 10.1177/2160763X12454423. [DOI] [PubMed] [Google Scholar]
- 83.Khomenko T., Kolodney J., Pinto J.T. New mechanistic explanation for the localization of ulcers in the rat duodenum: role of iron and selective uptake of cysteamine. Arch. Biochem. Biophys. 2012;525:60–70. doi: 10.1016/j.abb.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Armas D., Holt R.J., Confer N.F. A phase 1 pharmacokinetic study of cysteamine bitartrate delayed-release capsules following oral administration with orange juice, water, or omeprazole in cystinosis. Adv. Ther. 2018;35:199–209. doi: 10.1007/s12325-018-0661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pisoni R.L., Park G.Y., Velilla V.Q. Detection and characterization of a transport system mediating cysteamine entry into human fibroblast lysosomes. Specificity for aminoethylthiol and aminoethylsulfide derivatives. J. Biol. Chem. 1995;270:1179–1184. doi: 10.1074/jbc.270.3.1179. [DOI] [PubMed] [Google Scholar]
- 86.Medic G., van der Weijden M., Karabis A. A systematic literature review of cysteamine bitartrate in the treatment of nephropathic cystinosis. Curr. Med. Res. Opin. 2017;33:2065–2076. doi: 10.1080/03007995.2017.1354288. [DOI] [PubMed] [Google Scholar]
- 87.Devereux G., Steele S., Griffiths K. An open-label investigation of the pharmacokinetics and tolerability of oral cysteamine in adults with cystic fibrosis. Clin. Drug Invest. 2016;36:605–612. doi: 10.1007/s40261-016-0405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fidler M.C., Barshop B.A., Gangoiti J.A. Pharmacokinetics of cysteamine bitartrate following gastrointestinal infusion. Br. J. Clin. Pharmacol. 2007;63:36–40. doi: 10.1111/j.1365-2125.2006.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Matos D.G., Furnus C.C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- 90.Ray P.D., Huang B.-W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chopra V.S., Chalifour L.E., Schipper H.M. Differential effects of cysteamine on heat shock protein induction and cytoplasmic granulation in astrocytes and glioma cells. Mol. Brain Res. 1995;31:173–184. doi: 10.1016/0169-328x(95)00049-x. [DOI] [PubMed] [Google Scholar]
- 92.Jeitner T.M., Lawrence D.A. Mechanisms for the cytotoxicity of cysteamine. Toxicol. Sci. Off. J. Soc. Toxicol. 2001;63:57–64. doi: 10.1093/toxsci/63.1.57. [DOI] [PubMed] [Google Scholar]
- 93.Aruoma O.I., Halliwell B., Hoey B.M. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J. 1988;256:251–255. doi: 10.1042/bj2560251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Purdie J.W. A comparative study of the radioprotective effects of cysteamine, WR-2721, and WR-1065 in cultured human cells. Radiat. Res. 1979;77:303–311. [PubMed] [Google Scholar]
- 95.Nair C.K., Parida D.K., Nomura T. Radioprotectors in radiotherapy. J. Radiat. Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 96.Elmonem M.A., Veys K.R., Soliman N.A. Cystinosis: a review. Orphanet J. Rare Dis. 2016;11:47. doi: 10.1186/s13023-016-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilmer M.J., Schoeber J.P., van den Heuvel L.P. Cystinosis: practical tools for diagnosis and treatment. Pediatr. Nephrol. Berl. Ger. 2011;26:205–215. doi: 10.1007/s00467-010-1627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thoene J.G., Oshima R.G., Crawhall J.C. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J. Clin. Invest. 1976;58:180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bozdağ S., Gümüş K., Gümüş O. Formulation and in vitro evaluation of cysteamine hydrochloride viscous solutions for the treatment of corneal cystinosis. Eur. J. Pharm. Biopharm. 2008;70:260–269. doi: 10.1016/j.ejpb.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Besouw M., Tangerman A., Cornelissen E. Halitosis in cystinosis patients after administration of immediate-release cysteamine bitartrate compared to delayed-release cysteamine bitartrate. Mol. Genet. Metabol. 2012;107:234–236. doi: 10.1016/j.ymgme.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 101.Inano H., Onoda M., Suzuki K. Inhibitory effects of WR-2721 and cysteamine on tumor initiation in mammary glands of pregnant rats by radiation. Radiat. Res. 2000;153:68–74. doi: 10.1667/0033-7587(2000)153[0068:ieowac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 102.Wan X.-M., Zheng F., Zhang L. Autophagy-mediated chemosensitization by cysteamine in cancer cells. Int. J. Canc. 2011;129:1087–1095. doi: 10.1002/ijc.25771. [DOI] [PubMed] [Google Scholar]
- 103.Fujisawa T., Rubin B., Suzuki A. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PloS One. 2012;7 doi: 10.1371/journal.pone.0034437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki A., Bhardwaj R., Leland P. Cysteamine suppresses tumor metastasis by inhibiting activity of matrix metalloproteases without inducing toxicity in mouse models of human ovarian cancer. Canc. Res. 2017;77 4900–4900. [Google Scholar]
- 105.Nordlund J.J., Boissy R.E., Hearing V.J., editors. The Pigmentary System: Physiology and Pathophysiology. 1st ed. Oxford University Press; New York: 1998. [Google Scholar]
- 106.Rose P.T. Pigmentary disorders. Med. Clin. 2009;93:1225–1239. doi: 10.1016/j.mcna.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 107.Bastonini E., Kovacs D., Picardo M. Skin pigmentation and pigmentary disorders: focus on epidermal/dermal cross-talk. Ann. Dermatol. 2016;28:279–289. doi: 10.5021/ad.2016.28.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rigopoulos D., Gregoriou S., Katsambas A. Hyperpigmentation and melasma. J. Cosmet. Dermatol. 2007;6:195–202. doi: 10.1111/j.1473-2165.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 109.Davis E.C., Callender V.D. Postinflammatory hyperpigmentation. J. Clin. Aesthetic Dermatol. 2010;3:20–31. [PMC free article] [PubMed] [Google Scholar]
- 110.Nieuweboer-Krobotova L. Hyperpigmentation: types, diagnostics and targeted treatment options: Hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013;27:2–4. doi: 10.1111/jdv.12048. [DOI] [PubMed] [Google Scholar]
- 111.Ephrem E., Elaissari H., Greige-Gerges H. Improvement of skin whitening agents efficiency through encapsulation: current state of knowledge. Int. J. Pharm. 2017;526:50–68. doi: 10.1016/j.ijpharm.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 112.Chavin W., Schlesinger W. Some potent melanin depigmentary agents in the black goldfish. Naturwissenschaften. 1966;53:413–414. doi: 10.1007/BF00625789. [DOI] [PubMed] [Google Scholar]
- 113.Pathak M.A., Frenk E., Szabó G. Cutaneous depigmentation. Clin. Res. 1966;14:272–278. [Google Scholar]
- 114.Frenk E., Pathak M.A., Szabó G. Selective action of mercaptoethylamines on melanocytes in mammalian skin: experimental depigmentation. Arch. Dermatol. 1968;97:465–477. [PubMed] [Google Scholar]
- 115.Niu C., Aisa H.A. Upregulation of melanogenesis and tyrosinase activity: potential agents for vitiligo. Molecules. 2017;22:1303. doi: 10.3390/molecules22081303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Villarama C.D., Maibach H.I. Glutathione as a depigmenting agent: an overview. Int. J. Cosmet. Sci. 2005;27:147–153. doi: 10.1111/j.1467-2494.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 117.Kasraee B. Peroxidase-mediated mechanisms are involved in the melanocytotoxic and melanogenesis-inhibiting effects of chemical agents. Dermatology. 2002;205:329–339. doi: 10.1159/000066439. [DOI] [PubMed] [Google Scholar]
- 118.Karg E., Odh G., Wittbjer A. Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells. J. Invest. Dermatol. 1993;100:209S–213S. [PubMed] [Google Scholar]
- 119.Djurhuus R., Svardal A.M., Ueland P.M. Cysteamine increases homocysteine export and glutathione content by independent mechanisms in C3H/10T1/2 cells. Mol. Pharmacol. 1990;38:327–332. [PubMed] [Google Scholar]
- 120.Wilmer M.J., Kluijtmans L.A.J., van der Velden T.J. Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2011;1812:643–651. doi: 10.1016/j.bbadis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 121.Smit N.P., van der Meulen H., Koerten H.K. Melanogenesis in cultured melanocytes can be substantially influenced by L-tyrosine and L-cysteine. J. Invest. Dermatol. 1997;109:796–800. doi: 10.1111/1523-1747.ep12340980. [DOI] [PubMed] [Google Scholar]
- 122.Meier T., Issels R.D. Methods Enzymology, Vol. Vol. 252. Academic Press; 1995. [11] Promotion of cyst(e)ine uptake; pp. 103–112. [DOI] [PubMed] [Google Scholar]
- 123.Rendon M.I., Gaviria J.I. Review of skin-lightening agents. Dermatol. Surg. 2005;31:886–890. doi: 10.1111/j.1524-4725.2005.31736. [DOI] [PubMed] [Google Scholar]
- 124.Hsu C., Mahdi H.A., Pourahmadi M. Cysteamine cream as a new skin depigmenting product. J. Am. Acad. Dermatol. 2013;68 [Google Scholar]
- 125.Laouini A., Jaafar-Maalej C., Limayem-Blouza I. Preparation, characterization and applications of liposomes: state of the art. J. Colloid Sci. Biotechnol. 2012;1:147–168. [Google Scholar]
- 126.Zylberberg C., Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23:3319–3329. doi: 10.1080/10717544.2016.1177136. [DOI] [PubMed] [Google Scholar]
- 127.Akbarzadeh A., Rezaei-Sadabady R., Davaran S. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gharib R., Greige-Gerges H., Fourmentin S. Liposomes incorporating cyclodextrin–drug inclusion complexes: current state of knowledge. Carbohydr. Polym. 2015;129:175–186. doi: 10.1016/j.carbpol.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 129.Roman V., Bocquier F., Leterrier F. Radioprotective effect of cysteamine entrapped in liposomes orally administered to the mouse. C. R. Seances. Acad. Sci. III. 1982;295:191–193. [PubMed] [Google Scholar]
- 130.Jeitner T.M., Oliver J.R. Possible oncostatic action of cysteamine on the pituitary glands of oestrogen-primed hyperprolactinaemic rats. J. Endocrinol. 1990;127:119–127. doi: 10.1677/joe.0.1270119. [DOI] [PubMed] [Google Scholar]
- 131.Challa R., Ahuja A., Ali J. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Del Valle E.M.M. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–1046. [Google Scholar]
- 133.Ramnik S., Nitin B., Jyotsana M. Characterization of cyclodextrin inclusion complexes – a review. J. Pharmaceut. Sci. Technol. 2010;2:171–183. [Google Scholar]
- 134.Bibette J., Calderon F.L., Poulin P. Emulsions: basic principles. Rep. Prog. Phys. 1999;62:969–1033. [Google Scholar]
- 135.Coupland J.N., McClements D.J. Lipid oxidation in food emulsions. Trends Food Sci. Technol. 1996;7:83–91. [Google Scholar]
- 136.Bridle H. Waterborne Pathog. Academic Press; Amsterdam: 2014. Chapter Nine - nanotechnology for detection of waterborne pathogens; pp. 291–318. [Google Scholar]
- 137.Alaqad K., Saleh T.A. Gold and silver nanoparticles: synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016;6 1000384. [Google Scholar]
- 138.Liang X., Wei H., Cui Z. Colorimetric detection of melamine in complex matrices based on cysteamine-modified gold nanoparticles. Analyst. 2011;136:179–183. doi: 10.1039/c0an00432d. [DOI] [PubMed] [Google Scholar]
- 139.Cao R., Li B. A simple and sensitive method for visual detection of heparin using positively-charged gold nanoparticles as colorimetric probes. Chem. Commun. 2011;47:2865–2867. doi: 10.1039/c0cc05094f. [DOI] [PubMed] [Google Scholar]
- 140.Sun J., Ge J., Liu W. A facile assay for direct colorimetric visualization of lipopolysaccharides at low nanomolar level. Nano Res. 2012;5:486–493. [Google Scholar]
- 141.Jiang Y., Zhao H., Zhu N. A simple assay for direct colorimetric visualization of trinitrotoluene at picomolar levels using gold nanoparticles. Angew. Chem. Int. Ed. Engl. 2008;47:8601–8604. doi: 10.1002/anie.200804066. [DOI] [PubMed] [Google Scholar]
- 142.Kang J., Zhang Y., Li X. A rapid colorimetric sensor of clenbuterol based on cysteamine-modified gold nanoparticles. ACS Appl. Mater. Interfaces. 2016;8:1–5. doi: 10.1021/acsami.5b09079. [DOI] [PubMed] [Google Scholar]
- 143.Zhao D., Chen C., Lu L. A label-free colorimetric sensor for sulfate based on the inhibition of peroxidase-like activity of cysteamine-modified gold nanoparticles. Sensor. Actuator. B Chem. 2015;215:437–444. [Google Scholar]