Abstract
Background:
An important period in the care of patients with schizophrenia-spectrum disorders is when they transition from inpatient to outpatient services and are at increased risk for relapse and rehospitalization. Thus, we developed and examined the initial feasibility, acceptability, and clinical effects of an mHealth transitions of care intervention (Mobile After-Care Support; MACS) in an open trial.
Methods:
Ten adults with schizophrenia-spectrum disorders were recruited during their index psychiatric hospitalization and enrolled prior to discharge. Measures of feasibility, acceptability, and MACS targets were administered at baseline and a 1-month follow-up. Drawing on skills from Cognitive Behavioral Therapy for Psychosis (CBTp), MACS delivered brief assessments of clinically relevant variables, followed by just-in-time interventions for patients starting immediately post-discharge.
Results:
Individuals completed about one session per day on average as expected. Overall, measures of MACS usability and satisfaction were positive. T-test analyses showed that dysfunctional coping strategies significantly decreased from baseline to 1-month follow-up. Results also revealed statistically significant reductions in psychiatric symptoms over 1-month follow-up.
Conclusions:
This study demonstrates the feasibility and acceptability of MACS, a new app-based intervention targeting transitions of care for patients with psychosis. The field is turning to the use of mobile technology as a means of augmenting service delivery and providing real-time assessment and intervention for patients at risk. MACS is a promising adjunctive intervention that warrants further testing in a randomized controlled trial.
Keywords: mobile technology, psychiatric hospitalization, psychosis, schizophrenia, treatment adherence, cognitive behavior therapy for psychosis
1. Introduction
The global burden of schizophrenia-spectrum disorders is substantial, with estimated treatment costs exceeding $100 billion per year (1, 2). Indeed, psychosis is among the top 25 causes of disability worldwide (3). Schizophrenia-spectrum disorders are associated with significant functional impairments and high rates of relapse (4–6), often making treatment complicated and costly. These challenges are particularly salient when individuals with psychosis transition from inpatient to outpatient care. Compared with more stable outpatients, individuals with psychosis post-hospitalization often have more cognitive impairments, problems with treatment connection/engagement, housing insecurity, medication side effects, and suicidality (7). Recent psychiatric hospitalization predicts treatment nonadherence (8–10), and the transition from inpatient to outpatient services is associated with increased stress and premature treatment drop out (7, 11). Despite the post-discharge period being a time of elevated risk, clinical settings often lack feasible and effective services to support patients’ return to the community. Moreover, there is no gold standard intervention and delivery method that would be recommended to support treatment adherence and effective coping among these individuals post-discharge, suggesting the need for further research in this area.
1.1. Cognitive Behavioral Therapy for Psychosis (CBTp)
To date, adherence interventions for psychosis have produced promising, albeit mixed results (12–14). The most fruitful approaches have drawn on cognitive behavioral therapy for psychosis (CBTp) (13–15). CBTp teaches self-coping with illness by fostering active, planned, and effective problem solving to alleviate distress and improve functioning (16). Another key focus of CBTp is to support treatment engagement and medication/appointment adherence, which are essential to the management of schizophrenia (17). In addition to research showing that CBTp leads to decreased symptoms and improved functioning beyond medications alone (18), studies show that CBTp is effective at improving coping with illness (19). However, more work is needed to determine how to best deliver these interventions, particularly to help patients during the post-hospitalization period.
1.2. Digital mental health services for adults with psychosis
One promising pathway to efficiently support patients with psychosis as they return to the community following hospitalization might be through the delivery of CBTp interventions using digital mental health or mobile health (mHealth) services. mHealth, often rooted in ecological momentary assessment and intervention (20–22), refers to technology-based service protocols that can improve patients’ functioning and/or reduce symptoms (23). As shown in recent systematic reviews (24, 25), there is a growing body of research into digital mental health services for patients with psychosis, including studies supporting their feasibility, acceptability, and efficacy.
In terms of acceptability, qualitative feedback has shown that community-based patients with psychosis endorse the benefits of integrating mobile technology into clinical care, including improving patient-provider communication, as well as presenting an opportunity to trigger early intervention (26). Moreover, studies show that people with schizophrenia largely accept and can successfully complete mobile device-based assessment, with compliance rates typically comparable to those of nonclinical populations (27–33). Further, moderately high engagement (44%) with mHealth services has been observed for up to 6 months in a sample of individuals with psychosis (34).
mHealth interventions using CBTp principles for individuals with psychosis also show promising efficacy. For example, in a pilot trial of a text messaging-based intervention for community-dwelling adults with psychosis, results showed that the digital intervention lead to improved medication adherence, increased social interactions, and reduced severity of auditory hallucinations (28). Another study showed that text messaging helped individuals with psychosis to achieve their goals, such as attending outpatient treatment appointments or completing activities of daily living (35). In a study of community-based individuals, a smartphone-based intervention resulted in reductions in depression, general psychopathology, and psychotic symptoms (36). Most recently, a study that blended brief mobile intervention and face-to-face coping-focused therapy showed improved coping and reduced severity of auditory hallucinations (37).
In sum, data show that mental health interventions delivered through mobile devices are feasible and acceptable to patients with psychosis. Moreover, studies show that these interventions can produce significant reductions in symptoms and improve treatment adherence. However, most studies used relatively stable, community-based samples with psychosis. One study (34) focused on recently hospitalized individuals, but inclusion criteria were quite broad, allowing for hospital discharge to have occurred within the past 60 days, and patients were not transitioned directly from inpatient to outpatient care. Thus, the field is lacking systematic research focused on patients recruited during an index hospitalization and followed immediately post-discharge. This is an ideal period to leverage digital technology to deliver CBTp-based care, given that we know that these patients are at increased risk for treatment nonadherence and drop out, continued functional impairment, and the re-emergence of significant psychopathology shortly after they leave the hospital.
1.3. Rationale for the present study
The current study was a treatment development project to create a new mHealth intervention for patients with psychosis during the transition from inpatient to outpatient care. Thus, our aim was to examine the initial feasibility, acceptability, and possible effects of the newly developed mobile intervention (Mobile After-Care Support; “MACS”) for patients with psychosis post-hospitalization. MACS was developed as an application (“app”) to be used on participants’ smartphones. Using CBTp-based strategies, the app was designed to monitor patients’ treatment adherence and symptoms and to intervene by providing brief, just-in-time interventions to support treatment adherence and participants’ use of healthy coping skills to manage their illness. We explored if MACS would be feasible, measured by participants’ familiarity with and ability to use mobile devices, willingness to participate and remain in the study, and successful navigation of problems or issues encountered when using MACS. We also examined MACS’s acceptability, characterized by app engagement rates, ratings of usability and satisfaction, and qualitative feedback about patients’ experiences. Lastly, we examined directional changes in MACS’s intervention targets and outcomes to determine initial target engagement for testing in a future randomized controlled trial.
2. Material and methods
2.1. Participants
Ten adults with schizophrenia-spectrum disorders participated in this study. Inclusion criteria were: (a) currently hospitalized (inpatient psychiatric facility); (b) diagnosed with DSM5 criteria for schizophrenia or schizoaffective disorder based on the Structured Clinical Interview for DSM-5 (SCID-5; [38]); (c) 18 years or older; (d) prescribed oral antipsychotic medication upon discharge; and (e) able to speak and read English (materials written at a 5th grade reading level). Exclusion criteria were: (a) alcohol/drug use disorders at moderate or severe level based on SCID (mild substance use disorders were permitted); (b) planned discharge to supervised living setting or participation in formal outpatient adherence programs in which patients did not control their mediation administration (e.g., medication packaging); or (c) pregnant or had a medical condition contraindicating use of antipsychotic medications (e.g., dementia as indicated by patients’ medical charts). See Table 1 for summary of demographic and clinical characteristics of the sample.
Table 1.
Baseline demographic characteristics (n=10).
| n (%) | Mean (± SD) | |
|---|---|---|
| Age | 44.4 (±13.9) | |
| Gender (Female) | 6 (60.0%) | |
| Race | ||
| White | 5 (50.0%) | |
| African American/Black | 2 (20.0%) | |
| Multiple races | 3 (30.0%) | |
| Ethnicity (Latinx) | 1 (10.0%) | |
| Single (never married) | 5 (50.0%) | |
| Education (years) | 13.4 (±1.9) | |
| Household income (< $40,000/yr.) | 7 (87.5%) | |
| Full- or part-time employment | 4 (40.0%) | |
| Physical and/or psychiatric disability | 3 (30.0%) | |
| Retired | 1 (10.0%) | |
| Unemployed | 2 (20.0%) | |
| Primary Diagnosis | ||
| Schizophrenia | 6 (60.0%) | |
| Schizoaffective disorder (Bipolar) | 3 (30.0%) | |
| Schizoaffective disorder (Depressive) | 1 (10.0%) |
Note: Missing income data: n=2.
2.2. Procedures
Recruitment occurred during participants’ index inpatient admission at a private, acute-care psychiatric hospital in the northeast region of the U.S. The study was approved by the Institutional Review Board of the hospital. Electronic medical records for newly admitted patients were screened after obtaining a Protected Health Information waiver for this purpose. Participants completed a baseline assessment prior to hospital discharge to confirm eligibility and were then asked to complete a 1-month follow-up. Additional follow-ups were conducted for pilot purposes, but not examined here as 1-month was considered the target period for MACS treatment.
Follow-ups occurred in person whenever possible, but some (n=6) were conducted remotely for participants’ convenience. Research assistants were trained to initial interrater reliability (kappa > .80) on the interview-administered measures, with periodic checks to prevent against “drift.” Participants were compensated $30 for each assessment (baseline and 1-month). The MACS app was either downloaded onto the participant’s smartphone or if needed, a study device was provided with the app pre-loaded (n=4; 40%). All participants practiced responding to MACS app sessions to familiarize themselves with the program and troubleshoot technical problems prior to discharge.
2.3. Mobile After-care Support (MACS) app
The MACS app was programmed using an established mobile software service (ilumivu.com), which provided a secure, HIPAA-compliant application (Android or Apple IOS compatible). The protocol consisted of 3 randomly scheduled prompts during daytime hours (9am – 9pm). Additionally, users could initiate a MACS session “on demand.” Each session was designed to take 5–10 minutes to complete. Sessions began with brief assessments about coping, substance use, symptoms, treatment adherence, behavioral activation, and quality of life. Based on responses to these initial questions, participants were then prompted with individualized intervention skills. After obtaining releases of information from the individual, research reports, containing summarized MACS data, were sent to participants’ outpatient providers at baseline, two weeks later, and at the conclusion of the 1-month period. These reports explained that the individual was participating in the MACS study and summarized app-collected data related to symptoms endorsed.
Because MACS was designed as a mobile self-management intervention, we chose to focus on techniques that taught participants active coping strategies to manage illness-related distress and to foster adherence to medications and treatment appointments, given the key role these factors play in preventing relapse and enhancing long-term recovery. Primarily, MACS was constructed from common components adapted from CBTp studies, including those testing mobile interventions to improve self-coping and adherence behaviors (27, 28). Any reported treatment nonadherence during the initial mobile assessment was prioritized as a topic in need of intervention via MACS. Participants were asked to choose from a variety of possible reasons for nonadherence. If the reported reason was primarily logistical (e.g., ran out of pills), participants were instructed to contact their provider at the community clinic to address the problem. This information was also conveyed to providers through the periodic reports so that the community clinic could reach out to participants to address adherence issues. If nonadherence was attributed to medication concerns (e.g., does not believe medications help), MACS used CBTp techniques that encouraged participants to communicate concerns to providers, reminded them of costs vs. benefits of medication in terms of symptom management, and taught them to engage in other brief problem-solving strategies delivered through the app (39). If appointment nonattendance was reported, similar problem-solving strategies were suggested. The MACS app also provided interactive exercises designed to teach participants coping skill using CBTp exercises (e.g., “Is there another explanation for what is going on right now? Let’s explore some examples.” or “Try doing what you want despite what the voices say. Let’s practice how to do this now.”). Other domains that MACS targeted through CBTp interventions included: lack of social engagement/support, negative affect, low life satisfaction, and substance abuse.
2.4. Measures of feasibility and acceptability
We examined feasibility by assessing participants’ mobile device use and connectivity using a study-designed phone usage questionnaire. Participants’ need for additional MACS training or trouble-shooting during use of the app was cataloged to further quantify feasibility. In an exit interview at the 1-month follow-up, we asked participants about positive and negative aspects of using the app and how it affected them. Lastly, the following self-report measures were used to further assess feasibility and acceptability at the 1-month follow-up:
Client Satisfaction Questionnnaire-8 (CSQ-8; [40]).
The CSQ-8 is an 8-item reliable and valid measure designed to assess individuals’ satisfaction with services or an intervention. Higher scores indicated greater satisfaction. The CSQ-8 consistently shows high reliability (e.g., α = .93; (41).
System Usability Scale (SUS; [42]).
Initially developed to examine usability of products or services, the SUS is a reliable and valid 10-item self-report that was administered to specifically examine the usability of the MACS app. Example items included, “I would imagine that most people would learn to use this app very quickly.” In a study using a large collection of data and usability ratings (43), the SUS showed good reliability (α = .85).
The Usefulness, Satisfaction, and Ease of Use Questionnaire (USE; [44]).
The USE is a 30-item self-report measure found to be reliable and valid for assessing products or services. It examines four dimensions of usability related to the MACS app: (a) usefulness; (b) ease of use; (c) ease of learning; and, (d) satisfaction. A recent analysis by Gao and colleagues (45) showed very good reliability (α = .98).
2.5. Adherence, coping, functioning, and symptom measures.
At baseline and the 1-month follow-up, the following assessment measures were administered:
Antipsychotic Medication Beliefs and Attitudes Scale (AMBAS; [46]).
The AMBAS is a reliable and valid 12-item self-report that shows initial evidence of reliability and validity. It assesses medication beliefs and attitudes, including factors related to shame and stigma. Higher scores mean more positive medication beliefs.
Brief Adherence Rating Scale (BARS; [47]).
The BARS is an interviewer administered measure assessing the percentage of antipsychotic medication doses taken vs. prescribed over the past month. The BARS has shown good reliability (α = .92) when administered to individuals with psychosis (47).
Brief Coping Orientation to Problems Experienced (Brief COPE; [48]).
The Brief COPE is a 28-item self-report measure of various coping approaches, include problematic or maladaptive approaches. In this study, we used the three subscales constructed by Coolidge and colleagues (49): Emotion-focused coping strategies, Problem-focused strategies, and Dysfunctional coping strategies. The Brief COPE shows good internal consistency (48).
Brief Psychiatric Rating scale-18-item (BPRS; [50]).
The BPRS is an interviewer-rated measure of overall psychiatric symptoms, including anxiety, depression, and psychosis. It shows good validity for distinguishing symptoms associated with psychosis (51).
World Health Organization Disability Assessment Schedule 2.0 (WHODAS; [52]).
The WHODAS is a self-report measure of functional impairment. It probes for impairment in activities of daily living, cognition, mobility, self-care, and socialization. Higher scores indicate greater functional impairment. In a sample of adults with schizophrenia, the WHODAS showed high internal consistency and validity (53).
2.6. Statistical analyses
All statistical analyses were conducted in SPSS. We calculated descriptive statistics for measures of acceptability and feasibility. Bivariate correlational analyses were used to examine baseline demographic and clinical variables associated with MACS engagement. Within-subjects t-tests, as well as related effect sizes (Cohen’s d) and confidence intervals, were used to compare baseline to 1-month time points regarding outcomes. Summaries of exit interviews were compiled to illustrate pros and cons of MACS.
3. Results
3.1. Study recruitment and retention
See Figure 1 for CONSORT diagram depicting participant flow. Of the 15 patients approached for the study, 10 (66.7%) agreed to participate in the study. All 10 of these individuals completed the baseline assessment and were given the MACS app. Seven participants (70%) completed all measures administered at the 1-month follow-up and one additional participant partially completed 1-month measures. During the intervention, one participant (10%) was rehospitalized.
Figure 1.
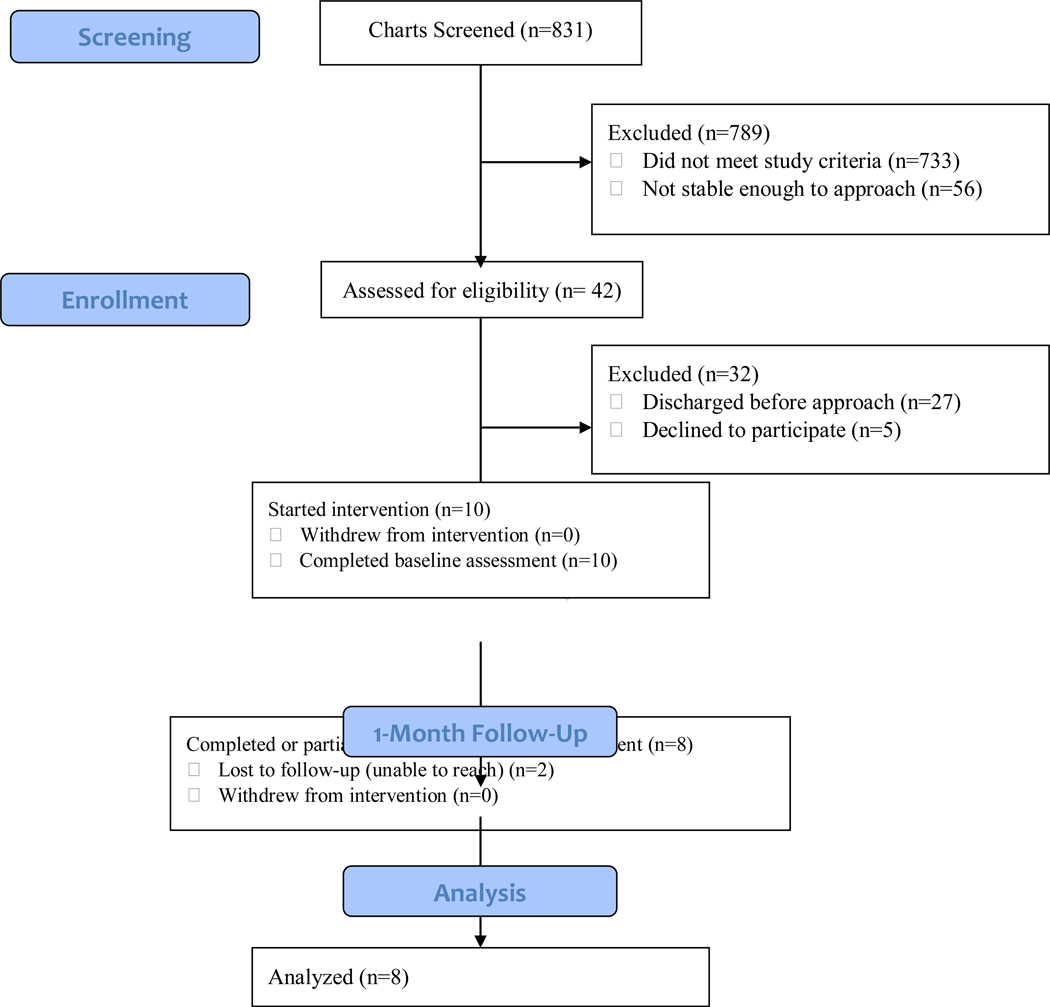
MACS participant flow.
3.2. Personal technology access/usage
At baseline, four participants (40%) did not own a smartphone (and were given a device to use during the study) and six participants (60%) did. A majority of participants reported using a mobile phone for ≥1 hour/day (n=7; 70%). Access to the internet and Wi-Fi were similarly high (n=9; 90%), although very few participants had an e-mail address (n=2; 20%).
3.3. MACS feasibility and completion
Participants were instructed to contact MACS staff to troubleshoot technical difficulties. Additionally, if non-usage of the app was observed for several days, study staff contacted the participant to determine the reason for this and address any issues. Some participants reported that the session did not show up correctly in the app and that the person was having difficulty navigating through the app, likely due to lack of technology fluency. Most issues were resolved quickly with minimal support.
A total of 275 MACS sessions were completed by participants. A majority of these sessions were completed when the participant was at home (78.9%). One participant did not complete any MACS sessions. Among the other nine participants, total engagement with the MACS app reflected, on average, approximately one session per day (M=28.0 sessions, SD=28.6). Incomplete (i.e., started, but not finished) MACS sessions were rare (M=2.6 sessions, SD=5.2). In correlational analyses of baseline demographics and other clinical variables in relation to MACS completion rates, results revealed that baseline BPRS score was significantly correlated with MACS completion rates, such that increased psychiatric severity was associated with increased completion rates (r=.73, p=.025). Otherwise, none of the these variables significantly correlated with MACS engagement (ps >.05).
3.4. MACS usability and acceptability ratings
Usability was measured with the CSQ-8, USE, and SUS measures at the conclusion of the 1-month MACS period. Mean item ratings for the CSQ-8 were above the midpoint on the 4-point scale (M=2.4, SD=0.2), suggesting overall positive satisfaction with MACS. Total SUS scores were also positive, with mean ratings of 75.4 (SD=31.8). Scores >68 represent “above average” usability (42). Overall USE ratings in each subscale trended to the positive range as well. See Table 2 for a summary of participants’ satisfaction ratings.
Table 2.
MACS app acceptability ratings (n=7).
| Mean (± SD) | Range | |
|---|---|---|
| CSQ-8 (by item) | 2.4 (±0.2) | 2.1 – 2.9 |
| SUS (total score) | 75.4 (±31.8) | 15 – 100 |
| USE - Ease of learning (by item) | 5.0 (±2.5) | 1 – 7 |
| USE - Ease of use (by item) | 4.8 (±2.2) | 1 – 6.8 |
| USE - Satisfaction (by item) | 4.4 (±1.9) | 1 – 6.3 |
| USE - Usefulness (by item) | 3.8 (±1.6) | 1 – 6 |
Note: CSQ-8= Client Satisfaction Questionnnaire-8; SUS= System Usability Scale; USE= The Usefulness, Satisfaction, and Ease of Use Questionnaire.
Participants chose to engage with a wide variety of MACS-provided coping skill interventions. The most commonly chosen MACS coping skills to focus on during the sessions related to coping with emotions (30.7%) followed by behavioral activation (21.6%). Coping skills related to psychotic symptoms also were particularly well-received. See Table 3 for summary of coping skills engagement and participants’ satisfaction with the chosen skill.
Table 3.
MACS app coping skills selected by participants at each session.
| Coping skill | n (%) | Skill Satisfaction (Mean (± SD)) | Skill Satisfaction (Range) |
|---|---|---|---|
| Emotion management | 74 (31.5%) | 3.08 (±1.08) | 1 – 5 |
| Behavior activation | 52 (22.1%) | 3.64 (±1.04) | 2 – 5 |
| Quality of life enhancement | 43 (18.3%) | 3.34 (±1.08) | 2 – 5 |
| Psychosis management | 40 (17.0%) | 4.34 (±1.13) | 1 – 5 |
| Social support enhancement | 20 (8.5%) | 3.60 (±0.75) | 2 – 5 |
| Substance use change | 6 (2.6%) | 3.33 (±1.34) | 2 – 5 |
At the conclusion of the 1-month MACS intervention, we conducted exit interviews to better understand participants’ experiences using the app. Feedback was mixed, but mainly positive. Most participants reported that they found the app “easy to use” and appreciated how the app prompted them to “think about how I’m feeling by checking in.” Some participants commented that the app had “too many sessions” and that the content could sometimes be “not as personable as receiving coping advice in-person or by phone.” Participants suggested that MACS could be improved by adding a wider array of coping skills and providing further training or explanation about coping skills.
3.5. MACS targets and clinical outcomes
Reported antipsychotic medication nonadherence and outpatient treatment nonadherence were minimal (4.7% and 3.3%, respectively). Only one participant noted having missed a treatment appointment; otherwise, participants reported being 100% adherent to outpatient appointments. T-test analyses comparing baseline to 1-month data showed that use of dysfunctional coping strategies significantly decreased during the 1-month period using MACS: t(7)=5.40, p=.002, d=1.45. Results also revealed a statistically significant reduction in psychiatric symptoms, as measured by the BPRS: t(7)=6.46, p=.002, d=2.13. Moreover, analyses supported expectations for directional improvements in other MACS outcomes, including improved functioning and increased positive medication beliefs, although these results were statistically nonsignificant. See Table 4 for summary of results.
Table 4.
Comparison of baseline and 1-month outcomes (n=7).
| Mean (± SD) | Mean (± SD) | t-test (p value) | Cohen’s d (95%CI) | |
|---|---|---|---|---|
| BPRS | 46.4 (±6.7) | 30.4 (±8.5) | 6.46 (<.001) | 2.13 (.39, 3.87) |
| WHODAS | 34.7% (±15.3%) | 28.5% (±21.2%) | .83 (.444) | .34 (.74, 1.43) |
| BARS | 96.9% (±8.8%) | 97.5% (±4.6%) | −.16 (.875) | .06 (−.76, .88) |
| AMBAS | 32.3 (±3.1) | 33.8 (±8.4) | −.36 (.733) | .40 (−.71, 1.51) |
| COPE - Dysfunctional coping | 26.9 (±5.3) | 18.0 (±4.1) | 5.40 (.002) | 1.45 (.05, 2.96) |
| COPE - Emotion-focused strategies | 24.4 (±7.6) | 24.4 (±8.7) | <.01 (>.999) | 0 (−.91, .91) |
| COPE - Problem-focused strategies | 16.6 (±4.9) | 14.6 (±6.8) | 1.35 (.225) | .34 (.60, 1.31) |
Note: 3 individuals did not complete the 1-month assessment. BARS= Brief Adherence Rating Scale; BPRS= Brief Psychiatric Rating scale-18-item; WHODAS= World Health Organization Disability Assessment Schedule 2.0; AMBAS= Antipsychotic Medication Beliefs and Attitudes Scale; COPE= Brief Coping Orientation to Problems Experienced.
4. Discussion
This study demonstrates the feasibility and acceptability of MACS, a new mHealth app-based intervention, designed to support treatment adherence and healthy coping among adults with psychosis immediately following hospital discharge. Despite being approached while hospitalized, a majority of patients agreed to participate in the study. The most highly rated coping skills offered by MACS were those related to managing symptoms of psychosis and facilitating behavioral activation. In addition, the most frequently chosen copings skills related to emotion regulation. This is likely reflective of the variety of symptoms individuals with psychosis continue to experience at hospital discharge, even if they are less acutely ill.
Participant feedback was mostly positive as many noted the benefits of being prompted to reflect on their symptoms and functioning, as well as in receiving brief CBTp-based support. Some participants expressed a desire for MACS’s coping skills to be more varied and that more support in using the coping skills would have been helpful. Otherwise, minimal troubleshooting was needed in which staff helped participants navigate MACS technical issues. Many of these problems were likely due to user error or low technology fluency, rather than problems with the app per se.
MACS completion rates were generally good, with individuals completing at least one session per day on average, which was the target rate for the study. Only one participant did not complete any MACS sessions. Correlational analyses showed that baseline psychiatric symptoms significantly predicted MACS session completion rates, with higher symptoms leading to higher MACS engagement. This relationship makes sense from a clinical perspective as individuals who might be acutely distressed could be more motivated to engage with MACS, particularly if they found it helpful. Although participants were prompted three times per day, responding to assessments and engaging in the interventions at least once daily far surpasses the weekly frequency of assessment and care provided on a typical outpatient schedule. Engagement with mHealth apps vary in this population, but published studies tend to define adequate engagement as participants completing at least 20% of prompted sessions (33, 37, 54). MACS is designed to be an adjunct to outpatient care, meaning that it will support other services, but should not fully replace more traditional treatment modalities.
Although this was a pilot study with a small sample, results suggested significant improvements in certain aspects of coping with illness and overall psychiatric symptoms. Adherence was high at baseline and remained high at follow-up. Inconsistent changes were observed for the other measures; although sample size was small and confidence intervals around effects were large. Overall, our findings extend prior mHealth work in psychosis (e.g., 29, 55) by further demonstrating the feasibility, acceptability, and potential efficacy of mHealth interventions in adults with psychosis. By initiating MACS at hospital discharge, this study built on prior work by targeting a novel, yet high risk time period.
4.1. Limitations
This was a small sample, which might not be representative of other patients with psychosis. Due to the longitudinal nature of the mHealth intervention, participants with relatively stable housing at discharge and reliable access to telephone communications and transportation were prioritized for recruitment. Furthermore, the study’s inclusion requirements of a SCID-5 diagnosis of schizoaffective disorder or schizophrenia excluded patients who were hospitalized with first psychotic episodes and those who were diagnosed with unspecified psychosis or schizophreniform disorder. In addition, the measures of medication adherence used were self-report and designed for oral medications only, and as a result, patients who were only prescribed long acting injectable antipsychotics were excluded from the study. Finally, clinical improvements reported here cannot necessarily be attributed to the MACS intervention because of the lack of a control group. Further testing is needed to examine MACS in a randomized controlled design now that it has been shown to be feasible and acceptable.
5. Conclusions
The present study is the first to our knowledge to specifically target treatment adherence and coping among hospitalized individuals with psychosis immediately upon discharge, using an mHealth approach. Currently available interventions for improving coping and medication/appointment adherence in psychosis have been shown to be efficacious, but are not routinely utilized in real-world clinical settings due to barriers related to feasibility, cost, and access. The field is turning to the use of mobile technology as a means of augmenting service delivery and providing real-time assessment and intervention in more efficient ways. However, there are notable gaps in existing research. Digital health interventions are a possible solution to these issues and they warrant further research.
Acknowledgments
Data used in the preparation of this manuscript were deposited in the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier: DOI: 10.15154/1519035 This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Author biography
Ethan Moitra is an Assistant Professor at the Warren Alpert Medical School of Brown University. He received his doctoral degree from Drexel University.
Hyun Seon Park is research staff at Butler Hospital. She received her bachelor’s degree from the University of Illinois – Urbana – Champaign. degree
Brandon A. Gaudiano is an Associate Professor at the Warren Alpert Medical School of Brown University. He received his doctoral degree from Drexel University.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Chong HY, Teoh SL, Wu DBC, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsych Dis Treat. 2016;12:357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin HJ, Mosweu I. The Societal Cost of Schizophrenia: A Systematic Review. Pharmacoeconomics. 2017;35(1):25–42. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feki R, Feki I, Smaoui N, Baati I, Abida I, Masmoudi J, et al. The Burden Among Caregivers of Patients with Chronic Psychoses. European Psychiatry. 2015;30:1380. [Google Scholar]
- 5.Foldemo A, Gullberg M, Ek A -C, Bogren L, Linköpings u, Institutionen för nervsystem och r, et al. Quality of life and burden in parents of outpatients with schizophrenia. Social Psychiatry and Psychiatric Epidemiology. 2005;40(2):133–8. [DOI] [PubMed] [Google Scholar]
- 6.Fulford D, Niendam TA, Floyd EG, Carter CS, Mathalon DH, Vinogradov S, et al. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophrenia research. 2013;147(1):125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, Weiden PJ. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216–22. [DOI] [PubMed] [Google Scholar]
- 8.Kreyenbuhl J, Slade EP, Medoff DR, Brown CH, Ehrenreich B, Afful J, et al. Time to discontinuation of first- and second-generation antipsychotic medications in the treatment of schizophrenia. Schizophr Res. 2011;131(1–3):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullins CD, Obeidat NA, Cuffel BJ, Naradzay J, Loebel AD. Risk of discontinuation of atypical antipsychotic agents in the treatment of schizophrenia. Schizophr Res. 2008;98(1–3):8–15. [DOI] [PubMed] [Google Scholar]
- 10.Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiat Res. 2010;176(2–3):109–13. [DOI] [PubMed] [Google Scholar]
- 11.Kimhy D, Harkavy-Friedman JM, Nelson EA. Identifying life stressors of patients with schizophrenia at hospital discharge. Psychiatr Serv. 2004;55(12):1444–5. [DOI] [PubMed] [Google Scholar]
- 12.Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. The Psychiatric clinics of North America. 2007;30(3):437–52. [DOI] [PubMed] [Google Scholar]
- 13.Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. Journal of Clinical Psychopharmacology. 2003;23(4):389–99. [DOI] [PubMed] [Google Scholar]
- 14.Zygmunt A, Olfson M, Boyer CA, Mechanic D. Interventions to improve medication adherence in schizophrenia. The American Journal of Psychiatry. 2002;159(10):1653–64. [DOI] [PubMed] [Google Scholar]
- 15.Farhall J, Thomas N. Cognitive and behavioural therapies for psychosis. Aust Nz J Psychiat. 2013;47(6):508–11. [DOI] [PubMed] [Google Scholar]
- 16.Farhall J, Greenwood KM, Jackson HJ. Coping with hallucinated voices in schizophrenia: a review of self-initiated strategies and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476–93. [DOI] [PubMed] [Google Scholar]
- 17.Ce Steel. CBT for schizophrenia: evidence-based interventions and future directions: John Wiley & Sons.; 2012. [Google Scholar]
- 18.Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523–38. [DOI] [PubMed] [Google Scholar]
- 19.Premkumar P, Peters ER, Fannon D, Anilkumar AP, Kuipers E, Kumari V. Coping styles predict responsiveness to cognitive behaviour therapy in psychosis. Psychiatry Res. 2011;187(3):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annu Rev Psychol. 2003;54:579–616. [DOI] [PubMed] [Google Scholar]
- 21.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psycho. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 22.Heron KE, Smyth JM. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. Brit J Health Psych. 2010;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham AK, Lattie EG, Mohr DC. Experimental Therapeutics for Digital Mental Health. Jama Psychiat. 2019;76(12):1223–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez-Jimenez M, Alcazar-Corcoles MA, Gonzalez-Blanch C, Bendall S, McGorry PD, Gleeson JF. Online, social media and mobile technologies for psychosis treatment: A systematic review on novel user-led interventions. Schizophr Res. 2014;156(1):96–106. [DOI] [PubMed] [Google Scholar]
- 25.Bell IH, Lim MH, Rossell SL, Thomas N. Ecological Momentary Assessment and Intervention in the Treatment of Psychotic Disorders: A Systematic Review. Psychiatr Serv. 2017;68(11):1172–81. [DOI] [PubMed] [Google Scholar]
- 26.Palmier-Claus JE, Rogers A, Ainsworth J, Machin M, Barrowclough C, Laverty L, et al. Integrating mobile-phone based assessment for psychosis into people’s everyday lives and clinical care: a qualitative study. Bmc Psychiatry. 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Depp CA, Mausbach B, Granholm E, Cardenas V, Ben-Zeev D, Patterson TL, et al. Mobile Interventions for Severe Mental Illness Design and Preliminary Data From Three Approaches. J Nerv Ment Dis. 2010;198(10):715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granholm E, Ben-Zeev D, Link PC, Bradshaw KR, Holden JL. Mobile Assessment and Treatment for Schizophrenia (MATS): A Pilot Trial of An Interactive Text-Messaging Intervention for Medication Adherence, Socialization, and Auditory Hallucinations. Schizophrenia Bulletin. 2012;38(3):414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granholm E, Loh C, Swendsen J. Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophrenia Bulletin. 2008;34(3):507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swendsen J, Ben-Zeev D, Granholm E. Real-Time Electronic Ambulatory Monitoring of Substance Use and Symptom Expression in Schizophrenia. Am J Psychiat. 2011;168(2):202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Zeev D, McHugo GJ, Xie H, Dobbins K, Young MA. Comparing Retrospective Reports to Real-Time/Real-Place Mobile Assessments in Individuals With Schizophrenia and a Nonclinical Comparison Group. Schizophr Bull. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards CJ, Cella M, Tarrier N, Wykes T. The optimisation of experience sampling protocols in people with schizophrenia. Psychiatry Res. 2016;244:289–93. [DOI] [PubMed] [Google Scholar]
- 33.Moitra E, Gaudiano BA, Davis CH, Ben-Zeev D. Feasibility and acceptability of posthospitalization ecological momentary assessment in patients with psychotic-spectrum disorders. Compr Psychiat. 2017;74:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Zeev D, Scherer EA, Gottlieb JD, Rotondi AJ, Brunette MF, Achtyes ED, et al. mHealth for Schizophrenia: Patient Engagement With a Mobile Phone Intervention Following Hospital Discharge. JMIR Ment Health. 2016;3(3):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pijnenborg GHM, Withaar FK, Brouwer WH, Timmerman ME, van den Bosch RJ, Evans JJ. The efficacy of SMS text messages to compensate for the effects of cognitive impairments in schizophrenia. Brit J Clin Psychol. 2010;49:259–74. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, Acceptability, and Preliminary Efficacy of a Smartphone Intervention for Schizophrenia. Schizophrenia Bulletin. 2014;40(6):1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell IH, Rossell SL, Farhall J, Hayward M, Lim MH, Fielding-Smith SF, et al. Pilot randomised controlled trial of a brief coping-focused intervention for hearing voices blended with smartphone-based ecological momentary assessment and intervention (SAVVy): Feasibility, acceptability and preliminary clinical outcomes. Schizophr Res. in press. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 39.Wenze SJ, Gaudiano BA, Weinstock LM, Tezanos KM, Miller IW. Adjunctive psychosocial intervention following Hospital discharge for Patients with bipolar disorder and comorbid substance use: A pilot randomized controlled trial. Psychiat Res. 2015;228(3):516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen D, Attkisson C, Hargreaves W, Nguyen T. Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning. 1979;2:197–207. [DOI] [PubMed] [Google Scholar]
- 41.Attkisson C, Zwick R. The client satisfaction questionnaire: Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning. 1982;5(3):233–7. [DOI] [PubMed] [Google Scholar]
- 42.Brooke J SUS-A quick and dirty usability scale. Usability Evaluation in Industry. 1996;194:4–7. [Google Scholar]
- 43.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the System Usability Scale. Int J Hum-Comput Int. 2008;24(6):574–94. [Google Scholar]
- 44.Lund AM. Measuring usability with the use questionnaire. Usability Interface. 2001;8(2):3–6. [Google Scholar]
- 45.Gao M, Kortum P, Oswald F. Psychometric Evaluation of the USE (Usefulness, Satisfaction, and Ease of use) Questionnaire for Reliability and Validity Proceedings of the Human Factors and Ergonomics Society 2018 Annual Meeting. 2018:1414–8. [Google Scholar]
- 46.Martins MJRV, Pinto AM, Castilho P, Macedo AF, Pereira AT, Bajouco M, et al. Assessing beliefs and attitudes towards antipsychotic medication from a recovery-based perspective: Psychometric properties of a new scale. Psychiat Res. 2019;273:325–30. [DOI] [PubMed] [Google Scholar]
- 47.Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1–3):60–9. [DOI] [PubMed] [Google Scholar]
- 48.Carver CS. You want to measure coping but your protocol’s too long: Consider the brief COPE. Int J Behav Med. 1997;4(1):92–100. [DOI] [PubMed] [Google Scholar]
- 49.Coolidge FL, Segal DL, Hook JN, Stewart S. Personality disorders and coping among anxious older adults. J Anxiety Disord. 2000;14(2):157–72. [DOI] [PubMed] [Google Scholar]
- 50.Ventura J, Lukoff D, Nuechteriein K, Liberman RP, Green MF, Shaner A. Brief psychiatric rating scale (BPRS): Expanded Version, Ver. 4.0. 1993. [Google Scholar]
- 51.Mueser KT, Curren PJ, McHugo GJ. Factor structure of the brief psychiatric rating scale in schizophrenia. Psychol Assessment. 1997;9(3):196–204. [Google Scholar]
- 52.WHO. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0) 2010.
- 53.McKibbin C, Patterson TL, Jeste DV. Assessing disability in older patients with schizophrenia - Results from the WHODAS-II. J Nerv Ment Dis. 2004;192(6):405–13. [DOI] [PubMed] [Google Scholar]
- 54.Oorschot M, Lataster T, Thewissen V, Lardinois M, Wichers M, van Os J, et al. Emotional Experience in Negative Symptoms of Schizophrenia-No Evidence for a Generalized Hedonic Deficit. Schizophrenia Bulletin. 2013;39(1):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Zeev D, Ellington K, Swendsen J, Granholm E. Examining a Cognitive Model of Persecutory Ideation in the Daily Life of People With Schizophrenia: A Computerized Experience Sampling Study. Schizophrenia Bulletin. 2011;37(6):1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


