Abstract
Sex steroid hormones such as 17β-estradiol (estradiol) regulate neuronal function by binding to estrogen receptors (ERs), including ERα and GPER1, and through differential production via the enzyme aromatase. ERs and aromatase are expressed across the nervous system, including in the striatal brain regions. These regions, comprising the nucleus accumbens core, shell, and caudate-putamen, are instrumental for a wide-range of functions and disorders that show sex differences in phenotype and/or incidence. Sex-specific estrogen action is an integral component for generating these sex differences. A distinctive feature of the striatal regions is that in adulthood neurons exclusively express membrane but not nuclear ERs. This long-standing finding dominates models of estrogen action in striatal regions. However, the developmental etiology of ER and aromatase cellular expression in female and male striatum is unknown. This omission in knowledge is important to address, as developmental stage influences cellular estrogenic mechanisms. Thus, ERα, GPER1, and aromatase cellular immunoreactivity was assessed in perinatal, pre-pubertal, and adult female and male rats. We tested the hypothesis that ERα, GPER1, and aromatase exhibits sex, region, and age-specific differences, including nuclear expression. ERα exhibits nuclear expression in all three striatal regions before adulthood and disappears in a region- and sex-specific time-course. Cellular GPER1 expression decreases during development in a region- but not sex-specific time-course, resulting in extranuclear expression by adulthood. Somatic aromatase expression presents at pre-puberty and increases by adulthood in a region- but not sex-specific time-course. These data indicate that developmental period exerts critical sex-specific influences on striatal cellular estrogenic mechanisms.
Keywords: rat, estrogen receptor, striatum, aromatase, sex differences, RRID AB_310305, RRID AB_1141090, RRID AB_566942
Graphical Abstract
Rat striatal regions express nuclear estrogen receptor α and GPER-1 early in development, while aromatase expression increases with age. a-f) Caudate-putamen micrographs depicting ERα positive cells or lack thereof females P3 (a), P20 (b), and adult (c); males P3 (d), P20 (e), and adult (f). Examples of ERα positive cells are marked by small white arrows.
Introduction
Estradiol can both permanently organize and temporarily modulate neurons and behavior (McCarthy & Arnold, 2011; Woolley, 2007). Estradiol can be produced not only by the gonads, but by neural tissue itself via expression of the enzyme aromatase (Balthazart, Choleris, & Remage-Healey, 2018; Krentzel & Meitzen, 2018). The presence of aromatase in a brain region suggests dynamic control of estrogen production, which can directly impact estrogen-sensitive neurons in a temporally rapid manner. Estrogen-sensitive neurons feature general loci of estradiol action: classical nuclear receptors and non-nuclear membrane receptors. Estradiol’s classical mechanism occurs by stimulating nuclear estrogen receptor (ER) α or β to directly induce changes in gene expression, which typically occurs over the course of hours to days. In contrast, estradiol can in seconds to minutes modulate neuronal electrical activity and other cellular and molecular attributes via receptors located on or near the plasma membrane. These membrane receptors can be, for example, membrane-associated ERα or β which signal through associated G-protein Coupled Receptors (GPCR) such as mGluRs (Meitzen & Mermelstein, 2011), or GPER1, which is itself a GPCR (Rudolph et al., 2016; Srivastava & Evans, 2013).
Many brain regions express membrane ERs, including the striatal brain regions: the caudate-putamen and the nucleus accumbens core and shell. A distinctive feature of the striatum compared to many other estrogen-sensitive brain regions is that in adulthood striatal neurons exclusively express functional membrane ERs such as ERα and GPER1 (Almey, Filardo, Milner, & Brake, 2012; Almey, Milner, & Brake, 2015, 2016; Becker & Hu, 2008; Foidart, Tlemcani, Harada, Abe-Dohmae, & Balthazart, 1995; Grove-Strawser, Boulware, & Mermelstein, 2010a; Le Saux, Morissette, & Di Paolo, 2006; Mermelstein, Becker, & Surmeier, 1996; Schultz et al., 2009; Stanic et al., 2014). No previous study has identified nuclear ER expression in the striatum. Sex-specific estradiol action has been shown to modulate numerous aspects of striatal neuron phenotype (Cao, Willett, Dorris, & Meitzen, 2018; Meitzen, Meisel, & Mermelstein, 2018; B. M. Peterson, Mermelstein, & Meisel, 2015a; Staffend, Loftus, & Meisel, 2011; Tozzi et al., 2015), neuromodulator signaling such as dopamine (Calipari et al., 2017; Di Paolo, 1994; Walker, Ray, & Kuhn, 2006; Yoest, Cummings, & Becker, 2014, 2018), striatal-influenced cognitive, social, emotional and premotor behaviors, as well as the relevant neuropsychiatric (Lorsch et al., 2018), motor (Krentzel & Meitzen, 2018), and addiction disorders (Becker 2019). This rich body of data has been dominated by models and discussions of the role of membrane ER. However, thorough investigations of ER compartmental expression in the striatum have been limited to adult rodents, and there is a paucity of information regarding membrane and nuclear ER expression across striatal region, developmental stage, and sex. This is an important limitation, as striatal regions are sensitive to perinatal estrogen exposure (Bonansco et al., 2018; Cao, Dorris, & Meitzen, 2016; Meitzen, Perry, et al., 2013), suggesting the presence of estrogen receptors early in development. A similar situation exists regarding aromatase expression. Though aromatase expression and function has been observed in striatal regions (Horvath, Roa-Pena, Jakab, Simpson, & Naftolin, 1997; Jakab, Horvath, Leranth, Harada, & Naftolin, 1993; McArthur, Murray, Dhankot, Dexter, & Gillies, 2007; Tozzi et al., 2015; Wagner & Morrell, 1996), a thorough analysis and comparison in the context of developmental stage, sex, and striatal region has not been performed. This lack of knowledge is unfortunate, as knowing whether aromatase and estrogen receptors are expressed are critical for generating a working model and hypotheses regarding striatal estrogen action.
Here we address this gap in knowledge by assessing the immunoreactivity of ERα, GPER1, and aromatase in both female and male rats across the striatal regions: caudate-putamen, nucleus accumbens core and shell. Immunoreactivity is evaluated just after birth, just before puberty, and during adulthood. We test the novel hypothesis that these estrogen-producing and estrogen-sensing proteins exhibit sex, region, and age-specific differences, including nuclear expression.
Methods
Animals:
All animal protocols were approved by the Institutional Animal Care and Use Committee at North Carolina State University. Male and female Sprague Dawley CD IGS rats were collected at ages P3, P20, and Adult (P142-157; n=3/sex/age; N=18, GPER1 and ERα) and P3, P20, and Adult (P60; n=5/sex/age; N=30, aromatase). Rats were born from timed-pregnant females purchased from Charles River Laboratories and housed with littermates and dam until weaning. After weaning (P20-21), animals were grouped housed in same-sex cages until experimental collection day. Animals were housed in a temperature and light-controlled room (23°C, 40% humidity, 12:12-hour light/dark cycle with lights on 7am-7pm) at the Biological Resource Facility of North Carolina State University. All cages are polysulfone Bisphenol A (BPA) free and filled with bedding manufactured from virgin hardwood chips (Beta chip, NEPCO, Warrengsburg, NY) to avoid endocrine disruptors present in corncob bedding (Mani, Reyna, Alejandro, Crowley, & Markaverich, 2005; Markaverich et al., 2002; Villalon Landeros et al., 2012). Glass-bottle water and soy protein-free rodent chow (2020X, Teklad, Madison, WI USA) were provided ad libitum. All animals were gonadally intact and estrous cycle was tracked and recorded on the day of brain extraction for adult females. Each stage of the estrous cycle was collected; however, comparisons between estrous cycle phases were underpowered and not further analyzed.
Antibodies (see Table 1):
Table 1:
Primary antibodies used in this study.
| Target | Manufacturer | Type | Immunogen | dilution |
|---|---|---|---|---|
| anti-estrogen receptor alpha | Millipore; RRID AB_310305 | Polyclonal, Host: rabbit | KLH-conjugated linear peptide corresponding to the C-terminus of rat Estrogen Receptor alpha (TYYIPPEAEGFPNTI). | 1:20,000 |
| anti-g protein estrogen receptor 1 | Abcam; RRID AB_1141090 | Polyclonal, Host: rabbit | KLH-conjugated synthetic peptide corresponding to amino acids 362-375 of Human GPCR GPR30 (DSTEQSDVRFSSAV). | 1:1000 |
| anti-aromatase (cytochrome p450) | Biorad; RRID AB_566942 | Monoclonal, Host: mouse | Synthetic peptide corresponding to amino acids 376 - 390 of human aromatase. | 1:100 |
anti-estrogen receptor alpha (ERα, C1355, polyclonal, Millipore; RRID AB_310305) – This antibody was selected because of its wide use in neuroscience publications and because the epitope is to the C-terminus (TYYIPPEAEGFPNTI)(McClellan et al., 2010). For brain immunohistochemistry, this antibody was previously validated by other groups where preabsorption occurred using a 15-aa sequence to the c-terminus (Burke, Letts, Krajewski, & Rance, 2006; Stanic et al., 2014), staining was similar to N-terminus antibody staining and mRNA studies (Burke et al., 2006), and western blot analysis revealed a single band at ~66kDa (Stanic et al., 2014). We a priori expected a negative signal for adult striatum as expression of ERα in the adult striatum is exclusive to extranuclear/membrane expression (Almey et al., 2012; Almey et al., 2015, 2016) and this technique is not sensitive enough to visualize membrane expression which may obscure the epitope. This antibody produced similar lack of expression in adult male mice striatum and cerebral cortex but positive in the arcuate nucleus of the hypothalamus (Agarwal et al., 2000). Therefore, we used the arcuate nucleus as a positive control for adult expression.
anti-g protein estrogen receptor 1 (GPER1, polyclonal, Abcam; RRID AB_1141090) – This antibody targeting the c-terminus (residues 362-375 DSTEQSDVRFSSAV) was selected for multiple reasons. First, immunoblotting and subcellular expression studies of GPER1 reveal many posttranslational modifications occur to redistribute GPER1 throughout the cell with several molecular weight bands dependent on expression pattern (Filardo & Thomas, 2012). Western blots using this antibody depicts these multiple bands and a specific blocking peptide showed preabsorption (Grassi, Ghorbanpoor, Acaz-Fonseca, Ruiz-Palmero, & Garcia-Segura, 2015). Second, this antibody has also been used for immunofluorescence in spinal cord (Zhang, Xiao, Zhang, Zhao, & Zhang, 2012) and brain (Klenke, Constantin, & Wray, 2016) demonstrating its usefulness for visualizing neural tissue.
anti-aromatase (aromatase, residues 376-390 human p450, clone H4, monoclonal, Biorad; RRID AB_566942) – There are very few commercial antibodies for aromatase that have been published for rat brain tissue. This antibody was selected primarily because it has been used and validated through western blotting in rat brain previously showing expression of the ~55kDa band (Castro, Sanchez, Torres, & Ortega, 2013). This same study found effects of BPA, an estrogenic endocrine disruptor, on aromatase protein expression that was also replicated via mRNA relative expression. Other studies using this antibody for changes in protein expression have also validated treated effects by measuring mRNA expression as well (Lu et al., 2007). The peptide sequence selected has been validated for detecting aromatase across multiple species (Turner et al., 2002).
Immunohistochemistry:
All animals were anesthetized with isofluorane and euthanized via rapid decapitation. Brains were quickly extracted and drop-fixed (also called immersion fixed) in 4% paraformaldehyde solution made in 0.1M phosphate buffer. This method of fixation was selected because of the difficulty of perfusing neonates. Since assessing sex differences in developmental trajectory was the major experimental goal, we selected a fixation method that enabled consistent experimental techniques across all sampled ages. Paraformaldehyde was prepared fresh the day of euthanasia. Brains were stored in 4% paraformaldehyde solution for 48-72 hours at 4°C. Brains were then transferred to a 30% sucrose solution made in 0.1M phosphate buffer and stored at 4 °C until sectioning. All brains were sectioned on a freezing microtome at 35-40μm and stored in cyroprotectant at −20 °C. Sections containing the striatal brain regions caudate-putamen (CP), nucleus accumbens core (AcbC) and shell (AcbSh) were selected for staining along with sections containing the cingulate cortex (Almey et al., 2014), arcuate nucleus of the hypothalamus (Chakraborty, Hof, Ng, & Gore, 2003), and medial amygdala (Roselli, Abdelgadir, Ronnekleiv, & Klosterman, 1998) for positive controls for GPER-1, estrogen receptor α and aromatase, respectively.
Sections were washed with 0.02M potassium phosphate buffer solution (KPBS) and then incubated with a 2% normal goat serum+0.03% Triton X-KPBS solution for 24 hours at 4 °C. Following blocking, sections were then incubated for 72 hours at 4 °C in respective primary antibody solutions: 1:1000 anti-g protein estrogen receptor 1 (GPER1, Abcam; RRID AB_1141090), 1:20,000 anti-estrogen receptor alpha (ERα, Millipore; RRID AB_310305), 1:100 anti-aromatase (Biorad; RRID AB 566942). Estrogen receptor β was not analyzed due to the lack of a validated primary antibody (Snyder 2010). Secondary incubation followed (1:200 for anti-rabbit goat Alexa 488 for ERα and GPER1 antibodies and 1:200 anti-mouse goat Alexa 555 for aromatase) for 90 minutes at room temperature. After washing in 0.02M KPBS, sections were mounted on slides with Citifluor (Electron Microscopy Sciences) for tissue preservation. Slides were stored in the dark at −20° C until imaging. We note that use of a drop fix protocol does not clear blood vessels of erythrocytes, other cells, and plasma. Several methods for fluorescently quenching the contents of blood vessels were attempted during the troubleshooting stages of this experiment, specifically protocols employing pretreatment with either glycine or hydrogen peroxide, but these techniques did not successfully eliminate the fluorescence of blood vessels and their contents. Fluorescent blood vessels were not included in any analysis.
Imaging:
All sections were imaged using a Leica TCS SPE confocal microscope with the LAS LAF software (Leica, Germany). Imaged sections of the caudate-putamen, nucleus accumbens core and shell were consistent across animals and were determined using anatomical landmarks including the corpus callosum and anterior commissure (Figure 1). The section of cingulate cortex imaged was within the same plain of section as the striatal regions. Target expression in both the arcuate nucleus of the hypothalamus and the medial amygdala were not quantified as these regions only serve as visual positive controls. All images were taken using the 40x objective and saved as z-stacks for the entire visible thickness of the sections (20-40μm). Gain and offset were kept consistent within a section, following a previously documented protocol (Ikeda, Krentzel, Oliver, Scarpa, & Remage-Healey, 2017). For images displayed in figures, contrast and brightness was uniformly altered across all images for presentation purposes.
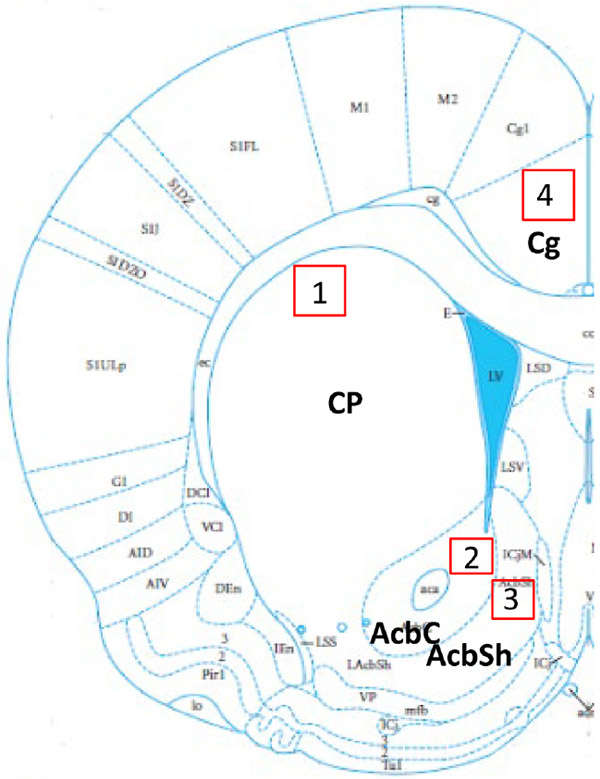
Figure 1: Anatomical locations.
Depiction of locations where subregions were imaged. Imaged areas are 1) caudate-putamen (CP), 2) nucleus accumbens core (AcbC), 3) nucleus accumbens shell (AcbSh), and 4) cingulate cortex (Cg). Adapted from Rat Brain in Stereotaxic Coordinates.
Cell quantification:
The principles of unbiased stereology were followed with specification for striatal tissue (Meitzen, Pflepsen, Stern, Meisel, & Mermelstein, 2011). Neuronal density was measured in both hemispheres and along the rostral-caudal axis of the CPu, AcbC, AcbSh, and cingulate cortex within the overall areas demarcated in Figure 1 (Figure 1). Within each demarcated area, for each section, a counting box (183 x 183 μm) was employed. This size box was used to minimize sampling variance by ensuring equal sampling of both patch and matrix. Patch and matrix medium spiny neurons have similar morphology and intrinsic membrane properties (Kawaguchi, Wilson, & Emson, 1989, 1990). The entire volume of the z-stack was quantified with cell counting markers placed consistently throughout the stacked images to ensure overcounting did not occur. For counting, cells fit an a priori defined criteria depending on the nature of the compartmental staining. Thus, the unit of count differed by target. For ERα, only the nucleus were considered a positive unit of count as extranuclear staining is not visible with this technique (see Almey et al. references for details of extranuclear expression). For GPER1, a shift in cellular compartment occurred with age, where younger animals (P3) exhibited a more nuclear appearance and older animals (P60+) exhibited an empty nucleus with staining in the cytoplasm. Thus, unit of count for GPER1 is considered positive as defined by a clear nucleus (P3), neuron shaped filled nucleus and body (P20), or empty nucleus surrounded by a circular stain (P60+). For aromatase, when expression was present (P20 and P60), the stain was consistent with an empty nucleus and filled cytoplasm.
For counting, all image files were first blinded for analysis, following an established protocol (Ikeda et al., 2017). Criteria for a positive cell were a fluorescent signal above background. For a quantification check control, a subset of images was selected to test saturation of the signal to determine that cells counted were above background signal. Criteria for nuclear expression were a circular and bright nucleus. Criteria for empty nucleus/cytoplasmic expression were an empty nucleus surrounded by a bright circular signal. Criteria for a filled cellular expression depicted by GPER1 positive cells at P20 were a tear-drop appearance which is the circular cell body and visible axon hillock. Overall, units of count were included only if they were fully in the visual field of view and were not bisected by any x or y border. Thus, units of count should be considered relative and an underestimate of actual unit count in reality. Units were counted through the entirety of the section (z plane). Counts per section were normalized by the dimensions of the image (183.33 μm x 183.33 μm x volume of z stack) and converted to mm3. Each section (2-3) per region per animal was then averaged for a representative density for that region of a single animal so that subject number was the final unit of replication. All cell counting was performed with experimenter blind to the age, sex, and brain region. Autofluorescence from blood vessels were identified via their elongated morphology and/or lack of nucleus and not counted. Cell counts were conducted using the LAS LAF software (Leica, Germany). Intracounter reliability of a single counter was determined by duplicating select images in the blinded image set and then calculating a coefficient of variation, which was set for a threshold of <15%.
Statistics:
Three-way ANOVAs were initially conducted to determine major main effects for sex, age, and region (Origin 2018). Two-way ANOVAs were then performed within a striatal region to test for potential Age by Sex interactions to further decompose the analysis (Graphpad Prism 8). Shapiro-Wilke tests were conducted for normality as they are more appropriate for small sample sizes across the ages for each sex of the ANOVAs that were run per region. All analyses passed (p>0.05) and were normal. Homogeneity of variance was tested for each ANOVA using the Brown-Forsythe test. Two of eleven two-way ANOVAs violated homogeneity of variance, and in these cases the Kruskall-Wallis and Dunn’s for multiple comparisons tests were employed. Tukey’s post-hoc tests were performed for all other ANOVAs. Statistical significance is reported for p<0.05, and trends are reported for p<0.10.
Results
Nuclear ERα are present in early development and disappear by adulthood in all striatal subregions in a sex-specific manner
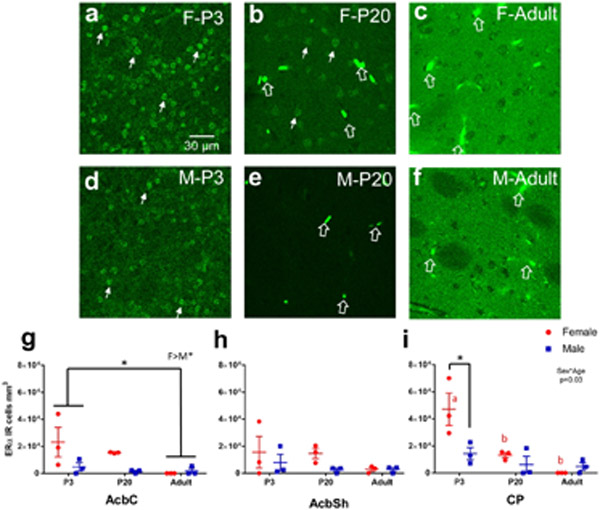
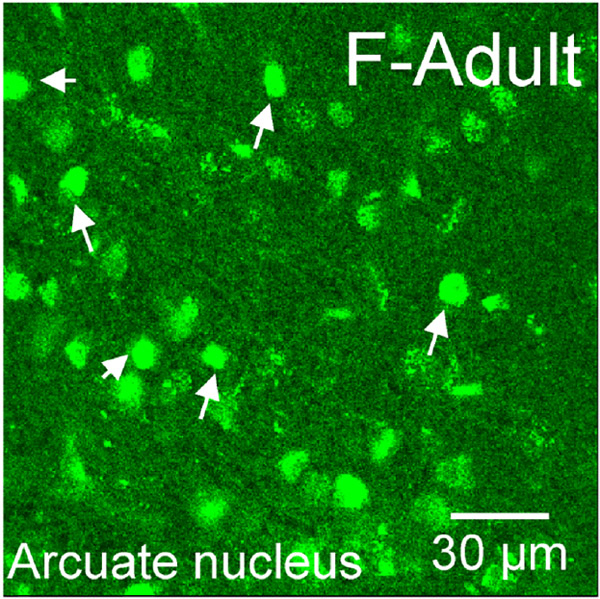
ERα immunoreactivity (IR) was assessed in males and females at P3, P20 and in adulthood in the caudate-putamen, nucleus accumbens core and shell (Figure 2). Nuclear expression patterns of ERα were present at P3 and P20, and then disappeared by adulthood (Figure 2a). Quantitative nuclear ERα-IR cell counts revealed differences by sex (F(1,36)=13.41, p=7.98x10-4), age (F(2,36)=14.23, p=2.79x10-5), and a significant sex*age interaction (F(2,36)=6.02, p=0.0056). Effects of region (F(2,36)=2.89, p=0.069) and age*region (F(2,36)=2.21, p=0.087) exhibited trends. To further analyze the sex*age relationship, we ran two-way ANOVAs within each striatal region. We found that in the AcbC, females exhibited more ERα-IR cells than males (Sex: F(1,12)=6.84, p=0.02) and there was a trending main effect for age (F(2,12)=3.81, p=0.050) where P3s exhibited more ERα-IR cells than adults (p=0.043, Figure 2g). For AcbSh, no significant effects were detected for age (F(2,12)= 1.27, p=0.32), sex (F(1,12)=2.03, p=0.18), or age*sex interaction (F(2,12)=0.57, p=0.58, Figure 2h). For CP, a significant age*sex interaction was detected (F(2,12)=5.26, p=0.023). P3 females showed more ERα-IR cells than P3 males (p=0.020), P20 females (p=0.020) and males (p=0.0041), and adult females (p=0.0012) and males (p=0.0031, Figure 2i). As a positive control, we also imaged the arcuate nucleus of the hypothalamus in the same adult animals to confirm nuclear ERα-IR was still being expressed in other brain regions. As expected, we detected intense, nuclear ERα-IR in the arcuate nucleus (Figure 3), demonstrating that the shift away from nuclear ERα expression in adulthood is accurate regarding the striatal regions.
Figure 2: Nuclear ERα present in early-life and disappears by adulthood in the striatal subregions.
a-f) Representative images of caudate-putamen depicting ERα-IR cells in females P3 (a), P20 (b), and adult (c) and males P3 (d), P20 (e), and adult (f). Examples of ERα-IR nuclei are marked by small, closed, white arrows. Examples of blood vessel/staining artifact around marked by large, open, white arrows. g) Cell counts of ERα-IR nuclei from each male (blue) and female (red) subject of the nucleus accumbens core (AcbC). Lines and error bars depict means and standard errors. h) Cell counts of ERα-IR nuclei from nucleus accumbens shell (AcbSh). i) Cell counts of ERα-IR nuclei from caudate-putamen (CP). * p<0.05 and red letters depict significant posthoc age comparisons for females.
Figure 3: Nuclear ERα present in adulthood in the arcuate nucleus.
Representative image of ERα-IR nuclei staining in an adult female arcuate nucleus to demonstrate that not all brain regions eliminate nuclear ERα in adulthood. Small, closed, white arrows point to examples of ERα-IR nuclei.
GPER1 immunoreactivity decreases during development in a region-dependent manner
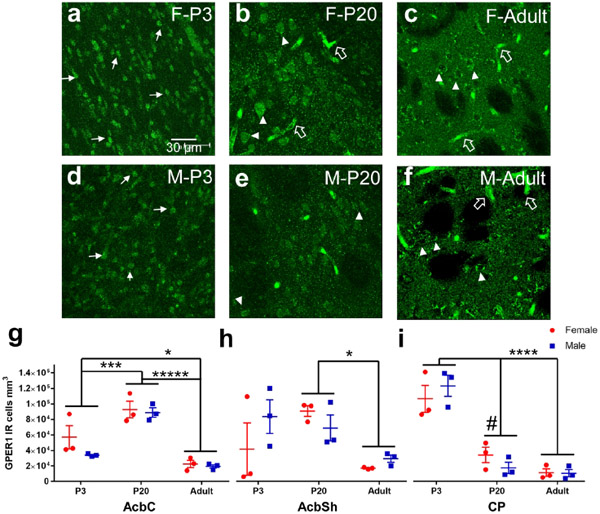
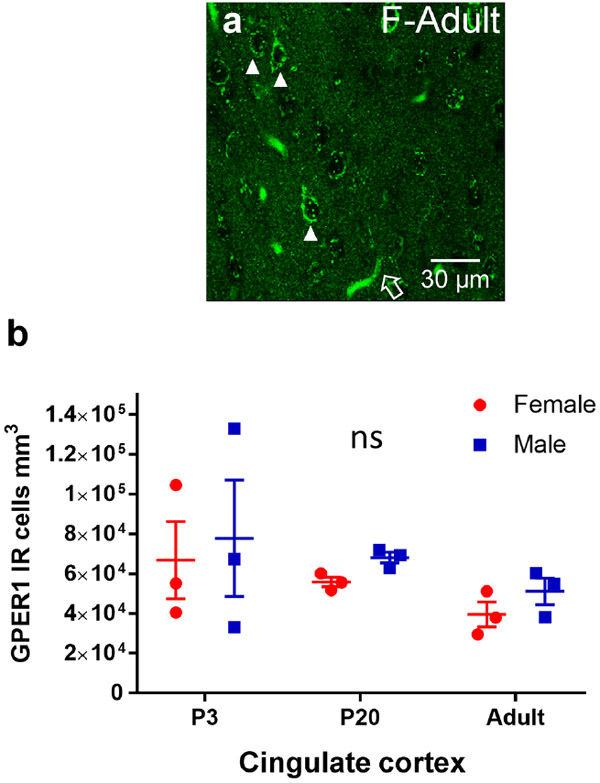
GPER1 was assessed in males and females at P3, P20 and in adulthood in the caudate-putamen, nucleus accumbens core and shell (Figure 4). GPER1 immunoreactivity exhibited strong differences across development. At P3, GPER1-IR cells in all three striatal brain regions exhibited a more nuclear like staining, with small, oblong puncta like fluorescence patterns (Figure 4a and d). In P20 striatal brain regions, GPER1-IR cells were more distributed across the internal components of the cells, with both the soma and nucleus exhibiting a fluorescent signal (Figure 4b and e). GPER1-IR in adults was present in the cytoplasm but not the nucleus (Figure 4c and f). Quantified GPER1-IR positive cell counts were first analyzed via a three-way ANOVA for age, sex, and region to determine initial relationships. GPER1-IR significantly differed with age (F(2,48)=29.4, p=4.59x10−9) and there was a significant age*region interaction (F(6,48)=10.1, p=3.32x10-7). To further analyze the age relationship, a two-way ANOVA for sex and age was performed per region. For nucleus accumbens core (AcbC) a significant age effect was detected (F(2,12)=37.2, p<0.0001) with a rise in GPER1-IR cells from P3 to P20 (Tukey’s HSD: P3 vs P20 p=0.0004). Adults showed the lowest number of GPER-IR cells (Tukey’s HSD: P3 vs. Adult p=0.028 and P20 vs. Adult p<0.001, Figure 4g). There was no effect of sex (Sex: F(1,12)=2.21, p=0.16 and Sex*Age: F(2,12)=0.95, p=0.42). For nucleus accumbens shell (AcbSh), we also detected a significant age effect (F(2,12)=5.18, p=0.024, Kruskal-Wallis p=0.042) where P20 striatal brain regions exhibited more GPER1-IR cells than adults (Dunn’s: P20 vs. Adult p=0.045, Figure 4h). There was no effect of sex (Sex: F(1,12)=0.52, p=0.48 and Sex*Age: F(2,12)=1.56, p=0.25). For caudate-putamen (CP), we detected a significant age effect (F(2,12)=55.2, p<0.0001, Kruskal-Wallis p<0.0001) that differed from the nucleus accumbens core and shell. P3 showed the most GPER1-IR cells compared to either P20 or Adults (Dunn’s: P3 vs. P20 p=0.069, P3 vs. Adult p=0.0011, and P20 vs. Adult p=0.58, Figure 4i). There was not a sex effect (Sex: F(1,12)=7.9x10-4, p=0.98 and Sex*Age: F(2,12)=1.20, p=0.33). Since age effects, especially reduction in adults, were the most robust findings, we also quantified GPER-IR cells in a non-striatal brain region, the cingulate cortex. We did not find any effects of age (F(2,12)=1.67, p=0.23), sex (F(1,12)=0.91, p=0.36), or age*sex interactions (F(2,12)=0.00083, p=0.99 , Figure 5b), indicating that changes in GPER1-IR cell density across age are robust to the striatum.
Figure 4: GPER1 changes cellular compartments and decreases in density with age.
a-f) Representative images of caudate-putamen depicting GPER1-IR cells in females at P3 (a), P20 (b), and adults (c) and males at P3 (d), P20 (e), and adults (f). Examples of GPER1-IR nuclei or soma+nuclei are marked by white triangles. Examples of cytoplasm only GPER1-IR are marked by white triangles. Examples of blood vessel/staining artifact around marked by large, open, white arrows. g) Cell counts of GPER1-IR cells from each male (blue) and female (red) subject of the nucleus accumbens core (AcbC). Lines and error bars depict means and standard errors. h) Cell counts of GPER1-IR cells from nucleus accumbens shell (AcbSh). i) Cell counts of GPER1-IR cells from caudate-putamen (CP).# p<0.10, * p<0.05, *** p<0.001, **** p<0.0001, ***** p<0.00001.
Figure 5: GPER1 does not have similar age dependent changes in cingulate cortex.
a) Representative image of cingulate cortex depicting GPER1-IR cells in an adult female. Examples of cytoplasm only GPER1-IR are marked by white triangles. Examples of blood vessel/staining artifact around marked by large, open, white arrows. b) Cell counts of GPER1-IR cells from each male (blue) and female (red) subject of the cingulate cortex. Ns=non-significant.
Aromatase immunoreactivity increases during development in a region-dependent manner
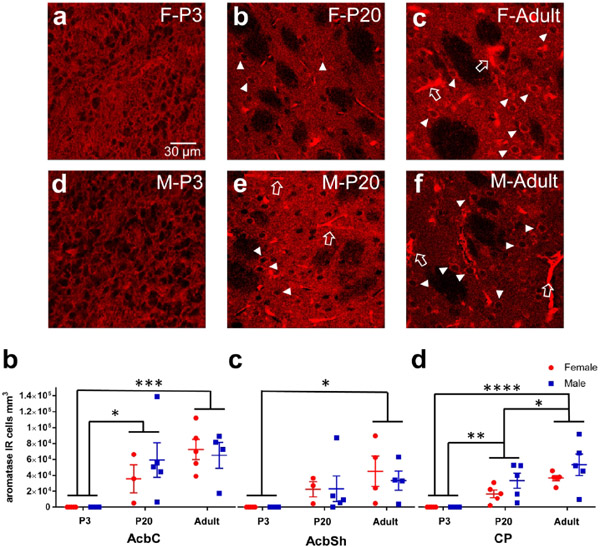
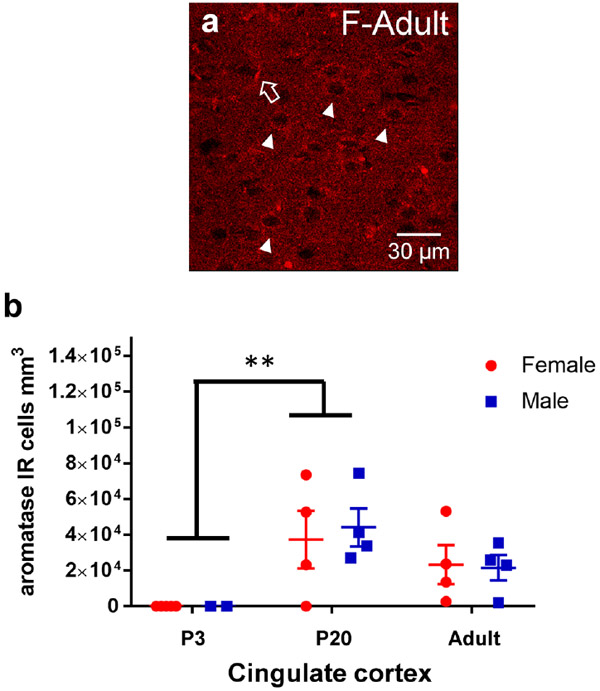
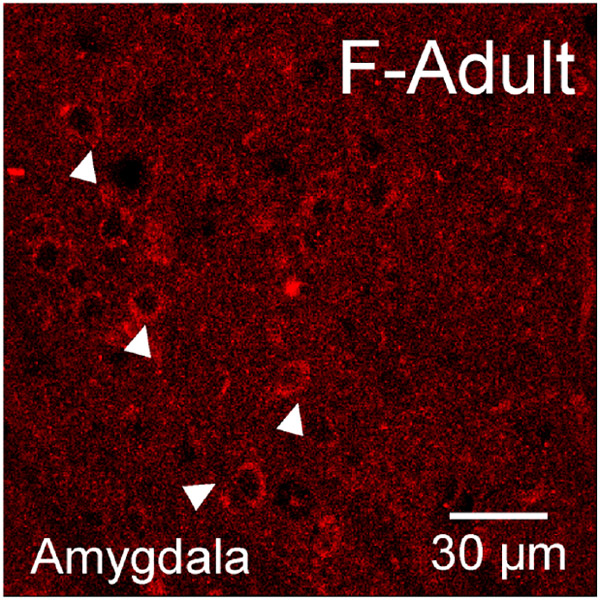
Aromatase was assessed in males and females at P3, P20 and in adulthood in the caudate-putamen, nucleus accumbens core and shell (Figure 6). Aromatase-IR somatic cell expression was quantified, which features a filled cytoplasm and empty nucleus (Figure 6b-c, e-f). Quantified aromatase-IR positive cell counts were first analyzed via a three-way ANOVA for age, sex, and region to determine initial relationships. A significant effect of age (F(2,78)=30.6, p=1.56x10-10), region (F(3,78)=3.40, p=0.022), and age*region (F(6,78)=2.22, p=0.049) was detected. There was no main effect of sex detected (F(1,78)=0.61, p=0.44). To deconstruct the age and regions effects further, we ran two-way ANOVAs for age and sex in each striatal region. In the AcbC, a significant age effect was detected (F(2,19)=10.63, p=0.0008) where aromatase-IR cells were more abundant in P20 and adult rats than P3 (Tukey’s HSD: P3 vs P20 p=0.019, P3 vs Adult p=0.006, Figure 6g). There was no effect of sex (F(1,19)=0.19, p=0.67). In the AcbSh, a significant age effect was also detected (F(2,18)=4.57, p=0.025) where aromatase-IR cells were greater in adult animals than in P3 animals (p=0.019, Figure 6h). There was no effect of sex (F(1,18)=0.12, p=0.73). In the CP, we found a significant age effect (F(2,24)=19.67, p<0.0001) where aromatase-IR cells abundance increased with age with adult rats exhibiting the highest cell density (Tukey’s HSD: P3 vs P20 p=0.0054, P3 vs Adult p<0.0001, P20 vs Adult p=0.027, Figure 6i). There was only a trend for a main effect of sex (F(1,24)=3.63, p=0.070). To determine if this increase in aromatase-IR cell density with age is robust to the striatum, aromatase-IR cell density was quantified in the cingulate cortex and a significant age effect was detected (F(2,17)=7.27, p=0.0052). In cingulate cortex, P3 aromatase-IR cells were decreased compared to P20 (p=0.0038) and there was no difference detected between P20 and Adults (p=0.18, Figure 7b). There was no effect for sex (F(1,17)=0.04, p=0.844). This indicates that the general developmental trajectory is shared between the assessed regions, but with select region-specific differences in the maturation. As a positive control for detecting the presence of aromatase, we also imaged the medial amygdala, and somatic immunoreactivity was detected similar to the striatal brain regions (Figure 8). Overall, these results indicate that aromatase expression generally increases in striatal regions across development.
Figure 6: Somatic aromatase-IR cells increases in density with age.
a-f) Representative images of caudate-putamen depicting aromatase-IR cells in females at P3 (a), P20 (b), and adults (c) and males at P3 (d), P20 (e), and adults (f). Examples of cytoplasm only aromatase-IR are marked by white triangles. Examples of blood vessel/staining artifact around marked by large, open, white arrows. g) Cell counts of aromatase-IR cells from each male (blue) and female (red) subject of the nucleus accumbens core (AcbC). Lines and error bars depict means and standard errors. h) Cell counts of aromatase-IR cells from nucleus accumbens shell (AcbSh). i) Cell counts of aromatase-IR cells from caudate-putamen (CP). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Figure 7: Somatic aromatase-IR cell density also increases with age in cingulate cortex.
a) Representative image from cingulate cortex depicting aromatase-IR cells in females. Examples of cytoplasm only aromatase-IR are marked by white triangles. Examples of blood vessel/staining artifact around marked by large, open, white arrows. b) Cell counts of aromatase-IR cells from each male (blue) and female (red) subject of the cingulate cortex. Lines and error bars depict means and standard errors. ** p<0.01
Figure 8: Somatic aromatase-IR in the medial amygdala.
Representative image from medial amygdala depicting aromatase-IR cells in females. Examples of cytoplasm only aromatase-IR are marked by white triangles.
Discussion
This paper reports multiple major novel findings. 1) Nuclear ERα is present in all three striatal regions only in early development. 2) Nuclear ERα-IR cells are more robust in females compared to males in the caudate-putamen and disappear in a sex-specific time course. 3) GPER1 transitions from multicompartmental cellular expression to cytoplasmic and membrane expression across development in all striatal regions. 4) GPER1-IR cells decrease by adulthood in the striatal regions of both sexes. 5) Aromatase somatic expression presents just before puberty and amplifies by adulthood in the striatal regions of both sexes. Collectively, these data indicate that early in development rat striatal neurons display nuclear ERα and GPER1 expression which then disappears before adulthood in a sex-specific time course concomitant with increased aromatase expression. This drastic transition away from nuclear expression indicates that development is a critical factor in determining how estradiol organizes and/or activates striatal neurons. Therefore, this novel discovery of nuclear estrogen receptors in the striatum not only instructs a paradigm shift in our understanding of how estradiol influences striatal function, but also potentially generates a model system useful for elucidating the mechanisms underlying switches in estrogen-sensing and estrogen-production in neural systems.
Estrogen receptors and aromatase dynamically change cell compartment and expression density across development
Canonically, the striatum has been considered a brain region that lacks nuclear estrogen receptors (Almey et al., 2012; Almey et al., 2015, 2016; Mermelstein et al., 1996; Schultz et al., 2009) and exhibits sparse estradiol binding (Pfaff & Keiner, 1973) however, most analyses of expression have been restricted to adulthood. We detected a present signal of nuclear ERα at P3 in both sexes and at P20 in females, indicating that early life may be a sensitive period of striatal sensitivity to estrogens stimulating nuclear-localized genomic mechanisms. Previous work in female postnatal day 10-12 forebrain has shown robust mRNA expression of ERα and dense estradiol binding in the caudate-putamen that has not been found in adults (Toran-Allerand, Miranda, Hochberg, & MacLusky, 1992), supporting our findings that pre-pubertal estrogen receptor expression is higher than adulthood. We did not detect any ERα-IR signal characteristic of somatic or nuclear expression in adulthood, which is consistent with previous reports that only detected somatic expression using viral overproduction techniques (Schultz et al., 2009) and studies which employed electron microscopy to detect ER expression on neuronal membranes and in non-nuclear cytoplasm (Almey et al., 2012; Almey et al., 2015, 2016). These papers definitively show that the ERα expressed in adult striatum is extranuclear. In our current study, we did not employ techniques with sufficient sensitivity to detect extranuclear membrane expression. Therefore, we cannot make definitive conclusions about sex or age differences in extranuclear expression. Future studies that utilize techniques such as RNA scope may aid in understanding how overall expression of ERα (as well as ERβ) mRNA changes across development in a sex dependent manner. Other future studies could target other aspects of protein concentration or function, perhaps using western blot or receptor binding techniques. Another future study could test a hypothesis directly predicted by the current study, i.e., which estrogen receptors are necessary for masculinization and feminization of striatal neurons, as previous studies have shown not only sex differences prior to puberty in striatal neuron function but also that estradiol is necessary for this sexual differentiation (Cao et al., 2016; Dorris, Cao, Willett, Hauser, & Meitzen, 2015). The presence of these sex differences are dependent on striatal brain region (present in the nucleus accumbens core and caudate-putamen but not nucleus accumbens shell (J. A. Willett et al., 2016)) so a region-specific approach will need to be utilized.
We also observed multicompartmental GPER1 expression across development. Perinatally, GPER1 expression appeared nuclear. Although GPER1 is a g-protein coupled receptor with a seven transmembrane domain (Srivastava & Evans, 2013; Waters et al., 2015), nuclear expression has been noted previously such as in cancer cells (Lappano & Maggiolini, 2018). To our knowledge, this is the first report of a nuclear-like expression of GPER1 in neural cells. By pre-puberty, the expression pattern of GPER1 changed, exhibiting comprehensive somal expression, both nuclear and extranuclear. In adulthood, GPER1 reached its canonical expression pattern of a filled soma and empty nucleus. This may suggest that as neurons mature, GPER1 cellular compartmentalization is also changing as well and increasingly placed into extranuclear membranes. Less cells overall expressed GPER1 in adulthood across the striatal brain regions. This is consistent with previous work in zebra finches that showed that GPER1 mRNA expression and immunoreactive-cells peak during adolescence and then decrease in adulthood in song-control nuclei (Acharya & Veney, 2012). This age dependent change was not consistent in all brain regions such as the cingulate cortex which was used as a non-striatal positive control for GPER1 expression (Almey et al., 2014). At all analyzed stages, we did not detect sex differences in GPER1 expression patterns or quantification per region. This does not indicate that sex is unimportant for GPER1 function. This study did not analyze overall protein expression, nor does it rule out sex-specific functional actions of GPER1. Previous work has shown that GPER1 can mediate sex-specific estradiol signaling (Oberlander & Woolley, 2016) even when cellular expression is similar (Krentzel, Macedo-Lima, Ikeda, & Remage-Healey, 2018).
This study finds that aromatase is expressed in the cytoplasm of somas of the striatal cells and that aromatase-IR cells increase with age in both sexes. Early work demonstrated that aromatase mRNA and protein exist in the striatum (Horvath et al., 1997; Jakab et al., 1993; Wagner & Morrell, 1996) and there is select functional evidence for neuroestrogen synthesis in the striatum (Bessa et al., 2015; McArthur et al., 2007; Morissette, Garcia-Segura, Belanger, & Di Paolo, 1992; Tozzi et al., 2015). Little attention has been given to quantifying aromatase expression across sex and multiple ages. Our study points to an increased capacity for striatal neurons to synthesize neuroestrogens in adulthood as compared to just after birth. One limitation of this study is that we did not definitively confirm the cells expressing aromatase are neurons, although thorough analysis across vertebrates including rodents has continually showed that aromatase is constitutively expressed in neurons and expression in glia occurs after injury to the brain (Duncan & Saldanha, 2019; Garcia-Segura et al., 1999). Aromatase also can be expressed in synaptic terminals (Saldanha, Schlinger, Micevych, & Horvath, 2004; Saldanha et al., 2000; Yague et al., 2006) which the visualization technique employed here is not sensitive enough to detect, so it cannot be ruled out that in early life the striatum contains terminal aromatase. Measuring aromatase activity and identifying which neuron types and cellular compartments express aromatase are likewise important future directions. These future analyses may also provide evidence for sex difference in striatal neuroestrogen production as similarity in somatic expression of aromatase between the sexes can still produce sex differences in activity of the enzyme (R. S. Peterson, Yarram, Schlinger, & Saldanha, 2005; Rohmann, Schlinger, & Saldanha, 2007).
Limitations to this study
This study by necessity encompassed several limitations. First, due to the lack of an available antibody, we were not able to assess whether ERβ also follows a similar developmental expression pattern (Snyder, Smejkalova, Forlano, & Woolley, 2010). Second, protein quantity was not directly assessed. This is because optical density measurements could not be made because of the autofluorescence associated with the presence of erythrocytes in blood vessels. Instead we performed a cell density analysis, which eliminates any possible influence of autofluorescence from nontargets. Assessing cell density coupled with the use of a drop fix technique, enabled developmental assessment of the target proteins in a cell-specific manner, including in neonate animals. The use of drop-fixation does encompass several salient limitations, although we acknowledge that all experimental techniques exhibit limitations. Specific to drop fix, this technique does not clear blood vessels, resulting in the blood vessel staining noted above. Binding of IgGs in blood by secondary antisera could well account for blood vessel staining. As can be seen in the images displayed, erythrocytes were stained by the secondary antibody. To account for this artifact, we employed strict criteria for what was considered a positive cell for all the antibodies utilized in this study to assure that overcounting of erythrocytes was not included in the analysis. Third, some groups had lower subject numbers (n=3) so statistical analyses were limited to larger effect sizes only. While many of the compartmental changes observed are discrete phenotypes across age, this does mean that smaller cell density differences between ages and regions may have been missed. Related to this point, it is possible that the drop fix technique could present issues in variability of fixation due to brain size. We do not believe that this particular limitation influenced experimental conclusions, as different patterns of staining were detected across development between striatal brain regions from the same brains, and also across the three targets. Fourth, we only quantified expression levels of the three targets using immunofluorescence of proteins. Changes in mRNA expression could validate overall expression of striatal cells. Given that a large component of the differences we observed are driven by cellular compartmentalization of the final protein expression, most mRNA assessment methods alone would not be appropriate for assessing those compartment changes. Future work to validate overall expression patterns however will need to be employed to determine whether cell density differences are due to either or both expression regulation and/or protein compartmentalization.
Hypothethical models for striatal estrogen signaling through development
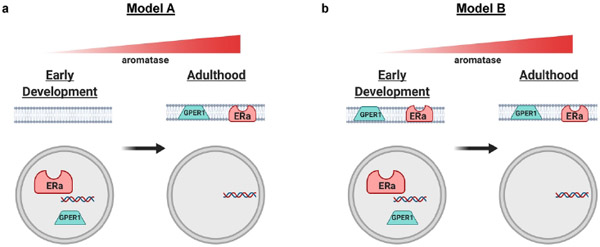
Interestingly, as aromatase expression rises, nuclear estrogen receptor localization decreases. This transition away from nuclear localization is a potential opportunity for elucidating fundamental mechanisms of estrogen receptor localization. Importantly, the current study was not designed to determine if extranuclear estrogen receptor expression is present in this system. Indeed, extranuclear estrogen receptors may or may not be present just after birth or prepuberty in the striatum. This leads to two possible models regarding the developmental transition away from nuclear estrogen receptors. (Figure 9). The first model (Figure 9A) posits that striatal neurons express both nuclear and extranuclear ERα and GPER1 just after birth and that the transition into adulthood to exclusively extranuclear expression is accomplished by the disappearance of nuclear estrogen receptors. This disappearance would be mediated via augmentation of post-translational modifications that favor extranuclear expression, as the same genes encode both membrane-associated and nuclear localized ERα and GPER1. The second model (Figure 9B) posits that striatal neurons exhibit exclusively nuclear expression of ERα and GPER1 just after birth. In this case, the transition to exclusively extranuclear expression in adulthood is mediated by turning on a developmental switch inducing post-translational modification mechanisms that favor membrane expression. One potential way to test these hypotheses would be to perform differential centrifugation to isolate cellular compartments and measure protein expression of ERα and GPER1 across development and sex (Tabatadze, Smejkalova, & Woolley, 2013).
Figure 9: Hypothetical models for estrogen receptor compartmentalization across development in the striatum.
These depict two hypotheses of how estrogen receptors ERα and GPER1 may exist in neuron cellular compartments from early development. Both models are based on this study’s current findings of transition from nuclear compartment to extranuclear compartments. Both models show a rise of aromatase expression with age. a) Model A depicts only nuclear expression of ERα and GPER1 in early development that switches to exclusively extranuclear expression by adulthood. b) Model B depicts both nuclear and extranuclear expression of ERα and GPER1 in early development, with nuclear expression disappearing by adulthood. Figure made using Biorender.
The most prominent and well investigated of these possible posttranslational modifications is palmitoylation, especially in the case of ERα and ERβ. Palmitoylation is a posttranscriptional process in which a palmityl group is added to specific target amino acid sequences expressed by a protein, in this case, an ERα. Palmitoylation controls the trafficking, cellular compartment localization, and interaction of ERα with other proteins via association with membrane lipid rafts and similar structures such as caveolins (Adlanmerini et al., 2014; Anbalagan, Huderson, Murphy, & Rowan, 2012; Anderson & Ragan, 2016; Hohoff et al., 2019; Lathe & Houston, 2018; Meitzen, Britson, Tuomela, & Mermelstein, 2019; Meitzen, Luoma, et al., 2013a; Pedram, Razandi, Deschenes, & Levin, 2012; Tonn Eisinger et al., 2018). In the striatum and hippocampus, palmitoylated ERα trigger signal transduction cascades from the neuronal plasma membrane through, at a minimum, activation of metabotropic glutamate receptors (mGluRs) (Boulware et al., 2005; Grove-Strawser, Boulware, & Mermelstein, 2010b; Miller, Krentzel, Patisaul, & Meitzen, 2019; B. M. Peterson, Mermelstein, & Meisel, 2015b; Song, Yang, Peckham, & Becker, 2019). Palmitoylation may or may not be relevant to GPER1. GPER1 is itself a G-protein coupled receptor (unlike ERα) and features seven transmembrane domains which allow embedding into phospholipid bilayers, such as the plasma membrane and endoplasmic reticulum, without extensive posttranscriptional modification. However, palmitoylation may be relevant for internal trafficking of GPER1 within the cell, and many G-protein coupled receptors are palmitoylated (Goddard & Watts, 2012). However, the literature regarding GPER1 and palmitoylation is limited.
Future investigations into the underlying mechanisms of this transition to test whether the posttranscriptional modification palmitoylation is necessary for transitioning ERα and GPER1 away from nuclear signaling in striatal neurons and if palmitoylation enzymes are upregulated during this transition phase in striatal neurons. Palmitoylation occurs via the activity of DHHC palmitoylacyltransferase enzymes. The specific palmitoylacyltransferase proteins DHHC-7 and −21 have been shown in vitro/ex vivo to transfer ERα from the nucleus to the membrane in hippocampal neurons (Meitzen et al., 2019; Meitzen, Luoma, et al., 2013b). One future direction is to block DHHC-7 and −21 to determine if this manipulation extends the window of nuclear estrogen receptor expression. We also observed a sex-specific time course for ERα nuclear expression where females showed more ERα-IR cells as well as this nuclear expression persisted until P20 which did not in males. It is possible that sex differences in select DHHC or caveolin gene expression may underlie these differences, similar to the hippocampal regions (Hohoff et al., 2019; Meitzen et al., 2019; Meitzen, Luoma, et al., 2013b). Quantifying DHHC expression and activity across development will be necessary to test this hypothesis further. Striatal neurons are also known to be sensitive to perinatal aromatization (Bonansco et al., 2018; Cao et al., 2016), and sensitive to fluctuations in sex steroid hormones in adulthood (Krentzel, Barrett, & Meitzen, 2019; Meitzen et al., 2018; Proano, Morris, Kunz, Dorris, & Meitzen, 2018; Jaime A. Willett et al., 2019; Wissman, May, & Woolley, 2012), though not in gross anatomical attributes (Meitzen et al., 2011; Wong, Cao, Dorris, & Meitzen, 2016). Aromatization of testosterone to estradiol early in life may also play a role in males, though this question requires further exploration.
One potential hypothesis for the changes in estrogen receptor compartmentalization of striatal neurons is that in early development, striatal neurons are preferentially sensitive to estrogenic signals instructing organizational mechanisms via genomic signaling. Thus, the presence of nuclear ERα early in life provides a potential mechanism for organizational effects of hormones and sex differences on striatal neuron physiology that have been previously reported (Cao et al., 2016). Nuclear ERα expression does not exclusively organize sexual differentiation of the brain as a role for membrane or extranuclear ERα has also been described (Khbouz et al.). It is unclear what the role of GPER1 in the nucleus may have in cell-signaling. Once in adulthood, the phenotype of estrogen receptors changes to extranuclear expression, providing the machinery for rapid, neuromodulatory estradiol signaling. Thus, the function of estradiol in the striatum may change across development. Interestingly, adults demonstrate the most robust aromatase expression in the striatum as well, demonstrating the capacity for striatal neurons to rapidly and dynamically produce their own estrogens. This relationship between aromatase and extranuclear estrogen receptor expression is similar to other brain areas exhibiting known rapid estradiol signaling such as the hippocampus of rodents (Kim, Szinte, Boulware, & Frick, 2016; Oberlander & Woolley, 2016; Sato & Woolley, 2016; Tabatadze et al., 2013; Tuscher et al., 2016) and the auditory forebrain of songbirds (Ikeda et al., 2017; Krentzel, Ikeda, Oliver, Koroveshi, & Remage-Healey, 2019; Krentzel et al., 2018; Remage-Healey, Dong, Maidment, & Schlinger, 2011; Remage-Healey, Dong, Chao, & Schlinger, 2012; Saldanha et al., 2000). Overall, the pattern of increased estrogen-producing cells and a change in the phenotype of estrogen-sensitive cells suggests that the striatum transforms in adulthood into a region targeted by rapid, neuromodulatory mechanisms of estrogenic signaling (Krentzel, Barrett, et al., 2019; Meitzen et al., 2018; Yoest, Quigley, & Becker, 2018).
Acknowledgements:
We thank Abcam for releasing information regarding the GPER1 antibody, and are especially grateful to Drs. Panthea Taghavi and Alex de Verteuil for their respective roles in this process. Additionally, Drs. Kevin Sinchak and Deepak Srivastava provided valuable advice regarding GPER1 immunofluorescence protocols, as did Dr. Heather Patisaul for the ERα protocol. We also thank Brian Horman for confocal training, and Kiersten Kronschnabel, Natalie Truby, and David Dorris for technical assistance.
ii. Funding Statement: This work was supported by the following funding sources: National Institutes of Health (NIH) Grants R01 MH-109471 (J.M.) and P30 ES-025128 (Center for Human Health and the Environment), and NC State University Provost Professional Experience Program and Start-up Funds.
Footnotes
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent: N/A
Permission to reproduce material from other sources: N/A
References
- Acharya KD, & Veney SL (2012). Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol, 72(11), 1433–1446. doi: 10.1002/dneu.22004 [DOI] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, … Lenfant F (2014). Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A, 111(2), E283–290. doi: 10.1073/pnas.1322057111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal VR, Sinton CM, Liang C, Fisher C, German DC, & Simpson ER (2000). Upregulation of estrogen receptors in the forebrain of aromatase knockout (ArKO) mice. Mol Cell Endocrinol, 162(1-2), 9–16. doi: 10.1016/s0303-7207(00)00227-6 [DOI] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, & Brake WG (2014). Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology, 155(11), 4422–4432. doi: 10.1210/en.2014-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, & Brake WG (2012). Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology, 153(11), 5373–5383. doi: 10.1210/en.2012-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, & Brake WG (2015). Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav, 74, 125–138. doi: 10.1016/j.yhbeh.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, & Brake WG (2016). Estrogen receptor alpha and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci Lett, 622, 118–123. doi: 10.1016/j.neulet.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbalagan M, Huderson B, Murphy L, & Rowan BG (2012). Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal, 10, e001. doi: 10.1621/nrs.10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, & Ragan MA (2016). Palmitoylation: a protein S-acylation with implications for breast cancer. NPJ Breast Cancer, 2, 16028. doi: 10.1038/npjbcancer.2016.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Choleris E, & Remage-Healey L (2018). Steroids and the brain: 50years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm Behav, 99, 1–8. doi: 10.1016/j.yhbeh.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Hu M (2008). Sex differences in drug abuse. Front Neuroendocrinol, 29(1), 36–47. doi: 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa A, Campos FL, Videira RA, Mendes-Oliveira J, Bessa-Neto D, & Baltazar G (2015). GPER: A new tool to protect dopaminergic neurons? Biochim Biophys Acta, 1852(10 Pt A), 2035–2041. doi: 10.1016/j.bbadis.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Bonansco C, Martinez-Pinto J, Silva RA, Velasquez VB, Martorell A, Selva MV, … Sotomayor-Zarate R (2018). Neonatal exposure to oestradiol increases dopaminergic transmission in nucleus accumbens and morphine-induced conditioned place preference in adult female rats. J Neuroendocrinol, 30(7), e12574. doi: 10.1111/jne.12574 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, & Mermelstein PG (2005). Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci., 25(20), 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, & Rance NE (2006). Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol, 498(5), 712–726. doi: 10.1002/cne.21086 [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, … Nestler EJ (2017). Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun, 8, 13877. doi: 10.1038/ncomms13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dorris DM, & Meitzen J (2016). Neonatal Masculinization Blocks Increased Excitatory Synaptic Input in Female Rat Nucleus Accumbens Core. Endocrinology, 157(8), 3181–3196. doi: 10.1210/en.2016-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Willett JA, Dorris DM, & Meitzen J (2018). Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation. Front Endocrinol (Lausanne), 9, 173. doi: 10.3389/fendo.2018.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro B, Sanchez P, Torres JM, & Ortega E (2013). Effects of adult exposure to bisphenol a on genes involved in the physiopathology of rat prefrontal cortex. PLoS One, 8(9), e73584. doi: 10.1371/journal.pone.0073584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, & Gore AC (2003). Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol, 466(3), 409–421. doi: 10.1002/cne.10906 [DOI] [PubMed] [Google Scholar]
- Di Paolo T (1994). Modulation of brain dopamine transmission by sex steroids. Rev Neurosci, 5(1), 27–41. doi: 10.1515/revneuro.1994.5.1.27 [DOI] [PubMed] [Google Scholar]
- Dorris DM, Cao J, Willett JA, Hauser CA, & Meitzen J (2015). Intrinsic excitability varies by sex in prepubertal striatal medium spiny neurons. J Neurophysiol, 113(3), 720–729. doi: 10.1152/jn.00687.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KA, & Saldanha CJ (2019). Central aromatization: a dramatic and responsive defense against threat and trauma to the vertebrate brain. Front Neuroendocrinol, 100816. doi: 10.1016/j.yfrne.2019.100816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, & Thomas P (2012). Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology, 153(7), 2953–2962. doi: 10.1210/en.2012-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A, Tlemcani O, Harada N, Abe-Dohmae S, & Balthazart J (1995). Pre- and post-translational regulation of aromatase by steroidal and non-steroidal aromatase inhibitors. Brain Res, 701(1-2), 267–278. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, & Hutchison JB (1999). Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience, 89(2), 567–578. doi: 10.1016/s0306-4522(98)00340-6 [DOI] [PubMed] [Google Scholar]
- Goddard AD, & Watts A (2012). Regulation of G protein-coupled receptors by palmitoylation and cholesterol. BMC Biol, 10, 27. doi: 10.1186/1741-7007-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi D, Ghorbanpoor S, Acaz-Fonseca E, Ruiz-Palmero I, & Garcia-Segura LM (2015). The Selective Estrogen Receptor Modulator Raloxifene Regulates Arginine-Vasopressin Gene Expression in Human Female Neuroblastoma Cells Through G Protein-Coupled Estrogen Receptor and ERK Signaling. Endocrinology, 156(10), 3706–3716. doi: 10.1210/en.2014-2010 [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, & Mermelstein PG (2010a). Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience, 170(4), 1045–1055. doi: 10.1016/j.neuroscience.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, & Mermelstein PG (2010b). Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience, 170, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohoff C, Zhang M, Ambree O, Kravchenko M, Buschert J, Kerkenberg N, … Zhang W (2019). Deficiency of the palmitoyl acyltransferase ZDHHC7 impacts brain and behavior of mice in a sex-specific manner. Brain Struct Funct, 224(6), 2213–2230. doi: 10.1007/s00429-019-01898-6 [DOI] [PubMed] [Google Scholar]
- Horvath TL, Roa-Pena L, Jakab RL, Simpson ER, & Naftolin F (1997). Aromatase in axonal processes of early postnatal hypothalamic and limbic areas including the cingulate cortex. J Steroid Biochem Mol Biol, 61(3-6), 349–357. [PubMed] [Google Scholar]
- Ikeda MZ, Krentzel AA, Oliver TJ, Scarpa GB, & Remage-Healey L (2017). Clustered organization and region-specific identities of estrogen-producing neurons in the forebrain of Zebra Finches (Taeniopygia guttata). J Comp Neurol. doi: 10.1002/cne.24292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Horvath TL, Leranth C, Harada N, & Naftolin F (1993). Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive "limbic ring" of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol, 44(4-6), 481–498. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, & Emson PC (1989). Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol, 62(5), 1052–1068. doi: 10.1152/jn.1989.62.5.1052 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, & Emson PC (1990). Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci, 10(10), 3421–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khbouz B, de Bournonville C, Court L, Taziaux M, Corona R, Arnal J-F, … Cornil CA Role for the Membrane Estrogen Receptor alpha in the sexual differentiation of the brain. European Journal of Neuroscience, n/a(n/a). doi: 10.1111/ejn.14646 [DOI] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, & Frick KM (2016). 17beta-Estradiol and Agonism of G-protein-Coupled Estrogen Receptor Enhance Hippocampal Memory via Different Cell-Signaling Mechanisms. J Neurosci, 36(11), 3309–3321. doi: 10.1523/JNEUROSCI.0257-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenke U, Constantin S, & Wray S (2016). BPA Directly Decreases GnRH Neuronal Activity via Noncanonical Pathway. Endocrinology, 157(5), 1980–1990. doi: 10.1210/en.2015-1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Barrett LR, & Meitzen J (2019). Estradiol rapidly modulates excitatory synapse properties in a sex and region-specific manner in rat nucleus accumbens core and caudate-putamen. J Neurophysiol. doi: 10.1152/jn.00264.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Ikeda MZ, Oliver TJ, Koroveshi E, & Remage-Healey L (2019). Acute neuroestrogen blockade attenuates song-induced immediate early gene expression in auditory regions of male and female zebra finches. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. doi: 10.1007/s00359-019-01382-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Macedo-Lima M, Ikeda MZ, & Remage-Healey L (2018). A membrane g-protein coupled estrogen receptor is necessary but not sufficient for sex-differences in zebra finch auditory coding. Endocrinology. doi: 10.1210/en.2017-03102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, & Meitzen J (2018). Biological Sex, Estradiol and Striatal Medium Spiny Neuron Physiology: A Mini-Review. Front Cell Neurosci, 12, 492. doi: 10.3389/fncel.2018.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappano R, & Maggiolini M (2018). GPER is involved in the functional liaison between breast tumor cells and cancer-associated fibroblasts (CAFs). J Steroid Biochem Mol Biol, 176, 49–56. doi: 10.1016/j.jsbmb.2017.02.019 [DOI] [PubMed] [Google Scholar]
- Lathe R, & Houston DR (2018). Fatty-acylation target sequence in the ligand-binding domain of vertebrate steroid receptors demarcates evolution from estrogen-related receptors. J Steroid Biochem Mol Biol, 184, 20–28. doi: 10.1016/j.jsbmb.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, & Di Paolo T (2006). ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology, 50(4), 451–457. doi: 10.1016/j.neuropharm.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Lorsch ZS, Loh YE, Purushothaman I, Walker DM, Parise EM, Salery M, … Nestler EJ (2018). Estrogen receptor alpha drives pro-resilient transcription in mouse models of depression. Nat Commun, 9(1), 1116. doi: 10.1038/s41467-018-03567-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Amleh A, Sun J, Jin X, McCullough SD, Baer R, … Hu Y (2007). Ubiquitination and Proteasome-Mediated Degradation of BRCA1 and BARD1 during Steroidogenesis in Human Ovarian Granulosa Cells. Molecular Endocrinology, 21(3), 651–663. doi: 10.1210/me.2006-0188 [DOI] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Alejandro MA, Crowley J, & Markaverich BM (2005). Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids, 70(11), 750–754. doi: 10.1016/j.steroids.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, … Faith R (2002). A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect, 110(2), 169–177. doi: 10.1289/ehp.02110169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur S, Murray HE, Dhankot A, Dexter DT, & Gillies GE (2007). Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem, 100(3), 678–692. doi: 10.1111/j.1471-4159.2006.04226.x [DOI] [PubMed] [Google Scholar]
- McCarthy MM, & Arnold AP (2011). Reframing sexual differentiation of the brain. Nat Neurosci, 14(6), 677–683. doi: 10.1038/nn.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KM, Stratton MS, & Tobet SA (2010). Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. J Comp Neurol, 518(14), 2710–2728. doi: 10.1002/cne.22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Britson KA, Tuomela K, & Mermelstein PG (2019). The expression of select genes necessary for membrane-associated estrogen receptor signaling differ by sex in adult rat hippocampus. Steroids, 142, 21–27. doi: 10.1016/j.steroids.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, … Mermelstein PG (2013a). Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology, 154, 4293–4304. doi: 10.1210/en.2013-1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, … Mermelstein PG (2013b). Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology, 154(11), 4293–4304. doi: 10.1210/en.2013-1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Meisel RL, & Mermelstein PG (2018). Sex Differences and the Effects of Estradiol on Striatal Function. Curr Opin Behav Sci, 23, 42–48. doi: 10.1016/j.cobeha.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, & Mermelstein PG (2011). Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat, 42(4), 236–241. doi: 10.1016/j.jchemneu.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Perry AN, Westenbroek C, Hedges VL, Becker JB, & Mermelstein PG (2013). Enhanced striatal beta1-adrenergic receptor expression following hormone loss in adulthood is programmed by both early sexual differentiation and puberty: a study of humans and rats. Endocrinology, 154(5), 1820–1831. doi: 10.1210/en.2012-2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Pflepsen KR, Stern CM, Meisel RL, & Mermelstein PG (2011). Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: differences between striatal region and brain hemisphere, but not sex. Neurosci Lett, 487(2), 177–181. doi: 10.1016/j.neulet.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, & Surmeier DJ (1996). Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci, 16(2), 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CK, Krentzel AA, Patisaul HB, & Meitzen J (2019). Metabotropic glutamate receptor subtype 5 (mGlu5) is necessary for estradiol mitigation of light-induced anxiety behavior in female rats. Physiol Behav, 112770. doi: 10.1016/j.physbeh.2019.112770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Garcia-Segura LM, Belanger A, & Di Paolo T (1992). Changes of rat striatal neuronal membrane morphology and steroid content during the estrous cycle. Neuroscience, 49(4), 893–902. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, & Woolley CS (2016). 17beta-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. J Neurosci, 36(9), 2677–2690. doi: 10.1523/JNEUROSCI.4437-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Deschenes RJ, & Levin ER (2012). DHHC-7 and −21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell, 23(1), 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, & Meisel RL (2015a). Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct, 220(4), 2415–2422. doi: 10.1007/s00429-014-0794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, & Meisel RL (2015b). Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct, 220, 2415–2422. doi: 10.1007/s00429-014-0794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, & Saldanha CJ (2005). Aromatase is presynaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci, 272(1576), 2089–2096. doi: 10.1098/rspb.2005.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, & Keiner M (1973). Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol, 151(2), 121–158. doi: 10.1002/cne.901510204 [DOI] [PubMed] [Google Scholar]
- Proano S, Morris HJ, Kunz LM, Dorris DM, & Meitzen J (2018). Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol, 120(3), 1356–1373 doi: 10.1152/jn.00263.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, & Schlinger BA (2011). Presynaptic Control of Rapid Estrogen Fluctuations in the Songbird Auditory Forebrain. Journal of Neuroscience, 31(27), 10034–10038. doi: 10.1523/jneurosci.0566-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, & Schlinger BA (2012). Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol, 107(6), 1621–1631. doi: 10.1152/jn.00749.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, & Saldanha CJ (2007). Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol, 67(1), 1–9. doi: 10.1002/dneu.20303 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, & Klosterman SA (1998). Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod, 58(1), 79–87. doi: 10.1095/biolreprod58.1.79 [DOI] [PubMed] [Google Scholar]
- Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, & Micevych PE (2016). Actions of Steroids: New Neurotransmitters. J Neurosci, 36(45), 11449–11458. doi: 10.1523/JNEUROSCI.2473-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, & Horvath TL (2004). Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J Comp Neurol, 469(4), 522–534. doi: 10.1002/cne.11035 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, & Schlinger BA (2000). Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol, 423(4), 619–630. [DOI] [PubMed] [Google Scholar]
- Sato SM, & Woolley CS (2016). Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. Elife, 5. doi: 10.7554/eLife.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, … Becker JB (2009). Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci, 29(6), 1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Smejkalova T, Forlano PM, & Woolley CS (2010). Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods, 188(2), 226–234. doi: 10.1016/j.jneumeth.2010.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Yang H, Peckham EM, & Becker JB (2019). Estradiol-Induced Potentiation of Dopamine Release in Dorsal Striatum Following Amphetamine Administration Requires Estradiol Receptors and mGlu5. eNeuro, 6(1). doi: 10.1523/eneuro.0446-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, & Evans PD (2013). G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol, 25(11), 1219–1230. doi: 10.1111/jne.12071 [DOI] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, & Meisel RL (2011). Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct, 215(3-4), 187–194. doi: 10.1007/s00429-010-0284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, & Boon WC (2014). Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors alpha and beta, and androgen receptors. PLoS One, 9(3), e90451. doi: 10.1371/journal.pone.0090451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Smejkalova T, & Woolley CS (2013). Distribution and posttranslational modification of synaptic ERalpha in the adult female rat hippocampus. Endocrinology, 154(2), 819–830. doi: 10.1210/en.2012-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Woolfrey KM, Swanson SP, Schnell SA, Meitzen J, Dell'Acqua M, & Mermelstein PG (2018). Palmitoylation of caveolin-1 is regulated by the same DHHC acyltransferases that modify steroid hormone receptors. J Biol Chem, 293(41), 15901–15911. doi: 10.1074/jbc.RA118.004167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Miranda RC, Hochberg RB, & MacLusky NJ (1992). Cellular variations in estrogen receptor mRNA translation in the developing brain: evidence from combined [125I]estrogen autoradiography and non-isotopic in situ hybridization histochemistry. Brain Res, 576(1), 25–41. doi: 10.1016/0006-8993(92)90606-a [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampa C, … Calabresi P (2015). Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci, 9, 192. doi: 10.3389/fncel.2015.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, … Saunders PT (2002). Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol, 172(1), 21–30. doi: 10.1677/joe.0.1720021 [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, & Frick KM (2016). Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav, 83, 60–67. doi: 10.1016/j.yhbeh.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, & Trainor BC (2012). Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-alpha expression in the brain. Endocrinology, 153(2), 949–953. doi: 10.1210/en.2011-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, & Morrell JI (1996). Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol, 370(1), 71–84. doi: [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, & Kuhn CM (2006). Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology, 31(6), 1193–1202. doi: 10.1038/sj.npp.1300915 [DOI] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, … Milner TA (2015). G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci, 35(6), 2384–2397. doi: 10.1523/JNEUROSCI.1298-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JA, Cao J, Johnson A, Patel OH, Dorris DM, & Meitzen J (2019). The estrous cycle modulates rat caudate-putamen medium spiny neuron physiology. European Journal of Neuroscience. doi: 10.1111/ejn.14506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JA, Will T, Hauser CA, Dorris DM, Cao J, & Meitzen J (2016). No Evidence for Sex Differences in the Electrophysiological Properties and Excitatory Synaptic Input onto Nucleus Accumbens Shell Medium Spiny Neurons. eNeuro, 3(1). doi: 10.1523/ENEURO.0147-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, May RM, & Woolley CS (2012). Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct, 217(2), 181–190. doi: 10.1007/s00429-011-0353-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JE, Cao J, Dorris DM, & Meitzen J (2016). Genetic sex and the volumes of the caudate-putamen, nucleus accumbens core and shell: original data and a review. Brain Struct Funct, 221(8), 4257–4267. doi: 10.1007/s00429-015-1158-9 [DOI] [PubMed] [Google Scholar]
- Woolley CS (2007). Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol, 47, 657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219 [DOI] [PubMed] [Google Scholar]
- Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, & Azcoitia I (2006). Aromatase expression in the human temporal cortex. Neuroscience, 138(2), 389–401. doi: 10.1016/j.neuroscience.2005.11.054 [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, & Becker JB (2014). Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem, 14(2), 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, & Becker JB (2018). Estradiol influences on dopamine release from the nucleus accumbens shell: Sex differences and the role of selective estradiol receptor subtypes. Br J Pharmacol. doi: 10.1111/bph.14531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, & Becker JB (2018). Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav. doi: 10.1016/j.yhbeh.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao X, Zhang XM, Zhao ZQ, & Zhang YQ (2012). Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: implications for pain hypersensitivity. J Biol Chem, 287(40), 33268–33281. doi: 10.1074/jbc.M112.368142 [DOI] [PMC free article] [PubMed] [Google Scholar]