Abstract
Alcohol use increases non-adherence to antiretroviral therapy (ART) among persons living with HIV (PLWH). Dynamic longitudinal associations are understudied. Veterans Aging Cohort Study (VACS) data 2/1/2008–7/31/16 were used to fit linear regression models estimating changes in adherence (% days with ART medication fill) associated with changes in alcohol use based on annual clinically-ascertained AUDIT-C screening scores (range −12 to +12, 0=no change) adjusting for demographics and initial adherence. Among 21,275 PLWH (67,330 observations), most reported no (48%) or low-level (39%) alcohol use initially, with no (55%) or small (39% ≤3 points) annual change. Mean initial adherence was 86% (SD 21%), mean annual change was −3.1% (SD 21%). An inverted V-shaped association was observed: both increases and decreases in AUDIT-C were associated with greater adherence decreases relative to stable scores (p<0.001, F (4, 21274)). PLWH with dynamic alcohol use (potentially indicative of alcohol use disorder) should be considered for adherence interventions.
Keywords: HIV, ART, antiretroviral therapy, adherence, alcohol use
RESUMEN
El consumo de alcohol aumenta el no-cumplimiento a la terapia antirretroviral (TARV) entre las personas que viven con VIH. No se han estudiado lo suficiente las dinámicas asociaciones longitudinales. Los datos del Estudio de la Envejecimiento de Cohorte de Veteranos (EECV) (1/2/2008–31/7/2016) fueron usados para encajar modelos de regresión lineal estimando los cambios en cumplimiento (% de días con medicaciones TARV surtidas) asociados con los cambios en el consumo de alcohol basado en los resultados anuales de las evaluaciones AUDIT-C, determinadas clínicamente, (una gama de −12 a +12, 0=cero cambio) adaptándose a las estadísticas demográficas y cumplimiento inicial. Entre 21,275 personas que viven con VIH (67,330 observaciones), la mayoría reportó ningún (48%) o bajos niveles del (39%) consumo de alcohol inicialmente, con ningún (55%) o muy pequeño (39% ≤ 3 puntos) cambio anual. la media inicial de cumplimiento fue 86% (DE 21%). La media de cambio anual fue −3.1% (DE 21%). Se observó una asociación de forma V invertida: tanto los aumentos como las disminuciones en AUDIT-C fueron asociados con mayor disminuciones de cumplimiento en comparación con resultados estables (p<0.001, F (4, 21274)). Personas que viven con VIH con el consumo dinámico de alcohol (potencialmente indicativo de un trastorno por consumo de alcohol) deben ser considerados por intervenciones de cumplimiento.
INTRODUCTION
The goal of antiretroviral treatment (ART) for human immunodeficiency virus (HIV) is to reduce HIV viral load to undetectable levels (i.e., viral suppression) and to prevent transmission of the virus (1). Although the imperative for 100% patient adherence to ART has decreased over time, a high level of adherence to ART is necessary for therapy to be effective (2–4). Poor ART adherence among persons living with HIV (PLWH) can result in disease progression (5, 6), transmission to others (7), and increased mortality (8, 9). A meta-analysis of 25 studies estimates that approximately 40% of PLWH in North America do not take the majority of their prescribed ART (10), highlighting the need to understand modifiable risk factors for nonadherence.
Alcohol use is common among PLWH and is a key modifiable contributor to multiple adverse HIV-related outcomes, including poor ART adherence (5). There is a strong cross-sectional dose-response relationship between alcohol use and ART adherence such that greater levels of alcohol use are associated with progressively worse adherence (11, 12) and lower rates of viral suppression (13, 14). Adherence to ART has been hypothesized to result from information about adherence (e.g., regimen, recommended use, side effects/interactions) and personal and social motivations for adherence, which work together to influence a variety of skills related to integrating ART into daily living (15). These skills are likely to be moderated by alcohol use (15). The dose-response relationship between alcohol use and ART adherence is specifically hypothesized to result from two mechanisms: 1) forgotten doses as a result of intoxication, and/or 2) intentionally skipping doses due to concern about the toxicity of alcohol-medication interactions (5, 16–20). The relationship of alcohol use to adherence may also be influenced by other factors associated with both, such as psychological processes (e.g., low health-related motivation and/or self-efficacy and depression (21, 22) and social factors (e.g., unstable housing, access to medical care; (15). Consistent with these hypotheses, a prior study of the temporal association between self-reported alcohol use and adherence over a 30-day window found that, among PLWH who use alcohol, doses were missed less frequently on non-drinking days compared to drinking days (23). If, indeed, these mechanisms account for the dose-response association, it would be reasonable to expect that changes in average alcohol use over longer periods of time could result in corresponding changes in ART adherence.
However, the literature examining these important relationships is very limited, with only two studies evaluating the association between changes in drinking and changes in adherence to ART in recruited samples (24, 25), and another evaluating changes in substance use generally with changes in ART adherence (26). No studies to our knowledge have looked longitudinally to evaluate whether individual-level incremental changes in alcohol use over time (in both directions) are associated with individual-level incremental changes in adherence in large, generalizable samples of PLWH who have not been recruited into studies. This additional work should be guided by previous findings (24), which suggest that relationships between changes in alcohol use and changes in ART adherence may vary depending on level of alcohol use and on HIV-specific treatment and health-related factors, including initial level of ART adherence and/or whether HIV viral load is detectable (24). Understanding these patterns can inform targeted interventions to improve ART adherence.
Use of Veterans Health Administration (VA) electronic health record (EHR) data enables detailed investigation of these issues. The VA is the nation’s largest provider of HIV care (27, 28), and has a performance measure requiring annual alcohol screening of all outpatients (29) using the validated Alcohol Use Disorders Identification Test Consumption (AUDIT-C) questionnaire (30, 31), which yields scores that correlate well with average alcohol consumption (32). Further, nearly all (96%−98%) PLWH receiving VA care report receiving ART through VA’s pharmacy (33), enabling ascertainment of ART adherence with VA pharmacy data, which captures all medications received by all VA patients (34, 35) with strong reliability for adherence refill measures (36, 37). Finally, VA is home to the Veterans Aging Cohort Study (VACS), a large, longitudinal, observational cohort study with ongoing enrollment that includes all PLWH receiving care nationally in the VA (27). Therefore, using VA electronic health record (EHR) data from VACS, we evaluated whether changes in the full spectrum of alcohol use (measured by AUDIT-C screening documented annually as part of clinical care in patients EHRs) over time were associated with commensurate changes in ART adherence among patients treated with ART. Building on prior research (24), we also evaluated whether the associations between change in alcohol use and change in adherence varied by baseline level of alcohol use and by indicators of optimal adherence at baseline.
METHODS
Data Source and Sample
Consistent with the cohort identification approach for our prior studies (13, 38), VA EHR data from VACS were extracted for all PLWH in the U.S. who received VA care nationally and had at least one documented AUDIT-C alcohol screen between 2/1/2008 (when alcohol screening data became widely available in VA’s Corporate Data Warehouse) and 5/1/2014. We followed this sample to identify those with a follow-up alcohol screen documented 9–15 months after an initial screen (to best capture annual screening). Follow-up screens were identified through 7/31/2016 for each initial screen; patients in the sample could contribute multiple pairs of alcohol screens. Finally, for the present study focused on ART adherence, we further limited the sample to those who had filled at least one ART prescription in the year prior to both initial and follow-up screens.
Measures
The independent variable—AUDIT-C change score—was calculated for each AUDIT-C pair. The AUDIT-C includes 3-items that have been shown to be reliable (39) and valid for identifying unhealthy alcohol use in Veteran and non-Veteran outpatients (30, 31, 40–42). The AUDIT-C asks about past-year alcohol consumption (frequency of consumption, quantity of typical consumption, and frequency of heavy episodic drinking) and results in a score ranging from 0 to 12 points; scores of 0 indicate no use. Increasing scores on the AUDIT-C are associated with parallel increases in consumption and symptoms of alcohol use disorder (32). Consistent with prior work (13, 38), the independent variable—AUDIT-C change score—was calculated as the initial AUDIT-C score minus the follow-up AUDIT-C score such that a “good” outcome (stable or decreased consumption) would have a positive value. Thus, for each pair of AUDIT-C screens, AUDIT-C change scores range from −12 to 12, with negative values indicating increased alcohol consumption, positive values reflecting decreased alcohol consumption, and 0 indicating no change.
The dependent variable—change in ART adherence—was calculated for each AUDIT-C pair using medication refill information from VA pharmacy data. In the VA (33), pharmacy refill adherence measures for ART are highly reliable (36, 37), and correlate with blood levels of medications (43). Therefore, consistent with previous work in VACS (44), initial and follow-up adherence scores were calculated as the proportion of days in the year prior to each alcohol screen that the patient had medication in their possession based on medication fill records in the EHR (43, 45). Change in ART adherence was calculated for each pair of AUDIT-C screens as the change in adherence scores (percent days adherent) between the year before the initial AUDIT-C and the year before the follow-up AUDIT-C, with negative values indicating decreased adherence, positive values reflecting increased adherence, and 0 indicating no change.
Three measures were used to stratify patients for assessment of subgroup variation in associations between changes in alcohol use and changes in ART adherence over time. Initial AUDIT-C scores were used to categorize patients into initial alcohol use risk groups: 0 points for non-drinking, 1–3 (1–2 for women) points for low-level, 4–5 (3–5 for women) points for mild, 6–7 points for moderate, and 8–12 points for severe alcohol use. A categorical measure was used to ensure adequate sample sizes in each group with cut points identified in alignment with prior research indicating increased risk of mortality associated with increasing risk group (9, 46). Patients were additionally categorized based on initial level of adherence (≥95% adherent versus <95% adherent) and by whether they had an indication of viral suppression (as a marker of optimal adherence to ART) at the time of the initial alcohol screen (HIV-RNA<500 versus ≥500 copies/ml). A threshold of ≥500 copies/ml was used because this has remained a target measure over time, and for some of the study timeframe this was the lowest level of detection.
Other Measures
Demographic characteristics at initial screen for each pair were used as covariates and included age (stratified as <50, 50–65, and >65), gender (male/female), and race/ethnicity (black, Hispanic, white, and other/unknown). Measures of comorbidity at the first initial (i.e., baseline) AUDIT-C were obtained for descriptive purposes and included depression, anxiety, other serious mental illness, stimulant use, opioid use, other drug use, alcohol use disorder, and any alcohol-specific medical condition (e.g., alcohol-related cirrhosis) in the prior year based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
Analyses
We first described patient characteristics at the time of the first initial AUDIT-C for patients included in the analytic sample. We assessed selection bias by comparing characteristics of patients not included in the sample due to lack of follow-up alcohol screening and/or not being on ART. We then summarized and compared mean baseline adherence levels across alcohol use risk groups at the time of the baseline AUDIT-C and tested the association using a non-parametric test for trend.
To evaluate the association between AUDIT-C change scores and change in ART adherence score, we fit linear regression models among all pairs of screens. AUDIT-C change scores were modeled flexibly using restricted cubic splines, which allow for a smooth association across all possible levels of AUDIT-C change without assuming linearity in the association (47). Models were first unadjusted and then adjusted for demographics and adherence to ART in the year prior to the initial AUDIT-C. The adjusted model was considered primary a priori. Though additional comorbidities were measured for descriptive purposes, they were not adjusted for in the models because they may be caused by heavier alcohol use, and may in turn lead to changes in adherence (i.e., mediate the associations of interest) (48–50). A robust sandwich estimator was used to calculate all standard errors to account for potential misspecification of the outcome distribution and non-independence as a result of multiple observations (screening pairs) per patient (51). To address potential selection bias (due to missing follow-up alcohol screening and/or missing adherence data), inverse probability weighting was used to weight the analytic sample back to the full sample of PLWH with at least one documented alcohol screen. Weights were estimated in the full sample of PLWH with an initial alcohol screen using logistic regression models in which an indicator for being in the analytic sample was regressed on patient characteristics at the time of initial screen.
Primary analyses tested if the change in adherence associated with changes in alcohol use over time differed from zero; the significance of the association was determined based on the significance of the spline terms tested using an overall (omnibus) Wald Test. For the primary model, we estimated and plotted the mean change in percent days adherent to ART for each AUDIT-C change score (−12 to +12). For these estimates, mean changes in ART adherence were obtained using fixed covariate values (mean age, male gender, black race, mean initial adherence).
Based on prior research (13, 25, 38), we hypothesized that both increases and decreases in AUDIT-C scores would be associated with decreased ART adherence but that increases in alcohol use may be associated with greater changes in adherence than decreases in alcohol use. Therefore, we conducted contrast tests to evaluate whether significant differences in mean estimated ART adherence changes were observed between: 1) PLWH remaining stable (AUDIT-C change score 0) and those with both increases and decreases of 2 and 5 points, and 2) PLWH with decreases in drinking (AUDIT-C change score +5) to those with similar increases and greater decreases in drinking (AUDIT-C change scores −5 and +8); and 3) those with large decreases (AUDIT-C change score +8) to those with large increases (AUDIT-C change score −8).
Next, to assess whether the association between changes in alcohol use and changes in ART adherence varied across subgroups of PLWH, we repeated our primary model stratified by initial alcohol use risk group, level of adherence (<95% versus ≥95%), and viral suppression (HIV-RNA<500 versus ≥500 copies/ml) as an indicator of clinically effective adherence. We also tested multiplicative interactions between each potential effect modifier and AUDIT-C change score spline terms. All analyses were conducted using Stata Statistical Software, release 14 (52). This study, including waivers of written consent and HIPAA authorization, was approved by the institutional review boards at VA Puget Sound and VA West Haven Healthcare Systems.
RESULTS
Sample Characteristics
Characteristics of the 21,275 PLWH (67,330 AUDIT-C screen pairs) who met eligibility criteria for the analytic sample are described in Table I. Patients were predominantly male and 50 years or older; approximately half were black race. One-third of the sample had a diagnosed depressive disorder, 10% had anxiety, and 10% had serious mental illness. Approximately 15% had a diagnosis for alcohol use disorder, 4% for opioid use disorder, nearly 2% had a diagnosis for a stimulant use disorder, and 8% had diagnoses for other drug use disorders. The majority of patients either reported no alcohol use (48%) or lower-level alcohol use (39%) at first initial screening. The mean percent days adherent was 85% (standard deviation [SD] 21%, see Table I). Over half (53%) of patients were optimally adherent to ART at the time of the first initial AUDIT-C (95% of days covered), and 88% had a suppressed viral load. Baseline AUDIT-C risk groups were associated with past-year percent days adherent such that progressively higher risk groups had progressively lower mean percent days adherent (Table II, p<0.001, z = −13.92 for non-parametric test for trend).
Table I.
Characteristics of VA Patients Living with HIV with at least one pair of annual alcohol screens and who filled at least One Prescription for Antiretroviral Treatment (ART) Medications in the Year Prior to an Initial and Follow-up AUDIT-C Screen (n=21,275 Patients): Estimates Derived at the Time of the First Initial AUDIT-C screening
| Characteristic | N | (%) |
|---|---|---|
| Total | 21,275 | (100.0) |
| Demographics | ||
| Gender (Female) | 556 | (2.6) |
| Age | ||
| <50 | 7,312 | (34.4) |
| 50 – 64 | 11,763 | (55.3) |
| ≥65 | 2,200 | (10.3) |
| Race/Ethnicity | ||
| Black | 10,177 | (47.8) |
| Hispanic | 1,775 | (8.3) |
| White | 8,737 | (41.1) |
| Other/Unknown | 586 | (2.8) |
| MH and SUD | ||
| Depressive Disorder | 6,875 | (32.3) |
| Anxiety Disorder | 2,067 | (9.7) |
| Serious Mental Illness | 2,152 | (10.1) |
| Stimulant Use Disorder | 349 | (16) |
| Opioid Use Disorder | 824 | (3.9) |
| Other Drug Use Disorder | 1,747 | (8.2) |
| Alcohol Use Disorder | 3,131 | (14.7) |
| Alcohol Use Severity | ||
| Initial Alcohol Use Risk Group | ||
| Non-Drinking (AUDIT-C=0) | 10,274 | (48.3) |
| Lower-Level Use (AUDIT-C=1–3, 1–2 women) | 8,384 | (39.4) |
| Medium-Level Use (AUDIT-C 4–5, 3–5 women) | 1,557 | (7.3) |
| High-Level Use (AUDIT-C 6–7) | 469 | (2.2) |
| Very High-Level Use (AUDIT-C 8–12) | 591 | (2.8) |
| HIV Clinical Measures | ||
| Suppressed Viral Load (n=16,934a) | 14,910 | (88.0) |
| Percent days ART adherent (mean, SD) | 85% | (21.1) |
| ≥95% ART Adherent | 11,290 | (53.1) |
n=4,341 with missing HIV RNA lab values
Table II.
Mean Initial Percent Days Adherent to Antiretroviral Treatment Across First Initial Alcohol Use Risk Groups Measured Using AUDIT-C Scores (n=21,275 patients)
| Initial Alcohol Use Risk Groups a | N | Initial Adherence (% days) Mean (SD) b |
|---|---|---|
| Non-Drinking (AUDIT-C=0) | 10,274 | 86.7% (20.4%) |
| Lower-Level Use (AUDIT-C=1–3, 1–2 women) | 8,384 | 85.2% (21.1%) |
| Medium-Level Use (AUDIT-C 4–5, 3–5 women) | 1,557 | 83.4% (22.0%) |
| High-Level Use (AUDIT-C 6–7) | 469 | 81.2% (23.0%) |
| Very High-Level Use (AUDIT-C 8–12) | 591 | 76.8% (25.8%) |
Defined consistent with clinically-relevant cut-points identifying increased risk of morbidity and mortality
non-parametric test for trend p<0.001, z = −13.92)
Association Between AUDIT-C Change and Change in Adherence
Among all observations (67,330 AUDIT-C screen pairs), change in AUDIT-C at annual follow-up screening was minimal: 55% of observations had no change (AUDIT-C change score=0) and 25% changed one point in either direction (13% decreased and 12% increased by one point). Only 6% of observations had an AUDIT-C change of more than 3 points in either direction.
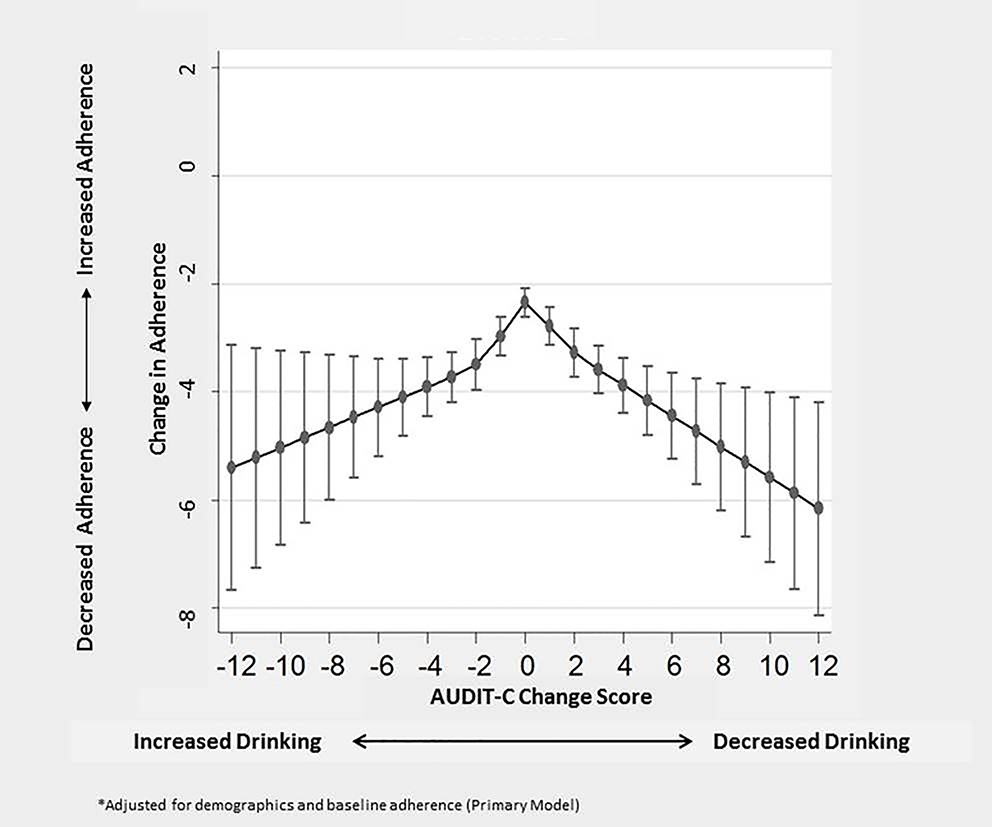
Mean change in adherence scores over time among all observations was −3.1% (SD 21) between the year prior to the initial AUDIT-C screen and the year prior to the follow-up screen. No association was observed between AUDIT-C change scores and change in ART adherence in unadjusted analyses (p-value for spline term = 0.40, F(4, 21274) = 1.02). However, in the adjusted primary model, AUDIT-C change scores were associated non-linearly with change issn ART adherence (p-value for spline term <0.001, F(4, 21274) = 13.20; Figure 1). Mean change in ART adherence estimated for each AUDIT-C change score from the primary model is presented in Figure 1 and depicts an inverted V-shaped association with both increases and decreases in AUDIT-C change score associated with greater decreases in adherence relative to stable AUDIT-C scores. Decreases in adherence were smallest among PLWH with no change in AUDIT-C score over time and increasingly greater for those with greater AUDIT-C change in either direction (increases or decreases). In contrast tests, all comparisons were significant except for between AUDIT-C change scores of +5 and 5+, and between change scores of + 8 and −8 (Table III).
Figure 1:
Adjusted* Mean Change in Percent Days Adherent to Antiretroviral Treatment (ART) Estimated for each AUDIT-C Change Score in a National Sample of VA Patients with HIV (n = 67,330 observations/pairs of screens)
Table III.
Association between AUDIT-C change scores and change in Adherence: Results of contrast tests for comparison of mean changes in adherence
| AUDIT-C Change Score | Predicted Change in Adherence | Comparison AUDIT-C Change Score | Predicted Change in Adherence for Comparison Change Score | Test Statistic | p-value | ||
|---|---|---|---|---|---|---|---|
| N | Δ (95% CI) | N | Δ (95% CI) | ||||
| 0 | 37,342 | −2.34 (−2.60, −2.08) | −2 | 3,009 | −3.49 (−3.95, −3.02) | 20.74 | <0.001 |
| −5 | 354 | −4.09 (−4.80, −3.38) | 22.26 | <0.001 | |||
| +2 | 3,347 | −3.27 (−3.71, −2.82) | 14.92 | 0.0001 | |||
| +5 | 391 | −4.15 (−4.79, −3.52) | 29.72 | <0.001 | |||
| +5 | 391 | −4.15 (−4.79, −3.52) | −5 | 354 | −4.09 (−4.80, −3.38) | −0.99 | 08977 |
| +8 | 174 | −5.01 (−6.18, −3.84) | 7.11 | 0.0077 | |||
| +8 | 174 | −5.01 (−6.18, −3.84) | −8 | 149 | −4.65 (−6.00, −3.31) | 0.16 | 0.6910 |
Stratified Analyses
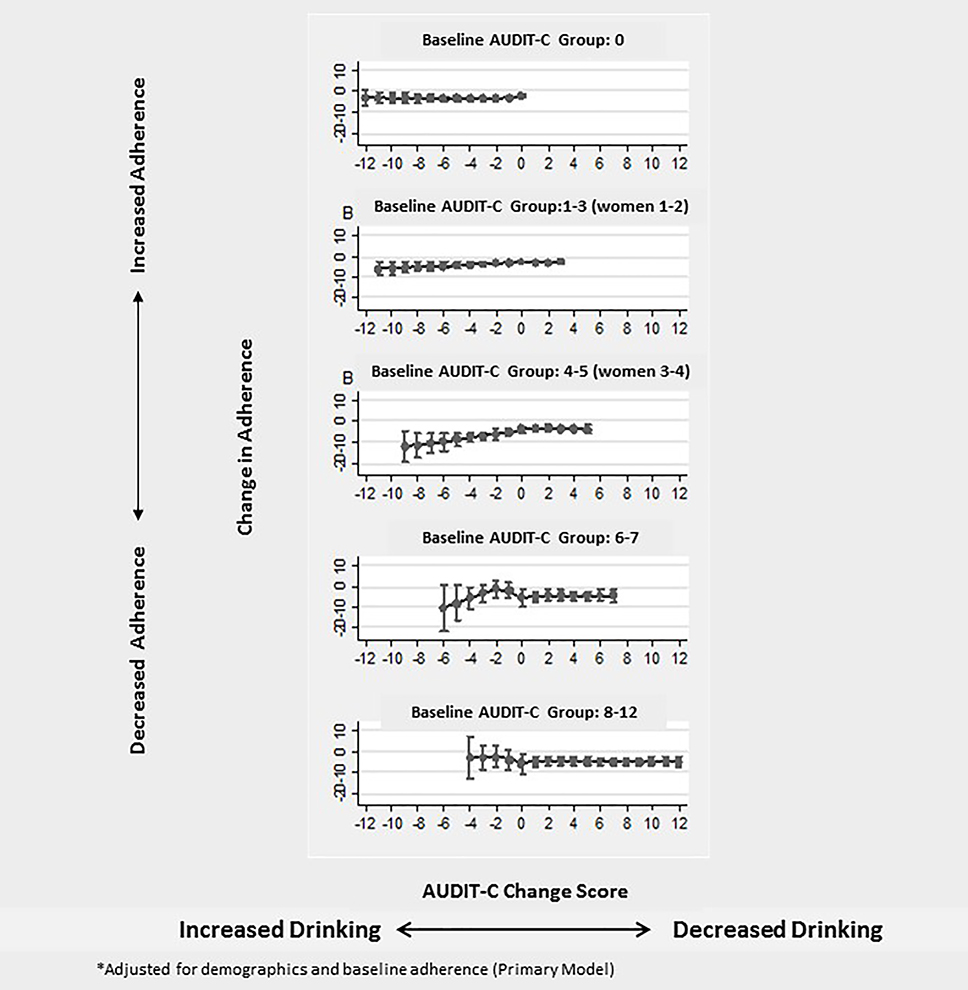
Results from assessment of subgroup variation identified no variation in the association between AUDIT-C change and change in ART adherence based on initial alcohol use risk groups (p-value for interaction=0.45). In stratified analyses, the association between AUDIT-C change and change in ART adherence was statistically significant for those with an initial AUDIT-C score in the three lowest risk groups (p=0.002; F (3, 12912) = 5.06 for AUDITC=0; p=.03 F(4, 12317) = 2.66 for AUDIT-C=1–3/1–2; and p=0.004 F(4, 3014) = 3.84 for AUDIT-C=4–5/3–5). Estimated mean changes in ART adherence for each AUDIT-C change score are presented in Appendix 1 stratified by initial AUDIT-C risk group.
Appendix 1:
Adjusted* Association between Change in AUDIT-C and Change in Percent Days Adherent to Antiretroviral Treatment in a National Sample of Patients Living with HIV: Stratified by Initial AUDIT-C Risk Group (n = 67,330 observations)
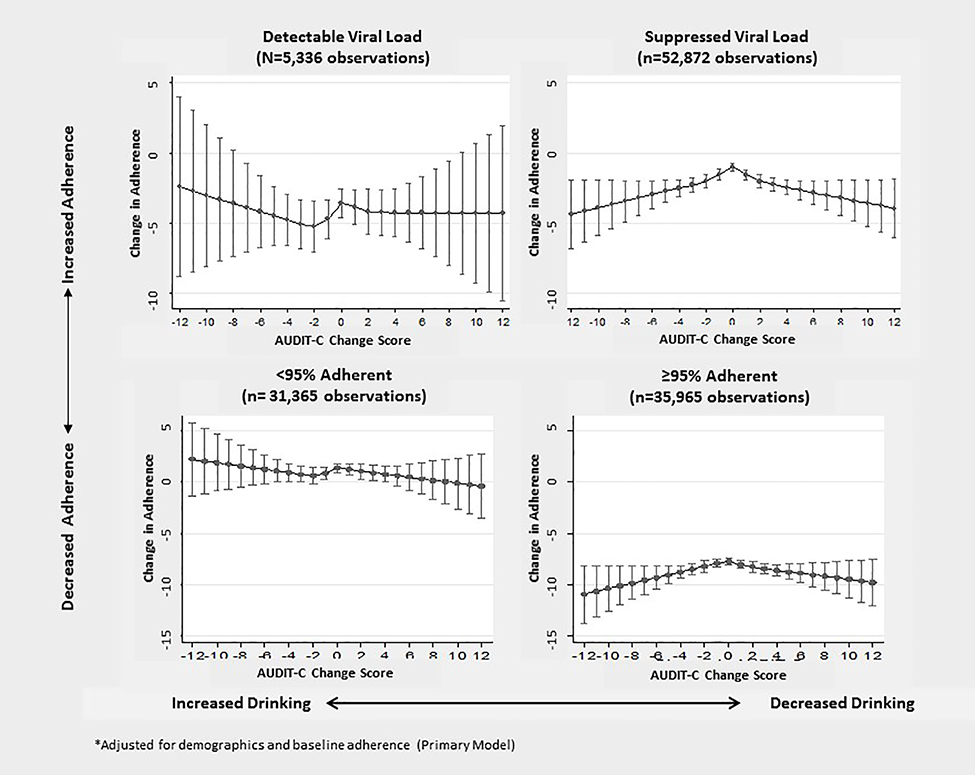
No statistical variation in the association between AUDIT-C change and change in ART adherence was identified across subgroups based on initial level of adherence or baseline HIV viral suppression (p-values for interaction terms 0.44 F(4, 19640) = 0.95 and 0.73 F(4, 19640) = 0.51, respectively). However, in stratified models, AUDIT-C change scores were associated non-linearly with adherence changes among those with initial adherence ≥95% (p=0.006 F(4, 14306) =3.60) but not <95% (p=0.40 F(4, 14864) =1.01) and among those with initially suppressed HIV viral loads (p-value for spline terms <0.001 F(4, 18566) =10.95) but not those with detectable viral loads (p=0.60 F(4, 3696) = 0.69). Estimated mean changes in ART adherence for each AUDIT-C change score are presented in Figure 2, stratified by initial adherence and viral load detectability. Results for those with suppressed viral load and high levels of initial ART adherence generally mirror those identified in the full analytic sample.
Figure 2:
Adjusted* Association between Change in AUDIT-C and Change in Percent Days Adherent to Antiretroviral Treatment in a National Sample of Patients with HIV: Stratified by initial Detectable versus Suppressed Viral Load (HIV RNA ≥500 versus <500 copies/ml) and initial Adherence (≥95% versus <95% days adherent) (n = 67,330 observations)
DISCUSSION
In this large national sample of PLWH, we evaluated whether changes in alcohol use over time—as reflected by repeat AUDIT-C screening collected as part of routine VA care—corresponded to changes in ART adherence. Though prior studies have addressed similar questions in smaller recruited samples using dichotomous adherence outcomes (24, 26) and/or categorical alcohol measures (25), this is the first to our knowledge to be conducted in a large clinical sample of PLWH (not biased by recruitment into a study) and the first to investigate the (potentially non-linear) association between incremental changes in alcohol use in both directions and incremental changes in adherence. We found that over time, ART adherence declined on average and, as expected, was sensitive to changes in documented alcohol use, such that greater increases in alcohol use were generally associated with greater declines in adherence. However, we also found that greater decreases in alcohol use based on the AUDIT-C screen were similarly associated with worse declines in adherence, suggesting that unstable alcohol use—possibly reflecting alcohol use disorder—may generally lead to worsening ART adherence. Additionally, though no significant variation in associations was observed across subgroups, in stratified analyses, associations between changes in alcohol use and changes in adherence were only observed in subsamples of PLWH with high initial levels of adherence and undetectable viral loads. For these subgroups, similar to findings in the entire sample, an inverted V-shaped relationship was identified such that stable alcohol use was associated with the smallest declines in ART adherence while increases and decreases both were associated with larger declines in a dose-response fashion.
Findings from the present study should be considered in light of findings from previous related research. One previous study evaluated associations between changes in substance use (generally) and changes in ART adherence (26), another evaluated whether increases in average weekly alcohol use were associated with changes in the likelihood of being 80% adherent to ART among women living with HIV (24), and a third evaluated whether changes in unhealthy drinking days were associated with adherence over time in an insured sample of PLWH recruited for an alcohol intervention trial (25). The present study’s findings are generally consistent with those of prior studies focused on changes in alcohol use and particularly similar to those of Barai et al. Specifically, in a small recruited sample of women receiving care in an urban HIV clinic, Barai et al. found that increases in alcohol use negatively impacted ART adherence only among women who started at high levels of adherence (≥80%) and who also consumed <8 drinks/week on average at baseline (24). While we found no statistically significant interaction with either initial drinking level or initial adherence, associations between changes in drinking and changes in adherence in our study were similarly limited to those with lower levels of initial alcohol use and those with high initial levels of adherence. Our findings build on this prior study (24) due to use of a large and non-recruited sample, which enabled us to investigate variability across multiple levels of initial alcohol use and demonstrate an association between changes in drinking documented in the EHR and changes in adherence in all but the 5% of those reporting the highest-level drinking (AUDIT-C ≥ 6).
Overall findings from the present study, as well as results in those with undetectable viral loads or high initial levels of adherence, are generally consistent with findings from our prior studies in VACS using similar methods (13, 38), which have assessed associations between changes in alcohol use and changes in HIV disease severity both based on clinical measures (CD4 count and HIV RNA viral load) (13) and a composite measure that predicts mortality (VACS Index 2.0) (38). These prior studies have found that stable alcohol use, largely representing mostly stable non- or low-level alcohol use, was associated with the greatest improvement in all measures of HIV severity, whereas both increases and decreases in drinking were associated with less improvement in markers of severity.
Findings regarding decreases in alcohol use in the present study are of interest. Several factors may contribute to this finding. A large portion of our sample started at non- or low-level alcohol use and did not change drinking over time, and a majority of our sample started with high levels of adherence. Although we stratified results by initial AUDIT-C group, we may be limited in estimating relationships among persons with large decreases in alcohol use and/or consistent high-level alcohol use. Further, large changes in drinking in both directions may be indicative of underlying alcohol use disorder, which is characterized by frequent changes in drinking and associated with multiple adverse outcomes (53–56). Because higher AUDIT-C scores correlate with stronger likelihood of alcohol use disorder (32), it is possible that the higher of a patient’s two AUDIT-C values is the strongest determinant of reductions in adherence, irrespective of the order of the values. Further, decreases in drinking in both directions may also be a proxy for changes in other health or life circumstances—such as worsening physical or mental health, financial insecurity, or unstable housing (15, 24)—that could also account for observed declines in ART adherence among those with decreases in drinking, as has been shown in prior work focused on other outcomes (57). Finally, under-reporting of alcohol use on the AUDIT-C at either time period may contribute to findings. Although the AUDIT-C is extensively validated, as implemented clinically in the VA, it may underestimate alcohol use (58, 59). Thus, it is possible that some of the changes in AUDIT-C scores reflected changes in reporting rather than changes in alcohol use
Though this study did not test the processes via which alcohol use may influence ART adherence, the global association identified is consistent with adherence theory suggesting alcohol use may be a key moderator of adherence processes (15). Further theory-driven study is needed to explore the mechanisms via which changes in alcohol use influence adherence over time in order to build on and inform integrated interventions addressing both outcomes (60–62) and build off prior ART adherence interventions that target reductions in alcohol use. This work should include exploration of factors such as shared psychological processes (e.g., low health-related motivation; low health-related self-efficacy, and/or depression) (21, 22), which could serve as “third factors” that contribute to the observed covariation in alcohol use and ART adherence.
This study is limited in several ways. First, given the conduct of the study among VA outpatients with HIV and ART prescriptions, findings may not be generalizable to PLWH who do not receive VA care or those not linked to healthcare. Further, the sample limited the conduct of gender-specific analyses due to relatively small numbers of women (especially those with heavy and/or changing alcohol use). Though beyond the scope of the present study, exploration of gender differences in associations between alcohol use and ART adherence over time may be necessary as alcohol use may have greater impact on HIV-related outcomes among women than men (63). Similarly, the samples of PLWH with markers of suboptimal adherence were relatively small, thus analyses in these subsamples may have been underpowered. Additionally, reliance on EHR data and use of pragmatic clinical measures may have limited measurement of alcohol use and ART adherence, resulting in some misclassification, and decreases in both alcohol use and adherence may reflect regression to the mean. Though AUDIT-C is a validated screen that corresponds well to alcohol consumption based on in-depth interview measures, and VA studies using a biomarker have suggested that on average AUDIT-C data from VA’s EHR is a valid marker of change, clinical AUDIT-C data are susceptible to under reporting due to nonstandard administration and stigma (58, 59, 64–66). Additionally, though over 96% of PLWH receiving VA care report receiving ART through VA’s pharmacy (67), the present study would have missed ART doses received outside VA.
Despite these limitations, the VA is the nation’s largest healthcare provider for PLWH; thus, use of VA data allows us to study nationally representative trends. The present study is the first to assess whether individual-level increases and decreases in alcohol use on a clinical screen are associated with continuous changes in adherence to ART over time in a large unrecruited sample. In this national sample of PLWH, ART adherence was sensitive to changes in AUDIT-C documented in the EHR, particularly among the majority of PLWH whose HIV was initially controlled (those with suppressed viral load and/or high levels of initial adherence). Of note, dynamic alcohol use, reflected by changes in AUDIT-C in both directions, was associated with greater decreases in adherence over time compared to stable AUDIT-C. Dynamic alcohol use may reflect underling alcohol use disorder, and findings may suggest that a patient’s highest AUDIT-C is of key importance with regard to predicting ART adherence and may be the most important intervention target. Though further work is needed to understand mechanisms for changes in adherence associated with changes in alcohol use, findings suggest that PLWH who exhibit high AUDIT-C scores at any time point and/or large changes in alcohol use may be at risk for clinical deterioration and ultimately may require adherence and alcohol use interventions. Given the known dangers of alcohol use for PLWH, and the lifesaving effects of ART, providers of HIV care should continue vigilant focus on supporting consistent non-drinking or low levels of alcohol use and consistent adherence to ART regimens. Although ART potency has increased over time, and PLWH with lower adherence appear to be benefiting (2, 4, 68), levels of adherence at which ART efficacy decreases are unclear, and all PLWH should be encouraged to maintain high levels of adherence.
ACKNOWLEDGMENTS
This research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA; R21AA022866–01) and supported by the COMpAAAS/Veterans Aging Cohort Study (U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799; U10 AA013566). Dr. Williams was supported by a Career Development Award from VA Health Services Research & Development (CDA 12–276) at the time of this study. Drs. Bradley and Satre are supported by NIAAA K24 awards (AA022128 and AA025703, respectively). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. This work was supported in part with resources and the use of facilities at the VA Puget Sound Health Care System in Seattle, WA. However, the contents of this manuscript do not necessarily represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: For this type of study, formal consent is not required. This study, including waivers of written consent and HIPAA authorization, was approved by the institutional review boards at VA Puget Sound and VA West Haven Healthcare Systems. All procedures were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
REFERENCES
- 1.Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am. 2014;28(3):371–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–41. [DOI] [PubMed] [Google Scholar]
- 3.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372–9. [DOI] [PubMed] [Google Scholar]
- 4.Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV Virologic Failure Rates Between Patients with Variable Adherence to Three Antiretroviral Regimen Types. Aids Patient Care STDS. 2015;29(7):384–8. [DOI] [PubMed] [Google Scholar]
- 5.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol use and Human Immunodeficiency Virus (HIV) Infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res. 2016;4 (10):2056–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7(4):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott-Sheldon LA, Carey KB, Kaiser TS, Knight JM, Carey MP. Alcohol Interventions for Greek Letter Organizations: A Systematic Review and Meta-Analysis, 1987 to 2014. Health Psychol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neblett RC, Hutton HE, Lau B, McCaul ME, Moore RD, Chander G. Alcohol consumption aong HIV-infected women: impact on time to antiretroviral therapy and survival. J Womens Health (Larchmt). 2011;20(2):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortego C, Huedo-Medina TB, Llorca J, Sevilla L, Santos P, Rodriguez E, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–96. [DOI] [PubMed] [Google Scholar]
- 11.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grodensky CA, Golin CE, Ochtera RD, Turner BJ. Systematic review: effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. J Stud Alcohol Drugs. 2012;73(6):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams EC, McGinnis KA, Bobb JF, Rubinsky AD, Lapham GT, Skanderson M, et al. Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort. Drug Alcohol Depend. 2018;189:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon A, Marshall BD, et al. Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS and behavior. 2019;23(1):140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5(4):193–203. [DOI] [PubMed] [Google Scholar]
- 16.Schensul SL, Ha T, Schensul JJ, Vaz M, Singh R, Burleson JA, et al. The Role of Alcohol on Antiretroviral Therapy Adherence Among Persons Living With HIV in Urban India. J Stud Alcohol Drugs. 2017;78(5):716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatch R, Emenyonu NI, Muyindike W, Kekibiina A, Woolf-King S, Hahn JA. Alcohol Interactive Toxicity Beliefs and ART Non-adherence Among HIV-Infected Current Drinkers in Mbarara, Uganda. AIDS Behav. 2017;21(7):1812–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalichman SC, Amaral CM, White D, Swetsze C, Kalichman MO, Cherry C, et al. Alcohol and adherence to antiretroviral medications: interactive toxicity beliefs among people living with HIV. J Assoc Nurses AIDS Care. 2012;23(6):511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalichman SC, Grebler T, Amaral CM, McNerey M, White D, Kalichman MO, et al. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. J Gen Intern Med. 2013;28(3):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellowski JA, Kalichman SC, Kalichman MO, Cherry C. Alcohol-antiretroviral therapy interactive toxicity beliefs and daily medication adherence and alcohol use among people living with HIV. AIDS Care. 2016;28(8):963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipira L, Williams EC, Huh D, Kemp CG, Nevin PE, Greene P, et al. HIV-Related Stigma and Viral Suppression Among African-American Women: Exploring the Mediating Roles of Depression and ART Nonadherence. AIDS Behav. 2019;23(8):2025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kekwaletswe CT, Jordaan E, Nkosi S, Morojele NK. Social Support and the Mediating Roles of Alcohol Use and Adherence Self-Efficacy on Antiretroviral Therapy (ART) Adherence Among ART Recipients in Gauteng, South Africa. AIDS Behav. 2017;21(7):1846–56. [DOI] [PubMed] [Google Scholar]
- 23.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–7. [DOI] [PubMed] [Google Scholar]
- 24.Barai N, Monroe A, Lesko C, Lau B, Hutton H, Yang C, et al. The Association Between Changes in Alcohol Use and Changes in Antiretroviral Therapy Adherence and Viral Suppression Among Women Living with HIV. AIDS Behav. 2017;21(7):1836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satre DD, Sarovar V, Leyden W, Hare CB, Catz SL, Bryant KJ, et al. Changes in Days of Unhealthy Alcohol Use and Antiretroviral Therapy Adherence, HIV RNA Levels, and Condomless Sex: A Secondary Analysis of Clinical Trial Data. AIDS Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–74. [DOI] [PubMed] [Google Scholar]
- 27.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 28.Department of Veterans Affairs. HIV/Hepatitis C QUERI Strategic Plan. 2010. http://www.queri.research.va.gov/about/strategic_plans/hiv.pdf. Washington, D.C.: U.S. Department of Veterans Affairs. [Google Scholar]
- 29.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 30.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 31.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–9. [DOI] [PubMed] [Google Scholar]
- 32.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37(8):1380–90. [DOI] [PubMed] [Google Scholar]
- 33.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60(3 Suppl):92S–123S. [DOI] [PubMed] [Google Scholar]
- 35.Aspinall SL, Sales MM, Good CB, Calabrese V, Glassman PA, Burk M, et al. Pharmacy Benefits Management in the Veterans Health Administration Revisited: A Decade of Advancements, 2004–2014. Journal of managed care & specialty pharmacy. 2016;22(9):1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846–57. [DOI] [PubMed] [Google Scholar]
- 37.Ren XS, Kazis LE, Lee A, Zhang H, Miller DR. Identifying patient and physician characteristics that affect compliance with antihypertensive medications. J Clin Pharm Ther. 2002;27:47–56. [DOI] [PubMed] [Google Scholar]
- 38.Williams EC, McGinnis KA, Tate JP, Matson TE, Rubinsky AD, Bobb JF, et al. HIV Disease Severity Is Sensitive to Temporal Changes in Alcohol Use: A National Study of VA Patients With HIV. J Acquir Immune Defic Syndr. 2019;81(4):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley KA, McDonell MB, Bush K, Kivlahan DR, Diehr P, Fihn SD. The AUDIT alcohol consumption questions: reliability, validity, and responsiveness to change in older male primary care patients. Alcohol Clin Exp Res. 1998;22(8):1842–9. [DOI] [PubMed] [Google Scholar]
- 40.Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med. 2008;23(6):781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2013;37 Suppl 1:E253–9. [DOI] [PubMed] [Google Scholar]
- 43.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814–23. [DOI] [PubMed] [Google Scholar]
- 44.Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21(12):1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. [DOI] [PubMed] [Google Scholar]
- 46.Kinder LS, Bryson CL, Sun H, Williams EC, Bradley KA. Alcohol screening scores and all-cause mortality in male Veterans Affairs patients. J Stud Alcohol Drugs. 2009;70(2):253–60. [DOI] [PubMed] [Google Scholar]
- 47.Khamis H, Kepler M. Multivariate cubic spline smoothing in multiple prediction. Comput Methods Programs Biomed. 2002;67(2):131–6. [DOI] [PubMed] [Google Scholar]
- 48.Holloway IW, Tan D, Dunlap SL, Palmer L, Beougher S, Cederbaum JA. Network support, technology use, depression, and ART adherence among HIV-positive MSM of color. AIDS Care. 2017;29(9):1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blashill AJ, Gordon JR, Safren SA. Depression longitudinally mediates the association of appearance concerns to ART non-adherence in HIV-infected individuals with a history of injection drug use. J Behav Med. 2014;37(1):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug Alcohol Depend. 2011;117(2–3):158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 52.StataCorp. Stata Statistical Software: Release 14, College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 53.Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36(7):931–41. [DOI] [PubMed] [Google Scholar]
- 54.Saha TD, Compton WM, Chou SP, Smith S, Ruan WJ, Huang B, et al. Analyses related to the development of DSM-5 criteria for substance use related disorders: 1. Toward amphetamine, cocaine and prescription drug use disorder continua using Item Response Theory. Drug Alcohol Depend. 2012;122(1–2):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89(1):82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105(5):817–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U shaped curve. Lancet. 1988;8623(2):1267–73. [DOI] [PubMed] [Google Scholar]
- 58.Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, et al. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med. 2011;26(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend. 2016;168:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naar-King S, Parsons JT, Murphy D, Kolmodin K, Harris DR. A multisite randomized trial of a motivational intervention targeting multiple risks in youth living with HIV: initial effects on motivation, self-efficacy, and depression. J Adolesc Health. 2010;46(5):422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parsons JT, Rosof E, Punzalan JC, Di Maria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: results of a pilot project. Aids Patient Care STDS. 2005;19(1):31–9. [DOI] [PubMed] [Google Scholar]
- 63.Matson TE, McGinnis KA, Rubinsky AD, Frost MC, Czarnogorski M, Bryant KJ, et al. Gender and alcohol use: influences on HIV care continuum in a national cohort of patients with HIV. AIDS. 2018;32(15):2247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, et al. Factors Underlying Quality Problems with Alcohol Screening Prompted by a Clinical Reminder in Primary Care: A Multi-site Qualitative Study. J Gen Intern Med. 2015;30(8):1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradley KA, Chavez LJ, Lapham GT, Williams EC, Achtmeyer CE, Rubinsky AD, et al. When Quality Indicators Undermine Quality: Bias in a Quality Indicator of Follow-Up for Alcohol Misuse. Psychiatr Serv. 2013;64(10):1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawkins EJ, Kivlahan DR, Williams EC, Wright SM, Craig T, Bradley KA. Examining quality issues in alcohol misuse screening. Subst Abus. 2007;28(3):53–65. [DOI] [PubMed] [Google Scholar]
- 67.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44(8 Suppl 2):S52–60. [DOI] [PubMed] [Google Scholar]
- 68.Moore RD, Bartlett JG. Dramatic decline in the HIV-1 RNA level over calendar time in a large urban HIV practice. Clin Infect Dis. 2011;53(6):600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]