Abstract
Background:
Exposure to household air pollution from solid fuel combustion for cooking and heating is an important risk factor for premature death and disability worldwide. Current evidence supports an association of ambient air pollution with cardiovascular disease but is limited for household air pollution and for cardiac function. Controlled exposure studies can complement evidence provided by field studies.
Objectives:
To investigate effects of short-term, controlled exposures to emissions from five cookstoves on measures of cardiac function.
Methods:
Forty-eight healthy adults (46% female; 20–36 years) participated in six, 2-h exposures (‘treatments’), including emissions from five cookstoves and a filtered-air control. Target fine particulate matter (PM2.5) exposure-concentrations per treatment were: control, 0 μg/m3; liquefied petroleum gas, 10 μg/m3; gasifier, 35 μg/m3; fan rocket, 100 μg/m3; rocket elbow, 250 μg/m3; and three stone fire, 500 μg/m3. Participants were treated in a set (pre-randomized) sequence as groups of 4 to minimize order bias and time-varying confounders. Heart rate variability (HRV) and cardiac repolarization metrics were calculated as 5-min means immediately and at 3 h following treatment, for analysis in linear mixed-effects models comparing cookstove to control.
Results:
Short-term differences in SDNN (standard deviation of duration of all NN intervals) and VLF (very-low frequency power) existed for several cookstoves compared to control. While all cookstoves compared to control followed a similar trend for SDNN, the greatest effect was seen immediately following three stone fire (β = − 0.13 ms {%}; 95% confidence interval = − 0.22, − 0.03%), which reversed in direction at 3 h (0.03%; − 0.06, 0.13%). VLF results were similar in direction and timing to SDNN; however, other HRV or cardiac repolarization results were not similar to those for SDNN.
Discussion:
We observed some evidence of short-term, effects on HRV immediately following cookstove treatments compared to control. Our results suggest that cookstoves with lower PM2.5 emissions are potentially capable of affecting cardiac function, similar to stoves emitting higher PM2.5 emissions.
Keywords: Cookstove, Fine particulate matter, Household air pollution, Healthy adult, Heart rate variability, Cardiac repolarization
1. Introduction
Air pollution is considered by the Global Burden of Disease Study to be the largest environmental contributor to premature death and disability (Stanaway et al., 2018). The study estimated a loss of 60 million disability-adjusted life years from individual exposure to household fine particulate matter (≤2.5 μm diameter; PM2.5) emitted from combustion of solid fuels (Stanaway et al., 2018). This burden of disease is particularly high in low- and middle-income countries, where a substantial proportion of the population relies on solid-fuel combustion for domestic energy purposes (cooking, heating, lighting) (Bonjour et al., 2013). Cardiovascular disorders, such as myocardial infarction and stroke, are a large contributor to the mortality attributed to PM2.5 exposure (Brook, Newby and Rajagopalan, 2018). Intervention studies seek to reduce PM2.5 exposure concentrations emitted from solid-fuel combustion by introducing improved cookstove designs (Thomas et al., 2015) and subsequently reducing the potential for an associated health burden (Lai et al., 2019).
Despite these efforts, scientific knowledge is lacking on the short- and long-term effects of household air pollution and its role in cardiovascular disease. While there are a number of field studies evaluating blood pressure (McCracken et al., 2007; Baumgartneret al., 2011, 2014), 2018; Clark et al., 2011, 2013; Dutta and Ray, 2012; Alexander et al., 2015, 2017; Neupane et al., 2015; Burroughs Pena et al., 2015; Norris ˜ et al., 2016), there is currently only one published field study on cardiac function and household air pollution (McCracken et al., 2011). That one field study, an intervention to reduce woodsmoke-derived household air pollution by switching cookstove type among users, provided mixed results for the impact on cardiac function: ST segment depression was improved with the intervention, but not high frequency (HF) or low frequency (LF) measures of heart rate variability (HRV) (McCracken et al., 2011).
Measurement of HRV provides an indication of change in the autonomic nervous system that can affect cardiac function and risk of adverse cardiovascular outcomes (Pope and Dockery, 2006). Chronically decreased HRV is associated with an increased risk of adverse cardiovascular events (Thayer, Yamamoto and Brosschot, 2010). Decreased HRV following exposure to ambient air pollution, as reflected by a decreased standard deviation of the time interval between heart contractions (SDNN), has been observed in multiple studies using ambulatory measures (Weichenthal, 2012; Weichenthal et al., 2014; Cole-Hunter et al., 2016, 2018; U.S. EPA, 2019). Cardiac repolarization can be measured to indicate short-term changes in the risk of cardiovascular-related disorders (Dekker et al., 2004). For example, ventricular repolarization (T-wave complexity) informs on the current state of the myocardium, and changes reflect vulnerability for cardiovascular morbidity and mortality (Zareba, Nomura and Couderc, 2001). Changes in cardiac repolarization (most commonly prolongation of heart rate-corrected QT interval, QTc) have been associated with short-term (hours to days) exposure to PM2.5 in ambient (observational) (Baja et al., 2010; Liao et al., 2010; Xu et al., 2019) and concentrated (controlled) (Devlin et al., 2014) atmospheres.
Field-based observational studies evaluating the health effects of exposure to household air pollution may be limited due to a number of factors, such as potential confounding by a subject’s ability to afford a lower-emissions stove. While controlled exposure studies do not necessarily represent real-world conditions, they may complement field studies in that the exposure event may be isolated and more carefully controlled (as a ‘treatment’). There are remarkably few controlled exposure studies for solid-fuel combustion emissions, and fewer on cardiac outcomes. One such study of diluted wood smoke (among healthy, non-smoking participants) clearly showed immediate reductions in HRV (e.g., SDNN) up to the end of the 1-h post-exposure period following treatment (2-h PM2.5 exposure concentration mean: 314 μg/m3) (Unosson et al., 2013). Meanwhile, another similar study suggested no such short-term (immediate) changes in other HRV metrics either immediately or at 20 h following treatment (3-h PM2.5 exposure concentration mean: 485 μg/m3), compared to control (Ghio et al., 2012).
Accordingly, the objective of the analysis presented here was to investigate short-term effects on cardiac function of healthy human adults following controlled exposure to air pollution emitted from various cookstove technologies. This analysis is part of the larger ‘STOVES’ (Sub-clinical Tests On Volunteers Exposed to Smoke) study. Importantly, cardiac function was not the primary outcome in this study, meaning that the study was not specifically designed (powered) to investigate a concentration–response relationship across cookstoves and this analysis is exploratory rather than addressing a predefined hypothesis.
2. Methods
The STOVES study methods and procedures have been published previously (Fedak et al., 2019, 2020; Walker et al., 2020b); however, below is a brief overview of those methods and the introduction of specific methods for this analysis.
2.1. Eligibility criteria
Young, healthy, never-smoker adults (18–35 years) of a normal to overweight body mass index (19–28 kg/m2) and weight greater than 49 kg (110 lb) were invited to participate in this study. A recruitment questionnaire was administered by study staff interview to determine participant health and eligibility status, followed by a physical exam conducted by a study physician at screening.
The following exclusion criteria were applied: prolonged exposure to commute-/traffic-related air pollution (defined by living outside of 20 miles [~32 km] from study location); regular exposure to smoke, dust, fumes, solvents, or regularly burned candles or incense within the last 3 months; history of heart disease, diabetes, or any chronic inflammatory or respiratory disease (such as asthma, arthritis, or severe allergies); ear or abdominal/thoracic surgery in the last month; spirometry value <70% of predicted value for age/sex; abnormal complete blood count, comprehensive metabolic panel, or lipid panel; diagnosis of kidney disease, systemic sclerosis, or recent diagnosis of cancer (i.e., current disease or have been in remission for <6 months); central intravenous line or port, or pacemaker; had a mastectomy; pregnancy, be breast-feeding, or planning a pregnancy within the next 6 months; regular intake of statins, anti-inflammatory medication, or certain other medications (as determined by study cardiologist); use of certain recreational drugs (including methamphetamines, cocaine, tetrahydrocannabinol, barbiturates, phencyclidine, amphetamines, opiates) within the last 3 months; history of claustrophobia; or, allergy to latex.
2.2. Study design
As described previously (Fedak et al., 2019), we used a cross-over repeated-measures design to evaluate five different cookstoves (‘treatments’), plus one filtered air treatment as a control, for their impact on HRV and repolarization. The different cookstoves were selected to represent widely available technologies that span a broad range of pollutant emissions. As previously reported, a sample size of 48 participants was chosen based primarily on statistical power calculations to detect changes in cardiovascular outcomes (Fedak et al., 2019). We enhanced statistical power by setting target PM2.5 exposure concentration ranges to be narrow and not over-lapping between different cookstove treatments.
Potential confounding factors were controlled for by study design (age, sex, temporally-varying factors) and by study protocol; participants served as their own control in analyses. As per protocol, participants were instructed to maintain a diet restricted to low-fat and low-cholesterol food for the 48-h period around each treatment (from the 24-h period prior to treatment until the end of the 24-h period following treatment). To facilitate this, consistent nourishment (i.e., diet-restricted lunch and snacks, and isotonic drinks, along with water) were provided to participants at the study site. For this same period, participants were instructed to abstain from all medications and dietary supplements (unless approved by the study physician). Further, for the 24-h period prior to treatment through the end of the 24-h follow up measure, participants were instructed to abstain from high-intensity exercise, alcohol, and caffeine. Deviations from this protocol were reported in the self-administered follow-up questionnaire (see Questionnaires, below).
All study procedures were approved by the Institutional Review Board of Colorado State University. Written informed consent was obtained from all study participants.
2.3. Study location
The study was conducted at the Powerhouse Energy Campus building of Colorado State University, Fort Collins, Colorado, USA. Treatments were administered in a Simulated Environmental Testing (SET) facility (see Controlled exposure treatments, below). The study schedule period ran from October 2016 to January 2018, with three “rounds” of participants (Round 1: October 2016 to April 2017; Round 2: April 2017 to August 2017; Round 3: August 2017 to January 2018) largely aligned to university semesters and covering the seasons of autumn/fall, spring, and summer.
2.4. Study session protocol
Study participants completed six treatment sessions involving a 7-h day including a 2-h treatment period. During the entire 7 h, participants were asked to avoid physical exertion (e.g., by using study building elevator instead of stairs to travel between floors). Cardiac function was measured at three time-points: immediately before, immediately after, and 3 h after this treatment period. Other health endpoints were measured at these time-points, and also at approximately 24 h post treatment (Fedak et al., 2019, 2020; Walker et al., 2020b). A total of four participants were scheduled to start participation on the same day of week and started at 30 min intervals, either 0730 h, 0800 h, 0830 h or 0900 h (maintained across sessions; e.g., Participant A participated in six sessions always starting on a Monday at 0800 h), with at least 2 weeks between sessions for a given participant. The six unique treatment orders (sequences) are deterministic and follow the Williams square design (Williams, 1949) – see Fig. 1 of Walker et al. (2020a, 2020b) for treatment sequences. In brief, the Williams square is a specialized balanced Latin square crossover. Each participant receives each of the six treatments. Individuals are randomly assigned to one of the six sequences. If a session was missed by a participant due to illness or unforeseen circumstances, that session (treatment type, ‘make-up’ session) was attempted at the end of each round.
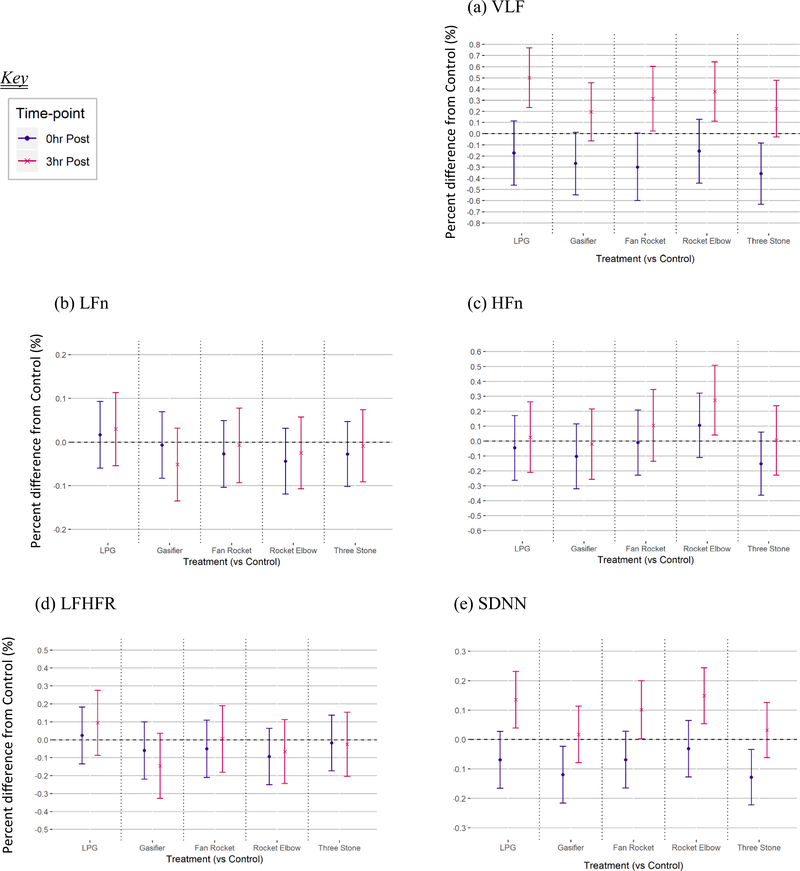
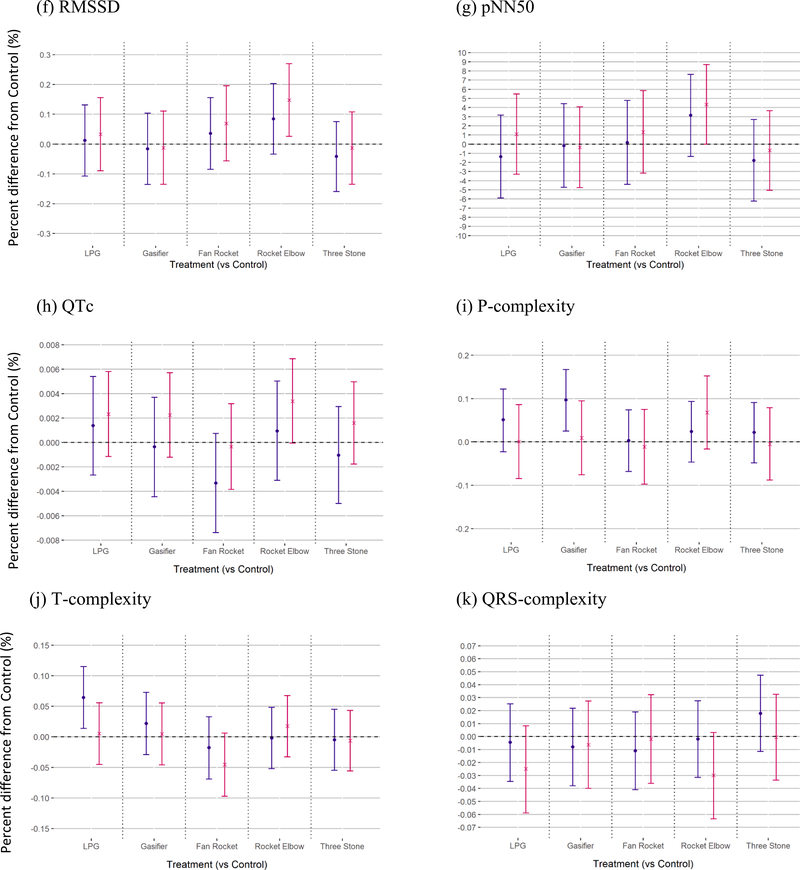
Fig. 1.
Short-term differences in cardiac function following short-term exposure to cookstove air pollution compared to control.
A trained technician monitored PM2.5, carbon monoxide, and oxygen concentrations, along with temperature and atmospheric pressure within the SET facility during operation for treatment periods. Real-time feedback of the SET facility system allowed levels of PM2.5, the exposure parameter of interest, to be maintained within pre-determined target ranges for each treatment.
Cookstove air pollution was diluted with HEPA (high-efficiency particulate air)-filtered air and the resultant mixture was drawn into the SET facility exposure chamber. The volumetric rate of flow for these airstreams was modulated (control system adjusted) to maintain PM2.5 target levels within the SET. PM2.5 was measured at 1-s intervals to avoid deviations greater than 5% from target as a 3-min rolling average throughout the treatment period. In the case of elevated concentrations, more filtered air was delivered, and vice versa. This dynamic delivery system was designed to provide similar levels of noise, relative humidity, and temperature (potential confounding variables) across treatments.
2.5. Controlled exposure treatments
Five different cookstove technologies were chosen to represent a range of emission levels that may be experienced with cookstove use in a real-world environment (World Health Organization, 2014; Pope et al., 2017). Cookstove emissions were administered as treatments within the SET facility housing up to four participants simultaneously, sitting at individual corners, in a main area of 2.7 m high by 3.5 m wide and 2.8 m long. The chamber main area had an anteroom to allow participant egress/ingress without affecting treatment concentrations. The chamber was also connected via piping to a fume hood which collected cookstove emissions and diluted PM2.5 concentrations to a target treatment range. Participants were assigned to treatment order at random.
A filtered-air treatment was administered as a control (targeted at 0 μg/m3 of PM2.5). The cookstoves, in order of increasing target PM2.5 exposure concentration representing respective technologies, were: liquefied petroleum gas (LPG), 10 μg/m3; gasifier, 35 μg/m3; fan rocket, 100 μg/m3; rocket elbow, 250 μg/m3, and; three-stone fire, 500 μg/m3. Target exposure concentrations were based a Monte-Carlo emissions/dispersion model (L’Orange et al., 2015) that utilized published emission factors for each stove-type (Jetter et al., 2012; L’Orange et al., 2012). Stoves were selected so that emission levels would be distinct and resultant exposure levels would span the range of WHO Interim Target Levels for household air pollution (World Health Organization, 2014). Emissions concentrations seen in homes for a given cookstove technology can vary greatly and depend on factors such as stove operation, home size, and air exchange rate. The target concentrations used in this study were set to represent levels that could realistically be found in homes using a given technology. Initial concentration ranges were estimated using emissions rates (Jetter et al., 2012; Bilsback et al., 2019) and a box-model approach to estimate concentration (L’Orange et al., 2015; Johnson et al., 2011). Target setpoints were then selected to fall within (or close to within) the estimations resulting from the box model. Continuous PM2.5 concentration was monitored in the SET using an aerosol monitor (DustTrak DRX 8533, TSI Incorporated, USA) that was calibrated to gravimetric PM2.5 filter samples collected for the full duration of each treatment (Pallflex Fiber Film T60A20; sample flow rate of 6 L/min using a Leland Legacy [SKC Inc, Eighty Four PA] sampling pump with integrated flow control). Additionally, concentrations of carbon monoxide and oxygen were measured continuously using nondispersive infrared spectrometry (Ultramat 23, Siemens AG, Munich, Germany) and paramagnetic analysis (Ultramat/Oxymat 6, Siemens AG, Munich, Germany), respectively. Background levels of gases were determined by monitoring in the same way for at least 5 min prior to conducting the treatment component of the study. Gas samples were drawn from a wall port and through a HEPA-filter. All continuous air quality measurements were made at a frequency of 1 s and subsequently averaged to a 3-min period. Additional characterization of the pollutant emissions, by stove treatment, was conducted outside of the participant treatment sessions; results for these characterizations are reported in the supplemental material of a related publication (Fedak et al., 2019).
Ambient pollution and meteorological information for Fort Collins were collected from regional monitors. For the 24-h period prior to each treatment, hourly measures of PM2.5 were downloaded from the U.S. Environmental Protection Agency’s Air Quality Data API (U.S. Environmental Protection Agency, 2018); meteorological data (relative humidity, temperature) were obtained from the Colorado State University Atmospheric Science Department’s Christman Field Weather Station (Colorado State University, 2018).
2.6. Measures of short-term differences in cardiac function
To monitor cardiac function (electrocardiography; ECG), an ambulatory 12-lead digital Holter recorder (H12+, Mortara Instruments Inc., Milwaukee, WI, USA) approved by the US Food and Drug Administration (US FDA, 2005) was applied to participants by a trained technician at the beginning of each study day. Participants were asked to remove hair prior to arriving, however a shaver was provided for this purpose. The skin receiving Holter lead electrodes were further prepped (cleaned of oils and dead skin cells) by an alcohol wipe and abrasive paste. Individual electrode signal strength was checked prior to initiating Holter recording. The Holter recorder stored raw continuous data (sampled at 1000 Hz) on a high-frequency memory card.
While the Holter recorded cardiac function continuously, measurement periods at each of the 3 time-points lasted 5 min (marked by study staff pushing the Holter ‘event’ button) and followed a 10-min rest period all while participants laid supine. The Holter was removed following measurement at the 3-h after-treatment time-point.
Raw continuous data were extracted from Holter memory cards and a full suite of cardiac function parameters were calculated by a trained research nurse using Holter manufacturer software (Mortara Instruments Inc.). The following quality-control procedure steps were implemented: (1) the trained research nurse, blinded to the exposure randomization, manually edited the sequence of ECG complexes to ensure proper labeling of each QRS complex and to check that ECG traces correspond to biologically plausible gross observations of the participant; (2) the measurement time-points recorded by the internal clock of the device (pushing the Holter ‘event’ button) were cross-referenced and verified by the technician-noted time recorded at the start and end of exposure, and the start and end of measurement time-points, and; (3) all parameters were plotted to determine sample distribution and existence of measurement outliers.
Prepared HRV metrics include both frequency- and time-domain measures. Frequency-domain measures are VLF (very-low frequency: 0.0033–0.04 Hz), LF (low frequency: 0.04–0.15 Hz), and HF (high frequency: 0.15–0.4 Hz) power, and the ratio of LF to HF (LFHFR). Time-domain measures are SDNN (standard deviation of the duration of all NN intervals), pNN50 (percent of consecutive NN intervals that differ by more than 50 ms), and RMSSD (square root of the mean of the squares of the differences between adjacent NN intervals). Prepared cardiac repolarization metrics include P-complexity (representing P-wave/atrial depolarization), QRS-complexity (representing QRS-wave/ventricular depolarization), T-complexity (representing T-wave/ventricular repolarization), and QTc (average length of QT interval, the period from start of QRS-wave to end of T-wave, corrected for heart rate by dividing by the square root of RR interval).
2.7. Questionnaires
To capture information on potential confounders, participants self-administered the following questionnaires: (1) a questionnaire collecting time-invariant information on participant demographic characteristics (e.g., race, ethnicity) once during the study; (2) a questionnaire collecting information on the preceding 24 h, including acute cardiac/allergy symptoms (as a potential health response, and for potential acute care concerns for an adverse response to the treatment), alcohol and caffeine consumption, diet, exposures to smoke and other air pollution, medication use, physical activity, sleep duration, and their mode of travel to the study facility; (3) a questionnaire collecting information on the preceding few hours (between time-points), including behavior (e.g., caffeine consumption), at each of the three time-points on each study day (alongside cardiac function measurements).
2.8. Statistical analysis
All statistical analyses were performed in R (Version 3.5.0, The R Foundation for Statistical Computing). Descriptive statistics for treatment and health outcome parameters were mean, standard deviation, minimum, and maximum values.
All cardiac function measurements were analyzed as 5-min averages, guided by previous work (Baja et al., 2010; Shields et al., 2013; Cole-Hunter et al., 2016, 2018). LF and HF power were normalized for heart rate (i.e., converted to LFn and HFn, as normalized units [n.u.] respectively) according to previous work (Voss et al., 2015). Model residuals were evaluated for normality, heteroscedasticity, and potential outliers (i.e., meeting assumptions for linear models, to determine if transformations were needed) by diagnostic plots (i.e., QQ and residuals vs fitted values). Prior to modelling, all measurement parameters were transformed using the natural logarithm (presented as “percent difference from control”), except for pNN50, which was square-root transformed due to the presence of zero values (presented as “difference from control”).
Linear mixed-effect models (lmer function in R) were used to compare cardiac function measurements between exposure and control treatments per time-point (Bates et al., 2015). The primary models contained a fixed categorical term for cookstove treatment type (rather than a continuous term for PM2.5 emission/exposure concentration), a fixed continuous term for baseline (immediately before treatment) measurement value, a random intercept for participant, and a random intercept for date of the controlled exposure. We included the baseline term to account for variations in the outcomes between treatment levels at the beginning of each study day (i.e., variations unrelated to the controlled exposures) (Vickers and Altman, 2001). We included the term for participant to account for repeated measures within each participant. We included the term for date to account for correlation that may occur between participants who were part of the same study session. A sensitivity analysis was performed on a subset of the dataset, including only participants who completed their treatments as originally scheduled (i.e., ‘in-sequence’); models in these analyses included terms for sequence and session number.
Ambient PM2.5, relative humidity, and temperature values over the prior 24 h were calculated as mean, standard deviation, and range (minimum, maximum), and compared across treatments by Kruskal-Wallis rank sum tests. PM2.5 was the only ambient pollutant investigated as it was the only treatment pollutant measured, and only for the prior 24 h as this coincides with other potential confounder information collected (e.g., caffeine consumption, physical activity).
In addition, we conducted sensitivity analyses to assess for potential confounding separately by ambient concentrations of PM2.5, ambient relative humidity, and ambient temperature (averaged for the 24-hr period prior to treatment) as untransformed covariates added to the primary model. Univariate analyses by treatment were previously conducted to determine self-reported frequency of participant non-compliance with restricted use of alcohol, caffeine and medication, as well as self-reported mode of transport to study facility and sleep quantity (Fedak et al., 2019).
Beyond LFn and HFn (normalizing LF and HF for heart rate), models were not adjusted for heart rate nor mean arterial blood pressure since Holter recording occurred while the participant was at rest (laying supine). Moreover, with the exception of LFn and HFn, adjusting HRV for heart rate may remove meaningful variance that can be attributable to autonomic and neurophysiological phenomena (de Geus et al., 2019).
3. Results
3.1. Participant sample
In total, 48 participants (22 females, 26 males) were included in the analyses due to satisfying inclusion criteria and participating in at least two of the six scheduled treatments. The mean body mass index was 23.4 kg/m2 (standard deviation: 2.2 kg/m2; range: 19.4–28.7 kg/m2), and the mean age was 27.5 years (3.6 years; 20.5–36.1 years) at onset of participation. Baseline (pre-treatment) levels of cardiac autonomic activity parameters, presented in Table 1, were within a normal, healthy range (Agelink et al., 2001).
Table 1.
Baseline (pre-treatment) five-minute means of heart rate variability and cardiac repolarization.
| Domain | Parameter | Unit | Base |
0hr |
3hr |
|||
|---|---|---|---|---|---|---|---|---|
| na | value* | na | value* | na | value* | |||
| HRV, Freq. | VLF | ms2 | 253 | 2829 ± 3372 (65, 26186) | 253 | 4214 ± 5976 (208, 49720) | 253 | 3386 ± 4710 (164, 40928) |
| LFn | n.u. | 272 | 0.61 ± 0.17 (0.09, 0.90) | 272 | 0.57 ± 0.17 (0.11, 0.89) | 272 | 0.60 ± 0.17 (0.13, 0.89) | |
| HFn | n.u | 272 | 0.39 ± 0.17 (0.10, 0.91) | 272 | 0.43 ± 0.17 (0.11, 0.89) | 272 | 0.40 ± 0.17 (0.11, 0.87) | |
| LFHFR | 1:1 | 272 | 2.13 ± 1.61 (0.10, 8.80) | 272 | 1.73 ± 1.25 (0.12, 8.38) | 272 | 2.07 ± 1.49 (0.15, 8.02) | |
| HRV, Time | SDNN | ms | 272 | 78.1 ± 38.3 (15, 265) | 272 | 92.3 ± 43.8 (24, 270) | 272 | 82.0 ± 38.7 (23, 243) |
| RMSSD | ms | 272 | 61.3 ± 43.8 (9, 280) | 272 | 78.3 ± 47.6 (16, 285) | 272 | 63.6 ± 40.8 (14, 267) | |
| pNN50 | % | 272 | 28.2 ± 22.3 (0, 74) | 272 | 37.3 ± 22.4 (0, 79) | 272 | 30.5 ± 22.9 (0, 80) | |
| Cardiac repolar. | QTc | ms | 272 | 417.3 ± 19.7 (376.0, 471.1) | 272 | 416.3 ± 20.6 (378.5, 482.2) | 272 | 418.2 ± 20.2 (379.4, 482.9) |
| P-comp. | (unitless) | 272 | 0.20 ± 0.10 (0.07, 0.73) | 272 | 0.02 ± 0.11 (0.04, 0.78) | 272 | 0.20 ± 0.12 (0.07, 0.82) | |
| QRS-comp. | (unitless) | 272 | 0.45 ± 0.18 (0.08, 1.05) | 272 | 0.46 ± 0.18 (0.09, 1.01) | 272 | 0.45 ± 0.18 (0.09, 1.17) | |
| T-comp. | (unitless) | 272 | 0.12 ± 0.05 (0.01, 0.38) | 272 | 0.11 ± 0.05 (0.02, 0.30) | 272 | 0.12 ± 0.05 (0.02, 0.33) | |
Abbreviations: S.D. = Standard deviation; n.u. = normalized units; VLF = Very low frequency (Power in range 0.0033–0.04 Hz); LFn = Low frequency (Power in range 0.04–0.15 Hz), corrected for heart rate; HFn = High frequency (Power in HF range 0.15–0.4 Hz), corrected for heart rate; LFHFR = Ratio of LF over HF; SDNN = Standard deviation of the duration of all NN intervals; RMSSD = Square root of the mean of the squares of the differences between adjacent NN intervals; pNN50 = Percent of consecutive NN intervals that differ by more than 50 ms; QTc = Interval of time between Q and T interval, corrected for heart rate; P-comp. = P-complexity (representing P-wave/atrial depolarization); QRS-comp. = QRS-complexity (representing QRS-wave/ventricular depolarization); T-comp. = T-complexity (representing T-wave/ventricular repolarization).
The sample size (n) shown here is discrepant from the 288 expected (of 48 participants completing 6 treatments): while a total of 48 participants were included in analyses, not all participants could complete all treatments or had data available for all time-points – some treatments or time-points were missed due to participant absence or technical difficulties and the inability to reschedule repeats within the study period.
Values are presented as mean (standard deviation [minimum.,maximum]).
Including allotted make-up sessions, all 6 controlled exposures (treatments) were completed by 39 (81% of total) participants, while at least 5 treatments were completed by 45 (94% of total) participants. Among the 288 participant-treatments that were scheduled (48 participants × 6 treatments), 272 (94%) were included in the primary analysis; 261 (91%) were included in the secondary (in-sequence sensitivity) analysis. Fifteen participant-treatments were missed due to either known but unavoidable scheduling conflicts or unforeseen circumstances (e.g., acute illness) and an inability to make up sessions within the allotted scheduling period. Additionally, one participant-treatment was not included due to a recording error on the Holter device. Within the 816 (272 participant-treatments × 3 time-points) possible data points, 5 are missing due to scheduling conflicts for participants (resulting in them leaving a study day without completing the three-hour follow-up time-point). This results in 811 (94% of a possible 864 [48 participants × 6 treatments × 3 time-points]) data points included in analyses.
3.2. Treatment conditions
Mean exposure levels (see Table 2) across the cookstove technologies tested ranged from 462 μg/m3 for the three stone fire (interquartile range, IQR: 76 μg/m3) to 8 μg/m3 for the LPG cookstove (IQR: 4 μg/m3); the mean exposure for the filtered-air control was 0.5 μg/m3 (IQR: 0.4 μg/m3). In general, each cookstove category closely matched (although was typically lower than) the target exposure level, with the largest difference of achieved versus target level occurring for the gasifier (11 μg/m3; 31% below target) and the smallest difference for the rocket elbow (9 μg/m3; 3.6% above target).
Table 2.
Exposure concentration two-hour means of PM2.5 (and corresponding CO) according to treatment target levels, and differences from target: group time-weighted means (of 1-s measurements), and their associated (absolute and percentage) variations from target.
| Treatment type | Number of Treatmentsa | Target Level, μg/m3 | Achieved Mean Level [IQR], μg/m3 | Difference of Achieved versus Target Level [IQR], μg/m3 (%) | Corresponding Mean [SD] of CO* (ppm) |
|---|---|---|---|---|---|
| Control | 47 | 0 | 0.5 [0.4] | 0.9 [0.4] (NA) | 2 [2] |
| LPG | 45 | 10 | 8 [4] | 3 [3] (− 26.8%) | 3 [1] |
| Gasifier | 43 | 35 | 46 [9] | 11 [10] (+30.8%) | 5 [3] |
| Fan rocket | 44 | 100 | 95 [14] | 11 [13] (− 10.9%) | 8 [2] |
| Rocket elbow | 45 | 250 | 255 [24] | 9 [17] (+3.6%) | 6 [2] |
| Three stone fire | 47 | 500 | 462 [76] | 42 [60] (− 8.5%) | 9 [4] |
Abbreviations: CO, carbon monoxide; IQR, interquartile range; LPG, liquefied petroleum gas; n, sample size; NA, not applicable.
Not all participants could complete all treatments – some treatments were missed due to participant absence or technical difficulties and the inability to reschedule repeats within the study period.
Mean (SD): CO did not have a target concentration. This row is showing the measured mean CO concentration at each exposure level.
Within a treatment session, a participant’s (corresponding 2-h) interquartile range of real-time (1-s resolution) measurements of PM2.5 exposure indicated that target concentration levels did not vary substantially across the 2-h period, resulting in non-overlapping average exposure concentration ranges between different cookstoves (Table 2).
Mean ambient levels of PM2.5, relative humidity, and temperature in the 24-h period prior to treatments were substantially different for some treatments. For example, mean PM2.5 and temperature for this 24-h period was a few units higher for fan-rocket compared to other treatments (Table 3).
Table 3.
Mean ambient conditions for the 24-h prior to treatment.
| Treatment | Number of Days | PM2.5, μg/m3 | Temperature, °C | Relative Humidity, % |
|---|---|---|---|---|
| All | 81 | 7 ± 4 (1, 19) | 10 ± 7 (− 8, 24) | 49 ± 14 (23, 81) |
| Control | 15 | 5 ± 3 (2, 13) | 7 ± 6 (− 7, 20) | 56 ± 13 (27, 80) |
| LPG | 13 | 8 ± 5 (3, 19) | 11 ± 5 (3, 24) | 47 ± 11 (29, 67) |
| Gasifier | 13 | 5 ± 3 (1, 11) | 7 ± 7 (− 6, 14) | 50 ± 12 (32, 69) |
| Fan rocket | 14 | 9 ± 4 (2, 18)** | 16 ± 6** (5, 23) | 42 ± 13 (23, 69) ** |
| Rocket elbow | 14 | 6 ± 2 (3, 9) | 13 ± 10 (− 8, 24) | 44 ± 13 (27, 67) |
| Three stone fire | 12 | 6 ± 3 (1, 12) | 7 ± 5 (− 3, 15) | 56 ± 14 (40, 81) |
Ambient parameter values are presented as Mean ± S.D. (Minimum, Maximum). Abbreviations: S.D. = standard deviation; PM2.5 = fine particulate matter (particles of <2.5 μm diameter); LPG = liquified petroleum gas.
Kruskal-Wallis rank sum testing outcome; significantly different value compared to values of other treatments:
p < 0.05
p < 0.01.
3.3. Short-term differences in cardiac function following cookstove treatments compared to control
3.3.1. Primary model outcomes
Results from our primary models indicate differences in overall HRV (as indicated by SDNN), but not cardiac repolarization, at some time points for some cookstoves. All model outcomes reported below are for cookstove treatments in comparison to control (filtered air) treatment at the same time point.
3.3.1.1. Heart rate variability.
All HRV primary model outcomes are presented in Fig. 1, with numerical values provided in Supplemental Table A1.
We observed a general trend for SDNN (as an overall indicator of HRV) to be lower immediately, and higher 3 h, following all cookstove treatments compared to control. The cookstoves eliciting the greatest short-term difference in SDNN following treatment compared to control, in order of decreasing magnitude of difference, were three stone fire, gasifier, LPG and fan rocket (equally), and rocket elbow. General trends for VLF were similar to those for SDNN, however not for any of the other frequency-domain HRV parameters following cookstove compared to control. See Fig. 1 and Supplemental Table A1. Specifically, we observed lower SDNN immediately following treatment for three stone fire compared to control (β = − 0.13%, 95% CI = − 0.22, − 0.03%) and gasifier compared to control (β = − 0.12 ms {%}, 95% CI = − 0.22, − 0.02%)%%.
We observed higher SDNN at 3 h following treatment for LPG compared to control (β = 0.14%, 95% CI = 0.04, 0.23%) and rocket elbow compared to control (β = 0.15%, 95% CI = 0.05, 0.24%). We also observed higher RMSSD (β = 0.15%, 95% CI = 0.03, 0.27%) and pNN50 (β = 4.34%, 95% CI = 0.00, 8.69%) at 3 h following rocket elbow compared to control. We did not note any differences in other time-domain HRV parameters. In the frequency domain of HRV at 3 h following rocket elbow compared to control, we observed both higher HFn (β = 0.27%, 95% CI = 0.04, 0.51%) and VLF (β = 0.38%, 95% CI = 0.11, 0.64%). See Supplemental Table A1 for all frequency-domain primary model results.
3.3.1.2. Cardiac repolarization.
All cardiac repolarization primary model outcome values are presented in Supplemental Table A1.
Overall, we observed little evidence of an association between cookstove treatments and cardiac repolarization. However, we observed QTc to be higher at 3 h following rocket elbow treatment compared to control (β = 0.003%; 95%CI = 0, 0.007%). See Fig. 1 and Supplemental Table A1. P-complexity was higher immediately following gasifier treatment compared to control (β = 0.10%; 95% CI = 0.03, 0.17%). T-complexity was also higher immediately following LPG treatment compared to control (β = 0.06%; 95%CI = 0.01, 0.12%). Other cardiac repolarization endpoints were not different among cookstove treatments compared to control.
3.3.2. Sensitivity analyses
We did not observe evidence of differences compared to the primary results when adjusting for the 24-h (pre-treatment) mean levels of ambient PM2.5, temperature, or humidity (see Supplemental material text and Tables A2, A3 and A4, respectively). Some notable differences were (at either 0 or 3 h) following fan rocket and rocket elbow treatments, for example: SDNN 3 h following fan rocket treatment compared to control when adjusted for ambient PM2.5 (β = 0.08%; − 0.04, 0.19%) versus unadjusted (primary model) (β = 0.10%; 0.00, 0.20%); SDNN immediately following fan rocket treatment compared to control when adjusted for ambient temperature (−0.12%; − 0.22, − 0.01%) versus unadjusted (primary model) (−0.07%; − 0.17, 0.03%). See Supplemental Tables A1–A3.
Further, we did not observe evidence of differences when analyses were performed on a subset of the dataset containing only those participants who completed their schedule in-sequence, including terms for sequence and session number: the biggest difference was seen for VLF 3 h following three-stone fire treatment compared to control among the subset (0.28%; 0.02, 0.53%) versus the full data set (0.22%; − 0.03, 0.48%)(see Supplemental Table A1 and A5).
4. Discussion
We observed evidence of short-term differences in some metrics of cardiac function following short-term exposures to cookstove air pollution compared to filtered air among healthy adults. We observed these short-term differences across the suite of cookstove types studied, with the differences not following an increasing monotonic concentration–response for fine particulate matter (PM2.5). The results suggest that stoves emitting relatively low amounts of PM2.5 could still be capable of producing short-term changes in some HRV metrics, similar to the effect from stoves emitting higher amounts of PM2.5. In general, the strongest evidence of a short-term difference occurred in the HRV time-domain parameter of SDNN (which is a metric indicative of overall HRV) and the HRV frequency-domain parameter of VLF. Across cookstoves, the strongest effect on cardiac function occurred following the rocket elbow cookstove, which was the second highest categorical PM2.5 exposure concentration (250 μg/m3). We observed differences in the direction and magnitude of change for parameters between time-points (from immediate to 3-h following exposure).
A recent meta-analysis of two controlled human exposure studies and two epidemiological panel studies found consistent evidence of a short-term pathophysiological (HRV) response (<6 h) following short-term exposures to ambient-sourced PM2.5 and UFP (Breitner et al., 2019). Similar to our results, they found responses varied across time-points – direction of response was not consistent and magnitude was not monotonic. While the reviewed studies found a general decrease and recovery (increase) in SDNN, two studies (‘REHAB’, ‘UPDIABETES’) saw the same general decrease immediately (first hour) following exposure; the remaining two studies (‘Augsburg Panel’, ‘UPCON’) found the opposite (Breitner et al., 2019). Like our study, none of the evaluated studies found evidence of changes in RMSSD or T-complexity within the first few hours following exposure.
A chronic lowering of HRV indicates an imbalance in cardiac autonomic function and an increased risk of adverse cardiovascular events including myocardial infarction and stroke (Thayer et al., 2010). The higher SDNN at 3 h following treatment (compared to control) across three of the five cookstove types suggests a short-term recovery (return to baseline value) or compensation (increase above baseline value) in cardiac autonomic function following exposure, as seen in similar previous analyses among healthy individuals (Magari et al., 2002; Wu et al., 2011; Huang et al., 2013) and coronary artery disease patients (Zanobetti et al., 2010).
Specifically, while Magari et al. (2002) report decreased SDNN up to a PM2.5 moving average period of 3 h, a recovery and eventual compensation was seen from the 4-h to 9-h period. Similarly, while statistically insignificant, Zanobetti et al. (2010) report decreased SDNN up to a PM2.5 moving average of 2 h, and compensation by the (following) 48-h period. Huang et al. (2013) also report decreased but recovered SDNN up to a (maximum) 1-h moving average period, possibly too brief to see compensation. However, Wu et al. (2011) report some reduction in SDNN and other HRV parameters across increasing moving average periods (from 0.5 to 4 h), however less sign of recovery and no compensation.
Some studies have shown only short-term increases (without first a decrease) in SDNN following PM exposure (Riediker et al., 2004; Shields et al., 2013), such as within 30 min (Shields et al., 2013) suggesting that cardiac autonomic activity may be affected immediately upon sensing PM deposition in the lower respiratory system (Rhoden et al., 2005).
Interestingly, Wu et al.’s (2010) study of real-time traffic-related PM2.5 exposure showed that short-term (30-min) exposures at lower concentrations (<50 μg/m3) increased SDNN, while higher concentrations decreased SDNN. Most of our (cookstove-emitted) PM2.5 exposure concentrations were greater than this reported turning point; however, we still observed decreased SDNN following our lowest categorical concentration (10 μg/m3), potentially explained by our longer (2-h) exposure period and therefore higher accumulated PM2.5 dose. Our observation suggests that effects on HRV, sometimes in opposite directions, may be explained now only by timing of exposure in relation to outcome but also by a non-monotonic exposure-response relationship (discussed below).
Observing differences for SDNN and VLF, while not other HRV metrics, following exposure may make sense as VLF is highly correlated with SDNN and is predominately modulated by parasympathetic activity through the baroreceptor reflex (Shaffer and Ginsberg, 2017). VLF is more strongly associated with all-cause mortality than other frequency domain measures (LF, HF), and low VLF has been associated with increased inflammation (physiological stress response) and cardiovascular health risk (Shaffer and Ginsberg, 2017). The absence of difference we observed in most of the suite of HRV or cardiac repolarization parameters, however, challenges the interpretation of our findings.
4.1. PM2.5 concentration-response
One could hypothesize that the physiological response to cookstove smoke exposure would have a monotonic relationship with PM2.5 concentration, in that larger concentrations elicit larger responses. In this study, the highest target concentration was approximately 50 times higher than the lowest target (i.e., 500 versus 10 μg/m3). However, we did not observe a proportionally greater magnitude of cardiac autonomic response following this greater concentration compared to lesser or the lowest concentration treatment. The difference in SDNN we observed immediately after gasifier (PM2.5: 35 μg/m3) compared to control was similar to that observed immediately after three stone fire compared to control, despite the latter treatment having over 10 times the concentration of PM2.5. Similarly, the difference in SDNN we observed immediately after rocket elbow (PM2.5: 250 μg/m3) compared to control was lower in magnitude to that observed immediately after fan rocket (100 μg/m3) compared to control, despite the latter delivering less than half the PM2.5 concentration of the former. This non-monotonic concentration–response relationship is consistent with our previous analyses (Fedak et al., 2019, 2020; Walker et al., 2020a, 2020b) and similar to our observations for other time-domain and frequency-domain measures of HRV, as well as cardiac repolarization. Short-term physiological effects may follow a threshold-response whereby any PM2.5 concentration level produces a binary response. Such a threshold-response is not suggested by previous studies evaluating short-term exposures (U.S. EPA, 2019). However, most of those studies are observational and not controlled human exposure studies.
4.2. Composition of cookstove emissions profiles
Improved cookstoves (e.g., rocket elbow) may substantially reduce emission concentrations of PM2.5 compared to traditional cookstoves (e. g., three stone fire), however other combustion-related emissions such as UFP have been seen to increase (de la Sota et al., 2018). Pollutants besides PM2.5 can vary in emission level depending on fuel or cookstove type used (Bilsback et al., 2019). In our previously published results, we see that concentrations of emissions such as carbon monoxide generally increased with cookstove-determined PM2.5 target concentrations (Fedak et al., 2019). However, compared to other treatments: ultrafine particle (UFP; particle aerodynamic diameter < 0.1 μm) and carbonyl concentrations were higher for LPG treatment; nitric oxide concentrations were highest for fan rocket treatment, although comparable to rocket elbow treatment, and; elemental carbon concentrations were higher for rocket elbow treatments (Fedak et al., 2019).
Studies on UFP emissions from cookstoves are scarce (Patel et al., 2016), yet UFP are considered an important exposure in addition to PM2.5 when studying adverse health effects (Chan et al., 2004; Pan et al., 2018). Our cookstove emission characterization test results do not point to a particle-size differentiation (difference in particle number concentrations, dominated by UFP) between cookstove treatments, despite some cookstoves (fan rocket, rocket elbow, three stone fire) burning the same fuel but at different rates to achieve different target PM2.5 concentrations (PM2.5: 100, 250, 500 μg/m3, respectively) (Fedak et al., 2019). However, our characterization measurements saw that the rocket elbow, while emitting half of the concentration of PM2.5 compared to three stone fire, emitted more than double the concentration (~100 μg/m3) of elemental carbon as well as the highest concentration of nitrous oxides (NOx/NO2) and acetone compared to all cookstoves tested (Fedak et al., 2019).
Higher proportions of combustion-derived elemental carbon and chemical emissions may partly explain our observed stronger differences in effect on several HRV parameters (SDNN, RMSSD, pNN50, HFn, LFn, VLF) following rocket elbow compared to other treatments. Black carbon (measured as elemental carbon) is a component of PM2.5 believed to have higher toxicity than total PM2.5, formed by incomplete combustion of solid fuel (Janssen et al., 2011, 2012). Recent evidence suggests that black carbon may adsorb and carry combustion-derived chemicals (such as complex hydrocarbons, carbonyls, including acetone) that interact with and modify the effect of PM mass on various sensitive tissues around the body (Janssen et al., 2011; Cassee et al., 2013; Niranjan and Thakur, 2017; Grady et al., 2018). A recent systematic review of cardiovascular endpoints including HRV stated that there is insufficient evidence to determine a difference in health effect between ambient black carbon and PM2.5 exposure (Kirrane et al., 2019). Some evidence does exist, however, to suggest a greater effect of black carbon on HRV (Schwartz, 2005) and cardiac repolarization (Baja et al., 2010; Xu et al., 2019). Impaired cardiac function has been associated with short-term exposure to traffic-related NO2 and NOx in some studies (Shields et al., 2013; Brook, Newby and Rajagopalan, 2018), but not all (Cole-Hunter et al., 2016, 2018; Laeremans et al., 2018), suggesting further research is needed, particularly with cookstove-related emissions.
4.3. Limitations of our study
Our analysis only considers a single 5-min measurement at two (post-exposure) time-points, immediately following and 3 h following treatment. This limitation does not allow us to capture a complete description of the overall impact of exposure on cardiac function over time, including instantaneous and next-day effects corresponding to exposure. Our selected time-points may not have captured the period in which a full effect, and therefore a clear exposure-response across cookstoves, is seen. An alternative design to be considered for future research would involve an equivalent protocol for participant measurements within the exposure facility, allowing the capture of instantaneous exposure effects. Such a design was not feasible with the available exposure facility, due to the controlled and space-limited area, and consideration of participant comfort (remaining supine). Similarly, we did not capture next-day (approximately 20 h following treatment) effects due to the removal of the Holter at the end of the first study day. This removal was to minimize participant burden and due to the expectation, based on current literature, that effects would be more immediate rather than lagged.
The different target PM2.5 concentration-exposure levels were set to be fairly representative of real-world cookstove technology emission/exposure levels that need to be addressed as intervention studies (World Health Organization, 2014; Pope et al., 2017), allowing some transferability of evidence to the field. However, using the same target PM2.5 concentration level across treatments would have allowed identification of differences in health effects due to composition of pollution mixtures at the same PM2.5 mass concentration and design/technology aspects (e. g., combustion efficiency, construction materials). As it is, both our combustion source (particle characteristics) and particle concentrations vary between experimental conditions, which reduces our ability to clearly interpret our findings compared to studies that examine across the same source or same concentration of particles. While our PM2.5 exposure gradient is larger than most studies making similar observations in cardiac function (Magari et al., 2002; Gong et al., 2008; Baja et al., 2010; McCracken et al., 2011; Tong et al., 2012; Shields et al., 2013; Unosson et al., 2013), an inadequate concentration or dose may explain the lack of consistent or notable differences between treatments or at all in some metrics such as QRS-complexity. Our study design was such to discern differences of exposure from different cookstove technologies or fuels (i.e., whole system), reflecting real-world conditions, rather than PM2.5 emission concentration alone or a clear exposure–response curve.
Due to the many cookstove technologies and cardiac function outcomes measured at multiple time-points, we must interpret our findings with caution due to multiple comparison tests being conducted. There may have been an issue with statistical power sufficiently capturing effects across the PM2.5 exposure gradient. Importantly, we may not have been adequately powered to detect small changes in cardiac function.
Finally, our study population did not consist of real-world cookstove users, nor were measurements made in real-world conditions – participants were screened to exclude regular air pollution exposures and underlying disease and were treated with intermittent rather than chronic exposure within a controlled environment. Moreover, our strict inclusion criteria, for the purpose of participant safety, limited the range of values for age, body mass index, smoking status, medical history, and other criteria which may modify measured effects. As such, our findings are not necessarily generalizable to real-world populations or environments of cookstove use. Underlying health conditions convey susceptibility for air pollution effects on the cardiac repolarization response (Xu et al., 2019). A similar exposure study design to ours, although focused on ambient UFP, also observed higher QTc and QRS-complexity among adults with metabolic syndrome, although the results were not statistically significant; stratifying the study population by a known genetic susceptibility (GSTM1) showed a greater (and statistically significant) increase in both metrics (Devlin et al., 2014).
4.4. Strengths of our study
To our knowledge, we have conducted the most comprehensive controlled human exposure study of cookstove emissions. We studied a relatively high number of cookstove technologies and cardiac autonomic activity endpoints. Moreover, we obtained a relatively high sample size of healthy adult participants that largely complied with consumption restrictions. We collected information on dietary intake of participants during participation, to note compliance of the requirement to restrict high-fat and high-cholesterol foods, which was satisfactory. The participant self-reported use of alcohol, caffeine, and medication was low throughout the study; bivariate analyses indicated no evidence of associations between these or other potentially confounding covariates (e.g., ambient PM2.5 and CO) and the various treatments (see Fedak et al., 2019 Data S2 and Tables S2–S11). Findings from controlled studies are more robust by the minimization of (or selection for) participant confounders (e.g., age, co-morbidities) and complement less internally valid observational studies of real-world use(r)s.
The main strength of our study comes from its design, being controlled by a specialized Latin (William’s) square structure for maintaining participant procession within a group of four, consistently (pending participant availability) across study dates and treatments. This design balanced treatments and first-order carryover effects, minimizing potential confounding associated with time-invariant factors at the personal level with each participant receiving each treatment and time-variant factors (e.g., age, ambient conditions, caffeine or alcohol consumption) and study experience (comfort), and also seasonal variation in ambient conditions.
Further still, participants themselves were blinded to the order of exposure to minimize bias in perception of treatment and a potential stress response, in anticipation of higher exposures, reflected in cardiac autonomic activity.
Finally, the administration of our treatments was controlled to maintain pre-determined (target) PM2.5 concentration levels. This control allowed a categorical analysis of cookstove type to augment evidence for real-world health implications when and where that cookstove type is used. This control/analysis was made possible by the presence of a trained technician and an automated modulatory exposure-delivery system not possible in a field study. Our controlled design avoided large fluctuations of exposure/emissions concentrations to help interpret our health responsesresponse, which is a major challenge in field studies due to the nature of cookstoves and combustion of solid fuel producing such fluctuations.
4.5. Conclusion
We find evidence to suggest that a short-term adverse difference in cardiac function, specifically in HRV (SDNN and VLF), can occur immediately following short-term exposures to even low or “improved” levels of cookstove-emitted PM2.5 among healthy adults. While the magnitude of our effect sizes was small, our findings point to a mechanism that may explain part of the public health burden from the global use of cookstoves. Exposure to household air pollution from cookstove emissions, including PM2.5, contributes substantially to global air pollution-attributable deaths; growing evidence supports that long-term exposure to PM2.5 magnifies cardiovascular health risk by triggering short-term cardiovascular dysfunction (Brook et al., 2018).
Future work could address two principal challenges highlighted by our study. First, there is a need to assess associations across longer exposure lags due to opposing effects at different lags, by following participants with Holter monitorsHolters applied over a 24-h period. Secondly, there is a need to investigate further the issue of a non-monotonic exposure-response that may be confounded by co-pollutants, by varying exposure levels using the same cookstove/fuel. These designs could be assisted by bridging consistencies across field studies of cookstove emissions, as well as both observational and experimental studies of ambient air pollution exposure (e.g., considering exposure windows of shorter or longer duration).
Supplementary Material
Acknowledgements
We acknowledge that this study would not be possible without our volunteer participants, the supervisory medical staff (study nurses and doctors) from Heart Center of the Rockies, and students of Colorado State University who helped to run our controlled exposure facility (in alphabetical order of surname: Evan Guiderra, Kate Gutekunst, Danny Stringer, and Lizette VanZyl).
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (NIH) under award number ES023688. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. At the time of submission, Tom Cole-Hunter receives funding in the form of a postdoctoral research fellowship from the Centre for Air pollution, energy, and health Research (CAR), a National Health and Medical Research Council (NHMRC) Centre for Research Excellence, under award number APP1116412. The authors certify that their freedom to design, conduct, interpret, and publish research is not compromised by any controlling sponsor, including CAR and NHMRC. That is, the funding sources had no involvement: in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The research described in this article has been reviewed by the Environmental Protection Agency and approved for publication. The contents of this article do not necessarily represent Agency policy nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106254.
References
- Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D, 2001. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin. Auton. Res. 11 (2), 99–108. [DOI] [PubMed] [Google Scholar]
- Alexander D, Larson T, Bolton S, Vedal S, 2015. Systolic blood pressure changes in indigenous Bolivian women associated with an improved cookstove intervention. Air Qual. Atmos. Health 8 (1), 47–53. [Google Scholar]
- Alexander D, Northcross A, Wilson N, Dutta A, Pandya R, Ibigbami T, Adu D, Olamijulo J, Morhason-Bello O, Karrison T, Ojengbede O, Olopade CO, 2017. Randomized controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. Am. J. Respir. Crit. Care Med. 195 (12), 1629–1639. [DOI] [PubMed] [Google Scholar]
- Baja ES, Schwartz JD, Wellenius GA, Coull BA, Zanobetti A, Vokonas PS, Suh HH, 2010. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study. Environ. Health Perspect. 118 (6), 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, et al. , 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 (1) 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, Bautista LE, 2011. Indoor air pollution and blood pressure in adult women living in rural China. Environ. Health Perspect. 119 (10), 1390–1395.21724522 [Google Scholar]
- Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M, 2014. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc. Natl. Acad. Sci. 111 (36), 13229–13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Carter E, Schauer JJ, Ezzati M, Daskalopoulou SS, Valois M-F, Shan M, Yang X, 2018. Household air pollution and measures of blood pressure, arterial stiffness and central haemodynamics. Heart 104 (18), 1515–1521. [DOI] [PubMed] [Google Scholar]
- Bilsback KR, Dahlke J, Fedak KM, Good N, Hecobian A, Herckes P, L’Orange C, Mehaffy J, Sullivan A, Tryner J, Van Zyl L, Walker ES, Zhou Y, Pierce JR, Wilson A, Peel JL, Volckens J, 2019. A laboratory assessment of 120 air pollutant emissions from biomass and fossil fuel cookstoves. Environ. Sci. Technol. 53 (12), 7114–7125. [DOI] [PubMed] [Google Scholar]
- Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, Lahiff M, Rehfuess EA, Mishra V, Smith KR, 2013. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ. Health Perspect. 121 (7), 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner S, Peters A, Zareba W, Hampel R, Oakes D, Wiltshire J, Frampton MW, Hopke PK, Cyrys J, Utell MJ, Kane C, Schneider A, Rich DQ, 2019. Ambient and controlled exposures to particulate air pollution and acute changes in heart rate variability and repolarization. Sci. Rep. 9 (1) 10.1038/s41598-019-38531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Newby DE, Rajagopalan S, 2018. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am. J. Hypertension 31 (1), 1–10. 10.1093/ajh/hpx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs Peña M, Romero KM, Velazquez EJ, Davila-Roman VG, Gilman RH, Wise RA, Miranda JJ, Checkley W, 2015. Relationship between daily exposure to biomass fuel smoke and blood pressure in high-altitude Peru. Hypertension 65 (5), 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassee FR, Héroux M-E, Gerlofs-Nijland ME, Kelly FJ, 2013. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhalation Toxicol. 25 (14), 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C-C, Chuang K-J, Shiao G-M, Lin L-Y, 2004. Personal exposure to submicrometer particles and heart rate variability in human subjects. Environ. Health Perspect. 112 (10), 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Bazemore H, Reynolds SJ, Heiderscheidt JM, Conway S, Bachand AM, Volckens J, Peel JL, 2011. A baseline evaluation of traditional cook stove smoke exposures and indicators of cardiovascular and respiratory health among Nicaraguan women. Int. J. Occup. Environ. Health 17 (2), 113–121. [DOI] [PubMed] [Google Scholar]
- Clark ML, Bachand AM, Heiderscheidt JM, Yoder SA, Luna B, Volckens J, Koehler KA, Conway S, Reynolds SJ, Peel JL, 2013. Impact of a cleaner-burning cookstove intervention on blood pressure in Nicaraguan women. Indoor Air 23 (2), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Hunter T, Weichenthal S, Kubesch N, Foraster M, Carrasco-Turigas G, Bouso L, Martínez D, Westerdahl D, de Nazelle A, Nieuwenhuijsen M, 2016. Impact of traffic-related air pollution on acute changes in cardiac autonomic modulation during rest and physical activity: a cross-over study. J. Expo Sci. Environ. Epidemiol. 26 (2), 133–140. [DOI] [PubMed] [Google Scholar]
- Cole-Hunter T, de Nazelle A, Donaire-Gonzalez D, Kubesch N, Carrasco-Turigas G, Matt F, Foraster M, Martínez T, Ambros A, Cirach M, Martinez D, Belmonte J, Nieuwenhuijsen M, 2018. Estimated effects of air pollution and space-time-activity on cardiopulmonary outcomes in healthy adults: a repeated measures study. Environ. Int. 111, 247–259. [DOI] [PubMed] [Google Scholar]
- Colorado State University, 2018. Atmospheric science department’s Christman Field Weather Station. Available at: https://www.atmos.colostate.edu/fccwx/fccwx_data_form.php.
- Dekker JM, Crow RS, Hannan PJ, Schouten EG, Folsom AR, 2004. Heart rate-corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle-aged men and women. J. Am. Coll. Cardiol. 43 (4), 565–571. [DOI] [PubMed] [Google Scholar]
- Devlin RB, et al. , 2014. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol. Sci. 140 (1), 61–72. 10.1093/toxsci/kfu063. [DOI] [PubMed] [Google Scholar]
- Dutta A, Ray MR, 2012. Prevalence of hypertension and pre-hypertension in rural women: a report from the villages of West Bengal, a state in the eastern part of India. Australian Journal of Rural Health 20 (4), 219–225. 10.1111/j.1440-1584.2012.01287.x. [DOI] [PubMed] [Google Scholar]
- Fedak KM, Good N, Walker ES, Balmes J, Brook RD, Clark ML, Cole-Hunter T, Devlin R, L’Orange C, Luckasen G, Mehaffy J, Shelton R, Wilson A, Volckens J, Peel JL, 2019. Acute effects on blood pressure following controlled exposure to cookstove air pollution in the STOVES study. JAHA 8 (14). 10.1161/JAHA.119.012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedak KM, Good N, Walker ES, Balmes J, Brook RD, Clark ML, Cole-Hunter T, Devlin R, L’Orange C, Luckasen G, Mehaffy J, Shelton R, Wilson A, Volckens J, Peel JL, 2020. Acute changes in lung function following controlled exposure to cookstove air pollution in the subclinical tests of volunteers exposed to smoke (STOVES) study. Inhalation Toxicol. 32 (3), 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJC, Gianaros PJ, Brindle RC, Jennings JR, Berntson GG, 2019. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 56 (2), e13287 10.1111/psyp.2019.56.issue-210.1111/psyp.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Soukup JM, Case M, Dailey LA, Richards J, Berntsen J, Devlin RB, Stone S, Rappold A, 2012. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup. Environ. Med. 69 (3), 170–175. [DOI] [PubMed] [Google Scholar]
- Gong Jr. [decea,H., Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, Cascio WE, Devlin RB, 2008. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhalation Toxicol. 20 (6), 533–545. [DOI] [PubMed] [Google Scholar]
- Grady ST, Koutrakis P, Hart JE, Coull BA, Schwartz J, Laden F, Zhang J, Gong J, Moy ML, Garshick E, 2018. Indoor black carbon of outdoor origin and oxidative stress biomarkers in patients with chronic obstructive pulmonary disease. Environ. Int. 115, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Deng F, Wu S, Lu H, Hao Y.u., Guo X, 2013. The impacts of short-term exposure to noise and traffic-related air pollution on heart rate variability in young healthy adults. J. Expo Sci. Environ. Epidemiol. 23 (5), 559–564. [DOI] [PubMed] [Google Scholar]
- Janssen NAH, Hoek G, Simic-Lawson M, Fischer P, van Bree L, ten Brink H, Keuken M, Atkinson RW, Anderson HR, Brunekreef B, Cassee FR, 2011. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM 10 and PM 2.5. Environ. Health Perspect. 119 (12), 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter J, Zhao Y, Smith KR, Khan B, Yelverton T, DeCarlo P, Hays MD, 2012. Pollutant emissions and energy efficiency under controlled conditions for household biomass cookstoves and implications for metrics useful in setting international test standards. Environ. Sci. Technol. 46 (19), 10827–10834. [DOI] [PubMed] [Google Scholar]
- Johnson, et al. , 2011. Modeling indoor air pollution from cookstove emissions in developing countries using a Monte Carlo single-box model. Atmospher. Environ. 10.1016/j.atmosenv.2011.03.044. [DOI] [Google Scholar]
- Kirrane EF, Luben TJ, Benson A, Owens EO, Sacks JD, Dutton SJ, Madden M, Nichols JL, 2019. A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ. Int. 127, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Orange C, Leith D, Volckens J, DeFoort M, 2015. A quantitative model of cookstove variability and field performance: Implications for sample size. Biomass Bioenergy 72, 233–241. [Google Scholar]
- L’Orange C, Volckens J, DeFoort M, 2012. Influence of stove type and cooking pot temperature on particulate matter emissions from biomass cook stoves. Energy Sustain. Develop. 16 (4), 448–455. [Google Scholar]
- de la Sota C, Lumbreras J, Pérez N, Ealo M, Kane M, Youm I, Viana M, 2018. Indoor air pollution from biomass cookstoves in rural Senegal. Energy Sustain. Develop. 43, 224–234. [Google Scholar]
- Laeremans M, Dons E, Avila-Palencia I, Carrasco-Turigas G, Orjuela JP, Anaya E, Cole-Hunter T, de Nazelle A, Nieuwenhuijsen M, Standaert A, Van Poppel M, De Boever P, Int Panis L, 2018. Short-term effects of physical activity, air pollution and their interaction on the cardiovascular and respiratory system. Environ. Int. 117, 82–90. [DOI] [PubMed] [Google Scholar]
- Lai AM, Carter E, Shan M, Ni K, Clark S, Ezzati M, Wiedinmyer C, Yang X, Baumgartner J, Schauer JJ, 2019. Chemical composition and source apportionment of ambient, household, and personal exposures to PM2.5 in communities using biomass stoves in rural China. Sci. Total Environ. 646, 309–319. [DOI] [PubMed] [Google Scholar]
- Liao D, Shaffer ML, Rodriguez-Colon S, He F, Li X, Wolbrette DL, Yanosky J, Cascio WE, 2010. Acute adverse effects of fine particulate air pollution on ventricular repolarization. Environ. Health Perspect. 118 (7), 1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magari SR, Schwartz J, Williams PL, Hauser R, Smith TJ, Christiani DC, 2002. The association between personal measurements of environmental exposure to particulates and heart rate variability. Epidemiology 13 (3), 305–310. [DOI] [PubMed] [Google Scholar]
- McCracken J, Smith KR, Stone P, Díaz A, Arana B, Schwartz J, 2011. Intervention to lower household wood smoke exposure in Guatemala reduces ST-segment depression on electrocardiograms. Environ. Health Perspect. 119 (11), 1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J, 2007. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ. Health Perspect. 115 (7), 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane M, Basnyat B, Fischer R, Froeschl G, Wolbers M, Rehfuess EA, 2015. Sustained use of biogas fuel and blood pressure among women in rural Nepal. Environ. Res. 136, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan R, Thakur AK, 2017. The toxicological mechanisms of environmental soot (black carbon) and carbon black: focus on oxidative stress and inflammatory pathways. Front. Immunol. 8 10.3389/fimmu.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C, Goldberg MS, Marshall JD, Valois M-F, Pradeep T, Narayanswamy M, Jain G, Sethuraman K, Baumgartner J, 2016. A panel study of the acute effects of personal exposure to household air pollution on ambulatory blood pressure in rural Indian women. Environ. Res. 147, 331–342. [DOI] [PubMed] [Google Scholar]
- Pan L.u., Wu S, Li H, Xu J, Dong W, Shan J, Yang X, Chen Y, Shima M, Deng F, Guo X, 2018. The short-term effects of indoor size-fractioned particulate matter and black carbon on cardiac autonomic function in COPD patients. Environ. Int. 112, 261–268. [DOI] [PubMed] [Google Scholar]
- Patel S, Leavey A, He S, Fang J, O’Malley K, Biswas P, 2016. Characterization of gaseous and particulate pollutants from gasification-based improved cookstoves. Energy Sustain. Develop. 32, 130–139. [Google Scholar]
- Pope CA III, Dockery DW, 2006. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manag. Assoc. 56 (6), 709–742. [DOI] [PubMed] [Google Scholar]
- Pope D, Bruce N, Dherani M, Jagoe K, Rehfuess E, 2017. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM 2.5 and CO: Systematic review and meta-analysis. Environ. Int. 101, 7–18. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, González-Flecha B, 2005. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim. Biophys. Acta (BBA) - General Subjects 1725 (3), 305–313. [DOI] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, Williams RW, Devlin RB, 2004. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am. J. Respir. Crit. Care Med. 169 (8), 934–940. [DOI] [PubMed] [Google Scholar]
- Schwartz J, 2005. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax 60 (6), 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP, 2017. An overview of heart rate variability metrics and norms. Front. Public Health 5 (September), 1–17. 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields KN, Cavallari JM, Hunt MJO, Lazo M, Molina M, Molina L, Holguin F, 2013. Traffic-related air pollution exposures and changes in heart rate variability in Mexico City: a panel study. Environ. Health 12 (1). 10.1186/1476-069X-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, et al. , 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Stu. The Lancet 392 (10159), 1923–1994. 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF, 2010. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 141 (2), 122–131. [DOI] [PubMed] [Google Scholar]
- Thomas E, Wickramasinghe K, Mendis S, Roberts N, Foster C, 2015. Improved stove interventions to reduce household air pollution in low and middle income countries: a descriptive systematic review. BMC Public Health 15 (1). 10.1186/s12889-015-2024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Rappold AG, Diaz-Sanchez D, Steck SE, Berntsen J, Cascio WE, Devlin RB, Samet JM, 2012. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution–induced cardiac effects and lipid changes in healthy middle-aged adults. Environ. Health Perspect. 120 (7), 952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N, et al. , 2012. Health effects of black carbon. Available at: http://ww.culturarsc.com/Medioambiente/Health-Effects-of-Black-Carbon.pdf (accessed: 24 November 2013). [Google Scholar]
- U.S. Environmental Protection Agency, 2018. Air quality data API. Available at: https://aqs.epa.gov/api.
- U.S. EPA, 2019. Integrated Science Assessment for Particulate Matter (Final Report, 2019). Washington, DC (United States). doi: EPA/600/R-19/188. [Google Scholar]
- Unosson J, Blomberg A, Sandström T, Muala A, Boman C, Nyström R, Westerholm R, Mills NL, Newby DE, Langrish JP, Bosson JA, 2013. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol 10 (1), 20 10.1186/1743-8977-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA, 2005. Mortara H12+ Holter Recorder. 510(k): Device Summary. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf5/K050896.pdf.
- Vickers AJ, Altman DG, 2001. Statistics Notes: Analysing controlled trials with baseline and follow up measurements. BMJ 323 (7321), 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A, et al. , 2015. Short-term heart rate variability—influence of gender and age in healthy subjects. PLOS ONE 10 (3), e0118308 10.1371/journal.pone.0118308 Edited by Hernandez AV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ES, Fedak KM, Good N, Balmes J, Brook RD, Clark ML, Cole-Hunter T, Devlin RB, et al. , 2020a. Acute differences in blood lipids and inflammatory biomarkers following controlled exposures to cookstove air pollution in the STOVES study. Int. J. Environ. Health Res. 2, 1–14. 10.1080/09603123.2020.1785402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ES, Fedak KM, Good N, Balmes J, Brook RD, Clark ML, Cole-Hunter T, Dinenno F, Devlin RB, L’Orange C, Luckasen G, Mehaffy J, Shelton R, Wilson A, Volckens J, Peel JL, 2020b. Acute differences in pulse wave velocity, augmentation index, and central pulse pressure following controlled exposures to cookstove air pollution in the Subclinical Tests of Volunteers Exposed to Smoke (SToVES) study. Environ. Res. 180, 108831 10.1016/j.envres.2019.108831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, 2012. Selected physiological effects of ultrafine particles in acute cardiovascular morbidity. Environ. Res. 115, 26–36. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Hatzopoulou M, Goldberg MS, 2014. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part. Fibre Toxicol. 11 (1), 70 10.1186/s12989-014-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, 1949. Experimental designs balanced for the estimation of residual effects of treatments. Austral. J. Sci. Res., Series A: Phys. Sci. 2 (149), 149–168. 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- World Health Organization, 2014. WHO indoor air quality guidelines: household fuel combustion. WHO Press; Available at: http://www.who.int/airpollution/publications/household-fuel-combustion/en/. [PubMed] [Google Scholar]
- Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X, 2011. The relationship between traffic-related air pollutants and cardiac autonomic function in a panel of healthy adults: a further analysis with existing data. Inhalation Toxicol. 23 (5), 289–303. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen J, Zhao Q, Zhang Y.i., Wang T, Feng B, Wang Y, Liu S, Yi T, Liu S, Wu R, Zhang Q, Fang J, Song X, Rajagopalan S, Li J, Brook RD, Huang W, 2019. Ambient air pollution is associated with cardiac repolarization abnormalities in healthy adults. Environ. Res. 171, 239–246. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, Speizer FE, 2010. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ. Health Perspect. 118 (3), 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareba W, Nomura A, Couderc JP, 2001. Cardiovascular effects of air pollution: what to measure in ECG? Environ. Health Perspect. 109 (suppl 4), 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.