Abstract
Millions of people worldwide with incurable liver disease die because of inadequate treatment options and limited availability of donor organs for liver transplantation. Regenerative medicine as an innovative approach to repairing and replacing cells, tissues, and organs, is undergoing a major revolution due to the unprecedented need of organs for patients around the world. Induced pluripotent stem cells have been widely studied in the field of liver regeneration and considered to be the most promising candidate therapies. This review will conclude the current state of efforts to derive hiPSCs for potential use in modelling and treatment on liver disease.
Keywords: induced pluripotent stem cells, liver diseases, liver transplantation, regeneration medicine, blastocyst complementation
Introduction
Tens of patients worldwide are affected by liver disease. Liver transplantation, the ultimate cell therapy, is presently the only proven treatment for many medically refractory liver diseases, including end-stage liver disease and many inherited liver diseases. However, there is a profound shortage of transplantable donor livers. According UNOS/OPTN data , there were 9675 liver donors in 2019 while the liver transplant waiting list number is about 13,000. This mismatch between donors and recipiemnts is most urgent for listed patient’s whose MELD score is >30 corresponding to >50% mortality in the next 90 days . Regenerative medicine, which focuses on innovative approaches to repairing and replacing cells, tissues, and organs, is undergoing a major revolution due to the unprecedented need of organs for patients around the world. Many patients with liver disease can benefit from cell therapy involving biologically active living cells, either by treatment of their liver disease or by prevention of their disease phenotype to get the liver donor for transplantation. Cell therapy, including hepatocyte transplantation and bioartificial liver (BAL) devices, has been proposed as a therapeutic alternative to the shortage of transplantable livers. Both BAL and hepatocyte transplantation are cellular therapies that avoid use of a whole liver. Hepatocytes are also widely used in drug screening and liver disease modelling. However, the demand for human hepatocytes heavily outweighs their availability [1, 2].
The recent discovery that induced pluripotent stem cells (iPSCs) can be derived from human somatic cells through forced expression of a few transcription factors has renewed hopes for regenerative medicine and in vitro disease modelling, as these cells are easily accessible. iPSC technology brings together the potential benefits of embryonic stem cells (ESCs) (ie, self-renewal, pluripotency) and addresses the 2 ethical concerns of ESCs: 1) iPSCs bypass the ethical concerns of embryo destruction since they are produced from somatic cells in vitro without embryonic tissues or oocytes, and 2) the immune-compatibility issues since their potential for generating patient-specific cell types for cell replacement therapy[3], It was shown that hepatocyte-like cells (HLCs) could be generated from iPSCs. Our previous study [4] and others [5] reporting the potential benefits of HLCs generated from human iPSCs (hiPSCs) have described their secretion of human albumin, alpha-1-antitrypsin (A1AT), and hepatocyte nuclear factor 4-alpha, synthesis of urea, and expression of cytochrome P450 enzymes in vitro. Therefore, in theory, hiPSCs could provide an unlimited source of human hepatocytes and hold great promise for applications in regenerative medicine, drug screening, and liver diseases modelling. Transplantation of hiPSC-derived HLCs could represent an alternative to liver transplantation for the treatment of acute liver failure (ALF) and chronic liver failure, liver cirrhosis, viral hepatitis, nonalcoholic fatty liver disease (NAFLD), and the correction of inherited metabolic liver disorders resulting from genetically deficient states. This review will focus on the current state of efforts to derive hiPSCs for potential use in modelling and treatment of liver disease. We will also highlight current applications of iPSCs in liver disease, future challenges and research frontiers.
Application of iPSCs in Liver Diseases
Regenerative Medicine
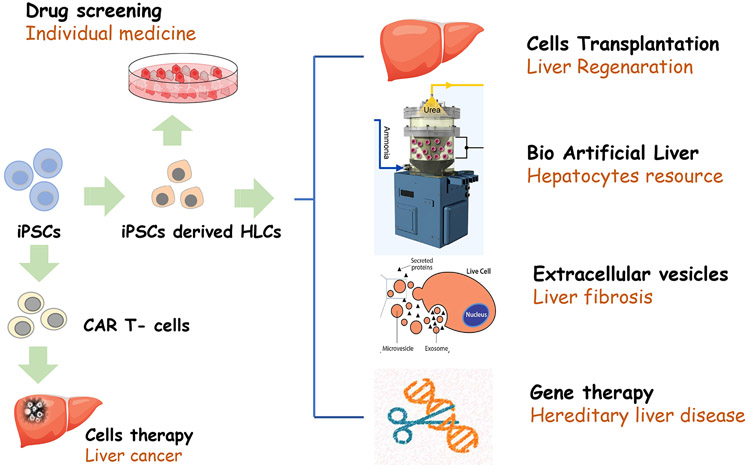
There are many potential applications of iPSCs in liver disease as illustrated in Figure 1. iPSC-derived HLCs from normal individuals can be used in the establishment of cell banks for applications in regenerative medicine. Results from various studies have demonstrated the therapeutic potential of iPSC-derived HLCs in liver diseases. Researchers reported that in vivo transplantation of HLCs derived from hiPSCs can rescue rodents from lethal fulminant hepatic failure [6], iPSC-derived HLCs have both the functional and proliferative potential for liver regeneration after transplantation in an ALF model, and enhance liver regeneration in mice [7]; reduce liver fibrosis in a mouse model [8]; and stabilize chronic liver disease in mice [9], Since the in vivo repopulation capacity of HLCs remains limited, Yuan et al demonstrated that the proliferation of hiPSC-derived HLCs can be enhanced by antibody-mediated modulation of c-Met signaling and facilitate hiPSC-derived HLC-based therapeutic applications for liver failure [10], For a human being, the possibility of generating hiPSC-derived HLCs from selected adults would facilitate the construction of libraries of cell lines with known genotypes, providing patients with a close HLA/MHC match, thereby minimizing the need for immunosuppression before cell engraftment[11].
Fig. 1.
Application of iPSCs technology for Liver Diseases
Bioartificial Liver
The shortage of donor livers for transplantation leads to approximately 40% of listed patients per year not receiving a liver transplant with a considerable number of these patients either dying or becoming too sick to transplant (www.unos.org). BAL is an extracorporeal supportive therapy developed to bridge patients with liver failure to liver transplantation or to recovery of the native liver. The BAL system removes toxins by filtration or adsorption (artificial liver) while performing biotransformation and synthetic functions of biochemically active hepatocytes. The main question in clinical application of these instruments is how to support them with enough functional hepatocytes. BAL doesn’t associate with the allogenic rejection, thus patient-specific hepatocytes are not needed.
To date, the different cell types that have been used in various BALs include primary porcine or human hepatocytes, cell lines, fetal liver cells, and stem cell-derived cells. While human hepatocytes are the preferred cells, primary human hepatocytes are not available in sufficient amounts needed for clinical BAL usage, and primary hepatocytes often retain their differentiated functions for a short duration in vitro. Human ESCs and iPSCs differentiated HLCs show great promise as cell sources for BAL devices. Patient-specific iPSC-derived HLCs transplantation can provide effective treatment to liver diseases; however, in acute situations, such as ALF, time to create, mature, and expand a patient’s cells into iPSCs and then HLCs may be prohibitive for ALF treatment, and with this condition, BAL is an ideal candidate treatment. The cell banks of normal individual-derived iPSCs with close HLA/MHC matched and then differentiation into HLCs could be used to create BAL for the temporary treatment of ALF.
The Fox group has reported an implantable BAL device containing HLCs derived from ESCs in a murine model of liver failure [12]. They differentiated mouse ESCs into HLCs by coculture with a combination of human liver nonparenchymal cell lines and cytokines. Treatment of 90% hepatectomized mice with a subcutaneously implanted BAL seeded with ESCs-derived HLCs improved liver function and prolonged survival. Although no reports have yet addressed functionality of HLCs derived from hiPSCs in a cell-based BAL, we believe hiPSC-derived HLCs are promising resource for BAL.
Extracellular Vesicles From iPSCs
Extracellular vesicles (EVs) are a heterogeneous collection of membrane-bound carriers with complex cargoes including proteins, lipids, and nucleic acids. Increasing evidence implicates EVs confer stability and can direct their cargoes to specific cell types, and as key players in intercellular and even interorgan communication. Progression of fibrosis and the development of cirrhosis are associated with the activation of hepatic stellate cells (HSCs), and the loss of liver homeostasis following injury. Povero et al tested the hypothesis that EVs isolated from iPSCs modulate HSC activation may have antifibrotic effects [13], Du and colleagues’ results also demonstrated that exosomes from hiPSC-derived mesenchymal stromal cells could alleviate hepatic I/R injury via activating sphingosine kinase and sphingosine-1-phosphate pathway in hepatocytes and promote cell proliferation [14]. These findings represent iPSCs potentially contributes to reduce or reverse liver fibrosis with the novel mechanism of EVs or exosomes.
Gene Therapy in Hereditary Liver Disease
The liver is affected by many types of diseases, including inherited metabolic disorders, because of deficiencies commonly found within a single gene as a consequence of heritable mutations in the genome. Hepatocyte transplantation has been performed as a treatment for inherited liver diseases, either for bridging to whole organ transplantation or for long-term correction of the underlying metabolic deficiency [15]. Development of iPSCs from patient somatic tissues and then differentiation into HLCs may provide a patient-specific hepatocyte source for treatment for inherited liver diseases. iPSCs-based gene/cell therapies have been applied in several animal models of liver-based metabolic disorders with encouraging results. Hemophilia B is an inherited deficiency in coagulation factor IX that leads to prolonged bleeding after injury. Wu et al reported that normal iPSC-derived HLC transplantation led to increased coagulation factor IX activity, improved thrombus generation, and better hemostasis parameters, and the transferred cells were localized in the liver in recipient hemophilia B mice [16]. Cell types other than HLCs derived from iPSCs were also used. Gunn rats bear a mutation within the uridine diphosphate glucuronosyltransferase-1a1 gene resulting in high serum bilirubin levels as seen in Crigler-Najjar syndrome. Spitzhorn and colleagues transplanted induced mesenchymal stem cells derived from normal hiPSCs into Gunn rats after partial hepatectomy. The transplanted mesenchymal stem cells differentiated into functional hepatocytes as evidenced by partially suppressed hyperbilirubinemia and expression of multiple human-specific hepatocyte markers [17].
In the case of monogenic inherited metabolic liver diseases, in which all the cells from the body initially carry the disease-causing mutation in their genomic DNA, a gene correction approach should be considered to generate disease-free autologous cells. Thus, a combination of cell and gene therapy is used. Simara et al and Garate et al have summarized the method for gene correction including zinc finger nucleases or transcription activator-like effector nucleases [18, 19].
Yusa and colleagues performed targeted gene correction of A1AT deficiency in hiPSCs with a combination of zinc finger nucleases and piggyBac technology [20], Their data showed that genetic correction of hiPSCs restored the structure and function of A1AT in subsequently derived liver cells in vitro and in vivo. Transplantation of these corrected iPSC-derived HLCs into immunodeficient mice was able to produce human albumin and the corrected A1AT protein. Their results provide the first proof of principle for the potential of combining hiPSCs with genetic correction to generate clinically relevant cells for autologous cell-based therapies. Wilson’s disease (WD) is an autosomal recessive inborn error of copper metabolism. Mutations in the ATP7B gene are responsible for WD, whose prevalence is 1 in 30,000 to 1 in 100,000 [21]. Zhang et al [22] describe the generation of iPSCs from a Chinese patient with WD that bears the R778L Chinese hotspot mutation in the ATP7B gene. They then performed gene correction in HLCs using a self-inactivating lentiviral vector that expresses codon optimized-ATP7B and these HLCs could reverse the functional defect in vitro. H1069Q substitution represents the most frequent mutation of ATP7B causing WD in the white population. Parisi et al reprogrammed skin fibroblasts of homozygous ATP7B-H1069Q patients into iPSCs and differentiated them into HLCs. Their results pinpoint rapid degradation as the major cause for loss of ATP7B function in H1069Q patients [23], Hence, these studies propose an attractive model for studying the pathogenesis of WD that may prove to be valuable for screening compounds or gene therapy approaches aimed to correct the abnormality. Primary hyperoxaluria type 1 is a rare autosomal recessive disorder of the liver metabolism due to functional deficiency of the peroxisomal enzyme alanine: glyoxylate aminotransferase. Esteve et al made patient-specific iPSCs and genetically defined autologous HLCs using a lentiviral vector encoding wild-type glyoxylate aminotransferase, and found that genetically modified HLCs exhibited substantial glyoxylate aminotransferase expression at both RNA and protein levels [24].
iPSCs Combine With Clustered Regularly Interspaced Short Palindromic Repeats and Associated Protein 9
The first generation of genome editing tools are protein-based nucleases, including zinc finger nucleases and transcription activator-like effector nucleases. Recently, we have seen the advent of the second generation of programmable genome editing tools based on RNA known as clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (Cas9). CRISPR-Cas9 technology can effectively and accurately realize the change of genetic material at the genome level, thus enabling precise editing of stem cell genomes to generate patient-specific disease models and development of personalized experimental systems. Biliary atresia (BA) is the most common cause of pediatric end-stage liver disease and the etiology is poorly understood. There is no effective therapy for BA partly due to lack of human BA models. Tian generated disease-specific iPSCs from 6 BA patients. While conducting studies involving the use of patient-derived iPSCs to clarify the unknown pathophysiology of rare genetic liver diseases, there might be confusion owing to unreliable data because of different genetic backgrounds of patients, lack of a phenotypic marker, various mutation patterns in the disease, and different multipotency of the iPSCs cell lines. Therefore, genetically engineered hiPSCs derived from healthy individuals are used to study diseases including liver disease [25]. Therefore, to determine the functional significance of BA-susceptibility genes identified by genome-wide association studies in biliary development, CRISPR-Cas9 system was used to created isogenic iPSCs with defined mutations in these genome-wide association studies BA loci (GPC1 and ADD3) from healthy iPSCs. Both the patient- and the KO-iPSCs showed deficiency in biliary differentiation along with increased fibrosis, the 2 key disease features of BA. These iPSCs can provide new human BA models for understanding the molecular basis of abnormal biliary development and opportunities to identify drugs that have therapeutic effects on BA [26].
The use of CRISPR-Cas9 to correct mutations in the stem cell genome, and then the mass production of functional cells available for transplantation through in vitro-directed differentiation technology, is also expected to achieve individualized cell replacement therapy for liver diseases. Homozygous familial hypercholesterolemia (FH) patients with 2 dysfunctional low-density lipoprotein receptor (LDLR) alleles are not as successfully treated with standard hypercholesterol therapies. Omer et al used CRISPR-Cas9 genome editing to permanently correct a 3-base pair homozygous deletion in LDLR exon 4 of patient-derived homozygous FH iPSCs. The genetic correction restored LDLR-mediated endocytosis in FH-HLCs and demonstrates the proof-of-principle that CRISPR-mediated genetic modification can be successfully used to normalize homozygous FH cholesterol metabolism deficiency at the cellular level [27].
iPSCs in Liver Disease Models
Many human liver diseases can be modeled using iPSCs as summarized in Table 1. Liver tissue from patients is difficult to obtain and only reveals the disease aftermath. Rodents and large models [28] of human congenital and acquired diseases provide a limited representation of human pathophysiology [29], since they do not always faithfully mimic human diseases, and most of them are imperfect [28]; therefore, there is still a clear need to adopt new advances in experimental techniques to develop. hiPSCs offer the ability to produce host-specific differentiated cells and thus have the potential to transform the study of liver disease. Disease modelling using iPSCs has been achieved for a variety of genetic diseases.
Table. 1.
iPSCs as platform for liver disease modeling
| iPSCs Resource | Diseases | Applications | Reference |
|---|---|---|---|
| iPSCs from Patient | Liver Fibrosis | Pathogenesias, mechanism(s), possible treatment | [27] |
| Hereditary Liver Disease | [28-35] | ||
| NAFLD | [36-41] | ||
| Cholangiopathies | [42-44] | ||
| iPSCs from Normal individual | HBV/HCV | Develop antiviral agents | [45] |
| HCV | [46-49] | ||
| HBV | [50-52] | ||
| Drug Discovery and Hepatoxicity Screening | [29,32,53-56] |
Modeling With iPSCs From Patient
Modeling in Liver Fibrosis
It is possible that the coculture of hiPSC-derived HLCs and hiPSC-derived HSC-like cells partially mimics the process of liver fibrosis [30], Coll et al showed that when 3-dimensional (3D) culture with hepatocytes, HSCs derived from hiPSCs were transformed into myofibroblasts via treatment with transforming growth factor-β, thioacetamide, and acetaminophen in vitro. Activated iPSC-derived HSCs secrete procollagen [30], which contributes to liver fibrosis.
Modeling in Hereditary Liver Disease
Monogenic metabolic disorders of the liver are an ideal platform to explore the complex gene-environment interactions and the role of genetic variation in the onset and progression of liver disease; use of human hepatocyte cultures may circumvent the problems of animal models of human diseases. Although many traditional cell-based models have been used to study pathogenesis and to screen for candidate drugs, none has used symptom-relevant human cell types since patient-specific primary hepatocytes are difficult to obtain. Disease-relevant cell types could accurately reflect disease pathogenesis in vitro. iPSCs generated from patients who have monogenic inherited liver diseases and HLCs derived from such iPSCs can be used as instruments to study the pathogenesis, disease mechanism(s), and possible cures for inherited liver disorders [31], [32].
Although current animal models, including the toxic milk mouse, ATP7B2/2 mouse, and Long-Evans Cinnamon rat, have provided very useful representation concerning the pathogenesis of WD, physiologic differences between species limit conclusions. Overeem et al generated urine cell-derived iPSCs from a patient with a homozygous mutation in ATP7B, a patient with a heterozygous mutation, and a patient with MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, keratodemia), and then differentiated toward HLCs. Their study demonstrates that these HLCs display architectural characteristics similar to those seen in the intact liver. The application of this methodology to cells from patients diagnosed with inherited copper metabolism-related liver diseases revealed unexpected and novel insights into patient mutation-specific disease mechanisms and drug responses [33].
Recently, several other liver-specific disease iPSCs, such as FH, glycogen storage diseases, Crigler-Najjar syndrome, hereditary tyrosinemia type 1, Gaucher disease, Niemann-Pick disease type C, A1AT deficiency, and alcoholic cirrhosis have been launched [34] [35] [36] [37] [38], Their results demonstrated that hiPSCs-derived HLCs can be generated from multiple patients of varied genetic and disease backgrounds, and their system is proved to be an efficient methodology for the early-stage safety and therapeutic screening of liver-targeted compounds of potential relevance to the pharmaceutical industry. The large array of iPSC lines created in these studies should permit more in-depth studies of disease phenotypes and the patient-derived HLCs from iPSCs can be used as suitable specific models to study the pathogenesis, mechanism(s), and possible treatment for inherited liver disorders.
Modeling in NAFLD
NAFLD/steatosis is a metabolic disease characterized by the incorporation of fat into hepatocytes. Nonalcoholic steatohepatitis, a serious form of NAFLD, may lead to cirrhosis and end-stage liver diseases. Poly (ADP-ribose) polymerase 1 (Parp1) has been reported to enhance cell reprogramming, and Chien et al demonstrated that forced expression of Oct4/Sox2/Klf4/Parp1 (OSKP) effectively promoted iPSC generation from senescent somatic cells from 18-month-old mouse. Intrasplenic transplantation of OSKP-iPSC-HLCs ameliorated the triglyceride over accumulation and hepatitis, prevented the production of inflammatory cytokines and oxidative substances, and reduced apoptotic cells in methionine/choline-deficient diet-fed mice [39], Graffmann and colleagues established and characterized iPSC lines from a 58-year-old, and a 35-year-old high-grade patients with NAFLD (70% steatosis) with homozygous wildtype PNPLA3 genotype, respectively [40] [41]. With an in vitro model for NAFLD based on HLCs differentiated from iPSCs, Graffmann et al induced fat storage in these HLCs and detected major expression changes of metabolism-associated genes, as well as an overall reduction of liver-related microRNAs. Their model recapitulates many metabolic changes that are characteristic for NAFLD [42], Metabolic derangements in hepatocytes controlled by SIRT1 play a role in the development of fatty liver in inbred animals, and Collin de I'Hortet et al generated hiPSCs with controllable expression of SIRT1. By differentiating these iPSCs into HLCs and knocking down SIRT1, they found increased fatty acid biosynthesis that exacerbates fat accumulation. To model human fatty livers, they repopulated decellularized rat livers and found that the hiPSC-derived liver tissue developed macrosteatosis, acquired proinflammatory phenotype, and shared a similar lipid and metabolic profiling to human fatty livers [43], iPSC-derived HLCs in NAFLD has been reviewed by Wruck et al [44].
Modeling in Cholangiopathies
Cholangiocytes are damaged in a variety of human diseases termed cholangiopathies, often causing advanced liver failure. Several groups have reported defined differentiation protocols that incorporate key signaling cues from normal biliary development to yield cholangiocyte-like cells from wild-type hiPSCs that demonstrate epithelial morphology in 2- and 3-dimensional culture, cholangiocyte markers, biliary gene expression profiles, and functional attributes consistent with biliary epithelium. Ghanekar and Kamath reported that mature, functional cholangiocytes can be derived from hiPSCs and utilized to model biliar diseases in vitro [45], Bile salt export pump (BSEP) plays an important role in hepatic secretion of bile acids and its deficiency results in severe cholestasis and liver failure. Mutation of the ABCB11 gene encoding BSEP induces BSEP deficiency and progressive familial intrahepatic cholestasis type 2 (PFIC2). Imagawa et al succeeded in establishing a PFIC2 model with iPSCs, which may be useful for its pathophysiologic analysis and drug development [46].
The transthyretin protein is thermodynamically destabilized by mutations in the transthyretin gene, promoting the formation of amyloid fibrils in various tissues Consequently, impaired autonomic organ function is observed in patients suffering from transthyretin-related familial amyloidotic polyneuropath (FAP). Hoepfner et al reprogrammed FAP patient fibroblasts to iPSCs and differentiated these cells into transthyretin-expressing HLCs. HLCs differentiated from FAP iPSCs and healthy control iPSCs secreted the transthyretin protein in similar concentrations. Mass spectrometry revealed the presence of mutant transthyretin protein in FAP HLC supernatants [47].
iPSCs From Normal Individuals in Infectious Diseases
Chronic liver diseases caused by either hepatitis B or C viruses (HBV/HBC) are a major health problem around the world. HBV and HCV infection has never been fully understood because there are few conventional models for hepatotropic virus infection. hiPSC-derived HLCs constitute a powerful tool for modeling hepatotropic pathogen infections in cell culture, providing an opportunity to elucidate the genetic basis of the mechanisms underlying cell susceptibility or resistance to viruses. In particular, normal individuals hiPSC-derived HLCs are an appropriate target for studying the interactions between the host and virus with hepatic tropism. Current iPSC models for HBV and HCV studies, and its relevance to the viral host cell interactions has been discussed by Bengrine and colleagues [48].
Sa-Ngiamsuntorn et al reported robust cell culture model for serum-derived HCV using iPSC-derived HLCs as host cells provides a remarkable system for investigating HCV life cycle, HCV-associated hepatocellular carcinoma development, and the screening for new anti-HCV drugs [49], Sakurai et al reported HCV genome-induced up-regulation of interferons and interferon-stimulated genes mediates a reduction in the HCV genome and protein levels in iPSC-derived HLCs [50], Schobel et al analyzed the suitability of iPSC-derived HLCs as a physiologically relevant model to study HCV replication in vitro. These HLCs supported the full HCV life cycle. Following HCV infection, interferon-stimulated gene expression is notably increased in HLCs. Wu and Dao Thi present a detailed stepwise protocol for the differentiation of stem cells to HLCs for HCV studies in cell culture. They also outline the use of an inducible CRISPR platform for the rapid and efficient modification of host factors of interest to better understand their function during HCV infection [51]. All these findings support iPSC-derived HLCs as a suitable model for HCV-host interaction regarding a functional innate immunity and lipoprotein synthesis [52].
Sakurai et al used hiPSCs-derived HLCs as an in vitro model of human HBV infection, and demonstrate that iPS-HLCs can be used as a promising in vitro HBV infection model [53]. Yuan and colleagues established a chimeric liver mouse model by engrafting the hiPSC-derived HLCs, which was capable of supporting HBV infection in vivo and evaluating the effects of antiviral drugs [54]. Wu et al reported that human iPSC-derived HLCs are permissive for noncell culture-adopted hepatitis E virus (HEV) in contrast to hepatoma cells. HEV replication induces an antiviral innate immune response in iPSC-derived HLCs [55]. Such models will advance our understanding of host-pathogen interactions and help to develop antiviral agents against HBV, HCV, and HEV.
Drug Discovery and Hepatoxicity Screening
An added benefit of iPSCs is that they can be used for drug screening research. The liver is the principle organ responsible for detoxification. Human primary hepatocytes have become a major liver model for hepatotoxicity tests. Unfortunately, as mentioned above, there is also a shortage of primary hepatocytes, and it is difficult to culture the hepatocytes in vivo without losing their special function. Today, approximately 70% of the top 20 pharmaceutical companies utilize stem cells in their research and among these, 64% use human ESCs or their derivatives. Human ESCs and their derivatives do not encompass all the variances within a population or all ethnicities. Since cells from many patients with different metabolism phenotypes can be tested, hiPSCs-derived HLCs have been expected to be used for individual new drug screening and optimization of patient-effective therapy and adverse effects of compounds during the drug discovery process [35] [56]. Cayo et al have used HLCs generated from homozygous FH iPSCs to identify drugs that can potentially be repurposed to lower serum low-density lipoprotein cholesterol [57]. Integration of patient-specific iPSC-based screening in early stages of drug development will help to more accurately predict drug effects in humans, thereby appreciably shortening the timeline and reducing the costs associated with clinical trials and high failure rates [58].
Moreover, the potential to make genetically corrected hiPSCs from diverse genetic subtypes from any number of diseases also allows for the development of reliable models for studying the development and progression of genetic diseases in vitro [58] [59]. For example, disease-causing gene correction in hiPSCs would make a perfect control for pharmaceutical in vitro comparative studies.
Drug adverse effects is a key part of the drug discovery process. Genova et al discussed recently developed disease models based on iPSCs, with a particular focus on available models of drugs' adverse effects, in particular hepatic/pancreatic toxicity [32].
Liver Cancer Treatment
T cells engineered to express chimeric antigen receptor (CAR) against the B cell antigen CD19 are achieving remarkable clinical effects on hematologic malignancies. The allogeneic transplantation approach is promising for broadened application of CAR-engineered T cell (CAR T-cell) therapy. iPSCs are one of the ideal cell sources for this approach. CAR-engineered iPSCs are demonstrated to give rise to CAR T-cell and exert their effector function. Ueda and Kaneko described the method to generate CAR-engineered iPSCs and differentiate them into T cells [60]. Although there are no reports addressing application of CAR T-cells derived from hiPSCs, we believe hiPSCs-CAR T-cells are promising resource for cancer treatment including liver cancer.
Challenges of iPSCs Application
Immature HLCs Derived From iPSCs
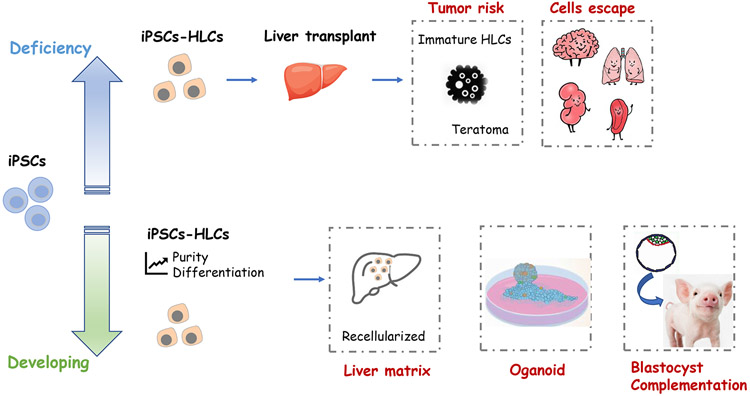
Major areas of iPSCs utilization can be divided into addressing deficiencies or cell shortages and new areas of cell therapy as outlines in Figure 2. For clinical application, differentiated cells should be assessed by comparing them with primary liver-derived cells for morphology and have functional hepatocyte properties such as nutrient processing, detoxification, plasma protein synthesis, and engraftment, after transplantation into a suitable animal model. So far, neither ESCs nor iPSCs can differentiate to fully mature hepatocytes in vitro. Researchers have termed such populations of cells derived from iPSCs or ESCs as HLCs; HLCs indicate only some of the properties of mature hepatocytes [61] [62]. Moreover, despite recent advances, the efficiency of human ESCs and iPSCs directed-differentiation into HLCs is highly variable, cell line-dependent. Since the undifferentiated iPSCs have the potential to form teratoma, research must be actively pursued to gain more information in order to clearly delineate the differentiation pathways of iPSC into specific cell types to ensure similar function and physiology.
Fig. 2.
Current challenges of iPSCs application in liver diseases
Strategies to Purify the HLCs Differentiated From iPSCs
The safety of clinical cell therapy using differentiated hiPSC derivatives must be improved through methods to remove the undifferentiated iPSCs and permit the transplantation of homogenous populations of a specific cell type [63]. To date, strategies for purifying a given cell population have used either a cell surface protein specific for the target cell population, such as a cell surface marker specific to hepatic progenitors, or lentivectors expressing a reporter gene under the control of a specific promoter [64]. For example, to purify HLCs from a heterogeneic population, elegant experiments by Basma et al [65] generated human HLCs through embryoid bodies, and purified these populations using fluorescence-activated cell sorting for the asialoglycoprotein receptor.
Yang et al [66] reported that they used lentivectors encoding green fluorescent protein (GFP) driven by the liver-specific apoliprotein A-II promoter to purify human hepatic progenitors. They first differentiated a human ESC line into hepatic progenitors using a chemically defined protocol. Subsequently, cells were transduced with GFP and sorted at day 16 of differentiation to obtain a cell population enriched in hepatic progenitor cells. After sorting, more than 99% of these apoliprotein A-II-GFP-positive cells expressed hepatoblast markers such as AFP and cytokeratin 19. When further cultured for 16 days, the sorted hepatoblasts underwent differentiation into more mature cells and exhibited hepatocyte properties such as albumin secretion. Moreover, they were devoid of viral DNA integration. Their strategy produces a novel tool that could be used not only for cell therapy but also for in vitro applications such as drug screening. The present strategy should also be suitable for the purification of a broad range of cell types derived from either iPSCs or adult stem cells.
Endodermal Progenitor Cell Lines From iPSCs
Besides the high variability of differentiation efficiency, the pluripotent nature of ESCs and iPSCs results in production of multiple cells types from different germ layers in most differentiation protocols. Thus, it is difficult to produce pure monolineage cultures of a desired cell type from iPSCs [67].
To establish definitive germ layer stem cell lines, such as definitive endoderm progenitor lines, is one of the effective methods to solve the problems of iPSCs differentiation. The spectrum of differentiation potential of definitive germ layer stem cells is relatively narrow; thus, the efficiency of directional differentiation from definitive germ layer stem cells to a specific cell types can be greatly increased. On the other hand, definitive germ layer stem cells have broader differentiation potential than tissue stem cells; thus, to establish a definitive germ layer stem cell line could obtain a variety of functional cell types, which is economical. Meanwhile, because definitive germ layer stem cell lines are less tumorigenic than ESCs, iPSCs, transient endoderm, and cannot form teratomas, thus is higher security. Endoderm stem cells can differentiate to the liver, pancreas, intestines, stomach, lung, and other organs cells; therefore, they have very important potential in clinical application. Scientists around the world have tried to establish a lot of work on stem cell lines from endoderm.
Because differentiation from iPSCs toward hepatic lineage cells mimics in vivo step-wise developmental processes, hiPSCs-derived hepatic progenitor-like cells (HPCs) might exist at an appropriate time point during similar in vitro differentiation steps. Yanagida and colleagues reported that after differentiating with defined cytokines, HPCs from hiPSCs can be highly purified using cell surface markers CD13 and CD133. Further investigation revealed that hiPSCs-derived HPCs exhibit a long-term proliferative potential and maintain bipotent differentiation toward hepatocytic cells and cholangiocytic cells [68]. In addition, mature hepatocytes can barely maintain a proliferative ability following cryopreservation, whereas their hiPSCs-derived HPCs have a highly proliferate ability even after cryopreservation. Cheng et al [69] generated self-renewing definitive endoderm progenitor lines from both human ESCs and iPSCs. These cells, termed endodermal progenitor (EP) cell lines for EP cells, display a proliferative capacity similar to ESCs yet lack teratoma-forming ability. In addition, EP cell lines generate endodermal tissues representing liver, pancreas, and intestine, both in vitro and in vivo. Thus, EP cell lines provide a powerful reagent to study how different gut tissues are specified from a common multipotent EP and to optimize monolineage differentiation. Moreover, creation of EP cells from ESCs or iPSCs may represent a strategy to optimize the production of pure, nontumorigenic cells for tissue replacement therapies.
Direct Induction of Functional HLCs
The induction of HLCs from iPSCs is a complicated process that would probably be replaced with the arrival of improved technology. Huang et al demonstrated the direct induction of functional HLCs (named as iHep cells) from mouse tail-tip fibroblasts by transduction of Gata4, Hnf1a, and Foxa3, and inactivation of p19Arf. iHep cells show typical epithelial morphology, express hepatic genes, and acquire hepatocyte functions. Notably, iHep cells showed an expression profile and hepatic function close to those of mature hepatocytes. The transplanted iHep cells repopulate the livers of fumarylacetoacetate hydrolase (FAH)-deficient mice and rescue almost half of recipients from death by restoring liver functions. More importantly, iHep cells do not seem to be prone to tumor formation [70]. Park et al investigated the effect of iHeps on acute liver injury and chronic hepatic fibrosis animal models induced by CCl4 intraperitoneal injection in BALB/C nude mice. Intrasplenic transplantation of iHeps substantially attenuated CCl4-induced acute liver injury. GFP-labeled iHeps migrated to the liver after intrasplenic transplantation and found that GFP and albumin were colocalized in migrated iHeps in the liver suggesting migrated iHeps were functional. In the chronic hepatic fibrosis mice experiment, transplantation of iHeps considerably attenuated liver fibrosis induced by CCl4 injection for 10 weeks [71]. Their studies provide a novel strategy to generate functional HLCs for the purpose of liver regenerative medicine.
hiPSCs-Derived Hepatic Stem Cells
Whereas some studies were able to correct hepatic defects in animal models, they focused on the most mature phenotype of HLCs derived from iPSCs and needed freshly prepared cells, which limits clinical applications of HLCs. Fourier et al report they produce hepatic stem cells from hiPSCs. They demonstrate the differentiation of hiPSCs into hepatic stem cells that (a) are produced using a differentiation protocol compatible with good manufacturing practices, (b) can be frozen, and (c) are sufficient to demonstrate in vivo therapeutic efficacy to extensively lower hyperbilirubinemia in a model of inherited liver disease, despite their immature phenotype. Thus, their approach provides major advances toward future clinical applications and would facilitate cell therapy manufacturing from human pluripotent stem cells [72].
In Vivo Differentiation of iPSCs
To date, no iPSCs-derived differentiation protocol has succeeded in yielding full function transplantable and high purity hepatocytes that fulfill both criteria-functional engraftment and response to proliferative stimuli in the diseased liver. Alternative strategies are needed to obtain mature hepatocytes. To exclude compensation by hepatocytes not derived from iPSCs, Espejel et al transferred wildtype mouse iPSCs into the embryos of FAH-deficient mice to generate chimeric mice, and demonstrated the ability of iPSCs to develop into hepatocytes in vivo, and to protect the FAH-deficient mice from developing hepatic failure [7]. In doing so, the authors were able to avoid the current limitations of in vitro hepatocyte differentiation protocols by allowing the iPSCs to undergo normal ontogenic development into mature hepatocytes in vivo.
Other possibilities for expansion and maturation of HLCs includes a genetically engineered large animal, similar but larger than the source of human hepatocytes in our study [73], to serve as an in vivo hepatocyte incubator [74]. Investigators have demonstrated that exposure to damaged liver tissue stimulates liver cell regeneration and can enhance homing and differentiation of stem cells to a hepatocyte phenotype. Our FAH-null heterozygote pig model which shows a damaged liver microenvironment may provide both a large number (~90% of a pig liver) and high function of human hepatocytes. The future success of ex vivo cell therapies depends on novel techniques to provide an abundant, high quality supply of functionally normal hepatocytes.
Low Efficiency of Engraftment
Specific human cells differentiated in vitro must be transplanted into rodent models to demonstrate their functionality. However, the following difficulties must be taken into account: the low efficiency of engraftment of transplanted cells into the host parenchyma, which do not proliferate under normal conditions in rodent livers. Alternatively, as the repopulation of the liver by donor hepatocytes has been demonstrated in animal models in which transplanted hepatocytes display a selective growth advantage over endogenous hepatocytes, such models could be used. For example, in some models, the survival and/or proliferation of native hepatocytes is impaired by a genetic or inherited inability to regenerate, as in FAH-deficient mice and urokinase (Alb-uPA) transgenic mice [75] [76]. These 2 types of mouse models have been crossed with immunodeficient mice with a different genetic background [77]. Furthermore, with a suitable animal model, HLCs generated from pluripotent or multipotent stem cells currently repopulate transplanted livers less efficiently than human adult hepatocytes (up to 80%) [73]. This also demonstrates that fully mature HLCs are eagerly needed to achieve higher engraftment efficiency to perform their function.
Moreover, when using conventional transplantation methods, ie, intrasplenic or portal venous infusion, it is difficult to control the engraftment efficiency and avoid unexpected engraftment in other organs because the transplanted cells are delivered into blood circulation before their liver engraftment. Nagamoto et al reported that transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure [78].
Decellularized Liver Matrix and Recellularized with iPSC-HLCs
It has been shown that efficient function of multiple cell types, including hepatocytes and islet hormone-producing cells, is dependent on matrix-producing cells and endothelial cells that provide a 3D support structure and sufficient vascularization [79] [80] [81]. Thus, liver extracellular matrix presents an ideal scaffold for stem-cell differentiation into hepatocytes [82] [83]. It is known that local environmental factors induce hepatocyte homing, differentiation, and proliferation, and studies indicate that stem cells may differentiate toward mature hepatocytes following transfer into an injured liver. Therefore, decellularized liver matrix has noteworthy potential as the scaffold for hepatocyte maturation.
Lorvellec compared hepatocyte differentiation of hiPSCs in a mouse decellularized liver scaffold (3D) with standard in vitro protocol (2D). Mouse livers were decellularized preserving microarchitecture, blood vessel network, and extracellular matrix. hiPSCs were primed towards the definitive endoderm. Cells were then seeded either in 3D or 2D cultures and the hepatocyte differentiation was continued. They demonstrate that decellularized liver scaffold improves hiPSCs differentiation into HLCs [84]. Abazari et al explored the effectiveness of decellularized amniotic membrane matrices for culturing of iPSCs and promoting their differentiation into HLCs. They found decellularized amniotic membrane scaffold might have a considerable potential in liver tissue engineering, because it can improve hepatic differentiation of hiPSCs which exhibited higher level of the hepatic marker and more stable metabolic functions [85].
Park and colleagues produced porcine iPSC-HLCs, which strongly expressed the hepatic markers and exhibited hepatic functionalities. Supplementation of extracellular matrix from porcine decellularized liver containing liver-derived growth factors stimulated the albumin expression of porcine iPSC-derived HLCs during differentiation procedures. The iPSC-derived HLCs were reseeded into decellularized liver scaffolds, and the recellularized liver scaffolds were transplanted into rats for a short term, and the grafts expressed hepatocyte markers and did not rupture. These results provide a foundation for development of bioengineered liver using stem cell and decellularized scaffolds [86].
Our group has shown recently that decellularized whole porcine livers revascularized with human umbilical vein endothelial cells and implanted heterotopically into immunosuppressed splenectomized pigs remained refused for up to 15 days. We also show that the human endothelial cells in the perfused revascularized bioengineered livers colonize the liver sinusoids and express sinusoidal endothelial markers similar to those in normal liver tissue. Revascularized liver scaffolds that can maintain blood perfusion at physiologic pressures might eventually help to overcome the chronic shortage of transplantable human livers [87].
Organoids
Takebe and his team grew the liver organ using hiPSCs, created by reprogramming human skin cells to an embryo-like state. The researchers first placed the iPSCs on growth plates in a specially designed medium; after 9 days, analysis showed that they contained a biochemical marker of maturing liver cells, called hepatocytes. At that key point, Takebe added 2 more types of cells known to help to recreate organ-like function in animals: endothelial cells, which line blood vessels, taken from an umbilical cord; and mesenchymal cells. Two days later, the cells assembled into a 5-mm-long, 3D tissue that the researchers labelled a liver bud, an early stage of liver development [88]. Thirty-three percent of cells from the hiPSC-derived liver buds are ALB positive. These buds quickly hooked up with nearby blood vessels and continued to grow rigorously after transplantation. The vascular networks of liver buds were similar in density and morphology to those of adult livers after transplantation. This is the first reported macroscopic functional human tissue derived from iPSCs [88], and then organoid cultures have emerged as an alternative in vitro system to recapitulate liver tissues in a dish.
Mun et al developed a novel hiPSCs-derived hepatocyte-like liver organoid that exhibited self-renewal (expandable and further able to differentiate) while maintaining their mature hepatic characteristics over long-term culture, which is critically advanced in terms of its generation method, functional performance, and application technologies [89]. Ouchi et al used 11 different healthy and diseased pluripotent stem cell lines, and developed a reproducible method to derive multicellular human liver organoids composed of hepatocyte-, stellate-, and Kupffer-like cells that exhibit transcriptomic resemblance to in vivo-derived tissues. Furthermore, organoids from patients with genetic dysfunction of lysosomal acid lipase phenocopied severe steatohepatitis, rescued by FXR agonism-mediated reactive oxygen species suppression [90].
Since organoid technology applications are limited in their capacity to generate functional hepatocytes in a reproducible and efficient manner, Akbari et al generated and characterized the hepatic organoid (eHEPO) culture system using hiPSCs-derived EpCAM-positive endodermal cells as an intermediate. eHEPOs can be produced within 2 weeks and expanded long term (>16 months) without any loss of differentiation capacity to mature hepatocytes. Starting from patient-specific iPSCs, they modeled citrullinemia type 1, a urea cycle disorder caused by mutations in the argininosuccinate synthetase enzyme. The disease-related ammonia accumulation phenotype in eHEPOs could be reversed by the overexpression of the wild-type argininosuccinate synthetase gene, which also indicated that this model is amenable to genetic manipulation [91].
Chimerism/Interspecies Blastocyst Complementation
Current bioengineering strategies to regenerate the liver have not been able to replicate its extraordinary cellular diversity and complex 3D arrangement, which are indispensable for life-sustaining metabolism and detoxification. An alternative approach to regenerate achimeric liver from donor cells is named blastocyst complementation (BC). This novel form of embryonic cell transplantation has made tremendous progress over the last 10 years [92].
The overall strategy is to establish a host which can be genetically modified and lacks a single or multiple lineage(s), thus providing a permissive niche for a donor stem cell to contribute to the absent lineage in the recipient (host) embryo [93] [94]. The donor stem cell is provided a competitive advantage as the mutant host is unable to form the lineage of interest without help from the donor cells, and/or a protective drug. The feasibility of this strategy has been amplified with the derivation and use of iPSCs, which can be readily obtained from a patient who would desire or need the organ. In other words, interspecies chimeras using iPSCs hold the potential to generate humanized organs for regenerative medicine by BC. In this fashion, the patient becomes both the donor and eventually the recipient. Furthermore, iPSCs lack the ethical issues associated with human ESCs. In addition to iPSCs, the ability to use gene-editing technologies such as zinc finger nucleases, transcription activator-like effector nuclease, and CRISPR-Cas9 technologies has further accelerated these research initiatives [95].
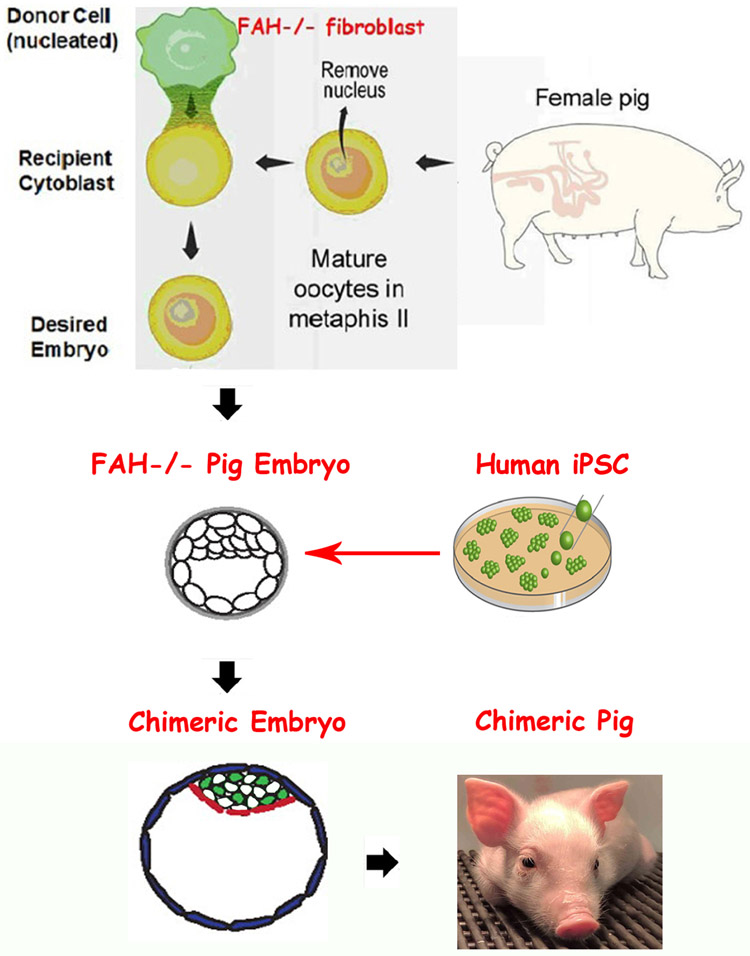
BC seems viable and appealing option in liver transplantation study. For liver BC, several candidate genes like Hhex, Tbx3, OC-1 and OC-2 have been reported [96]. Unbalanced expression of these genes leads to liver failure during embryo stage, and provides a permissive niche for hepatocyte engraftment and expansion. It was also shown that the livers of FAH-deficient pigs provide a selective advantage for growth of normal (FAH+) hepatocytes, which prompt FAH can be also a candidate gene for liver BC, as illustrated in Figure 3. [97]. This way could obtain high donor cells chimera pig efficiently, according our recently blastocyst complementation study.
Fig. 3.
Blastocyst complementation with hiPSC (Nyberg group from Mayo Clinic).
Methods to enhance the chimeric potential of donor cells have been attempted through inhibiting apoptosis until the developmental stage matches that of the host. Recent reports suggest that developmental synchronization between host embryo and donor cells appears to be required, with naive-iPSCs and intermediate-iPSCs contributing to chimeras. So, considering the anatomic and physiologic similarity that exists between human and livestock animal organs, pigs are particularly good animals to use for this purpose [98]. However, human naive (or inner-cell mass-like) PSCs can be derived and propagated through the use of customized culture conditions. The HLCs derived from human iPSCs could provide a stable source of hepatocytes for BC [99].
3D Bioprinting
Modern 3D bioprinting technologies allied with autologous iPSCs-derived grafts could represent a relevant tissue engineering approach to treat end-stage liver disease patients. Faulkner-Jones et al report the first investigation into the bioprinting of hiPSCs, their response to a valve-based printing process, as well as their post-printing differentiation into HLCs. HLCs differentiated from both hiPSCs and hESCs sources were bioprinted and the cells were positive for hepatocyte markers and have morphology that was also found to be similar to that of hepatocytes. Their work demonstrates that the valve-based printing process is gentle enough to print human pluripotent stem cells (both hESCs and hiPSCs) while either maintaining their pluripotency or directing their differentiation into specific lineages [100]. Goulart et al evaluated the impact of using single cell dispersion (2D differentiation) of iPSC-derived HLCs versus using iPSC-derived HLCs spheroids (3D cell culture), both in combination with nonparenchymal cells (eg, mesenchymal and endothelial cells), into final liver tissue functionality. Their results indicate the advantage of using spheroid-based bioprinting, contributing to improve current liver bioprinting technology towards future regenerative medicine applications and liver physiology and disease modeling [101].
The functional maturation and preservation of HLCs derived from hiPSCs are essential to personalized in vitro drug screening and disease study. Ma and colleagues present a 3D hydrogel-based triculture model that embeds hiPSC-derived HPCs with human umbilical vein endothelial cells and adipose-derived stem cells in a microscale hexagonal architecture. Their 3D triculture model shows both phenotypic and functional enhancements in the hiPSC-derived HPCs over weeks of in vitro culture. Specifically, they find improved morphologic organization, higher liver-specific gene expression levels, increased metabolic product secretion, and enhanced cytochrome P450 induction [102].
Cholangiopathies are rare diseases of the bile duct with high mortality rates. The current treatment for cholangiopathies is liver transplantation. Many researchers have used hiPSCs as a source for cholangiocyte-like cell generation and have incorporated advances in bioprinting to create artificial bile ducts for implantation and transplantation. This has allowed the field to move dramatically forward in studies of biliary regenerative medicine. In Buisson et al’s review, the authors provide an overview of cholangiocytes, the organogenesis of the bile duct, cholangiopathies, and the current treatment and advances that have been made that are opening new doors to the study of cholangiopathies [103].
The application of bioprinting technology in tissue engineering enables the development of a 3D biomimetic liver model which could be used for animal-free drug development, personalized medicine, and disease modeling.
Conclusion
iPSCs present possibilities for in vitro modeling, improving research on diseases, drug development, tissue engineering, the development of BAL, and a foundation for producing autologous cell therapies that would avoid immune rejection and enable correction of gene defects prior to cell transplantation, therefore, opening up new opportunities for medical advances. However, there are still several major obstacles that need to be overcome before iPSCs successfully reach the bedside. Extensive characterization of the functionality of iPSCs-derived hepatic cells and their functional equivalence with in vivo counterparts needs to be extensively investigated and remains to be formally demonstrated; the application of the benefits that iPSCs offer is also limited by the ability to derive fully functional disease-relevant somatic cells; and major challenges remain in defining pathways that efficiently lead to pure and functional populations of many liver disease-relevant cells. Additionally, quick generation and differentiation protocol for emergent usage are needed. Before autologous or allogeneic cell therapy can be applied to human patients, a thorough preclinical assessment of iPSCs in suitable large-animal models is required, to ensure that the proposed treatment with iPSC-derived cells is both safe and effective. It has reported recently that transplantation of undifferentiated iPSCs demonstrated T-cell-dependent immune response in recipient syngeneic mice due to the abnormal expression of antigens following genetic manipulation. Therefore, critical aspects need to be further addressed, including the long-term safety, tolerability, and efficacy of the iPSC-based treatments, as well as their carcinogenic potential. It is paramount to conduct larger and well-designed clinical trials to fully establish the safety profile of such therapies and to define the target patient groups with efficacy assessed by standardized protocols. Despite limitations, iPSC-derived hepatocytes remain a promising population for liver cell therapies.
Moreover, engineered donor grafts derived from iPSCs, including recellularized biomatrix and liver organoids produced, may someday provide organs for liver transplantation. Furthermore, generation of transplantable human organs derived from human-animal chimerae may become possible. These results highlight the enormous therapeutic potential for treating organ failure. Nowadays, most stem cell-related studies in the field of liver disease are still based on basic research and preclinical research. There are many scientific, medical, ethical, political, financial, and other challenges yet to be solved. For the benefit of patients and future generations, scientists need to translate the achievements of regenerative medicine into clinical application in the future.
Acknowledgment
Funding from Mayo Foundation for Medical Education and Research, National Natural Science Foundation of China are gratefully acknowledged.
Funding Sources
This work was supported by grant(s) from National Natural Science Foundation of China (81570565 to Y. Yu), the Mayo Foundation for Medical Education and Research, National Institutes of Health (R01 DK10667), and Minnesota Regenerative Medicine.
Footnotes
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Yu Y, Wang X, and Nyberg SL, Potential and Challenges of Induced Pluripotent Stem Cells in Liver Diseases Treatment. J Clin Med, 2014. 3(3): p. 997–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uygun BE, Yarmush ML, and Uygun K, Application of whole-organ tissue engineering in hepatology. Nat Rev Gastroenterol Hepatol, 2012. 9(12): p. 738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yagi H, et al. , Embryonic and induced pluripotent stem cells as a model for liver disease. Crit Rev Biomed Eng, 2009. 37(4–5): p. 377–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, et al. , Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem Cell Research, 2012. 9: p. 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Si-Tayeb K, et al. , Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology, 2010. 51(1): p. 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YF, et al. , Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology, 2011. [DOI] [PMC free article] [PubMed]
- 7.Espejel S, et al. , Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. The Journal of clinical investigation, 2010.120(9): p. 3120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asgari S, et al. , Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem cell reviews, 2013. 9(4): p. 493–504. [DOI] [PubMed] [Google Scholar]
- 9.Choi SM, et al. , Liver engraftment potential of hepatic cells derived from patient-specific induced pluripotent stem cells. Cell cycle, 2011.10(15): p. 2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan L, et al. , Agonist c-Met Monoclonal Antibody Augments the Proliferation of hiPSC-derived Hepatocyte-Like Cells and Improves Cell Transplantation Therapy for Liver Failure in Mice. Theranostics, 2019. 9(7): p. 2115–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao T, et al. , Immunogenicity of induced pluripotent stem cells. Nature, 2011. 474(7350): p. 212–5. [DOI] [PubMed] [Google Scholar]
- 12.Soto-Gutierrez A, et al. , Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol, 2006. 24(11): p. 1412–9. [DOI] [PubMed] [Google Scholar]
- 13.Povero D, et al. , Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight, 2019. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, et al. , Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem, 2017. 43(2): p. 611–625. [DOI] [PubMed] [Google Scholar]
- 15.Fox IJ, et al. , Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med, 1998. 338(20): p. 1422–6. [DOI] [PubMed] [Google Scholar]
- 16.Wu YM, et al. , Hepatocyte-Like Cells Derived From Mouse Induced Pluripotent Stem Cells Produce Functional Coagulation Factor IX in a Hemophilia B Mouse Model. Cell Transplant, 2016. 25(7): p. 1237–46. [DOI] [PubMed] [Google Scholar]
- 17.Spitzhorn LS, et al. , Transplanted Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Support Liver Regeneration in Gunn Rats. Stem Cells Dev, 2018. [DOI] [PMC free article] [PubMed]
- 18.Simara P, Motl JA, and Kaufman DS, Pluripotent stem cells and gene therapy. Transl Res, 2013. 161(4): p. 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garate Z, et al. , New frontier in regenerative medicine: site-specific gene correction in patient-specific induced pluripotent stem cells. Hum GeneTher, 2013. 24(6): p. 571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusa K, et al. , Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature, 2011. 478(7369): p. 391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ala A, et al. , Wilson's disease. Lancet, 2007. 369(9559): p. 397–408. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, et al. , Rescue of ATP7B function in hepatocyte-like cells from Wilson's disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet, 2011. 20(16): p. 3176–87. [DOI] [PubMed] [Google Scholar]
- 23.Parisi S, et al. , Characterization of the most frequent ATP7B mutation causing Wilson disease in hepatocytes from patient induced pluripotent stem cells. Sci Rep, 2018. 8(1): p. 6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteve J, et al. , Generation of induced pluripotent stem cells-derived hepatocyte-like cells for ex vivo gene therapy of primary hyperoxaluria type 1. Stem Cell Res, 2019. 38: p. 101467. [DOI] [PubMed] [Google Scholar]
- 25.Kakinuma S and Watanabe M, Analysis of the mechanism underlying liver diseases using human induced pluripotent stem cells. Immunol Med, 2019. 42(2): p. 71–78. [DOI] [PubMed] [Google Scholar]
- 26.Tian L, et al. , Biliary Atresia Relevant Human Induced Pluripotent Stem Cells Recapitulate Key Disease Features in a Dish. J Pediatr Gastroenterol Nutr, 2019. 68(1): p. 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omer L, et al. , CRISPR Correction of a Homozygous Low-Density Lipoprotein Receptor Mutation in Familial Hypercholesterolemia Induced Pluripotent Stem Cells. Hepatol Commun, 2017.1(9): p. 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asgari S, et al. , Induced pluripotent stem cells: a new era for hepatology. J Hepatol, 2010. 53(4): p. 738–51. [DOI] [PubMed] [Google Scholar]
- 29.Ordonez MP and Goldstein LS, Using human-induced pluripotent stem cells to model monogenic metabolic disorders of the liver. Semin Liver Dis, 2012. 32(4): p. 298–306. [DOI] [PubMed] [Google Scholar]
- 30.Coll M, et al. , Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell, 2018. 23(1): p. 101–113 e7. [DOI] [PubMed] [Google Scholar]
- 31.Pournasr B and Duncan SA, Modeling Inborn Errors of Hepatic Metabolism Using Induced Pluripotent Stem Cells. Arterioscler Thromb Vase Biol, 2017. 37(11): p. 1994–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genova E, et al. , Induced Pluripotent Stem Cells as a Model for Therapy Personalization of Pediatric Patients: Disease Modeling and Drug Adverse Effects Prevention. Curr Med Chem, 2018. 25(24): p. 2826–2839. [DOI] [PubMed] [Google Scholar]
- 33.Overeem AW, et al. , Pluripotent stem cell-derived bile canaliculi-forming hepatocytes to study genetic liver diseases involving hepatocyte polarity. J Hepatol, 2019. 71(2): p. 344–356. [DOI] [PubMed] [Google Scholar]
- 34.Ghodsizadeh A, et al. , Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem cell reviews, 2010. 6(4): p. 622–32. [DOI] [PubMed] [Google Scholar]
- 35.Rashid ST, et al. , Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. The Journal of clinical investigation, 2010.120(9): p. 3127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soga M, et al. , HPGCD outperforms HPBCD as a potential treatment for Niemann-Pick disease type C during disease modeling with iPS cells. Stem Cells, 2015. 33(4): p. 1075–88. [DOI] [PubMed] [Google Scholar]
- 37.Rashid ST, et al. , Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest, 2010.120(9): p. 3127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kholodenko IV, et al. , Isolation of Induced Pluripotent Cells from Stromal Liver Cells of Patients with Alcoholic Cirrhosis. Bull Exp Biol Med, 2017.163(4): p. 535–541. [DOI] [PubMed] [Google Scholar]
- 39.Chien Y, et al. , Improvement of non-alcoholic steatohepatitis by hepatocyte-like cells generated from iPSCs with Oct4/Sox2/Klf4/Parp1. Oncotarget, 2018. 9(26): p. 18594–18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graffmann N, et al. , Establishment and characterization of an iPSC line from a 58years old high grade patient with nonalcoholic fatty liver disease (70% steatosis) with homozygous wildtype PNPLA3 genotype. Stem Cell Res, 2018. 31: p. 131–134. [DOI] [PubMed] [Google Scholar]
- 41.Graffmann N, et al. , Establishment and characterization of an iPSC line from a 35years old high grade patient with nonalcoholic fatty liver disease (30-40% steatosis) with homozygous wildtype PNPLA3 genotype. Stem Cell Res, 2018. 31: p. 113–116. [DOI] [PubMed] [Google Scholar]
- 42.Graffmann N, et al. , Modeling Nonalcoholic Fatty Liver Disease with Human Pluripotent Stem Cell-Derived Immature Hepatocyte-Like Cells Reveals Activation of PLIN2 and Confirms Regulatory Functions of Peroxisome Proliferator-Activated Receptor Alpha. Stem Cells Dev, 2016. 25(15): p. 1119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collin de I'Hortet A, et al. , Generation of Human Fatty Livers Using Custom-Engineered Induced Pluripotent Stem Cells with Modifiable SIRT1 Metabolism. Cell Metab, 2019. 30(2): p. 385–401 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wruck W, et al. , Concise Review: Current Status and Future Directions on Research Related to Nonalcoholic Fatty Liver Disease. Stem Cells, 2017. 35(1): p. 89–96. [DOI] [PubMed] [Google Scholar]
- 45.Ghanekar A and Kamath BM, Cholangiocytes derived from induced pluripotent stem cells for disease modeling. Curr Opin Gastroenterol, 2016. 32(3): p. 210–5. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa K, et al. , Generation of a bile salt export pump deficiency model using patient-specific induced pluripotent stem cell-derived hepatocyte-like cells. Sci Rep, 2017. 7: p. 41806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoepfner J, et al. , In vitro modelling of familial amyloidotic polyneuropathy allows quantitative detection of transthyretin amyloid fibril-like structures in hepatic derivatives of patient-specific induced pluripotent stem cells. Biol Chem, 2017. 398(8): p. 939–954. [DOI] [PubMed] [Google Scholar]
- 48.Bengrine A, et al. , Modeling of HBV and HCV hepatitis with hepatocyte-like cells. Front Biosci (Schol Ed), 2016. 8: p. 97–105. [DOI] [PubMed] [Google Scholar]
- 49.Sa-Ngiamsuntorn K, et al. , A robust model of natural hepatitis C infection using hepatocyte-like cells derived from human induced pluripotent stem cells as a long-term host. Virol J, 2016.13: p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakurai F, et al. , Hepatitis C virus-induced innate immune responses in human iPS cell-derived hepatocyte-like cells. Virus Res, 2017. 242: p. 7–15. [DOI] [PubMed] [Google Scholar]
- 51.Wu X and Dao Thi VL, Embryonic or Induced Pluripotent Stem Cell-Derived Hepatocellular Systems for HCV Culture. Methods Mol Biol, 2019.1911: p. 121–135. [DOI] [PubMed] [Google Scholar]
- 52.Schobel A, Rosch K, and Herker E, Functional innate immunity restricts Hepatitis C Virus infection in induced pluripotent stem cell-derived hepatocytes. Sci Rep, 2018. 8(1): p. 3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakurai F, et al. , Human induced-pluripotent stem cell-derived hepatocyte-like cells as an in vitro model of human hepatitis B virus infection. Sci Rep, 2017. 7: p. 45698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan L, et al. , A Chimeric Humanized Mouse Model by Engrafting the Human Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cell for the Chronic Hepatitis B Virus Infection. Front Microbiol, 2018. 9: p. 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, et al. , Pan-Genotype Hepatitis E Virus Replication in Stem Cell-Derived Hepatocellular Systems. Gastroenterology, 2018.154(3): p. 663–674 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medine CN, et al. , Developing high-fidelity hepatotoxicity models from pluripotent stem cells. Stem Cells Transl Med, 2013. 2(7): p. 505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cayo MA, et al. , A Drug Screen using Human iPSC-Derived Hepatocyte-like Cells Reveals Cardiac Glycosides as a Potential Treatment for Hypercholesterolemia. Cell Stem Cell, 2017. 20(4): p. 478–489 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi SM, et al. , Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Flepatology, 2013. 57(6): p. 2458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuba-Surma EK, et al. , Stem cells as a novel tool for drug screening and treatment of degenerative diseases. Curr Pharm Des, 2012. 18(18): p. 2644–56. [DOI] [PubMed] [Google Scholar]
- 60.Ueda T and Kaneko S, In Vitro Differentiation of T Cell: From CAR-Modified T-iPSC. Methods Mol Biol, 2019. 2048: p. 85–91. [DOI] [PubMed] [Google Scholar]
- 61.Yu Y, et al. , Hepatocyte-like cells differentiated from human induced pluripotent stem cells: Relevance to cellular therapies. Stem Cell Res, 2012. 9(3): p. 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yi F, Liu GH, and Izpisua Belmonte JC, Human induced pluripotent stem cells derived hepatocytes: rising promise for disease modeling, drug development and cell therapy. Protein Cell, 2012. 3(4): p. 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hentze H, et al. , Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res, 2009. 2(3): p. 198–210. [DOI] [PubMed] [Google Scholar]
- 64.Hedlund E, et al. , Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells, 2007. 25(5): p. 1126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basma H, et al. , Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology, 2009.136(3): p. 990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang G, et al. , Integration-deficient lentivectors: an effective strategy to purify and differentiate human embryonic stem cell-derived hepatic progenitors. BMC Biol, 2013.11: p. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murry CE and Keller G, Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell, 2008.132(4): p. 661–80. [DOI] [PubMed] [Google Scholar]
- 68.Yanagida A, et al. , An In Vitro Expansion System for Generation of Human iPS Cell-Derived Hepatic Progenitor-Like Cells Exhibiting a Bipotent Differentiation Potential. PLoS One, 2013. 8(7): p. e67541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng X, et al. , Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell, 2012. 10(4): p. 371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang P, et al. , Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature, 2011. 475(7356): p. 386–9. [DOI] [PubMed] [Google Scholar]
- 71.Park S, et al. , The therapeutic potential of induced hepatocyte-like cells generated by direct reprogramming on hepatic fibrosis. Stem Cell ResTher, 2019.10(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fourrier A, et al. , Regenerative cell therapy for the treatment of hyperbilirubinemic Gunn rats with fresh and frozen human induced pluripotent stem cells-derived hepatic stem cells. Xenotransplantation, 2019: p. el2544. [DOI] [PubMed] [Google Scholar]
- 73.Azuma H, et al. , Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol, 2007. 25(8): p. 903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hickey RD, et al. , Efficient production of Fah-null heterozygote pigs by chimeric adeno-associated virus-mediated gene knockout and somatic cell nuclear transfer. Hepatology, 2011. 54(4): p. 1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhim JA, et al. , Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci USA, 1995. 92(11): p. 4942–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meuleman P, et al. , Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology, 2005. 41(4): p. 847–56. [DOI] [PubMed] [Google Scholar]
- 77.Dianat N, et al. , Human pluripotent stem cells for modelling human liver diseases and cell therapy. Curr Gene Ther, 2013. 13(2): p. 120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagamoto Y, et al. , Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol, 2016. 64(5): p. 1068–75. [DOI] [PubMed] [Google Scholar]
- 79.Matsumoto K, et al. , Liver organogenesis promoted by endothelial cells prior to vascular function. Science, 2001. 294(5542): p. 559–63. [DOI] [PubMed] [Google Scholar]
- 80.Konstantinova I and Lammert E, Microvascular development: learning from pancreatic islets. Bioessays, 2004. 26(10): p. 1069–75. [DOI] [PubMed] [Google Scholar]
- 81.Hammar E, et al. , Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes, 2004. 53(8): p. 2034–41. [DOI] [PubMed] [Google Scholar]
- 82.Snykers S, et al. , In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells, 2009. 27(3): p. 577–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flaim CJ, Chien S, and Bhatia SN, An extracellular matrix microarray for probing cellular differentiation. Nat Methods, 2005. 2(2): p. 119–25. [DOI] [PubMed] [Google Scholar]
- 84.Lorvellec M, et al. , Mouse decellularised liver scaffold improves human embryonic and induced pluripotent stem cells differentiation into hepatocyte-like cells. PLoS One, 2017.12(12): p. e0189586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abazari MF, et al. , Decellularized amniotic membrane Scaffolds improve differentiation of iPSCs to functional hepatocyte-like cells. J Cell Biochem, 2019. [DOI] [PubMed] [Google Scholar]
- 86.Park KM, et al. , Decellularized Liver Extracellular Matrix as Promising Tools for Transplantable Bioengineered Liver Promotes Hepatic Lineage Commitments of Induced Pluripotent Stem Cells. Tissue Eng Part A, 2016. 22(5–6): p. 449–60. [DOI] [PubMed] [Google Scholar]
- 87.Shaheen MF, et al. , Sustained perfusion of revascularized bioengineered livers heterotopically transplanted into immunosuppressed pigs. Nat Biomed Eng, 2019. [DOI] [PMC free article] [PubMed]
- 88.Takebe T, et al. , Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature, 2013. 499(7459): p. 481–4. [DOI] [PubMed] [Google Scholar]
- 89.Mun SJ, et al. , Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol, 2019. 71(5): p. 970–985. [DOI] [PubMed] [Google Scholar]
- 90.Ouchi R, et al. , Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab, 2019. 30(2): p. 374–384 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akbari S, et al. , Robust, Long-Term Culture of Endoderm-Derived Hepatic Organoids for Disease Modeling. Stem Cell Reports, 2019. 13(4): p. 627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meier RPH, et al. , Xenotransplantation: back to the future? Transpl Int, 2018. 31(5): p. 465–477. [DOI] [PubMed] [Google Scholar]
- 93.Garry DJ and Garry MG, Interspecies Chimeras and the Generation of Humanized Organs. Circ Res, 2019. 124(1): p. 23–25. [DOI] [PubMed] [Google Scholar]
- 94.Hirabayashi M, Goto T, and Hochi S, Pluripotent stem cell-derived organogenesis in the rat model system. Transgenic Res, 2019. 28(3–4): p. 287–297. [DOI] [PubMed] [Google Scholar]
- 95.Wu J, et al. , Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell, 2017. 168(3): p. 473–486 el5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crane AT, et al. , Interspecies Organogenesis for Human Transplantation. Cell Transplant, 2019. 28(9–10): p. 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borel F, et al. , Survival Advantage of Both Human Hepatocyte Xenografts and Genome-Edited Hepatocytes for Treatment of alpha-1 Antitrypsin Deficiency. Mol Ther, 2017. 25(11): p. 2477–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagashima H and Matsunari H, Growing human organs in pigs-A dream or reality? Theriogenology, 2016. 86(1): p. 422–6. [DOI] [PubMed] [Google Scholar]
- 99.Levine S and Grabel L, The contribution of human/non-human animal chimeras to stem cell research. Stem Cell Res, 2017. 24: p. 128–134. [DOI] [PubMed] [Google Scholar]
- 100.Faulkner-Jones A, et al. , Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication, 2015. 7(4): p. 044102. [DOI] [PubMed] [Google Scholar]
- 101.Goulart E, et al. , 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication, 2019. [DOI] [PubMed] [Google Scholar]
- 102.Ma X, et al. , Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci USA, 2016. 113(8): p. 2206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buisson EM, et al. , Regenerative Medicine of the Bile Duct: Beyond the Myth. Int J Stem Cells, 2019. 12(2): p. 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]