Abstract
Light Sheet Fluorescence Microscopy (LSFM) provides a rapid and complete three-dimensional image of the cochlea. The method retains anatomical relationships - on a micron scale - between internal structures such as hair cells, basilar membrane and modiolus with external surface structures such as the round and oval windows. Immunolabeled hair cells were used to visualize the spiraling basilar membrane in the intact cochlea without time intensive dissections or additional histological processing; yet material prepared for LSFM could be rehydrated, the basilar membrane dissected out and re-imaged at higher resolution with the confocal microscope. In immersion-fixed material, details of the cochlear vasculature were seen throughout the cochlea. Hair cell counts (both inner and outer) as well as frequency maps of the basilar membrane were comparable to those obtained by other methods, but with the added dimension of depth. The material provided measures of angular, linear and vector distance between characteristic frequency regions along the basilar membrane. Thus, LSFM provides a unique ability to rapidly image the entire cochlea in a manner applicable to model and interpret physiological results. Furthermore, the three-dimensional organization of the cochlea can be studied at the organ and cellular level with LSFM, and this same material can be taken to the confocal microscope for detailed analysis at the subcellular level.
Keywords: confocal microscopy, electrocochleography, outer hair cells, inner hair cells, auditory nerve, RRID: AB_10015251, RRID: AB_2534017, RRID:SCR_007370, RRID:SCR_003070, RRID:SCR_002465
Graphical Abstract
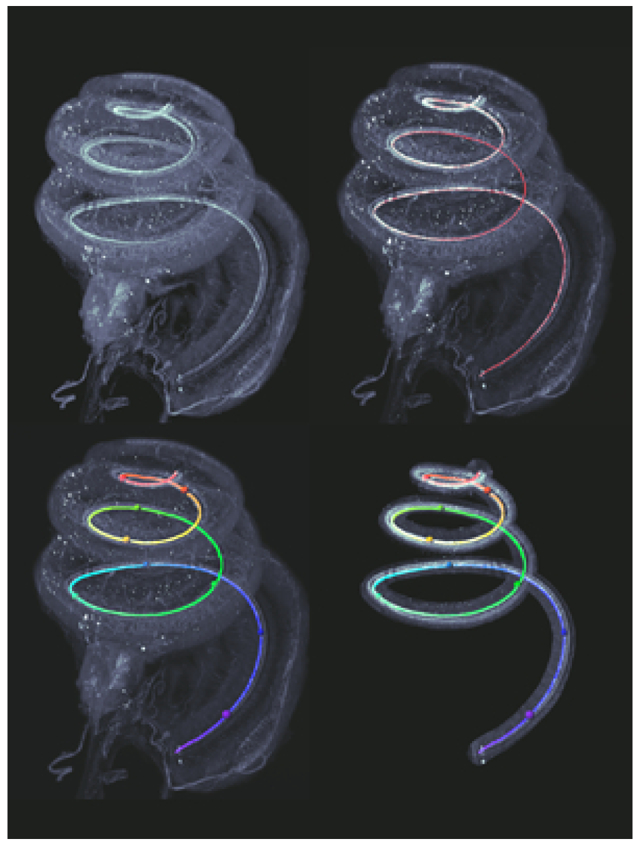
Light sheet microcopy is used to map frequency along the basilar membrane within intact cochleae, retaining spatial orientation and relationships with internal and external structural landmarks. Three-dimensional measures of how frequency domains relate to one another gives insight into how the geometric organization of the cochlea may influence its physiology.
1. INTRODUCTION
A major consideration in interpreting cochlear function is its complex three-dimensional structure. Along with its spiral shape there are systematic variations in size and arrangement of its internal components (e.g., basilar membrane, tectorial membrane and scala) that provide for the separation of sounds into constituent frequency channels. The primary transformation of sound into a neural frequency code lies in the physical properties of the basilar membrane and the sensory hair cells within the organ of Corti that together create a tonotopically arranged sensory organ. Many reconstructive techniques have been employed to analyze cochlear structure, ranging from tissue sections and flat-mounts to surface preparations (e.g., Bohne & Harding, 1993; Chamberlain, 1977; Guild, 1921; Hardie, MacDonald, & Rubel, 2004; Kopecky, Johnson, Schmitz, Santi, & Fritzsch, 2012; Liu, Gao, Yin, Luo, & Lu, 2007; Retzius, 1884; Santi, 1986; Wada, Sugawara, Kobayashi, Hozawa, & Takasaka, 1998). Confocal microscopy, with its ability to resolve very small structures, including synapses of the auditory nerve onto hair cells, together with the range of immunocytochemical tools available to resolve multiple structures in detail, has become an important tool for investigating cochlear structure (Batrel et al., 2017; Kujawa & Liberman, 2009; Liberman, Wang, & Liberman, 2011; Zhang, Engler, Koepcke, Steenken, & Koppl, 2018). However, to prepare material for standard confocal microscopy requires detailed dissections of the basilar membrane and organ of Corti such that the large-scale, three-dimensional structure is generally lost. Recently, advances in confocal method (longer working distance) have enabled three-dimensional views of restricted regions of the cochlea (MacDonald & Rubel, 2008; Risoud et al., 2017; Wrzeszcz, Reuter, Nolte, Lenarz, & Scheper, 2013). An alternative approach is light sheet microscopy -- a technique with an experimental history originally developed by Siedentopf and Zsigmondy (1903) and initially applied to the physical sciences (see Huisken & Stainier, 2009; Magde, 1976; Mappes et al., 2012) that has emerged as a powerful tool for the study of biological structure in three-dimensions. Its use in biology began with studies of the cochlea (Voie, 2002; Voie, Burns, & Spelman, 1993; Voie & Spelman, 1995), who developed a method to optically section the intact cochlea using planar illumination in combination with a fluorescent dye (rhodamine B isothiocyanate), and further refined by Huisken et al (2004) for imaging Medaka and Drosophila embryos.
Since these initial applications to biological tissue, the technique has evolved thorough multiple design iterations (see reviews by Girkin & Carvalho, 2018; Huisken & Stainier, 2009; Keller & Dodt, 2012; Kromm, Thumberger, & Wittbrodt, 2016; Santi, 2011; Shah, Weber, & Huisken, 2017) although each can be classified under the global term of light sheet fluorescence microscopy (LSFM; Santi, 2011). These techniques, previously limited to investigators proficient in microscope construction (e.g., Becker, Jährling, Kramer, Schnorrer, & Dodt, 2008; Buytaert & Dirckx, 2007; Dodt et al., 2007; Jährling, Beckera, Kramerc, & Dodt, 2008; Keller & Stelzer, 2008; Power & Huisken, 2017; Santi, 2011; Santi et al., 2009; Voie, 2002; Voie et al., 1993; Voie & Spelman, 1995) have recently become available for wider use through commercial vendors (e.g., LaVision and others; see Brown, Pastras, Curthoys, Southwell, & Van Roon, 2016; Levy et al., 2019). Similar to confocal microscopy, LSFM depends on laser illumination of the sample, but the laser source is distributed across a plane (x, y dimensions) through which the sample is moved in the z-dimension, rather than a single point source moving in a raster-like pattern. With this technique, detailed anatomy can be resolved at about 1-3 μm, while preserving intact the three-dimensional structure of the entire cochlea. Here, we apply this technique to the cochlea of the gerbil. We describe the processing steps required, show that processing tissue for LSFM does not preclude further examination with standard confocal microscopy, and illustrate how the cochlea’s three-dimensional frequency structure can be preserved, displayed, and measured.
2. MATERIALS AND METHODS
Cochleae harvested from 12 adult Mongolian gerbils (Meriones unguiculatus) were used in this study. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, following the standards of the National Institutes of Health and Committee on Care and Use of Laboratory Animals.
2.1. General Procedures
Briefly, our LSFM procedures were: 1) harvested cochleae were decalcified, 2) excess temporal bone was trimmed away, 3) the tissue was immunostained and 4) dehydrated and cleared to transparency for LSFM examination. The crucial step for LSFM is tissue clearing because the sample must be transparent. For the cochlea, the main issue is the presence of bone covering not only the outside (otic capsule) but also internally between cochlear turns (interscalar septum), the osseous spiral lamina and the modiolus. The decalcification step prior to clearing is a standard requirement for cochlear histology and during this step boney structures can be dissected away if necessary, although the extent to which bone is removed can come at a cost to structural integrity, as we will describe.
2.2. Animal Handling
The sample included eight untreated animals and four animals that received ototoxic injections of furosemide and kanamycin (FK, 100 mg/kg furosemide, intraperitoneal; Merck NADA#34-478, Kenilworth, NJ, 200 mg/kg kanamycin, subcutaneous; Sigma-Aldrich PHR1487, St. Louis, MO) with a seven day post-injection survival to ablate outer hair cells (see Pappa et al., 2019). The animals were euthanized with Nembutal (100 mg/kg sodium pentobarbital; Oak Pharmaceuticals, Lake Forest, IL). Four animals were perfusion-fixed (intracardial) with a 0.1M phosphate buffer (PB) rinse to remove blood, followed by fixative (4% paraformaldehyde in PB). For all other animals, following euthanasia the temporal bones were immediately removed and immersed in fixative solution. For both immersion and perfusion fixed material, the bulla was opened, the round window punctured with a sharp probe and the stapes bone removed to allow fixative unobstructed access to the internal chambers of the cochlea. All temporal bones remained in fixative for at least 48 hours prior to being decalcified with 10% EDTA (Sigma-Aldrich E-5134, St. Louis, MO) for 3–4 days.
2.3. Immunolabeling for Light Sheet Fluorescence Microscopy
Following decalcification, hair cells of the cochlea were immunostained (four after removal of the otic capsule; ten with the otic capsule intact) with all steps carried out at room temperature. Decalcified cochleae were rinsed (3 x 15 min) in phosphate buffered saline (PBS; 0.9% saline in 0.1M PB) then transferred to blocker solution (5% normal donkey serum with 1% Triton X 100 in PBS) for 2 hours. Specimens were then moved into primary antibody solution (1:200) in blocker overnight. To label hair cells the primary antibody used was anti-myosin VIIA, raised in rabbit (Proteus Biosciences; 25-6790, Ramona, CA, RRID:AB, 10015251). The next day specimens were rinsed in PBS (3 x 15 min) and transferred to secondary antibody solution (1:500) in blocker for 2 hours in the dark. The secondary used was donkey-anti-rabbit tagged with Alexa Fluor 568 (ThermoFisher; A10042, Waltham, MA, RRID:AB_2534017). Post-secondary, tissue was again rinsed in PBS (3 x 15 min) and either dehydrated immediately or stored overnight in PBS prior to dehydration.
2.4. Light Sheet Imaging.
For LSFM, tissues were dehydrated though a series of graded ethanol solutions (30%, 50%, 70%, 90% and 100%) for 2 hours each, then changed to fresh 100% ethanol overnight. The specimens were then cleared to transparency in MSBB (5:3 methyl salicylate:benzyl benzoate; Spalteholz, 1911) for 2 hours and then fresh MSBB overnight. Finally, the specimens were transferred to dibenzyl ether (DBE) overnight. Prior to LSFM, they were mounted onto an acrylic stand with a two-part silicon adhesive (MPK Enterprises; 1125, Huntington Beach, CA). Specimens were examined using a commercially available system (LaVision UltraMicroscope II Light Sheet Microscope, LaVision BioTec GmbH, Bielefeld, Germany). The mounted cochlea was placed into the microscope’s imaging chamber (containing DBE; refractive index = 1.562), and images were captured using a sCMOS detector (Andor Neo 5.5 sCMOS camera). Acquired images were imported to a workstation and analyzed using Bitplane Imaris 9.2 software (Oxford Instruments, Concord, MA, RRID:SCR_007370).
The LaVision UltraMicroscope II system used has an Olympus MVPLAPO 2x/0.5 objective with a corrected dipping cap (5.7 mm working distance; a 10 mm working distance cap was available but not used in the present study), equipped with an optical zoom yielding a magnification range of 1.26 – 12.6x. The resulting x-y resolution at the detector ranges from 10.32 μm (1.26x) to 1.02 μm (12.6x). This configuration provides a working distance long enough to easily image an entire gerbil cochlea. We typically imaged the gerbil cochlea at magnifications of 4 – 12.6x (detector resolution of 3.24 – 1.02 μm respectively). Resolution in the z-dimension is dependent upon the numerical aperture chosen for the light sheet optics (from 0.0148 – 0.148). We most often used a laser numerical aperture in the range of 0.026 – 0.076, yielding a light sheet thickness (in z-dimension) of 28 −10 μm. To avoid oversampling in the z-dimension, we used a step size that was ⅓ to ½ of the sheet thickness. Myosin VIIa labeled hair cells were imaged with a 561 nm laser and a Chroma ET600/50m emission filter. One specimen (not immunolabeled) was imaged specifically for autofluorescence with a 488 nm laser and a Chroma ET525/50m emission filter.
2.5. Confocal Imaging
Tissue was prepared in two ways for examination with the confocal microscope. One set of samples was obtained following the initial tissue fixation and decalcification procedures described above, however the basilar membrane (BM) was immediately dissected out in toto and prepared as a flat-mount. The basilar membrane was immunostained as above, except that after rinsing of the secondary antibody, the tissue was mounted in glycerol between two #1.5 coverslips. The second set of samples came from cochleae previously prepared and imaged for LSFM. To acquire this tissue, light sheet cochleae (already immunostained) were rehydrated back to buffer, the BM carefully dissected free and flat-mounted between coverslips.
Tissue was examined using a Zeiss 700 laser scanning confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) at magnifications from 5x to 63x oil immersion. To image fine details of the BM, we used a Plan Apochromat 63X/1.4 oil objective, excited the sample with a 555 nm laser, and collected the emission with the dichroic beam splitter set at 555 nm and a 560 nm long pass filter. Pixel size was 0.198 μm in x-y and z-stack step size was 1 μm. We typically used 2x averaging and took care to adjust gain and laser power to avoid saturation of signal in any regions of interest. Images were acquired in 12-bit data mode, viewed and manipulated with ImageJ software (National Institutes of Health, https://imagej.nih.gov/ij/,RRID:SCR_003070): when needed, high magnification image stacks were deconvolved with AutoQuant software (version 3.1; Media Cybernetics, Rockville, MD, RRID:SCR_002465), using default settings and saved as 16-bit unsigned integer Bitplane Imaris 5.5 files.
3. RESULTS
We sought to generate a complete three-dimensional representation of the basilar membrane as it ascends from base to apex, while retaining its relationships (again in three-dimensions) with internal and external anatomical landmarks. Our approach to reach this goal are described in three parts: 1) stages of tissue preparation; 2) microscopic observations, progressively going from the three-dimensional organization at the organ level (entire cochlea), to tissue level (thick sections including the basilar membrane, adjacent structures and vasculature) to the cell level (hair cells of the basilar membrane), 3) a demonstration of the ability to return the material used for LSFM to a state suitable for confocal microscopy if higher resolution is desired and 4) application of the frequency map of the basilar membrane described for the gerbil (Müller, 1996) in the preserved three-dimensional structure.
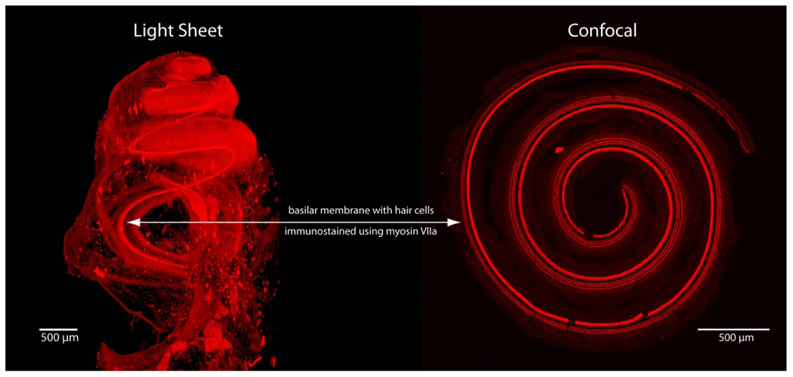
To demonstrate the main differences between LSFM and confocal microscopy, in Figure 1 we show examples of images that can be obtained from each method. The cochleae are from two different animals, each immunolabeled with antibodies to myosin VIIa. The image using LSFM (Figure 1, left) is of the entire cochlea while the confocal image (Figure1, right) is of a dissected basilar membrane and organ of Corti. Both were imaged at 5x. The light sheet image was acquired in 5 minutes, while the confocal image took 30 minutes to scan a 120 μm thick cochlear flat-mount. The light sheet images provide a three-dimensional view of the cochlea from base to apex, while the confocal image is collapsed in the z-axis such that a three-dimensional representation is restricted to the depth of the basilar membrane/organ of Corti complex.
Figure 1.
Examples of images obtained from light sheet and confocal microscopes (z-stack maximum intensity projection), both imaged at 5x magnification. Each image was obtained from a perfusion-fixed cochleae immunolabeled with antibodies to myosin VIIa to label hair cells. The dissection process for confocal imaging loses the 3-D architecture that is maintained with LSFM.
3.1. Preparation of the cochlea for Light Sheet Microscopy
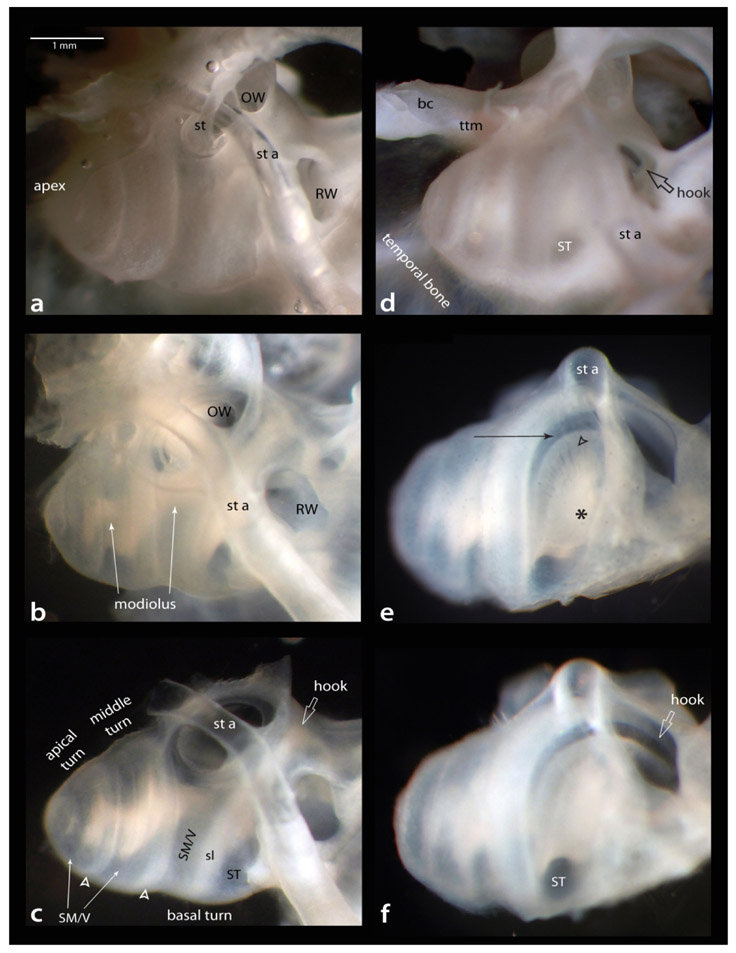
The temporal bone from a perfusion-fixed animal is shown in Figure 2, panels a-c are from a lateral view and d-f are rotated to view the round window (RW); panels a and d are before, and the remaining panels are after decalcification. Prior to decalcification, we remove the stapes (st in a). After decalcification (b), the otic capsule becomes translucent such that many internal structures can be observed. In b note that, in gerbil, the modiolus bends (white arrows) and does not conform to a single axis throughout its apical to basal extent (Chamberlain, 1977). Next, we remove excess bone from the medial (brain side) to decrease interference during imaging (c, compare with b; note: macroscopic images lack a distinct view of Reissner’s membrane, concealing the boundary separating scala media from scala vestibuli). Also in c, note the scala tympani and media/vestibuli (marked in the basal turn) separated by the less transparent spiral ligament (sl), and that while the scala media/vestibuli remains near the surface in the middle and apical turns the scala tympani is less visible due to close apposition to the interscalar septum (hollow arrowheads). In d, the hook region of the basilar membrane (hollow arrow) can be seen through the RW prior to decalcification. For e, the imaging focus is through the scala tympani of the basal turn; note the modiolus (asterisk) and fascicles of auditory nerve fibers (hollow arrowhead) en route to the basilar membrane (arrow) of the basal turn. In f, the focus is through the RW to show the hook as it continues into the vestibule. Also note the scala tympani as it exits the basal turn beginning its ascent towards the apex. This specimen could now proceed to the immunostaining step or be further dissected as described below.
Figure 2.
Decalcification steps. Two views of a perfusion-fixed left cochlea: a-c lateral view; d-f rotated to a view the round window (arrow in d). Panels a and d are before decalcification, all others after decalcification. Decalcified material is sufficient to see many structural details, for example in e, asterisk marks the modiolus containing the auditory nerve, from which fascicles of the auditory nerve (hollow arrowhead) course through the osseous spiral lamina to reach the basilar membrane (arrow). Further descriptive details in text. Scale bar in a same for b-f. bc; boney canal for stapedial artery’s intracranial entrance; OW, oval window; RW, round window; sl, spiral ligament; SM/V, region occupied by scala media and vestibuli; ST, scala tympani; st, stapedial bone; st a, stapedial artery; ttm, tensor tympani muscle.
An important question is whether to remove the otic capsule, either to improve visualization of internal structures and increase accessibility for antibodies, or to leave it intact and retain spatial relationships with external surface landmarks. As we will show, there is a loss of structural integrity after its removal, but the issue of antibody penetration does not appear to be critical. Thus, in most cases we prefer to leave the otic capsule intact. However, for our initial LSFM preparations, we removed the otic capsule in order to allow all parts of the cochlea access to the antibody. In Figure 3a, the otic capsule and adjacent temporal bone has been dissected away, leaving the spiral ligament (sl) intact, and revealing the vestibular canals (anterior, horizontal and posterior), which are important to leave in place as a purchase for dissecting the capsule. In b, a more lateral view, the locations of both the oval and round windows are visible with the spiral ligament of the basal turn (sl) coursing between them. When rotated as if viewed through the RW (c), the osseous spiral lamina (osl) and basilar membrane (bm) of all three turns come into better view. At this point, the specimen could be immunostained and continue to the next processing step for LSFM. However, for confocal microscopy, the basilar membrane must first be dissected away from the lateral wall and spiral ligament, and then away from the osseous spiral lamina (d). This can be done as a complete spiral in most well-fixed cases. Here the spiral is shown floating in buffer in its natural three-dimensional orientation prior to immunostaining and compression as a flat-mount. Note that the modiolus and spiral lamina (including spiral ganglion cells) remains intact and could be further processed if so desired. This technically difficult dissection is not required with LSFM.
Figure 3.
Views of the left labyrinth after dissection of the otic capsule. In a, as viewed from above; b, from the lateral side; c, as tilted to a view through the round window. In d, the basilar membrane is shown after being dissected free of the spiral ligament, osseous spiral lamina and modiolus. Scale bar in a same for b and c. aa, ampulla of the anterior canal; ant, lat and post canals; anterior, lateral and posterior vestibular canals; bm, basilar membrane; la, ampule of the lateral canal; osl; osseous spiral lamina; OW, oval window; pa, ampule of the posterior canal; RW, round window; sl, spiral ligament.
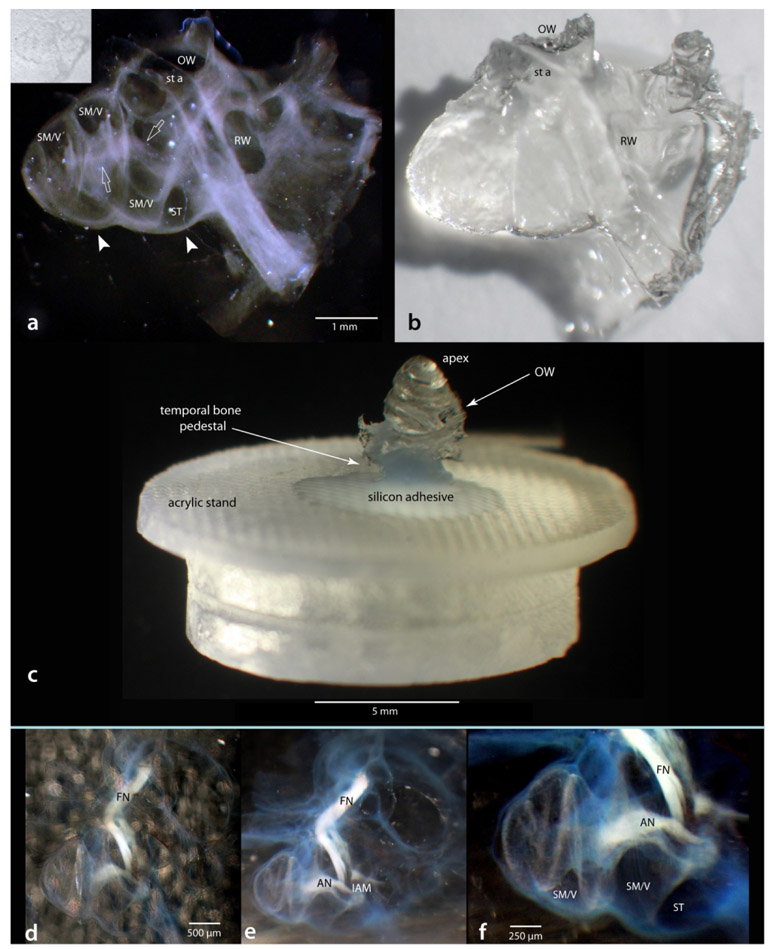
Following immunostaining, the bone must be made transparent before LSFM imaging can begin. The clearing agent and its use were described in the methods. Figure 4 shows examples with the otic capsule intact. When in the clearing agent, the cochlea becomes nearly invisible and difficult to view with transmitted light. When viewed with light from above, its presence can only be seen by the faint shadow it casts against a white background (inset in a). However, when illuminated from the side (Figure 4a), a darkfield effect can be achieved and internal features become pronounced. Once removed from the clearing solution, the cochlea appears crystalline (b).
Figure 4.
Example of a cochlea cleared and mounted for LSFM. Appearance of a cleared cochlea in (a) and out (b) of the clearing solution; same specimen as Figure 2. The inset in a is a brightfield image to show that without using incident light from the side the cleared cochlea is almost invisible. For LSFM imaging the cochlea is mounted in an “apex-up” position on a custom made acrylic stand (c). Leaving a sprig of auditory nerve (AN) or facial nerve (FN) attached can assist during preparation steps (d-f). Scale in a same for b; scale in d same for e. IAM, internal acoustic meatus; OW, oval window; RW, round window; SM/V, region occupied by scala media and vestibuli; ST, scala tympani; st a, stapedial artery.
The last step prior to imaging is to mount the cleared specimens in a way suitable for the light sheet imaging chamber. The details of mounting a particular specimen is specific to the imaging device used and to the best physical orientation needed to minimize the amount of tissue the laser must penetrate before reaching the fluorophores. In our case, the best physical placement was to use a “pedestal” of temporal bone to orient the cochlea in an “apex up” position. The specimens were then attached to an acrylic stand with silicon adhesive as shown in Figure 4c. As a side note on tissue handling: leaving a sprig of nerve attached (auditory or facial) can be of assistance in maneuvering the sample between solutions. In Figure 4c-f, the auditory and facial nerves can easily be seen with a light source at approximately 45 degrees to the specimen (d) and even better with the light source at a 90 degree angle to the cleared specimen (e), and at higher magnification (f).
3.2. Imaging and Image Processing
After mounting the cleared specimen, it is positioned in the light sheet microscope and imaged using the appropriate laser excitation wavelength; here we show results from immunostaining and autofluorescence (though we have also used other fluorescently tagged markers such as phalloidin or rhodamine B isothiocyanate). Examples of screenshots, taken from similar locations but different magnification during imaging parameter set-up, are shown in Figure 5a and b. In a, at 5x magnification, the x-y resolution at the detector is 2.6 μm, while in b, at 10x magnification the resolution is 1.3 μm. Resolution in the z-axis is dependent upon the numerical aperture of the laser (see Ariel, 2018 for a more detailed discussion on technical aspects of LSM). For both a and b, the numerical aperture was 0.034, yielding a sheet thickness of 21 μm, thus the only difference between a and b is magnification. To compare resolutions under these conditions, c and d are images (enlarged in Imaris) from a and b, showing that inner and outer hair cells are resolvable with either magnification. For further comparison, images from another case are shown captured at 5x (e; numerical aperture = 0.026, sheet thickness = 28 μm) vs captured at 12.6x (f; numerical aperture = 0.076, sheet thickness = 10 μm). In short, using higher magnifications and larger sheet numerical apertures lead to images with higher detail.
Figure 5.
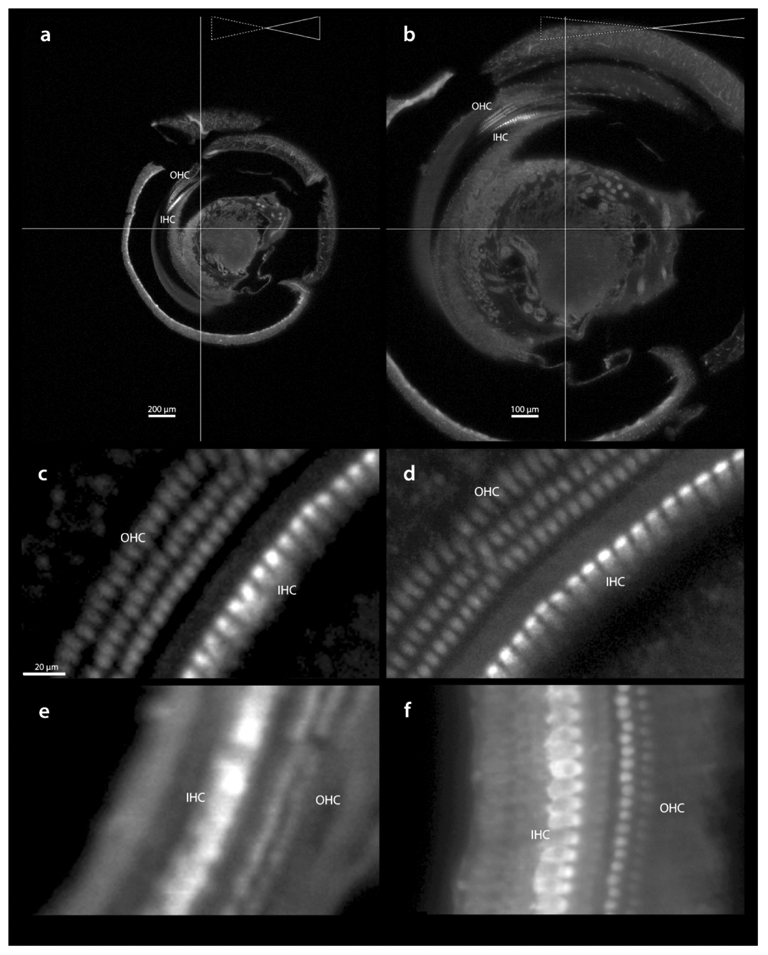
Acquisition of light sheet images. In a and b, similar regions from a specimen captured at 5x (a) and 10x (b) magnification, using a laser thickness of 21 μm. Enlarged regions of the basilar membrane (c and d from a and b respectively) show the level of detail resolved in 5x vs 10x images. The region of brightest intensity is the cuticular plate, which is rich in myosin VIIa. To compare resolutions, other specimens were imaged at 5x with laser thickness of 28 μm (e) and 12.6x with laser thickness 10 μm (f). Scale in c same for d-f. IHC, inner hair cells; OHC, outer hair cells.
However, there is a trade-off, in time and storage space for collecting high vs. low-resolution images. To image a gerbil cochlea from apex to base at 5x (aperture = 0.026 or 0.034) takes 5 - 10 minutes and generates 250 - 500 images (each image approximately 11 MB in size). At 12.6x it can take 2 hours or more and generate in excess of 5,000 images. This is due to the cochlea being too wide for a single apex-to-base scan at higher magnifications (note clipped edges in Figure 5b) and requires taking a series of tiled stacks (e.g., 3x3 grid in the x-y plane), that are stitched together later using Imaris Stitcher software. If appropriate to the goal of a study, imaging only a specific region of interest at high magnification can significantly reduce acquisition time.
Figure 6 shows a LSFM imaged cochlea rendered in Imaris software to view the entire cochlea (a) or optically sectioned in three orthogonal planes relative to the specimen’s orientation on the mounting stand (b-d). This cochlea was immersion fixed and had its otic capsule removed prior to immunostaining with myosin VIIa. The antibody labeled both inner and outer hair cells, with the inner hair cells being particularly bright. Note in 6a that labeled hair cells can be seen as they ascend from base to apex. A useful feature offered by the Imaris software is the ability to optically section the specimen in any plane and at any thickness (using the Imaris extended sectioning tool). The orthogonal planes (b-d) are maximum intensity projections representing 300 μm-thick sections that more clearly show labeled hair cells in each turn of the cochlear spiral and emphasize the spatial arrangements that exist within the cochlea. For example, a section that includes the extreme basal region (b), demonstrates the distant location of its hair cells relative to the modiolus as compared to those in higher turns. Similarly, in c labeled hair cells can be visualized in all three cochlear turns, while the entrance of the auditory nerve into the modiolus is shown in the section in d. Furthermore, given this cochlea was fixed by immersion, it preserved the appearance of vascular structures (described further below) and many other features of cochlear anatomy are visible through autofluorescence, such as the spiral ligament, stria vascularis and the auditory nerve.
Figure 6.
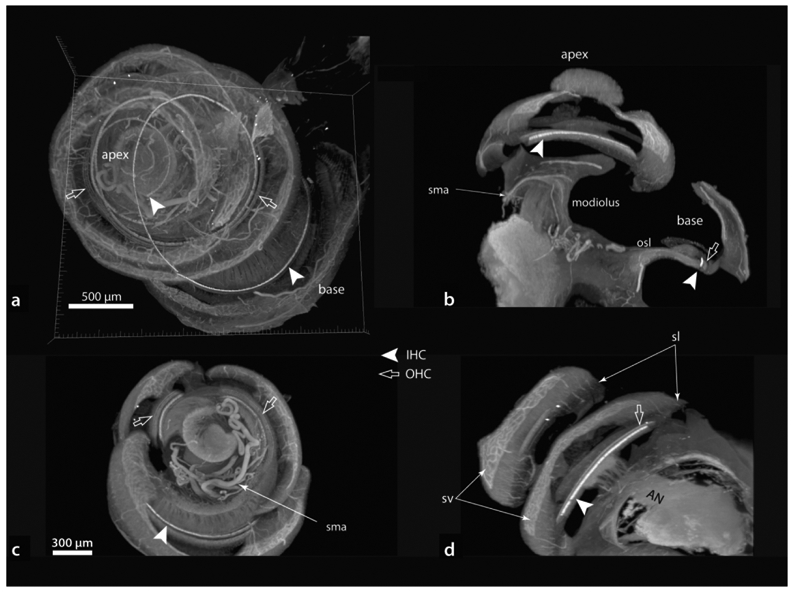
Examples of three-dimensionally rendered cochlea (a) and orthogonally re-sliced images (b–d; scale in c same for b and d). This specimen was immersion fixed and otic capsule removed prior to immunostaining. Note the spiral modiolar artery (sma) in b and c. Additional specific details in text. AN, auditory nerve; IHC, inner hair cells; OHC, outer hair cells; osl; osseous spiral lamina; sl, spiral ligament; sv, stria vascularis.
Another example using the ability to slice in any plane is shown in Figure 7, where a 100 pm-thick section (Figure 7a) contains myosin labeled inner and outer hair cells, dendrites of spiral ganglia cells, their cell bodies within Rosenthal’s canal (SGC), and their axons coursing to the auditory nerve (Aud Nv). More details emerged as the thickness of the slab was increased to 250 μm (7b) and 500 μm in 7c. For example, greater extents of the osseous spiral lamina (osl), spiral ganglion cells within Rosenthal’s canal (between hollow arrows) and the vascular bed lying next to the auditory nerve and spiral ganglia are appreciable (arrows).
Figure 7.

Series of images progressively increasing in thickness from 100 μm (a) to 250 μm (b) to 500 μm (c). Note changes in anatomical details observable as thickness and relative depth increase, such as spiral ganglion cells (SGC) within Rosenthal’s canal (a) as they expand to form their spiraling structure (hollow arrow heads in b and c). Solid arrowhead points to the same vessel in a-c; solid arrows indicate the vascular bed within the modiolus. Scale in b same for c. Aud Nv, auditory nerve; IHC, inner hair cells; OHC, outer hair cells; osl; osseous spiral lamina; sl, spiral ligament; sv, stria vascularis.
Although removing the otic capsule ensures that all parts of the cochlea have equal access to the antibody (the hair cells specifically), we noticed two artifacts associated with its removal (Figure 8). These artifacts were intermittent and their locations unpredictable. They include deformations (hollow arrows) that could be related to the alcohol dehydration or reflect where the specimen was resting on surfaces during the processing stages. Also, there could be curling of the spiral ligament, possibly due to the alcohol treatment, along its free edge (hollow arrowheads). The origins of both types of artifact lie in the lateral wall being structurally unsupported beyond the osseous spiral lamina. These artifacts could potentially be reduced by less aggressive otic capsule dissection, leaving the interscalar septum intact for support, or restricting bone removal to just that overlying the scala vestibuli.
Figure 8.
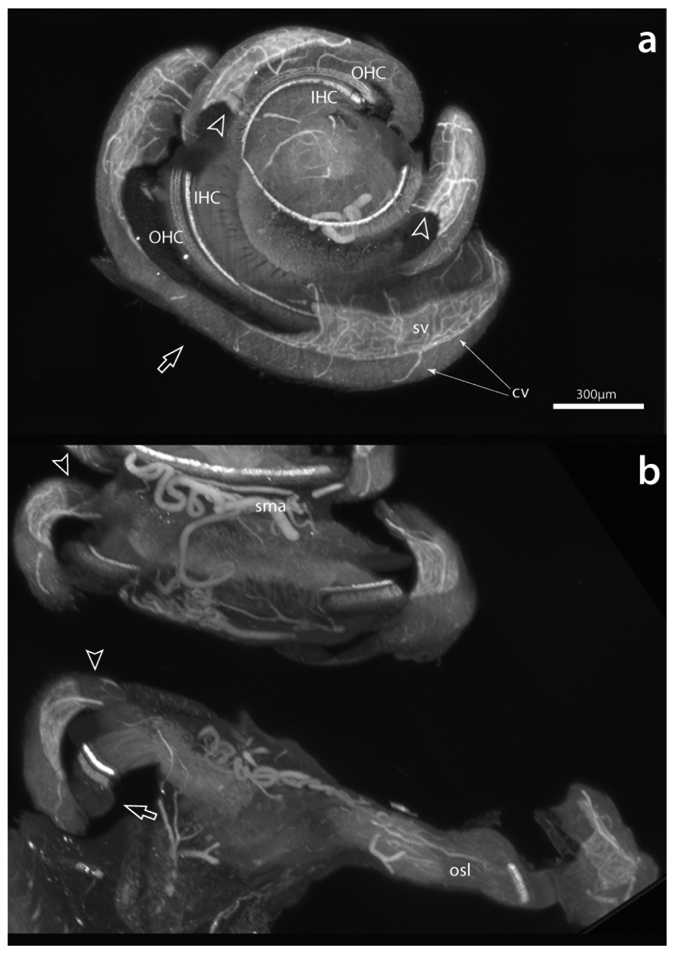
Two forms of artifact observed in specimens with the otic capsule removed. Curling of the free margin of the spiral ligament (hollow arrowheads) likely resulted from the alcoholic dehydration steps, while a buckling disfigurement (hollow arrow) is possibly due to dehydration or reflects the cochlea resting on a surface during preparation steps. Scale same for a and b. cv, collecting venules; IHC, inner hair cells; OHC, outer hair cells; osl; osseous spiral lamina; sma, spiral modiolar artery; sv, stria vascularis.
To avoid these artifacts, another approach is to prepare the specimens with the otic capsule intact. For this, we needed to establish whether gentle perfusion, consisting of a small stream of antibody solution (a few ul), through the small puncture made in the RW at the time of fixation (with the oval window opened via stapes removal), could reliably reach all regions of the cochlea. In Figure 9 we show material from three animals to demonstrate that antibody solutions can reach the apex and all other parts of the cochlea in capsule-intact preparations. Figures 9a-c show sections through each cochlear turn in a perfusion fixed animal obtained from the original “apex up” orientation at the time of imaging. Hair cells in the apex (a) are well labeled, as are those in the middle (b) and basal turns (c). Also note that the antibodies marked not only auditory hair cells, but also vestibular hair cells of the saccule (hollow arrow). Furthermore, the immunolabeling procedure works with both immersion and perfusion fixed material. For comparison, a peri-modiolar section (orthogonally re-sliced image from an “apex up” orientation) and a three-dimensional rendering of a perfusion fixed cochlea are illustrated in d and e, while those from an immersion fixed specimen are shown in f and g. Each case shows robust immunolabeling throughout the cochlea. Differences in the type of results obtained between the two methods of fixation are that immersion fixation (f and g) preserves the blood in vessels and thus makes the vasculature visible by autofluorescence (e.g., asterisks in f; sv in f and g), whereas perfusion fixation (d and e) provides a better view of hair cells with less interference. Regardless of fixation method, inner and outer hair cells are readily viewed in three-dimensions or in cross section. Additionally, these sections (d and f) demonstrate that the spiral ganglia, tunnel of Corti, auditory nerve and Reissner’s membrane are visible. Similar to the case shown in Figure 6 (capsule removed), labeled hair cells can be seen spiraling up the organ of Corti from basal through apical turns, however, specimens with an intact otic capsule maintain an accurate three-dimensional relationship with the external surface of the cochlea. Furthermore, as shown in Figure 10, systemic pretreatment with FK did not interfere with LSFM processing, promote gross tissue or vascular distortions, nor impede the ability to immunolabel hair cells (a, FK treated animal; b, normal animal). This Figure also illustrates the retention of anatomical relationships internal to the cochlea, such as the spiral ganglion and many vascular elements, including the spiral modiolar artery, radiating arterioles and the stria vascularis.
Figure 9.
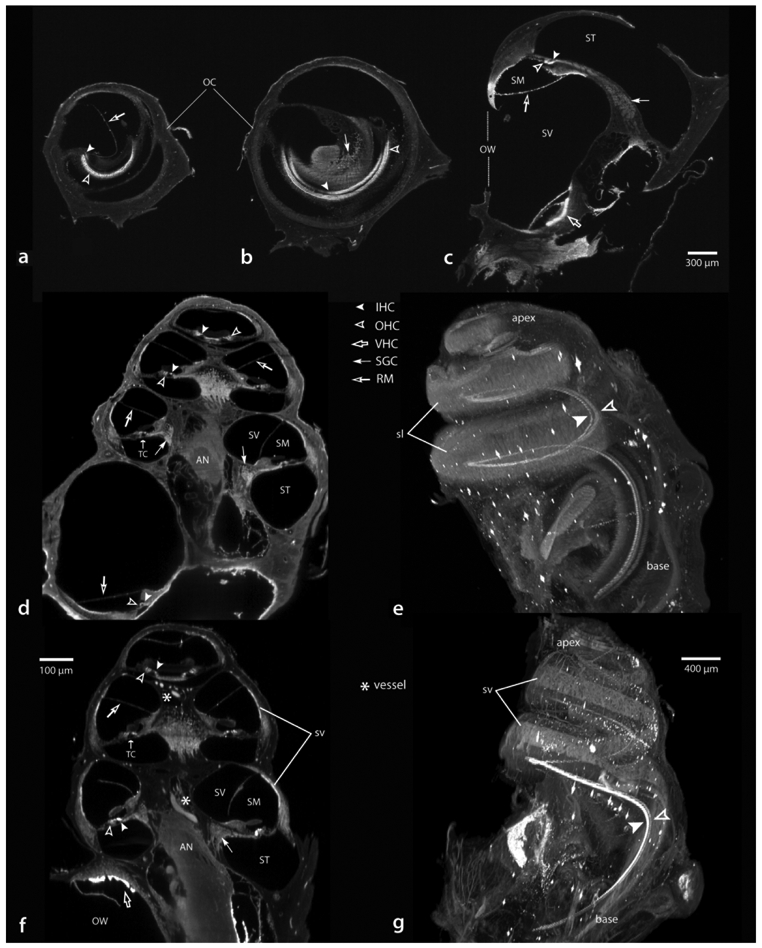
Examples from otic capsule intact specimens showing antibody solutions had complete access to hair cells throughout each turn of the cochlea in perfusion (a-e) or immersion (f and g) fixed specimens. Crosssections from the apical (a), middle (b) and basal (c) turns showing labeled hair cells. Two other cases viewed in a nominal perimodiolar orientation (d and f) and when each is rendered in three-dimensions (e and g, respectively). Scale for a-c in c. AN, auditory nerve; IHC, inner hair cells; OHC, outer hair cells; OC, otic capsule; OW, oval window; RM, Reisner’s membrane; SGC, spiral ganglion cells; sl, spiral ligament; SM, scala media; ST, scala tympani; SV, scala vestibule; sv, stria vascularis; TC, tunnel of Corti; VHS, vestibular hair cells.
Figure 10.

Systemic pre-treatment with furosemide/kanamycin did not affect the quality of material prepared for LSFM. Images from the middle turn of a furosemide/kanamycin treated animal (a) compared to a normal animal (b). Inner hair cells (IHC) are well labeled in both animals, as are outer hair cells (OHC) with the exception of those missing due to the drug treatment (a). No degradation of vascular elements, such as the radiating arterioles (ra) or stria vascularis (sv) were noted. sma, spiral modiolar artery; SGC, spiral ganglion cells.
3.3. Imaging the vasculature of the cochlea
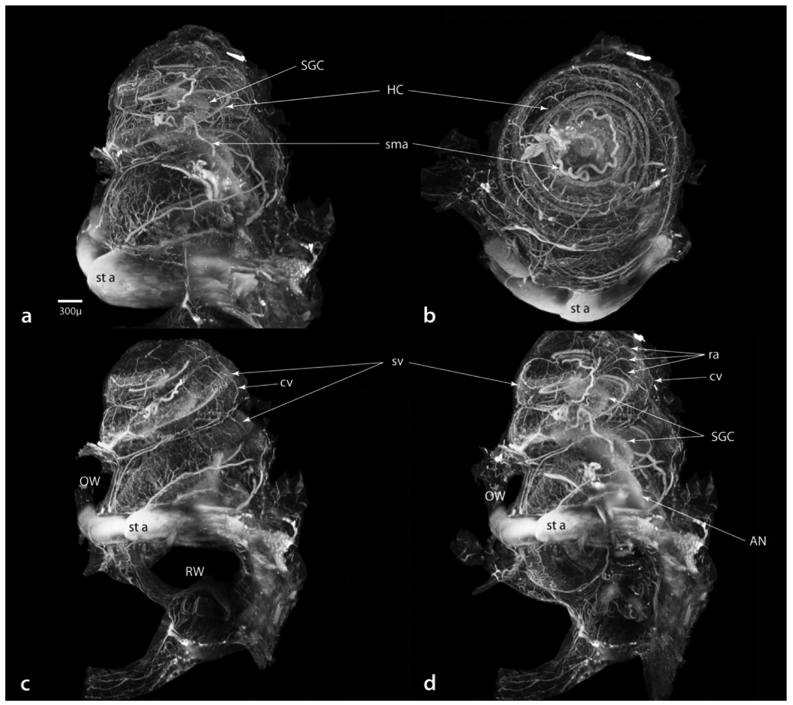
Various levels of cochlear vasculature that can be observed by autofluorescence in immersion fixed LSFM material are illustrated in Figure 11. These “negative” images improve the appearance of the vessels. Figure 11a, is an exterior view of the cochlea and b is a slice through the lateral wall, both showing the pattern of radiating arterioles (ra), collecting veins (cv) and the dense vascular patterns of the stria vascularis (sv). In c, proceeding internally, smaller caliber vessels (hollow arrowheads point to examples) emanating from the spiral modiolar artery, course through and supply the ganglion cells, continuing on to the osseous spiral lamina and hair cells. In this Figure, a portion of the organ Corti has been digitally sectioned away (in the apical and basal turns), to reveal elements that are located beneath the hair cells. In particular, note the bifurcating artery visible in the basal turn (hollow arrow in c), shown at higher magnification in d. In d, the small caliber artery can be traced (hollow arrowheads) from the spiral modiolar artery (sma) to its point of bifurcation (hollow arrow) where one branch forms a vessel of the tympanic lip (solid arrowhead) while the other branch continues past the inner hair cells as a vessel of the basilar membrane (solid arrow).
Figure 11.

Autofluorescence of cochlear vasculature in material stained for myosin VIIa. For these images the look-up table values have been inverted to enhance visualization of vascular elements. Progressing from lateral (a and b) to internal (c and d) the vascular features of the lateral wall and internal cochlear structures can be imaged in remarkable detail. In c, the cochlear turns are indicated: A, apical; M, middle; and B, basal. Also in c, hollow arrowheads indicate small caliber vessels within the osseous spiral lamina (osl), the hollow arrow points to a bifurcating vessel shown at higher magnification in d. In d, the vessel can be traced (hollow arrowheads) from its origin at the spiral modiolar artery (sma) to its bifurcation (hollow arrow) becoming a vessel of the tympanic lip (solid arrowhead) and a vessel of the basilar membrane (solid arrow). In e, an enlarged image shows the intricate arrangement of anastomoses (e.g., arrowheads) between radiating arterioles (ra) and collecting venules (cv) within the stria vascularis Scale for b same as in a. ES, external sulcus; IHC, inner hair cells; OHC, outer hair cells; SGC, spiral ganglion cells sl, spiral ligament.
Vascular elements of the dense arterio-venous anastomoses within the stria vascularis are shown in 11e. Here, radial arterioles arching over the scala vestibuli enter the stria vascularis where they form a dense network of capillary anastomoses with the collecting venules, which coalesce to form a larger vein along the external boundary of the scala tympany (see 11 a and b). The solid arrowheads in 11e trace one of several possible examples of the torturous route taken between a radiating arteriole and a collecting venule.
The previous Figures have shown vascular and non-vascular structures excited with a 561 nm laser, which is optimal for observing hair cells labeled with the Alexa Fluor-568 secondary antibody. In Figure 12 we show an immersion fixed, non-immunolabeled cochlea imaged with a 488 nm laser, to optimize autofluorescence of formaldehyde-fixed tissue (Leischner, Schierloh, Zieglgansberger, & Dodt, 2010). Figure 12a and b are a lateral and apical view, respectively, while c is rotated to show the round window and d is a slightly changed depth of focus in the same orientation. This Figure illustrates the level of vasculature detail in context with other aspects of cochlear structure. For example, note in a and b that the spiral modiolar artery (sma) is visible alongside the spiral ganglion cells, while c and d show the radiating arterioles and collecting venules of the stria vascularis. Thus, simply imaging for autofluorescence with LSFM reveals abundant information about three-dimension organizational relationships among many structures of interest in the cochlea.
Figure 12.
Three-dimensional images of an immersion fixed, otic capsule intact cochlea viewed at a wavelength of 488 nm to enhance formaldehyde autofluorescence. This specimen was not immunostained. Viewed from lateral (a), apical (b) and after rotation to the round window (c) perspectives, then changing the depth of view to just inside the round window (d). Not only is the vasculature depicted in great detail, but also the spatial relationships that exist between vascular elements and internal structures such as hair cells (HC), the spiral ganglia (SGC) and auditory nerve (AN). Scale in a same for all images. cv, collecting venules; OW, oval window; ra, radiating arterioles; RW, round window; sma, spiral modiolar artery; SGC, spiral ganglion cells; st a, stapedial artery; sv, stria vascularis.
3.4. Turning light sheet material into confocal microscope material.
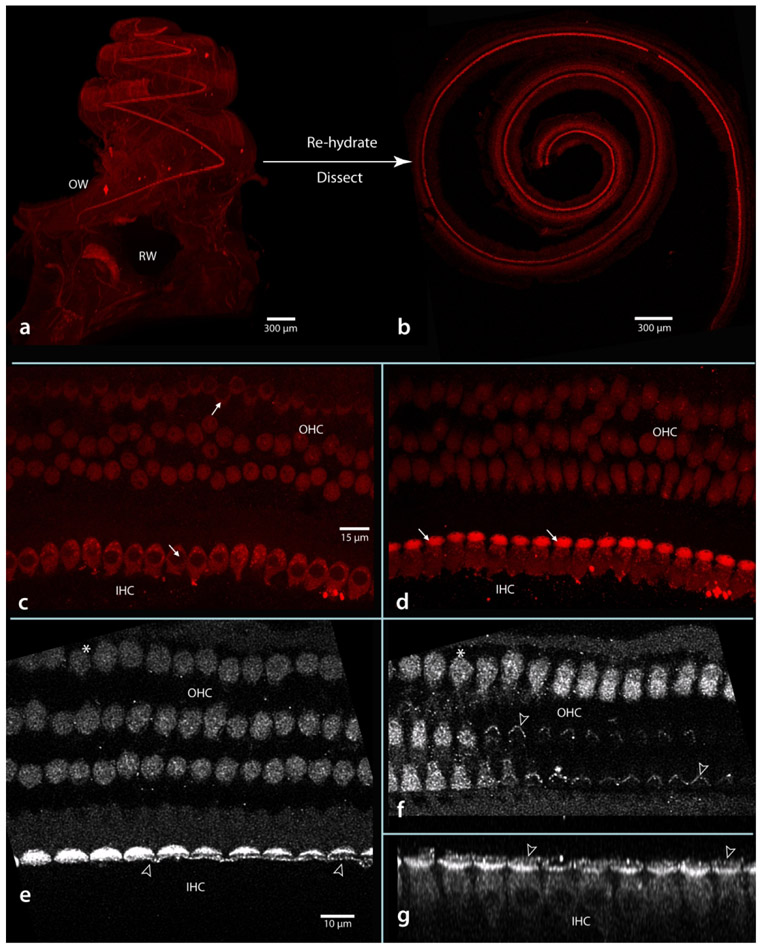
A trade-off of using LSFM to produce detailed images of the intact cochlea might be that it requires sacrificing the higher resolutions possible with confocal microscopy. However, as shown in Figure 13, this is not the case because LSFM material remains structurally intact and can be reprocessed for high-resolution confocal microscopy. Here, a specimen imaged previously with LSFM (a) was re-hydrated to buffer, the basilar membrane dissected free and flat-mounted for confocal microscopy (b). At high magnification (63x oil), confocal imaging shows the subcellular integrity of hair cells (c-g). In c, note that cellular membranes of both inner and outer hair cells are intact, as are the nuclei of both cell types (arrows), while in d (a higher focal plane from the same image stack), the intensely reactive cuticular plates (arrows) of inner hair cells are evident. A segment that has been deconvolved and enlarged shows the stereocilia of both inner and outer hair cells (e and f respectively, hollow arrowheads), while in g the row of inner hair cells has been re-sliced in a plane orthogonal to that of e, showing the row of inner hair cells, their nuclei, and stereocilia extending from the cuticular plates. Thus, even after LSFM processing, fine subcellular details remain intact and visible, including the fragile stereocilia of hair cells. Note that the flat-mount confocal images shown in Figure 13 c-g show the same pattern of myosin VIIa staining as originally described for hair cells by (Hasson, Heintzelman, Santos-Sacchi, Corey, & Mooseker, 1995).
Figure 13.
Material prepared originally for LSFM can be rehydrated and the basilar membrane (with organ of Corti complex) dissected free for further confocal examination. The basilar membrane as it appears in a light sheet image before rehydration (a) and in a confocal image after dissection and flat-mounting (b). Panel b is a single image snap-shot taken prior to examination at 63x oil (c-g). In the higher magnification images there was no observable membrane disruption; nuclei of both inner and outer hair cells (IHC, OHC) were evident (arrows in c) as were cuticular plates of inner hair cells (arrows in d). Deconvolved and enlarged images (e and f) show outer and inner hair cells at two focal levels (asterisk at upper left marks same outer hair cell in e and f). Here notice stereocilia of both inner and outer hair cells was preserved (hollow arrowheads in e and f). In g, the stack has been re-sliced to show the row of inner hair cells, their soma, nuclei, cuticular palates and stereocilia (hollow arrowheads point to same inner hair cells in e and g). Scale in c same for d; in e same for f and g.
3.5. Uses of three-dimensional cochlear images
LSFM of the gerbil cochlea is useful for producing a cochleogram of inner and outer hair cells and describing the frequency distribution along the basilar membrane, with the added benefits that these now have depth and accurate three-dimensional coordinates. In addition, the technique can be used to guide surgical access to specific frequency ranges within the gerbil cochlea, versus those that are inaccessible due to being deep within the bone. For these analyses we relied upon Bitplane Imaris software’s image visualization and rendering engines.
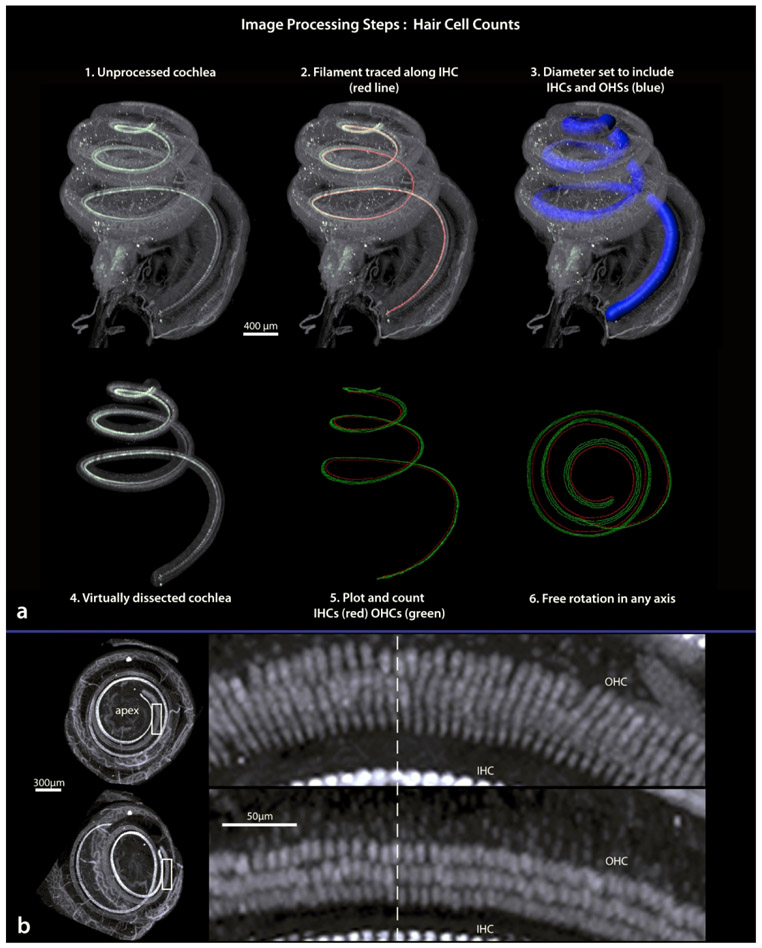
Steps involved in our method for hair cell counting are outlined in Figure 14a. Step 1 shows a cochlea from a normal animal immunostained with myosin VIIa. Using the ‘filament’ tool in Imaris we trace along the inner hair cells from base to apex, shown as a red line in step 2. The filament then serves as the center of a 100 μm diameter “tunnel”, large enough to include inner and outer hair cells (step 3) which we virtually dissect (step 4). Specifically, we convert the filament to a string of spots (using a custom function provided by Matthew Gastinger, Bitplane) and from those spots perform a distance transformation, creating a new data channel with intensity equivalent to distance from the filament, in microns. A surface is created based on that channel, including any voxels with an intensity of 100 or smaller; this surface is the 100 gm diameter tunnel. The original data channel can be masked within that tunnel, creating a virtual dissection of the basilar membrane. This dissection simplifies visualization of hair cells and can be freely rotated in any direction to assist in counting hair cells at any location along the spiraling basilar membrane. We manually counted cells with the Imaris spot function, by mouse clicking to produce a red spot (inner) or green spot (outer) over each hair cell. Step 5 shows the result; the mapped hair cells (inner vs outer) are stored in separate channels, and like the membrane, are preserved in three-dimensions and freely rotatable in any direction. Step 6 shows a view looking down the central axis similar to that of a basilar membrane flat-mount prepared for confocal microscopy (Figures 1b and 13b).
Figure 14.
Procedures for virtually dissecting a three-dimensional basilar membrane (a; steps 1-4) then plotting the location of hair cells (steps 5 and 6). The top left image in b shows the upper two turns of the cochlea as looking down through the apex, the lower left image is after rotation away from apex. Boxed areas are shown to the right. Here note that from an apex down view, the orientation of outer hair cells obscure accurate identification of individual cells, while after rotation individual cells can be recognized. Dashed line marks the same location in both images. IHC, inner hair cells; OHC, outer hair cells.
While inner hair cells have a relatively uniform orientation across regions of the basilar membrane, outer hair cells dramatically change their orientation, and in the apical turn they tend to lie on their sides and can obscure one another (Lim, 1980; Spoendlin, 1970). However, free rotation of the LSFM images allows for different regions of the basilar membrane to be viewed at different angles. In Figure 14b, outer hair cells at apical regions “lie on their sides” when viewed in an apex-up orientation (14b, top). Rotation away from apex-up (14b, bottom) changes the perspective such that individual outer hair cells within each of the three rows become easily distinguishable.
In the normal animal shown in Figure 14a, the number of inner hair cells was 1,081 and the number of outer hair cells was 4,546. These values are similar to those obtained by counting the number of hair cells in a cochlear flat-mount stained with hematoxylin, where inner hair cells numbered 1,130 and outer hair cells numbered 4,590 (counted by two observers blind to each other’s findings). The close approximation of our cell counts from light sheet material to those obtained with a standard staining procedure for routine light microscopy suggests that the light sheet material is adequate for cell counting, and is further evidence that our antibody solutions had equal access to hair cells along all turns of the cochlea.
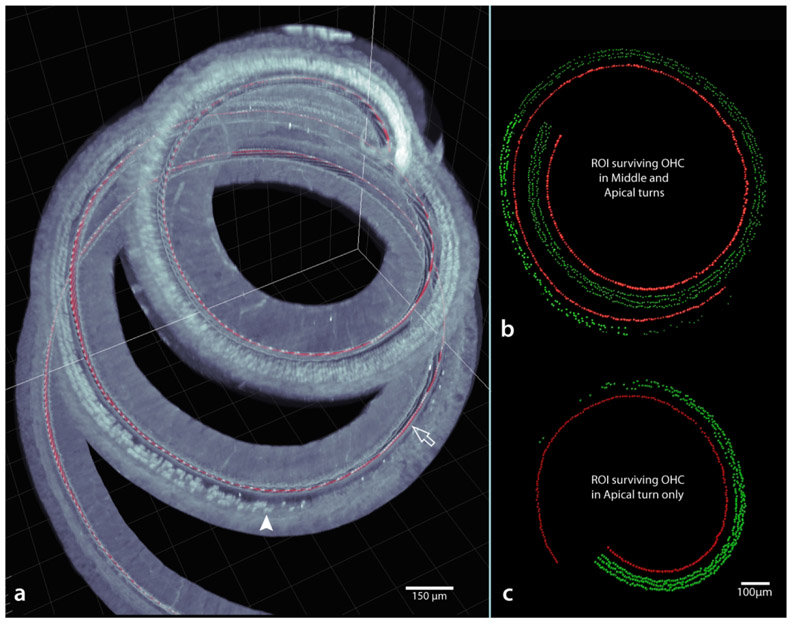
Figure 15a shows an example of a case with a reduced number of outer hair cells, due to prior treatment with a combination of furosemide and kanamycin (FK; see methods). In this case, after tracing a filament along the cuticular plates of immunolabeled inner hair cells, the regions of preserved, diminishing, and absent outer hair cells are clearly visible even in this low magnification image. When regions of interest are imaged at higher power the transition from complete preservation to complete loss of outer hair cells could be quantified (two examples shown in Figures 15b and c; for the case shown in c the transition occurs at a more apical region than b).
Figure 15.
Example of a virtually dissected basilar membrane (a) from an animal treated with an ototoxic injection of furosemide/kanamycin. Note the filament tracing (red line) along the inner hair cells (hollow arrow) and the location of surviving outer hair cells (e.g., arrowhead) are visible even in this 5x-imaged specimen. However, for accurate counting, materials imaged at 12.6x (through at least the region of interest) are preferred. In b and c, the regions of interest derived from two other injected cases are illustrated. Note that unlike a typical cytocochleogram, as a result of the image processing steps (Figure 14a), hair cells have z coordinates as well as x and y. Scale same for b and c.
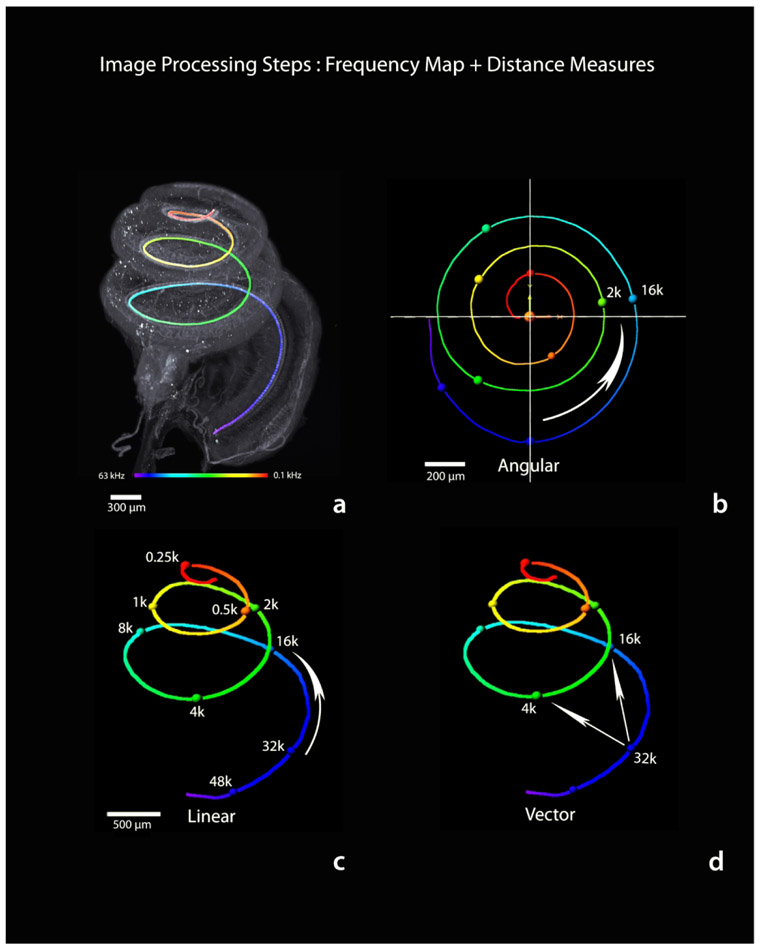
A three-dimensional frequency map of the basilar membrane was created through application of the gerbil frequency-to-distance transformation (Müller, 1996) to the traced filament (see Figure 14, step 2). The filament was first converted to a series of spot objects representing, on average, a center-to- center distance of 1.3 pm (using this method the mean basilar membrane length measured at the inner hair cells averaged 11.48 mm; n = 14, SD = 0.67; median length = 11.55 mm). Next, distance along the filament was color-coded as shown in Figure 16a according to the frequency-distance transformation, resulting in a three-dimensional frequency map that could then extracted and freely rotated in any axis (Figure 16 b-d). The positions of octave frequencies are indicated by colored spheres and distances between them represented in terms of angular, linear, and vector coordinates as indicated numerically in Table 1. The values listed in Table 1 are with respect to 32 kHz as the apical end of the hook region - beginning of the ascending spiral - of the basilar membrane away from the RW. To measure angular distance, the frequency map was rotated to a view from the apex down that best visually approximates the center of the spiral and a new x,y,z origin defined by the center of the spiral. Then an x-axis was defined by a line from the beginning (base) to end (apex) of the basilar membrane; the y-axis made perpendicular; the z-axis was along the height of the cochlea. From this new reference frame, the arctangent2 function was applied to the distance data, and angular distance derived -- similar to the angular measures used to study the human cochlea (see Biedron, Westhofen, & Ilgner, 2009; Xu, Xu, Cohen, & Clark, 2000). Linear distance is simply distance along the basilar membrane between two frequencies, while vector distance represents the shortest direct three-dimensional distance between frequency points.
Figure 16.
Procedure for creating a three-dimensional frequency map of the basilar membrane (a) and measuring distance along that map (b-d). The inner hair cell filament trace (Figure 14 step 2) is converted to color-coded distance and the frequency-distance transformation is applied (a). For convenience in mapping across animals, spheres are placed at common octave frequency locations. Like hair cell counting, the frequency map can be extracted and freely rotated along any axis. This map may be used to make specific frequency related distance measurements, such as angular, linear or vector distance between any two frequencies. See also Table 1. Scale in c same for d.
Table 1. Distance Measures Obtained via Light Sheet all relative to the RW at 32K CF frequency region.
Numerical listing of three-dimensional relative distance measures derived as shown in Figure 16. This specimen had a total basilar membrane length of 11.41 mm.
| CF frequency kHz |
Angular deg |
Linear mm |
Vector mm |
|---|---|---|---|
| 32 | 0 | 0 | 0 |
| 16 | 99.16 | 1.50 | 1.37 |
| 8 | 206.18 | 3.04 | 2.03 |
| 4 | 320.90 | 4.52 | 1.37 |
| 2 | 460.33 | 6.01 | 1.90 |
| 1 | 593.87 | 7.34 | 2.15 |
| 0.5 | 748.43 | 8.49 | 1.84 |
| 0.25 | 899.10 | 9.41 | 2.36 |
Frequency mapping can also be used to describe and chart the pattern of outer hair cell loss in FK animals as shown Figure 17. In this figure, LSFM images of the outer hair cells transition region within the apical turn (a, image with otic capsule intact; b, the virtually dissected basilar membrane after frequency mapping). Once the frequency map has been applied, the regions of outer hair cell loss can be defined by frequency. Briefly, proceeding from the apex, a normal compliment of outer hair cells exists across three rows through 316 Hz, by 357 Hz the sporadic disorganization across rows is evident, progressively worsening from 483 Hz to 570 Hz as the three rows diminish to two rows. Sporadic clustering of hair cells begins at 607 Hz, with the transition zone complete by 702 Hz and no outer hair cells located basal to that, with the exception of a lone surviving outer hair cell at 885 Hz. This figure also shows the value of virtual basilar membrane extraction eliminating the distraction of overlying tissue.
Figure 17.
Outer hair survival after ototoxic injection can be described by frequency distribution. An LSFM image of the apical turn (a) shows the location of surviving outer hair cells (e.g. hollow arrows) and a distant lone survivor (solid arrow in a and b). Hollow arrowheads point out the intact IHC row. Following the steps outlined for filament tracing, distance transformation and basilar membrane extraction, the frequency-mapped region of interest is shown in b. Here, the degree of outer hair cell loss can be described by frequency, ranging from a full compliment of cells at 316 Hz through the last surviving cell at 885 Hz. Scale same for a and b.
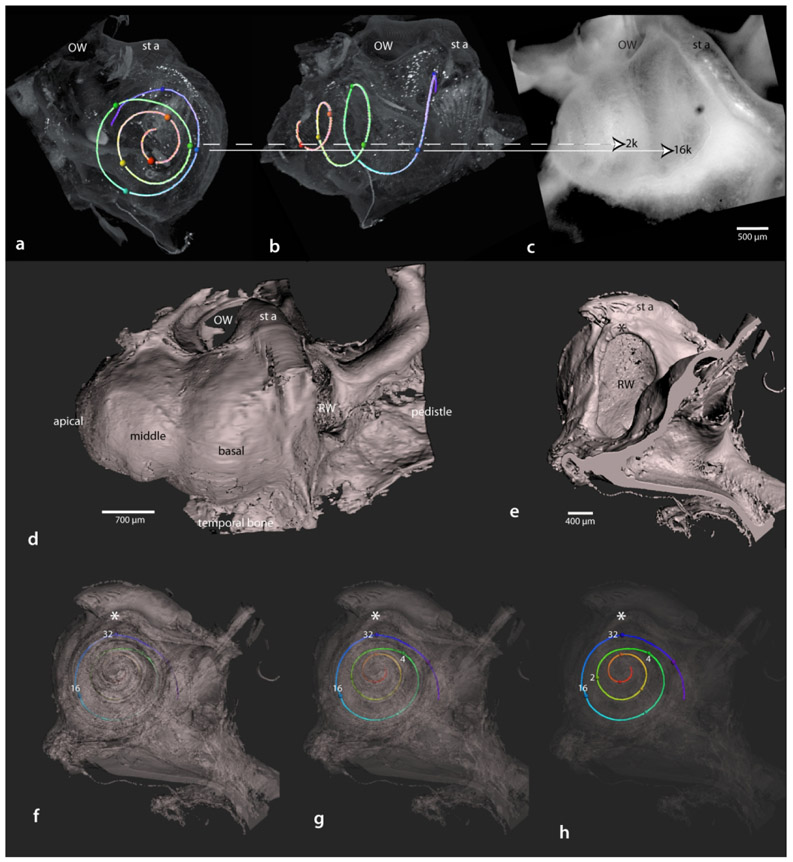
By rendering the frequency map within otic capsule intact specimens, the preparations retain their spatial relationships with external surface features of the cochlea, including landmark structures such as the oval and round windows and the stapedial artery. These external landmarks are useful for planning approaches to frequency regions of the cochlea that can be accessed during surgery. Figure 18 shows the frequency map within the intact cochlea from an apical (a) and lateral (b) view. Note the location of 2 kHz and 16 kHz at the lateral edge of the cochlea; these two frequency domains are easily accessible at surgery (c), while a wide range of intervening frequencies are less accessible on the medial side. It is common practice to record cochlear responses from the round window niche. To visualize what is “seen” by the round window, a three-dimensional imaged cochlear surface rendering (d), along with its frequency map, were rotated into a position similar to a surgical exposure of the round window (e). In f-h, the opacity of the rendering is slowly diminished to “see” the frequency distribution through the round window. Note that 32 kHz is located within the upper lateral quadrant of the round window niche, just below the crest of the round window niche (asterisks). Also note the very end of the “hook” lies medial to the round window, where is resides close to the vestibular canals.
Figure 18.
Application of the three-dimensional frequency map for geometry based physiological investigations. Frequency maps from otic capsule intact specimens retain relationships with external structures, as viewed from the apex (a) or laterally (b). When aligned with a typical surgical view of the cochlea (c), frequency specific targets can be identified for study. In d, a surface rendered cochlea is rotated to view the round window (e) oriented as for placement of an electrocochleographic recording electrode. In f-h, opacity is incrementally decreased allowing for a geometric interpretation of what the electrode “sees” through the round window as depth of view reaches the apex. Specific frequencies are labeled at 32, 16, 4 and 2 kHz. Asterisk in e-h denotes the crest of the round window niche. Note in d the bone pedestal used for mounting the cochlea to the acrylic stand (as in Figure 4c). Scale for a and b shown in c; scale for e-h shown in e. OHC, outer hair cells; OW, oval window; RW, round window; st, stapedial bone; st a, stapedial artery.
4. DISCUSSION
We have shown that LSFM provides a rapid three-dimensional imaging solution for the gerbil cochlea at the micron level with minimal histological distortions. A benefit compared to other three-dimensional methods such as micro CT or MRI is that, like confocal microscopy, it allows for fluorescent immunostaining of relevant structures. It improves upon confocal microscopy using flat-mounted tissue by maintaining the relevant structures within an intact anatomy demonstrating their relationships with both internal and external landmarks. Fortunately, the higher resolution afforded by confocal microscopy, which is needed to view subcellular structures remains available because material prepared for LSFM can be rehydrated and further dissected as needed. LSFM material is amenable to counting hair cells, charting their distribution and applying a frequency map along the basilar membrane – all while maintaining accurate three-dimensional anatomical relationships.
Previous studies using LSFM to investigate the cochlea in mouse and guinea pig, using image segmentation procedures (e.g., Santi, Rapson, & Voie, 2008; Voie et al., 1993; Voie & Spelman, 1995) have shown that many features of the cochlea can be detailed in a quantitative manner -- such as volumetric measures of the spiral ganglion (Johnson, Schmitz, & Santi, 2011), the scalar chambers and their contents (e.g., Brown et al., 2016; Johnson et al., 2011; Kopecky et al., 2012; Lo et al., 2017; Voie & Spelman, 1995), including the basilar membrane (Buytaert, Johnson, Dierick, Salih, & Santi, 2013; Santi et al., 2008; Schmitz, Johnson, & Santi, 2014) . In mice, LSFM has been used to count myosin labeled hair cells and rhodamine-stained spiral gangion cells (Schmitz et al., 2014), and to show outer hair cells immunostained with antibodies to prestin (Santi, 2011). Additonally, in guinea pig, LSFM has been used study endolymphatic movement subsequent to injection of fluorescein isothiocyanate (FITC)-dextran into the scala media (Brown et al., 2016), to study aspects of the round window and cochlear aqueduct (Hofman, Segenhout, Albers, & Wit, 2005) and to evalate the efficacy of round window vs cochleostomy based cochlear implant trajectories (Lo et al., 2017). In mouse, rat and human, decellularized cochleae were examined with LSFM to investigate potential frameworks for stem cell growth (Santi & Johnson, 2013)
In gerbil, Buytaert et al. (2011) used LSFM to provide a detailed description of the middle ear cavity and the ossicular chain. Here, using LSFM of the gerbil cochlea, we demonstrate that the cochlea’s three-dimensional frequency structure can be preserved, displayed, and measured. These measures are of great interest to physiologists investigating the cellular origins of the complex waveforms generated by the cochlea in response to acoustic (e.g., Forgues et al., 2014; Pappa et al., 2019) or electrical (e.g., Li, Lu, Zhang, Hansen, & Li, 2020; Wiegner, Wright, & Vollmer, 2016) stimuli, for monitoring changes in intracochlear pressure levels at defined frequency locations (e.g., Kale & Olson, 2015) and for interpreting the results of cochlear implant studies (e.g., Ahmad et al., 2012; Canfarotta et al., 2020; DeMason et al., 2012; Stakhovskaya, Sridhar, Bonham, & Leake, 2007). Typically, electrocochleographic recordings are taken from the round window in gerbils (e.g., Batrel et al., 2017; Fontenot, Giardina, & Fitzpatrick, 2017; Forgues et al., 2014; He, Porsov, Kemp, Nuttall, & Ren, 2012; Henry, 1995) as well as for other species including human – recently becoming a common metric for evaluating insertion damage and outcomes in cochlear implant patients (e.g., Abbas, Tejani, Scheperle, & Brown, 2017; Adunka et al., 2016; Campbell et al., 2016; Choudhury et al., 2012; Fontenot et al., 2019; Giardina et al., 2019; Giardina et al., 2018; Riggs et al., 2017; Scott et al., 2016). Given these recordings are from an ascending helix (and its associated frequency representation) spiraling around the modiolus, a persistent question has been: how does the cochlear geometry affect recordings taken at the round window or from within the cochlea? To adequately address this question, we need a three-dimensional map of the basilar membrane that retains spatial geometric relationships with other anatomical structures. With the ability of LSFM to visualize the internal constituents of the cochlea - the spiraling basilar membrane in particular - we have a means to explore such questions. Furthermore, the procedures outlined for placing a frequency map into an intact cochlea are applicable to any species where a Greenwood-like frequency-distance transformation is available (Greenwood, 1990).
4.1. Methods specific to the cochlea
Regardless of species, or microscopic method (long working distance confocal or LSFM), any preparation requiring clearing the cochlea to a transparent state follows a similar sequence of steps – fix, decalcify, dehydrate and clear. If desired, immunostaining occurs after decalcification (e.g., MacDonald & Rubel, 2008), while en-bloc staining, such as rhodamine B isothiocyanate, is done post-dehydration (e.g., Voie et al., 1993). One exception being the decellularized preparation of fresh tissue described by Santi and Johnson (2013) where the fixation step was omitted as it interfered with the action of their detergent solutions.
Histological techniques typically involve procedures that introduce tissue distortions. We found that for the gerbil cochlea, if the otic capsule were removed obvious structural distortions were present; however, if the capsule was left intact these distortions were minimized. Presence of the otic capsule did not restrict access to the myosin antibody when introduced by gentle perfusion through the round window (and allowed to exit thorough the oval window) leaving delicate structures such as Reissner’s membrane intact. Similarly, round window perfusion was used by Santi and Johnson (2013) to immunolabel structures with antibodies to collagen and laminin. We do not know if immunolabeling by simple diffusion would be successful for LSFM in gerbils. However, the procedure developed by MacDonald and Rubel (2008) in the mouse and used in gerbil by Risoud et al. (2017) for long working distance confocal imaging, immunolabeled the entire cochlea by passive diffusion after opening the round and oval windows in conjunction with small holes made in the otic capsule over the helicotrema apically and the scala vestibuli basally. In their LSFM mouse study, Schmitz, Johnson and Santi (2014) followed the procedures of MacDonald and Rubel (2008) to label hair cells, so this could be feasible for gerbil LSFM as well, if the study allows for small punctures to be made in the otic capsule.
An important consideration for the cochlea is fixation – whether by quick immersion in fixative or by cardiac perfusion. Immersion is a viable method for the cochlea in many species, including the gerbil, where the cochlea can be rapidly extracted and membranes opened to allow introduction of fixative (see Henson, 1978). If vasculature is an integral part of the study, immersion fixation is clearly indicated. Otherwise, the reduction in autofluorescence by washing out blood through perfusion may be the optimal choice for many investigations.
Unlike flat-mounted basilar membrane material used for confocal microscopy, LSFM requires tissues to be dehydrated and cleared, with dehydration likely to cause some degree of shrinkage. To address the amount of shrinkage with dehydration, Richter, Edge, He, and Dallos (2000) compared basilar membrane length in fixed vs fixed and dehydrated tissue showing a change in the mean length from 11.4 (n=3) to 11.1 mm (n=3), or roughly a 3% difference. In our cases the mean basilar membrane length was 11.48 (n = 14), suggesting that the dehydration steps similarly introduced little shrinkage. In fixed mouse cochleae, Buytaert et al. (2013) found that LSFM processing steps resulted in an overall shrinkage of 4.23%. Various studies have looked at shrinkage resulting simply from tissue fixation. For example, studies comparing fixed vs unfixed ocular tissue (Tran et al., 2017) or mixed tissue samples (Ferguson, Bryant, & Ito, 1999) found formalin or paraformaldehyde fixationinduced shrinkage to be less than 2%. Although we made no attempt to quantify shrinkage in the present study, the literature would suggest that we experienced less than 5% shrinkage.
Ours in now at least the eighth report to measure the length of the basilar membrane in gerbils. Previous studies used dissections or surface preparations (Edge et al., 1998; Richter et al., 2000; Sokolich, Hamernik, Zwislocki, & Schmiedt, 1976; Tarnowski, Schmiedt, Hellstrom, Lee, & Adams, 1991), section reconstruction (Müller, 1996; Plassmann, Peetz, & Schmidt, 1987) or scanning electron microscopy (Lovell et al., 2012). We now add a measure of basilar membrane length derived from LSFM. Unfortunately, across studies, there is no standard landmark to use for a definitive measure of length. Previous studies have used the tunnel of Corti, the spiral ligament, or inner pillar cells, while we used immunolabeled inner hair cells as a template to measure length. Regardless of the method used, there is a general agreement across studies supporting a length of approximately 11.5 mm as best representing the “average” basilar membrane length. However, in the comparative study by Plassmann, Peetz, Schmidt (1987), length was measured at two defined basilar membrane anchor points, an inner anchor (spiral lamina) and an outer anchor (spiral ligament). The average inner length was 10.9 mm and the outer length 15 mm, a difference of 4.1 mm. Both are measures of basilar membrane length but point to the nature of the problem of defining an “average” length - given the basilar membrane is not a unidimensional linear structure, but rather a spiraling planar surface with an inner and outer radius. With this physical construct, measures of length depend on the needs of the study and structures under investigation. Fortunately, for mapping frequency distributions, this is not an issue as the distance-frequency maps are based on relative rather than absolute distance (Greenwood, 1990; Müller, 1996). The variance observed in our length measures (SD = 0.67; n = 14) is larger than many other studies but is similar to those with a sample size close to ours (e.g., Müller, 1996; SD = 0.54; n = 10). As noted by Bohne and Carr (1979) in the chinchilla, variance in length measures increase with sample size and thus provide a better estimate of variance in the population, reiterating the necessity for using relative distance as the metric for across animal comparisons.
4.2. Comparison with other methods for imaging the cochlea
The classic methods used to study cochlear anatomy include histological sectioning, dissected flat-mounts, scanning and transmission electron microscopy. Three-dimensional reconstructions from sections are time-consuming, require sophisticated techniques for registration and are impossible for ex situ flat-mount material. Radiological approaches such as CT, micro CT, synchrotron radiation imaging, MRI and optical tomography are capable of imaging the cochlea in three-dimensions, but in general, radiological methods find their best utility in human clinical or translational applications rather than in basic science studies, although micro CT and optical tomography have recently become accessible for non-human investigations (Buytaert et al., 2013; Iyer et al., 2016). Optical coherence tomography has the distinct advantage that it can be used in living tissue (as can LSFM, e.g., Huisken et al., 2004; Keller et al., 2010) to make functional measurements over time (Tona et al., 2014), and MRI using a small bore and high strength magnet have been applied to several species providing quantitative information about the dimensions of cochlear ducts, for example (Thorne et al., 1999). However, the typically low contrast images obtained with these tomographic techniques limits their ability to isolate details of many structures of interest, and they are not generally compatible with immunocytochemical probes, although recent advances in methodology have incorporated use of fluorescent probes for optical tomography (e.g., Nolte et al., 2017). In human cadaveric material, contrast enhancement with osmium improves images quality in micro-CT scans for three-dimensional reconstructions with resolution on the order of 5 −10 μm (Glueckert et al., 2018; van den Boogert et al., 2018) and synchrotron radiation imaging techniques generate impressive three-dimensional cochlear images with resolutions of 9 μm (Helpard, Rohani, Ladak, & Agrawal, 2020; Iyer et al., 2018; Lareida et al., 2009; H. Li et al., 2020). However, as few facilities offer synchrotron capabilities, it remains ill-suited for routine use in animal studies.
Currently, confocal microscopy dominates studies of cochlear anatomy because of its high resolution and ability to visualize immunolabeled structures of interest, such as synaptic ribbons and terminals (Wan, Gomez-Casati, Gigliello, Liberman, & Corfas, 2014; Zhang et al., 2018). A drawback is that the material is in the form of dissected flat-mounts, which no longer retain context with the rest of the cochlea. Although confocal microscope arrangements with longer working distance objectives do allow for a three-dimensional image (MacDonald & Rubel, 2008; Wrzeszcz et al., 2013) the technique suffers from incomplete laser penetration through the entire depth of the specimen (e.g., Reynaud, Krzic, Greger, & Stelzer, 2008). In contrast, LSFM provides a complete view of the cochlea, with good cellular resolution, but is insufficient for imaging subcellular details such as synaptic elements or stereocilia. To bridge this gap, we showed that LSFM material can be rehydrated, the basilar membrane dissected out, flat-mounted and viewed with a standard confocal microscope. Other studies have demonstrated that LSFM material can be post-processed for examination with common histological techniques. In a post-LSFM cochlea, embedded and sectioned in celloidin for light microscopy, revealed the level of cellular detail to be equivalent to that of a normal control cochlea (Johnson, Cureoglu, O'Malley, & Santi, 2014), while decellularized cochlea specimens prepared for LSFM (although lacking cellular detail) can nonetheless be re-processed and observed with scanning electron microscopy techniques (Santi et al., 2016). Retention of cellular detail has also been shown in cochleae cleared and prepared for long working distance confocal microscopy, then epoxy embedded and sectioned for examination with light or epi-fluorescence microscopes (MacDonald and Rubel, 2008). These observations, together with our re-hydration and flat-mount imaging results suggest that materials prepared for LSFM can readily be returned to a state compatible for examination by other microscopic techniques.
In principle it should be possible to replace the flat-mounted confocal material back within its original context. However, the distortions produced by further dissection and flattening of the spiraling basilar membrane between coverslips impose challenges that make precise three-dimensional realignment difficult to overcome. Perhaps the most unique property of LSM images analyzed with Imaris software is the ability to freely rotate a three-dimensional cochlea in any direction, to optically section the cochlea in any plane, at any angle and at any thickness. These properties are indispensable for tracing cochlear vasculature, for counting hair cells and for constructing a three-dimensional frequency map of the basilar membrane.
Vasculature of the Cochlea
The spatial organization of the cochlea’s vasculature supply is demonstrated in immersion fixed material (Figures 6 - 12), where blood and vascular tissue autofluoresce brightly (Dodt et al., 2007; Leischner et al., 2010; Rich et al., 2013; Viegas, Martins, Seco, & do Carmo, 2007). In three-dimensional space, the course and relationships of the spiral modiolar artery and vein are quite evident as these vascular structures traverse across each cochlear turn. The intricate anastomoses within the stria vascularis, radiating arterioles, collecting venules and vascular elements of the osseous spiral lamina can all be visualized. These vascular details were also demonstrated by Santi and Johnson (2013) in their decellularized preparations using antibodies directed against collagen and laminin. The images of these structures rival those obtained by corrosion cast methods (Tange & Wijburg, 1986) or injection of contrast dyes (Jones-Mumby & Axelsson, 1984). Recently, Jiang et al. (2019) injected Lectin-DyLight 649 intravenously into mice and obtained excellent results imaging the cochlea using confocal microscopic techniques. Alternatively, endothelial markers such as PECAM (Lee, Cho, & Lee, 2015) or cardiac injection of FITC (Brown et al., 2016) could also be used to examine vascular patterns in LSFM prepared material. In mouse, cardiac perfusion with tomato-lectin conjugated with FITC allowed for intricate three-dimensional vascular patterns of the brain to studied with LSFM (Jährling, Becker, & Dodt, 2009) and this labeling method holds promise for the cochlea also.
4.3. Counting Hair Cells
We counted cells by mouse clicking to produce a red or green spot over each cell; however, the process could easily be automated using Imaris or ImageJ – with two caveats. First is the necessity of rotating the image to provide a clear view of outer hair cells (see Figure 14b). While this is easily accomplished on-the-fly during manual counting, automated counting would require diligent segmentation of the basilar membrane across different rotation angles. The second problem is the method of automatic cell detection. Too lenient a criterion leads to over-counting (artifacts of similar size and intensity would be included in the counts) or too strict a criterion could leave cells uncounted. Either way, a human eye would need to check the automated counts for accuracy to exclude artifacts or include cells that escaped the automated process. Given these caveats, and the need for human oversight, it is almost as simple to just click the mouse to count while inspecting the cells as it is to make segments and recheck automated counts. However, the method of choice for counting cells would be at the user’s discretion.
This method of counting hair cells was equally applicable in animals that had received FK injections to chemically ablate outer hair cells (see Figures 10, 15 and 17). In mouse, Schmitz et al (2014) used FK injections to study ototoxic effects on the cochlea, at drug concentrations and survival times (28 days) sufficient to reduce both inner and outer hair cell populations along with spiral ganglion cells. They counted hair cells (normal and FK treated) in a manner similar to ours, and illustrated the patten of hair cell loss in three-dimensional reconstructions along with the associated loss of spiral ganglion cells. These observations show LSFM to be a powerful tool to investigate not only normal material but also experimentally manipulated cochleae.
4.4. Application of the frequency map
Results from the frequency mapping of LSFM imaged cochleae are amenable to measures of angular, linear, or vector distance relative to specific frequency locations (Figure 16 and Table 1). Together, these measures help conceptualize the geometrical organization of the cochlea. For example, in Table 1 compare the distance measures from 32 kHz to 16 and 4 kHz. Note that 4 kHz is 3.02 mm and 221.74 degrees further away from 32 kHz than is 16 kHz, yet in terms of vector distance they are identical – each being 1.37 mm away. Similarly, the CF regions of 2 and 16 kHz are adjacent to one another, though located in separate turns of the cochlea. In terms of vector distance from a round window electrode (at 32 kHz), they differ only by 0.53 mm, yet they are separated from each other by a linear distance of 4.51 mm. We find these measures of interest because a major reason we have undertaken a three-dimensional description of cochlear anatomy and frequency is to begin to determine the spatial distributions of hair cell and neural sources that contribute to the responses observed with round-window electrocochleography. There are important hints in the literature that purely linear measurements that consider the currents as flowing through a tube to reach the recording site at the round window may not be entirely correct. For example, Davis, Gernandt, Riesco-MacClure, and Covell (1949) used the term “crosscountry” to explain their observations on microphonics recorded from different turns of the cochlea, suggesting that conduction across the thin bone separating scala vestibuli of one turn and the scala tympani of the adjacent turn may be “as easy as” the longer route around the modiolus. Thus, the electrical activity of one turn could affect that observed in the immediately adjacent turn. Similarly, (Bekesy, 1951) in establishing his “electroanatomy” of the cochlea emphasized the necessity to study the geometric arrangements within the cochlea to adequately understand its electrical properties and suggesting, as did Davis et al, that crossconduction between turns of the cochlea must exist. Dallos, Schoeny, and Cheatham (1971) confirmed this, demonstrating that the phase and magnitude of microphonics could be influenced by electrical activity in the immediately adjacent turns, particularly among the more apical turns. While many tabulations exist comparing measurements of the resistivities among and between various cochlear elements (Finley, Wilson, & White, 1990; Frijns, de Snoo, & Schoonhoven, 1995; Hanekom & Hanekom, 2016; Honrubia, 1976; Wong et al., 2016) we are unaware of any that measure the resistance across the interscalar septum separating the scala vestibuli of one turn from the immediately adjacent scala tympani of the next higher turn.
Our measures of cochlear geometry increase understanding the relationships that exist between adjacent turns and frequency location, assisting in the determination of whether or not “cross country” interactions promote constructive or destructive interference in distant recordings taken at the round window (see Nuttall & Dolan, 1991). Additionally, our 3-dimensional frequency map illustrates where frequencies “go around the bend” of the modiolus relative to one-another to provide insights into how physiological responses recorded at the round window (or elsewhere within the scala tympani) might be affected by curvature of the cochlea (see Dallos, 1969). A better understanding of these potential three-dimensional interactions is of specific interest to cochlear implant designers (Hanekom & Hanekom, 2016; Micco & Richter, 2006; Sasmal & Grosh, 2019). Knowledge of geometric relationships will be increasingly important as cochlear implant recordings are now taken internal to the cochlea – from contacts of the implant device itself -- in addition to the RW recordings (e.g., Campbell et al., 2016; Giardina et al., 2019; Harris, Riggs, Giardina, et al., 2017; Harris, Riggs, Koka, et al., 2017; Koka & Litvak, 2017; Koka, Saoji, & Litvak, 2017; Riggs et al., 2019; Saoji et al., 2019). Furthermore, given that no two cochleae are identical (e.g., Erixon, Hogstorp, Wadin, & Rask-Anderson, 2008) even small variation in three-dimensional architecture, in terms of frequency domains relative to other structures or to one another, can be reduced by a geometrically correct frequency map derived from each individual subject in physiology studies, thus increasing accuracy in the interpretation of physiological measures.
4.5. A Three-Dimensional Cochlea.
Three-dimensional imaging of the cochlea, the basilar membrane in particular, has evolved from artistic renditions of gross anatomical observations (Breschet, 1836; DuVerney, 1683) to graphical reconstruction from histological sections (Guild, 1921; Schuknect, 1953). More recently, sophisticated computer-generated models have been developed to investigate the three-dimensional organization of cochlear structure, relying on MRI, tomographic scans or histological sections to establish the models parameters (Frijns, Briaire, & Grote, 2001; Frijns et al., 1995; Kawano, Seldon, & Clark, 1996; Plassmann et al., 1987; Sieber et al., 2019; Spelman, 1990). The great advantage of LSFM over these previous techniques is it renders a complete three-dimensional cochlea, basilar membrane included, in a comparatively simple manner requiring no tedious histological processing or integration into a computer model beyond the import of a stack of images into Imaris. More importantly for the present purposes, frequencies can be easily mapped onto the basilar membrane, retaining its proper anatomical orientation. From these maps, several relevant geometric measures can be rapidly obtained and offer a promising new approach to examine the structure-function relationships that exist within the coiled cochlea.
Acknowledgements.
Research supported by Auditory Disease Research Fund held by the Department of Otolaryngology/Head and Neck Surgery at UNC Chapel Hill. Core facilities used to prepare this report were supported in part by the North Carolina Biotech Center Institutional Support Grant 2016-IDG-1016; The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center and the Neuroscience Microscopy Core, supported by funding from the NIH-NINDS Neuroscience Center Support Grant P30 NS045892 and the NIH-NICHD Intellectual and Developmental Disabilities Research Center Support Grant U54 HD079124.
Footnotes
Conflict of Interest. The authors declare no conflicts of interest.
Data Availability Statement. An example 2-D image stack and some 3-D analysis tools are available at https://fitzpatricklab1.web.unc.edu/. Additional data is available on request from the authors.
References
- Abbas PJ, Tejani VD, Scheperle RA, & Brown CJ (2017). Using Neural Response Telemetry to Monitor Physiological Responses to Acoustic Stimulation in Hybrid Cochlear Implant Users. Ear Hear, 38(4), 409–425. doi: 10.1097/AUD.0000000000000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adunka OF, Giardina CK, Formeister EJ, Choudhury B, Buchman CA, & Fitzpatrick DC (2016). Round window electrocochleography before and after cochlear implant electrode insertion. Laryngoscope, 126(5), 1193–1200. doi: 10.1002/lary.25602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FI, Choudhury B, De Mason CE, Adunka OF, Finley CC, & Fitzpatrick DC (2012). Detection of intracochlear damage during cochlear implant electrode insertion using extracochlear measurements in the gerbil. Laryngoscope, 122(3), 636–644. doi: 10.1002/lary.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel P (2018). UltraMicroscope II - A User Guide. Chapel Hill, NC. doi: 10.17615/C69M15. [DOI] [PubMed] [Google Scholar]
- Batrel C, Huet A, Hasselmann F, Wang J, Desmadryl G, Nouvian R, . . . Bourien J (2017). Mass Potentials Recorded at the Round Window Enable the Detection of Low Spontaneous Rate Fibers in Gerbil Auditory Nerve. PLoS One, 12(1), e0169890. doi: 10.1371/journal.pone.0169890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Jährling N, Kramer ER, Schnorrer F, & Dodt HU (2008). Ultramicroscopy: 3D reconstruction of large microscopical specimens. J Biophotonics, 1(1), 36–42. doi: 10.1002/jbio.200710011 [DOI] [PubMed] [Google Scholar]
- Bekesy v. G. (1951). The Coarse Pattern of the Electrical Resistance in the Cochlea of the Guinea Pig (Electroanatomy of the Cochlea). J. Acoust. Soc. Am, 23, 18–28. [Google Scholar]
- Biedron S, Westhofen M, & Ilgner J (2009). On the number of turns in human cochleae. Otol Neurotol, 30(3), 414–417. doi: 10.1097/MAO.0b013e3181977b8d [DOI] [PubMed] [Google Scholar]
- Bohne BA, & Carr CD (1979). Location of structurally similar areas in chinchilla cochleas of different lengths. J Acoust Soc Am, 66(2), 411–414. doi: 10.1121/1.383092 [DOI] [PubMed] [Google Scholar]
- Bohne BA, & Harding GW (1993). Combined organ of Corti/modiolus technique for preparing mammalian cochleas for quantitative microscopy. Hear Res, 71(1-2), 114–124. [DOI] [PubMed] [Google Scholar]
- Breschet G (1836). Recherches anatomiques et physiologiques sur I'organe de I’ouie et sur I'auddition dans I'homme et les animaus vertebres. . Paris: J.-B: Baillière. [Google Scholar]
- Brown DJ, Pastras CJ, Curthoys IS, Southwell CS, & Van Roon L (2016). Endolymph movement visualized with light sheet fluorescence microscopy in an acute hydrops model. Hear Res, 339, 112–124. doi: 10.1016/j.heares.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Buytaert JA, & Dirckx JJ (2007). Design and quantitative resolution measurements of an optical virtual sectioning three-dimensional imaging technique for biomedical specimens, featuring two-micrometer slicing resolution. J Biomed Opt, 12(1), 014039. doi: 10.1117/1.2671712 [DOI] [PubMed] [Google Scholar]
- Buytaert JA, Johnson SB, Dierick M, Salih WH, & Santi PA (2013). MicroCT versus sTSLIM 3D imaging of the mouse cochlea. J Histochem Cytochem, 61(5), 382–395. doi: 10.1369/0022155413478613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buytaert JA, Salih WH, Dierick M, Jacobs P, & Dirckx JJ (2011). Realistic 3D computer model of the gerbil middle ear, featuring accurate morphology of bone and soft tissue structures. J Assoc Res Otolaryngol, 12(6), 681–696. doi: 10.1007/s10162-011-0281-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Sly D, Iseli C, Wei B, Briggs R, & O'Leary S (2016). Intraoperative Real-time Cochlear Response Telemetry Predicts Hearing Preservation in Cochlear Implantation. Otol Neurotol, 37(4), 332–338. doi: 10.1097/MAO.0000000000000972 [DOI] [PubMed] [Google Scholar]
- Canfarotta MW, Dillon MT, Buss E, Pillsbury HC, Brown KD, & O’Connell BP (2020). Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear. doi: 10.1097/AUD.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SC (1977). Neuroanatomical aspects of the gerbil inner ear: light microscope observations. J Comp Neurol, 171(2), 193–204. doi: 10.1002/cne.901710205 [DOI] [PubMed] [Google Scholar]
- Choudhury B, Fitzpatrick DC, Buchman CA, Wei BP, Dillon MT, He S, & Adunka OF (2012). Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol, 33(9), 1507–1515. doi: 10.1097/MAO.0b013e31826dbc80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P (1969). Comments on the Differential-Electrode Technique. J Acoust Soc Am, 45, 999–1007. [Google Scholar]
- Dallos P, Schoeny ZG, & Cheatham MA (1971). On the limitations of cochlear-microphonic measurements. J Acoust Soc Am, 49(4), Suppl 2:1144–1154. [PubMed] [Google Scholar]
- Davis H, Gernandt BE, Riesco-MacClure JS, & Covell WP (1949). Aural microphonics in the cochlea of the guinea pig. J. Acoust. Soc. Am, 21, 502–510. [Google Scholar]
- DeMason C, Choudhury B, Ahmad F, Fitzpatrick DC, Wang J, Buchman CA, & Adunka OF (2012). Electrophysiological properties of cochlear implantation in the gerbil using a flexible array. Ear Hear, 33(4), 534–542. doi: 10.1097/AUD.0b013e3182498c28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Leischner U, Schierloh A, Jährling N, Mauch CP, Deininger K, . . . Becker K (2007). Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods, 4(4), 331–336. doi: 10.1038/nmeth1036 [DOI] [PubMed] [Google Scholar]
- DuVerney JG (1683). Traité de l'organe de l'ouïe; contenant la structure, les usages et les maladies de toutes lesparties de l'oreille. Paris: E. Michallet. [Google Scholar]
- Edge RM, Evans BN, Pearce M, Richter CP, Hu X, & Dallos P (1998). Morphology of the unfixed cochlea. Hear Res, 124(1-2), 1–16. doi:S0378-5955(98)00090-2 [pii] [DOI] [PubMed] [Google Scholar]
- Erixon E, Hogstorp H, Wadin K, & Rask-Anderson H (2008). Variational Anatomy of the Human Cochlea: Implications for Cochlear Implantation. Otol. Neurotol, 30, 14Y22. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Bryant JT, & Ito K (1999). Three-dimensional computational reconstruction of mixed anatomical tissues following histological preparation. Med Eng Phys, 21(2), 111–117. doi: 10.1016/s1350-4533(99)00032-6 [DOI] [PubMed] [Google Scholar]
- Finley CC, Wilson BS, & White MW (1990). Models of Neural Responsiveness to Electrical Stimulation In J.M. M & F.A. S (Eds.), Cochlear Implants (pp. 55–98). New York: Springer. [Google Scholar]
- Fontenot TE, Giardina CK, Dillon MT, Rooth MA, Teagle HF, Park LR, . . . Fitzpatrick DC (2019). Residual Cochlear Function in Adults and Children Receiving Cochlear Implants: Correlations With Speech Perception Outcomes. Ear Hear, 40(3), 577–591. doi: 10.1097/AUD.0000000000000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK, & Fitzpatrick DC (2017). A Model-Based Approach for Separating the Cochlear Microphonic from the Auditory Nerve Neurophonic in the Ongoing Response Using Electrocochleography. Front Neurosci, 11, 592. doi: 10.3389/fnins.2017.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgues M, Koehn HA, Dunnon AK, Pulver SH, Buchman CA, Adunka OF, & Fitzpatrick DC (2014). Distinguishing hair cell from neural potentials recorded at the round window. J Neurophysiol, 111(3), 580–593. doi: 10.1152/jn.00446.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns JH, Briaire JJ, & Grote JJ (2001). The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otol Neurotol, 22(3), 340–349. doi: 10.1097/00129492-200105000-00012 [DOI] [PubMed] [Google Scholar]
- Frijns JH, de Snoo SL, & Schoonhoven R (1995). Potential distributions and neural excitation patterns in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res, 57(1–2), 170–186. doi: 10.1016/0378-5955(95)00090-q [DOI] [PubMed] [Google Scholar]
- Giardina CK, Brown KD, Adunka OF, Buchman CA, Hutson KA, Pillsbury HC, & Fitzpatrick DC (2019). Intracochlear Electrocochleography: Response Patterns During Cochlear Implantation and Hearing Preservation. Ear Hear, 40(4), 833–848. doi: 10.1097/AUD.0000000000000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina CK, Khan TE, Pulver SH, Adunka OF, Buchman CA, Brown KD, . . . Fitzpatrick DC (2018). Response Changes During Insertion of a Cochlear Implant Using Extracochlear Electrocochleography. Ear Hear, 39(6), 1146–1156. doi: 10.1097/AUD.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin J, & Carvalho M (2018). The light-sheet microscopy revolution. Journal of Optics, 20, 1–20. [Google Scholar]
- Glueckert R, Johnson Chacko L, Schmidbauer D, Potrusil T, Pechriggl EJ, Hoermann R, . . . Handschuh S (2018). Visualization of the Membranous Labyrinth and Nerve Fiber Pathways in Human and Animal Inner Ears Using MicroCT Imaging. Front Neurosci, 12, 501. doi: 10.3389/fnins.2018.00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD (1990). A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am, 87(6), 2592–2605. [DOI] [PubMed] [Google Scholar]
- Guild SR (1921). A graphic reconstruction method for the study of the organ of Corti. Anat. Rec, 22, 140–157. [Google Scholar]
- Hanekom T, & Hanekom JJ (2016). Three-dimensional models of cochlear implants: A review of their development and how they could support management and maintenance of cochlear implant performance. Network, 27(2-3), 67–106. doi: 10.3109/0954898X.2016.1171411 [DOI] [PubMed] [Google Scholar]
- Hardie NA, MacDonald G, & Rubel EW (2004). A new method for imaging and 3D reconstruction of mammalian cochlea by fluorescent confocal microscopy. Brain Res, 1000(1-2), 200–210. doi: 10.1016/j.brainres.2003.10.071 [DOI] [PubMed] [Google Scholar]
- Harris MS, Riggs WJ, Giardina CK, O'Connell BP, Holder JT, Dwyer RT, . . . Adunka OF (2017). Patterns Seen During Electrode Insertion Using Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol Neurotol, 38(10), 1415–1420. doi: 10.1097/MAO.0000000000001559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MS, Riggs WJ, Koka K, Litvak LM, Malhotra P, Moberly AC, . . . Adunka OF (2017). Real-Time Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol Neurotol, 38(6), e107–e113. doi: 10.1097/MAO.0000000000001425 [DOI] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, & Mooseker MS (1995). Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl AcadSci U S A, 92(21), 9815–9819. doi: 10.1073/pnas.92.21.9815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Porsov E, Kemp D, Nuttall AL, & Ren T (2012). The group delay and suppression pattern of the cochlear microphonic potential recorded at the round window. PLoS One, 7(3), e34356. doi: 10.1371/journal.pone.0034356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpard LW, Rohani SA, Ladak HM, & Agrawal SK (2020). Evaluation of Cochlear Duct Length Measurements From a 3D Analytical Cochlear Model Using Synchrotron Radiation Phase-Contrast Imaging. Otol Neurotol, 41(1), e21–e27. doi: 10.1097/MAO.0000000000002420 [DOI] [PubMed] [Google Scholar]
- Henry KR (1995). Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hear Res, 90(1-2), 176–184. [DOI] [PubMed] [Google Scholar]
- Henson MM (1978). The basilar membrane of the bat, Pteronotus p. parnellii. Am J Anat, 153(1), 143–157. doi: 10.1002/aja.1001530109 [DOI] [PubMed] [Google Scholar]
- Hofman R, Segenhout JM, Albers FW, & Wit HP (2005). The relationship of the round window membrane to the cochlear aqueduct shown in three-dimensional imaging. Hear Res, 209(1-2), 19–23. doi: 10.1016/j.heares.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Honrubia V, Strelioff D and Sitko S . (1976). Electroanatomy of the cochlea: Its role in cochlear potential measurements In Ruben RJ, Elberling C and Salomon G (Ed.), Electrocochleography (pp. 23–39). Baltimore: University Park Press. [Google Scholar]
- Huisken J, & Stainier DY (2009). Selective plane illumination microscopy techniques in developmental biology. Development, 136(12), 1963–1975. doi: 10.1242/dev.022426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, & Stelzer EH (2004). Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science, 305(5686), 1007–1009. doi: 10.1126/science.1100035 [DOI] [PubMed] [Google Scholar]
- Iyer JS, Batts SA, Chu KK, Sahin MI, Leung HM, Tearney GJ, & Stankovic KM (2016). Micro-optical coherence tomography of the mammalian cochlea. Sci Rep, 6, 33288. doi: 10.1038/srep33288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer JS, Zhu N, Gasilov S, Ladak HM, Agrawal SK, & Stankovic KM (2018). Visualizing the 3D cytoarchitecture of the human cochlea in an intact temporal bone using synchrotron radiation phase contrast imaging. Biomed Opt Express, 9(8), 3757–3767. doi: 10.1364/BOE.9.003757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling N, Becker K, & Dodt HU (2009). 3D-reconstruction of blood vessels by ultramicroscopy. Organogenesis, 5(4), 227–230. doi: 10.4161/org.5.4.10403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling N, Beckera K, Kramerc E, & Dodt H-U (2008). 3D-Visualization of nerve fiber bundles by ultramicroscopy. Medical Laser Application, 23, 209–215. [Google Scholar]
- Jiang H, Wang X, Zhang J, Kachelmeier A, Lopez IA, & Shi X (2019). Microvascular networks in the area of the auditory peripheral nervous system. Hear Res, 371, 105–116. doi: 10.1016/j.heares.2018.11.012 [DOI] [PubMed] [Google Scholar]
- Johnson SB, Cureoglu S, O'Malley JT, & Santi PA (2014). Comparison of traditional histology and TSLIM optical sectioning of human temporal bones. Otol Neurotol, 35(7), 1145–1149. doi: 10.1097/MAO.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Schmitz HM, & Santi PA (2011). TSLIM imaging and a morphometric analysis of the mouse spiral ganglion. Hear Res, 278(1-2), 34–42. doi: 10.1016/j.heares.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mumby CJ, & Axelsson A (1984). The vascular anatomy of the gerbil cochlea. Am J Otolaryngol, 5(2), 127–137. [DOI] [PubMed] [Google Scholar]
- Kale SS, & Olson ES (2015). Intracochlear Scala Media Pressure Measurement: Implications for Models of Cochlear Mechanics. Biophys J, 109(12), 2678–2688. doi: 10.1016/j.bpj.2015.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, & Clark GM (1996). Computer-aided three-dimensional reconstruction in human cochlear maps: measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal's canal. Ann Otol Rhinol Laryngol, 105(9), 701–709. doi: 10.1177/000348949610500906 [DOI] [PubMed] [Google Scholar]
- Keller PJ, & Dodt HU (2012). Light sheet microscopy of living or cleared specimens. Curr Opin Neurobiol, 22(1), 138–143. doi: 10.1016/j.conb.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Keller PJ, Schmidt AD, Santella A, Khairy K, Bao Z, Wittbrodt J, & Stelzer EH (2010). Fast, highcontrast imaging of animal development with scanned light sheet-based structured-illumination microscopy. Nat Methods, 7(8), 637–642. doi: 10.1038/nmeth.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PJ, & Stelzer EH (2008). Quantitative in vivo imaging of entire embryos with Digital Scanned Laser Light Sheet Fluorescence Microscopy. Curr Opin Neurobiol, 18(6), 624–632. doi: 10.1016/j.conb.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Koka K, & Litvak LM (2017). Feasibility of Using Electrocochleography for Objective Estimation of Electro-Acoustic Interactions in Cochlear Implant Recipients with Residual Hearing. Front Neurosci, 11, 337. doi: 10.3389/fnins.2017.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K, Saoji AA, & Litvak LM (2017). Electrocochleography in cochlear implant recipients with residual hearing: comparison with audiometric thresholds. Ear and hearing, 38(3), e161–e167. doi: 10.1097/AUD.0000000000000385 [DOI] [PubMed] [Google Scholar]
- Kopecky B, Johnson S, Schmitz H, Santi P, & Fritzsch B (2012). Scanning thin-sheet laser imaging microscopy elucidates details on mouse ear development. Dev Dyn, 241(3), 465–480. doi: 10.1002/dvdy.23736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromm D, Thumberger T, & Wittbrodt J (2016). An eye on light-sheet microscopy. Methods Cell Biol, 133, 105–123. doi: 10.1016/bs.mcb.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Kujawa SG, & Liberman MC (2009). Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci, 29(45), 14077–14085. doi:29/45/14077 10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lareida A, Beckmann F, Schrott-Fischer A, Glueckert R, Freysinger W, & Müller B (2009). High-resolution X-ray tomography of the human inner ear: synchrotron radiation-based study of nerve fibre bundles, membranes and ganglion cells. J Microsc, 234(1), 95–102. doi: 10.1111/j.1365-2818.2009.03143.x [DOI] [PubMed] [Google Scholar]
- Lee C, Cho H-H, & Lee S (2015). Expression of Angiogenic Molecules in Cochlear Vasculature. Amer. J. Clin. Exp.Med, 3, 279–283. [Google Scholar]
- Leischner U, Schierloh A, Zieglgansberger W, & Dodt HU (2010). Formalin-induced fluorescence reveals cell shape and morphology in biological tissue samples. PLoS One, 5(4), e10391. doi: 10.1371/journal.pone.0010391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RB, Marquarding T, Reid AP, Pun CM, Renier N, & Oviedo HV (2019). Circuit asymmetries underlie functional lateralization in the mouse auditory cortex. Nat Commun, 10(1), 2783. doi: 10.1038/s41467-019-10690-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Schart-Moren N, Rohani SA, Ladak HM, Rask-Andersen H, & Agrawal S (2020). Synchrotron Radiation-Based Reconstruction of the Human Spiral Ganglion: Implications for Cochlear Implantation. Ear Hear, 41(1), 173–181. doi: 10.1097/AUD.0000000000000738 [DOI] [PubMed] [Google Scholar]
- Li Q, Lu T, Zhang C, Hansen MR, & Li S (2020). Electrical stimulation induces synaptic changes in the peripheral auditory system. J Comp Neurol, 528(6), 893–905. doi: 10.1002/cne.24802 [DOI] [PubMed] [Google Scholar]
- Liberman LD, Wang H, & Liberman MC (2011). Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J Neurosci, 31(3), 801–808. doi:31/3/801 10.1523/JNEUROSCI.3389-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DJ (1980). Cochlear anatomy related to cochlear micromechanics. A review. J Acoust Soc Am, 67(5), 1686–1695. doi: 10.1121/1.384295 [DOI] [PubMed] [Google Scholar]
- Liu B, Gao XL, Yin HX, Luo SQ, & Lu J (2007). A detailed 3D model of the guinea pig cochlea. Brain StructFunct, 212(2), 223–230. doi: 10.1007/s00429-007-0146-0 [DOI] [PubMed] [Google Scholar]
- Lo J, Sale P, Wijewickrema S, Campbell L, Eastwood H, & O'Leary S J (2017). Defining the Hook Region Anatomy of the Guinea Pig Cochlea for Modeling of Inner Ear Surgery. Otol Neurotol, 38(6), e179–e187. doi: 10.1097/MAO.0000000000001446 [DOI] [PubMed] [Google Scholar]
- Lovell JM, Brosch M, Budinger E, Goldschmidt J, Scheich H, Tschorn S, . . . Zuschratter W (2012). Scanning and Transmission Electron Microscope Examination of Cochlea Hair and Pillar Cells from the Ear of the Mongolian Gerbil (Meriones unguiculatus). Anat. Physiol, 2, 1–6. [Google Scholar]
- MacDonald GH, & Rubel EW (2008). Three-dimensional imaging of the intact mouse cochlea by fluorescent laser scanning confocal microscopy. Hear Res, 243(1-2), 1–10. doi: 10.1016/j.heares.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magde D (1976). Chemical Kinetics and Fluorescence Correlation Spectroscopy. Quarterly Reviews of Biophysics, 9(1), 35–47. doi: 10.1017/S0033583500002146 [DOI] [PubMed] [Google Scholar]
- Mappes T, Jahr N, Csaki A, Vogler N, Popp J, & Fritzsche W (2012). The invention of immersion ultramicroscopy in 1912-the birth of nanotechnology? Angew Chem Int Ed Engl, 51(45), 11208–11212. doi: 10.1002/anie.201204688 [DOI] [PubMed] [Google Scholar]
- Micco AG, & Richter CP (2006). Electrical resistivity measurements in the mammalian cochlea after neural degeneration. Laryngoscope, 116(8), 1334–1341. doi: 10.1097/01.mlg.0000231828.37699.ab [DOI] [PubMed] [Google Scholar]
- Müller M (1996). The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res, 94(1–2), 148–156. [DOI] [PubMed] [Google Scholar]
- Nolte L, Tinne N, Schulze J, Heinemann D, Antonopoulos GC, Meyer H, . . . Ripken T (2017). Scanning laser optical tomography for in toto imaging of the murine cochlea. PLoS One, 12(4), e0175431. doi: 10.1371/journal.pone.0175431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall AL, & Dolan DF (1991). Cochlear microphonic enhancement in two tone interactions. Hear Res, 57(2), 235–245. [DOI] [PubMed] [Google Scholar]
- Pappa AK, Hutson KA, Scott WC, Wilson JD, Fox KE, Masood MM, . . . Fitzpatrick DC (2019). Hair cell and neural contributions to the cochlear summating potential. J Neurophysiol, 727(6), 2163–2180. doi: 10.1152/jn.00006.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann W, Peetz W, & Schmidt M (1987). The cochlea in gerbilline rodents. Brain Behav Evol, 39(1-2), 82–101. [DOI] [PubMed] [Google Scholar]
- Power RM, & Huisken J (2017). A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat Methods, 14(4), 360–373. doi: 10.1038/nmeth.4224 [DOI] [PubMed] [Google Scholar]
- Retzius G (1884). Das Gehörorgan der Wirbelthiere. Morphologisch-histologische Studien. BandII. Das Gehörorgan der Reptilien, der Vögel undder Säugethiere Stockholm: Samson & Wallin [Google Scholar]
- Reynaud EG, Krzic U, Greger K, & Stelzer EH (2008). Light sheet-based fluorescence microscopy: more dimensions, more photons, and less photodamage HFSP J, 2(5), 266–275. doi: 10.2976/1.2974980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RM, Mummert M, Gryczynski Z, Borejdo J, Sorensen TJ, Laursen BW, . . . Fudala R (2013). Elimination of autofluorescence in fluorescence correlation spectroscopy using the AzaDiOxaTriAngulenium (ADOTA) fluorophore in combination with time-correlated single-photon counting (TCSPC). Anal Bioanal Chem, 405(14), 4887–4894. doi: 10.1007/s00216-013-6879-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Edge R, He DZ, & Dallos P (2000). Development of the gerbil inner ear observed in the hemicochlea. J Assoc Res Otolaryngol, 1(3), 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs WJ, Dwyer RT, Holder JT, Mattingly JK, Ortmann A, Noble JH, . . . Adunka OF (2019). Intracochlear Electrocochleography: Influence of Scalar Position of the Cochlear Implant Electrode on Postinsertion Results. Otol Neurotol, 40(5), e503–e510. doi: 10.1097/MAO.0000000000002202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs WJ, Roche JP, Giardina CK, Harris MS, Bastian ZJ, Fontenot TE, . . . Fitzpatrick DC (2017). Intraoperative Electrocochleographic Characteristics of Auditory Neuropathy Spectrum Disorder in Cochlear Implant Subjects. Front Neurosci, 11, 416. doi: 10.3389/fnins.2017.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risoud M, Sircoglou J, Dedieu G, Tardivel M, Vincent C, & Bonne NX (2017). Imaging and cell count in cleared intact cochlea in the Mongolian gerbil using laser scanning confocal microscopy. Eur Ann Otorhinolaryngol Head Neck Dis, 134(4), 221–224. doi: 10.1016/j.anorl.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Santi PA (1986). Organ of Corti surface preparations for computer-assisted morphometry. Hear Res, 24(3), 179–187. doi: 10.1016/0378-5955(86)90017-1 [DOI] [PubMed] [Google Scholar]
- Santi PA (2011). Light sheet fluorescence microscopy: a review. J Histochem Cytochem, 59(2), 129–138. doi: 10.1369/0022155410394857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, Aldaya R, Brown A, Johnson S, Stromback T, Cureoglu S, & Rask-Andersen H (2016). Scanning Electron Microscopic Examination of the Extracellular Matrix in the Decellularized Mouse and Human Cochlea. J Assoc Res Otolaryngol, 17(3), 159–171. doi: 10.1007/s10162-016-0562-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, & Johnson SB (2013). Decellularized ear tissues as scaffolds for stem cell differentiation. J Assoc Res Otolaryngol, 14(1), 3–15. doi: 10.1007/s10162-012-0355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, Johnson SB, Hillenbrand M, GrandPre PZ, Glass TJ, & Leger JR (2009). Thin-sheet laser imaging microscopy for optical sectioning of thick tissues. Biotechniques, 46(4), 287–294. doi: 10.2144/000113087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, Rapson I, & Voie A (2008). Development of the mouse cochlea database (MCD). Hear Res, 243(1-2), 11–17. doi: 10.1016/j.heares.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji AA, Patel NS, Carlson ML, Neff BA, Koka K, Tarigoppula VSA, & Driscoll CL (2019). Multi-frequency Electrocochleography Measurements can be Used to Monitor and Optimize Electrode Placement During Cochlear Implant Surgery. Otology & Neurotology, 40(10), 1287–1291. doi: 10.1097/MAO.0000000000002406 [DOI] [PubMed] [Google Scholar]
- Sasmal A, & Grosh K (2019). Unified cochlear model for low- and high-frequency mammalian hearing. Proc Natl Acad Sci U S A, 116(28), 13983–13988. doi: 10.1073/pnas.1900695116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz HM, Johnson SB, & Santi PA (2014). Kanamycin-furosemide ototoxicity in the mouse cochlea: a 3-dimensional analysis. Otolaryngol Head Neck Surg, 150(4), 666–672. doi: 10.1177/0194599813519071 [DOI] [PubMed] [Google Scholar]
- Schuknect HF (1953). Techniques for study of cochlear function and pathology in experimental animals. Arch. of Otolaryngology, 58, 377–397. [DOI] [PubMed] [Google Scholar]
- Scott WC, Giardina CK, Pappa AK, Fontenot TE, Anderson ML, Dillon MT, . . . Fitzpatrick DC (2016). The Compound Action Potential in Subjects Receiving a Cochlear Implant. Otol Neurotol, 37(10), 1654–1661. doi: 10.1097/MAO.0000000000001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah G, Weber M, & Huisken J (2017). Light SheetMicroscopy In Kubitscheck U (Ed.), Fluorescence Microscopy: From Principles to Biological Applications, Second Edition. (pp. 243–265). VCH Verlag: Wiley. [Google Scholar]
- Sieber D, Erfurt P, John S, Santos GRD, Schurzig D, Sorensen MS, & Lenarz T (2019). The OpenEar library of 3D models of the human temporal bone based on computed tomography and micro-slicing. SciData, 6, 180297. doi: 10.1038/sdata.2018.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedentopf H & Zsigmondy R (1903). Über Sichtbarmachung und Größenbestimmung ultramikoskopischer Teilchen, mit besonderer Anwendung auf Goldrubingläser. Ann. Phys 10, 1–39. [Google Scholar]
- Sokolich WG, Hamernik RP, Zwislocki JJ, & Schmiedt RA (1976). Inferred response polarities of cochlear hair cells. J Acoust Soc Am, 59(4), 963–974. [DOI] [PubMed] [Google Scholar]
- Spalteholz W (1911). Ober das Durchsichtigmachen yon menschlichen und tierischen Prfiparaten und seine theoretischen Bedingungen. Leipzig, S. Hirzel. [Google Scholar]
- Spelman FA (1990). Determination of Tissue Impedances of the Inner Ear: Models and Measurements In M. J. M. a. S. F.A. (Ed.), Cochlear Implants (pp. 35–53). New York: Springer. [Google Scholar]
- Spoendlin H (1970). Structural basis of peripheral frequency analysis In R. a. S. Plomp GF (Ed.), Frequency Analysis and Periodicity Detection in Hearing: The Proceedings of the International Symposium on Frequency Analysis and Periodicity Detection in Hearing (pp. 2–40). Leiden: A.W. Sijthoff. [Google Scholar]
- Stakhovskaya O, Sridhar D, Bonham BH, & Leake PA (2007). Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol, 8(2), 220–233. doi: 10.1007/s10162-007-0076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange RA, & Wijburg FA (1986). The vasculature of the external wall of the gerbil's inner ear. Hear Res, 23(2), 135–139. doi:0378-5955(86)90010-9 [pii] [DOI] [PubMed] [Google Scholar]
- Tarnowski BI, Schmiedt RA, Hellstrom LI, Lee FS, & Adams JC (1991). Age-related changes in cochleas of mongolian gerbils. Hear Res, 54(1), 123–134. [DOI] [PubMed] [Google Scholar]
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW Jr., & Gewalt SL (1999). Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope, 109(10), 1661–1668. doi: 10.1097/00005537-199910000-00021 [DOI] [PubMed] [Google Scholar]
- Tona Y, Sakamoto T, Nakagawa T, Adachi T, Taniguchi M, Torii H, . . . Ito J (2014). In vivo imaging of mouse cochlea by optical coherence tomography. Otol Neurotol, 35(2), e84–89. doi: 10.1097/MAO.0000000000000252 [DOI] [PubMed] [Google Scholar]
- Tran H, Jan NJ, Hu D, Voorhees A, Schuman JS, Smith MA, . . . Sigal IA (2017). Formalin Fixation and Cryosectioning Cause Only Minimal Changes in Shape or Size of Ocular Tissues. Sci Rep, 7(1), 12065. doi: 10.1038/s41598-017-12006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogert T, van Hoof M, Handschuh S, Glueckert R, Guinand N, Guyot JP, . . . van de Berg R (2018). Optimization of 3D-Visualization of Micro-Anatomical Structures of the Human Inner Ear in Osmium Tetroxide Contrast Enhanced Micro-CT Scans. Front Neuroanat, 12, 41. doi: 10.3389/fnana.2018.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas MS, Martins TC, Seco F, & do Carmo A (2007). An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur J Histochem, 51(1), 59–66. [PubMed] [Google Scholar]
- Voie AH (2002). Imaging the intact guinea pig tympanic bulla by orthogonal-plane fluorescence optical sectioning microscopy. Hear Res, 171(1-2), 119–128. doi: 10.1016/s0378-5955(02)00493-8 [DOI] [PubMed] [Google Scholar]
- Voie AH, Burns DH, & Spelman FA (1993). Orthogonal-plane fluorescence optical sectioning: three-dimensional imaging of macroscopic biological specimens. J Microsc, 170(Pt 3), 229–236. doi: 10.1111/j.1365-2818.1993.tb03346.x [DOI] [PubMed] [Google Scholar]
- Voie AH, & Spelman FA (1995). Three-dimensional reconstruction of the cochlea from two-dimensional images of optical sections. Comput Med Imaging Graph, 19(5), 377–384. doi: 10.1016/0895-6111(95)00034-8 [DOI] [PubMed] [Google Scholar]
- Wada H, Sugawara M, Kobayashi T, Hozawa K, & Takasaka T (1998). Measurement of guinea pig basilar membrane using computer-aided three-dimensional reconstruction system. Hear Res, 120(1–2), 1–6. doi: 10.1016/s0378-5955(98)00007-0 [DOI] [PubMed] [Google Scholar]
- Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, & Corfas G (2014). Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife, 3. doi: 10.7554/eLife.03564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegner A, Wright CG, & Vollmer M (2016). Multichannel cochlear implant for selective neuronal activation and chronic use in the free-moving Mongolian gerbil. J NeurosciMethods, 273, 40–54. doi: 10.1016/j.jneumeth.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Wong P, George S, Tran P, Sue A, Carter P, & Li Q (2016). Development and validation of a highfidelity finite-element model of monopolar stimulation in the implanted guinea pig cochlea. IEEE Transactions on Biomedical Engineering, 63(1), 188–198. Doi: 10.1109/TBME.2015.2480601 [DOI] [PubMed] [Google Scholar]
- Wrzeszcz A, Reuter G, Nolte I, Lenarz T, & Scheper V (2013). Spiral ganglion neuron quantification in the guinea pig cochlea using Confocal Laser Scanning Microscopy compared to embedding methods. Hear Res, 306, 145–155. doi: 10.1016/j.heares.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Xu J, Xu SA, Cohen LT, & Clark GM (2000). Cochlear view: postoperative radiography for cochlear implantation. Am J Otol, 21(1), 49–56. [PubMed] [Google Scholar]
- Zhang L, Engler S, Koepcke L, Steenken F, & Koppl C (2018). Concurrent gradients of ribbon volume and AMPA-receptor patch volume in cochlear afferent synapses on gerbil inner hair cells. Hear Res, 364, 81–89. doi: 10.1016/j.heares.2018.03.028 [DOI] [PubMed] [Google Scholar]