Abstract
Introduction.
The combination of PD-1/PD-L1 immune checkpoint blockade (ICB) and chemotherapy has revolutionized the treatment of advanced non-small cell lung cancer (NSCLC), but the mechanisms underlying this synergy remain incompletely understood. In this study, we explored the relationships between neoadjuvant chemotherapy and the immune microenvironment (IME) of resectable non-small cell lung cancer (NSCLC) in order to identify novel mechanisms by which chemotherapy may enhance the effect of ICB.
Methods.
Genomic, transcriptomic and immune profiling data of 511 patients treated with neoadjuvant chemotherapy followed by surgery (NCT) vs upfront surgery (US) were compared to determine differential characteristics of the IMEs derived from whole exome sequencing (NCT=18; US=73), RNA microarray (NCT=45; US=202), flow cytometry (NCT=17; US=39), multiplex immunofluorescence (NCT=10; US=72), T cell receptor (TCR) sequencing (NCT=16 and US=63) and circulating cytokines (NCT=18; US=73).
Results.
NCT was associated with increased infiltration of cytotoxic CD8+ T cells and CD20+ B cells. Moreover, NCT was associated with increases in CD8+CD103+ and CD4+CD103+PD-1+TIM3− tissue resident memory T cells. Gene expression profiling supported memory function of CD8+ and CD4+ T cells. However, NCT did not impact TCR clonality, richness or tumor mutational burden. Finally, NCT was associated with decreased plasma BDNF (TrkB) at baseline and week 4 post-surgery.
Conclusions.
Our study supports that, in the context of resectable NSCLC, neoadjuvant chemotherapy promotes anti-tumor immunity through T and B cell recruitment in the IME, and through a phenotypic change towards cytotoxic and memory CD8+ and CD4+ memory helper T cells.
Keywords: non-small cell lung cancer, neoadjuvant chemotherapy, cytotoxic T cell, tissue resident memory T cell, B cell
Introduction
Lung cancer is the leading cause of cancer-related death in the United States [1]. Chemotherapy has been one main modality in the treatment of metastatic lung cancer [2] and remains the standard of care for resectable non-small cell lung cancer (NSCLC) in the preoperative (neoadjuvant) or postoperative (adjuvant) setting [3, 4]. The molecular mechanisms by which chemotherapy kills cancer cells remain incompletely understood. Although many studies have suggested apoptosis directly induced by chemotherapy agents is the main mechanism [5], emerging evidence from in vivo studies has implied that immune mediated cell killing may also play an important role as well [6, 7].
Immune checkpoint blockade (ICB) targeting the PD-1/PD-L1 axis has revolutionized the treatment of numerous solid tumors including NSCLC [8–12]. However, the response rate to single-agent ICB remains ~12–20% in unselected patients [13]. Recently, the combination of ICB with chemotherapy has been shown to significantly improve treatment efficacy compared to chemotherapy [14–17]. However, the mechanisms by which chemotherapy augments the effect of ICB remain incompletely understood.
A large body of pre-clinical evidence suggests that cytotoxic agents can modulate the tumor immune microenvironment (IME) through various mechanisms, such as inhibition of tumor-induced immune suppression, direct stimulation of T cell responses, and immunogenic cell death [6, 7, 18]. In particular, platinum agents such as cisplatin have been shown to: 1) increase dendritic cells (DCs) while depleting myeloid-derived suppressor cells (MDSCs), thus favoring immune effector responses in melanoma mouse models [19]; 2) increase neoplastic cells’ susceptibility to killing by cytotoxic T cells and NK cells [20] and; 3) induce the upregulation of mannose-6-phosphate receptors on the surface of tumor cells, thereby increasing permeability to granzyme B [21].
A recent study conducted by our group showed that NSCLC patients who received neoadjuvant chemotherapy followed by surgery (NCT), as compared to patients who received upfront surgery (US), had overall higher levels of immune infiltration, including higher densities of CD3+ lymphocytes and CD68+ tumor-associated macrophages (TAMs) in their tumors [22]. In patients that underwent NCT, higher levels of CD3+CD4+ lymphocytes and TAMs were associated with better survival. However, in that study, we mainly focused on the impact of NCT on IME using multiplex immunofluorescence (mIF). How chemotherapy affects the genomic landscape of NSCLC and how these molecular changes interact with the IME remains to be elucidated. To better address the effects of NCT, we analyzed multi-omics molecular and immune profiling data from patients with resectable NSCLC who underwent NCT or US with curative intent. Our study objectives were to: 1) further our understanding of the impact of NCT on the IME; 2) identify specific subsets of immune cells that are modulated by NCT and; 3) discover novel mechanisms by which chemotherapy may enhance anti-tumor immune response and, consequently, increase the effect of ICB.
Materials and Methods
Patients and datasets.
Available data from three independent cohorts (n = 511) of localized and resected NSCLC at the University of Texas MD Anderson Cancer Center (including the ImmunogenomiC prOfiling of NSCLC (ICON) and PROSPECT cohorts) were evaluated. The tumor IME was compared between US and NCT patients using multiple platforms: 1) tumor infiltrating lymphocyte immune profiling using flow cytometry (NCT=17; US=39); 2) multiplex immunofluorescence (mIF) (NCT=10; US=72); 3) differential gene expression using RNA microarray (NCT=45; US=202); 4) T cell receptor (TCR) variable CDR3β chain sequencing (NCT=16 and US=63) and; 5) tumor mutational burden (TMB) using available Whole Exome Sequencing (WES) data (NCT=40; US=61). Plasma circulating cytokine profiling was also analyzed (NCT=18; US=73).
Clinical metadata comparison for NCT and US groups.
Clinical characteristics between NCT and US patient groups of two resectable NSCLC cohorts (i.e., ICON and PROSPECT) were compared using Student’s t-test, Pearson’s Chi-squared test, and Fisher’s exact test. The appropriate statistical test for each clinical variable was chosen based on the clinical data types and statistical assumptions. Significance was defined as p-value < 0.05. Significant clinical variables were used to fit the binary logistic regression model followed by AIC stepwise model selection. All analyses were performed using R version 3.5.1 (Boston, MA, USA) [23].
Tumor Infiltrating Lymphocytes (TILs) flow cytometry analysis.
The BD™ Medimachine System (BD biosciences) was used to disaggregate fresh tumor tissue in order to create single cell suspensions for flow cytometry staining. Using the following fluorochrome-conjugated monoclonal antibodies, surface staining was performed in 1x DPBS with 1% bovine serum albumin for 30 min on ice: CD45 (BUV395, Clone HI30, Cat. No. 563792, BD Biosciences), CD3 (PerCP-Cy5.5, Clone SK7, Cat. No. 340949, BD Biosciences), CD8 (AF 700, Clone RPA-T8, Cat. No. 557945, BD Biosciences), CD4 (BUV496, Clone SK3, Cat. No. 564651, BD Biosciences), PD-1 (BV650, Clone EH12, Cat. No. 564104, BD Biosciences), TIM3 (BV605, Clone F38–2E2, Cat. No. 345018, Biolegend), CD103 (BV711, Clone Ber-Act8, Cat. No. 563162, BD Biosciences), CTLA4 (BV786, Clone BNI3, Cat. No. 563931, BD Biosciences), GITR (AF 488, Clone eBioAITR, Cat. No. 53–5875-42, eBioscience), LAG3 (PE, Clone 3DS223H, Cat. No. 12–2239-42, eBioscience), CD56 (PE-Cy7, Clone B159, Cat. No. 557747, BD Biosciences), ICOS (BV421, Clone C398.A4, Cat. No. 313524, Biolegend), and CD25 (APCFire/750, Clone BC96, Cat. No. 302642, Biolegend). Cells were then fixed and permeabilized using eBioscience™ Foxp3/Transcription Factor Staining Buffer Set (Cat. No. 00–5523-00, ThermoFisher) according to the manufacturer’s instructions, and stained using FOXP3 (PE-eFluor610, Cat. No. 61–4776-42, eBioscience) and Ki67 (APC, Clone 20Raj1, Cat. No. 17–5699-42, eBioscience) anti-human antibodies. Data acquisition was conducted using a BD Fortessa X20 and analyzed using FlowJo Software version 10.5.3 (Tree Star, Inc.). Using LIVE/DEAD™ Fixable Yellow Dead Cell Stain dye (Cat. No. L-34968, Life Technologies), dead cells were excluded from the analysis. The abundances of each studied immune marker across the samples were quantified as fractions of the CD3+ cells, and samples with outliers were removed from the dataset using the Tukey’s algorithm. The Mann-Whitney U test without correction for multi-testing was used to find markers that are significantly different (p-value < 0.05) between the US and NCT patient groups. Box plots for the obtained results were generated using Graph Pad (version 8.1.2; San Diego, CA, USA). The multivariable analysis was further used to assess the contribution of confounding clinical characteristics including smoking status, clinical staging, age, and histology on association of each selected immune marker with NCT. The analysis was implemented in R (version 3.6.0; Boston, MA, USA) using function lm() to fit the following formula: “selected immune marker ~ smoking status + clinical staging + age + histology + chemotherapy”.
Vectra multiplex immunofluorescence staining and analysis.
Multiplex immunofluorescence (mIF) staining (Vectra panel) was performed on tumor samples using methods that have been previously described and validated [24]. In brief, four micrometer-thick formalin fixed, paraffin embedded tumor sections were stained using an automated staining system (BOND-RX; Leica Microsystems, Buffalo Grove, IL) using two mIF panels. Panel 1: pancytokeratin (clone AE1/AE3, dilution 1:300, Dako, Santa Clara, CA), PD-L1 (clone E1L3N, dilution 1:3000, Cell Signaling Technology, Danvers, MA), CD68 (clone PG-M1, dilution 1:450, Dako, Santa Clara, CA), CD3 (Cat. No. IS503, dilution 1:100, Dako, Santa Clara, CA), CD8 (clone C8/144B, dilution 1:300, Thermo Fisher Scientific, Waltham, MA), and PD-1 (clone EPR4877–2, dilution 1:250, Abcam, Cambridge, MA). Panel 2: pancytokeratin (clone AE1/AE3, dilution 1:300, Dako, Santa Clara, CA), CD57 (natural killer cells, clone HNK-1, dilution 1:40; BD Biosciences, San Jose, CA); CD45RO (memory T cells, clone UCHL1, ready to use; Leica Biosystems, Buffalo Grove, IL); granzyme B (cytotoxic lymphocytes; clone F1, ready to use; Leica Biosystems), FOXP3 (regulatory T cells, clone 206D, dilution 1:50; BioLegend, San Diego, CA); and CD20 (B-cell lymphocytes, dilution 1:100; Dako, Santa Clara, CA). All the markers were stained in sequence using their respective fluorophore containing in the Opal 7 kit (Cat. No. NEL797001KT; Akoya Biosciences/PerkinElmer, Waltham, MA) [25]. The stained slides were scanned using a multispectral microscope, Vectra 3.0 imaging system (Akoya Biosciences/PerkinElmer), under fluorescence conditions in low magnification at 10x. After being scanned in low magnification, a pathologist selected five regions of interest (ROI) (each ROI, 0.3345 mm2) to cover 1.65mm2 of the tumor tissue using the phenochart 1.0.9 viewer (Akoya Biosciences/PerkinElmer). The ROIs were analyzed by a pathologist using InForm 2.4.0 image analysis software (Akoya Biosciences/PerkinElmer) in two different compartments: tumor nests and tumor stroma. Marker colocalization was used to identify malignant cells (AE1/AE3+), PD-L1+ malignant cells (AE1/AE3+PD-L1+), and populations of T cells (CD3+), cytotoxic T cells (CD3+CD8+), antigen-experienced T cells (CD3+CD8+PD-1+), cytotoxic T cells antigen-experienced (CD3+CD8+PD-1+), macrophages (CD68+), and macrophages expressing PD-L1 (CD68+PD-L1+) with panel 1 and memory T-cell lymphocytes (CD45RO+), natural killer cells (CD57+), regulatory cells (FOXP3+), and B cells (CD20+) with panel 2. Densities of each colocalized cell population were quantified as the average and the final data was expressed as number of cells/mm2. Malignant cells and macrophages expressing PD-L1 were also expressed in percentages. All data was consolidated using R studio 3.5.3 (Phenopter 0.2.2 packet, Akoya Biosciences/PerkinElmer; Boston, MA, USA) and SAS 7.1 Enterprise.
The Mann-Whitney U test without correction for multiple testing was used to find markers that are significantly different (p-value < 0.05) between the US and NCT patient groups. Box plots for the obtained results were generated using Graph Pad (version 8.1.2). The multivariable analysis was further used to assess the contribution of confounding clinical characteristics including smoking, clinical staging, age and histology on association of each selected immune marker with NCT. The analysis was implemented in R (version 3.6.0; Boston, MA, USA) using function lm() to fit the following formula: “selected immune marker ~ smoking + clinical stage + age + histology + chemotherapy”.
RNA Microarray Gene Set Enrichment Analysis.
Gene Set Enrichment Analysis (GSEA) was conducted on available tumor RNA microarray data comparing NCT and US patients using GSEA software [26] and the Molecular Signatures Database (MSigDB) v6.2. The C7 (immunologic) gene set collection was used for this analysis [27]. Gene sets with less than 15 genes or more than 500 genes were excluded from the analysis. The genes were ranked based on their p-values from logistic regression models, which take into account the four clinical variables identified as potential confounding factors for the effect of NCT: age, smoking status, histology and clinical staging. The Type I error rate was controlled using False Discovery Rate (FDR). All analyses were performed using R version 3.5.1 (Boston, MA, USA) [23].
T cell receptor variable β chain sequencing analysis.
Tumor sequencing of the CDR3 regions of human TCR-β chains was performed by immunoSEQ as recommended (Adaptive Biotechnologies, Seattle, WA). Clonality, a measure of T cell reactivity, and richness, a measure of T cell diversity, were calculated using the Analyzer platform. T cell density in fresh-frozen tissue samples was calculated by normalizing TCR-β template counts to the total amount of DNA used for TCR sequencing assuming 6.6pg of DNA per cell [28, 29].
Tumor Mutational Burden analysis.
TMB analysis was based on available tumor WES data. Details about WES mutational calling and analysis pipeline can be found in the Supplementary Methods section. Briefly, TMB was defined as the total number of non-synonymous somatic mutations divided by the total covered exonic region. TMB data is presented as mutations / megabase (Mb). A multivariate linear regression analysis was used to test the correlation between TMB and NCT, clinical staging, age, histology and smoking status. The confounding factors were assessed using the multivariable analysis as described in the “Vectra multiplex immunofluorescence staining and analysis” section.
Circulating cytokine analysis.
Peripheral blood samples were collected and plasma was stored at −80°C until analysis. For cytokines, chemokines and angiogenic factors (CAF) analysis, plasma samples were processed and analyzed using multiplexed magnetic bead-based assays (EMD Bioscience Research Reagents, Temecula, CA, USA) as previously published [30, 31]. For all CAF analyses, duplicate samples were analyzed and the mean was reported and used in biomarker analysis. The CAFs included: sCD40L, EGF, Eotaxin/CCL11, FGF-2, Flt-3 ligand, Fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, sMCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, TGF-α, TNF-α, TNF-β, VEGF (MilliporeSigma, Burlington, MA, USA) and sBTLA, GITR, sHVEM, IDO, LAG-3, PD-1, PD-L1, PD-L2, TIM-3, CD28, CD80, 4–1BB, sCD27, and CTLA-4 (ProcartalPlex, ThermoFisher Scientific, Waltham, MA, USA).
Student’s t-test was used for comparing the expression of circulating cytokines between NCT and US groups at four different time points: 1) baseline (i.e., immediately before surgery); 2) 24 hours post-surgery; 3) 4 weeks post-surgery and; 4) 4 months post-surgery. Type I error rate was controlled using FDR. Significant markers were the ones with p-value < 0.05 and fold change > 1.2. All analyses were performed using R version 3.5.1 (Boston, MA, USA). The confounding factors were assessed using the multivariable analysis as described in the “Vectra multiplex immunofluorescence staining and analysis” section above.
Results
NCT and US groups are clinically different in terms of age, histology, smoking status, and clinical staging.
Clinicopathologic characteristics of the patients included in each dataset are described in Supplementary Tables 1–6. Patients with NSCLC undergoing NCT or US are inherently different because NCT is usually delivered to patients with later stages of disease (stage II-III). To identify potential confounding factors for the effect of NCT, we compared clinical variables between the NCT and US groups. Univariate and multivariate analyses are detailed in Supplementary Tables 7 and 8, respectively. From all significant parameters identified using multivariate analyses, clinical stage, age, histology and smoking history were retained for all further multivariate analyses based on biological and surgical timepoint relevance.
Flow cytometry immune profiling of TILs reveals increased cytotoxic CD8+ T cells, CD8+ CD103+ and CD4+ CD103+ PD-1+ TIM3− tissue resident memory T cells in the tumor IME of NCT patients, as compared with US patients.
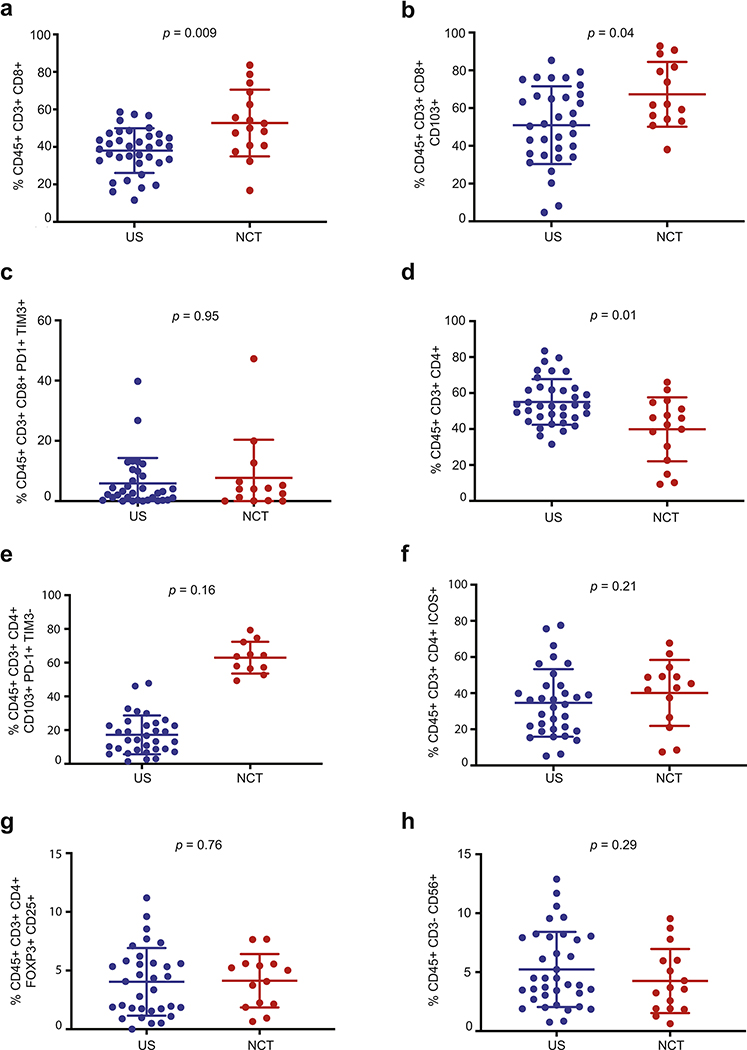
To investigate the impact of NCT in the tumor IME, we compared NCT (n=17) and US (n=39) patients by flow cytometry (Supplementary Figure 1). First, univariate (p=0.004; Supplementary Table 9) and multivariate (p=0.009; Supplementary Table 9 and Figure 1a) analyses revealed a statistically significant correlation between NCT and increased CD3+ CD8+ cytotoxic T cells. Moreover, these analyses showed an increase in CD3+ CD8+ CD103+ tissue resident memory T cells in univariate (p=0.021; Supplementary Table 9) and multivariate (p=0.039; Supplementary Table 9 and Figure 1b) analyses. Considering stage, histology, and smoking status could have profound impact on the tumor molecular and immune features, we further compared NCT vs US using specific clinical subgroups (controlling for stage, histology and smoking status) large enough for analysis: 1) patients with non-squamous histology, ever smoking status and clinical stage 2 or 3 disease; 2) patients with squamous histology, ever smoking status and clinical stage 2 and 3; 3) patients with non-squamous histology, ever smoking status and clinical stage 2 only disease and; 4) patients with squamous histology, ever smoking status and clinical stage 2 only disease. Trends for NCT-related changes in CD3+ CD8+ cytotoxic T cells were retained in all subgroups (Supplementary Figures 2a, 3a, 4a and 5a). Similarly, NCT-associated changes in tissue resident memory CD8+ CD103+ T cells also remained the same in all subgroups: (Supplementary Figures 2b, 3b, 4b and 5b). Taken together, these results suggested that neoadjuvant chemotherapy may be associated with increased cytotoxic CD3+CD8+ T cells and tissue resident memory CD8+CD103+ T cells, as compared with US patients.
Figure 1. Flow cytometry immune profiling of TILs reveals increased cytotoxic CD8+ T cells, CD8+ CD103+ and CD4+ CD103+ PD-1+ TIM3− tissue resident memory T cells in the tumor IME of NCT patients, as compared with US patients.
To investigate the impact of NCT in the tumor IME, we compared NCT (n=17) and US (n=39) patients using flow cytometry-based TILs analysis. Multivariate analyses revealed a statistically significant correlation between NCT and increased CD3+ CD8+ cytotoxic T cells (p=0.009; Figure 1a). Moreover, these analyses showed an increase in CD3+ CD8+ CD103+ tissue resident memory T cells in multivariate analyses (p=0.039; Figure 1b). However, CD3+ CD8+ PD-1+ TIM3+ T cells were not significantly different between the two groups by univariate analyses (p=0.95; Figure 1c). NCT-related CD3+ CD8+ T cell increase was associated with a concomitant decrease of the CD3+ CD4+ T cell percentage in multivariate analyses (p=0.01; Figure 1d), as well as an increase in tissue resident memory CD3+ CD4+ CD103+ PD-1+ TIM3− T cells in multivariate analyses (p=0.162; Figure 1e). However, CD3+ CD4+ ICOS+ effector T cells (p=0.21; Figure 1f) and CD3+ CD4+ FOXP3+ CD25+ Tregs (p=0.76; Figure 1g) were not significantly different between the two groups by univariate analyses. Finally, CD3− CD56+ Natural Killer (NK) cells were not significantly different between the two groups by univariate analyses (p=0.29; Figure 1h).
Interestingly, CD3+ CD8+ PD-1+ TIM3+ T cells were not significantly different between the two groups by univariate analyses (p=0.95; Figure 1c).
Furthermore, flow cytometry results also showed that the NCT-related CD3+ CD8+ T cell increase was associated with a concomitant decrease of the CD3+ CD4+ T cell percentage in both univariate (p=0.007; Supplementary Table 9) and multivariate (p=0.01; Supplementary Table 9 and Figure 1d) analyses, as well as a significant increase in tissue resident memory CD4+ CD103+ PD-1+ TIM3− T cells in univariate (p=0.011; Supplementary Table 9) and the same trend in multivariate analyses (p=0.162; Supplementary Table 9 and Figure 1e). Our multivariate model results were further reinforced by the fact that NCT-associated changes in tissue resident CD4+ CD103+ PD-1+ TIM3− were also retained in all above-mentioned 4 clinical subgroups (Supplementary Figures 2c, 3c, 4c and 5c).
Finally, CD3+ CD4+ ICOS+ effector T cells (p = 0.21; Figure 1f), CD3+ CD4+ FOXP3+ CD25+ Tregs (p=0.76; Figure 1g) and CD3− CD56+ Natural Killer (NK) (p=0.29; Figure 1h) were not significantly different between the two groups by univariate analyses.
Tumor multiplex IF imaging reveals increased cytotoxic CD8+ T cells and CD20+ B cells in the tumor IME of NCT patients, as compared with US patients.
Multiplex IF imaging (NCT=10; US=72) confirmed that, when compared with US patients, NCT patients had increased CD3+ cells by univariate (p=0.089; Supplementary Table 10) and multivariate (p=0.026; Supplementary Table 10 and Figure 2a and 2c) analyses. Similarly, the NCT group had increased CD3+CD8+ T cells in univariate (p=0.092; Supplementary Table 10) and multivariate (p=0.009; Supplementary Table 10 and Figure 2b and 2c) analyses. Multiplex IF analyses also revealed that the NCT group had higher CD20+ B cells by univariate (p=0.080; Supplementary Table 10) or multivariate (p=0.014; Supplementary Table 10 and Figure 2d and 2e) analyses. Further subgroup analyses in previously mentioned 4 clinical subgroups controlling histology, stage and smoking status demonstrated the same trends regarding NCT-related changes in CD3+ (Supplementary Figures 6a, 7a, 8a and 9a), NCT-associated changes in CD3+ CD8+ cells (Supplementary Figures 6b, 7b, 8b and 9b) and NCT-associated changes in CD20+ cells (Supplementary Figures 6c and 8c) except for CD20+ cells for squamous cell histology subgroups because of missing data (Supplementary Figures 7c and 9c).
Figure 2. Tumor multiplex IF imaging reveals increased cytotoxic CD8+ T cells and CD20+ B cells in the tumor IME of NCT patients, as compared with US patients.
Multiplex IF imaging (NCT=10; US=72) confirmed that the NCT group had increased CD3+ cells by multivariate (p=0.026; Figure 2a and 2c) analyses. Similarly, the NCT group had increased CD3+CD8+ T cells by multivariate (p=0.009; Figure 2b and 2c) analyses. Multiplex IF analyses also revealed that the NCT group had higher CD20+ B cells after multivariate analyses (p=0.014; Figure 2d and 2e).
RNA microarray GSEA supports memory functions of CD8+ and CD4+ T cells in NCT patients, as compared with US patients.
Complete GSEA results are described in Supplementary Table 11 and Supplementary Table 12. After adjustment for age, histology, smoking status and clinical staging, we found that within the top 20 upregulated immune pathways in the NCT group, 6 were related to CD8+ T cells, 9 were related to CD4+ T cells, and 2 were related to B cells. Within these top pathways, GSE15750_DAY6_VS_DAY10_TRAF6KO_EFF_CD8_TCELL_UP (q<0.01; Figure 3a), GSE36476_CTRL_VS_TSST_ACT_40H_MEMORY_CD4_TCELL_OLD_DN (q<0.01; Figure 3b) and GSE36476_CTRL_VS_TSST_ACT_72H_MEMORY_CD4_TCELL_YOUNG_DN (q<0.01; Figure 3c) were upregulated in the NCT group, yielding support for CD8+ and CD4+ T cell memory functions being increased following NCT. Finally, the upregulation of GSE21063_WT_VS_NFATC1_KO_8H_ANTI_IGM_STIM_BCELL_UP (q<0.01; Figure 3d) provides support for the activation of B cells following NCT.
Figure 3. RNA microarray GSEA supports memory functions of CD8+ and CD4+ T cells in NCT patients, as compared with US patients.
A GSEA was conducted using available RNA microarray data (NCT=45; US=202) to determine dominant immune transcriptomic pathways associated to NCT. First, NCT was associated with the development of memory function for both CD8+ T cells (q<0.01) (Figure 3a) and CD4+ T cells (q<0.01) (Figure 3b and 3c). NCT was also associated with the activation of B cells (q<0.01; Figure 3d). Positively correlated genes (“na_pos”) are associated to the NCT group, while negatively correlated genes (“na_neg”) are associated to the US group.
TCR clonality and richness are consistent between NCT and US groups.
Considering the increased CD8+ T cell infiltration following NCT we sought to further characterize the T cell repertoire. To do so, we performed sequencing of the variable CDR3 region of the β chain of the T cell receptor involved in antigen binding in 15 NCT and 63 US patients. Richness, a measure of diversity, was not significantly different by univariate (p=0.58) and multivariate analyses (p=0.44; Supplementary Figure 10b). Similarly, clonality, a measure of T cell reactivity, was not significantly different by univariate (p=0.74) or multivariate analyses (p=0.58; Supplementary Figure 10c).
NCT does not impact TMB.
TMB was evaluated in 40 NCT and 61 US patients with available WES data. In univariate analyses, we observed a statistically significant difference in TMB between the two groups (p=0.033). However, this difference did not remain significant following multivariate analyses (p=0.342; Supplementary Figure 11), and smoking history was the only significant variable within the multivariate model (p=0.003).
NCT is associated with decreased plasma BDNF (TrkB) at baseline and week 4 post-surgery, and with decreased plasma sCD27 at week 4 post-surgery, as compared with US patients.
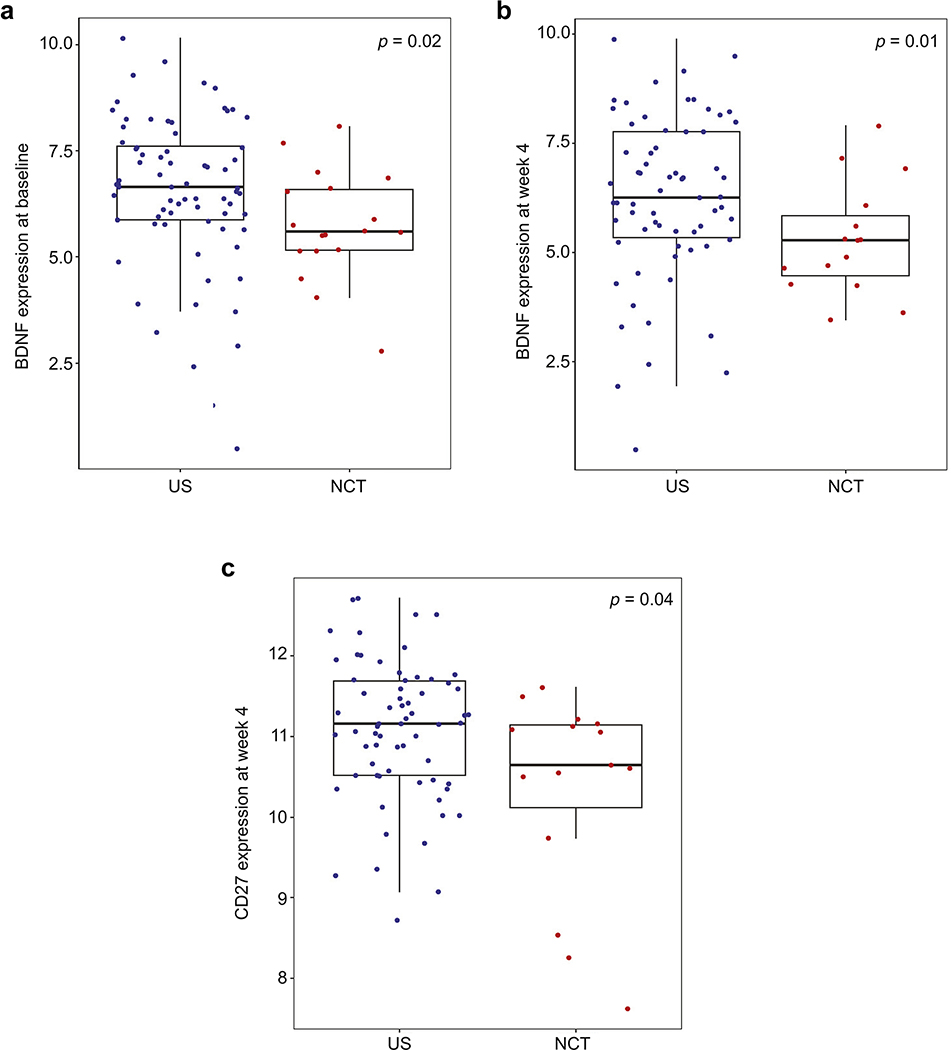
To better understand the systemic immune effects of NCT, we measured circulating plasma cytokines from 18 NCT and 73 US patients. CAFs were assessed at four different timepoints: 1) baseline (i.e., immediately before surgery); 2) 24 hours post-surgery; 3) 4 weeks post-surgery and; 4) 4 months post-surgery. Using univariate analyses, NCT was associated with increased sMCP-1 (p=0.026) and decreased BDNF (p=0.039) at baseline. NCT was also correlated with increased sHVEM (p=0.02), decreased BDNF (p=0.025) and decreased sCD27 (p=0.044) at week 4. Following multivariate analyses, only decreased peripheral BDNF (TrkB) at baseline (p=0.02; Figure 4a) and week 4 (p=0.01; Figure 4b) and decreased plasma sCD27 at week 4 (p=0.04; Figure 4c) remained statistically significant.
Figure 4. NCT is associated with decreased plasma BDNF (TrkB) at baseline and week 4 post-surgery, and with decreased plasma sCD27 at week 4 post-surgery, as compared with US patients.
Multiple plasma circulating cytokines from 18 NCT and 73 US patients were measured at four different timepoints: 1) baseline (i.e., immediately before surgery); 2) 24 hours post-surgery; 3) 4 weeks post-surgery and; 4) 4 months post-surgery. Following multivariate analyses, NCT was associated with decreased peripheral BDNF (TrkB) at baseline (p=0.02; Figure 4a) and week 4 (p=0.01; Figure 4b), and with decreased plasma sCD27 at week 4 (p=0.04; Figure 4c).
Discussion
Lake & Robinson have previously highlighted multiple mechanisms by which chemotherapy may augment the efficacy of immunotherapy: antigen threshold and presentation, T cell response and trafficking, target destruction, generation of memory and external regulation of the previous steps [32]. By comparing tumors from resectable NSCLC patients treated with NCT or US, we were able to further address these mechanisms.
When compared with US patients, flow cytometry and tumor mIF analyses showed an association between NCT and increased infiltration of cytotoxic CD8+ T cells and revealed a novel association between NCT and CD8+ CD103+ and CD4+ CD103+ PD-1+ TIM3− tissue resident memory T cells, which were confirmed by multivariate analyses and subgroup analyses focusing on specific clinical stages. Furthermore, GSEA from gene expression profiling also supported memory function of CD8+ and CD4+ T cells as well. CD8+ CD103+ TILs have previously been associated with increased overall survival in early stage NSCLC patients [33]. A recent study also reported that CD8+ CD103+ CD39+ TILs were enriched for tumor-reactive cells in primary as well as metastatic tumors [34]. This particular TIL subset was shown to efficiently kill autologous tumor cells in an MHC-class I-dependent manner. Therefore, our results suggest that beneficial effects of neoadjuvant chemotherapy may be mediated in part by CD8+ CD103+-mediated tumor cell killing, although further mechanistic studies would be needed to confirm this hypothesis. Moreover, pre-clinical data demonstrated that mice vaccinated with cisplatin-treated ovarian cancer cells had enhanced antitumor immunity and prolonged survival that was largely dependent on CD4+ T-cell mediated immune responses [35]. Similar findings were observed in the context of resectable NSCLC treated with NCT, where increased CD4+ T cell infiltration at the time of surgery was associated with longer OS [22]. Our results show for the first time that these associations may be explained by increased CD4+ CD103+ PD-1+ TIM3− tissue resident memory T cells, rather than increased CD4+ ICOS+ effector T cells or decreased Tregs. These results indicate that the generation of memory may be a central element by which NCT may enhance tumor immunity. Our results also show an association between NCT and increased immune cell infiltration in the IME, but the T cell repertoire does not appear to be affected as TCR-β clonality and richness were no different between the groups. However, the negative T cell repertoire findings may be consequent to bulk tumor TCR-β sequencing, as opposed to isolated T cell subpopulations. Collectively, these results suggest that chemotherapy can increase T cell expansion and priming into the IME and promote phenotypic changes to T cells that indicate that memory may be a central element by which NCT may enhance anti-tumor immunity.
Furthermore, tumor multiplex IF results highlighted increased CD20+ B cells in the IME following NCT, which was also supported by specific disease stage subgroup analyses as well as top immunologic pathways identified by the GSEA results. To our knowledge, this is the first report of this finding in the context of resectable human NSCLC. Although there are conflicting results regarding the effects of chemotherapy on humoral immunity [6], chemotherapy agents such as low-dose cyclophosphamide have been reported to increase B cells both at the pre-clinical [36] and clinical level [37]. Interestingly, a recent study described the promotion of neoadjuvant anti-PD-1 immunotherapeutic responses by B cells, which suggests that chemotherapy and anti-PD-1 combinations may synergize to enhance the effect of this immune cell subpopulation [38].
Very few circulating plasma cytokines were differentially expressed in NCT patients, suggesting that NCT acts predominantly within the tumor. However, the fact that NCT was associated with sustained decrease of BDNF (TrkB) circulating levels certainly warrants further investigation given that BDNF has been correlated with metastasis in the context of lung adenocarcinoma [39].
As a retrospective study, our study has several important inherent limitations, which should be taken into consideration when interpreting the data. First, we were limited by the availability of specimens, particularly from patients who received NCT, so some datasets included only a small number of NCT patients, thus limiting statistical power. Second, despite the fact that all NCT regimens were platinum-based, the heterogeneity of chemotherapy regimens in this study prevented us from drawing agent-specific conclusions. Third, the small number of NCT patient datasets could not provide the statistical power necessary to differentiate the IME between NCT responders and non-responders. Fourth, the correlative analyses conducted on the surgical tumor samples occurred approximately three weeks after the last dose of NCT; the extent to which the observed changes persist beyond that time point is currently unknown. Finally, the ideal study to investigate the impact of chemotherapy on molecular and immune features would be on paired specimens before and after chemotherapy rather than the independent cohorts in the current study. Unfortunately, finding high-quality paired specimens turned out to be extremely challenging, mainly due to the scarcity of adequate pre-chemotherapy biopsies. These pre-chemotherapy diagnostic biopsies tended to be very small, most of which have already been exhausted after pathologic diagnosis, immunohistochemistry staining and molecular profiling. Additionally, the decay of these small FFPE specimens tends to be much worse than for large surgical specimens. We attempted analyses on several paired specimens that we were able to identify without success. Nevertheless, our findings were consistent in multivariate analyses and subgroup analyses controlling important confounding factors, which suggests that these observed changes may reflect the effect from chemotherapy.
In conclusion, our results show an association between NCT and increased immune cell trafficking into the tumor IME, which can potentially be related to improved antigen presentation as the systemic cytokine milieu and T cell profiling did not denote substantial increased activation of immune cells. Together with the current knowledge that chemotherapy and PD-1/PD-L1 inhibitors show synergistic activity for the treatment of NSCLC, our results suggest that potential combinations including T cell activating agents (TLR9, STING, and IL-10 agonists) may represent promising approaches to further augment anti-tumor immune response for the treatment of NSCLC.
Supplementary Material
Acknowledgements.
This work used the Tissue Biospecimen and Pathology Resource and Research Histopathology Facility at MD Anderson. This project was in part supported by the Translational Molecular Pathology-Immunoprofiling Lab (TMP-IL) at the Department of Translational Molecular Pathology, the University of Texas MD Anderson Cancer Center. The authors appreciate the assistance of Elliana Young with the Department of Institutional Analytics and Informatics at MD Anderson. We thank all the dedication of our research nurses Mary Ann Gianan and Craig DeGraaf, consent team May Celestino, blood team Patrice Lawson, Heather Napoleon, Mayra Vasquez and Eric Prado, and all thoracic OR nurses and anesthesiologists at MD Anderson Cancer Center, without whom this work could not have been completed.
Financial Support: This work was supported by the generous philanthropic contributions to The University of Texas MD Anderson Lung Cancer Moon Shots Program, the Gil and Dody Weaver Foundation and Bill and Katie Weaver Charitable Trust, the MD Anderson Cancer Center Support Grant P30 CA01667, MD Anderson Physician Scientist Program and the Cancer Prevention and Research Institute of Texas Multi-Investigator Research Award grant. P.O. Gaudreau was supported by the Fonds de Recherche Québec–Santé’s (FRQS) Resident Physician Health Research Career Training Program (32667). The funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Disclosures. D.L. Gibbons has received research funding from AstraZeneca, Astellas, Janssen, Ribon Therapeutics and Takeda and has participated in advisory boards for AstraZeneca and Sanofi. S.G. Swisher has participated in advisory committees for Ethicon and for the Peter MacCallum Cancer Center. J.V. Heymach has received research support from AstraZeneca, Bayer, GlaxoSmithKline, and Spectrum; participated in advisory committees for AstraZeneca, Boehringer Ingelheim, Exelixis, Genentech, GlaxoSmithKline, Guardant Health, Hengrui, Lilly, Novartis, Specrtum, EMD Serono, and Synta; and received royalties and/or licensing fees from Spectrum. T. Cascone has received speaker’s fees from the Society for Immunotherapy of Cancer and Bristol-Myers Squibb and research funding to MD Anderson Cancer Center from Boehringer Ingelheim and Bristol-Myers Squibb. J. Zhang served on advisory board for AstraZeneca and Geneplus and received speaker’s fees from BMS, Geneplus, OrigMed, Innovent, grant from Merck, outside the submitted work. No potential conflicts of interest are disclosed by the other authors.
List of Abbreviations
- CAF
Chemokines and Angiogenic Factors
- FDR
False Discovery Rate
- ICB
Immune Checkpoint Blockade
- ICON
ImmunogenomiC prOfiling of NSCLC
- mIF
Multiplex Immunofluorescence
- IME
Immune Microenvironment
- NSCLC
Non-Small Cell Lung Cancer
- OS
Overall Survival
- RFS
Recurrence-Free survival
- ROI
Region Of Interest
- TCR
T Cell Receptor
- TILs
Tumor Infiltrating Lymphocytes
- TMAs
Tumor-Associated Macrophages
- TMB
Tumor Mutational Burden
- US
Upfront Surgery
- WES
Whole Exome Sequencing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2019. CA Cancer J Clin, 2019. 69(1): p. 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, et al. , Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol, 2017. 35(30): p. 3484–3515. [DOI] [PubMed] [Google Scholar]
- 3.Group N.M.-a.C., Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet, 2014. 383(9928): p. 1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pignon JP, et al. , Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol, 2008. 26(21): p. 3552–9. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S and Debatin KM, Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene, 2006. 25(34): p. 4798–811. [DOI] [PubMed] [Google Scholar]
- 6.Bracci L, et al. , Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ, 2014. 21(1): p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfirschke C, et al. , Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity, 2016. 44(2): p. 343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rittmeyer A, et al. , Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet, 2017. 389(10066): p. 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J, et al. , Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med, 2015. 373(2): p. 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, et al. , Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med, 2015. 373(17): p. 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, et al. , Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med, 2016. 375(19): p. 1823–1833. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, et al. , Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet, 2016. 387(10027): p. 1540–50. [DOI] [PubMed] [Google Scholar]
- 13.Qin H, et al. , New advances in immunotherapy for non-small cell lung cancer. Am J Transl Res, 2018. 10(8): p. 2234–2245. [PMC free article] [PubMed] [Google Scholar]
- 14.West H, et al. , Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2019. 20(7): p. 924–937. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi L, et al. , Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med, 2018. 378(22): p. 2078–2092. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, et al. , Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med, 2018. 379(21): p. 2040–2051. [DOI] [PubMed] [Google Scholar]
- 17.Langer CJ, et al. , Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol, 2016. 17(11): p. 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zitvogel L, et al. , Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity, 2013. 39(1): p. 74–88. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, et al. , Preconditioning chemotherapy with cisplatin enhances the antitumor activity of cytokine-induced killer cells in a murine melanoma model. Cancer Biother Radiopharm, 2012. 27(3): p. 210–20. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann-Leitner ES and Abrams SI, Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother, 2001. 50(9): p. 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, et al. , Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest, 2010. 120(4): p. 1111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra ER, et al. , Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer, 2018. 6(1): p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R: A language and environment for statistical computing. 2018, Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 24.Parra ER, et al. , Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci Rep, 2017. 7(1): p. 13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra ER, Francisco-Cruz A, and Wistuba II, State-of-the-Art of Profiling Immune Contexture in the Era of Multiplexed Staining and Digital Analysis to Study Paraffin Tumor Tissues. Cancers (Basel), 2019. 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, et al. , Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A, 2005. 102(43): p. 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godec J, et al. , Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation. Immunity, 2016. 44(1): p. 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuben A, et al. , Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nature Communications, 2020. 11(1): p. 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuben A, et al. , TCR Repertoire Intratumor Heterogeneity in Localized Lung Adenocarcinomas: An Association with Predicted Neoantigen Heterogeneity and Postsurgical Recurrence. Cancer Discov, 2017. 7(10): p. 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanrahan EO, et al. , Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol, 2010. 28(2): p. 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heymach JV, et al. , Effect of low-fat diets on plasma levels of NF-kappaB-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev Res (Phila), 2011. 4(10): p. 1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake RA and Robinson BW, Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer, 2005. 5(5): p. 397–405. [DOI] [PubMed] [Google Scholar]
- 33.Djenidi F, et al. , CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol, 2015. 194(7): p. 3475–86. [DOI] [PubMed] [Google Scholar]
- 34.Duhen T, et al. , Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun, 2018. 9(1): p. 2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JE, et al. , Cancer cells containing nanoscale chemotherapeutic drugs generate antiovarian cancer-specific CD4+ T cells in peritoneal space. J Immunother, 2012. 35(1): p. 1–13. [DOI] [PubMed] [Google Scholar]
- 36.Liu P, et al. , Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J Immunother, 2010. 33(1): p. 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berd D, Maguire HC Jr., and Mastrangelo MJ, Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res, 1984. 44(11): p. 5439–43. [PubMed] [Google Scholar]
- 38.Helmink BA., et al. , B cells and tertiary lymphoid structures promote immunotherapy response. Nature, 2020. 577(7791): p. 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinkevicius KW, et al. , Neurotrophin receptor TrkB promotes lung adenocarcinoma metastasis. Proc Natl Acad Sci U S A, 2014. 111(28): p. 10299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.