Abstract
Background:
Early life exposure to neurotoxicants and non-chemical psychosocial stressors can impede development of prefrontal cortical functions that promote behavioral regulation and thereby may predispose to adolescent risk-taking related behaviors (e.g., substance use or high-risk sexual activity). This is particularly concerning for communities exposed to multiple stressors.
Methods:
This study examined the relation of exposure to mixtures of chemical stressors, non-chemical psychosocial stressors, and other risk factors with neuropsychological correlates of risk-taking. Specifically, we assessed psychometric measures of both adverse behavioral regulation and adaptive attributes among adolescents (age ~ 15 years) in the New Bedford Cohort (NBC), a sociodemographically diverse cohort of 788 children born 1993–1998 to mothers residing near the New Bedford Harbor Superfund site. The NBC includes biomarkers of prenatal exposure to organochlorines and metals; sociodemographic, parental and home characteristics; and periodic neurodevelopmental assessments. We modelled exposure mixtures using multi-dimensional smooths within generalized additive models.
Results:
Children of younger mothers with lower IQ who were exposed prenatally to higher polychlorinated biphenyls and lead had poorer anger control. This pattern was not apparent for children of older mothers with higher IQs. Direction of associations between increased hyperactivity and prenatal levels of organochlorine mixtures differed by maternal age and depression symptoms. Higher cord blood Pb levels, in conjunction with poorer HOME scores, were associated with poorer self-esteem when mothers had fewer depression symptoms.
Conclusions:
Analyses suggest that prenatal chemical exposures and non-chemical factors interact to contribute to neuropsychological correlates of risk-taking behaviors in adolescence. By simultaneously considering multiple factors associated with adverse behavioral regulation, we identified potential high-risk combinations that reflect both chemical and psychosocial stressors amenable to intervention.
Keywords: Risk-taking behavior, Mixtures, Metals, Organochlorines, Prenatal exposures, Adolescents
1. Introduction
The prefrontal cortex and its associated inhibitory functions are not fully developed until well into early adulthood. Consequently, impulsive and reward-driven areas of the brain may dominate in adolescents and young adults and predispose these age groups to engage in high risk/reward activities. Poor response inhibition and impulsive behaviors on psychometric testing have been associated with health risk behaviors such as high-risk sexual activity and binge drinking (Nydegger et al., 2014; Betancourt et al., 2012; Watson et al., 2014). Similarly, teens with externalizing behavior (e.g., aggression, hyperactivity) have increased prevalence of health-risk behaviors (Ferdinand and Verhulst, 1994; Sharma et al., 2014; Skinner et al., 2015).
In adolescence, risk-taking is multifactorial, and differs by sex, family sociodemographics, peer behavior, parental behavior, and community factors including neighborhood crime and safety (Wiehe et al., 2013; Prendergast et al., 2019). In addition, exposure to neurotoxicants that impede development of the prefrontal cortex may contribute to risk-taking and its neuropsychological correlates, including diminished executive functions such as impulse control and reward response-related behaviors. Lead (Pb) is probably the most studied example, with early life and childhood exposures associated with increased risk of violence, aggression and delinquency (Needleman et al., 2002, 1996; Nkomo et al., 2017).
While there is limited research assessing specific risk-taking related behaviors and chemicals other than Pb, there is evidence linking other neurotoxic metals and polychlorinated biphenyls (PCBs) with key neuropsychological correlates of risk-taking (Sable and Schantz, 2006). Biomarkers of prenatal PCB exposure have been associated with impaired response inhibition in school-aged children born to Great Lakes fish consumers (Jacobson and Jacobson, 2003; Stewart et al., 2005, 2006) and hyperactive/impulsive behavior in our study cohort at age 8 (Sagiv et al., 2010). Similarly, prenatal methylmercury (MeHg) exposure has been associated with impaired response inhibition at school age (Stewart et al., 2006).
Adaptive individual attributes (e.g., self-esteem) may protect against behavior related to risk-taking but may also suffer adverse impacts from exposure to chemicals or non-chemical psychosocial factors (Zimmerman, 2013). Studies of adaptive factors among adolescents have shown that high levels of self-esteem (Rivers et al., 2013) and academic success (Vagi et al., 2013) are associated with strong parenting and positive home and community environments. Few studies have examined the effects of multiple prenatal exposures on development of subsequent adaptive skills that may protect against risk-taking related behaviors.
Thus, although there is mechanistic and epidemiologic support for hypothesized associations of some neurotoxicants with adverse behavioral regulation, to our knowledge, there are no prior published studies that have prospectively addressed the impact of multiple prevalent neurotoxicants on neuropsychological correlates of risk-taking among adolescents. The association with neurotoxicants is further complicated by the simultaneous role that non-chemical factors such as the quality of the child’s home environment, parenting, and contextual measures likely play in the targeted behaviors. Our objective was to address this gap in the existing literature by examining the relation of mixtures of simultaneous exposure to chemical stressors and non-chemical factors with neuropsychological correlates of risk-taking and adaptive (protective) attributes among adolescents participating in the New Bedford Cohort (NBC) study. Given that manifestations of risk-taking related behavior in adolescents can result in substantial social and medical costs, improved understanding of the multiple factors hypothesized to contribute to such behaviors is a public health priority (Conegundes et al., 2019; Sipsma et al., 2017; Schawo et al., 2017).
2. Methods
2.1. Study population
The NBC is a prospective birth cohort of 788 children born 1993–1998 to mothers residing in one of four Massachusetts towns (New Bedford, Acushnet, Fairhaven, Dartmouth) surrounding the PCB and metal contaminated New Bedford Harbor (NBH) Superfund site (Korrick et al., 2000; U.S. Environmental Protection Agency. New Bedford Harbor Cleanup, 2018). This study was originally designed to assess the relation of prenatal and early life chemical exposures with subsequent neurodevelopment. In addition to residence near the NBH, study eligibility criteria included maternal age 18 +years and fluency in English or Portuguese. Newborns (approximately equal numbers of boys and girls) were recruited at the time of birth from one of the main hospitals serving the New Bedford area (St. Luke’s Hospital) and, as study eligibility included a newborn exam (and vaginal delivery), they were generally healthy, low risk births. New Bedford is a diverse low-income city with approximately 95,000 residents, 23% of whom live below the poverty level (Census Bureau, 2018). In addition, the New Bedford area has among the highest rates of teen pregnancy (3.5 times the state average) (Massachusetts Department of Public Health (MADPH) Teen Births Massachusetts:, 2013) and admission to substance abuse treatment facilities (twice the state average) (Massachusetts Department of Public Health (MADPH) Southeast Massachusetts Regional Health Dialogue Presentation, 2007; Massachusetts Department of Public Health (MADPH) Substance Abuse Treatment Admissions Statistics for State and City Town, 2018) in Massachusetts; thus, it is a community for which risk-taking related behaviors have a substantial adverse impact.
2.2. Behavior Assessment
Children in the NBC have completed periodic in-person neurodevelopmental assessments since birth, including extensive assessments at approximately age 15 years conducted between 2008 and 2014. Eligibility criteria for inclusion in these adolescent evaluations included: residence in the study area for in-person testing, intact cognition (i.e., able to undergo standardized testing), available biomarkers of prenatal chemical exposure, and available contact information. Of the 788 newborns enrolled in the NBC, 660 (84%) met these eligibility criteria and 528 (80% of those eligible) participated in 15-year assessments. Eligible study families were sent letters of invitation followed by a phone call to ascertain interest. Those who did not respond to letters or phone calls were sent at least one additional recruitment letter.
The NBC is a largely neurotypical general population sample, so clinical manifestations of risk-taking behaviors (for example, substance use disorders or teen pregnancy) are relatively rare. As a result, this analysis relied on dimensional measures of behavioral skills that represent neuropsychological correlates of risk-taking. These measures have a number of advantages such as characterizing fundamental behavioral liabilities shared by many risk-taking behaviors as well as enhancing statistical power compared with clinical outcomes. Specifically, behavioral outcomes for this analysis were assessed using the Behavior Assessment System for Children, 2nd Edition, Self-Report of Personality (BASC-2 SRP) for adolescents (age 12–21 years), a standardized behavioral rating scale (Reynolds and Kamphaus, 2004). 525 of the 528 NBC participants assessed as adolescents completed a BASC-2 SRP paper questionnaire during their study in-person neurodevelopmental assessment. The adolescent BASC-2 SRP uses the frequency of self-reported behaviors on 176 questions to generate up to 27 different behavioral scales (or scores) that characterize a range of maladaptive and adaptive behavioral phenotypes and emotional functions. We studied a subset of BASC-2 SRP behavioral scales each of which integrated information from 7 to 17 questions, depending on the scale. These included maladaptive (anger control, hyperactivity, and sensation seeking) and adaptive (interpersonal relations, self-reliance, self-esteem, and ego strength) behaviors chosen, a priori, to represent measures potentially associated with risk-taking. A higher BASC-2 score for maladaptive behaviors indicates worse behavior whereas a higher score for adaptive behaviors indicates better adaptive skills. This instrument generally has excellent reliability; most study measures had coefficient alpha values from 0.70 and 0.85 and test–retest reliability estimates from 0.61 to 0.78. The BASC-2 SRP provides raw scores as well as age and sex adjusted T-scores standardized to a mean of 50 with a standard deviation of 10. Because of potential loss of information that may occur when raw scores are truncated to create T-scores, we used raw scores for our analyses and adjusted our models for the adolescents’ age and sex.
2.3. Assessment of chemical exposures and non-chemical factors
Umbilical cord serum samples were analyzed for 51 individual PCB congeners, dichlorodiphenyldichloroethylene (DDE), and hexachlorobenzene (HCB). In addition, cord blood Pb; maternal peripartum toenail metals (arsenic (As), selenium (Se), and Mn); and peripartum maternal total hair Hg levels were measured. NBC cord blood samples were collected at birth with one aliquot centrifuged for removal of the serum fraction and a second aliquot collected for whole blood metal analyses. Collection of these prenatal exposure biomarkers and chemical analysis methods are described elsewhere (Sagiv et al., 2010, 2012; Korrick et al., 2000) and include excellent reproducibility and sensitivity; detection limits and percentage of samples below the limit of detection (LOD) for the exposures included in our analyses are presented in Supplemental Table S1. Only 0.6 to 7.4% of biomarker values were below the LOD except for HCB (30.0% <LOD) and PCB congener 180 (16.8% <LOD). Because of insufficient volume, lipid content could not be measured in NBC cord serum, so organochlorine concentrations are expressed on a wet weight basis as ng/g serum. Cord whole blood Pb levels were measured using isotope dilution (ID) inductively coupled plasma-mass spectrometry (ICP-MS) with excellent sensitivity and precision (<5%). All of our analyses (including both descriptive statistics and exposure mixture models) used instrumental readings for organochlorine and metal values below the detection limit to optimize statistical power and avoid bias associated with censoring at the method detection limit.
Questionnaires, home visits (to assess the quality of the home environment and parenting), maternal depression symptoms, maternal IQ, and medical record reviews were used to ascertain non-chemical risk factors and covariate measurements (Sagiv et al., 2012; Shoaff et al., 2019). Specifically, maternal depression was measured using the Beck Depression Inventory, 2nd edition (BDI-II), where lower scores indicate fewer and less intense symptoms of depression (Beck et al., 1996). Maternal IQ was measured using the Kaufman Brief Intelligence Test (KBIT) (Kaufman and Kaufman, 1990). The quality of the home environment was measured using the Home Observation for Measurement of the Environment (HOME), which uses a combination of structured questions and examiner observation to assess the support and stimulation available to the child from their home environment including from exchanges with family and interactions with objects (Caldwell and Bradley, 1985). Higher scores are indicative of a better home environment and parenting; a lower score reflects a less optimal or poorer home environment.
2.4. Variable selection
We identified a priori potential predictors of behavior based on existing literature (Table 1). Prior to constructing mixture models, we first determined which variables were associated with each outcome using univariate linear models. Variables that resulted in greater than one standard deviation change in the outcome were retained for inclusion in the final adjusted mixture model for that outcome.
Table 1.
Characteristics of 525 New Bedford Cohort mother–child pairs (born 1993–1998) with child self-reported Behavior Assessment System for Children, 2nd Edition (BASC-2) behavioral assessments done at approximately age 15 years.
| Select Characteristics | n | % | Median | Mean (SD) |
|---|---|---|---|---|
| Child Characteristics | ||||
| Age at examination, years | 15.5 | 15.6 (0.7) | ||
| Sex | ||||
| Male | 250 | 47.6 | ||
| Female | 275 | 52.4 | ||
| Race/ethnicity (5 values missing) | ||||
| Non-Hispanic White | 360 | 68.6 | ||
| Non-Hispanic African American | 38 | 7.2 | ||
| Hispanic | 55 | 10.5 | ||
| Non-Hispanic Other | 67 | 12.8 | ||
| Ever breastfed study child (16 values missing) | ||||
| Yes | 261 | 49.7 | ||
| No | 248 | 47.2 | ||
| Hobel perinatal risk score (5 values missing) | 15.5 | 15.4 (10.6) | ||
| Birthweight, grams (5 values missing) | 3433 | 3434 (445) | ||
| Gestational age, weeks (6 values missing) | 39.8 | 39.8 (1.3) | ||
| Maternal Characteristics | ||||
| Age at child’s birth, years | 26.6 | 26.7 (5.3) | ||
| Marital status at child’s birth (14 values missing) | ||||
| Never married/divorced/widowed | 209 | 39.8 | ||
| Married | 302 | 57.5 | ||
| Education at child’s birth (16 values missing) | ||||
| <12th grade | 75 | 14.3 | ||
| High school graduation or higher | 434 | 82.7 | ||
| Smoking during pregnancy (44 values missing) | ||||
| Yes | 125 | 23.8 | ||
| No | 356 | 67.8 | ||
| Illicit drug use in year prior to birth (82 values missing) | ||||
| Yes | 56 | 10.7 | ||
| No | 387 | 73.7 | ||
| Foreign-born (76 values missing) | ||||
| Yes | 89 | 17.0 | ||
| No | 360 | 68.6 | ||
| Race/ethnicity (25 values missing) | ||||
| Non-Hispanic White | 395 | 75.2 | ||
| Non-Hispanic African American | 23 | 4.4 | ||
| Hispanic | 40 | 7.6 | ||
| Non-Hispanic Other | 42 | 8.0 | ||
| IQ (11 values missing) | 99.0 | 98.4 (10.4) | ||
| Depression (BDI-II score) at 15-yr exam (10 values missing) | 5.0 | 7.6 (8.8) | ||
| Other Household Characteristics | ||||
| Father’s education at child’s birth (23 values missing) | ||||
| <12th grade | 125 | 23.8 | ||
| High school graduation or higher | 377 | 71.8 | ||
| HOME score at 8-year exam (48 values missing) | 47 | 46 (5.2) | ||
| Annual household income at child’s birth (17 values missing) | ||||
| <$20,000 | 163 | 31.0 | ||
| ≥$20,000 | 345 | 65.7 | ||
| Chemical Exposure Measures | ||||
| Cord serum ∑PCB4 (ng/g, 14 missing values) | 0.19 | 0.26 (0.32) | ||
| Cord serum ρ,ρ’-DDE (ng/g, 14 missing values) | 0.31 | 0.53 (1.10) | ||
| Cord serum HCB (ng/g, 14 missing values) | 0.023 | 0.026 (0.024) | ||
| Cord blood Pb (μg/dL, 24 values missing) | 1.15 | 1.50 (1.38) | ||
| Peripartum maternal hair Hg (ng/g, 168 values missing) | 448 | 619 (577) | ||
| Peripartum maternal toenail As (μg/g 219 values missing) | 0.068 | 0.099 (0.107) | ||
| Peripartum maternal toenail Se (μg/g, 219 values missing) | 1.02 | 1.06 (0.23) | ||
| Peripartum maternal toenail Mn (μg/g, 219 values missing) | 0.28 | 0.43 (0.45) |
Abbreviations: As, arsenic; BDI-II, Beck Depression Inventory, 2nd edition; DDE, dichlorodiphenyldichloroethylene; HCB, hexachlorobenzene; Hg, mercury; HOME, Home Observation for Measurement of the Environment; Pb, lead; ∑PCB4, the sum of four prevalent polychlorinated biphenyl congeners 118, 138, 153, and 180; Mn, manganese; SD, standard deviation; Se, selenium.
Note: chemical biomarker values < LOD were assigned the instrumental concentration value.
We included child’s age and sex in all models to standardize the BASC-2 raw scores. For chemical exposures, log transformed cord serum measures of DDE, HCB, the sum of four prevalent PCB congeners (congeners 118, 138, 153, and 180, ΣPCB4), cord blood Pb, and maternal hair Hg were included in at least one of the final mixture models for each outcome; other metal biomarkers tested but not associated with any of the outcomes in the univariate linear models were maternal peripartum toenail Mn, Se, and As. Non-chemical factors included in at least one of the final mixture models were maternal characteristics (age at child’s birth, depression symptoms at the time of the child’s 15-year exam, IQ) and the HOME at the child’s 8- year exam. We also considered the following categorical variables: parental education, income, and marital status at child’s birth; child race/ethnicity, maternal race/ethnicity and if she was foreign-born; breast feeding of study child, maternal smoking during pregnancy and illicit drug use in the year prior to birth. Birth weight, gestational age, and a modified version of the Hobel perinatal risk score (Hobel et al., 1973) were also tested but not associated with any of the outcomes.
2.5. Statistical analyses
Semi-parametric additive mixture models were generated for each outcome using a multivariable loess smooth term for mixtures of continuous predictors in addition to the categorical variables within a generalized additive model (GAM) framework (Vieira et al., 2005; Webster et al., 2006). GAMs, like generalized linear models, are robust to different outcome distributions and have the advantage of simultaneously assessing multiple non-linear associations (Hastie and Tibshirani, 1990; Wood, 2006). The resulting models were mapped by predicting outcomes for varying combinations of two variables in the mixture on the X and Y axes, while holding any other variables in the multi-dimensional mixture smooth constant. To reduce the impact of edge effects in the smooth, chemical exposure concentrations and maternal depression symptoms were predicted from the 5th to 95th percentile of the data distribution due to data sparseness (Vieira et al., 2017). Other variables were predicted at the value (minimum or maximum for continuous data) or category that resulted in worse adverse behavior scores or better adaptive individual attribute scores. Only children with complete data were included in each analysis. Analyses for each outcome were also stratified by sex. We used the R package MapGAM (Vieira et al., 2018) to run the spatial analyses and create the maps (R software, version 3.4.4). We calculated a global - P value for the significance of the mixture smooth term in the models via permutation tests using the deviance statistics for the permuted models with and without the mixture smooth term (Wood, 2006; Vieira et al., 2017). The institutional review boards of the University of California at Irvine (2017–3834), Boston University Medical Campus (H-36787), and Brigham and Women’s Hospital (2008P001663/BWH) approved the research. Written parental informed consent and adolescent assent were obtained before study evaluations.
3. Results
A total of 525 NBC participants completed the BASC-2-SRP behavioral rating scale at a median age of 15.5 years (range 14 to <18 years). Select characteristics of the children are presented in Table 1 and demonstrate sociodemographic diversity consistent with the New Bedford area. Distributions of the adverse and adaptive behaviors are summarized in Table 2. Children with missing data (Table 1) were excluded from the analyses; thus, the number of participants in each outcome analysis varies depending on the covariates in the model. We also present characteristics of the children stratified by sex (Supplemental Table S2). The effect estimates and 95% confidence intervals for the association between categorical covariates included in the final adjusted mixture models and the outcomes are presented in Tables 3 and 4, respectively. For analysis of anger control, four chemical exposures and four continuous non-chemical factors were identified as potentially predictive and included in two different models given limitations in the number of covariates that can be included as multivariable smoothed terms in GAMs: log cord blood Pb, log cord serum ΣPCB4, maternal age at child’s birth, and maternal IQ (Model 1, p =0.047) and log cord serum HCB, log maternal hair Hg, BDI-II score, and HOME score (Model 2, p < 0.001). Continuous chemical exposures and non-chemical factors were distributed between the two models so that correlations among the measures in each smooth were minimized.
Table 2.
Distributions of self-reported adverse behavior and adaptive attribute scores on the Behavior Assessment System for Children, 2nd Edition (BASC-2) for New Bedford Cohort adolescents.
| Self-reported outcome | n | Raw scores |
t-scores |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | Mean | SD | Median | Range | ||
| Anger control | 405, 313 | 9.7 | 6.9 | 8 | 0–36 | 49.0 | 11.1 | 46 | 33–89 |
| Hyperactivity | 428 | 5.8 | 3.6 | 5 | 0–21 | 50.1 | 11.0 | 48 | 33–90 |
| Sensation seeking | 436 | 10.9 | 4.5 | 11 | 1–24 | 48.4 | 10.4 | 48 | 26–79 |
| Self-esteem | 389 | 15.1 | 4.5 | 17 | 1–20 | 50.2 | 11.0 | 55 | 15–62 |
| Ego strength | 383 | 33.3 | 7.7 | 36 | 7–43 | 49.0 | 10.7 | 52 | 12–63 |
| Self-reliance | 400 | 15.0 | 3.6 | 15 | 3–22 | 50.3 | 10.2 | 50 | 16–70 |
| Interpersonal relations | 400 | 16.3 | 2.8 | 17 | 2–19 | 53.2 | 8.7 | 55 | 10–62 |
Note: Different n’s reflect the complete case data available for each outcome specific multivariable model. Anger control n’s correspond to Models 1 and 2, respectively.
SD - standard deviation.
Table 3.
Effect estimates and 95% confidence intervals for parametric covariates in final adjusted models of mixtures and neuropsychological correlates of risk-taking related behaviors assessed using the Behavior Assessment System for Children, 2nd Edition (BASC-2) in New Bedford Cohort adolescents.
| Anger control (1)1 | Anger control (2)2 | Hyperactivity3 | Sensation seeking4 | |
|---|---|---|---|---|
| Child’s age at examination, years | −0.26 (−1.32, 0.81) | −0.18 (−1.28, 0.92) | −0.46 (−0.99, 0.08) | 0.30 (−0.35, 0.95) |
| Child’s sex – Male | −0.77 (−2.10, 0.55) | 0.15 (−1.26, 1.56) | 0.46 (−0.22, 1.13) | 1.78 (0.95, 2.61) |
| Mother’s education at child’s birth < High School | −0.24 (−2.41, 1.93) | −0.37 (−2.89, 2.14) | 0.20 (−0.84, 1.24) | NI5 |
| Father’s education at child’s birth < High School | 0.36 (−1.33, 2.05) | 0.88 (−0.89, 2.65) | 0.95 (0.10,1.80) | 0.69 (−0.35, 1.73) |
| Mother unmarried at child’s birth | −1.16 (−3.01, 0.69) | 0.21 (−1.73, 2.15) | −0.70 (−1.62, 0.23) | NI |
| Annual household income at child’s birth <$20,000 | 1.53 (−0.24, 3.30) | 0.55 (−1.41, 2.50) | 0.25 (−0.65, 1.14) | NI |
| Mother’s race/ethnicity6 | ||||
| Non–Hispanic African American | −2.82 (−7.09, 1.45) | −4.29 (−9.14, 0.56) | −2.19 (−4.36, −0.02) | −1.98 (−4.25, 0.29) |
| Hispanic | −1.20 (−5.05, 2.65) | −1.05 (−5.05, 2.95) | −0.14 (−2.07, 1.79) | −0.57 (−2.28, 1.14) |
| Non-Hispanic Other | −1.41 (−4.35, 1.54) | −2.57 (−5.85, 0.72) | −1.49 (−3.00, 0.01) | −1.21 (−2.66, 0.24) |
| Child’s race/ethnicity6 | NI | |||
| Non-Hispanic African American | 2.76 (−0.65, 6.17) | 3.12 (−0.58, 6.83) | 0.57 (−1.14, 2.28) | |
| Hispanic | 1.22 (−2.25, 4.69) | 1.66 (−1.99, 5.31) | 0.73 (−1.02, 2.49) | |
| Non-Hispanic Other | 0.66 (−1.82, 3.14) | −0.68 (−3.50, 2.15) | 0.06 (−1.18, 1.31) | |
| Mother was foreign-born | 1.20 (−0.70, 3.10) | 1.78 (−0.17, 3.72) | NI | NI |
| Mother smoked during pregnancy | 1.83 (0.20, 3.46) | 1.14 (−0.54, 2.82) | 0.40 (−0.41, 1.22) | NI |
| Mother used illicit drug(s) in year prior to birth | 0.98 (−1.23, 3.20) | 1.62 (−0.69, 3.93) | 0.58 (−0.53, 1.69) | 0.91 (−0.39, 2.21) |
| Mother ever breastfed study child | −0.21 (−1.62, 1.19) | −1.00 (−2.48, 0.48) | NI | −0.49 (−1.36, 0.37) |
Model smooth term includes: log cord serum ∑PCB4, log cord blood Pb, maternal age at child’s birth and maternal IQ.
Model smooth term includes: log cord serum HCB, log maternal hair Hg, maternal depression symptoms (BDI-II), and HOME score.
Model smooth term includes: log cord serum ∑PCB4, DDE and HCB; maternal age at child’s birth, and maternal depression symptoms (BDI-II).
Model smooth term includes: maternal age at child’s birth and maternal IQ.
NI indicates variable that was not included in the final adjusted mixtures model for that outcome.
Reference = Non-Hispanic white.
Table 4.
Effect estimates and 95% confidence intervals for parametric covariates in final adjusted models of mixtures and adaptive attributes assessed using the Behavior Assessment System for Children, 2nd Edition (BASC-2) in New Bedford Cohort adolescents.
| Self–esteem1 | Ego strength2 | Self–reliance3 | Interpersonal Relations4 | |
|---|---|---|---|---|
| Child’s age at examination, years | 0.34 (−0.33, 1.01) | 1.06 (−0.11, 2.24) | 0.69 (0.13, 1.25) | 0.22 (−0.21, 0.65) |
| Child’s sex - Male | 2.04 (1.20, 2.87) | 1.67 (0.24, 3.10) | −0.27 (−0.96, 0.42) | −0.23 (−0.77, 0.30) |
| Mother’s education at child’s birth < High School | NI5 | 0.64 (−1.90, 3.18) | −0.40 (−1.57, 0.78) | NI |
| Father’s education at child’s birth < High School | NI | 0.17 (−1.66, 2.01) | −0.06 (−0.94, 0.82) | −0.35 (−1.03, 0.33) |
| Mother unmarried at child’s birth | NI | 0.43 (−1.61, 2.48) | −0.09 (−1.07, 0.90) | −0.06 (−0.82, 0.70) |
| Annual household income at child’s birth <$20,000 | NI | −0.11 (−2.11, 1.88) | −0.002 (−0.96, 0.96) | −0.24 (−0.98, 0.50) |
| Mother’s race/ethnicity6 | NI | |||
| Non-Hispanic African American | 4.32 (−0.37, 9.00) | 1.33 (−0.94, 3.60) | 1.49 (−0.27, 3.25) | |
| Hispanic | 0.39 (−3.72, 4.50) | 0.25 (−1.73, 2.24) | 0.91 (−0.63, 2.45) | |
| Non-Hispanic Other | 2.50 (−0.71, 5.71) | 0.93 (−0.63, 2.49) | 1.03 (−0.18, 2.25) | |
| Child’s race/ethnicity6 | NI | |||
| Non-Hispanic African American | −1.49 (−5.19, 2.21) | −0.50 (−2.31, 1.31) | 0.04 (−1.37,1.45) | |
| Hispanic | −1.80 (−5.64, 2.05) | −1.02 (−2.84, 0.81) | 0.11 (−1.30, 1.53) | |
| Non-Hispanic Other | −2.72 (−5.41, −0.03) | −1.41 (−2.69, −0.13) | −0.58 (−1.58, 0.42) | |
| Mother was foreign-born | NI | −1.51 (−3.52, 0.49) | −0.40 (−1.32, 0.53) | 0.26 (−0.44, 0.95) |
| Mother smoked during pregnancy | NI | −1.59 (−3.39, 0.21) | −0.20 (−1.05, 0.64) | −0.40 (−1.05, 0.25) |
| Mother used illicit drug(s) in year prior to birth | −1.27 (−2.53, 0.00) | −2.33 (−4.71, 0.05) | −0.84 (−1.99, 0.30) | −0.36 (−1.24, 0.53) |
| Mother ever breastfed study child | NI | 0.17 (−1.35, 1.69) | NI | NI |
Model smooth term includes: log cord blood Pb, maternal depression symptoms (BDI-II), HOME score.
Model smooth term includes: log cord blood Pb, maternal age at child’s birth, maternal depression symptoms (BDI-II), maternal IQ, and HOME score.
Model smooth term includes: maternal age at child’s birth, maternal depression symptoms (BDI-II), and HOME score.
Model smooth term includes: maternal age at child’s birth, maternal depression symptoms (BDI-II) and HOME score.
NI indicates variables were not included in the final adjusted mixtures model for that outcome.
Reference = Non-Hispanic white.
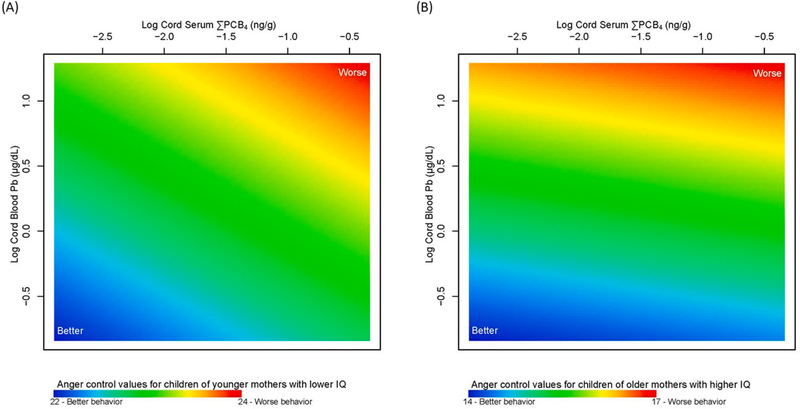
We compared anger scores from Model 1 for varying levels of cord serum ΣPCB4 and cord blood Pb predicted at maternal age of 18 years and IQ score of 57 (Fig. 1A) and maternal age of 40 and IQ score of 124 (Fig. 1B). Results suggest that children of younger mothers with lower IQ who were also exposed to higher levels of cord serum ΣPCB4 and cord blood Pb had worse (higher scores) anger control (Fig. 1A). This pattern was attenuated for children of older mothers with higher IQ, with higher Pb exposure largely determining worse anger control (Fig. 1B). These results highlight the potential importance of non-chemical factors (lower maternal age at the child’s birth and lower maternal IQ) in enhancing the adverse impact of multiple chemical exposures on anger control. The range of predicted anger control scores for Model 1 was 14–24, so an increase from 22 to 24 (Fig. 1A) represents a 20% increase in worse anger control (Supplementary Figure S1; Supplemental Table S3). In sex-stratified analyses, boys had consistently higher scores (worse anger control) compared to girls (Supplementary Figure S2). While worse anger control among boys was associated with exposure to higher levels of cord serum ΣPCB4, worse anger control among girls was associated with exposure to higher cord blood Pb but at lower levels of cord serum ΣPCB4. However, neither association was statistically significant (p =0.33 for boys and p =0.20 for girls).
Fig. 1. Association between adolescent self-reported anger control and mixture of log cord serum ΣPCB4 (ng/g), log cord blood Pb (μg/dL), maternal age at child’s birth and maternal IQ in the New Bedford Cohort (anger control Model 1).
Anger control scores are presented for varying log cord serum ΣPCB4 and log cord blood Pb levels predicted at (A) low maternal age (18 years) and low maternal IQ of 57 and (B) high maternal age (40 years) and high maternal IQ of 124. Higher anger control values (red) are indicative of worse behavior and lower values (blue) are indicative of better behavior.
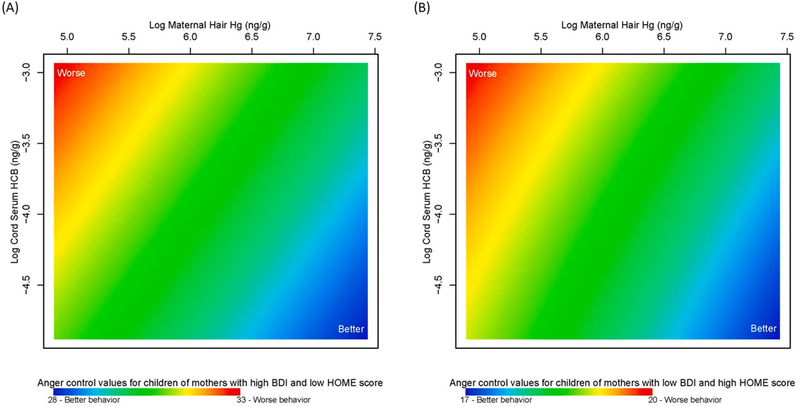
We examined the relationship between anger control and prenatal maternal hair Hg and cord serum HCB levels (Model 2) predicted at a BDI-II score of 47 and HOME score of 28 (Fig. 2A) and BDI-II score of 0 and HOME score of 57 (Fig. 2B). We observed that higher HCB levels and lower Hg levels were associated with worse anger control for children born to mothers with more depression symptoms and poorer HOME scores (Fig. 2A). The relationship was similar for children born to mothers with no depression symptoms and better HOME scores, however anger control values were not as high (range of 17–20 compared to 28–33; Fig. 2B). Overall, the range of predicted anger control scores for Model 2 was 14–33 and more maternal depression symptoms (higher BDI-II) consistently were associated with worse anger control (Supplementary Figure S3; Supplemental Table S4). Again, boys had higher scores (worse anger control) compared to girls (Supplementary Figure S4). However, for girls born to mothers with more depression symptoms and poorer HOME scores, worse anger control was associated with higher HCB levels and higher Hg levels, not lower Hg levels, as was observed in overall and male-stratified analyses. Also of note, better anger control was associated with higher HCB levels among girls born to mothers with no depression symptoms and better HOME scores (Supplementary Figure S4B3; p =0.009 for boys and p =0.010 for girls).
Fig. 2. Association between adolescent self-reported anger control and mixture of log maternal hair Hg (ng/g), log cord serum HCB (ng/g), maternal depression symptoms (BDI-II) and home environment quality (HOME score) in the New Bedford Cohort (anger control Model 2).
Anger control values are presented for varying log maternal hair Hg levels and log cord serum HCB predicted at (A) high BDI-II score of 47 (more depression symptoms) and low HOME score of 28 (poor home environment) and (B) low BDI-II score of zero (no depression symptoms) and high HOME score of 57 (good home environment). Higher anger control values (red) are indicative of worse behavior and lower values (blue) are indicative of better behavior.
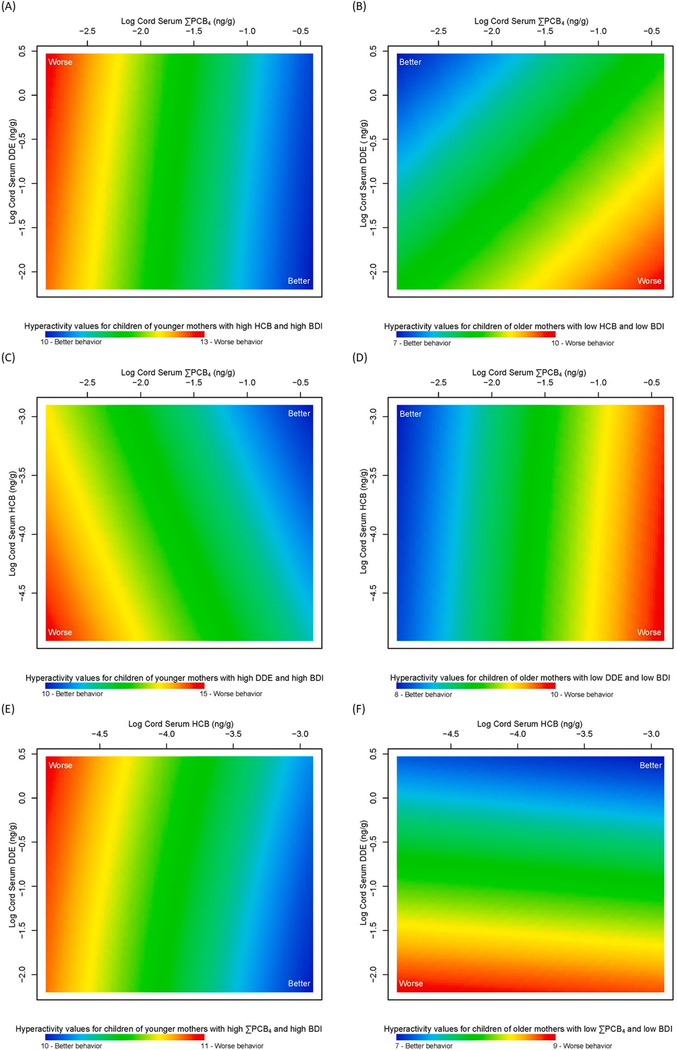
The final mixture model for hyperactivity included log cord serum organochlorine levels for ΣPCB4, DDE, and HCB; maternal age at the child’s birth; and maternal depression symptoms (p < 0.001). When predicted for children with higher HCB levels born to younger mothers (18 years of age) with more depression symptoms (BDI-II score of 47), hyperactivity was worse at lower ΣPCB4 levels across all DDE levels (Fig. 3A). However, when predicted for children with lower HCB levels born to older mothers (40 years of age) with no depression symptoms, adverse associations were seen with higher ΣPCB4 and lower DDE levels (Fig. 3B). Worse hyperactivity was also observed for children with higher ΣPCB4 exposure across all HCB levels (Fig. 3D compared to Fig. 3C) born to older mothers with lower DDE levels and no depression symptoms. The pattern for hyperactivity also differed when HCB and DDE levels were allowed to vary (Fig. 3E and 3F) depending on the mother’s age and depression symptoms. Overall, the range of predicted hyperactivity scores was 7–15; hyperactivity was worse for children with higher ΣPCB4 levels born to younger mothers with no depression symptoms (Supplementary Figure S5A) or to older mothers with more depression symptoms (Supplementary Figure S5B; Supplemental Table S5), but not for children born to younger mothers with more depression symptoms. Results of sex-stratified analyses for males were generally similar to the overall analyses (p = 0.002). Patterns of associations with ΣPCB4, DDE, and HCB differed for females compared to overall analyses, regardless of maternal characteristics (Supplementary Figure S6), but were not statistically significant (p =0.18). Hyperactivity was consistently worse among girls exposed to higher HCB and DDE levels, but lower ΣPCB4, and unlike anger control, hyperactivity scores were similar for girls and boys. Sensation seeking was not associated with any chemical exposures; the final mixture model of non-chemical factors included maternal age and IQ. Although sensation seeking was worse with lower maternal age and IQ, the mixture term was not statistically significant (Supplementary Figure S7, Supplemental Table S6; p =0.40).
Fig. 3. Association between adolescent self-reported hyperactivity and mixture of log cord serum ΣPCB4 (ng/g), DDE (ng/g), and HCB (ng/g), maternal age at child’s birth and maternal depression symptoms (BDI-II) in the New Bedford Cohort.
Hyperactivity values are presented for varying log cord serum ΣPCB4 and DDE levels predicted at (A) low maternal age (18 years), high log cord serum HCB (−2.9 ng/g), and high BDI-II score of 47 (more depression symptoms) and (B) high maternal age (40 years), low log cord serum HCB (−4.9 ng/g), and low BDI-II score of 0 (no depression symptoms). Hyperactivity values are also presented for varying log cord serum ΣPCB4 and HCB levels predicted at (C) low maternal age (18 years), high log cord serum DDE (0.5 ng/g), and high BDI-II score of 47 (more depression symptoms) and (D) high maternal age (40 years), low log cord serum DDE (−2.2 ng/g), and low BDI-II score of 0 (no depression symptoms) and for varying log cord serum HCB and DDE levels predicted at (E) low maternal age (18 years), high log cord serum ΣPCB4 (0.4 ng/g), and high BDI-II score of 47 (more depression symptoms) and (F) high maternal age (40 years), low log cord serum ΣPCB4 (−2.9 ng/g), and low BDI-II score of 0 (no depression symptoms). Higher hyperactivity values (red) are indicative of worse behavior and lower values (blue) are indicative of better behavior.
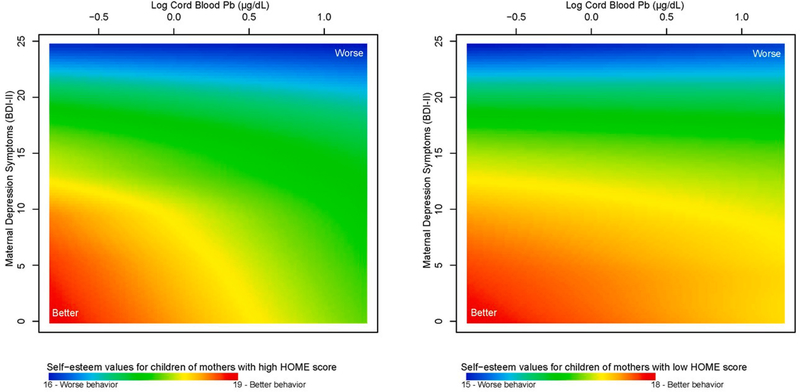
We also examined adaptive individual attributes (self-esteem, ego strength, self-reliance and interpersonal relations), with higher scores being indicative of better adaptive skill. Chemical exposures were not associated with self-reliance (Supplementary Figure S8; Supplemental Table S7) or interpersonal relations (Supplementary Figure S9; Supplemental Table S8); these behavioral phenotypes were associated with maternal age at birth, HOME score, and maternal depression symptoms. For self-esteem, the mixture model included a multivariable smooth of log cord blood Pb, maternal BDI-II, and HOME score. Fig. 4 presents self-esteem scores across varying log cord blood Pb levels and BDI-II, predicted for better and worse home environments (HOME scores of 57 and 28, respectively; p < 0.001). Children of mothers with fewer depression symptoms, lower cord blood Pb and better HOME scores had the highest self-esteem (maximum value 19, Fig. 4A). For children of mothers with poorer HOME scores, self-esteem did not vary appreciably across cord blood Pb levels (Fig. 4B). Self-esteem was consistently lowest (minimum value 7, Supplementary Figure S10A; Supplemental Table S9) among children of mothers with more depression symptoms and poorer HOME scores. Higher cord blood Pb levels, in conjunction with poorer HOME score, were associated with poorer self-esteem when mothers had fewer depression symptoms (Supplementary Figure S10B; Supplemental Table S9). Results from sex-stratified analyses were generally similar to results from models of all children (Supplementary Figure S11; p =0.006 for boys and p < 0.001 for girls). Maximum scores (better, more adaptive behavior) were lower for girls with poorer HOME scores compared to boys in similar environments. However, unlike boys whose self-esteem did not vary appreciably across cord blood Pb levels and had better self-esteem when maternal depression symptoms were low (Supplementary Figure S11B2), girls had worse scores at low maternal depression symptoms if cord blood Pb levels were high (Supplementary Figure S11B3).
Fig. 4. Association between adolescent self-reported self-esteem and mixture of log cord blood Pb (μg/dL), maternal depression symptoms (BDI-II), and home environment quality (HOME score) in the New Bedford Cohort.
Self-esteem values are presented for varying log cord blood Pb levels and maternal BDI-II (a high BDI-II is indicative of more depression symptoms) predicted for (A) a good home environment (high HOME score of 57) and (B) a poor home environment (low HOME score of 28). Higher self-esteem values (red) are indicative of better behavior and lower values (blue) are indicative of worse behavior.
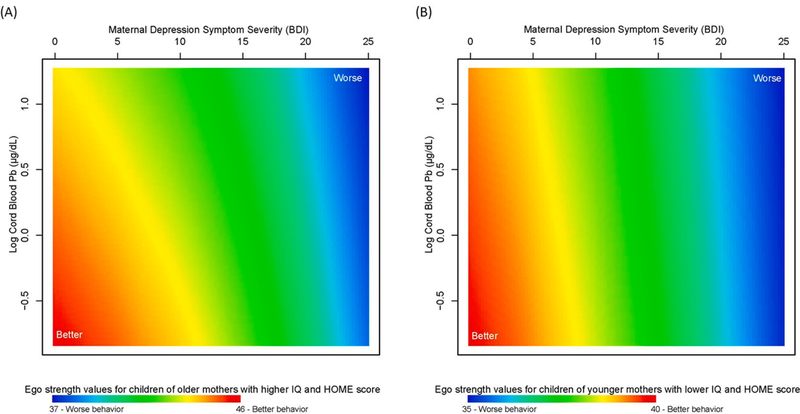
The final model for ego strength included a multivariable smooth of BDI-II, log cord blood Pb, maternal age at child’s birth, maternal IQ, and HOME score (p < 0.001). Ego strength scores improved with fewer maternal depression symptoms and lower cord blood Pb levels for children of older mothers (40 years of age) with higher IQ of 124 and a better HOME score of 57 (Fig. 5A) but did not vary with Pb levels for children of younger mothers (18 years of age) with lower IQ of 57 and a poorer HOME score of 28 (Fig. 5B). This pattern of associations was also true for Pb levels with varying HOME scores (Supplementary Figure S12). The greatest predicted ego strength (maximum value 48) was among children of older mothers with higher IQ, fewer depression symptoms, lower Pb levels, and better HOME scores (Supplementary Figure S12C; Supplemental Table S10); the worst ego strength (minimum value 29) was also among children of older mothers with higher IQ, but with more depression symptoms, higher Pb levels, and poorer HOME scores (Supplementary Figure S8D; Supplemental Table S10). Results were similar for sex-stratified analyses (Supplementary Figure S13; p < 0.001 for boys and p < 0.001 for girls), although girls born to younger mothers with lower IQ and poorer home scores had lower ego strength scores than boys (maximum of 37 for girls compared to 40 for boys).
Fig. 5. Association between adolescent self-reported ego strength and mixture of maternal depression symptoms (BDI-II), log cord blood Pb (μg/dL), maternal age at child’s birth, home environment quality (HOME score), and maternal IQ in the New Bedford Cohort.
Ego strength values are presented for varying maternal BDI-II (a high BDI-II is indicative of more depression symptoms) and log cord blood Pb levels predicted at (A) high maternal age (40 years), high HOME score of 57 (good home environment), and high maternal IQ of 124 and (B) low maternal age (18 years), low HOME score of 28 (poor home environment), and low maternal IQ of 57. Higher ego strength values (red) are indicative of better behavior and lower values (blue) are indicative of worse behavior.
4. Discussion
In this study, we examined the relationship of adolescent self-reported adverse behavior and adaptive attributes with mixtures of chemical exposures, non-chemical psychosocial stressors, and other non-chemical factors. This is one of the few (if not the only) studies to examine the association of simultaneous exposure to multiple chemical and non-chemical factors with adverse behaviors reflecting neuropsychological correlates of adolescent risk-taking, as well as with adaptive skills that may protect against risk-taking. Previous studies have primarily examined exposure to individual chemicals; e.g., prenatal exposures to PCBs, Pb and Hg have each been associated with impairment of impulse control prior to adolescence (Cory-Slechta et al., 2002; Stewart et al., 2005, 2006). Pb has also been associated with long-term high-risk behaviors, including delinquency during adolescence (Needleman et al., 1990; Needleman, 2009). In a recent study, prenatal exposure to PCB congener 66 was found to be associated with smoking and drinking among adolescents (Dickerson et al., 2019). To address this gap in the literature, we used state-of-the-art methods to characterize the complex relationship of real-world exposure mixtures with select behavioral phenotypes in adolescence, an age at which risk-taking behaviors often manifest.
Despite differences in our analytic approach and in the age at testing as well as specific outcomes measured in the current study compared to earlier studies, there is general agreement between our results and findings associating neuropsychological correlates of risk-taking behavior with prenatal PCB, Pb, and Hg exposures. However, the interpretation of the chemical exposure-outcome relationships observed in our mixture analyses is more complex than in individual exposure analyses, and the magnitude and direction of our associations often depended on the presence of non-chemical stressors in the mixture, which previous studies have not considered.
Although the directions of most associations were as expected, results indicated that the associations with chemical exposures differed by maternal and home characteristics. Two high-risk scenarios by which chemical exposures and non-chemical factors appeared to interact emerged from some of our results. In the first scenario, an adverse association of chemical exposure with behavior was only observed when non-chemical factors were optimized for adverse impact. In the second scenario, the presence of an apparently dominant risk factor for adverse behavior (e.g., maternal depression symptoms or a poor home environment) precluded detection of any additional adverse impacts from chemical exposures. Both scenarios by which the considered risk factors interacted highlight the importance of including non-chemical factors when examining the relationship of health measures with mixtures of chemical exposures (Padilla et al., 2013; Clougherty et al., 2018).
To illustrate the first high-risk combination, anger control was worse at higher levels of both cord serum ΣPCB4 and cord blood Pb, but this was only apparent for children of younger mothers with lower IQ. The second scenario suggests the potential for a “signal-to-noise ratio” problem wherein the presence of an apparently strong risk factor for a given behavior may make it difficult to ascertain any modest additional contribution from a more-weakly associated exposure. For example, the adverse association of cord serum ΣPCB4 with hyperactivity was only apparent for children of older mothers who had no depression symptoms. This pattern with maternal depression was also observed for cord blood Pb and self-esteem. However, the relationships of these behaviors with ΣPCB4 and Pb levels, respectively, were no longer apparent (or went in the opposite direction from what was expected) with greater maternal depression symptoms. Results also support the potential for the relative impact of chemical exposure mixtures compared to non-chemical risk factors to vary by behavior.
Other associations with chemical exposures and behavior outcomes were also opposite of what was expected. While the adverse association of cord serum HCB with anger control was consistent for different levels of non-chemical factors, the worst anger controls scores were observed at low levels of maternal hair Hg exposure. However, anger control skills declined with increasing maternal hair Hg for children in a good home environment with mothers who had high depression symptoms compared to mothers with no symptoms. For hyperactivity, we observed little impact on scores by varying cord serum HCB and there was an inverse association with cord serum DDE. One possible explanation for the apparent beneficial associations of DDE is potential confounding by maternal diet wherein healthful diets (for example diets high in fish and fresh produce) may result in higher DDE exposure levels (Davidson et al., 2008). In addition, HCB had a relatively high proportion of values less than the LOD (30%). Non-differential exposure measurement error for concentrations below the LOD may have contributed to a null bias in HCB analyses. Although in some cases only modest changes in predicted behavior scores were associated with differences in chemical and/or non-chemical mixture exposures, even small shifts in a population’s distribution of continuous behavioral health measures can have a disproportionate impact on the frequency of clinical disease in that population (for example, the frequency of clinical behavioral disorders) (Bellinger, 2004, 2012). Thus, these subtle shifts in scores represent increased risk of adverse behavior from exposure mixtures on a population-level, a risk that can be ameliorated with appropriate population-level mitigation efforts.
Better adaptive behavioral phenotypes were all associated with better home environments and fewer maternal depression symptoms, again emphasizing the importance of non-chemical factors on these health outcomes. Among the chemical exposures examined, only cord blood Pb was a significant predictor and only for self-esteem and ego-strength, wherein higher cord blood Pb levels were associated with poorer self-esteem and ego-strength in adolescence.
Given prior evidence that exposures may have differential impacts on boys and girls (Sagiv et al., 2012; Torres-Rojas and Jones, 2018; Winneke et al., 2014; Claus Henn et al., 2018), we conducted sex-stratified analyses for study outcomes associated with biomarkers of chemical exposure. Although these analyses were underpowered to detect sexual dimorphism in associations, associations with maladaptive behaviors among boys were generally similar to overall analyses, whereas associations in girls differed from the overall findings. Given the complexity of the exposure mixtures being considered, it is difficult to determine whether this apparent divergence of associations between boys and girls represents increased (or decreased) sensitivity to the adverse impacts of particular chemical or non-chemical exposures. One can only conclude that, for risk of maladaptive behavior, there may be differential response to exposure mixtures in boys compared to girls. Conversely, where adaptive behavior was assessed, most exposure mixture associations were similar in boys and girls.
The exact biological mechanisms by which individual metals and organochlorines cause neurobehavioral toxicity are uncertain. Furthermore, there are limited data assessing putative mechanisms by which chemical and non-chemical mixtures may impact behavior. However, a number of our targeted chemical exposures – e.g., PCBs, lead, MeHg – have been shown to interfere with monoamine neurotransmitters (e.g., dopamine) in the prefrontal cortex (PFC) and select subcortical nuclei which, in turn, can lead to impairment of impulse control and reward-response functions that underly some risk taking behaviors (Dreiem et al., 2010; Kern et al., 2010; Meyer et al., 2015; Perez-Fernandez et al., 2019; Seegal et al., 1990). In addition, both neurotoxic metals (e.g. lead) and early life stress exposures can enhance systemic glucocorticoid production which, in turn, alters brain dopaminergic functions in these same brain regions (Sobolewski et al., 2018). The potential for both chemical exposures and non-chemical factors to adversely impact PFC dopamine-related functions supports the biological plausibility of our observed interactions among chemical and non-chemical exposure mixtures in determining behavior.
While there are numerous approaches to identifying and analyzing mixtures (Braun et al., 2016; Huang et al., 2018; Kalloo et al., 2018; Kapraun et al., 2017), our application of generalized additive modeling has several strengths. GAMs allow for analyzing non-linear associations with mixtures by smoothing multiple stressors while simultaneously adjusting for other covariates. In addition, we are able to visualize the relationship between varying levels of two mixture components by mapping cross-sections of the results. Lastly, this approach is readily available via the user-friendly MapGAM package in R. Similar to other statistical approaches, GAMs are limited by sparseness of data in multiple dimensions, and there are limitations in the number of variables that can be incorporated as multivariable smooth terms, depending on sample size and the distributional properties of the smoothed variables. While our analyses can provide a different epidemiologic perspective for the interaction of chemical and non-chemical risk-factors, our approach does not inform whether mixture effects are additive, synergistic or antagonistic.
Our dataset included extensive prenatal chemical biomarkers and non-chemical risk factors collected through adolescence, but we did not have post-natal chemical exposure biomarkers. While it would have been interesting to examine effect modification by exposures occurring in childhood, the lack of these variables in our analysis does not impact the associations observed with prenatal exposures and behavioral outcomes. Exposures occurring after birth cannot affect prenatal exposures, indicating they are not confounders (Rothman et al., 2008). It is possible that post-natal exposures are also affected by a shared cause of prenatal exposures that is not explained by the other measured risk factors, and in this scenario, the impact on the associations observed is less clear.
Our study tested associations with several outcomes. With multiple comparisons, there is a concern that significant findings may be due to chance. Of the 18 total tests conducted, 14 (78%) were statistically significant, which exceeded the 5% type 1 error rate that we would have expected under the null hypothesis of no associations. In addition, because our BASC-2 measures reflect related maladaptive (or adaptive) behaviors, we expected relatively concordant findings across these multiple outcomes. Our generally concordant results mitigate against the likelihood that findings are the result of multiple comparisons.
We also had three variables with more than 10% missingness, which reduced the same size of our analyses. There is the potential for bias due to differences between participants included and excluded from these analyses. For our anger control analyses (Model 2), which had the most missingness, we see minimal differences in exposures and outcome between those with missing as compared to non-missing data, but participants excluded because of missing data were more likely to be racial minorities, to have parents with less education, and to have other characteristics reflecting socioeconomic disadvantage (Supplemental Table S11). We also note that there are some differences in characteristics of the 788 mother–child pairs recruited at time of birth compared to the 525 who remained in the study and participated in the self-reported behavioral assessments at approximately age 15 years. Consistent with predictors of study retention in prospective cohorts (Carter et al., 2012), those remaining in the study tended to have characteristics reflecting greater socioeconomic advantage (Supplemental Table S12). Given these differences, those remaining in the study tended to have characteristics reflecting greater socioeconomic advantage (Supplemental Table S12). Given these differences, there is the potential for selection bias from loss to follow-up and missing information, but the direction of this bias is unclear.
Another limitation of the study is the generalizability of our results to other populations. The relationship between the mixtures of interest and self-reported behaviors are for adolescents living in the New Bedford area who were born during the 1990 s. Associations may differ for other behavioral assessments (e.g., functional psychometric tests or teacher reported behavioral check lists), for adolescents living in other geographic areas, or for populations born in different time periods. Additional studies are needed that investigate mixtures of chemical exposures and non-chemical factors in other vulnerable populations.
In spite of these limitations, our analytic strategy allowed us to identify a broad set of potentially remediable risk factors for behaviors reflecting neuropsychological correlates of adolescents’ risk-taking. For example, in addition to environmental exposure mitigation, our findings substantiate the potential for family mental health supports to promote children’s healthy behavioral development, including promotion of resilience to chemical exposures. Given the potential for substantial morbidity in association with adolescents’ risk-taking related behavior, informing possible prevention strategies is a valuable endeavor. The complex and non-linear associations that we identified reinforced the importance of multi-stressor exposure characterization, coupled with methods for mixtures epidemiology like GAMs, to maximize the benefits of future interventions across all population groups.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42 ES007381, P42 ES005947, and R01 ES014864].
Abbreviations:
- As
arsenic
- BASC-2
Behavior Assessment System for Children, 2nd Edition
- BDI-II
Beck Depression Inventory, 2nd edition
- DDE
dichlorodiphenyldichloroethylene
- GAMs
Generalized Additive Models
- HCB
hexachlorobenzene
- Hg
mercury
- HOME
Home Observation for Measurement of the Environment
- Pb
lead
- ΣPCB4
the sum of four prevalent polychlorinated biphenyl congeners 118, 138, 153, and 180
- Mn
manganese
- SD
standard deviation
- Se
selenium
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106199.
References
- Beck A, Steer R, Brown G, 1996. Beck Depression Inventory-II. The Psychological Corporation, San Antonio. [Google Scholar]
- Bellinger DC, 2004. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ. Res 95 (3), 394–405. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, 2012. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ. Health Perspect 120 (4), 501–5076. [DOI] [PubMed] [Google Scholar]
- Betancourt LM, Brodsky NL, Brown CA, et al. , 2012. Is executive cognitive function associated with youth gambling? J. Gambl. Stud 28, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF, 2016. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ. Health Perspect 124, A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH, 1985. Home Observation for Measurement of the Environment. Dorsey, New York. [Google Scholar]
- Carter KN, Imlach-Gunasekara F, McKenzie SK, Blakely T, 2012. Differential loss of participants does not necessarily cause selection bias. Aust. N Z J. Publ. Health 36 (3), 218–222. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Austin C, Coull BA, Schnaas L, Gennings C, Horton MK, Hernández-Ávila M, Hu H, Téllez-Rojo MM, Wright RO, Arora M, 2018. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ. Res 161, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Levy JI, 2018. Psychosocial and Chemical Stressors In: Rider C, Simmons J (Eds.), Chemical Mixtures and Combined Chemical and Nonchemical Stressors. Springer, Cham. [Google Scholar]
- Conegundes L, Valente JY, Cogo-Moreira H, Martins CB, Andreoni S, Sanchez ZM, 2019. Transition from nonuse to use of alcohol or binge drinking among adolescents: Secondary analysis of a randomized controlled trial. Addict. Behav 102, 106159. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Brockel BJ, O’Mara DJ, 2002. Lead Exposure and Dorsomedial Striatum Mediation of Fixed Interval Schedule-Controlled Behavior. Neurotoxicology. 23, 313–327. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain J, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. , 2008. Neurodevelopmental Effects of Maternal Nutritional Status and Exposure to Methylmercury from Eating Fish during Pregnancy. Neurotoxicology. 29 (5), 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson AS, Ransome Y, Karlsson O, 2019. Human prenatal exposure to polychlorinated biphenyls (PCBs) and risk behaviors in adolescence. Environ. Int 129, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Okoniewski RJ, Brosch KO, Miller VM, Seegal RF, 2010. Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers Alter Striatal Dopamine Neurochemistry in Synaptosomes from Developing Rats in an Additive Manner. Toxicol. Sci 118 (1), 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand RF, Verhulst FC, 1994. The prediction of poor outcome in young adults: comparison of the Young Adult Self-Report, the General Health Questionnaire and the Symptom Checklist. Acta Psychiatr. Scand 89, 405–410. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ, 1990. Generalized Additive Models. Chapman & Hall Ltd, London, United Kingdom. [Google Scholar]
- Hobel CJ, Hyvarinen MA, Okada DM, Oh W, 1973. Prenatal and intrapartum high-risk screening. Am. J. Obstet. Gynecol 117, 1–9. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang A, Morello-Frosch R, et al. , 2018. Cumulative Risk and Impact Modeling on Environmental Chemical and Social Stressors. Curr. Environ. Health Rpt 5, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, 2003. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr 143, 780–788. [DOI] [PubMed] [Google Scholar]
- Kalloo G, Wellenius G, McCandless L, et al. , 2018. Profiles and Predictors of Environmental Chemical Mixture Exposure among Pregnant Women: The Health Outcomes and Measures of the Environment Study. Environ. Sci. Technol 52, 10104–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapraun DF, Wambaugh JF, Ring CL, Tornero-Velez R, Setzer RW, 2017. A method for identifying prevalent chemical combinations in the U.S. population. Environ. Health Persp 125 (087017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N, 1990. Brief intelligence test. American Guidance Service, Circle Pines, MN. [Google Scholar]
- Kern CH, Stanwood GD, Smith DR, 2010. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 64 (5), 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrick SA, Altshul LM, Tolbert PE, Burse VW, Needham LL, Monson RR, 2000. Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. J. Expo Anal. Environ. Epidemiol 10, 743–754. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health (MADPH) Southeast Massachusetts Regional Health Dialogue Presentation (2007) https://www.mass.gov/files/documents/2016/07/qa/southeast-region-present.ppt Accessed December 16, 2018.
- Massachusetts Department of Public Health (MADPH) Substance Abuse Treatment Admissions Statistics for State and City & Town, 2018. https://www.mass.gov/lists/substance-abuse-treatment-admissions-statistics Accessed December 16, 2018.
- Massachusetts Department of Public Health (MADPH) Teen Births Massachusetts: 2013. https://www.mass.gov/files/documents/2016/07/rn/teen-births-2013.pdf Accessed December 16, 2018.
- Meyer AE, Miller MM, Nelms Sprowles JL, Levine LR, Sable HJ, 2015. A comparison of presynaptic and postsynaptic dopaminergic agonists on inhibitory control performance in rats perinatally exposed to PCBs. Neurotoxicol. Teratol 50, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman H, 2009. Low Level Lead Exposure: History and Discovery. Ann Epidemiol 19, 235–238. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN, 1990. The long-term effects of exposure to low doses of lead in childhood. N. Engl. J. Med 322 (2), 83–88. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB, 1996. Bone lead levels and delinquent behavior. JAMA 275, 363–369. [PubMed] [Google Scholar]
- Needleman HL, McFarland C, Ness RB, Fienberg SE, Tobin MJ, 2002. Bone lead levels in adjudicated delinquents. A case control study. Neurotoxicol. Teratol 24, 711–717. [DOI] [PubMed] [Google Scholar]
- Nkomo P, Mathee A, Naicker N, Galpin J, Richter LM, Norris SA, 2017. The association between elevated blood lead levels and violent behavior during late adolescence: The South African Birth to Twenty Plus cohort. Environ. Int 109, 136–145. [DOI] [PubMed] [Google Scholar]
- Nydegger LA, Ames SL, Stacy AW, Grenard JL, 2014. Response inhibition moderates the association between drug use and risky sexual behavior. Subst. Use Misuse 49, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla CM, Deguen S, Lalloue B, et al. , 2013. Cluster analysis of social and environment inequalities of infant mortality. A spatial study in small areas revealed by local disease mapping in France. Sci. Total Environ 454–455, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fernandez C, Flores P, Sánchez-Santed F, 2019. A Systematic Review on the Influences of Neurotoxicological Xenobiotic Compounds on Inhibitory Control. Front. Behav. Neurosci 13, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast LE, Toumbourou JW, McMorris BJ, Catalano RF, 2019. Outcomes of Early Adolescent Sexual Behavior in Australia: Longitudinal Findings in Young Adulthood. J. Adolesc. Health 64, 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW, 2004. Behavior Assessment System for Children, second edition (BASC-2) 2004. AGS Publishing: Circle Pines, MN. [Google Scholar]
- Rivers SE, Brackett MA, Omori M, Sickler C, Bertoli MC, Salovey P, 2013. Emotion skills as a protective factor for risky behaviors among college students. J. Coll. Stud. Dev 54, 172–183. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL, 2008. Modern epidemiology. 3. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Sable HJK, Schantz SL, 2006. In: Levin ED, Buccafusco JJ (Eds.), Animal Models of Cognitive Impairment Frontiers in Neuroscience.
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA, 2010. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol 171, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Amarasiriwardena C, Korrick SA, 2012. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch. Pediatr. Adolesc. Med 166, 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawo S, Bouwmans C, van der Schee E, Hendriks V, Brouwer W, Hakkaart L, 2017. The search for relevant outcome measures for cost-utility analysis of systemic family interventions in adolescents with substance use disorder and delinquent behavior: a systematic literature review. Health Qual. Life Outcomes 15, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Shain W, 1990. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol. Appl. Pharmacol 106 (1), 136–144. [DOI] [PubMed] [Google Scholar]
- Sharma L, Markon KE, Clark LA, 2014. Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychol. Bull 140, 374–408. [DOI] [PubMed] [Google Scholar]
- Shoaff JR, Calafat AM, Schantz SL, Korrick SA, 2019. Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environ. Res 172, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipsma HL, Canavan M, Gilliam M, Bradley E, 2017. Impact of social service and public health spending on teenage birth rates across the USA: an ecological study. BMJ Open 7 (5), e013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner SR, Robinson M, Smith MA, et al. , 2015. Childhood behavior problems and age at first sexual intercourse: a prospective birth cohort study. Pediatrics 135, 255–263. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Conrad K, Marvin E, Allen JL, Cory-Slechta DA, 2018. Endocrine active metals, prenatal stress and enhanced neurobehavioral disruption. Horm. Behav 101, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, 2005. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol. Teratol 27, 771–780. [DOI] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, 2005. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol. Teratol 27, 771–780. [DOI] [PubMed] [Google Scholar]
- Stewart P, Sargent DM, Reihman J, Gump B, Lonky E, Darvill T, et al. , 2006. Response Inhibition During Differential Reinforcement of Low Rates (DRL) Schedules May Be Sensitive to Low-Level Polychlorinated Biphenyl, Methylmercury, and Lead Exposure in Children. Environ. Health Perspect 114 (12), 1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, et al. , 2006. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ. Health Perspect 114, 1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rojas C, Jones BC, 2018. Sex Differences in Neurotoxicogenetics. Front. Genet 9, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau, 2013–2017 American Community Survey 5-Year Estimates. https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml Accessed December 16, 2018.
- U.S. Environmental Protection Agency. New Bedford Harbor Cleanup. https://www.epa.gov/new-bedford-harbor/harbor-cleanup#Why Accessed December 16, 2018.
- Vagi KJ, Rothman EF, Latzman NE, Tharp AT, Hall DM, Breiding MJ, 2013. Beyond correlates: A review of risk and protective factors for adolescent dating violence perpetration. J. Youth Adolesc 42, 633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira VM, Fabian MP, Webster TF, Levy JI, Korrick SA, 2017. Spatial variability in ADHD-related behaviors among children born to mothers residing near the New Bedford Harbor Superfund Site. Am. J. Epidemiol 185, 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira VM, Bartell SM, MapGAM, Bliss RY, 2018. Mapping Smoothed Effect Estimates from Individual-Level Data (R package, version 1.2). R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Vieira V, Webster T, Weinberg J, Aschengrau A, Ozonoff D, 2005. Spatial analysis of lung, colorectal, and breast cancer on Cape Cod: an application of generalized additive models to case-control data. Environ. Health 14, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TD, Sweeney JF, Louis H, 2014. Neurocognitive, psychological and behavioral correlates of binge drinking and use of alcohol with caffeinated beverages in college-aged adults. Am. J. Drug Alcohol. Abuse 40, 58–66. [DOI] [PubMed] [Google Scholar]
- Webster T, Vieira V, Weinberg J, Aschengrau A, 2006. Method for mapping population-based case-control studies: an application using generalized additive models. Int. J. Health Geogr 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehe SE, Kwan MP, Wilson J, Fortenberry JD, 2013. Adolescent health-risk behavior and community disorder. PLoS ONE 8, e77667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G, Ranft U, Wittsiepe J, Kasper-Sonnenberg M, Fürst P, Krämer U, Seitner G, Wilhelm M, 2014. Behavioral sexual dimorphism in school-age children and early developmental exposure to dioxins and PCBs: a follow-up study of the Duisburg Cohort. Environ. Health Perspect 122 (3), 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN, 2006. Generalized Additive Models: An Introduction with R. Chapman & Hall Ltd, London, United Kingdom. [Google Scholar]
- Zimmerman MA, 2013. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ. Behav 40, 381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.