Abstract
The cave bear (Ursus spelaeus s.l.) was an iconic extinct bear that inhabited the Pleistocene of Eurasia. The cause of extinction of this species is unclear and to identify the actual factors, it is crucial to understand its feeding preferences. Here, we quantified the shape descriptor metrics in three-dimensional (3D) models of the upper teeth (P4–M2) of the cave bear to make inferences about its controversial feeding behaviour. We used comparative samples, including representatives of all living bear species with known diets, as a template. Our topographic analyses show that the complexity of upper tooth rows in living bears is more clearly associated with the mechanical properties of the items consumed than with the type of food. Cave bears exhibit intermediate values on topographic metrics compared with the bamboo-feeder giant panda (Ailuropoda melanoleuca) and specialists in hard mast consumption (Ursus arctos and Ursus thibetanus). The crown topography of cave bear upper teeth suggests that they could chew on tough vegetal resources of low quality with high efficiency, a characteristic that no living bear currently displays. Our results align with a climate-driven hypothesis to explain the extinction of cave bear populations during the Late Pleistocene.
Keywords: cave bears, dental topography, feeding behaviour, evolution
1. Introduction
The cave bear (Ursus spelaeus s.l.) is an iconic extinct bear, making up a part of the megafauna that inhabited the Pleistocene of Eurasia, whose extinction causes are controversial. One key aspect of understanding why these cave bears became extinct relates to their feeding behaviour [1]. Some authors propose that cave bears were adapted to feed exclusively on vegetal resources from 100 000 to 20 000 years ago [2], without evidence of a dietary shift towards omnivory at a time of lowered vegetation productivity during the Last Glacial Maximum [3]. Both the lack of dietary flexibility and possible human competition are proposed to be critical factors in explaining the extinction of cave bears [4,5]. Recently, Pérez-Ramos et al. [6] demonstrated that large paranasal sinuses were likely selected in cave bears to overcome longer hibernation periods, but the sinuses also compromised the skulls biomechanically. This biomechanical restriction in the skull of cave bears led to a lack of dietary flexibility typical of omnivorous bears. Cave bears exhibited a unique gradual increase in their tooth-root area values of maxillary teeth (P4–M2), the values of their most posterior molars approaching those of the bamboo-feeder giant panda (Ailuropoda melanoleuca) [7]. Despite this, the topography of maxillary tooth crown surfaces in cave bears has never been studied in detail. Therefore, the motivation of our study was to make inferences on cave bear feeding preferences by investigating the topography of their maxillary teeth [6].
Tooth shape correlates with feeding behaviour because it plays a key role in the mechanical breakdown of food and release of nutrients during chewing [8]. In recent years, the analysis of the three-dimensional (3D) surface of tooth crowns has allowed the quantification of shape descriptors of tooth crown topographies that are associated with masticatory performance and feeding behaviour in mammals [8–10].
Here, we apply a 3D dental topographic analysis to quantify the dental topographic curvature (Dirichlet normal energy, DNE), relief (surface relief index, RFI) and complexity (using orientation patch count rotated, OPCR) of tooth crown surfaces of maxillary postcanine series in living bears. Our main goal is to explore the relationship between these topographic shape descriptors and feeding behaviour. Finally, we use this information as a template to make dietary inferences in the cave bear (U. spelaeus s.l.) and to discuss possible extinction causes.
2. Material and methods
We sampled the maxillary tooth rows from P4 to M2 of 86 individuals, including a total of 258 teeth, belonging to extant and extinct bear species from different museum collections (see electronic supplementary material, table S1). Our sample includes all known extinct species and subspecies of cave bears (U. spelaeus s.l.) [11]: U. ingressus and U. spelaeus (U. spelaeus spelaeus, U. spelaeus eremus and U. spelaeus ladinicus) [12]. We used a comparative sample data of all living bear species: A. melanoleuca, Helarctos malayanus, Melursus ursinus, Tremarctos ornatus, U. americanus, U. maritimus, U. thibetanus and U. arctos. Tooth rows with cracked or chipped teeth were discarded, and only fully occluding unworn or lightly worn teeth were considered in the analyses.
High-resolution polyurethane dental replicas of each maxillary series (P4–M2) were produced from polyvinylsiloxane-base moulds and scanned using a Roland LPX-600 at 0.2 mm resolution following Figueirido et al. (2017) [13] (see electronic supplementary material). Subsequently, we measured a set of 3D-topographic shape metrics from triangulated polygonal mesh data for each tooth row using MorphoTester [9]: (i) DNE [14]; (ii) RFI [15] and (iii) complexity, using OPCR [16,17].
DNE quantifies the amount of bending across a surface, reflecting the relative surface curvature and undulation, irrespective of structure size or orientation [9,14]. Higher DNE values represent sharpened edges and troughs [14]. RFI was calculated as a simple ratio of the 3D crown surface area (3da) divided by the two-dimensional (2D) projected surface area (2da) [9,15], providing the relative tooth crown height [17,18]. Finally, OPCR was used to quantify complexity with a minimum patch size of five polygons [9]. OPCR was calculated by dividing the occlusal surface into contiguous patches of equal orientation (45° sectors). The number of patches was counted and averaged following eight successive rotations of 5.625° around the z-axis [9].
To explore the association of size with DNE, RFI and OPCR, we regressed these metrics against the 2da (mm2) of each tooth row (P4–M2) as a proxy for size (both log-transformed). We also computed phylogenetically independent contrast analyses [19] of DNE, RFI, OPCR and 2da using the R package GEIGER [20], and we regressed the contrasts for the three topographic shape metrics on the contrast for 2da.
We assembled a phylogenetic tree using Mesquite [21] to explore the phylogenetic patterns of DNE, RFI and OPCR. The relationships of living bears were taken from the previous study [22] based on ancient mitogenomes, and the phylogenetic relationships of cave bears were based on the previous phylogenetic analyses [23].
We calculated Pagel's lambda statistic for continuous traits [24] to assess the phylogenetic signal in DNE, RFI and OPCR, and the phylogenetic variation of the three variables was illustrated by traitgrams [25] using the R package Phytools [26].
To test the association between DNE, RFI, OPCR and feeding behaviour in living bears, we used previously established dietary groupings [7]. The eight species of living bears were classified into three broad dietary categories to facilitate the ecomorphological comparisons: (i) omnivores, feeding less than 50% soft mast; more than 15% hard mast (U. arctos and U. thibetanus); (ii) folivores-frugivores, feeding more than 50% soft mast and less than 15% hard mast (U. americanus and T. ornatus) and (iii) faunivores, feeding more than 50% of animal protein, either insects (H. malayanus and M. ursinus) or vertebrates (U. maritimus). The giant panda (A. melanoleuca) was assigned to an independent category [7] owing to its highly specialized diet based on bamboo [27]. We assessed the relationship of DNE, RFI, OPCR and feeding behaviour using a phylogenetic ANOVA [28] with GEIGER [20], and assuming Brownian motion. We used 1000 replicates to test for statistical significance. Finally, a principal component analysis (PCA) on the variance–covariance matrix of DNE, RFI and OPCR (converted to Z-scores) was performed with PAST 4.02 [29] to calculate the specific patterns from topographic metrics that account for most of the variability observed among dietary groupings.
3. Results
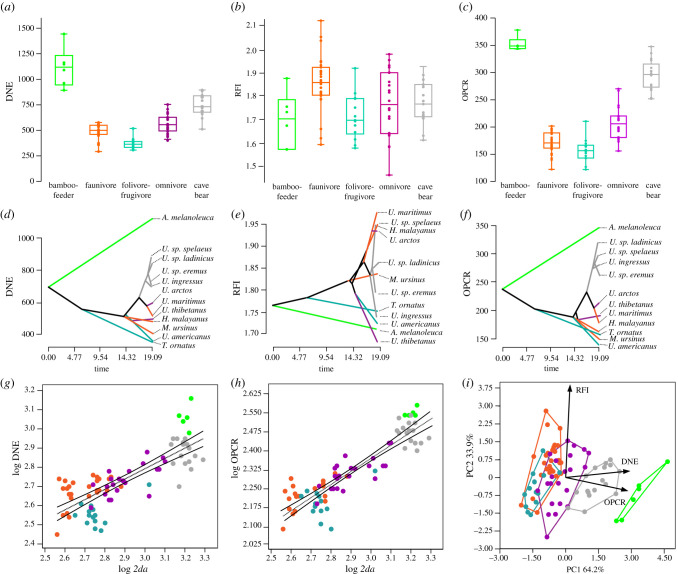
The giant panda (A. melanoleuca) showed the highest DNE values, followed by cave bears (figures 1a, 2a, table 1). The omnivorous bears exhibited intermediate DNE values, followed by the faunivores. The folivores-frugivores reached the lowest DNE values among the sample (figure 1a). The same trend among dietary groupings was observed for OPCR (figures 1c, 2c); however, the RFI value (figure 1b) did not distinguish among dietary groupings (figure 2b). The results of the phy-ANOVA indicated that both DNE and OPCR are significantly associated with feeding behaviour in living taxa, even when taking phylogeny into account (DNE: F = 44.509, pphy = 0.00693; OPCR: F = 44.909, pphy = 0.00597), but this was not the case for RFI (F = 0.4725, pphy = 1.7265).
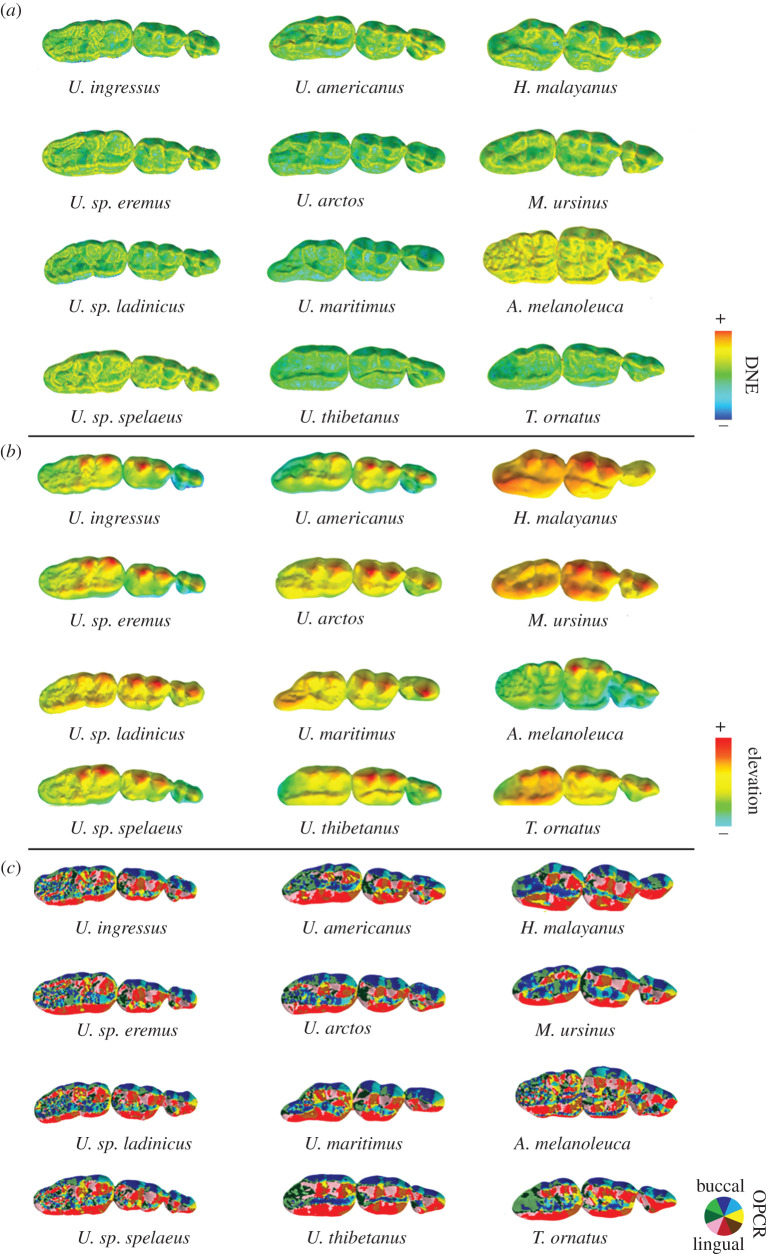
Figure 1.
Occlusal views of upper tooth row (P4–M2) triangulated meshes displaying morphometric maps for (a) Dirichlet normal energy (DNE), (b) elevation for surface relief index (RFI) calculation and (c) orientation patch count rotated (OPCR) topographic metrics applied with MorphoTester [9]. Only one specimen per species is shown. Warmer (red) and cooler (blue) colours for DNE and elevation maps indicate higher and lower curvature and crown height, respectively. OPCR complexity maps from triangulated meshes indicate surface orientation patches (see colour wheel). Mesial: right.
Figure 2.
Results of dental topographic analysis. Box-plots for (a) DNE, (b) RFI and (c) OPCR by feeding behaviour in living taxa and cave bears. Boxes enclose 25%–75% percentile values, the horizontal bar indicates the median value and whiskers denote range. Individual values are plotted. Traitgrams for (d) DNE, (e) RFI and (f) OPCR in living taxa and cave bears (the same colour coding as feeding behaviour is used). Bivariate plots for (g) DNE and (h) OPCR against two-dimensional surface area (2da). Dotted lines denote linear regressions; 95% prediction intervals are shown. (i) Bivariate plot depicted from the scores on the first two eigenvectors obtained in PCA computed from the three topographic (DNE, RFI and OPCR) metrics. Convex hulls show the distribution limits of each dietary group considered. The labelled rays show the loadings for topographic metrics onto PC1 and PC2 axes. In green, the bamboo-feeder giant panda; in orange, the faunivores; in purple, the omnivores (hard mast specialists); in dark green, the folivore-frugivores (soft mast specialists); and in grey, the cave bears.
Table 1.
Summary statistics (mean ± 1 s.d.) for DNE, RFI, OPCR and surface area (2da) obtained from the upper P4–M2 dental series in living and extinct bears.
| taxon | n | DNE | RFI | OPCR | surface area (2da) |
|---|---|---|---|---|---|
| A. melanoleuca | 6 | 1121.34 ± 195.8 | 1.69 ± 0.1 | 354.15 ± 12.9 | 1601.62 ± 76.8 |
| H. malayanus | 11 | 481.85 ± 78.7 | 1.86 ± 0.1 | 164.45 ± 19.6 | 440.09 ± 64.2 |
| M. ursinus | 5 | 409.34 ± 56.3 | 1.72 ± 0.1 | 172.13 ± 23.7 | 454.77 ± 68.1 |
| T. ornatus | 7 | 357.60 ± 37.6 | 1.72 ± 0.1 | 162.02 ± 10.6 | 518.87 ± 40.3 |
| U. americanus | 7 | 364.58 ± 69.9 | 1.70 ± 0.08 | 152.89 ± 29.6 | 639.73 ± 181.2 |
| U. arctos | 12 | 597.27 ± 91.8 | 1.85 ± 0.1 | 220.97 ± 27.8 | 972.97 ± 207.2 |
| U. maritimus | 11 | 520.37 ± 40.5 | 1.88 ± 0.1 | 186.28 ± 13.6 | 629.53 ± 63.7 |
| U. thibetanus | 10 | 494.58 ± 65.5 | 1.67 ± 0.1 | 192.25 ± 23.3 | 647.28 ± 62.7 |
| U. sp. ladinicus† | 4 | 854.44 ± 48.6 | 1.80 ± 0.07 | 328.06 ± 19.5 | 1537.39 ± 119.8 |
| U. sp. spelaeus† | 3 | 816.72 ± 71.1 | 1.86 ± 0.02 | 306.43 ± 7.3 | 1429.08 ± 74.9 |
| U. sp. eremus† | 4 | 693.52 ± 90.5 | 1.74 ± 0.04 | 279.90 ± 22.02 | 1587.02 ± 135.6 |
| U. ingressus† | 6 | 699.09 ± 95.8 | 1.70 ± 0.08 | 289.88 ± 28.5 | 1597.43 ± 170.1 |
The phylogenetic signals of both DNE (λ = 0.973; p = 0.01) and OPCR (λ = 0.985; p = 0.02) were statistically significant, but RFI was statistically independent of phylogeny (λ = 6.6107 × 10−5; p > 0.05). This pattern was also confirmed by the traitgrams (figure 2d–f), as the distribution of both DNE and OPCR preserves a clear phylogenetic structure across species, but RFI distribution does not seem to be influenced by phylogeny [25].
The bivariate regressions of both DNE and OPCR against the surface area (2da) of the upper tooth rows (figure 2g–h) indicated that variation in both topographic metrics is significantly influenced by tooth size (DNE: R2 = 0.632, p < 0.001; OPCR: R2 = 0.812, p < 0.001). By contrast, the bivariate regression of RFI against 2da was not statistically significant (R2 = 0.010; p = 0.355), which indicated that RFI is not influenced by tooth size. The association between the contrasted OPCR and 2da (R2 = 0.5207; F = 10.87; p = 0.008) was statistically significant. However, the association between the contrasted RFI and 2da (R2 = 0.2216; F = 2.847, p = 0.122), as well as DNE and 2da (R2 = 0.2855; F = 3.996; p = 0.073) were not statistically significant.
The morphospace depicted from the PCA performed from topographic metrics (DNE, RFI and OPCR) yielded two significant eigenvectors, which jointly explained 98% of the original variance (figure 2i; electronic supplementary material, table S2, electronic supplementary material). The factor loadings of the variables on each eigenvector indicated that although both the giant panda and the cave bears were characterized by high values of DNE and OPCR, the faunivores and folivores-frugivores were characterised by low values of both variables. Omnivores were characterized by intermediate values.
4. Discussion
Among living and extinct bears, phylogenetic signal is present on dental topographic curvature and complexity but not on relief, which indicates a clear phylogenetic structure on both curvature and complexity. Moreover, the phylogenetic ANOVA demonstrated that both variables are significantly associated with feeding behaviour, whereas topographic relief is not.
Dental topographic curvature, relief and complexity are associated with size among species (figure 2g,h) but, as indicated by the independent contrast analysis, this association does not result from phylogenetic correlation for complexity. Previous authors have demonstrated that cave bears follow a different trend of increasing tooth-root areas across maxillary dental series [7] from any living species; cave bears exhibit a continuous increase from P4 to M2 in tooth-root area values, reaching M2 values similar to those of the giant panda (A. melanoleuca). Our results suggest that this trend of increasing tooth-root areas across P4 to M2 of cave bears [7] also entails an increase in curvature and complexity in their upper teeth crown surfaces. In general, a larger tooth can process more food with each bite, either through a larger area of contact or through the formation of a longer blade [30–35].
The PCA performed from the shape descriptor metrics revealed an ordination according to feeding behaviour (figure 2i). The giant pandas and cave bears show extreme positive scores on PC1 (i.e. high OPCR and DNE values), whereas intermediate scores were observed for the omnivores that specialize in feeding on a hard mast (i.e. fruits and seeds with a hard protective covering, including acorns and pine seeds [36], and roots and tubercles). By contrast, the faunivores and folivores-frugivores exhibited extreme negative scores. This overlap between the folivores-frugivores and the faunivores likely relates to the fact that folivores-frugivores are soft mast specialists (feeding mainly on fleshy fruits or strobili that are softer than hard mast items e.g. [36]). Therefore, based on our findings, topographic metrics are more closely correlated with the mechanical properties of the material than with the type of food ingested.
The PCA also shows evidence that cave bears combine values of curvature and complexity in a unique manner among living bears, as evidenced by their position in the empty space of figure 2i. We hypothesized that cave bears were feeding on a resource that possessed intermediate mechanical properties to bamboo and hard mast. No living bear currently exploits this feeding resource, most likely because it was only present in the high-alpine biomes that cave bears inhabited. Given that both complexity and curvature are highly influenced by tooth size, we also hypothesize that increasing upper tooth surface areas also increase the values of both topographic variables, and probably, also their chewing efficiency.
In any case, our results indicate that giant pandas show the highest values of curvature and complexity among the sample, which should relate to their peculiar diet, feeding on bamboo [27,37]. Moreover, previous authors [38] have demonstrated that primate species with the most complex teeth are those that consume extremely fibrous vegetation, such as bamboo-eating lemurs. The second group with the highest values of complexity is the cave bears, which may indicate that they were probably feeding on hard materials of low quality, such as highly fibrous vegetal resources.
Although hypothesizing on the specific vegetal resource that cave bears were specialized to feed on is tempting, our results indicate that cave bears were specialized to feed upon tough vegetal resources of the high-alpine biome, which supports the climate-driven hypothesis to explain their extinction at the beginning of the Last Glacial Maximum when the primary productivity that the cave bear fed upon was dramatically lowered [3–5].
Supplementary Material
Acknowledgements
We would like to thank American Museum of Natural History (New York, USA), the Natural Museum of Scotland, the Museum für Naturkunde, the University of Vienna Institution of Palaeontology, and collections of the Valladolid University for allowing us data acquisition. We appreciate the fossil material reviewed by Professor Gernot Rabeder (University of Vienna) and Dr Paco Pastor (University of Valladolid, Spain). The University of Málaga imaging centre scanned the dental moulds and two anonymous reviewers improved the rigour of the manuscript contents.
Data accessibility
Pérez-Ramos A, Romero A, Rodriguez E, Figueirido B. 2020. Data from: Three-dimensional dental topography and feeding ecology in the extinct cave bear. Dryad Digital Repository (doi:10.5061/dryad.95x69p8j9).
Authors' contribution
B.F. conceived the study; B.F., A.P.-R. and A.R. designed research. A.P.-R., A.R. and E.R. collected data. B.F., A.R. and A.P.-R. analysed data. B.F. wrote the paper with the input of A.R., A.P.-R. and E.R. All authors agree to be held accountable for the content of the manuscript and approve the final version.
Competing interests
We declare we have no competing interests.
Funding
This study has been funded by the Spanish Ministry of Economy and Competitiveness-MEC (CGL2012-37866, CGL2015-68300P) and Junta de Andalucía (UMA18-FEDERJA-188) to B.F.
References
- 1.Figueirido B, van Heteren AH. 2019. The story continues: recent advances on the life and death of the Pleistocene cave bear. Hist. Biol. 31, 405–409. ( 10.1080/08912963.2018.1436426) [DOI] [Google Scholar]
- 2.Bocherens H. 2019. Isotopic insights on cave bear palaeodiet. Hist. Biol. 31, 410–421. ( 10.1080/08912963.2018.1465419) [DOI] [Google Scholar]
- 3.Terlato G, Bocherens H, Romandini M, Nannini N, Hobson KA, Peresani M. 2018. Chronological and isotopic data support a revision for the timing of cave bear extinction in Mediterranean Europe. Hist. Biol. 31, 474–484. ( 10.1080/08912963.2018.1448395) [DOI] [Google Scholar]
- 4.Stiller M, et al. 2010. Withering away−25,000 years of genetic decline preceded cave bear extinction. Mol. Biol. Evol. 27, 975–978. ( 10.1093/molbev/msq083) [DOI] [PubMed] [Google Scholar]
- 5.Mondanaro A, et al. 2019. Additive effects of climate change and human hunting explain population decline and extinction in cave bears. Boreas 48, 605–615. ( 10.1111/bor.12380) [DOI] [Google Scholar]
- 6.Pérez-Ramos A, Tseng ZJ, Grandal-D'Anglade A, Rabeder G, Pastor FJ, Figueirido B. 2020. Biomechanical simulations reveal a trade-off between adaptation to glacial climate and dietary niche versatility in European cave bears. Sci. Adv. 6, eaay9462 ( 10.1126/sciadv.aay9462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Ramos A, Kornelius K, Van Heteren AH, Rabeder G, Grandal-D'Anglade A, Pastor FJ, Serrano FJ, Figueirido B. 2019. A three-dimensional analysis of tooth-root morphology in living bears and implications for feeding behaviour in the extinct cave bear. Hist. Biol. 31, 461–473. ( 10.1080/08912963.2018.1525366) [DOI] [Google Scholar]
- 8.Lucas PW. 2004. Dental functional morphology: How teeth work. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Winchester JM. 2016. MorphoTester: an open source application for morphological topographic analysis. PLoS ONE 11, e0147649 ( 10.1371/journal.pone.0147649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans AR. 2013. Shape descriptors as ecometrics in dental ecology. Hystrix 24, 133–140. ( 10.4404/hystrix-24.1-6363) [DOI] [Google Scholar]
- 11.Baca M, Popović D, Stefaniak K, Marciszak A, Urbanowski M, Nadachowski A, Mackiewicz P. 2016. Retreat and extinction of the Late Pleistocene cave bear (Ursus spelaeus sensu lato). Sci. Nat. 103, 11–12. ( 10.1007/s00114-016-1414-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knapp M. 2019. From a molecules' perspective – contributions of ancient DNA research to understanding cave bear biology. Hist. Biol. 31, 442–447. ( 10.1080/08912963.2018.1434168) [DOI] [Google Scholar]
- 13.Figueirido B, Pérez-Ramos A, Schubert BW, Serrano F, Farrell AB, Pastor FJ, Neves Aline A, Romero A. 2017. Dental caries in the fossil record: a window to the evolution of dietary plasticity in an extinct bear. Sci. Rep. 7, 1–7. ( 10.1038/s41598-017-18116-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunn JM, Boyer DM, Lipman Y, St Clair EM, Jernvall J, Daubechies I. 2011. Comparing Dirichlet normal surface energy of tooth crowns, a new technique of molar shape quantification for dietary inference, with previous methods in isolation and in combination. Am. J. Phys. Anthropol. 145, 247–261. ( 10.1002/ajpa.21489) [DOI] [PubMed] [Google Scholar]
- 15.Boyer DM. 2008. Relief index of second mandibular molars is a correlate of diet among prosimian primates and other euarchontan mammals. J. Hum. Evol. 55, 1118–1137. ( 10.1016/j.jhevol.2008.08.002) [DOI] [PubMed] [Google Scholar]
- 16.Evans AR, Wilson GP, Fortelius M, Jernvall J. 2007. High-level similarity of dentitions in carnivorans and rodents. Nature 445, 78–81. ( 10.1038/nature05433) [DOI] [PubMed] [Google Scholar]
- 17.Pineda-Munoz S, Lazagabaster IA, Alroy J, Evans AR. 2017. Inferring diet from dental morphology in terrestrial mammals. Meth. Ecol. Evol. 8, 481–491. ( 10.1111/2041-210X.12691) [DOI] [Google Scholar]
- 18.Berthaume MA, Winchester J, Kupczik K. 2019. Effects of cropping, smoothing, triangle count, and mesh resolution on dental topographic metrics. PLoS ONE 14, e0216229 ( 10.1371/journal.pone.0216229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 20.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 21.Maddison WP, Maddison DR. 2018. Mesquite: a modular system for evolutionary analysis. Version 3.51. See http://www.mesquiteproject.org.
- 22.Krause J, et al. 2008. Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary. BMC Evol. Biol. 8, e220 ( 10.1186/1471-2148-8-220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiller M, Molak M, Prost S, Rabeder G, Baryshnikov G, Rosendahl W, Germonpré M. 2014. Mitochondrial DNA diversity and evolution of the Pleistocene cave bear complex. Quatern. Int. 339, 224–231. ( 10.1016/j.quaint.2013.09.023) [DOI] [Google Scholar]
- 24.Blomberg SP, Garland T Jr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. (doi:0.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 25.Münkemüller T, Lavergne S, Bzeznik B, Dray S, Jombart T, Schiffers K, Thuiller W. 2012. How to measure and test phylogenetic signal. Methods Eco. Evol. 3, 743–756. ( 10.1111/j.2041-210X.2012.00196.x) [DOI] [Google Scholar]
- 26.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 27.Figueirido B, Tseng ZJ, Martín-Serra A. 2013. Skull shape evolution in durophagous carnivorans. Evolution 67, 1975–1993. ( 10.1111/evo.12059) [DOI] [PubMed] [Google Scholar]
- 28.Garland T Jr, Dickerman AW, Janis CM, Jones JA. 1993. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292. ( 10.1093/sysbio/42.3.265) [DOI] [Google Scholar]
- 29.Hammer Ø, Harper DA, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 9. [Google Scholar]
- 30.Evans AR, Pineda-Munoz S. 2018. Inferring mammal dietary ecology from dental morphology. In Methods in paleoecology (eds Croft D, Su D, Simpson S), pp. 37–51. Cham, Switzerland: Springer. [Google Scholar]
- 31.Vizcaíno SF, Bargo MS, Cassini GH. 2006. Dental occlusal surface area in relation to food habits and other biologic features in fossil Xenarthrans. Ameghiniana 43, 11–26. [Google Scholar]
- 32.Janis CM. 1990. Correlation of cranial and dental variables with dietary preferences in mammals: a comparison of macropodoids and ungulates. Mem. Queensl. Mus. 28, 349–366. [Google Scholar]
- 33.Janis CM. 1995. Correlations between craniodental morphology and feeding behavior in ungulates: reciprocal illumination between living and fossil taxa. In Functional morphology in vertebrate paleontology (ed Thomason JJ.), pp. 78–98. Cambridge, NJ: Cambridge University Press. [Google Scholar]
- 34.Janis CM, Constable E. 1993. Can ungulate craniodental features determine digestive physiology? J. Vert. Paleontol. 13, 43A. [Google Scholar]
- 35.Mendoza M, Janis CM, Palmqvist P. 2002. Characterizing complex craniodental patterns related to feeding behaviour in ungulates: a multivariate approach. J. Zool. 258, 223–246. ( 10.1017/S0952836902001346) [DOI] [Google Scholar]
- 36.Janis CM, Fortelius M. 1988. On the means whereby mammals achieve increased functional durability of their dentitions, with special references to limiting factors. Biol. Rev. 63, 197–230. ( 10.1111/j.1469-185X.1988.tb00630.x) [DOI] [PubMed] [Google Scholar]
- 37.Mattson DJ. 1998. Diet and morphology of extant and recently extinct northern bears. Ursus 10, 479–496. [Google Scholar]
- 38.Eronen JT, Zohdy S, Evans AR, Tecot SR, Wright PC, Jernvall J. 2017. Feeding ecology and morphology make a bamboo specialist vulnerable to climate change. Curr. Biol. 27, 3384–3389. ( 10.1016/j.cub.2017.09.050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pérez-Ramos A, Romero A, Rodriguez E, Figueirido B. 2020. Data from: Three-dimensional dental topography and feeding ecology in the extinct cave bear. Dryad Digital Repository (doi:10.5061/dryad.95x69p8j9).