Abstract
Background
Shingrix is a non-live recombinant vaccine approved by the Food and Drug Administration (FDA) in 2017 to prevent shingles and postherpetic neuralgia in immunocompetent adults age 50 years and older. A myriads of local and systemic reactions due to the vaccine have been reported, but diffuse erythematous maculopapular rash and neurological symptoms have not yet been reported in English literature.
Methods
Using Google and PubMed, we searched for relevant case reports and journal articles describing adverse effects related to shingrix vaccination.
Results
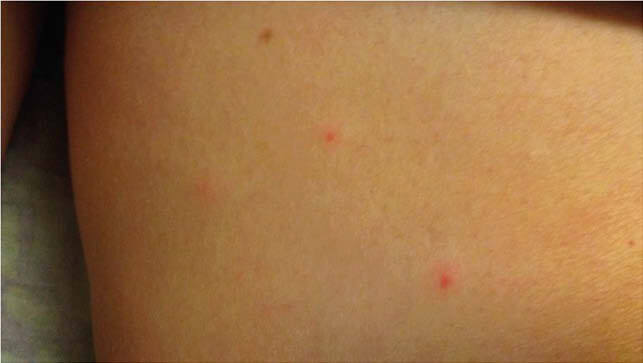
A 54-year female without significant past medical history presented with diffuse erythematous maculopapular rash, itching and a feeling of weakness in both legs. Her symptoms started with itching and erythematous macular rash at the site of shingles shot followed by headache, myalgia, and malaise which did not improved much with Benadryl. Next day, she felt numbness and weakness in both legs. On the third day, she awoke with diffuse red rash on the face, trunk, lower extremities, fewer lesions on upper extremities. Her review of systems was negative except as mentioned. on examination, she was found to have diffuse erythematous maculopapular itchy rash as shown in Fig 1, but no sensory, motor, cranial never or cerebellar signs. Infectious disease was consulted who recommended IV acyclovir considering early varicella with given morphology. The morphology of lesions did not change over a period of times and VZV PCR of lesions came negative hence acyclovir was discontinued after three days. Her symptoms and rash improved over the hospital stay with supportive treatment and was discharged home on day fifth of admission.
Fig. 1A, Rash over face
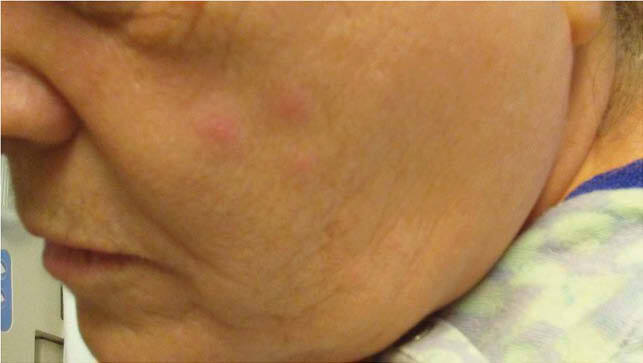
Fig. 1B, Rash over right thigh
Conclusion
The safety of shingrix was evaluated by the pool data from eight clinical trials of more than 10,000 participants. Among the study population, 9.4% had local injection-site reactions including pain, redness, and swelling and 10.8% had systemic events including myalgia, fatigue, headache, shivering, fever, and gastrointestinal symptoms. The nature and duration of rash described in our patient has not been reported in english literature including these clinical trials. Noticing new reactions with broad use of new vaccine is conceivable.
Disclosures
All Authors: No reported disclosures


