Abstract
The incidence of factor IX (FIX) inhibitors in severe hemophilia B (SHB) is not well defined. Frequencies of 3-5% have been reported but most studies to date have been small, including patients with different severities, and without prospective follow up for inhibitor incidence. The study objective was to investigate the inhibitor incidence in patients with SHB followed up for to 500 exposure days (ED), the frequency of allergic reactions, and the relationship with genotypes. Consecutive previously untreated patients (PUP) with SHB enrolled into the PedNet cohort were included. Detailed data was collected for the first 50 ED, followed by the annual collection of the inhibitor status and allergic re-actions. The presence of inhibitors was defined by at least two consecutive positive samples. Additionally, data on FIX gene mutation was collected. One hundred and fifty-four PUP with SHB were included; 75% were followed up until 75 ED, and 43% until 500 ED. Inhibitors developed in 14 patients (seven high-titer). The median number of ED at inhibitor manifestation was 11 (interquartile range [IQR]: 6.5-36.5). The cumulative inhibitor incidence was 9.3% (95% Confidence Interval [CI]: 4.4-14.1) at 75 ED, and 10.2% (95% CI: 5.1-15.3) at 500 ED. Allergic reactions occurred in four (28.6%) inhibitor patients. Missense mutations were most frequent (46.8%) overall but not associated with inhibitors. Nonsense mutations and deletions with large structural changes comprised all mutations among inhibitor patients and were associated with an inhibitor risk of 26.9% and 33.3%, respectively. In an unselected, well-defined cohort of PUP with SHB, the cumulative inhibitor incidence was 10.2% at 500 ED. Nonsense mutations and large deletions were strongly associated with the risk of inhibitor development. The ‘PedNet Registry’ is registered at clinicaltrials.gov; identifier: NCT02979119.
Introduction
Hemophilia A (HA) and B (HB) are X-linked inherited bleeding disorders, characterised by deficiency of factor VIII (FVIII) and factor IX (FIX), respectively. HB occurs at a frequency of about 1 in every 25-30.000 newborn males, comprising about 15% of all patients with hemophilia. 1 Patients with severe HB (SHB) have a FIX level <0.01 IU/mL and account for approximately 30-40% of persons with HB.2,3 Both hemophilia subtypes suffer from recurrent joint bleeds, soft-tissue bleeds, and muscle bleeds. Several studies in adult patients reported a more severe phenotype for HA.4,5 The treatment history has a large effect on the joint outcome of hemophilia, even though the reason for these different outcomes in adults is still unclear. A study in a large cohort of children did not detect differences in the age of the first joint bleed.6 Longer follow-up of patients with SHA and SHB on primary prophylaxis will provide the answers whether clear phenotypic differences exist.
A clear difference between hemophilia subtypes has been observed in inhibitor frequency, with studies reporting incidences of 1-5% in HB2,7-11 compared to 25-35% in HA.12,13 Moreover, in HB, FIX inhibitors occur almost exclusively in the severe form,11 while FVIII inhibitors occur in both SHA and non-severe HA.14 The inhibitor incidence in HB is not well defined. Due to small numbers of patients with HB, sufficiently large cohorts of previously untreated patients (PUP) are difficult to obtain. Thus, no representative prospective studies reporting the inhibitor incidence are available to date.15 Many studies reporting on the inhibitor frequency in HB included all severities and even the definition of SHB varied between <0.01 and <0.02 IU/mL. The period of highest risk for inhibitor development is believed to be during the first 50-75 exposure days (ED) which can take many years during on demand therapy. Studies on HB published before widespread use of prophylaxis might have missed late inhibitors.
Gene defects such as large deletions and nonsense mutations have been reported to increase the risk of inhibitors in HB.7,9,16-19 The high proportion of missense mutations in HB resulting in circulating amounts of FIX antigen is considered as one of the factors responsible for the overall lower risk of inhibitors.7,9,17,19 The lower proportion of the severe phenotype of HB compared to HA may be another reason.11 Moreover, the similarity between FIX and the other vitamin K-dependent factors (FII, FVII, FX) has been hypothesized to render therapeutic FIX less immunogenic.20
Although the development of an inhibitor in HB is a rare event, it is associated with significant morbidity, related not only to a higher risk of hemorrhagic complications but also to the frequent occurrence of allergic reactions. 16,21-23 How often allergic reactions occur in association with inhibitor manifestation is unclear. Nephrotic syndrome often complicates immune tolerance induction treatment in HB and might be one reason for the low success rates of 30-35%.24 International registries on HB reported on the risk factors for inhibitors such as genotypes but the selection of patients in such registries makes it difficult to draw definitive conclusions on their impact.9,16-18
The objective of our study was to investigate inhibitor development in SHB patients followed up for up to 500 ED, the frequency of allergic reactions at inhibitor manifestation, and the effect of FIX genotype on the inhibitor risk.
Methods
Patients
The PedNet study is a birth cohort enrolling all PUP with HA and HB (FVIII or FIX less than 25%) born after January 2000 treated at participating centers. For the current study, we included consecutive PUP with SHB (FIX activity <0.01 IU/mL) followed until January 1, 2018, if informed consent was available, data quality was sufficient, and they had been exposed to FIX concentrate. A list of the participating centers that contributed to this study can be found in the Appendix. Patients who were referred to the participating centers because of the presence of an inhibitor were excluded. Patients were enrolled and followed according to the PedNet study protocol, NCT02979119. Study approval was obtained from the institutional review boards of every center. Written informed consent was obtained from the parents or guardians of all participants.
Measurements
Baseline information on diagnosis of HB, including age at diagnosis, basal FIX plasma level, FIX gene mutation, ethnicity, family history of hemophilia, family history of inhibitors were collected in uniform web-based case report forms. During the first 50 ED, detailed information on each ED, dose, product type and reason for treatment was collected. After 50 ED, treatment data was collected in annual follow-up forms per individual patient. This included all changes in the treatment regimens, bleeds and surgeries, and inhibitor development, and other adverse events. An ED was defined as a calendar day on which one or more infusions of FIX were given.
Genetic analysis
Characterization of mutations in the FIX gene was performed locally at the participating centers and the reports on genotype were centrally evaluated and adapted to the Human Genome Variation Society nomenclature.25 In order to assess the association of the FIX genotype with inhibitor development, mutations were categorized according to the mutation type (point mutation, deletion, duplication, insertion, polymorphism, complex) and the mutation effect (missense, nonsense, frameshift, large structural changes [>50 bp], small structural changes [<50 bp] in frame, silent, splice site mutation, promotor abnormalities).
Outcomes
The primary outcome of the study was development of a clinically relevant inhibitor, defined as at least two positive inhibitor titers combined with a decreased in vivo FIX recovery. Inhibitor positivity was defined according to the cut-off level in the individual laboratory of each center, the highest cut-off level used being 0.6 Bethesda Units per mL (BU/mL). Secondary outcome was development of a high-titer inhibitor, defined as the occurrence of an inhibitor with a peak titer of more than 5 BU/mL. The number of ED at inhibitor development was defined as the last ED before the date of the first positive inhibitor test. After a single positive inhibitor test, all subsequent tests and recovery measurements were collected. For this study, additional information was collected regarding the occurrence of allergic reactions at the time of inhibitor development.
Follow-up data on the clinical course of patients who developed an inhibitor has been collected and will be reported in a separate manuscript.
Data analyses
We used Kaplan-Meier curve survival analysis methods with the number of ED as the time variable. All patients were included in the analyses and censored at the ED of their last follow-up. In the analyses using “all clinically relevant inhibitors” as the outcome, censoring occurred at the last ED. In the analyses with “high titer inhibitor development” as the outcome, censoring occurred at the last exposure day in non-inhibitor patients and at the last ED before inhibitor development in patients with low-titer inhibitors. Continuous variables are summarized by median and interquartile range (IQR). Inhibitor incidences are reported as percentage with 95% Confidence Interval (95% CI). Comparisons of categorial variables between groups were done by Fisher’s exact test.
Results
Of a total of 168 consecutive PUP with SHB identified in PedNet centers during the study period, 14 were excluded for lack of consent (n=2), insufficient data quality (n=5), loss-to-follow-up (n=1) or because they were not yet treated with a FIX product (n=7). Thus, 154 (91.6%) patients were included in this analysis. The demographic information of study patients is reported in Table 1. The median age at first treatment was 0.8 years, the age at the time of study evaluation was 9.6 years. About half of the patients had a positive family history for hemophilia at diagnosis. Caucasian ethnicity was present in more than 80% of the patients. Seventy-seven percent of patients were followed up until 50 ED, 75% until 75 ED, 68% until 150 ED, and 43% until 500 ED.
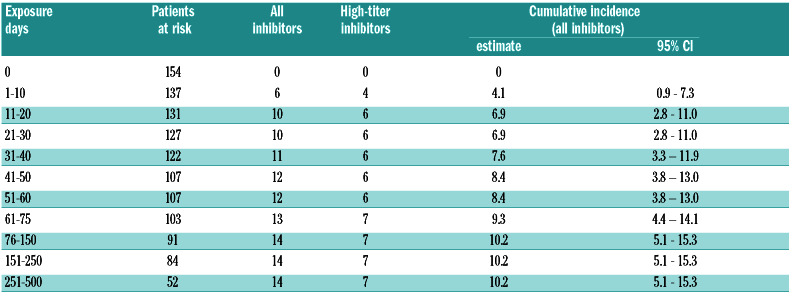
Inhibitors were diagnosed in 14 patients, seven were classified as high-titer and 7 as low-titer. The median number of ED at inhibitor manifestation was 11 (IQR: 6.5-36.5), the median age was 23.2 months (IQR: 12.1-37.1). The cumulative inhibitor incidence at 75 ED was 9.3% (95 % CI: 4.4-14.1) for all inhibitors and 5% for high-titer inhibitors. Between 76 and 500 ED, only 1 low-titer inhibitor was diagnosed at 121 ED. The cumulative inhibitor incidence at 500 ED was 10.2% (95% CI: 5.1-15.3) (Table 2 and Figure 1).
A positive family history of FIX inhibitors was significantly more frequent (21%) among inhibitor patients compared to non-inhibitor patients (2%) (Table 1). A recombinant FIX product was used initially in 75% and 71% of non-inhibitor and inhibitor patients, respectively. Peak treatment episodes of at least 5 days at initial FIX exposure occurred in 15% and 14%, respectively. The median age at the start of prophylaxis was 1.5 years for non-inhibitor patients and 1.3 years for inhibitor patients, but only five inhibitor patients had started prophylaxis before developing the inhibitor. Surgery performed before ED20 (and before inhibitor development) occurred in 34% and 29% of non-inhibitor and inhibitor patients, respectively.
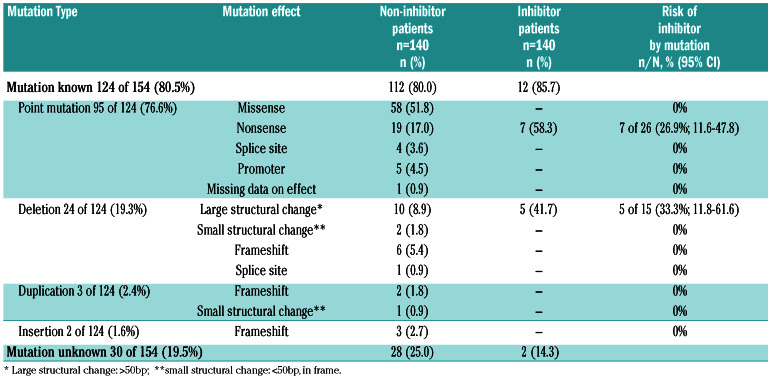
The FIX gene mutation genotypes were known from 124 of 154 (80.5%) patients (Table 3). Overall, the most frequent mutation type were point mutations in 95 of 124 (76.6%) patients, leading to missense mutations in 58 of 124 (46.8%) and nonsense mutations in 26 of 124 (21.0%) patients. Deletions were found in 24 of 124 (19.4%) patients, causing large structural changes in 15 of 124 (12.1%) and frameshift in 6 of 124 (4.8%) patients. Duplications were found in 2.4% and insertions in 1.6% of patients. For more details on the mutation type and effect see Table 3.
Table 1.
Demographic information of study participants comparing patients.

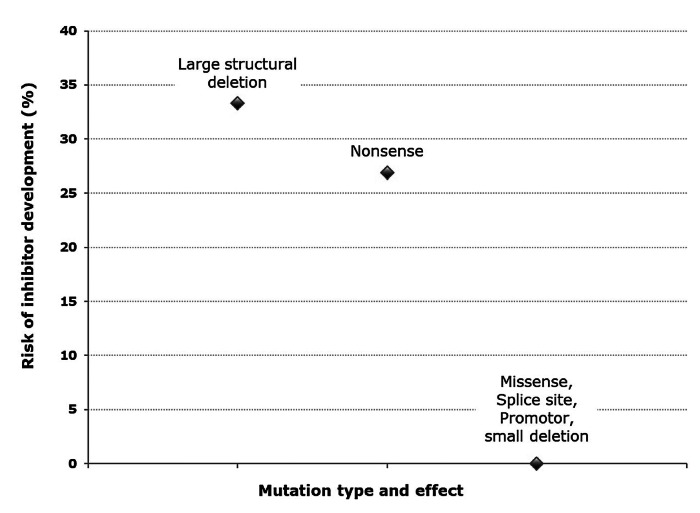
Among inhibitor patients, the most frequent mutations were nonsense mutations in 7 of 12 (58.3%), four patients with low-titer and three patients with high-titer inhibitors, and deletions with large structural changes in 5 of 12 (41.7%), three patients with low-risk and two with high-risk inhibitors. No other mutations were present among inhibitor patients. In two inhibitor patients, the genotype was not known (no genetic report was available in one; no mutation was identified in spite of repeated analysis in the other). Figure 2 displays the risk of inhibitor development by mutation. The inhibitor risk for deletions with large structural change was 33.3% (95% CI: 11.8-61.6) and for nonsense mutations 26.9% (95% CI: 11.6-47.8). For all other mutations, the inhibitor risk was zero.
Allergic reactions at the time of inhibitor development were observed in 4 of 14 (28.6%, 95% CI: 8.4-58.1) patients, one patient with a high-titer inhibitor and three patients with low titer inhibitors (Table 3). Of these four patients, two patients had nonsense mutations, one had a large deletion and one had an unknown mutation.
Discussion
In this unselected, prospectively followed birth cohort of 154 PUP with SHB, 14 patients developed an inhibitor. The cumulative inhibitor incidence at 75 ED was 9.3% for all inhibitors and 5% for high-titer inhibitors. At 500 ED, the cumulative inhibitor incidence for all inhibitors had increased to 10.2%. Previous large databases of HB patients in general reported inhibitor frequencies of 1.3-2.8%2,8-10 while studies focusing on SHB patients reported inhibitor frequencies of 3.8-4.9%.2,7,8,11 However, these registries reported inhibitor prevalences rather than prospectively following patients for inhibitor incidence. In the European Hemophilia Safety Surveillance (EUHASS), five inhibitors were reported among 72 PUP with SHB (6.9%).26 A recent systematic review of PUP studies in SHB reported a summary inhibitor incidence of 10.2%.27 However, most studies included in this analysis were small (patient numbers between 7 and 72), with inhibitor frequencies from individual studies varying between 5-14%.
Table 2.
Inhibitor development in relation to number of exposure days.
Figure 1.
Kaplan-Meier survival curve of inhibitor development until 500 exposure days
How can we interpret the higher incidence of inhibitors in SHB found in our study compared to most previous reports? The PedNet registry represents an unselected birth cohort. In contrast, patients with bleedings in the neonatal period will not be eligible for licensing PUP studies.28 In the current study on SHB patients, we found that 52% of patients had a negative family history, similar to prospective studies in SHA patients.12,13 This frequency is much higher than the 30% of patients reported in the literature. Patients with a negative family history are usually diagnosed after the onset of bleeding, potentially increasing their risk for inhibitors.12
In our cohort of patients born after 2000, all received primary prophylaxis, which enabled us to follow them until 500 ED. This long-term follow-up might also have resulted in detecting a higher total number of inhibitors compared to other studies.27 Another reason for the higher incidence in our study may be attributed to more frequent testing for inhibitors. Inhibitors have received much more attention in the last decades and frequent testing for inhibitors has become standard of care. In SHA, increased frequencies of inhibitors have been observed since the nineties.12,13 Our group reported that significantly more low-titer inhibitors were diagnosed in SHA after the year 2000.29 In the current study of SHB, low-titer inhibitors comprised about half of all inhibitors, and the incidence of high-titer inhibitors was 5%, which is more in line with previous reports.2,7,10,11
Figure 2.
Risk of inhibitor development by mutation
Table 3.
Factor IX mutations in study participants comparing patients without and with inhibitors.
A positive family history for inhibitors has been recognized as an important risk factor, in our patients this was confirmed in 21% of the inhibitor patients. Higher incidences of inhibitors for SHB where reported in Sweden and Ireland that might have been influenced by the inclusion of families with more high-risk mutations or a founder effect.19,30 No inhibitor patients in our study were related which excludes this so-called “founder” effect.
The relative frequency of FIX gene mutation types/effects in our cohort was comparable to those reported from other registries.9,17 We found 46.8% missense mutations in SHB patients, in contrast to only 11.4% missense mutations among SHA patients in the RODIN study.12 A higher prevalence of missense mutations in SHB compared to SHA has previously been reported.7,9,12,17,18,31 Missense mutations were only found in non-inhibitor patients, consistent with previous reports.7,9,16-19 We hypothesize that the high proportion of missense mutations in HB is a key reason for the overall lower inhibitor incidence compared to HA. Two mutation types/effects were strongly associated with inhibitor development, comprising all mutations among the inhibitor patients: nonsense mutations and deletions with large structural changes both representing null-mutations. Patients with these high-risk mutations had a 27% and 33% risk of developing an inhibitor, respectively. The findings from our representative PUP cohort confirm previous reports,7,9,16-19 suggesting a strong association between absent endogenous FIX protein due to gross and complete gene deletions and inhibitor development.32
For gross FIX gene defects resulting in absent endogenous FIX protein, the observed risk of inhibitor development (27-33%) is similar to HA due to gross FVIII gene mutations resulting in absent endogenous FVIII protein (inhibitor risk around 35%).33
Treatment-related factors, such as the type of FIX product, peak treatment episodes at the initial exposure to FIX concentrate, time of the start of prophylaxis, or surgery, were not associated with inhibitor development. However, we must be cautious in interpreting these findings, as the power to identify or rule out determinants of inhibitor development is limited with the small number of inhibitor patients.
Allergic reactions are more frequently observed in HB compared to HA. Frequencies of allergic reactions reported in the literature for HB vary considerably between 4-60% of inhibitor patients.10,16,21-23 In our cohort, allergic reactions at the time of inhibitor manifestation were observed in four (29%) patients, one with a high-titer and three with low-titer inhibitors. Of these patients, two patients had nonsense mutations, one had a large deletion, and one had an unknown mutation. Large deletions have previously been reported to be associated with allergic reactions.16,22 Data on the course and effect of immune tolerance induction in our cohort are currently collected and will be reported in a future manuscript.
Our study has some limitations, such as the lack of racial diversity which limits generalisability to some extent. The determination of hemophilia severity as well as inhibitor testing was done in local laboratories at the individual centers. A potential disadvantage is insufficient standardization, however, the advantage of this pragmatic approach is better feasibility resulting in a more representative cohort. Although we report the largest consecutive cohort of PUP with SHB to date, its size still does not allow to comprehensively evaluate whether there is an influence of treatment-related risk factors on inhibitor development.
In conclusion, our study in an unselected cohort of PUP with SHB found a cumulative inhibitor incidence of 10.2% at 500 ED. Missense mutations were the most frequent mutation type but these were not associated with inhibitors, while nonsense mutations and large deletions were significantly associated with an increased risk of inhibitor development.
Supplementary Material
Acknowledgments
We thank all members of the PedNet study group who are listed in the Appendix for providing the data on their HB patients. We thank Ella van Hardeveld and Marloes de Kovel for maintaining the data base and supporting the analysis.
Appendix
PedNet study group members and centers in Europe: A Santamaria Ortiz, Unitat Hemofilia, Hospital Vall d’Hebron, Barcelona, Spain; MT Alvarèz Román, Unidad de Coagulopatías, Hopital Universitario La Paz, Madrid, Spain, M Bührlen, Gesundheit Nord, Klinikum Bremen Mitte, Prof.-Hess- Kinderklinik, Bremen, Germany; HM van den Berg, PedNet Hemophilia Research Foundation, Baarn, the Netherlands; M Carvalho, S. Imuno-hemoterapia, Centro Hospitalar São João, Porto, Portugal; E Chalmers, Department of Hematology, Royal Hospital for Sick Children, Yorkhill, Glasgow, UK; H Chambost, Aix Marseille Univ, INSERM, INRA, C2VN, and APHM, La Timone Children Hospital, Center for Bleeding Disorders, Marseille, France; A Rosa Cid, Unidad de Hemostasia y Trombosis, Hospital Universitario y Politécnico La Fe, Valencia, Spain; S Claeyssens, Centre Regional d’Hemophilie, Centre Hospitalo Universitaire, Toulouse, France; T Stamm Mikkelsen, Department of Pediatrics, University Hospital of Aarhus at Skejby, Aarhus, Denmark; C Escuriola, HZRM Hämophilie Zentrum Rhein Main GmbH, Mörfelden-Walldorf, Germany; K Fischer, Van Creveld Kliniek, University Medical Center Utrecht, Utrecht, the Netherlands; C Van Geet, Catholic University of Leuven, Campus Gasthuisberg, Service of Pediatric Hematology, Leuven, Belgium; H Glosli, Oslo University Hospital HF, Oslo, Norway; N N Gretenkort Andersson, Department of Clinical Sciences, Division of Pediatrics, Lund University, Lund, and Center for Thrombosis and Haemostasis, Skåne University Hospital, Malmö, Sweden; R Kobelt, Hämophiliezentrum, Wabern and Children's Hospital of the University of Bern, Bern,Switzerland; C Königs, J .W. Goethe University Hospital, Department of Pediatrics, Frankfurt, Germany; K Kurnik, Dr. V. Haunersches Kinderspital, University of Munich, Munich, Germany; R Liesner, Hemophilia Center, Department of Hematology, Great Ormond Street Hospital for Children, London, UK; R Ljung, Department of Clinical Sciences, Division of Pediatrics, Lund University, Lund, Sweden; A Mäkipernaa, Children’s Hospital, University Central Hospital and University of Helsinki, Helsinki, Finland; C Male, Department of Pediatrics, Medical University of Vienna, Vienna, Austria; A Molinari, Dipartimento di Ematologia ed Oncologia, Unità Trombosi ed Emostasi, Ospedale Pediatrico Giannina Gaslini, Genova, Italy; W Muntean*, Universitäts-Klinik für Kinder- und Jugendheilkunde, Graz, Austria; B Nolan, Department of Pediatric Hematology, Children’s Health Ireland at Crumlin, Dublin, Ireland; J Oldenburg, Institut für Experimentelle Hämatologie und Transfusionsmedizin, Universitätsklinikum Bonn, Bonn, Germany; R Pérez Garrido*, Hospital General Unidad de Hemofilia, Hospitales Universitarios Virgen del Rocio, Sevilla, Spain; H Platokouki, Hemophilia-Hemostasis Unit, St. Sophia Children’s Hospital, Athens, Greece; A Rafowicz, Centre de Référence pour le Traitement des Maladies Hémorragiques (CRTH), Hôpital Bicêtre, Kremlin Bicêtre, Paris, France; S Ranta, Department of Pediatrics, Clinic of Coagulation Disorders, Karolinska Hospital, Stockholm,Sweden; E Santagostino, ME Mancuso, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione, IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy; A Thomas*, Royal Hospital for Sick Children, Edinburgh, UK and J Motwani, Department of Hematology, The Children’s Hospital, Birmingham, UK.
PedNet Study group members and centers in Israel.
GK, National Hemophilia Center, Ministry of Health, Sheba Medical Center, Hashomer, Israel.
PedNet Study group members and centers in Canada.
MC, Division of Hematology/Oncology, Hospital for Sick Children, Toronto, Ontario and GR, Division of Hematology/Oncology, Hôpital St Justine, Montréal, Quebec, Canada.
*No longer participating as a PedNet center.
References
- 1.Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016; 388(10040):187-197. [DOI] [PubMed] [Google Scholar]
- 2.Katz J. Prevalence of factor IX inhibitors among patients with haemophilia B: results of a large-scale North American survey. Haemophilia. 1996;2(1):28-31. [DOI] [PubMed] [Google Scholar]
- 3.Goodeve AC. Hemophilia B: molecular pathogenesis and mutation analysis. J Thromb Haemost. 2015;13(7):1184-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulman S, Eelde A, Holmstrom M, Stahlberg G, Odeberg J, Blomback M. Validation of a composite score for clinical severity of hemophilia. J Thromb Haemost. 2008;6(7):1113-1121. [DOI] [PubMed] [Google Scholar]
- 5.Tagariello G, Iorio A, Santagostino E, et al. Comparison of the rates of joint arthroplasty in patients with severe factor VIII and IX deficiency: an index of different clinical severity of the 2 coagulation disorders. Blood. 2009;114(4):779-784. [DOI] [PubMed] [Google Scholar]
- 6.Clausen N, Petrini P, Claeyssens-Donadel S, Gouw SC, Liesner R. Similar bleeding phenotype in young children with haemophilia A or B: a cohort study. Haemophilia. 2014; 20(6):747-755. [DOI] [PubMed] [Google Scholar]
- 7.Belvini D, Salviato R, Radossi P, et al. Molecular genotyping of the Italian cohort of patients with hemophilia B. Haematologica. 2005;90(5):635-642. [PubMed] [Google Scholar]
- 8.Miller CH, Benson J, Ellingsen D, et al. F8 and F9 mutations in US haemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia. 2012;18(3):375-382. [DOI] [PubMed] [Google Scholar]
- 9.Rallapalli PM, Kemball-Cook G, Tuddenham EG, Gomez K, Perkins SJ. An interactive mutation database for human coagulation factor IX provides novel insights into the phenotypes and genetics of hemophilia B. J Thromb Haemost. 2013; 11(7):1329-1340. [DOI] [PubMed] [Google Scholar]
- 10.Castaman G, Bonetti E, Messina M, et al. Inhibitors in haemophilia B: the Italian experience. Haemophilia. 2013;19(5):686-690. [DOI] [PubMed] [Google Scholar]
- 11.Puetz J, Soucie JM, Kempton CL, Monahan PE. Prevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection database. Haemophilia. 2014; 20(1):25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046-4055. [DOI] [PubMed] [Google Scholar]
- 13.Calvez T, Chambost H, d'Oiron R, et al. Analyses of the FranceCoag cohort support differences in immunogenicity among one plasma-derived and two recombinant factor VIII brands in boys with severe hemophilia A. Haematologica. 2018;103(1):179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckhardt CL, van Velzen AS, Peters M, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood. 2013;122(11):1954-1962. [DOI] [PubMed] [Google Scholar]
- 15.Santoro C, Quintavalle G, Castaman G, et al. Inhibitors in hemophilia B. Semin Thromb Hemost. 2018;44(6):578-589. [DOI] [PubMed] [Google Scholar]
- 16.Chitlur M, Warrier I, Rajpurkar M, Lusher JM. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997-2006). Haemophilia. 2009; 15(5):1027-1031. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Miller CH, Payne AB, Craig Hooper W. The CDC hemophilia B mutation project mutation list: a new online resource. Mol Genet Genomic Med. 2013;1(4):238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saini S, Hamasaki-Katagiri N, Pandey GS, et al. Genetic determinants of immunogenicity to factor IX during the treatment of haemophilia B. Haemophilia. 2015; 21(2):210-218. [DOI] [PubMed] [Google Scholar]
- 19.Martensson A, Letelier A, Hallden C, Ljung R. Mutation analysis of Swedish haemophilia B families - high frequency of unique mutations. Haemophilia. 2016;22(3):440-445. [DOI] [PubMed] [Google Scholar]
- 20.Warrier I. Factor IX antibody and immune tolerance. Vox Sang. 1999;77(Suppl 1):S70-71. [DOI] [PubMed] [Google Scholar]
- 21.Warrier I, Ewenstein BM, Koerper MA, et al. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediatr Hematol Oncol. 1997;19(1):23-27. [DOI] [PubMed] [Google Scholar]
- 22.Thorland EC, Drost JB, Lusher JM, et al. Anaphylactic response to factor IX replacement therapy in haemophilia B patients: complete gene deletions confer the highest risk. Haemophilia. 1999;5(2):101-105. [PubMed] [Google Scholar]
- 23.Recht M, Pollmann H, Tagliaferri A, Musso R, Janco R, Neuman WR. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia. 2011;17(3):494-499. [DOI] [PubMed] [Google Scholar]
- 24.Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood. 1997;89(3):1115-1116. [PubMed] [Google Scholar]
- 25.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer K, Lassila R, Peyvandi F, et al. Inhibitor development in haemophilia according to concentrate. Four-year results from the European HAemophilia Safety Surveillance (EUHASS) project. Thromb Haemost. 2015;113(5):968-975. [DOI] [PubMed] [Google Scholar]
- 27.Franchini M, Santoro C, Coppola A. Inhibitor incidence in previously untreated patients with severe haemophilia B: a systematic literature review. Thromb Haemost. 2016;116(1):201-203. [DOI] [PubMed] [Google Scholar]
- 28.Fischer K, Ljung R, Platokouki H, et al. Prospective observational cohort studies for studying rare diseases: the European PedNet Haemophilia Registry. Haemophilia. 2014; 20(4):e280-286. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg HM, Hashemi SM, Fischer K, et al. Increased inhibitor incidence in severe haemophilia A since 1990 attributable to more low titre inhibitors. Thromb Haemost. 2016;115(4):729-737. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins PV, Egan H, Keenan C, et al. Mutation analysis of haemophilia B in the Irish population: increased prevalence caused by founder effect. Haemophilia. 2008;14(4):717-722. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz J, Astermark J, Menius ED, et al. F8 haplotype and inhibitor risk: results from the Hemophilia Inhibitor Genetics Study (HIGS) Combined Cohort. Haemophilia. 2013;19(1):113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138(3):305-315. [DOI] [PubMed] [Google Scholar]
- 33.Schwaab R, Brackmann HH, Meyer C, et al. Haemophilia A: mutation type determines risk of inhibitor formation. Thromb Haemost. 1995;74(6):1402-1406. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.