Abstract
Outcomes after relapse of childhood B-acute lymphoblastic leukemia (B-ALL) are poor, and optimal therapy is unclear. The children’s Oncology Group study AALL0433 evaluated a new platform for relapsed ALL. Between March 2007 and October 2013 AALL0433 enrolled 275 participants with late bone marrow or very early isolated central nervous system (iCNS) relapse of childhood B-ALL. Patients were randomized to receive standard versus intensive vincristine dosing; this randomization was closed due to excess peripheral neuropathy in 2010. Patients with matched sibling donors received allogeneic hematopoietic cell transplantation (HCT) after the first three blocks of therapy. The prognostic value of minimal residual disease (MRD) was also evaluated in this study. The 3-year event free and overall survival (EFS/OS) for the 271 eligible patients were 63.6±3.0% and 72.3±2.8% respectively. MRD at the end of Induction-1 was highly predictive of outcome, with 3-year EFS/OS of 84.9±4.0% and 93.8±2.7% for patients with MRD <0.1%, versus 53.7±7.8% and 60.6± 7.8% for patients with MRD ≥0.1% (P<0.0001). Patients who received HCT versus chemotherapy alone had an improved 3-year disease-free survival (77.5±6.2% vs. 66.9 + 4.5%, P=0.03) but not OS (81.5±5.8% for HCT vs. 85.8±3.4% for chemotherapy, P=0.46). Patients with early iCNS relapse fared poorly, with a 3-year EFS/OS of 41.4±9.2% and 51.7±9.3%, respectively. Infectious toxicities of the chemotherapy platform were significant. The AALL0433 chemotherapy platform is efficacious for late bone marrow relapse of B-ALL, but with significant toxicities. The MRD threshold of 0.1% at the end of Induction-1 was highly predictive of the outcome. The optimal role for HCT for this patient population remains uncertain. This trial is registered at clinicaltrials.gov (NCT# 00381680).
Introduction
Childhood acute lymphoblastic leukemia (ALL) is highly curable,1,2 but 10% of patients will not survive, primarily because of relapse. After relapse, B-lymphoblastic phenotype, longer first remission, and isolated extramedullary relapse have been associated with improved chances of salvage.3-6 Minimal residual disease (MRD) post re-Induction has also been shown to be predictive of outcome.7,8 Previous studies have shown that up to one-half of patients with late marrow or very early isolated extramedullary (IEM) relapse can be cured.5,9,10,11,12 However, this group is heterogeneous, and it is challenging to distinguish which patients require therapies beyond chemotherapy, particularly hematopoietic cell transplantation (HCT).
The Children’s Oncology Group (COG)11-14 and other international cooperative groups9,15,16,17,18 have used highly varied chemotherapy approaches for relapsed ALL with largely similar outcomes. Although HCT has been successfully utilized in relapsed ALL,19-21 there are limited and conflicting data whether HCT is superior to conventional chemotherapy for late marrow or IEM relapse.22 Patients with HLA-matched sibling donors were previously reported to have better outcomes than those from unrela-ted/ alternative donors due to decreased toxicities. However, more recent reports suggest similar HCT outcomes regardless of the donor type.20,23
COG AALL0433 (NCT# 00381680) for intermediaterisk first relapse of B-ALL was instituted to evaluate the efficacy of a hybrid platform, combining elements from prior COG studies for both bone marrow and IEM relapse.24-26 Eligible patients overlap considerably with the S2 group from the Berlin, Franfurt, Muenster (BFM) relapse trials.15 This report details results from AALL0433.
Methods
Patient characteristics
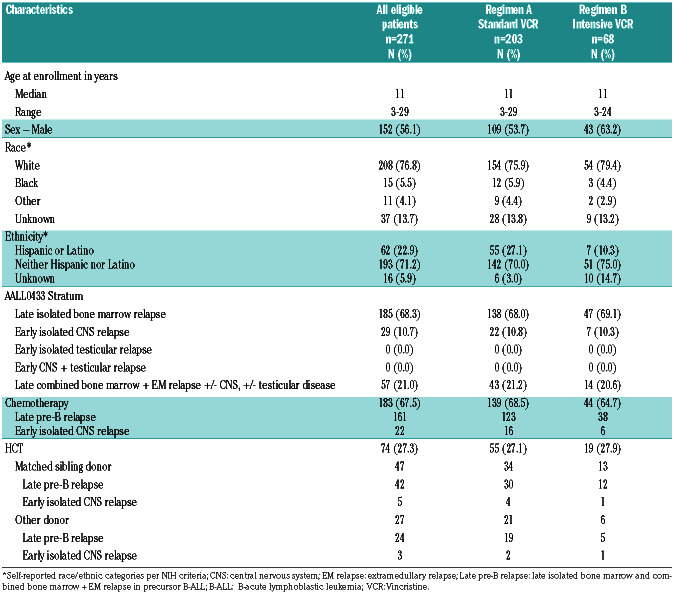
AALL0433 enrolled subjects age 1-29.99 years with intermediate- risk relapse of B-ALL between March 2007 and October 2013. Eligible patients had late bone marrow or combined marrow/ EM relapse ≥36 months from diagnosis, or very early IEM (CNS/testicular) relapse <18 months from diagnosis. Bone marrow relapse was defined as an M3 marrow (>25% blasts) occurring after attaining complete remission (CR) without concurrent CNS/testicular relapse. Isolated CNS (iCNS) relapse was defined as cerebrospinal fluid having ≥5 cells/microliter with blasts or clinical or radiologic signs of CNS leukemia. Biopsy was required to establish the diagnosis of isolated testicular relapse. Combined relapse was defined as a CNS and/or testicular relapse with concurrent M2 (≥5% blasts) or M3 marrow. AALL0433 was approved by the National Cancer Institute (NCI) and Institutional Review Boards of participating institutions. Informed consent was obtained in accordance with Department of Health and Human Services Guidelines.
Treatment
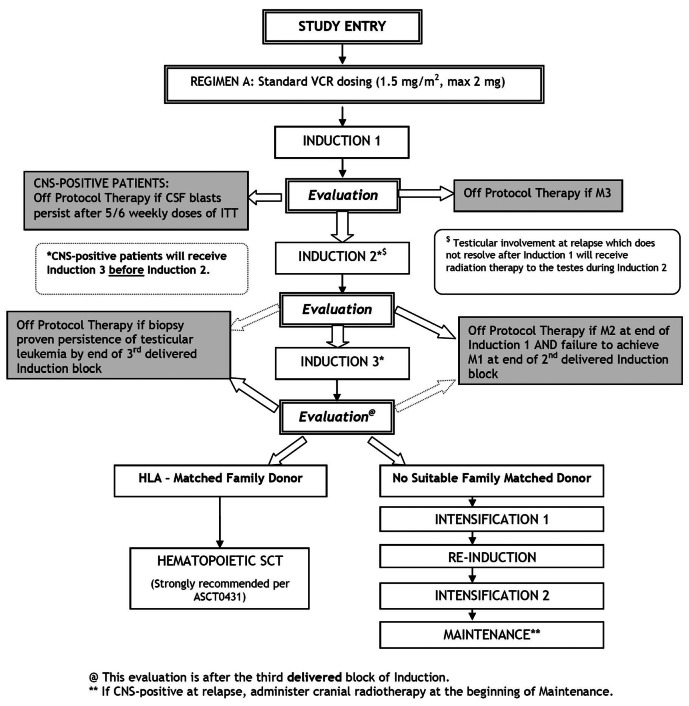
AALL0433 utilized chemotherapy beginning with three intensive blocks from COG AALL01P2 [24], followed by intensification, reinduction, and maintenance from COG 9412/AALL02P225, 26 (Figure 1 and Online Supplementary Table S1). The duration of therapy was 2 years. iCNS and combined marrow/CNS patients who did not proceed to HCT received 18 Gy cranial radiotherapy at the beginning of maintenance. Testicular relapse patients with residual involvement (biopsyproven) after Induction-1 were to receive 24 Gy radiotherapy.
Patients were randomized at enrollment to either standard (Arm A, 1.5 mg/m2, maximum 2 mg) or intensive (Arm B, 2 mg/m2, maximum 2.5 mg) vincristine dosing. Randomization was suspended in June 2010 after scheduled interim monitoring revealed excess peripheral neuropathy in Arm B meeting predefined stopping rules. The amended study (without randomization) re-opened in November 2010. Since there were no differences in outcomes for Arm A pre- and post- randomization closure, outcome data for the standard arm were pooled
All patients with an HLA matched sibling donor were recommended to proceed to HCT after completing three treatment blocks. HCT regimens were not prescribed, and were given at the discretion of treating centers; patients were encouraged to enroll on concurrent COG HCT study ASCT0431.19 Unrelated/alternative donor HCT was not included in AALL0433, but outcome data were collected for those who underwent unrelated/alternative donor HCT per investigator discretion.
MRD assessment
MRD was performed at two COG reference laboratories using previously described flow cytometric methods.27-29 MRD was measured at the end of Induction-1 and Induction-3. Results were blinded to investigators, as one study objective was to establish prognostic MRD thresholds for patients with relapse.
Toxicity assessment
Data on adverse events and clinically significant laboratory findings are summarized using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Adverse event reporting was supplemented with NCI’s Adverse Event Expedited Reporting System (AdEERS) and MedWatch reports.
Statistical analysis
Primary outcomes were 3-year event-free survival (EFS) and overall survival (OS) rates. EFS was defined as time from enrollment to first event (induction failure, induction death, death in remission, relapse, or second malignant neoplasm [SMN] or date of last contact for those who were event-free). OS was calculated as time from enrollment to death or last contact for those alive. Disease-free survival (DFS) and OS for patients on the standard arm were compared between those receiving chemotherapy versus HCT (matched sibling or other donor) post achieving second complete remission (CR2), where CR2 was defined as having <5% blasts in the bone marrow morphologically. For analyses involving HCT in CR2, DFS/OS were defined from date of CR2; and DFS times for chemotherapy patients were adjusted by median time to HCT for the cohort.
Survival rates were estimated using the Kaplan-Meier method with standard errors of Peto et al.30,31 The two-sided log-rank test was used for the comparison of the survival curves. The cumulative incidence function for competing risks was used to calculate cumulative incidence rates, and comparisons were made using the K-sample test.32 Comparison of proportions was done using the c2 test or Fisher’s Exact test. P-values <0.05 were considered statistically significant for all comparisons. All analyses were performed using SAS® version 9.4. Graphics were generated using R (http://www.R-project.org, version 3.4.0). Data frozen on 06/30/2018 are included in this report.
Results
Participants
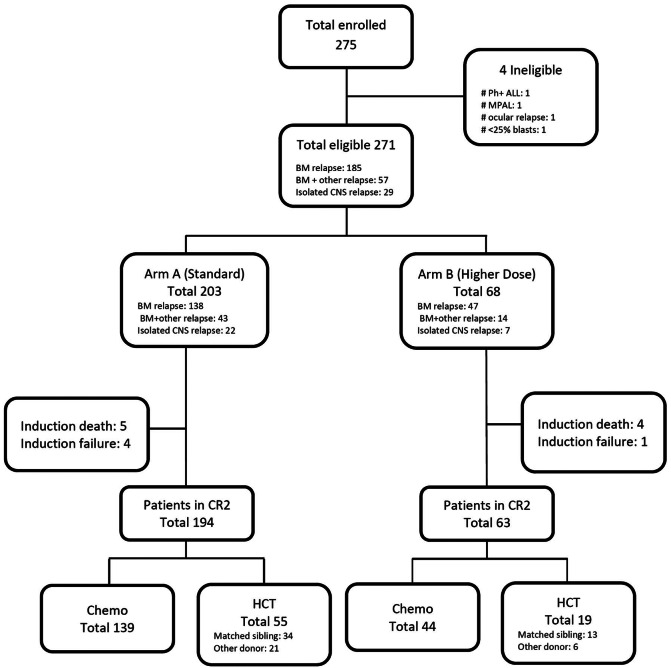
AALL0433 enrolled 275 subjects between March 2007 and October 2013; four patients were ineligible, not meeting inclusion criteria. The median follow-up time was 4.13 years (range: 0.05–9.91). The patient flow and characteristics are given in Figure 2 and Table 1. Of 271 eligible patients, 185 had bone marrow, 57 had combined marrow/ EM, and 29 had very early iCNS relapse. No patients with isolated or combined testicular relapse were enrolled. Nine patients died in Induction-1. Five patients went off protocol therapy due to induction failure (three with M3 marrow after Induction-1, two failing to achieve an M1 marrow [< 5% blasts] after Induction-2). Three patients who achieved CR2 died in Induction-3. Two hundred and fifty-seven patients (95%) achieved CR2, and 254 completed the threeblock induction in CR2. One hundred and eighty-three patients (161 late marrow/combined, 22 iCNS) chemotherapy/ radiotherapy per protocol, and seventy-four patients underwent HCT in CR2 (51 late marrow, 16 combined, and seven iCNS relapses). Fourty-seven patients received matched sibling donor HCT as outlined in the protocol but 27 underwent alternative donor HCT. The median time to transplant from CR2 was 102 days (range: 52-379) for late marrow and 88 days (range: 27-253) for iCNS relapse.
Figure 1.
Schema. CNS: central nervous system; VCR: Vincristine; CSF: cerebrospinal fluid; ITT: intention-to-treat ; HLA: human leukocyte antigen; SCT: stem cell transplant.
Treatment outcome
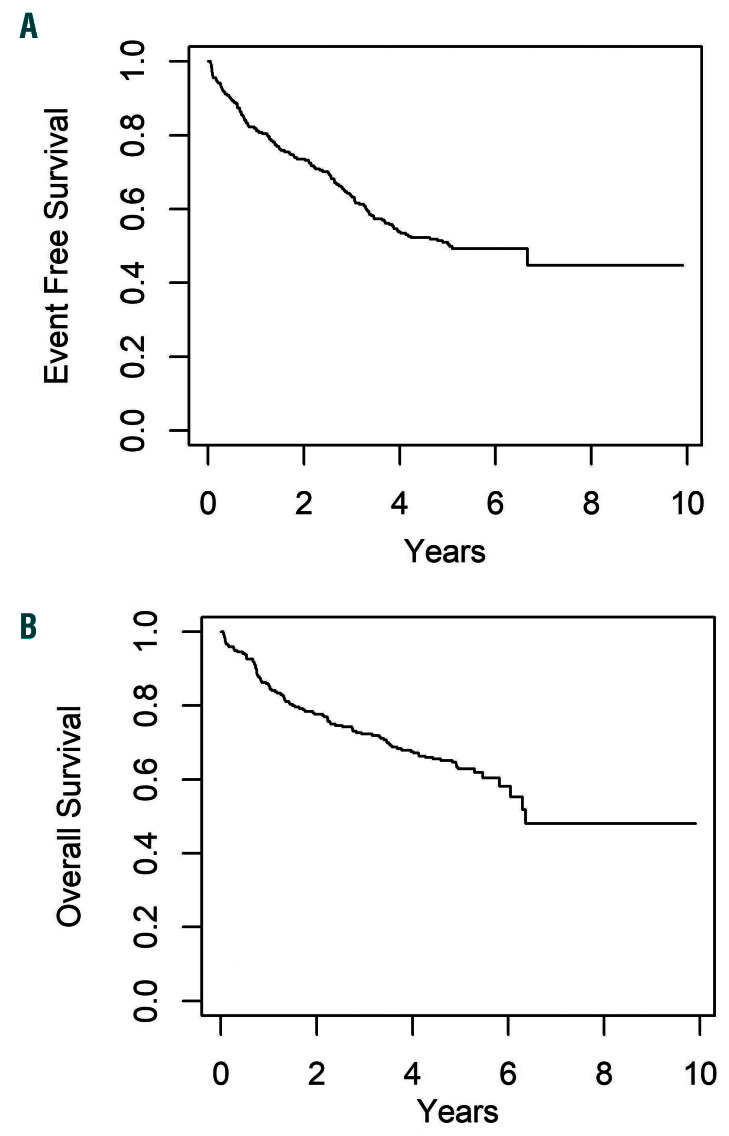
The EFS/OS for all 271 eligible patients were, 63.6±3.0% and 72.3±2.8% at 3 years, and 51.0±3.5% and 62.9±3.3% at 5 years, respectively (Figure 3). The overall EFS/OS for patients with bone marrow/combined relapse were 66.3±3.1% and 74.8±2.9% at 3 years, and 52.5±3.7% and 65.1±3.5% at 5 years. Younger patients at the time of enrollment had superior outcomes compared to older patients, with 3-year EFS and OS of 68.7±3.6% and 76.2±3.3% respectively for patients less than 13 years old versus 54.1±5.3% and 65.1±5.1% for patients greater than 13 years (P=0.0037 for EFS, P=0.0009 for OS).
The 3-year cumulative incidence of isolated bone marrow second relapse was 14.7±2.5%, considering other sites of relapse, induction failure, induction death, death in remission and SMN as competing risks. The 3-year cumulative incidence of remission death was 6.0±1.7%, considering induction events (induction deaths and induction failure), relapse and SMN as competing events. Six patients developed SMN (all therapy-related myeloid leukemia or myelodysplastic syndrome).
Establishment of MRD threshold
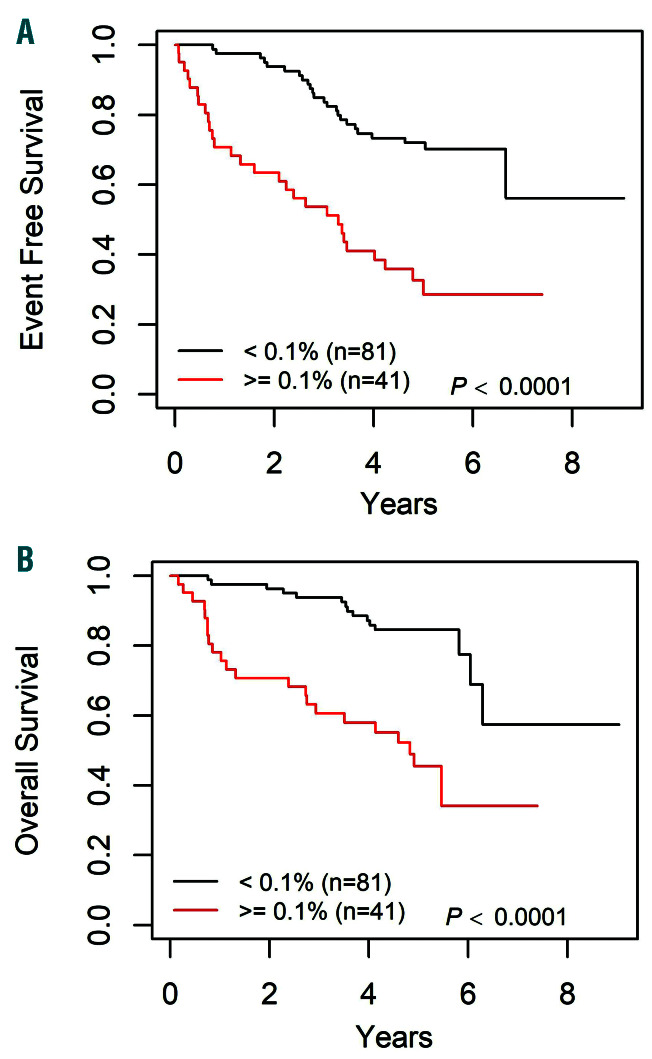
The outcomes of patients with bone marrow or combined marrow/ EM relapse treated on the standard arm were stratified by MRD levels after Induction-1. Of 122 patients with data available, 62 (51%) had MRD <0.01%, 41 (34%) had MRD >0.1%, and 19 (16%) had levels between 0.01-0.1%. The 3-year EFS/OS were 84.9±4.0% and 93.8±2.7% for patients with MRD <0.1%, versus 53.7 ±7.8% and 60.6±7.8% for patients with MRD >0.1% (P<0.0001) (Figure 4). The most prognostic MRD threshold was 0.1%, as there were no significant differences in outcome between patients with MRD <0.01% versus those with intermediate MRD levels (0.01-0.1%) (Online Supplementary Figure S1A).
Figure 2.
CONSORT Diagram. BM: bone marrow; HCT: hematopoietic cell transplantation; CR2: second complete remission; CNS: central nervous system; Ph-ALL: Philadelphia chromosome–like acute lymphoblastic leukemia; MPAL: mixed phenotype acute leukemia.
Table 1.
Patient characteristics.
Seventy of the above patients had end of Induction-3 MRD data; 62 (88.6%) were <0.1% and 8 (11.4%) were ≥0.1%. The eight patients with MRD >0.1% had inferior outcomes with 3-year EFS 37.5±17.1% and OS 50.0±17.7% compared to 85.1±4.6% and 95.1±2.8% for those <0.1% (P=0.0007 for EFS; P=0.0105 for OS) (Online Supplementary Figure S1B).
Hematopoietic cell transplantation
The DFS/OS for patients with bone marrow/combined relapse who received chemotherapy alone versus HCT were compared. The DFS analyses for patients receiving chemotherapy were adjusted by median time to transplant, resulting in 10 chemotherapy patients (six relapses, three deaths, one withdrawal of consent) being excluded from these analyses. There were no significant differences in the DFS/OS between patients receiving matched sibling donor HCT versus other HCT (Online Supplementary Figure S2); further analyses combined all HCT patients regardless of the donor source.
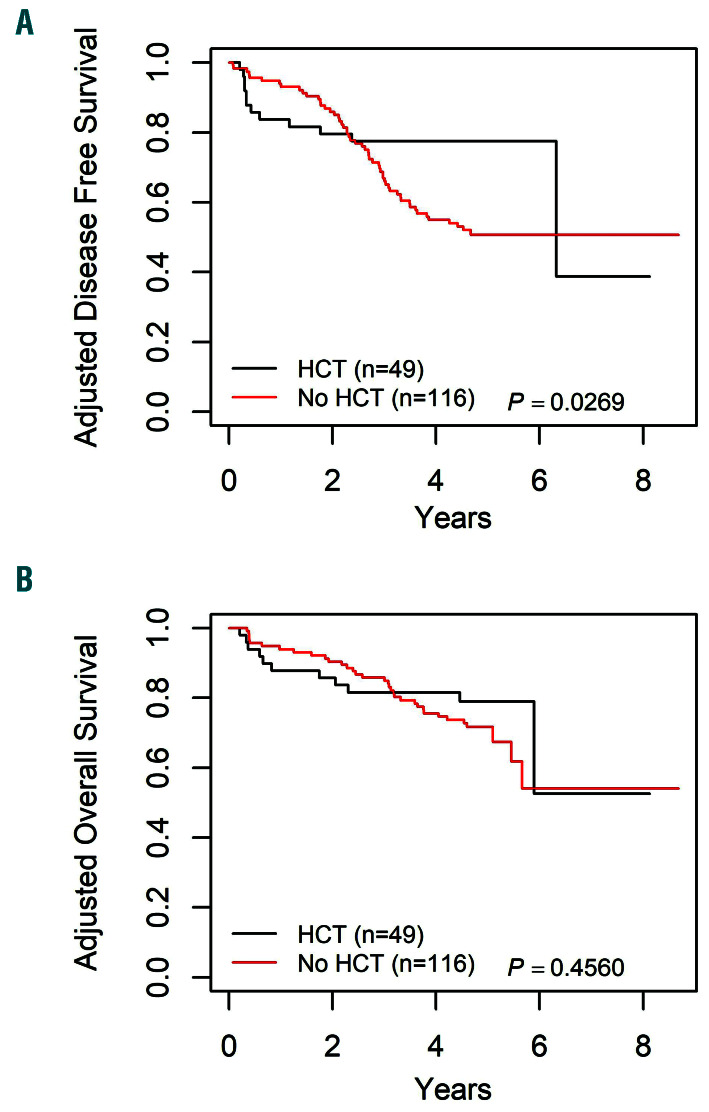
Analyzing patients on the standard arm alone, a significant improvement in DFS was seen for HCT over chemotherapy, with a 3-year DFS of 77.5±6.2% versus 66.9±4.5% (P=0.03) (Figure 5). However, this did not correspond with improved OS (81.5±5.8% for HCT vs. 85.8±3.4% for chemotherapy; P=0.46). When patients on both vincristine dose arms were included, the 3-year DFS maintained a trend towards significance (73.1±5.6% for HCT vs. 65.6±4.0%, P=0.08).
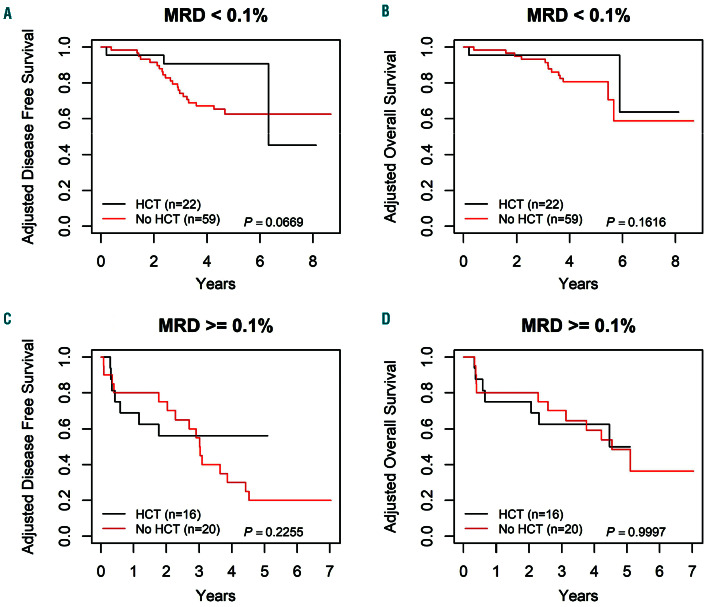
The HCT outcomes at different MRD thresholds were also examined (Figure 6). In patients with MRD <0.1% at the end of Induction-1, HCT showed a trend of improved 3-year DFS of 90.7±6.5% versus 74.1±5.8% for chemotherapy (P=0.07). The difference in 3-year OS was not significant, 95.5±4.7% for HCT versus 93.1±3.4% for chemotherapy (P=0.16). In patients with MRD >0.1% after Induction-1, no benefit was seen for HCT over chemotherapy for 3-year DFS (HCT 56.3±13.2%, chemotherapy 55.0±11.1, P=0.23) or 3-year OS (HCT 62.5±12.8%, chemotherapy 70.0±10.3%, P=1.00).
Figure 3.
Overall outcomes for all eligible patients. (A) Event-free survival (EFS) curves, 3-year and 5-year EFS: 63.6±3.0%, and EFS 51.0%±3.5%, respectively. (B) Overall survival (OS), 3-year and 5-year OS 72.3±2.8%, and OS 62.9±3.3%, respectively.
Figure 4.
Survival curves by 0.1% minimal residual disease (MRD) threshold after induction-1 for late bone marrow relapse patients on standard arm (Arm A), with available MRD data. (A) Event-free survival (EFS) curves by 0.1% MRD threshold, 3-year EFS: 84.9±4.0% for patients with MRD <0.1% versus 53.7±7.8% for patients with MRD ≥0.1% (P<0.0001). (B) Overall survival (OS) curves by 0.1% MRD threshold, 3-year OS: 93.8±2.7% for patients with MRD <0.1% and 60.6±7.8% for patients with MRD ≥0.1% (P<0.0001).
Isolated CNS relapse
The 3-year EFS/OS for eligible patients with very early iCNS relapse were 41.4±9.2% and 51.7±9.3%, respectively. No deaths or induction failures were observed during the three induction blocks for iCNS patients. After completion of Induction-3, seven patients were treated with HCT, and 22 continued chemotherapy/radiotherapy. After adjusting DFS/OS times for the chemotherapy cohort by median time to transplant from CR2 (88 days), one chemotherapy patient relapsed before that and was excluded from the DFS/OS analyses. The 3-year DFS/OS for transplanted patients (n=7) were 71.4±17.0% and 71.4±17.1%, compared to 28.6±9.9% and 42.9±10.8% for those who received chemotherapy/radiotherapy (n=21), respectively (P=0.12 for DFS, P=0.18 for OS) (Online Supplementary Figure S3). Three patients of the 14 of 28 patients who relapsed, recurred in the CNS, 10 in bone marrow (BM) alone, and one patient in both BM/CNS.
Vincristine randomization
Planned interim toxicity analysis in 2010 showed an increase in grade 3 or higher peripheral neuropathy in the higher-dose vincristine arm 14 of 68 (21%) compared to 3 of 69 (4%) in the control arm (P=0.004). The difference was primarily reported in patients >13 years old, with an incidence of 9 of 26 (25%) in the higher-dose arm compared to 1 of 21 (5%) in the control arm. There was no significant difference in patients <13, with 2 of 48 (4.2%) in the standard versus 5 of 42 (11.9%) in the higher-dose arm, P=0.245. These results led to the suspension, followed by closure of the vincristine randomization as there was no evidence of an improved MRD response at the time of interim analysis (data not shown). At final analysis, there was a trend toward inferiority of the higher dose arm, with a 3-year EFS of 70.1±3.5% versus 55.1±6.5% for standard versus intensive dosing (P=0.091), and OS of 78.6 ±3.1% versus 63.6±6.3% (P=0.071) (Online Supplementary Figure S4).
Infection/deaths
Eighteen on-study deaths (6.6%) due to toxicity were reported in patients receiving chemotherapy. All were due to infection and their sequelae (Online Supplementary Table S2). Nine occurred in Induction-1, giving an induction death rate of 3.3%. An additional nine deaths occurred in patients achieving CR2 – all followed highdose cytarabine in the Induction-3 (n=3) or Intensification-2 phases (n=6). Grade 3 or higher bloodstream infections were also common in these phases, with incidences of 23.6%, 29%, and 30.6% in Induction- 1, Induction-3, and Intensification-2, respectively.
Discussion
Despite the high success rate of treatment for newly diagnosed childhood B-ALL, survival for those relapsing after frontline treatment remains poor. In contrast to newly-diagnosed B-ALL, where therapy is similar across international study groups, therapies used for relapse are highly variable. Divergent therapeutic approaches, smaller patient numbers for clinical trials, and varying utilization of HCT make it difficult to compare trial results from different cooperative study groups.
We report the outcomes from the first Phase 3 trial for BM and very early IEM relapse of B-ALL from the Children’s Oncology Group. The 3-year EFS and OS of 63.6% and 72.3% from AALL0433 are similar to the overall results reported from the UK ALLR3 trial, which revealed 3-year progression free survival of 60% and OS of 72%.18 However, 57% of patients in the R3 study underwent HCT in the sescond remission as opposed to continued chemotherapy. In contrast, less than one third of patients enrolled on AALL0433 received HCT. While the overall results are broadly similar between studies, the significant differences in the percentage of patients recei-ving HCT in the second remission makes it challenging to compare the relative efficacy of the different chemotherapy regimens.
Although the overall outcomes from AALL0433 are similar to those reported by other groups, significant infectious toxicities occurred even after Induction-1, with half of treatment-related deaths occurring in CR2. The toxicity death rate from the current study is similar to the 5% (4 of 76) death rate from the 9412 protocol which served as the framework for AALL0433,25 and also within the range reported in recent trials for relapsed ALL.10,17,24
Higher vincristine doses were hypothesized to improve outcomes. Capping of vincristine dose was adopted from clinical observations from the 1960’s,33 but results in a relatively lower dose (based on body surface area) for many older patients with ALL, who are known to have a higher risk of relapse. However, vincristine intensification was not feasible in this non-blinded randomization due to an unacceptably high incidence of peripheral neuropathy, particularly among patients older than 13 years. Patients on this trial had significant prior vincristine exposure during primary therapy, which may have been a cofactor. Although the randomization was stopped before meeting statistical endpoints, the higher dose arm trended toward inferior outcomes. Genetic predictors of vincristine neuropathy were explored as a secondary study objective, and polymorphisms in CEP72 were discovered to be associa-ted with neuropathy incidence.34
MRD has been shown to be one of the most important predictors of outcome in newly diagnosed ALL, but prognostic threshold values after relapse are less clear. The end-induction MRD threshold of 0.01% is highly prognostic in frontline ALL trials from COG and European cooperative groups27,29,35,36 and was also highly prognostic in the AIEOP REC 2003 relapse trial.8 However, the UKALL R3 relapse protocol used the same 0.01% threshold, and showed improved progression-free survival for those receiving mitoxantrone (66%) versus idarubicin (46%) despite no difference in MRD clearance between arms.17,18 A higher MRD threshold of 0.1% was highly predictive of outcome in BFM REZ P95/96/2002.7,37 The same 0.1% threshold was the strongest predictor of outcome on AALL0433, and is currently being used to riskstratify patients on the successor COG relapse study AALL1331 (NCT02101853). It is feasible that the optimal MRD threshold used to stratify risk groups may be higher in relapse than in newly diagnosed ALL.
Figure 5.
Survival curves by hematopoietic cell transplantation versus chemotherapy alone, for late bone marrow relapse patients on Arm A. (A) Adjusted disease-free survival (DFS) curves by hematopoietic cell transplantation (HCT) versus chemotherapy alone, 3-year DFS: 77.5±6.2% for HCT and 66.9±4.5% for chemotherapy alone (P=0.03). One relapse occurred after 6 years, where only two patients had more than 6 years of follow-up, which resulted in DFS for HCT dropping from 77.5% to 38.8%. (B) Adjusted overall survival (OS) curves by HCT versus chemotherapy alone, 3-year OS: 81.5±5.8% for HCT and 85.8±3.4% for chemotherapy alone (P=0.46).
The indications for HCT after late BM or early IEM relapse of B-ALL are unclear, as randomized trials have not been feasible in this population. Although there was an improved 3-year DFS for patients receiving HCT over chemotherapy in AALL0433, this did not result in improved OS. Sub-analysis showed improved DFS with HCT in patients with MRD <0.1% after Induction-1, but no improvement in those with MRD ≥0.1%, and no improvement in OS for HCT over chemotherapy regardless of the MRD level.
This is contrary to other reports where HCT improved outcomes for patients with poor MRD response.38,39 Lower pre-transplant MRD levels correlate with improved HCT outcomes,19,40 but it is less clear how MRD kinetics immediately after induction (typically several months before HCT) relate to eventual outcomes. The emergence of immunotherapeutic options (such as blinatumomab and CAR T-cell therapies) during AALL043341-43 may have improved the chances of salvage and prolonged OS for patients with a second relapse.
Figure 6.
Survival curves by hematopoietic cell transplantation versus chemotherapy alone, for late bone marrow relapse patients on Arm A, using a 0.1% minimal residual disease threshold. (A) Adjusted disease-free survival (DFS) curves for minimal residual disease (MRD) <0.1%, by hematopoietic cell transplantation (HCT) versus chemotherapy alone, 3-year DFS: 90.7±6.5% for HCT and 74.1±5.8% for chemotherapy alone (P=0.07). (B) Adjusted overall survival (OS) curves for MRD <0.1%, by HCT versus chemotherapy alone, 3-year OS: 95.5±4.7% for HCT and 93.1±3.4% for chemotherapy alone (P=0.16). (C) Adjusted disease-free survival (DFS) curves for MRD ≥0.1%, by HCT versus chemotherapy alone, 3-year DFS: 56.3±13.2% for HCT and 55.0± 11.1% for chemotherapy alone (P=0.23). (D) Adjusted OS curves for MRD ≥0.1%, by HCT versus chemotherapy alone, 3-year OS: 62.5±12.8% for HCT and 70.0±10.3% for chemotherapy alone (P=1.00).
The AALL0433 regimen has significant toxicity. AALL0433 includes 45,000 mg/m2 of high-dose methotrexate, 12,200 mg/m2 of cyclophosphamide, 2,900 mg/m2 of etoposide, and five courses of high-dose cytarabine, which were associated with nine deaths in second remission. As broadly similar results have been seen in other recent trials for this patient population with regimens with lower toxicity,5,6 further intensification of traditional chemotherapeutic agents is unlikely to improve outcomes for relapsed ALL.
This study has several limitations. Measurement of MRD at the time AALL0433 opened was performed as a research test (with blinded results), but became common clinical practice by the time of study closure, leading some investigators to pursue HCT off study. AALL0433 was not powered to formally compare HCT and chemotherapy, especially for iCNS patients where numbers were small (although a similar trend favoring HCT for iCNS patients was also noted in ALL R3).44 Additionally, vincristine do-sing was not blinded to investigators or families, so reporting bias is feasible in the incidence of neuropathy reported on the higher dose arm.
Newer therapies for relapsed ALL including immunotherapies and small molecule inhibitors, are being actively investigated.45 Many of these therapies have shown impressive early results in patients with a second or later relapse and merit testing in patients at the time of the first relapse. The COG AALL1331 relapse trial includes a randomization to intensive post-induction chemotherapy blocks (based upon UKALL R3) versus replacement with blinatumomab. Immunotherapies will also be tested in upcoming COG trials for patients with newly diagnosed ALL (NCT #’s 03914625, 03959085, 03876769). Ultimately, the goal of curing more patients will result from preventing relapses, and not treating them.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Rochelle Yanofsky, Elizabeth Raetz, Mignon Loh, Susan Conway, Catherine Shannon, and the other members of the AALL0433 committee for their contributions to this work. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia.
Funding Statement
Funding: This study was supported by grants U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173 and U10 CA180899 from the National Institutes of Health, and by St. Baldrick’s Foundation.
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children's Oncology Group. J Clin Oncol. 2012; 30(14):1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015; 33(27):2938-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. 1998;102(2):423-438. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen K, Devidas M, Sheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22(12):2142-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14(6):e205-217. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Moretta F, Rutella S. Management of relapsed acute lymphoblastic leukemia in childhood with conventional and innovative approaches. Curr Opin Oncol. 2013:25(6):707-715. [DOI] [PubMed] [Google Scholar]
- 7.Eckert C, von Stackelberg A, Seeger K, et al. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALLREZ BFM P95/96. Eur J Cancer. 2013;49(6):1346-1355. [DOI] [PubMed] [Google Scholar]
- 8.Paganin M, Zecca M, Fabbri G, et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed ‘high-risk’ acute lymphoblastic leukemia. Leukemia. 2008;22(12):2193-2200. [DOI] [PubMed] [Google Scholar]
- 9.Lawson SE, Harrison G, Richards S, et al. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000; 108(3):531-543. [DOI] [PubMed] [Google Scholar]
- 10.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2009;28(14):2339-2347. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan GR, Rivera GK, Pollock BH, et al. Alternating drug pairs with or without periodic reinduction in children with acute lymphoblastic leukemia in second bone marrow remission: a Pediatric Oncology Group study. Cancer. 2000;88(5):1166-1174. [DOI] [PubMed] [Google Scholar]
- 12.Kelly ME, Lu X, Devidas M, et al. Treatment of relapsed precursor-B acute lymphoblastic leukemia with intensive chemotherapy: POG (Pediatric Oncology Group) study 9411 (SIMAL 9). J Pediatr Hematol Oncol. 2013;35(7):509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abshire TC, Pollock BH, Billett AL, et al. Weekly polyethylene glycol conjugated Lasparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 2000;96(5):1709-1715. [PubMed] [Google Scholar]
- 14.Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG- 1941. J Clin Oncol. 2006;24(19):3150-3156. [DOI] [PubMed] [Google Scholar]
- 15.Henze G, von Stackelberg A, Eckert C. ALLREZ BFM--the consecutive trials for children with relapsed acute lymphoblastic leukemia. Klin Padiatr. 2013;225(Suppl 1): S73-78 . [DOI] [PubMed] [Google Scholar]
- 16.Roy A, Cargill A, Love S, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130(1):67-75. [DOI] [PubMed] [Google Scholar]
- 17.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker C, Krishnan S, Hamadeh L, et al. Outcomes of patients with childhood B-cell precursor acute lymphoblastic leukaemia with late bone marrow relapses: long-term follow-up of the ALLR3 open-label randomised trial. Lancet Haematol. 2019; 6(4):e204-e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: A phase 3 Children's Oncology Group / Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123(13):2017-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors - the ALL-SCT-BFM-2003 trial. J Clin Oncol. 2015;33(11):1265-1274. [DOI] [PubMed] [Google Scholar]
- 21.Uderzo C, Valsecchi MG, Bacigalupo A, et al. Treatment of childhood acute lymphoblastic leukemia in second remission with allogeneic bone marrow transplantation and chemotherapy: ten-year experience of the Italian Bone Marrow Transplantation Group and the Italian Pediatric Hematology Oncology Association. J Clin Oncol. 1995; 13(2):352-358. [DOI] [PubMed] [Google Scholar]
- 22.Pulsipher MA, Peters C, Pui CH. High risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant. 2011;17(Suppl 1):S137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacMillan M, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(Suppl 9):S16-22. [DOI] [PubMed] [Google Scholar]
- 24.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: a Children's Oncology Group study. J Clin Oncol. 2008;26(24):3971-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barredo JC, Devidas M, Lauer SJ, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a Pediatric Oncology Group study. J Clin Oncol. 2006;24(19):3142-3149. [DOI] [PubMed] [Google Scholar]
- 26.Barredo JC, Hastings C, Lu X, et al. Isolated late testicular relapse of B-cell acute lymphoblastic leukemia treated with intensive systemic chemotherapy and response-based testicular radiation: a Children's Oncology Group study. J Pediatr Blood Cancer. 2018; 65(5):e26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111(12):5477-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borowitz MJ, Pullen DJ, Shuster JJ, et al. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors: a Children's Oncology Group study. Leukemia. 2003;17(8):1566-1572. [DOI] [PubMed] [Google Scholar]
- 29.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood. 2015;126(8):964-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 31.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Br J Cancer. 1977;35(1):1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statistics, 1988;16(3):1141-1154. [Google Scholar]
- 33.McCune JS, Lindley C. Appropriateness of maximum-dose guidelines for vincristine. Am J Health Syst Pharm. 1997;54(15):1755-1758. [DOI] [PubMed] [Google Scholar]
- 34.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313(8):815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206-3214. [DOI] [PubMed] [Google Scholar]
- 36.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809-818. [DOI] [PubMed] [Google Scholar]
- 37.Eckert C, Groeneveld-Krentz S, Kirschner-Schwabe R, et al. Improving stratification for children with late bone marrow B-cell acute lymphoblastic leukemia relapses with refined response classification and integration of genetics. J Clin Oncol. 2019; 37(36):3493-3506. [DOI] [PubMed] [Google Scholar]
- 38.Eckert C, Henze G, Seeger K, et al. Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. 2013;31(21):2736-2742. [DOI] [PubMed] [Google Scholar]
- 39.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377-384. [DOI] [PubMed] [Google Scholar]
- 40.Pulsipher MA, Langholz B, Wall DA, et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested? Bone Marrow Transplant. 2015; 50(9):1173-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016; 34(36):4381-4389. [DOI] [PubMed] [Google Scholar]
- 42.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014; 371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masurekar AN, Parker CA, Shanyinde M, et al. Outcome of central nervous system relapses in childhood acute lymphoblastic leukaemia--prospective open cohort analyses of the ALLR3 trial. PLoS One. 2014; 9(10):e108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierro J, Hogan LE, Bhatla T, Carroll WL. New targeted therapies for relapsed pediatric acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2017;17(8):725-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.