Abstract
Background
The recent outbreak of Coronavirus Disease 2019 (COVID-19) is a public health emergency of international concern. In China, Wuhan, Hubei Province was the epicenter. The disease is more severe in patients with high comorbidities and dialysis patients fall into this category.
Methods
In this report, we reviewed the whole course of the epidemic emerged in the HD center of Wuhan NO.1 Hospital by 28 February 2020. We compared the differences on the epidemiological characteristics and clinical features between patients surviving from COVID-19 and patients who died.
Result
In this hospital, at time of the present report, 627 patients were on chronic hemodialysis and the prevalence of affected cases was 11.8% (74/627).The median age of the COVID-19-positive patients was 63 years (range 31–88), with an almost even gender distribution (females accounted for 54.4%).The most frequent presenting symptom was cough (41.9%), followed by fatigue (24.2%), fever (17.2%) and dyspnea (14.8%); 22.4% of the cases were and asymptomatic. Fourteen of the 74 patients died (19%), as for presenting symptoms, cough was more frequent in patients who died (P < 0.05). Surviving patients had higher levels of phosphate and albumin, and lower levels of C-reactive protein (CRP). Chest CT scan was positive in all cases, including in asymptomatic ones, and revealed in about three fourths of the cases bilateral (76.2%) lesions; in each lung lesions were multiple in about half of the cases of the cases (52.3%). After diagnosis, median time to death was 7 days in the 14 patients who died, with a range between 4 and 18 days.
Conclusion
This preliminary, single Center study identifies hemodialysis patients as a population at high risk of severe, and deadly COVID-19 infection. While classic baseline clinical conditions, including age, kidney disease and gender, are not significantly associated with survival in COVID-19 infected hemodialysis, our study also suggests a significant association between risk of and death, poor nutritional status and less than optimal metabolic balance.
Keywords: Hemodialysis, COVID-19, Epidemic, Death, Clinical features
Introduction
The World Health Organization recently declared the outbreak of Coronavirus Disease 2019 (COVID-19) was a public health emergency of international concern [1], and Wuhan, the former hardest-hit city in central China’s Hubei Province [2]. It has been revealed in epidemiological research that people who suffer from chronic diseases like diabetes, hypertension and cardiovascular diseases and elderly people are not only more susceptible to the infection of COVID-19, but also tend to be severely struck by the disease [3]. In the light of these findings, hemodialysis (HD) patients mostly also suffer from chronic diseases like diabetes and hypertension, and have a higher mobility, a higher necessity for congregation, and a weaker immune system than ordinary people [4]. These made HD patients one of the most susceptible populations to COVID-19 and provided grounds for more concern to be poured into the health conditions of HD patients during the outbreak.
Methods
Study design and participants
We retrospectively analyzed the epidemics process in the HD center of Wuhan No.1 Hospital, a total of 74 maintenance hemodialysis patients in this study until 28 February 2020, diagnosis of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program (5th edition), which was published by the National Health Commission of China [5], the suspected case of COVID-19 is defined as the one has the epidemiological history or clinical presentations of fever, respiratory symptoms, or decreased white blood cells or lymphocytes count. The clinical diagnosed case is recognized when the suspect case displays the imaging features of pneumonia. A confirmed case of COVID-19 was defined as a positive result on high-throughput sequencing or real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens [6]. Depending on the guidance of the government, all patients were transferred to the corresponding designated hospitals for treatment after infection and disease assessment and classification. We followed-up all patients and recorded their prognosis. Only the clinical diagnosed cases and confirmed cases were included in this study. To ensure that no one infected patient was left out, every hemodialysis patient was examined by blood routine and Chest CT tests, that’s how asymptomatic cases were found.
Data collection
The medical records of patients were analyzed by the research team of the Department of Wuhan No.1 Hospital, Epidemiological, clinical, laboratory, and radiological characteristics were obtained with data collection forms from their electronic medical records stored in the hospitals HIS (hospital information system).The datum was reviewed by a trained team of physicians. During the period of follow-up, the death happened among these patients were recorded and the presumed cause of death was proposed by the research team, based on the time.
Statistical analysis
All analyses were done with SPSS software. Measurement data were expressed using the median (M) and interquartile range (P25, P75) values, Enumeration data were described as number (%). The measurement data between two groups were compared with Mann–Whitney U test, while the count data were compared with χ2 test. Binary logistic regression is utilized to analyze the risk factors of death for HD patients infected with COVID-19. A P value of less than 0.05 is considered as a significant difference.
Results
Epidemiological characteristics and clinical features
By 28 February 2020, 627 patients were on chronic hemodialysis and the prevalence of affected cases was 11.8% (74/627), The median age of the COVID-19 positive patients was 63 years (range 31–88), with an almost even gender distribution (females accounted for 54.4%); in keeping with what is observed in our general dialysis population, the most common primary disease is glomerulonephritis (33%), followed by hypertension (27%), and diabetes (26%); lupus nephritis was recorded in 5.5% of the cases. The most frequent presenting symptom was cough (42%), followed by fatigue (24.2%), fever (17.2%) and dyspnea (14.8%); 22.4% of the cases were asymptomatic (Table 1).
Table 1.
Epidemiological characteristics and clinical features [n (%), M (P25, P75)]
| Total | Surviving group | Death group | Χ2/Z | P | |
|---|---|---|---|---|---|
| Features | N = 74 | n = 60 | n = 14 | ||
| Age (years) | 63.00 (57.00–71.50) | 63.00 (57.00–72.00) | 63.00 (59.50–72.00) | − 0.309 | 0.757 |
| Age groups (years) | |||||
| ≤ 40 | 1 (1.3) | 0 (0) | 1 (7.1) | 2.661 | 0.264 |
| 40–65 | 41 (55.2) | 35 (58) | 6 (42.9) | ||
| ≥ 66 | 32 (43.4) | 25 (41.9) | 7 (50.0) | ||
| Gender | |||||
| Male | 34 (45.6) | 25 (41.9) | 9 (61.5) | 1.411 | 0.235 |
| Female | 40 (54.4) | 35 (58.1) | 5 (38.5) | ||
| Primary diseases | |||||
| Primary glomerulonephritis | 24 (32.8) | 23 (38.7) | 1 (7.7) | 6.738 | 0.150 |
| Diabetes | 19 (25.6) | 14 (22.6) | 5 (38.5) | ||
| Hypertension | 20 (27.0) | 17 (29.0) | 3 (23.1) | ||
| Lupus nephritis | 4 (5.5) | 2 (3.2) | 2 (15.4) | ||
| Others | 7 (9.3) | 4 (6.5) | 3 (21.4) | ||
| Clinical features | |||||
| Fever | 13 (17.2) | 12 (19.4) | 1 (7.7) | 0.931 | 0.335 |
| Cough | 31 (41.9) | 21 (35.5) | 10 (69.2) | 4.207 | 0.040 |
| Vomiting | 2 (2.6) | 2 (3.2) | 0 (0) | 0.429 | 0.512 |
| Diarrhea | 3 (4.1) | 2 (3.2) | 1 (7.7) | 0.421 | 0.516 |
| Fatigue | 18 (24.2) | 14 (22.6) | 4 (30.8) | 0.328 | 0.567 |
| Anorexia | 5 (6.7) | 4 (6.5) | 1 (7.7) | 0.022 | 0.882 |
| Dyspnea | 11 (14.8) | 8 (12.9) | 3 (23.1 | 0.709 | 0.400 |
| Asymptomatic | 17 (22.4) | 15 (25.8) | 1 (7.7) | 1.847 | 0.174 |
| HD-related indicators | |||||
| Hemodialysis age | 57.00 (36.00–72.00) | 67 (36.00–85.25) | 51.00 (29.00–59.00) | − 1.479 | 0.139 |
| Dry weight | 59.25 (53.33–67.13) | 59.00 (52.00–66.00) | 60.00 (56.35–72.50) | − 1.107 | 0.268 |
| Vascular access | |||||
| AVF | 51 (69.2) | 43 (71.0) | 9 (61.5) | 0.375 | 0.540 |
| CVC | 23 (30.8) | 17 (29.0) | 5 (38.5) | ||
| Weekly dialysis duration | 9.35 (8.00–10.40) | 9.60 (7.90–10.80) | 8.80 (8.20–10.00) | − 0.813 | 0.416 |
| UFV | 1.60 (0.97–2.15) | 1.48 (0.91–2.18) | 1.80 (1.45–2.10) | − 0.673 | 0.501 |
| UFR | 0.67 (0.42–0.89) | 0.61 (0.40–0.82) | 0.86 (0.35–1.02) | − 0.943 | 0.346 |
| spKt/V | 1.32 (1.30–1.40) | 1.32 (1.30–1.40) | 1.32 (1.30–1.36) | − 0.333 | 0.739 |
| SBP | 139.00 (126.00–167.50) | 142.00 (126.00–171.50) | 78.00 (70.50–90.50) | − 1.009 | 0.313 |
| DBP | 78.00 (70.50–89.00) | 133.50 (112.00–147.50) | 78.50 (70.50–87.00) | 1.0126 | 0.900 |
AVF arteriovenous fistula, CVC central venous catheters, UFV ultrafiltration volume, UFR ultrafiltration rate, spKt/V urea removal index. spKt/V: − Ln (R − 0.008 × t) + (4–3.5 × R) × UF/W (Among which Ln: natural logarithm. R: BUN after hemodialysis/BUN before hemodialysis. UF ultrafiltration volume. t duration of hemodialysis. W patient’s weight after hemodialysis). SBP systolic blood pressure, DBP diastolic blood pressure
Fourteen of the 74 patients died (19%). The baseline characteristics of the patients who died were comparable to those of survivors, as for median age (63 years in both subsets), male–female ratio and distribution of kidney diseases causing end stage kidney disease (ESKD). It may be worth noticing, however, that 2/4 patients affected by systemic lupus and 5/19 patients affected by diabetes deceased; due to the small numbers, differences are not statistically significant (P > 0.05). As for presenting symptoms, cough was more frequent in patients who died (69.2 vs. 41.9% in survivors. (P < 0.05), but other epidemiological characteristics and clinical features no significant difference (Table 1).
Laboratory and chest CT scan features
Among 74 cases of HD patients infected with COVID-19, 16 patients (21.2%) presented a decrease in white blood cells (WBC), 46 cases (62.5%) presented a decrease in lymphocytes. The mean laboratory features were likewise non-specific; Leukopenia at presentation was more marked in patients who died, but the difference is not statistically significant [5.66 (IQR 3.95–7.90) 109/L versus 7.28 (IQR 4.76–8.46) 109/L]. As a rule patients presented a mild-to-moderate increase in C-Reactive protein (CRP), with a median of 16.95 (IQR 3.75–39.28) mg/L; CRP was significantly higher in patients who died than in survivors [85.40 mg/L (IQR 32.18–156.25) versus 12.95 (IQR 3.23–22.98) mg/L; P = 0.006]. Surviving patients had higher levels of phosphate and albumin, suggesting a better baseline nutritional status (albumin level: 4.03 (IQR 3.7–4.2)g/dL in survivors versus 3.46 (IQR 3.05–4.0) in the patients who died, P = 0.008; phosphate level 1.69 (IQR 1.23–2.35) mmol/l in survivors versus 1.33 (IQR 0.86–1.71) mmol/l in patients who died, P = 0.017) and a better PTH control [128.95 (IQR 96.16–407.00] pg/ml in survivors versus 314.85 (IQR 186.65–469.48) pg/mL in patients who died, P = 0.084) (Table 2).
Table 2.
Results of laboratory and chest CT scan [n (%), Median (P25, P75)]
| Total N = 74 | Surviving group n = 60 | Death group n = 14 | Statistical value | P | |
|---|---|---|---|---|---|
| White blood cells (109/L) | 6.18 (4.25–7.90) | 5.66 (3.95–7.90) | 7.28 (4.76–8.46) | − 0.722 | 0.470 |
| White blood cells (109/L) | |||||
| < 4 | 16 (21.2) | 14 (22.6) | 2 (15.4) | 0.297 | 0.862 |
| 4–10 | 53 (72.1) | 43 (71.0) | 11 (76.9) | ||
| > 10 | 5 (6.7) | 4 (6.5) | 1 (7.7) | ||
| Neutrophils (109/L) | 4.28 (3.00–6.26) | 4.03 (2.92–6.22) | 5.26 (3.72–7.35) | − 1.338 | 0.181 |
| Lymphocytes (109/L) | 0.86 (0.70–1.20) | 0.86 (0.72–1.11) | 0.85 (0.59–1.34) | − 0.013 | 0.990 |
| Lymphocytes (109/L) | |||||
| < 1 | 46 (62.5) | 39 (64.5) | 8 (53.8) | 0.440 | 0.507 |
| ≥ 1 | 28 (37.5) | 21 (35.5) | 6 (46.2) | ||
| Hemoglobin (g/L) | 102.00 (91.00–114.00) | 104.00 (94.00–114.00) | 91.00 (80.50–113.50) | − 1.403 | 0.160 |
| Hemoglobin (g/L) | |||||
| < 100 | 30 (40.4) | 21 (35.5) | 9 (61.5) | 2.534 | 0.111 |
| ≥ 100 | 44 (59.6) | 39 (64.5) | 5 (38.5) | ||
| Platelets (109/L) | 138.00 (105.00–177.75) | 138.00 (116.00–178.00) | 121.00 (83.50–189.50) | − 0.733 | 0.463 |
| C-reactive protein (mg/L) | 16.95 (3.75–39.28) | 12.95 (3.23–22.98) | 85.40 (32.18–156.25) | − 2.732 | 0.006 |
| Glutamic pyruvic transaminase (U/L) | 8.50 (6.00–12.00) | 9.00 (7.00–13.00) | 6.00 (5.00–10.50) | − 1.148 | 0.251 |
| Glutamic oxaloacetic transaminase (U/L) | 14.00 (11.25–20.75) | 15.00 (10.00–20.00) | 13.00 (11.50–22.50) | − 0.180 | 0.857 |
| Albumin (g/L) | 39.05 (36.15–40.90) | 40.30 (37.40–42.30) | 34.60 (30.50–40.00) | − 2.650 | 0.008 |
| Albumin (g/L) | |||||
| < 35 | 11 (15.4) | 3 (5.0) | 8 (53.8) | 9.899 | 0.002 |
| ≥ 35 | 63 (84.6) | 57 (95.0) | 6 (46.2) | ||
| Serum potassium (mmol/L) | 4.40 (3.63–4.88) | 4.40 (37.40–42.30) | 4.20 (3.40–4.70) | − 1.031 | 0.303 |
| Serum calcium (mmol/L) | 2.14 (1.93–2.31) | 2.17 (1.87–2.41) | 2.08 (1.98–2.20) | − 0.823 | 0.410 |
| Serum calcium (mmol/L) | |||||
| < 2.10 | 34 (45.6) | 25 (41.9) | 9 (61.5) | 2.510 | 0.285 |
| 2.10–2.54 | 32 (43.2) | 27 (45.2) | 5 (38.5) | ||
| > 2.54 | 8 (10.5) | 8 (12.9) | 0 (0) | ||
| Blood phosphorus (mmol/L) | 1.62 (1.15–2.07) | 1.69 (1.23–2.35) | 1.33 (0.86–1.71) | − 2.380 | 0.017 |
| Blood phosphorus (mmol/L) | |||||
| < 1.13 | 15 (20.3) | 10 (16.1) | 5 (38.5) | 4.862 | 0.088 |
| 1.13–1.78 | 28 (38.2) | 21 (35.5) | 7 (46.2) | ||
| > 1.78 | 31 (42.2) | 29 (48.4) | 2 (15.4) | ||
| Parathyroid hormone (pg/ml) | 258.50 (114.63–465.35) | 314.85 (186.65–469.48) | 128.95 (96.16–407.00) | − 1.726 | 0.084 |
| Parathyroid hormone (pg/ml) | |||||
| < 150 | 21 (28.4) | 12 (19.4) | 9 (61.5) | 8.313 | 0.016 |
Chest CT scan was positive in all cases, including in asymptomatic ones, and revealed in about three-fourths of the cases bilateral (76.2%) lesions; in each lung lesions were multiple in about half of the cases of the cases (52.3%) (Table 2).
Survival curve for dead patients
After diagnosis, median time to death was 7 days in the 14 patients who died, with a range between 4 and 18 days (Fig. 1).
Fig.1.
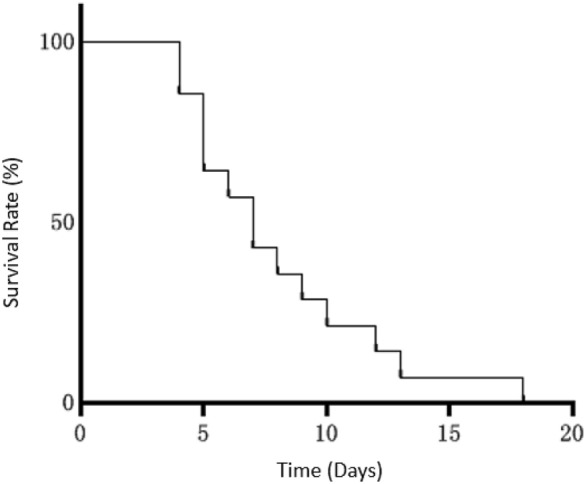
Survival curve of dead patients
Discussion
Epidemiological research revealed that elderly people or patients suffering from chronic diseases are more susceptible to COVID-19 and are subject to a relatively high death risk [7]. HD patients tend to have a weak immune system, and their frequent trips to hospital expose them under a higher risk of viral infection. These factors, adding that the hemodialysis room where HD patients stay is a crowded, enclosed space, make HD patients one of the most susceptible populations to COVID-19. Research results showed that HD patients get a pneumonia incidence rate 14–16 times of ordinary people [8]. On the other hand, we observed a significantly small number of epidemiological reports on HD patients.
Results showed that HD patients in this research have an incidence rate of 11.8% (74/627). The median age of 74 HD patients infected with COVID-19 is 63 years old, older than that in most published research [9, 10]. Cough (41.9%) was the most common incipient clinical symptoms in this research, followed by fatigue, fever and dyspnea, while anorexia, diarrhea and vomiting were among the less common symptoms, which was slightly different from existing reports that featured fever as the most common symptoms on COVID-19 patients [6, 10, 11]. In addition, 22.4% confirmed COVID-19 patients were asymptomatic, which suggests certain difficulty in the early identification and screening of COVID-19 patients in the clinical aspects. The lung imaging scans of confirmed patients mostly present bilateral opacity (76.2%), multiple opacity (52.3%), and patchy opacity (63.6%). While some existing research reported multiple ground-glass lesion as the typical lung lesion in CT scans of COVID-19 patients [12], this research found that ground-glass changes in patients’ lung CT scans claim a relatively small proportion (9.1%). Relevant guidelines [5, 13] stated that COVID-19 patients in early stage presented the imaging features of multiple small patchy opacity or interstitial changes, followed by bilateral multiple ground-glass opacity and even pulmonary consolidation in severe cases. In this regard, screening work and examination of lung CT scans should be conducted as early as possible to achieve timely detection of the disease and quarantine for confirmed patients and contain the potential spread of COVID-19 in HD patients.
This research surveyed 14 death cases, with a median survival time of 7 days and the cough being the most prominent symptom for death group. In terms of clinical features, the CRP level in dead patients was higher than surviving patients. The group of deceased patients also presented a significantly lower level of albumin and phosphate, two indicators of the patient’s nutritional status, indicating that it’s imperative to ensure sufficient nutrition for patients infected with COVID-19. In addition, this research found dead patients have a lower PTH standard-reaching rate than surviving patients, which runs consistent with the findings of other studies that a PTH level lower than the reference range can be a significant predictive factor leading to the death of patients [14]. Therefore, increasing attention should be paid to measuring PTH level of COVID-19 patients to ensure timely intervention and treatment of the disease.
This research is a single-center study with a relatively small sample size. During our analysis, for the purpose of avoiding the omission of patients with false negative results in RT-PCR, we used the diagnostic criteria in the Revised Diagnosis and Treatment Planning of the Novel Coronavirus Pneumonia (the Fifth Trial Edition) and included both clinically diagnosed cases and confirmed cases, which consequently resulted in an increase in the false positive rate in patients. In addition, given that the therapies received by HD patients after they were confirmed with COVID-19 could hardly be traced, this research lacks the description for treatment measures. Supplementary contents and corrections to the findings of this article could be made in follow-up studies as the outbreak evolves.
Author contribution
YM and LC drafted the manuscript and processed statistical data; CT; HL; DH; DH; DC; XH; FC collected the epidemiological and clinical data; FX revised the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yonglong Min, Li Cheng share co-first authorship.
References
- 1.Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020 doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jernigan DB. Update: public health response to the coronavirus disease 2019 outbreak—United States, February 24, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(8):216–219. doi: 10.15585/mmwr.mm6908e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020 doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaier M, Leick A, Uhlmann L, et al. End-stage renal disease, dialysis, kidney transplantation and their impact on CD4(+) T-cell differentiation. Immunology. 2018;155(2):211–224. doi: 10.1111/imm.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(2020) Office of the National Health Commission and office of the National Administration of Chinese Traditional Medicine. Diagnosis and Treatment Planning of the Novel Coronavirus Pneumonia (the Fifth Trial Edition; Revised Edition). 2020
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6):1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2019 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 10.1136/bmj.m792 [DOI] [PubMed]
- 12.She J, Jiang J, Ye L, et al. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. ClinTransl Med. 2020;9(1):19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am SocNephrol. 2015;10(1):98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]


