Abstract
Coronavirus disease 2019 (COVID-19) has emerged as a significant public health emergency in recent times. It is a respiratory illness caused by the novel virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was initially reported in late December 2019. In a span of 6 months, this pandemic spread across the globe leading to high morbidity and mortality rates. Soon after the identification of the causative virus, questions concerning the impact of environmental factors on the dissemination and transmission of the virus, its persistence in environmental matrices, and infectivity potential begin to emerge. As the environmental factors could have far-reaching consequences on infection dissemination and severity, it is essential to understand the linkage between these factors and the COVID-19 outbreak. In order to improve our current understanding over this topic, the present article summarizes topical and substantial observations made regarding the influences of abiotic environmental factors such as climate, temperature, humidity, wind speed, air, and water quality, solid surfaces/interfaces, frozen food, and biotic factors like age, sex, gender, blood type, population density, behavioural characteristics, etc. on the transmission, persistence, and infectivity of this newly recognized SARS-CoV-2 virus. Further, the potential pathways of virus transmission that could pose risk to population health have been discussed, and the critical areas have been identified which merits urgent research for the assessment and management of the COVID-19 outbreak. Where possible, the knowledge gaps requiring further investigation have been highlighted.
Keywords: SARS-CoV-2, COVID-19, Environmental factors, Transmission, Persistence, Infectivity, Cold chain transportation
Introduction
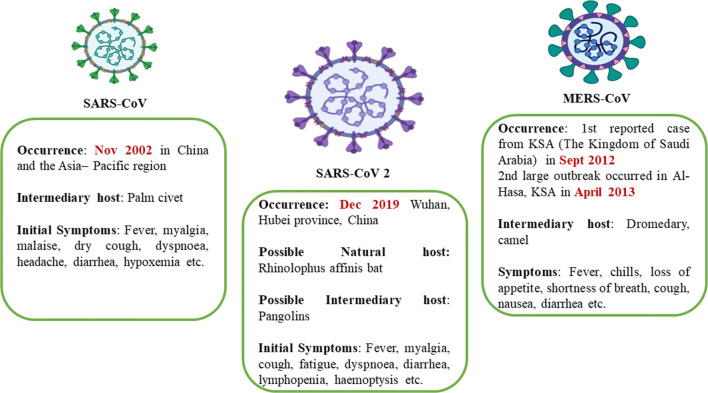
The recent outbreak of the novel coronavirus disease 2019 (COVID-19) has become a public health emergency of international concern in the real sense. COVID-19 is a respiratory disease known to be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Peng et al. 2020; Siddiqui et al. 2020a). On 31st December 2019, China was the first country who notifies WHO about the occurrence of this disease in Wuhan City, Hubei Province of China (China CDC 2020; WHO 2020a). Soon after that report, other countries like Thailand, Japan, and the Republic of Korea, also reported similar cases (WHO 2020a). Since then, the cases of COVID-19 have been increasing, leading to high morbidity and mortality rates across the globe. In recent decades, this pandemic is the 3rd major outbreak, after the SARS (severe acute respiratory syndrome) outbreak in 2002–2003 and the MERS (Middle East respiratory syndrome) outbreak in 2012 (Adhikari et al. 2020; Nghiem et al. 2020) (Fig. 1). The outbreak ranges for COVID-19 are much greater than the previous epidemics. As of December 5, 2020, more than 65,257,760 confirmed cases, including 1,513,179 deaths, have been registered worldwide due to COVID-19, affecting 218 countries and territories around the world and two international conveyances (3:02 pm CEST, 5 December 2020, WHO 2020b; https://www.worldometers.info/coronavirus/. Accessed 15 July 2020).
Fig. 1.
Major disease outbreaks in recent decades
One of the primary concerns of this outbreak is the relationship between environmental factors and the transmission, stability/vitality, and infectivity of the virus (Qu et al. 2020; Bontempi et al. 2020). The influence of environmental factors on COVID-19 prevalence could have across-the-board consequences for public health as well as for pandemic mitigation policies and strategies (Eslami and Jalili 2020; Usman et al. 2020). It is now confirmed that SARS-CoV-2 is primarily transmitted through respiratory droplets and close contact with the infected person and contaminated objects (WHO, 2020c). Though human to human transmission has been recognized as the main pathway of SARS-CoV-2 infection, however, the possible environmental spread via bioaerosols, aquatic environment, faecal-oral route, etc., is still under investigation (Eslami and Jalili 2020; Usman et al. 2020). In relation to SARS-CoV-2, the association of climatic factors such as temperature, humidity, precipitation, wind speed, solar radiation, etc. with virus prevalence and spread is much complicated and is currently under debate (Oliveiros et al. 2020; Ahmadia et al. 2020; Coccia 2020a; Yao et al. 2020). The link between air pollution and COVID-19 prevalence has also been highlighted in several studies, suggesting air pollution as an additional co-factor of SARS-CoV-2 lethality (Conticini et al. 2020; Coccia 2020a; Wu et al. 2020c). The particulate matter has been identified as potential carriers for viral particles, increasing the possibility of virus diffusion over long distances affecting COVID-19 prevalence in the communities (Martelletti and Martelletti 2020). The association between concentration of air pollutants like PM10 and PM2.5, SO2, NO2, CO, O3, and COVID-19 fatality rates specifies that population residing in highly polluted areas like metropolitan cities are more susceptible to respiratory diseases and are at much greater risk (Zhu et al. 2020; Ogen 2020). Recently, viral RNA has been reported in sewage/wastewater, raising the concern over contamination of receiving water bodies and exposure risk to human health associated with waterways (Wu et al. 2020a; Medema et al. 2020; Ahmed et al. 2020). Also, the detection of viable viral particles in faces and stool samples signifies the possibility of faecal-oral transmission as an additional pathway of infection (Heller et al. 2020; Senapati et al. 2020). Although airborne transmission of SARS-CoV-2 via contaminated wastewater has not been established yet, however, the previous super-spreading event of SARS in Hong Kong housing block (Peiris et al. 2003) points to the likelihood of its transmission via exposure to aerosolized wastewater (Usman et al. 2020). The persistence of SARS-CoV-2 on solid surfaces is highly variable, ranging from a few hours up to several days (Kampf et al. 2020). A limited number of clinical studies investigated the aerosol and surface distribution of SARS-CoV-2 in a hospital environment (Guo et al. 2020; Ong et al. 2020; Liu et al. 2020c). Their findings revealed the ubiquitous presence of the virus in air and surface samples and the potential of virus transmission through aerosols was proposed. Further, research is still needed to study the viability and infectivity of SARS-CoV-2 on solid surfaces/interfaces. Another mode of viral transmission via cold chain transportation in the frozen food industry grabbed worldwide attention after several incidences of COVID-19 re-emergence at various places (Liu et al. 2020d; Han et al. 2020b). Besides these factors, biotic factors like age structure, gender, blood type, population density, behavioural characteristics, cultural characteristics, and hygiene practices have also been known to significantly impact viral transmission, persistence, and infectivity in the environment (Coccia 2020a; Bontempi et al. 2020).
The present article summarizes topical and substantial observations made regarding the influence of various environmental abiotic factors (climatic factors, air quality, aquatic environment, surfaces/interfaces) and biotic factors (age, gender, blood type, population density, behavioural characteristics, etc.), on the transmission, prevalence, stability, and infectivity of SARS-CoV-2. Where possible, the knowledge gaps requiring further investigation have been highlighted.
Characteristics of SARS-CoV2
SARS-CoV-2 is an enveloped, non-segmented, positive-sense, single-stranded RNA virus, approximately 65–125 nm in diameter (Shereen et al. 2020). It belongs to the family of Coronaviridae, which is further divided into subfamily Orthocoronavirinae. This subfamily belongs to four genera, i.e. alpha-coronavirus, beta-coronavirus, gamma-coronavirus, and delta-coronavirus (Ye et al. 2020). Till date, seven types of human coronaviruses (HCoVs) are known that can infect the human. These include HCoV-229E and HCoV-OC43, severe acute respiratory syndrome-CoV (SARS-CoV), HCoV-NL63, HCoV-HKU1, Middle East respiratory syndrome-CoV (MERS-CoV), and novel SARS-CoV-2 (Mohammadi et al. 2020; Hasoksuz et al. 2020; Decaro and Lorusso 2020; Siddiqui et al. 2020b). CoVs can infect humans and animals such as camels, cattle, cats, civets, bats, and other animals, causing respiratory, hepatic, neurologic, and gastrointestinal diseases (Mohammadi et al. 2020). SARS-CoV-2 has been reported to be genetically similar to the SARS-CoV and is similarly supposed to cross the species barrier from animal to human (Yuen et al. 2020). Although its specific origin is yet to be determined, the studies suggest bats as the natural reservoir hosts of SARS-CoV-2 and pangolins as the possible intermediary hosts (Liu et al. 2020a). Much like SARS-CoV, SARS-CoV-2 uses the angiotensin-converting enzyme ACE2 (a membrane exopeptidase) as a receptor to gain entrance into human cells (Xu et al. 2020b). The chief characteristics of SARS-CoV-2 are represented in Table 1. The clinical symptoms of this virus include high fever, chills, dry cough, dyspnoea, fatigue, and lymphopenia. Other symptoms like diarrhoea, myalgia, expectoration, and haemoptysis has also been observed in the infected person. In severe cases, the patients may develop pneumonitis, acute lung damage, acute respiratory distress syndrome. In old age patients, serious complications such as heart failure, respiratory failure, and liver failure are most likely to occur (Pal et al. 2020; Zu et al. 2020; Huang et al. 2020).
Table 1.
Characteristics of SARS-CoV-2
| Particulars | Characteristics |
|---|---|
| Family | Coronaviridae |
| Sub family | Orthocoronavirinae |
| Order | Nidovirales |
| Genera | Four: αCoV, β-CoV, γ-CoV, and δ-CoV |
| RNA | Linear, single-stranded RNA genomes of positive polarity |
| Infection | |
| α-CoV and β-CoV | Infect the respiratory, gastrointestinal, and CNS of humans and mammals |
| γ-CoV and δ-CoV | Infect the birds |
| β-CoV | SARS-CoV (2002–2003) |
| MERS-CoV (2012) | |
| 2019-nCoV (2019) | |
| Nucleotide sequence similarity | 79% with SARS-CoV and 50% with MERS-CoV |
| Natural host | Bat; 96.2% genome sequence similarity to RaTG13, a bat coronavirus detected in, Rhinolophus affinis |
| Intermediate hosts | Pangolins; 99% genome sequence similarity with pangolin-CoV |
| Protein structure | Spike protein (S-protein) |
| Receptor | Human angiotensin converting enzyme 2 (ACE2) |
| Binding affinity |
High affinity between ACE2 and SARS-CoV-2 S protein Population with higher expression of ACE2 might be more susceptible to COVID-19 |
Environmental factors
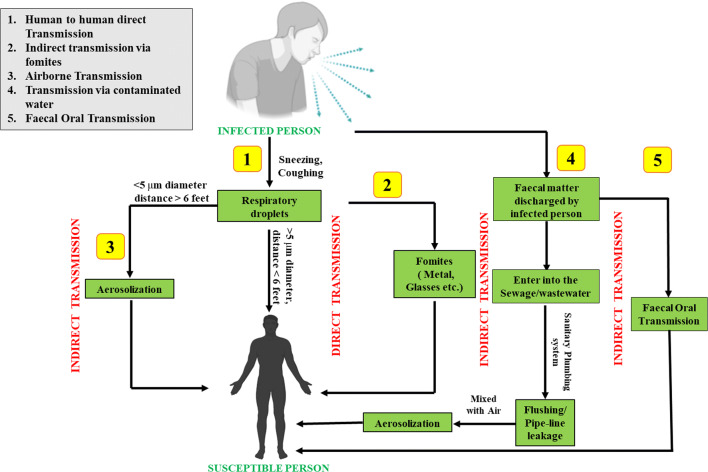
The transmission of a virus is influenced by various factors such as virus infectivity, host behaviours, host defence mechanism, environmental factors, etc. (Pica and Bouvier 2012). The modes by which the virus is transmitted from one person to another are very critical for understanding the impact of the environment on transmission, survival, and infectivity of the virus. The major pathway of virus transmission is contact transmission, which includes direct and indirect ways (Teymoori-Rad et al. 2020). Direct transmission refers to the transfer of viruses from an infected person to another person without any intermediate object, whereas indirect transmission is the transfer through contact with a contaminated intermediate object. Another major pathway/mode is airborne transmission, which encompasses droplet spray transmission and aerosol transmission. In droplet spray transmission, the virus is transferred via air droplets produced at the time of breathing/sneezing/coughing/talking, followed by its deposition on the mucous membrane (Pica and Bouvier 2012; WHO 2020c; Teymoori-Rad et al. 2020). In the case of SARS-CoV-2, human to human transmission (direct and indirect, both) is the primary mechanism of infection when a person is in an incubation state; parallelly, there are other cases where individuals remain infectious while being asymptomatic (superspreaders) (WHO 2020c; Yuen et al. 2020; Cave 2020). The present concern regarding the transmission of SARS-CoV-2 is not limited to direct contact with infected people or indirect contact with infected surfaces, but recent studies suggested transmission via droplets spray and aerosols (Teymoori-Rad et al. 2020). In aerosols, SARS-CoV-2 remains viable up to 3 h, while on solid surfaces, it could survive up to 72 h though the infectious titre gets reduced (van Doremalen et al. 2020). In highly polluted areas, the accelerated transmission dynamics of SARS-CoV-2 is major because of air pollution to human transmission (airborne viral infectivity) rather than human-to-human transmission (Coccia 2020a). Besides, some studies reported fragments of viral RNA in faeces and anal swabs of infected persons (Holshue et al. 2020; Xiao et al. 2020), indicating the possibility of newer routes of transmission like faecal-oral transmission. Also, the likelihood of environmental dissemination of SARS-CoV-2 via water, bioaerosols, and food should be taken into consideration. Figure 2 depicts possible transmission routes of SARS-CoV-2 in different environmental matrices. Apart from these transmission modes, pre-symptomatic and asymptomatic transmission have been identified as another pathway of COVID-19 infection (Zhang 2020). The findings of Arons et al. (2020) indicate that pre-symptomatic residents may contribute to the widespread transmission of SARS-CoV-2 in nursing facilities. Gao et al. (2020), Xu et al. (2020a), and Kronbichler et al. (2020) also displayed asymptomatic transmission as a source of COVID-19 infection.
Fig. 2.
Various possible transmission routes of SARS-CoV-2 in environment
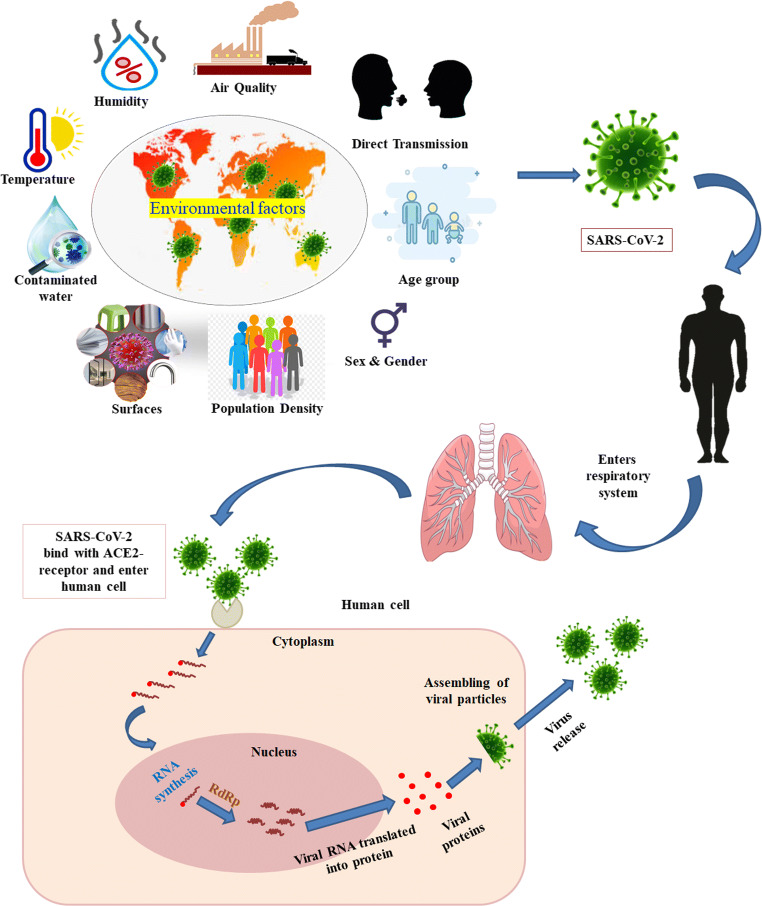
The transmission intensity, persistence in the environmental matrices, and infection potential of the virus could be influenced by a variety of factors. Among them, some are common factors that remain the same for all the regions. These include biological properties of SARS-CoV-2, its incubation state, effects on infected and susceptible persons, etc., while there are some factors wherein the longevity, transmissivity, and infectivity of SARS-CoV-2 will vary from region to region. These include pollutant concentration levels in the air, meteorological conditions, atmospheric conditions, and topography affecting pollutant dispersion, population density, age structure, gender, blood type, hygiene procedures, health status, health care facilities, economic wealth, cultural characteristics, personal protection measures, etc. (Coccia 2020a, Bontempi et al. 2020) (Fig. 3). The following sections summarize recent and significant observations made regarding the influence of varied abiotic and biotic factors on SARS-CoV-2 transmission, persistence, and infectivity and discuss the existing knowledge gaps.
Fig. 3.
Influence of Environmental factors on SARS-CoV-2 infection
Abiotic factors
Climate
Recent studies argued the role of different factors, including meteorological variables, health care facilities, age structure, sex, population density, immunity, etc., in transmission intensity and survival of SARS-CoV-2 (Wang et al. 2020a, Coccia 2020a, Bontempi et al. 2020). Previously in the case of influenza, MERS, and SARS transmissions, besides the biotic factors, weather parameters like seasonal variability of temperature, solar radiation, along relative humidity showed immense importance (Dalziel et al. 2018; Shaman et al. 2010, 2011; Chattopadhyay et al. 2018; Gardner et al. 2019; Tan et al. 2005; Otter et al. 2016). Likewise, it is evidenced from recent studies that COVID-19 positive cases within certain regions are related to weather parameters like specific humidity and mean temperature (Sajadi et al. 2020; Araujo and Naimi 2020). However, some studies demonstrated limited effects of climate of a certain location on COVID-19 outbreak within local communities (O'Reilly et al. 2020). All of these latest studies are confounding as it is also pertaining that worldwide travelling network of people resulted in the delayed outbreak of COVID-19 in various regions having a warmer climate (Lai et al. 2020). Till July 5, 2020, globally, 218 countries and territories had been affected by COVID-19 transmissions, among which several areas experienced active community transmissions. All of these countries and territories represent different climatic zones expanding from hot and humid to dry and colder regions. Hence, it is imperative to contextualize the relatedness of meteorological conditions in response to SARS-CoV-2 survival and spreading.
Basically, virus-induced respiratory infections mostly depend on the physiological status of the host, persistence, dynamics, and ability of the virus to infect the host, and atmospheric dispersion trends where meteorological variables, especially humidity and atmospheric temperature, are the primary regulators. Weather patterns are dependent on land surface features, latitudes, and their interactivity with the earth systems. In temperate regions, aerosols can be effective virus transmitters during winter due to a relatively lower temperature and humidity level, while in tropical areas during the monsoon season, hot and humid conditions reduce the virus transmission via aerosols (Tamerius et al. 2013). According to the earlier study, temperate climate facilitates the survival and transmission of the virus rather than tropical climate, which is in accordance with the common viral respiratory infections (Bloom-Feshbach et al. 2013). Other viruses belonging to the Coronaviridae family like SARS CoV and MERS CoV preferred cold climates having less humidity and lower temperature (Casanova et al. 2010). It is also reported that higher relative humidity (> 95%) and a specific range of temperature (28–33 °C) cannot influence the effectivity of SARS-CoV-2 as it poses a comparatively stable structure (Chan et al. 2011). Consistent community spreading of COVID-19 along a specific latitude (30–50° N corridor) was recorded at a mean temperature of 5–11 °C along with limited specific (3–6 g/kg) and absolute humidity (4–7 g/m3) (Sajadi et al. 2020). However, a recent study adopting a generalized additive modelling approach using meteorological parameters in nine Asian cities showed inconsistent relationships among the confirmed COVID-19 positive daily cases and humidity and average temperature (He et al. 2020). A negative association between COVID-19 occurrence and atmospheric temperature and absolute humidity was also reported in Hubei Province China, during the cold and dry winter season, where a 1-unit increment in minimum temperature reduced the COVID-19 positive cases by 72.47 units (Li et al. 2020). Long ago, it was reported that higher UV irradiance has the potential to limit the outbreak of SARS coronavirus within humans and the environment (Duan et al. 2003). On the contrary, the current research in Chinese cities revealed that solar radiation and the higher temperature had no such association with the transmission ability of COVID-19 (Yao et al. 2020). Currently, worldwide studies are going on rapidly to find out the actual climatic impact on survival and the spreading of COVID-19, which has no geographical barrier. Scientific outputs of such recent studies are summarized in Table 2.
Table 2.
Literature survey on COVID-19 and meteorological conditions
| Study area | Time span | Meteorological variables | Inferences | References |
|---|---|---|---|---|
| 31 provinces of China | January 23 to March 1, 2020 | Temperature, relative humidity, precipitation, and wind speed | Doubling time of COVID-19 cases was associated with temperature and relative humidity but precipitation and wind speed didn’t have any influence. | Oliveiros et al. 2020 |
| Non-tropical countries | January 20 to March 19, 2020 | Temperature and humidity |
Absolute humidity (AH) is much important weather variable regarding COVID-19 transmissions as compared with temperature and relative humidity. Maximum COVID-19 incidents were observed at 4-9 g/m3 AH and 3-17 °C temperature. |
Bukhari and Jameel 2020 |
| USA | January 1 to April 9, 2020 | Absolute humidity (AH) and temperature | AH is more significant weather variable and maximum COVID-19 cases reported within 4–6 g/m3 AH and 4–11 °C temperature. | Gupta et al. 2020 |
| All the affected countries | January, 2020 | Temperature | Temperature influenced the COVID-19 infection intensities. | Wang et al. 2020b |
| China | January, 2020 | Absolute humidity (AH) and temperature | COVID-19 outbreak was associated with AH and temperature. However, weather changes would not affect infection transmission intensity and exponential increase in positive cases. | Luo et al. 2020 |
| Wuhan, China | Historical datasets of 2002 to 2003 and 2015 to 2019 | Temperature, humidity and precipitation |
13–24 °C temperature, 50%-80% of humidity and < 30 mm rainfall/month facilitates the survival of 2019-nCoV. Cold climate may eliminate the virus. |
Bu et al. 2020 |
| Entire globe | January 22, to April 6, 2020 | Temperature, precipitation, wind speed, solar radiation, and water vapour pressure | Warming velocity and precipitation pattern mostly influenced the COVID-19 transmissions as compared with temperature. | Chiyomaru and Takemoto 2020 |
| Brazil | February 27 to April 1, 2020 | Temperature | Negative linear relationship was analysed between a specific temperature range (16.8 to 27.4 °C) and COVID-19 daily infections. | Prata et al. 2020 |
| China | January 23, to February 29, 2020 | Temperature |
Linear relationship was found in between daily COVID-19 positive cases and average temperature (threshold limit 3 °C). 4.861% rise in daily positive cases was also recorded with increase in 1 °C temperature. |
Xie and Zhu 2020 |
| Iran | February 19 to March 22, 2020 | Temperature, precipitation, humidity, wind speed, and solar radiation | Low wind speed, less amount of solar radiation and humidity promotes the survival rate of COVID-19 virus. | Ahmadia et al. 2020 |
| China | January and February, 2020 | Weather data (temperature, solar radiation and precipitation | Influences of weather on COVID-19 survival and transmissions are limited which does not refer the extinction of the pandemic during summer. | Byass 2020 |
| Italy | February 1 to April 1, 2020 | Average temperature, moisture %, wind speed, days of rain and fog | Low wind speed, High moisture % and no. of fog days, high air pollution level accelerates transmission dynamics of viral infectivity. | Coccia 2020b |
| Iran | February 15 to March 22, 2020 | Average temperature, population size | Average temperature has low sensibility while population size has high sensitivity to the transmission rate of COVID-19. No evidence of lower transmission rate in warmer climate in comparison with cold/moderate climates was obtained. | Jahangiri et al. 2020 |
These research studies include short-duration data sets, small sample size, and different protocols for computations. Thus, definite results from all of these studies are limited. However, through this systematic review of current research, we can document that there is no such evidence regarding the specific relationship between survival and spreading of COVID-19 and weather variables. However, absolute humidity and the daily average temperature may have influences on COVID-19 outbreaks in some specific locations. Yet long-term investigations in this line are necessary to understand the association between COVID-19 outbreaks and climate.
Air quality
The respiratory viral infections can be transmitted across distances by aerosols; hence viral diffusion via airways represents a significant pathway of disease spread. Depending on the expert’s background, several definitions of airborne transmission is present in the scientific literature (Bontempi 2020b); however, WHO refers airborne transmission as the presence of microorganisms within droplet nuclei (particles having < 5-μm diameter) which remains suspended in the air for a longer period of time, while remaining infective and be transmitted over distances greater than 1–2 m (WHO report 2020). Previous reports related to indoor case studies of SARS indicated that airborne transmission is the chief route of infection (Li et al. 2005; Booth et al. 2005; Olsen et al. 2003). Looking at the similarity of SARS-CoV-1 and SARS-CoV-2, the likelihood of transmission of SARS-CoV-2 via airways cannot be ruled out. It has been reported that SARS-CoV-2 could survive in aerosol for a few hours. In the study conducted by van Doremalen et al. (2020), the virus-laden aerosol was generated through a three-jet collision nebulizer and fed into a Goldberg drum. The finding revealed that SARS-CoV-2 remain viable in aerosols for 3 h, with a drop in infectious titre from 103.5 to 102.7 TCID50/litre of air (tissue-culture infectious dose). This reduction was reported similar to SARS-CoV-1 reduction. A couple of studies confirmed the existence of SARS-CoV-2 RNA in air samples collected from Wuhan Hospitals (Liu et al. 2020c) and Nebraska University Hospital (Santarpia et al. 2020). Contrary to these findings, all the air samples were tested negative in a study carried out in a hospital in Iran (Faridi et al. 2020) and an outbreak centre in Singapore (Ong et al. 2020). Although the evidence regarding the airborne transmission of SARS-CoV-2 is scarce, still the role it played in SARS-CoV-1 and MERS-CoV epidemiology signifies airborne transmission as a possible additional factor for COVID-19 outbreaks. As a precautionary measure, WHO recommended universal masking in public for controlling the infection at the source thereby impeding the disease outbreak; however, the effectiveness of the masks against airborne transmission of virus droplets/aerosols is still not apparent (Zhai 2020; Esposito et al. 2020). Drake (2020) revealed that the cloth masks could reduce exposure by 50%, while surgical and N95 masks reduce exposure up to 75% and 99%, respectively (Drake 2020). The air simulation experiment also showed that though the facial masks cannot block the viral transmission completely, however, they do provide a significant degree of protection against airborne transmission (Ueki et al. 2020). Taking into account the global shortage of surgical and N95 masks, Esposito et al. (2020) advocated the use of cloth masks as a sustainable alternative. However, the relationship between the protective efficiency of the masks and the components of viral droplets/aerosols needs to be investigated further.
Air pollution has been identified as an important risk factor in cases of respiratory infections; thus, this section aims at assessing the impact of the air pollutants on the transmission, persistence, and infectivity of SARS-CoV-2. The air pollutants induce their own distinctive toxic/hazardous effect in the respiratory/cardiovascular system of the person, also some of them are potent oxidants, and in those conditions when a person got the viral infection, the situation becomes more intensified, leading to a chain of immune disorders and diseases in the exposed person. Air pollutants are also known to suppress early immune responses to infection in the body. The impact of the air pollutants can be assessed by the fact that the SARS-infected patients residing in areas having a high air pollution index had an 84% higher risk of mortality in comparison with those who are living in areas having a lower air pollution index (Cui et al. 2013). Similarly, Kan et al. (2005) found a positive relationship between air pollution and SARS mortality. The positive correlation between high concentrations of air pollutants and fatalities due to respiratory viral infections has been established in several other epidemiological analysis. In fact, Lombardy, and Emilia Romagna regions of Italy, which witnessed the highest COVID-19 fatalities in the world, are among Europe’s most polluted areas (Conticini et al. 2020). Their findings suggested that atmospheric pollution should be considered an additional co-factor of SARS-CoV-2 lethality. Similarly, Coccia (2020a) observed that in North Italy, those cities which had greater than 100 days of air pollution (exceeding the PM10 standard values), the average number of infected persons was very high, while the cities having less than 100 days of air pollution witnessed a much lesser average number of infected persons. The study also discovered that the transmission dynamics of SARS-CoV-2 are highly associated with air pollution in cities accompanied with low wind speed. It was further suggested that hot and sunny weather enhances transmission of SARS-CoV-2 infectivity, while sunny days and summer season helps in coping up with viral infectivity owing to higher generation of Vit D that enhances a person’s immunity. In another study, the relationship between historical exposure to air pollution exposure and the COVID-19 mortality rate was investigated and found that even a small increase (1 휇g/m3) in the concentration of PM2.5 raises the COVID-19 mortality rate by 8%. (Wu et al. 2020a). Contrary to these findings, in the study of Bontempi (2020a) wherein PM10 concentration and COVID-19 cases in Lombardy (Italy) were assessed from February 10 to March 27, 2020, no association between PM10 values and COVID-19 diffusion mechanism was established. Besides air pollution and other factors, Bontempi (2020b) speculated commercial exchanges between Italy and China, as one of the contributing factors responsible for the initial diffusion of the virus in Northern Italy.
Among the air pollutants, particulate matter (PM10 and PM2.5), ozone, sulphur dioxide, nitrogen dioxide, and carbon monoxide have been reported to affect the respiratory airways, intensifying the vulnerability and the severity of the infections. Also, these pollutants are likely to assist the prolonged existence of the viral particles in favourable climate settings. A recent study suggested that the presence of these pollutants at higher concentration level along with certain climatic conditions may assist in the elongated longevity of SARS-CoV-2 in the air, which in turn will open the possibility of its indirect transmission in addition to the direct contact transmission (Frontera et al. 2020). Similarly, the possibility of SARS-CoV-2 for the exploitation of “highways” made up of atmospheric particulates has been hypothesized in another article (Sanità di Toppi et al. 2020). In the same line, Martelletti and Martelletti (2020) hypothesized that the air pollutant particles act as fertile territory for the SARS virus, and in a linear association, the virus lasts for a longer period and turns out to be more hostile in an immune system already injured by hazardous air pollutants. This is evidenced from a recent study held in Italian Northern Regions by SIMA (Società Italiana di Medicina Ambientale). The study depicted that the COVID-19 effect was more pronounced in those areas which had an excessive concentration of PM10 and PM2.5 (Setti et al. 2020). It was further explained that the particulate matter in the atmosphere serves as a carrier or transporter for virus particles enabling them to float in the airflows for a larger period of time, promoting its diffusion to longer distances. In airflows, the virus particles could survive for hours to days (Martelletti and Martelletti 2020). When a person inhales SARS-CoV-2 laden-PM, the virus enters into the deeper tracheobronchial and alveolar regions, causes cytokine storm syndrome leading to the death of the person infected and also increase the possibility of its transmission (Mehta et al. 2020). Pro-inflammations, injuries, and fibrosis from inhaled particulate matter in combination with cytokine storm induced by SARS-Cov-2 infection could further enhance the infection severity. The association of PM with increased respiratory morbidity and mortality is well documented in literature; however, the potential effect of PM on SARS-CoV-2 diffusion has been proposed only as an experimental evidence of potential risks and has not been confirmed yet (Qin et al. 2020; Bontempi 2020b). Hence, more studies are needed to better understand the interaction of SARS-CoV-2 with particulate matter.
Recently Zhu et al. (2020) found a positive association between the concentration of five air pollutants, i.e. PM2.5, PM10, CO, NO2, and O3, and daily cases of COVID-19 in 120 cities of China, however, SO2 concentration was reported to be negatively associated with COVID-19. Similarly, another study revealed that long-term exposure to NO2 is an important contributor to the mortalities caused by the SARS-CoV-2 across European countries (Ogen 2020). The fatality data was collected from several regions in Italy, Spain, France, and Germany, and spatial distribution of tropospheric NO2 was obtained using Sentinel-5P satellite. The findings revealed that the areas having the highest concentration of NO2 together with downward airflow recorded the highest fatalities. Apart from NO2 concentration, the topography of the study area along with the conditions of temperature inversion (limiting the dispersion of NO2), further aggravated the problem affecting the population even more severely.
All these pieces of evidence advocate that SARS-CoV-2 is highly prevalent and lethal in the areas having a high level of air pollution; thus, the inhabitants residing in areas with a higher concentration of air pollutants experiencing chronic exposure are more susceptible to respiratory diseases and hence are at more risk. Along with the concentration of contaminants in the atmosphere and biotic factors, other factors such as topographical features, airflows, atmospheric conditions also play a critical role in determining the SARS-CoV-2 longevity, transmissivity, and infectivity. Besides, the mechanism through which the air pollutants modify the viral pathogenesis after inhalation needs an in-depth experimental investigation to verify the impact of the COVID-19 pandemic. Atop, far-sighted measures for reducing the pollution load from the environment is much more critical.
Water and wastewater
The spread of SARS-CoV-2 through waterways is a newer possibility which must be explored in depth. The contamination of water supplies with faeces has always been a risk for human health. In several previous studies, pathogenic viruses have been held responsible for different water-borne diseases. Contaminated water supplies could act as a vehicle for spreading virus particles, providing an opportunity for disease outbreaks. Although limited information is available over the environmental persistence of SARS-Cov-2, however in the last two decades, several studies have reported the persistence and survival of other coronaviruses in the aquatic environment at different temperatures. For instance, Wang et al. (2005a) spiked the SARS-CoV in hospital wastewater, sewage, tap water and found that the virus could survive for 2 days at 20 °C and ≥ 14 days at 4 °C in all the spiked matrices. Similarly, Gundy et al. (2009) spiked Human Coronavirus (229E), ATCC-740, and Feline Infectious Peritonitis Virus (FIPV), ATCC-990, in tap water and sludge effluent and demonstrated 99.9% reduction over 10 days in tap water at 23 °C and over 100 days at 4 °C, whereas in sewage, 99.9% reduction was reported between 2 and 3 days at 23 °C. In a similar line, Casanova et al. (2010) spiked surrogate coronavirus named Transmissible Gastroenteritis Virus (TGEV) and Murine Hepatitis Virus (MHV) in MiliQ water, lake water, and human sewage and found that the TGEV significantly reduced (99.9%) at 25 °C in MiliQ water after 33 days, lake water samples after 13 days and sewage after 14 days, while no significant reduction was observed at 4 °C in MiliQ water after 49 days and a slight reduction was observed in other two samples. Similarly, MHV was found to be reduced by 99.9% at 25 °C in MiliQ water after 26 days, lake water samples, and sewage after 10 days, however at 4 °C, no significant reduction was observed except in the case of sewage samples. These studies indicated that higher temperature inactivates the virus, probably via protein denaturation and increased activity of extracellular enzymes. The study of Ye et al. (2016), wherein Murine hepatitis virus (MHV) was spiked in municipal wastewater, also confirmed that the virus persistence decreases with the increase in the temperature. Likewise, the persistence of coronavirus in stools and urine samples have also been reported. Previously in 2005, Wang et al. (2005a) revealed that SARS-CoV could persist in faeces samples for 3 days and in urine samples for 17 days at 20 °C, while at a lower temperature (4 °C), it could persist for 17 days in faeces. It was further confirmed in a study by Weber et al. (2016), wherein the virus was observed to persist in stool samples for 4 days. In sewage, SARS-CoV remains viable for 14 days and 2 days at 48 °C and 20 °C, respectively, and its RNA could be detected for 8 days although the virus itself becomes inactive Wang et al. (2005b). The persistence of SARS-CoV in stool and sewage has also been displayed in studies of Cheng et al. (2004), Hung et al. (2004), and Wang et al. (2005c). These studies imply that CoVs from the faecal discharge of infected persons may remain infectious in wastewater/sewage for a long period of time. Since these studies underlined the persistence of coronaviruses in water environment and faeces, and the concerns over the possible transmission of SARS-CoV-2 through wastewater/sewage has been raised.
Both viable and non-viable SARS-CoV-2 and their debris like RNA fragments, mRNA, or capsid subunits could enter wastewater via shedding of bodily excreta, including saliva, sputum, and faeces of infected persons. This is evidenced from the recent studies of Bowser (2020) and Pan et al. (2020) who revealed the existence of SARS-CoV-2 in human faeces. Similarly, Wang et al. (2020c) & Wu et al. (2020b) also detected viable SARS-CoV-2 in infected persons’ faeces. In fact, the viral RNA in the stools of an infected person was reported in the range of 16.5 to 100% at a concentration up to 6.8 log10 genome copies/g of stool (Chen et al. 2020b; Lo et al. 2020; Han et al. 2020a; Lescure et al. 2020). It was also observed that SARS-CoV-2 RNA could persist in faeces for 22 days, while for respiratory airways and serum, this duration is of 18 days and 16 days, respectively (Zheng et al. 2020a). Subsequently, in sewage and sewage sludge, SARS-CoV-2 RNA showed its presence (Medema et al. 2020; Ahmed et al. 2020; Peccia et al. 2020; Rimoldi et al. 2020). Similar studies reporting the presence of SARS-CoV-2 in wastewater have been displayed in Table 3. All the scientific evidence uncovering the viral load of SARS-CoV-2 in wastewater projected it as a potential instrument for wastewater-based epidemiology (WBE). In this regard, monitoring of SARS-CoV-2 in wastewater has been proposed as a complementary approach for the investigation of virus circulation in the community at different places and times (Haramoto et al. 2020; Ahmed et al. 2020; Medema et al. 2020). The wastewater/sewage surveillance using WBE could be used to establish trends in current outbreaks, identification of new outbreaks, and the prevalence of infections. It could also serve as an early warning system for community-wide emergence, subsidence, or elimination of COVID-19 (La Rosa et al. 2020; Daughton 2020). The actual status of the outbreak could be estimated using this approach, as it covers the excrement from both asymptomatic and symptomatic patients, which may otherwise go unnoticed during clinical surveillance (Kumar et al. 2020). Further, it could help in the identification of the infected individuals in WBE-revealed hotspots (Hart and Halden 2020). It is important to highlight that the wastewater/sewage surveillance offers an economical solution for tracking the outbreaks and could help in real-time monitoring and forecasting the emergence of any other pandemic of similar nature; however, its precision and sensitivity is still not clear (Kumar et al. 2020).
Table 3.
Presence of SARS-CoV-2 in wastewater
| Study area | Water matrix | Sample volume | Time span | Virus concentration and detection method | Inferences | References |
|---|---|---|---|---|---|---|
| Massachusetts, USA | Sewage | 18th March to 25th March 2020 | Initial testing was done with PCR using primers specific for the SARS-CoV-2 S gene followed by US CDC primer/probe sets targeting the N1, N2, and N3 loci of the SARS-CoV-2 nucleocapsid gene | SARS-CoV-2 was detected in all the 10 samples with approximately 100 genomic copies/ml | Wu et al. 2020a* | |
| Southeast Queensland, Australia | Untreated wastewater (sewage) | 100–200 ml | 24th February 2020 to 1st May 2020 | Viruses was concentrated via two methods: (i) direct RNA extraction from electronegative membranes and (ii) ultrafiltration followed by detection with RT-qPCR with two different primer-probe sets for nucleocapsid protein gene | Out of nine samples, two were tested positive; one positivity for each concentration method (not the same sample) but with only one set of primers and at very low titres: 1.2 and 1.9 genomic copies/100 ml | Ahmed et al. 2020 |
| Region of Murcia (Spain) | Wastewater | 200 ml | 12 March to 14 April 2020 | Aluminium hydroxide adsorption-precipitation concentration method was used and RT-qPCR diagnostic panel validated by US CDC was used for detection | SARS-CoV-2 RNA was detected in two out of eighteen secondary water samples and all twelve tertiary water samples were tested as negative | Randazzo et al. 2020 |
| Paris, France | Raw and treated wastewater | 5th March to 23rd April 2020 | Viral concentrate was lysed and extracted using PowerFecal Pro kit (QIAGEN) on QIAsymphony automated extractor; Confirmed by RT-qPCR on viral RdRp gene | All the samples tested positive for SARS-CoV-2 genomes | Wurtzer et al. 2020* | |
| Milan and Rome, Italy | Influent sewage | 250 ml | February and April 2020 | Concentration was done using two-phase (PEG-dextran method) separation; developed novel nested PCR assay specific for SARS-CoV-2 analysis | 50% samples were tested positive and one of them was present in the sample that was collected just a few days after the first case of SARS-CoV-2 in Italy. | La Rosa et al. 2020 |
| Bozeman, Montana, USA | Raw sewage | 500 ml | 23rd March to 27th March, 2020; 30th March to 3rd April 2020 | The samples were concentrated with Corning Spin-X UF concentrators & RNeasy Mini Kit extracted RNA. RT-qPCR was done using N1 and N2 primer pairs and probes from 2019-nCoV CDC EUA Kit | All the seven samples tested positive for SARS-CoV-2. Composite sampling is suggested as the most reliable method for calculating viral conc. In water over time. | Nemudryi et al. (2020)* |
| Netherlands | 100–200 ml | 5th February to 16th March 2020 | Samples were filtered and concentrated by centrifugation. Four primer sets were selected, i.e. N1–N3 for nucleocapsid protein gene and envelope protein (E) gene against two separate SARS-CoV-2 genes | 77.8% samples foundpositive after reporting of the first case of COVID 19 in Netherlands. | Medema et al. (2020)* | |
| Yamanshi Prefecture, Japan | Wastewater and river water | 200–5,000 ml | 17th March to 7th May 2020 | Concentration and extraction was done using electronegative membrane-vortex (EMV) method and adsorption-direc tRNA extraction method | SARS-CoV-2 detected in 20% of the sec. wastewater with a conc. of 2.4 × 103 copies/L. All the sample of influent and river were tested negative. EMV method was found superior | Haramoto et al. 2020* |
*The data has been retrieved from medRxiv as preliminary reports, which had not yet been peer-reviewed
Although the presence of SARS-CoV-2 RNA has been established in faeces and wastewater/sewage, still, the risk for exposed population in association with the water cycle is debatable, as the pathogenicity of the viral particles in these matrices has not been reported yet. Furthermore, the influence of environmental variables like temperature, light exposure, organic matter, presence of other microorganisms, rainfall events, hydraulic retention time in sewers, etc., on COVID-19 prevalence also warrants thorough investigation (Randazzo et al. 2020). Furthermore, the presence of SARS-CoV-2 in faeces necessitates a detailed investigation of faecal-oral transmission. Regarding this, Heller et al. (2020) proposed a framework for the faecal-oral hypothesis revealing the possible ways through which virus can be transmitted from faeces to mouth organ, infecting the respiratory tract and or gastrointestinal tract of the exposed person. The primary ways include transmission through water wherein a person is exposed to the virus via ingestion of contaminated water (waterborne) or water-washed, which could be prevented via a sufficient supply of water for maintaining domestic and personal hygiene. The other route is faecal-oral non-bacterium infections, which spread due to inadequate hygiene practices. Insect vectors add another route of transmission. Besides, a water-cleaning category has been included wherein the infection could spread via contacting contaminated water used to clean the surfaces. Senapati et al. (2020) supported this hypothesis of faecal-oral transmission. In their study, the rectal swab and corresponding nasopharyngeal swab was collected from 12 COVID-19 patients and tested for SARS-CoV-2 genome. Of the total samples, 80% asymptomatic and 28% symptomatic were tested positive for the genome indicating the faecal dissemination of the SARS-CoV-2 genome. These studies suggest that the faecal-oral transmission should be taken into account while preparing strategies to mitigate the virus outbreak. Also, the mechanism of SARS-CoV-2 interaction with the gastrointestinal tract merits urgent research.
Although the presence of SARS-CoV-2 has been confirmed in wastewater, its persistence in various types of water at different temperature conditions warrants further investigation. Moreover, the possibility of virus transmission (airborne) from contaminated wastewater through virus-laden aerosols generated during wastewater flushing needs to be explored. A previous study over SARS epidemic cluster in Amoy Gardens, Hong Kong, demonstrated how the small droplets containing viruses from the contaminated sewage leads to infection outbreak in the community residing in a high-rise housing estate (Peiris et al. 2003). Similarly, Gormley et al. (2017) displayed the cross-transmission of aerosol-laden pathogens via building sanitary plumbing system airstreams. Recently, Ong et al. (2020) proposed the likelihood of SARS-CoV-2 transmission through bioaerosols generated from toilet flushing. Looking at the substantial load of SARS-CoV-2 in wastewater plumbing system together with the probability for airborne transmission owing to virus aerosolization, the wastewater plumbing systems should be taken into consideration as a possible transmission route for COVID-19 infection (Gormley et al. 2020).
With the increasing number of evidence of SARS-CoV-2 presence in wastewater, the concern over possible contamination of receiving water bodies (groundwater, rivers, lakes, sea) has also been raised. In order to check that, effective disinfection of the wastewater at the point of the source has to be ensured before their discharge into the other environmental compartments. Also, the monitoring of wastewater/sewage should be carried out through the implementation of a surveillance system for measuring the presence and prevalence of COVID-19 in communities. Another important concern is the detection of SARS-CoV-2. At present, standardized methods for SARs-CoV-2 like enveloped virus is lacking; thus, there is a need for developing efficient concertation and detection method of the virus in water samples. Also, in detection methods like RT-PCR, ELISA, both viable and non-viable virus particles along with their degraded products should be taken into account, to provide the actual status of virus loadings in sewage/wastewater (Daughton 2020). The judgement of viral infectivity/viability in contaminated matrices is also an important aspect of research.
Solid surfaces
On solid surfaces like aluminium, metal, glasses, plastics, etc., viruses like SARS-CoV, MERS-CoV, or HCoV could persist for hours to days that in turn increases the possibility of virus transmission via touching (Kampf et al. 2020; Otter et al. 2016). The stability of SARS-CoV-2 on solid surfaces and the interfaces is reported to be highly inconsistent, varying from few hours up to as high as 9 days, depending on virus strain and varying conditions of temperature, humidity, type of surface (smooth or rough), etc. (Kampf et al. 2020). To investigate the persistence of SARS-CoV-2 on solid surfaces, van Doremalen et al. (2020) applied SARS-CoV and SARS-CoV-2 on copper, cardboard, stainless steel, and plastic and then kept them at 21–23 °C and 40% relative humidity for 7 days. In comparison with copper and cardboard, SARS-CoV-2 showed more stability on the plastic surfaces, followed by stainless steel. As far as viability is concerned, SARS-CoV-2 stayed viable for 72 h and 24 h on plastic and stainless-steel surface, respectively, while on the surfaces of copper and cardboard, no viable virus was observed after 4 h and 24 h, respectively. It was further reported that the viability of the virus decreases with the increase in time. A clinical study investigated aerosol and surface distribution of SARS-CoV-2 in hospital wards of Wuhan, China (Guo et al. 2020). The surface and air samples were collected from an intensive care unit (ICU), having 15 patients, and a general COVID-19 ward (GW) having 24 patients. The virus showed wide distribution in air and on object surfaces such as floors, soles of ICU medical staff shoes, computer mice, trash cans, sickbed handrails, doorknobs, sleeves cuffs, gloves, patient masks. In comparison with the general ward, the concentration of virus was found much higher in the intensive care unit. Ong et al. (2020) also reviewed the presence of SARS-CoV-2 in air and surface objects (toilet bowls, sinks, air outlet fans, door handle) present in isolation rooms of three patients admitted to a COVID-19-outbreak centre in Singapore. The surface samples were collected before and after routine cleaning of the high-touch areas. Most of the pre-cleaning surface samples were tested positive, whereas the post-cleaning samples showed negative results. This evidence advocates that routine cleaning procedures using effective disinfectant could limit the surface contamination of SARS-CoV-2. Contrary to previous findings, in this study, the air samples were found negative. The reason may be ascribed to the small sample size and inconsistent methodology. Another clinical study was carried out in two Wuhan Hospitals by Liu et al. (2020c) for investigating the aerodynamics of SARS-CoV-2. Their findings revealed the presence of high loads of SARS-CoV-2 viral RNA in aerosols collected from apparel changing rooms of the medical staff and a mobile toilet room of the COVID-19 patients, which was poorly ventilated, while the concentration was very low in isolation wards and ventilated patient rooms. The study further suggested that proper ventilation, less crowded space, proper sanitization of protective apparel, and timely disinfection of the toilets could help in containing the surface contamination. Though these studies confirmed the presence of SARS-CoV-2 on varied surfaces, however, the information over infectivity of the virus over solid surfaces/interfaces is yet to be explored.
Frozen food and cold chain transportation
Recent findings indicating the presence of SARS-CoV-2 in frozen food and packaging surface has raised the concern over the cold chain transportation of the virus. The first such report came from Beijing’s Xinfadi market when SARS-CoV-2 was detected on imported salmon’s surface (Han et al. 2020b). Later, in China, several cases reported the existence of SARS-CoV-2 on food packaging materials, food storage locations, surfaces of frozen foods such as shrimps, chicken wings, seafood, etc. (SMHC 2020; Liu et al. 2020d). A similar case has been reported in Qingdao Port, wherein 421 surface swab samples of cod outer package were tested for the presence of SARS-CoV-2, and 50 of them were tested positive (Liu et al. 2020d). All of these evidences suggest foodborne transmission via cold chain transportation as a potential pathway of SARS-CoV-2 transmission (Han et al. 2020b). In response to the recurrent detection of SARS-CoV-2 in frozen foods and packaging surfaces, China suspended the import of frozen food products from Europe initially, and later from the USA, Germany, and Brazil where most seafood processing workers were found infected (Han et al. 2020b; Siregar 2020). Though foodborne transmission was speculated as a possible risk factor in previous studies, it grabbed the attention of the regulatory authorities and the consumers only after reporting of the recent incidences of outbreak re-emergence at various places linked with cold chain transportation (Sharma et al. 2020). Notably, the lower temperature has been associated with the spread of COVID-19 as a favourable condition, but their impact on frozen and packaged food has been greatly disregarded since the onset of this viral disease (Pang et al. 2020). This ignorance led to the occurrence of a number of cases regarding the presence of SARS-CoV-2 in frozen foods and their potential impacts worldwide (Liu et al. 2020d; Han et al. 2020b). In the UK, a poultry processing unit and a food establishment were critically disrupted due to the emergence of COVID-19 (Wales 2020). Similarly, Tuna canneries in Portugal and Ghana were suspended after the workers tested positive for SARS-CoV-2 (Thomas 2020). In another episode in Germany, more than 15000 labours, working for a slaughterhouse tested positive for the viral infection resulting in the lockdown of two districts, populated with more than 60,000 people (Thomas 2020; Fisher et al. 2020).
The low-temperature conditions which are maintained at cold storages, for keeping the food products fresh for a longer period, also provide favourable environment for the extended survival of the virus. More than a few studies have demonstrated the prolonged persistence of SARS-CoV-2 at lower temperature conditions. For instance, Chin et al. (2020) assessed the stability of SARS-CoV-2 at 4 °C and reported great viral stability, exhibiting 0.7 log-unit reduction of infectious titre after 2 weeks of incubation. Likewise, SARS-CoV-2 was found to be stable on polypropylene disks at 4 °C up to 48 h of duration (Matson et al. 2020). The study of Kratzel et al. (2020) was also found in agreement with the findings of Matson et al. (2020). In order to investigate the stability of SARS-CoV-2 in food samples at lower temperatures, Fisher et al. (2020) spiked 200 μl of 3 × 106) TCID50/ml SARS-CoV-2 in the pieces of salmon, chicken, and pork and kept them refrigerated (4 °C) and frozen temperature (− 20 °C and − 80 °C) for 21 days. The titre of SARS-CoV-2 was observed to persist on the food material at all three temperatures for the entire duration of the experiment, indicating that the virus could retain its virulence in cold storage conditions for up to three weeks. This signifies that SARS-CoV-2 could survive transport and storage conditions and thus represent a significant risk to the individual working in the cold chain industry. In the absence of personal safety measures, an individual could contract infection with the contaminated food product or packaging via contact route and could serve as a carrier of the virus, increasing the possibilities of viral spread. Moreover, tracing the contamination link is challenging since the spread transmits through a complicated ‘farm-to-table’ life cycle (Han et al. 2020b). In this regard, it is imperative that all the workers and employers connected with the business of frozen and packaged foods stay vigilant during the working hours and follow all the safety guidelines and protocols recommended by health agencies for protection from COVID-19 (Zhao 2020).
Although foodborne transmission has not been fully explored yet, it important to underline that the contaminated cold storage food could serve as a long-range carrier of SARS-CoV-2, presenting a systematic risk of its transmission across the regions and countries via cold chain industries. In order to put a check on this pathway, the regulatory authorities must reinforce strict scrutiny and quarantine rules for the imported frozen foods along with the emphasis on the adoption of proper safety measures in the food supply chain.
Biotic factors
Age factor
Although individuals of each age group are infected with SARS-CoV-2, however, the effects are much differentiated, and the chief deciding factors include age, gender, and health conditions. Old age has been identified as one of the predictors for high mortality among the population due to COVID-19. Others include low lymphocyte count, high C-reactive protein, and high D-dimer levels. The rate of mortality has been observed to increases gradually with age around 60 years of age. For instance, in China, the mortality rate was reported to be 0.2% only in the persons up to 40 years of age, while it increased up to 8% in the person between 70–79 years of age, and the highest mortality rate of 14.8% was reported from 80 years of age (China CDC 2020). Similarly, in Europe, 89% of mortality was reported in patients ≥ 65 years of age (Weekly surveillance report COVID-19, euro.who.int). Individuals with comorbidities such as diabetes, high blood pressure, hypertension, chronic respiratory disease, cardiovascular disease, cancer, etc., have a higher fatality rate than the average (Kluge 2020). In New York state, 86.2%, and in Europe, 95% of deaths due to COVID-19 were reported to have at least one comorbidity with cardiovascular disease, the leading comorbidity (Franki 2020; Weekly surveillance report COVID-19, euro.who.int). As per WHO-China joint mission report on COVID-19 (2019), patients with no comorbid conditions had a crude fatality rate (CFR) of 1.4%, while patients with comorbid conditions experienced much greater CFR rates (13.2%) for those with cardiovascular disease, 9.2% for diabetes, 8.4% for hypertension, 8% for chronic respiratory disease, and 7.6% for cancer. Caramelo et al. (2020) also made a similar observation regarding the greatest risk to elderly patients, even higher than those having any comorbidity, and cardiovascular disease was reported to be the riskiest. Also, in infected persons over 60 years of age, greater clinical manifestations, higher severity & longer disease courses have been noticed (Liu et al. 2020b). In a study, the age dependence in susceptibility to SARS-CoV-2 infection and in clinical symptoms was shown (Davies et al. 2020). It was reported that the individuals > 20 years are more susceptible to virus infection in comparison with individuals < 20 years of age. Further, it was reported that clinical symptoms manifest in 21% of infections in 10-19-year-old individuals, increasing to 69% of infections in individuals > 70 years of age (Davies et al. (2020). Though the children are susceptible to infection, they are more likely to be asymptomatic or paucisymptomatic, referred to as sub-clinical infections. The involvement of sub-clinical infections in the transmission of COVID-19 is uncertain yet, thus represents a crucial topic to be taken into account in further investigations (Bi et al. 2020; Davies et al. 2020). Also, a systematic evaluation of age could help in the establishment of risk stratification for all COVID-19 patients worldwide.
Sex and gender
Sex and gender also played a crucial role in the COVID-19 outbreak. Jin et al. (2020) investigated the role of gender in morbidity and mortality in COVID-19 patients and found that males are comparatively more at risk in comparison with females, irrespective of their age. In China, the females were found to be less susceptible towards this virus, having a fatality rate of 1.7% in comparison with males having a 2.8% fatality rate (China CDC 2020). Similarly, in Europe, 57% of the COVID-19 infected individuals and 72% of dead persons belonged to the male community (Weekly surveillance report COVID-19, euro.who.int). Though the exact reason behind these differences is not known yet, sex-based immunological differences may account for the lesser susceptibility of females to COVID-19 infection (Chen et al. 2020a). Oestrogen present in the female body stimulates the immune system making them more resistant to the attack of virus, whereas the testosterone in males suppresses the immune system, putting them at risk. Also, the females have two X chromosomes, which have the genes responsible for the recognition of pathogens, and the males have only one, the female body could better combat the virus attack (Cunningham 2020). Besides, gender-specific behavioural differences such as a relatively lesser prevalence of smoking and drinking in females and males developing comorbid conditions such as cardiovascular disease, hypertension, etc., at a younger age in comparison with the females, could have contributed to the greater mortalities in males (Gebhard et al. 2020). It is crucial to understand the influence of sex and gender on SARS-CoV-2 prevalence and to incorporate them into preparedness and response efforts of health interventions.
Blood type
The blood groups are known to have a significant association with infection severity, acting as receptors for pathogenic microbes. The linkage between the blood types and host susceptibility towards varied infections depends on the expression of blood group antigens (Cooling 2015). Previous findings have revealed that the ABO blood group polymorphism plays a significant role in disease acquisition and infection severity (Lindesmith et al. 2003; Cheng et al. 2005; Cooling 2015; Chen et al. 2016). The presence of an association between blood type and susceptibility towards norovirus and Helicobacter pylori infections (Lindesmith et al. 2003; Boren et al. 1993) provoked the exploration of possible linking among the coronavirus infections and blood type and the first report highlighting the relationship between the blood type and SARS infection, came during SARS-CoV outbreak in 2002, in Hong Kong population (Cheng et al. 2005). The findings revealed that the person having O blood type were less susceptible towards SARS-CoV infection in comparison with those having other blood types. A similar kind of association has been observed in the case of COVID-19. For instance, Zhao et al. (2020) studied ABO blood group distribution among SARS-CoV-2 infected patients in three hospitals in China and observed blood group A to be associated with a higher risk of COVID-19 acquisition and mortality in comparison with non-A blood group whereas blood group O showed the lowest risk of viral infection and severity. Likewise, in another study, the percentage of blood group A in COVID-19 patients was observed to be substantially higher in comparison with the controls (0.38 vs. 0.32%, P < 0.001), whereas the percentage of blood group O was found to be lower as compared with the controls (0.26 vs. 0.34%, P < 0.001) (Liet al., 2020). One of the studies carried out in Denmark also reported the positive relationship of the O blood type with the lower risk of SARS-CoV-2 infection (RR 0.87, 95% CI 0.83–0.91) (Barnkob et al. 2020). Among the population classified according to gender, Fan et al. (2020) reported that the females with blood type A were associated with greater susceptibility to COVID-19. Similarly, several other studies indicated that blood group A or AB is highly susceptible to SARS-CoV-2 infection having a greater risk for requiring mechanical ventilation, continuous renal replacement therapy (CRRT), and prolonged ICU length of stay, compared with blood groups O or B (Fan et al. 2020; Hoiland et al. 2020; Hultström et al. 2020). Likewise, the Rh-negative blood group was also reported to be linked with a lower risk for SARS-CoV-2 infection and COVID-19 severity (Ray et al. 2020).
While ACE2 has been considered the primary receptor of SARS-CoV-2 in the host cell (Lan et al. 2020), Arend (2020) suggested that the SARS-CoV-2 more preferably binds to the host cell through the formation of a hybrid structure, dominated by viral serine. The resulting intermediate structure gets replaced by carbohydrates particularly to the ABO blood groups. SARS-CoV-2 via hybridization of the ABO(H) blood groups or by mimicking the glycosylation metabolic pathways bind to the cell surfaces of the blood group. As the viral serine targets the saccharides of blood groups A, B, and AB in the glycosylation process, the non-O blood group person seems to develop relatively more disease symptoms than O blood groups. Silva-Filho et al. (2020) hypothesized that mostly antigen A and partly antigens B and AB promote the production of sialoside clusters in the target cells via cis carbohydrate-carbohydrate interactions, which in turn amplifies the interaction of these cells with the SARS-CoV-2 by enhancing the feasibility of binding of the N-terminal domain (NTD) and receptor-binding domain (RBD) to CD147 and ACE2 receptors, respectively. Besides ACE2, transmembrane proteins such as CD147 and TMPRSS2 (Transmembrane Protease Serine type 2) has also been recognized as significant players in SARS-CoV-2-human cell interaction (Wang et al. 2020d; Matsuyama et al. 2020).
In contrast to these findings, Latz et al. (2020) found no correlation between the blood type and SARS-CoV-2 infection severity leading to intubation or death. Nevertheless, statistical analysis displayed that individuals with B or AB blood type and Rh-positive individuals were relatively more likely to test positive in comparison with O blood type and Rh-negative individuals, respectively. The findings of Zietz et al. (2020) were also in partial agreement with Latz et al.’s observations. Hoiland et al. (2020) suggested that the variation in the findings could be ascribed to the widely different mortality rates. The variations either due to the factors specific to the disease or the system-level community health, also represent a valid concern regarding the comparability with studies of similar nature (Hoiland et al. 2020). Although these studies supported the association of ABO blood type and COVID-19, but most of these have reported several limitations such as small sample size, regional selection bias, presence of other potential diseases, reliance over preliminary data, variability in study design and selected population, etc. (Fan et al. 2020, Liet al., 2020; Barnkob et al. 2020; Zhao et al. 2020) and thus cannot be considered conclusive.
The recent findings exploring the interlinking of SARS-CoV-2 infection with ABO blood group system are present at their preliminary stage, and the results are controversial, thus determining an individual’s susceptibility based on their ABO blood group affiliation will be too early at this stage. Further in-depth investigations are required to explore the molecular mechanism underlying the susceptibility, which could lead towards a better understanding of COVID-19 pathophysiology and its kinetics.
Population density
High population density act as a catalyst for the transmission and subsequent spread of COVID-19. As per WHO safety guidelines, a person should keep more than a 1-m distance from a person who is coughing and sneezing, but this becomes much difficult in places having higher population-densities. Highly dense slum areas are more prone to infection, as the people residing there live in close association with each other in unhygienic conditions without proper access to safe water and sanitation. Limited space, low hygiene standards, and shared community facilities (water taps, common toilets, etc.) make the slum areas much vulnerable towards infection and transmission of COVID-19. High-density urban agglomerations are also the hotspots of COVID-19 infection, where the pace of the spread of the disease has been observed to be much higher. For instance, large metropolitan cities like New York, London registered the highest number of COVID-19 cases and associated fatalities in the US and UK (Desai 2020). Similarly, in China, the virus spread much faster in densely populated cities as compared with less crowded cities. A positive relationship of population density with an effective reproductive number (R values) of COVID-19, has been found in a study in China. As per their report, in the pre-lockdown state, a rise in 1000 people/km2 population density is associated with a 0.1188 increase in the R value (Wang et al. 2020a). As urbanization will continue to grow up in the countries, the government should reinspect their urban strategies to combat pandemics of similar nature in the future. Population mobility, which has a direct relationship with population density, is another factor affecting the COVID-19 outbreak (Ahmadia et al. 2020). In India, thousands of migrant workers return to their villages in lockdown situations, spreading the infection to rural areas that do not have adequate medical facilities, making them even more vulnerable to the COVID-19 crisis. Besides population density, other factors like health care facilities, adherence to the quarantine policies, social distancing, etc. play a much bigger role in determining the transmission intensity of COVID-19.
Behavioural variables
Recently smoking and obesity have also been linked to COVID-19 as a potential risk factor. Although the effects of COVID-19 on an obese person is not fully described. However, it is suggested that in infected persons with increased abdominal obesity, the pulmonary function is affected adversely because of reduced diaphragmatic excursion, making the ventilation even more problematic (Dietz and Santos 2020). Moreover, enhanced inflammatory cytokines further contribute to the infection (Decaro and Lorusso 2020). A cohort study implies that the severity of COVID-19 is associated with body mass index (BMI), having the highest impact on patients with BMI ≥ 35 (Simonnet et al. 2020). It is a well-known fact that smoking adversely affects the lung’s functionality, impedes the body’s responsiveness to infections, and suppresses immunity; thus, it is likely that the habit of smoking makes a person more susceptible to COVID-19 infection. In this line, Zheng et al. (2020b) and Zhao et al. (2020) analysed data of COVID-19 patients and revealed a significant association between smoking and the severity of the infection. In contrast, Vardavas and Nikitara (2020) found a non-significant relationship between smoking and disease severity. Few studies reported that ACE2 is expressed explicitly in the lungs of smokers and patients with chronic obstructive pulmonary disease, thus enhancing the risk of infection (Brake et al. 2020; Cai 2020; Wang et al. 2020e), while downregulation of ACE2 with smoking is also reported (Oakes et al. 2018). The relationship between smoking and SARS-CoV-2 infectivity has not been fully understood yet, thus needs further investigation.
Conclusion and future perspective
The novel coronavirus disease (COVID-19) outbreak has posed unprecedented threats and challenges to community health worldwide. In this critical time, one of the major concerns that have gained attention globally is the spread of COVID-19 through environmental media and the influence of the environmental factors on disease transmissivity/transmissibility, persistency, and pathogenicity. Recent studies have shown possible transmission of SARS-CoV-2, the causative agent of COVID-19, through air and water media. Few studies also highlighted the persistence of the SARS-CoV-2 in environmental matrices from few hours to days, however, the period up to which SARS-CoV-2 remains infectious in natural conditions needs to be researched further. The possibility of airborne and waterborne transmission also received much attention after discovering the presence of virus particles and viral RNA in environmental samples; however, its involvement in environmental circulation has not been confirmed yet and merits urgent research. Apart, there is a lot of debate over the association of COVID fatalities with climatic factors. To gain insights over this, it is critical to understand the links between climate, weather, pollution, and epidemics. Long-term research in this line will help anticipate the advent of any other possible pandemics in the future.
Faecal-oral transmission is another major concern specifically in developing countries. Although current knowledge is very scarce and fragmentary in this regard, its possibility as an additional route of spread cannot be ruled out. Thus, it is critical that government agencies worldwide encourage the development of sewage monitoring capabilities that could help in forecasting the emergence of any other pandemic of similar nature. Indeed, wastewater-based epidemiology (WBE) should be used to monitor and manage public health in real time, on a global scale. Furthermore, upgrading the existing water and wastewater treatment infrastructures and efficient disinfection at the point of the source could help in containing the spread of the virus. Additionally, high-sensitivity methods for precise quantitation of the SARS-CoV-2 in environmental samples merits urgent research. The infectivity of SARS-CoV-2 over solid surfaces and interfaces is another area requiring in-depth investigation, till then to contain the spread of infection, methods for large-scale disinfection of the virus in diverse environmental settings should be developed. The transmission of SARS-CoV-2 via contaminated frozen food and packaging surfaces represents a newer possibility which must be investigated with high attention. Furthermore, biotic factors like population density, mobility, age structure, gender stratification, blood type, along with urbanization, medical care facilities, individual health status and immunity, hygiene practices, sanitation, behavioural variables, social preferences, etc., should be taken into account in the pandemic’s analysis.
It is important to highlight here that SARS-CoV-2 has many potential transmission pathways, some of them have been established, and many others are yet to be confirmed. Also, the persistence and infectivity of SARS-CoV-2 under different environmental conditions has not been fully explored yet. Furthermore, the inter-relationship of the abiotic and biotic factor and its potential impact on the kinetics of this pandemic at the local, regional, and global scale is still not clear. These knowledge gaps need to be addressed to enable the policy makers for establishing the frameworks, risk mitigation strategies, and precautionary control measures. Henceforth, it is imperative to develop an interdisciplinary research approach involving collaboration among medical professionals, policymakers, economists, epidemiologists, engineers, environmentalists, socialists, etc., that could help in gaining a clear concept of COVID-19 prevalence and dynamics in the community. In summary, a better understanding of the influences of different environmental factors on SARS-CoV-2 prevalence and its fate in environmental matrices could help in the development of suitable strategies for mitigating the exponential spread of COVID-19.
Acknowledgements
The authors are deeply indebted to the Head, Department of Environmental Science, Central University of Rajasthan, for providing all necessary facilities for this work.
Authors contributions
Kumar S and Singh R: conceptualization, original manuscript writing, editing; Kumari N, Karmakar S, Behera M, Siddiqui AJ, Rajput VD, Bauddh K: investigation, resources, data collection; Kumar N and Minkina T: review and editing
Data availability
Not Applicable.
Compliance with ethical standards
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sanjeev Kumar and Ritu Singh contributed equally to this work.
References
- “COVID-19 weekly surveillance report” (2020) Data for the week of 22 - 28 Jun 2020 (Epi week 26) www.euro.who.int.https://www.euro.who.int/en/healthtopics/healthemergencies/coronaviruscovid-19/ weekly-surveillance-report. Accessed on 5th July 2020
- Adhikari S P, Meng S, Wu Y J, Mao Y P, Ye R X, Wang Q Z, ... & Zhou H (2020) Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Diseases Poverty, 9(1): 1–12 [DOI] [PMC free article] [PubMed]
- Ahmadia M, Sharifi A, Dorosti S, Ghoushchi SJ, Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci Total Environ. 2020;729:138705. doi: 10.1016/j.scitotenv.2020.138705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O'Brien JW, Tscharke B. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo MB, Naimi B (2020) Spread of SARS-CoV-2 Coronavirus likely to be constrained by climate. medRxiv. 10.1101/2020.03.12.20034728
- Arend P (2020) Why blood group A individuals are at risk whereas blood group O individuals are protected from SARS-CoV-2 (COVID-19) infection: A hypothesis regarding how the virus invades the human body via ABO(H) blood group-determining carbohydrates. Immunobiology:152027 [DOI] [PMC free article] [PubMed]
- Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Tanwar S. Pre-symptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnkob MB, Pottegård A, Støvring H, Haunstrup TM, Homburg K, Larsen R, Hansen MB, Titlestad K, Aagaard B, Møller BK, Barington T (2020) Reduced prevalence of SARS-CoV-2 infection in ABO blood group O. Blood Adv 4(20):4990–4993 [DOI] [PMC free article] [PubMed]
- Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, Gao W. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, Viboud C. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8:3–4. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. Commercial exchanges instead of air pollution as possible origin of COVID-19 initial diffusion phase in Italy: more efforts are necessary to address interdisciplinary research. Environ Res. 2020;186:109775. doi: 10.1016/j.envres.2020.109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E, Vergalli S, Squazzoni F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ Res. 2020;188:109814. doi: 10.1016/j.envres.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth TF, Kournikakis B, Bastien N, Ho J, Kobasa D, Stadnyk L, Mederski B. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren T, Falk P, Roth K, Larson G, Normark S (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262(5141):1892–1895 [DOI] [PubMed]
- Bowser AD (2020) Coronavirus may cause environmental contamination through faecal shedding. Medscape Medical News. https://www.medscape.com/viewarticle/926390. Accessed 15 July 2020
- Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu J, Peng DD, Xiao H, Yue Q, Han Y, Lin Y, Hu G, Chen J (2020) Analysis of meteorological conditions and prediction of epidemic trend of 2019-nCoV infection in 2020. Med RXIV. 10.1101/2020.02.13.20022715
- Bukhari Y, Jameel Y (2020) Will coronavirus pandemic diminish by summer? SSRN Electron J. 10.2139/ssrn.3556998
- Byass P. Eco-epidemiological assessment of the COVID-19 epidemic in China, January–February 2020. Glob Health Action. 2020;13:1760490. doi: 10.1101/2020.03.29.20046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G (2020) Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv, 2020020051. 10.1101/2020.02.05.20020107
- Caramelo F, Ferreira N, Oliveiros B (2020) Estimation of risk factors for COVID-19 mortality-preliminary results. medRxiv. 10.1101/2020.02.24.20027268.t
- Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76(9):2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave E. COVID-19 super-spreaders: definitional quandaries and implications. Asian Bioethics Rev. 2020;12:1–8. doi: 10.1007/s41649-020-00118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Peiris J, Lam S, Poon L, Yuen K, Seto WH. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay I, Kiciman E, Elliott JW, Shaman JL, Rzhetsky A. Conjunction of factors triggering waves of seasonal influenza. Elife. 2018;7:e30756. doi: 10.7554/eLife.30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yang S-H, Xu H, Li J-J (2016) ABO blood group system and the coronary artery disease: an updated systematic review and metaanalysis. Sci Rep 6(1):23250 [DOI] [PMC free article] [PubMed]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, Yang J. The presence of SARS CoV2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC, Lau CS, Lim WW. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cheng G, Chui CH, Lau FY, Chan PK, Ng MH, Sung JJ, Wong RS (2005) ABO blood group and susceptibility to severe acute respiratory syndrome. Jama 293(12):1447–1451 [DOI] [PubMed]
- Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H, Chan MCW, Peiris M, Poon LLM. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China CDC (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China, 2020; The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Chinese Center for Disease Control and Prevention CCDC Weekly / Vol. 2 / No. x [PMC free article] [PubMed]
- Chiyomaru K, Takemoto K (2020) Global COVID-19 transmission rate is influenced by precipitation seasonality and the speed of climate temperature warming. medRxiv. 10.1101/2020.04.10.20060459
- Coccia M (2020a) Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci Total Environ 729:138474 [DOI] [PMC free article] [PubMed]
- Coccia M (2020b) Transmission dynamics of COVID-19 in polluting environment: mechanisms of air pollution-to-human transmission and human-to-human transmission. medRxiv, 20055657. doi:10.1101/2020.04.06.20055657
- Conticini E, Frediani B, Caro D (2020) Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environmental Pollution, 114465. 10.1016/j.envpol.2020.114465 [DOI] [PMC free article] [PubMed]
- Cooling L (2015) Blood groups in infection and host susceptibility. Clin Microbiol Rev 28(3):801–870 [DOI] [PMC free article] [PubMed]
- Cui Y, Zhang ZF, Froines J, Zhao J, Wang H, Yu SZ, Detels R. Air pollution and case fatality of SARS in the People’s Republic of China: an ecologic study. Environ Health. 2013;2:15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A (2020) COVID-19 kills more men than women. The immune system may be why. Science News. Accessed on 5th July 2020.
- Dalziel BD, Kissler S, Gog JR, Viboud C, Bjornstad ON, Metcalf JE, Grenfell BT. Urbanization and humidity shape the intensity of influenza epidemics in US cities. Science. 2018;362:75–79. doi: 10.1126/science.aat6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci Total Environ. 2020;726:138149. doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NG, Klepac P, Liu Y. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Decaro N, Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet Microbiol. 2020;244:108693. doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D (2020) Urban densities and the Covid-19 Pandemic: Upending the Sustainability Myth of Global Megacities. ORF Occasional Paper 4;244(4)
- Dietz W, Santos BC. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28(6):1005–1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- Drake J (2020) Protecting against airborne transmission of COVID-19. Forbes
- Duan SM, Zhao XS, Wen RF, Huang JJ, Pi GH, Zhang SX, Han J, Bi SL, Ruan L, Dong XP, Team S R Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. 2003;16(3):246–255. [PubMed] [Google Scholar]
- Eslami H, Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10(1):92. doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Principi N, Leung CC, Migliori GB. Universal use of face masks for success against COVID-19: evidence and implications for prevention policies. Eur Respir J. 2020;55:2001260. doi: 10.1183/13993003.01260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Zhang W, Li B, Li D-J, Zhang J, Zhao F (2020) Association Between ABO Blood Group System and COVID-19 Susceptibility in Wuhan. Front Cell Infect Microbiol 10. 10.3389/fcimb.2020.00404 [DOI] [PMC free article] [PubMed]
- Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Momeniha F. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Reilly A, Zheng A, Cook A, Anderson D (2020) Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. BioRxiv. 10.1101/2020.08.17.255166
- Franki, Richard (2020) “Comorbidities the rule in New York’s COVID-19 deaths”. www.the-hospitalist.org. Society of Hospital Medicine. Accessed on 5th July 2020
- Frontera A, Martin C, Vlachos K, Sgubin G. Regional air pollution persistence links to covid19 infection zoning. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, Ma K (2020) A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 10.1016/j.jmii.2020.05.001 [DOI] [PMC free article] [PubMed]
- Gardner EG, Kelton D, Poljak Z, Van Kerkhove M, Von Dobschuetz S, Greer AL. A case-crossover analysis of the impact of weather on primary cases of Middle East respiratory syndrome. BMC Infect Dis. 2019;19(1):113. doi: 10.1186/s12879-019-3729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):1–13. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M, Aspray TJ, Kelly DA, Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PLoS One. 2017;12(2):e0171556. doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M, Aspray TJ, Kelly DA. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob Health. 2020;8(5):e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy PM, Gerba CP, Pepper IL. Survival of coronaviruses in water and wastewater. Food Environ Virol. 2009;1(1):10. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, Zhang MY. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China. Emerg Infect Dis. 2020;26(7):10–3201. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Raghuwanshi GS, Chanda A. Effect of weather on COVID-19 spread in the US: a prediction model for India in 2020. Sci Total Environ. 2020;728:138860. doi: 10.1016/j.scitotenv.2020.138860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MS, Seong MW, Heo EY, Park JH, Kim N, Shin S, Choi EH. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin Infect Dis. 2020;71:2236–2239. doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhang X, He S, Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ Chem Lett. 2020;1:1–12. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E, Malla B, Thakali O, Kitajima M (2020) First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. medRxiv. 10.1101/2020.06.04.20122747 [DOI] [PMC free article] [PubMed]
- Hart OE, Halden RU. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M, Kilic S, Sarac F. Coronaviruses and SARS-COV-2. Turkurhesh J Med Sci. 2020;50:549–556. doi: 10.3906/sag-2004-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Chin Y, Huang J, He Y, Akinwunmi BO, Yu S, Ming WK (2020) Meteorological factors and domestic new cases of coronavirus disease (COVID-19) in nine Asian cities: a time-series analysis. medRxiv. 10.1101/2020.04.15.20066613
- Heller L, Mota CR, Greco DB. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci Total Environ. 2020;729:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiland RL, Fergusson NA, Mitra AR, Griesdale DEG, Devine DV, Stukas S, Cooper J, Thiara S, Foster D, Chen LYC, Lee AYY, Conway EM, Wellington CL, Sekhon MS (2020) The association of ABO blood group with indices of disease severity and multiorgan dysfunction in COVID-19. Blood Adv 4(20):4981–4989 [DOI] [PMC free article] [PubMed]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Diaz G. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultström M, Persson B, Eriksson O, Lipcsey M, Frithiof R, Nilsson B (2020) Blood type A associates with critical COVID-19 and death in a Swedish cohort. Crit Care 24:496. 10.1186/s13054-020-03223-8 [DOI] [PMC free article] [PubMed]
- Hung IF, Cheng VC, Wu AK, Tang BS, Chan KH, Chu CM, Wong MM, Hui WT, Poon LL, Tse DM, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10(9):1550–1557. doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangiri M, Jahangiri M, Najafgholipour M. The sensitivity and specificity analyses of ambient temperature and population size on the transmission rate of the novel coronavirus (COVID-19) in different provinces of Iran. Sci Total Environ. 2020;728:138872. doi: 10.1016/j.scitotenv.2020.138872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, Liu S, Yang J-K. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan HD, Chen BH, Fu CW, Yu SZ, Mu LN. Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomed Environ Sci. 2005;18:1–4. [PubMed] [Google Scholar]
- Kluge H H P (2020) Statement–Older people are at highest risk from COVID-19, but all must act to prevent community spread. Retrieved from World Health Organization website: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/statements/statement-older-people-are-at-highest-risk-from-covid-19,-but-all-must-act-to-prevent-community-spread. Accessed 23 July 2020
- Kratzel A, Steiner S, Todt D, V’kovski P, Brueggemann Y, Steinmann J, Steinmann E, Thiel V, Pfaender S. Temperature-dependent surface stability of SARS-CoV-2. J Inf Secur. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Shin JI. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Patel AK, Shah AV, Raval J, Rajpara N, Joshi M, Joshi CG. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Iaconelli M, Mancini P, Ferraro GB, Veneri C, Bonadonna L, Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Bogoch II, Ruktanonchai NW, Watts A, Lu X, Yang W, Tatem AJ (2020) Assessing spread risk of Wuhan novel coronavirus within and beyond China, January-April 2020: a travel network-based modelling study. medRxiv. 10.1101/2020.02.04.20020479
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581(7807):215–220 [DOI] [PubMed]
- Latz CA, DeCarlo C, Boitano L, Png CYM, Patell R, Conrad MF, Eagleton M, Dua A (2020) Blood type and outcomes in patients with COVID-19. Ann Hematol 99(9):2113–2118 [DOI] [PMC free article] [PubMed]
- Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, Enouf V. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang X, Yu ITS, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang L, Ren Z, Xing C, Qiao P, Chang B (2020) Meteorological factors correlate with transmission of 2019-nCoV: proof of incidence of novel coronavirus pneumonia in Hubei Province, China. medRxiv. 10.1101/2020.04.01.20050526
- Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R (2003) Human susceptibility and resistance to Norwalk virus infection. Nat Med 9(5):548–553 [DOI] [PubMed]
- Liu P, Jiang J-Z, Wan X-F, Hua Y, Li L, Zhou J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16(5):e1008421. doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mao B, Liang S, Yang JW, Lu HW, Chai YH, Wang L, Zhang L, Li QH, Zhao L, He Y, Gu XL, Ji XB, Li L, Jie ZJ, Li Q, Li XY, Lu HZ, Zhang WH, Song YL, Shanghai Clinical Treatment Experts Group for COVID-19 (2020) Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J. 2020;55(5):2001112. doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Liu X (2020c) Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 10.1101/2020.03.08.982637v1
- Liu P, Yang M, Zhao X, Guo Y, Wang L, Zhang J et al (2020d) Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: Successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosafety Health. 10.1016/j.bsheal.2020.11.003 [DOI] [PMC free article] [PubMed]
- Lo IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X, Sin NN. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Majumder MS, Liu D, Poirier C, Mandl KD, Lipsitch M, Santillana M (2020) The role of absolute humidity on transmission rates of the COVID-19 outbreak. MedRxiv. 10.1101/2020.02.12.20022467 [DOI] [PMC free article] [PubMed]
- Martelletti L, Martelletti P (2020) Air pollution and the novel Covid-19 disease: a putative disease risk factor. SN Compr Clin Med, 1–5 [DOI] [PMC free article] [PubMed]
- Matson MJ, Yinda CK, Seifert SN, Bushmaker T, Fischer RJ, van Doremalen N, Lloyd-Smith JO, Munster VJ. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg Infect Dis. 2020;26(9):2276–2278. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F, Sakata M, Tahara M, Kutsuna S, Ohmagari N, Kuroda M, Suzuki T, Kageyama T, Takeda M (2020) Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci 117(13):7001–7003 [DOI] [PMC free article] [PubMed]
- Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A (2020) Presence of SARS-Coronavirus-2 in sewage. medRxiv. 10.1101/2020.03.29.20045880 [DOI] [PubMed]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID -19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Meskini M, do Nascimento Pinto AL (2020) Novel coronavirus (COVID-19) overview. J Public Health. 10.1007/s10389-020-01258-3 [DOI] [PMC free article] [PubMed]
- Nemudryi A, Nemudraia A, Surya K, Wiegand T, Buyukyoruk M, Wilkinson R, Wiedenheft B (2020) Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. medRxiv. 10.1101/2020.04.15.20066746 [DOI] [PMC free article] [PubMed]
- Nghiem LD, Morgan B, Donner E, Short MD. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud Chem Environ Eng. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin-angiotensin system. Am J Phys Regul Integr Comp Phys. 2018;315(5):895–906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to the coronavirus (COVID-19) fatality rate. Sci Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveiros B, Caramelo L, Ferreira NC, Caramelo F (2020) Role of temperature and humidity in the modulation of the doubling time of COVID-19 cases. medRxiv. 10.1101/2020.03.05.20031872
- Olsen SJ, Chang HL, Cheung TYY, Tang AFY, Fisk TL, Ooi SPL, Hsu KH. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly KM, Auzenbergs M, Jafari Y, Liu Y, Flasche S, Lowe R. Effective transmission across the globe: the role of climate in COVID-19 mitigation strategies. Lancet Planet Health. 2020;4(5):e172. doi: 10.1016/S2542-5196(20)30106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3):e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhang D, Yang P, Poon LL, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Ren L, Wu S, Ma W, Yang J, Di L, Du J. Cold-chain food contamination as the possible origin of Covid-19 resurgence in Beijing. Natl Sci Rev. 2020;7:1861–1864. doi: 10.1093/nsr/nwaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J, Zulli A, Brackney DE, Grubaugh ND, Kaplan EH, Casanovas-Massana A, Weinberger DM (2020) SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv. 10.1101/2020.05.19.20105999
- Peiris JSM, Chu CM, Cheng VCC. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Song W, Ding Z, Guan Q, Yang X, Xu Q, Xia Y. Linking key intervention timings to rapid declining effective reproduction number to quantify lessons against COVID-19. Front Med. 2020;14:623–629. doi: 10.1007/s11684-020-0788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica N, Bouvier NM. Environmental factors affecting the transmission of respiratory viruses. Curr Opin Virol. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata DN, Rodrigues W, Bermejo PH. Temperature significantly changes COVID-19 transmission in (sub) tropical cities of Brazil. Sci Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Liang P, Wu C, Wang G, Xu Q, Xiong X, et al. Longitudinal survey of microbiome associated with particulate matter in a megacity. Genome Biol. 2020;21(1):1–11. doi: 10.1186/s13059-020-01964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G, Li X, Hu L, Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ Sci Technol. 2020;54(7):3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G (2020) SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Research, 115942 [DOI] [PMC free article] [PubMed]
- Ray JG, Schull MJ, Vermeulen MJ, Park AL (2020) Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness: A Population-Based Cohort Study. Ann Intern Med 24:M20-4511. 10.7326/M20-4511 [DOI] [PMC free article] [PubMed]
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (2020) 16-24 February 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed 23 July 2020
- Rimoldi SG, Stefani F, Gigantiello A, Polesello S, Comandatore F, Mileto D, Pagani C (2020) Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. medRxiv. 10.1101/2020.05.01.20086009 [DOI] [PMC free article] [PubMed]
- Sajadi MM, Habibzadeh P, Vintzileos A, Shokouhi S, Miralles-Wilhelm F, Amoroso A (2020) Temperature and latitude analysis to predict potential spread and seasonality for COVID-19. SSRN, 3550308. 10.2139/ssrn.3550308 [DOI] [PMC free article] [PubMed]
- Sanità di Toppi L, Sanità di Toppi L, Bellini E. Novel coronavirus: how atmospheric particulate affects our environment and health. Challenges. 2020;11(1):6. doi: 10.3390/challe11010006. [DOI] [Google Scholar]
- Santarpia JL, Rivera DN, Herrera V, Morwitzer MJ, Creager H, Santarpia GW, Lawler JV (2020, medRxiv) Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 10.1101/2020.03.23.20039446v2
- Senapati S, Kshatri JS, Prasad P, Turuk J, Pati S, Parida A (2020) A pilot study to investigate the fecal dissemination of SARS-CoV-2 virus genome in COVID-19 patients in Odisha, India. medRxiv. 10.1101/2020.05.26.20113167
- Setti L, Passarini F, De Gennaro G, Di Gilio A, Palmisani J, Buono P, Fornari G, Perrone M, Pizzalunga A, Barbieri P, Rizzo E, Miani A. (2020) Position Paper Relazione circa l’effetto dell’inquinamento da particolato atmosferico e la diffusione di virus nella popolazione. SIMA - Società Italiana di Medicina Ambientale, 1–5.
- Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8:e1000316. doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Goldstein E, Lipsitch M. Absolute humidity and pandemic versus epidemic influenza. Am J Epidemiol. 2011;173(2):127–135. doi: 10.1093/aje/kwq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Jinadatha C, Lichtfouse E. Environmental chemistry is most relevant to study coronavirus pandemics. Environ Chem Lett. 2020;18:993–996. doi: 10.1007/s10311-020-01017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenzhen Municipal Health Commission (SMHC) (2020) Detection of SARS-CoV-2 on an imported chicken wing sample. https://wjw.sz.gov.cn/yqxx/content/post_7998108.html. Accessed on 4 Dec 2020
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AJ, Jahan S, Ashraf SA, Alreshidi M, Ashraf MS, Patel M, Adnan M (2020a) Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2. J Biomol Struct Dyn 1–14 [DOI] [PMC free article] [PubMed]
- Siddiqui AJ, Danciu C, Ashraf SA, Moin A, Singh R, Alreshidi M, Medical Hypotheses, ... & Badraoui R (2020b) Plants-derived biomolecules as potent antiviral phytomedicines: new insights on ethnobotanical evidences against coronaviruses. Plants, 9(9):1244 [DOI] [PMC free article] [PubMed]
- Silva-Filho JC, de Melo CGF, de Oliveira JL (2020) The influence of ABO blood groups on COVID-19 susceptibility and severity: A molecular hypothesis based on carbohydrate-carbohydrate interactions. Med Hypotheses 144:110155 [DOI] [PMC free article] [PubMed]
- Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, LICORN and the Lille COVID-19 and Obesity study group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siregar T (2020) Could frozen food transmit COVID-19? Medical Xpress. https://medicalxpress.com/news/2020-11-frozen-food-transmit-covid-.html. Accessed 4 Dec 2020
- Tamerius JD, Shaman J, Alonso WJ, Bloom-Feshbach K, Uejio CK, Comrie A, Viboud C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9(3):e1003194. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Mu L, Huang J, Yu S, Chen B, Yin J. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J Epidemiol Communi Health. 2005;59(3):186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teymoori-Rad M, Samadizadeh S, Tabarraei A, Moradi A, Shahbaz MB, Tahamtan A. Ten challenging questions about SARS-CoV-2 and COVID-19 [published online ahead of print, 2020 Jun 30] Expert Rev Respir Med. 2020;14:881–888. doi: 10.1080/17476348.2020.1782197. [DOI] [PubMed] [Google Scholar]
- Thomas L (2020) Fresh and frozen food can harbor SARS-CoV-2 for at least 21 days. News medical life sciences. https://www.news-medical.net/news/20200819/Fresh-and-frozen-food-can-harbor-SARS-CoV-2-for-at-least-21-days.aspx. Accessed 4 Dec 2020
- Ueki H, Furusawa Y, Iwatsuki-Horimoto K, Imai M, Kabata H, Nishimura H, & Kawaoka Y (2020) Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. MSphere, 5(5) [DOI] [PMC free article] [PubMed]
- Usman M, Farooq M, Hanna K. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ Sci Technol. 2020;54(13):7758–7759. doi: 10.1021/acs.est.0c02777. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Lloyd-Smith JO. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Nikitara K (2020) COVID-19 and smoking: a systematic review of the evidence. Tobacco induced diseases, 18. 10.18332/tid/119324 [DOI] [PMC free article] [PubMed]
- Wales (2020) Coronavirus: almost 100 staff at food factories test positive. BBC News. https://www.bbc.com/news/uk-wales-3091149?intlink_from_url=&link_location=live-reporting-story. Accessed 4 Dec 2020
- Wang XW, Li JS, Jin M, Zhen B, Kong QX, Song N, Si BY. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126(1-2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Li J, Guo T, Zhen B, Kong Q, Yi B, Zhu X. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci Technol. 2005;52(8):213–221. doi: 10.2166/wst.2005.0266. [DOI] [PubMed] [Google Scholar]
- Wang XW, Li JS, Guo TK. Excretion and detection of SARS coronavirus and its nucleic acid from digestive system. World J Gastroenterol. 2005;11(28):4390–4395. doi: 10.3748/wjg.v11.i28.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Luo Q, Chen R, Chen T, Li J (2020e) Susceptibility analysis of COVID-19 in smokers based on ACE2. 10.20944/preprints202003.0078.v
- Wang J, Tang K, Feng K, Lin X, Lv W, Chen K, Wang F (2020a) High temperature and high humidity reduce the transmission of COVID-19. SSRN. 10.2139/ssrn.3551767
- Wang M, Jiang A, Gong L, Luo L, Guo W, Li C, Zheng J, Li C, Yang B, Zeng J, Chen Y, Zheng K, Li H (2020b) Temperature significant change COVID-19 transmission in 429 cities. MedRxiv. 10.1101/2020.02.22.20025791
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (2020c) Detection of SARS-CoV-2 in different types of clinical specimens. Jama 323(18):1843–1844 [DOI] [PMC free article] [PubMed]
- Wang K, Chen W, Zhou YS, Lian JQ, Zhang Z, Du P, Gong L, Zhang Y, Cui HY, Geng JJ, Wang B et al (2020d) SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv. 10.1101/2020.03.14.988345
- Weber DJ, Rutala WA, Fischer WA, Kanamori H, Sickbert-Bennett EE. Emerging infectious diseases: focus on infection control issues for novel coronaviruses (Severe Acute Respiratory Syndrome-CoV and Middle East Respiratory Syndrome-CoV), hemorrhagic fever viruses (Lassa and Ebola), and highly pathogenic avian influenza viruses, A (H5N1) and A (H7N9) Am J Infect Control. 2016;44(5):e91–e100. doi: 10.1016/j.ajic.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020a) Strategic partnership for international health regulations (2005) and health security (SPH); COVID-19 as a Public Health Emergency of International Concern (PHEIC) under the IHR. https://extranet.who.int/sph/covid-19-public-health-emergency-international-concern-pheic-under-ihr. Accessed on 23rd July 2020
- World Health Organization (2020b) WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/. Accessed on 24th July 2020
- World Health Organization (2020c) Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 27 March 2020 (No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.1)
- Wu F, Xiao A, Zhang J, Gu X, Lee WL, Kauffman K, Duvallet C (2020a) SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 10.1101/2020.04.05.20051540 [DOI] [PMC free article] [PubMed]
- Wu Y, Guo C, Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Nethery RC, Sabath BM, Braun D, Dominici F (2020c) Exposure to air pollution and COVID-19 mortality in the United States. medRxiv. 10.1101/2020.04.05.20054502 [DOI] [PMC free article] [PubMed]
- Wurtzer S, Marechal V, Mouchel JM, Maday Y, Teyssou R, Richard E, Moulin L (2020) Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 10.1101/2020.04.12.20062679 [DOI] [PMC free article] [PubMed]
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dental Res. 2020;99(8):989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Pan J, Liu Z, Meng X, Wang W, Kan H, Wang W. No Association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur Respir J. 2020;55(5):2000517. doi: 10.1183/13993003.00517-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Ellenberg RM, Graham KE, Wigginton KR. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ Sci Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: the most important research questions. Cell and Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J. Facial mask: a necessity to beat COVID-19. Build Environ. 2020;175:106827. doi: 10.1016/j.buildenv.2020.106827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX. SARS-CoV-2: air/aerosols and surfaces in laboratory and clinical settings. J Hosp Infect. 2020;105(3):577–579. doi: 10.1016/j.jhin.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao (2020) Exporting meat products to china during COVID 19: what you need to know. China briefing from diezan shiera & associates. https://www.china-briefing.com/news/exporting-meat-products-to-china-during-covid-19-what-you-need-to-know/. Accessed 4 Dec 2020
- Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, Lin S. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92:1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Chen W. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Ye C. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Jingu X, Huang F, Li C. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietz M, Tatonetti NP (2020) Testing the association between blood type and COVID-19 infection, intubation, and death. MedRxiv. 10.1101/2020.04.08.20058073 [DOI] [PMC free article] [PubMed]
- Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;21:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.