Hemophilia B is almost exclusively caused by mutations of the F9 gene and inherited in an X-linked recessive manner.1 The amino acid residue arginine of factor IX (FIX) with CpG sequences2 is a hot spot for mutations,3 involving CG to TG or CA transitions and causing hemophilia B.4 Interestingly, for the coding sequence of residue Arg338 of FIX, only the transition CGA to TGA, which causes nonsense mutations, had been identified in a patient with severe hemophilia B,5 whereas the other type of CpG transition predicting the missense mutation p.Arg338Gln has been strangely absent from the list of F9 mutations reported in the hemophilia B database. It had been suspected that the particular type of amino acid replacement at this site may lead to conditions other than hemophilia.6,7
In a Chinese patient with deep vein thrombosis (DVT), we identified a F9 mutation at the same site as that in FIX Padua, but differently from this latter, the arginine is changed to a polar amino acid residue glutamine (p.Arg384Gln, HGVS numbering; or p.Arg338Gln, legacy numbering) due to the nucleotide transition c.1151G>A. A coagulation function assay with both the patient’s plasma and recombinant FIX p.Arg338Gln (p.R338Q) showed that the mutation also increased FIX activity. When the FIX with the p.R338Q modification was expressed in vivo after being transferred with self-complementary adeno-associated virus (scAAV) vectors, the deficiency of FIX in a mouse model of hemophilia B was restored, and the bleeding phenotype was significantly ameliorated.
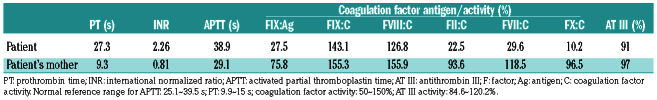
A 13-year old boy was referred to Ruijin Hospital for consultation regarding recurrent DVT. The patient was well and had no history of other major disorders. The two episodes of DVT had affected both femoral and popliteal veins of his left leg and the diagnosis was confirmed by ultrasound studies. He had no history of left leg trauma and no vascular abnormality was identified in the imaging study. There was no confirmed family history of venous thromboembolism. The patient was being treated with warfarin and this had not been discontinued during the study because of the potential risk of DVT recurrence. The study was approved by the ethics committee of Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine. Written consent was acquired from family members and the patient’s mother on behalf of both parents. Under treatment with warfarin, the activity of three other K-dependent coagulation factors, factors II, VII and X, was significantly suppressed in the patient; however, his plasma maintained highly elevated FIX activity (FIX:C around 143.1% of normal). In addition, the plasma FIX antigen (FIX:Ag) level was much lower, at approximately 27.5% of normal. The patient’s mother also had relatively higher FIX clotting activity (FIX:C 155.3%) and her FIX:Ag was 75.8% of normal (Table 1). Thus, the ratios between FIX:C and FIX:Ag were significantly higher for both the patient and his mother, being 5.20 and 2.06, respectively. The antithrombin III (AT III) level was within the normal range and lupus anticoagulant and antiphospholipid antibodies were negative in both the patient and his mother. Although we were unable to determine the patient’s protein C (PC) and protein S (PS) levels accurately, because of his treatment with warfarin, the levels of PC and PS were both within normal ranges in the mother (data not shown). The patient’s father was not available for analysis.
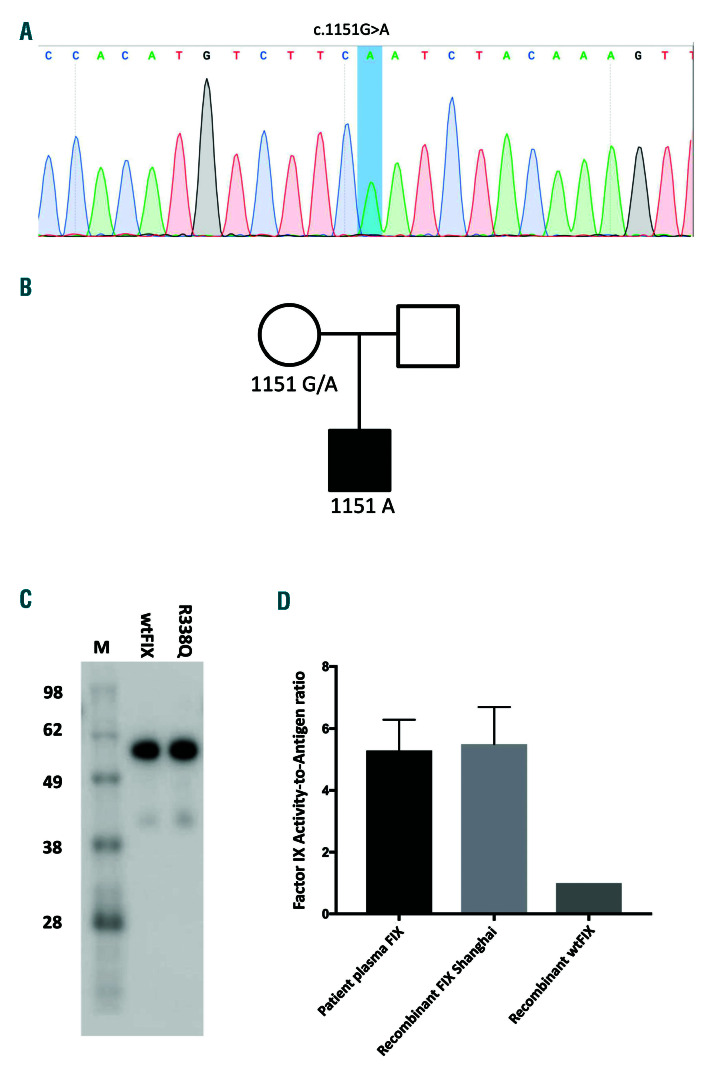
The coding sequence and flanking regions of the F9 gene were amplified by polymerase chain reaction and directly sequenced. The genes coding for PS, PC, AT III and common genetic thrombotic risk factors, including the factor V Leiden mutation and prothrombin G20210G/A, were screened. Direct sequencing of the F9 gene revealed a nucleotide substitution c.1151G>A (Figure 1A), which predicated a missense mutation p.Arg338Gln (p.R338Q). The genetic variant was traced back to the patient’s mother (Figure 1B). This pedigree was negative for both factor V Leiden and the prothrombin G20210A mutation. Analysis of the genes for PS, PC and AT III failed to reveal any pathogenic changes (data not shown).
The F9 cDNA containing the p.R338Q mutation were inserted into the mammalian expression vector pCDNA3.1 and transfected into HEK293T cells using lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) to produce FIX (the methods are described in the Online Supplementary File). The amount and coagulation activity of FIX were determined by enzyme-linked immunosorbent assay and an activated partial thromboplastin time-based clotting assay. Recombinant FIX was subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis and detected by western blotting using a FIX-specific antibody (PAHFIX-S, Haematologic Technologies, Essex Junction, VT, USA). As shown in Figure 1C, the recombinant FIX Shanghai had a similar migration pattern as that of the wild-type FIX and the mutation increased the FIX activity by approximately 5- fold as determined by the ratio of FIX:C to FIX:Ag (Figure 1D), which was similar to the change of FIX activity observed in the patient.
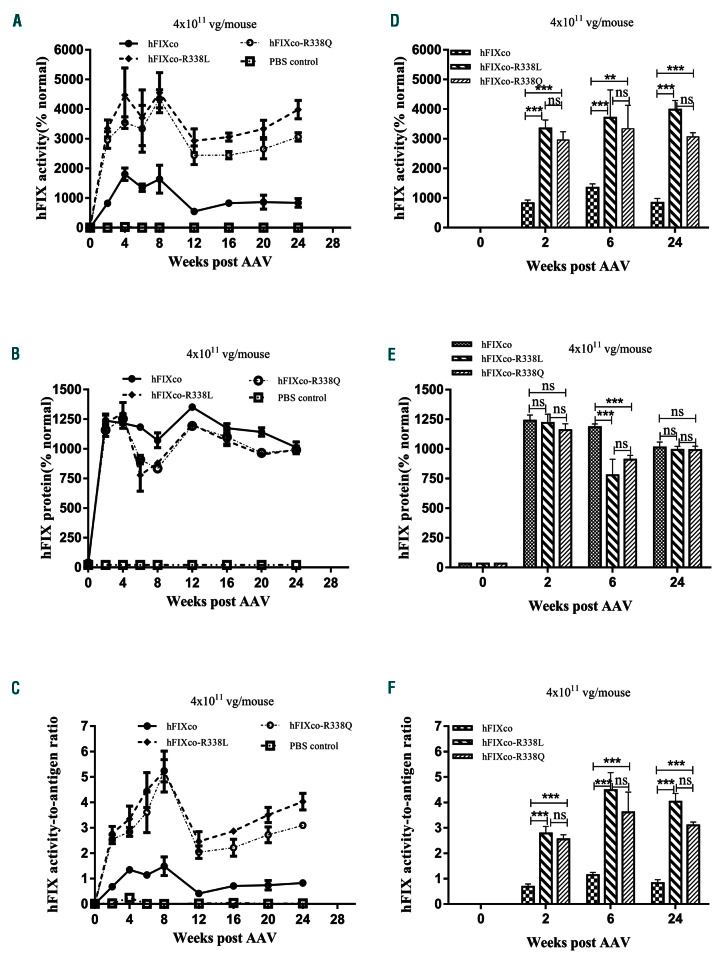
We further investigated the expression and function of FIX p.R338Q in a murine model of hemophilia B using both high and low doses of recombinant adeno-associated virus (rAAV) vectors, with wild-type FIX and FIX p.R338L. The plasmid scAAV-TTR-hFIXco (AAV-hFIXco) was constructed by cloning codon-optimized human FIX (hFIXco) cDNA8 under the control of the human transthyretin (TTR) promoter in a scAAV vector plasmid, and with two inverted terminal repeat sequences of AAV2 flanking the human FIX gene expression cassettes. The plasmids scAAV-TTR-hFIXcoR338Q (AAV-hFIXco- R338Q) and scAAV-TTR-hFIXcoR338L (AAV-hFIXco- R338L) were obtained by site-directed mutagenesis of the plasmid AAV-hFIXco at p.R338L and p.R338Q, respectively. Recombinant AAV type 8 (rAAV8) vectors were generated by triple plasmid transfection (methods provided in the Online Supplementary File). Two vector doses of approximately 5×1010 and 4×1011 rAAV8 viral genomes (vg), which were equivalent to 2×1012 or 1.6×1013 vg/kg in a clinical setting, were diluted in 200 mL of phosphatebuffered saline and injected into 4- to 8-week old hemophilia B mice via the tail vein (n=5 mice per group). FIX:Ag and FIX:C were determined in the mice by enzyme-linked immunosorbent assay and an activated partial thromboplastin-based assay, using pooled normal human plasma as the standard. Twentyfour weeks after injection of AAV vectors packed with either of the FIX species, the FIX:C of FIX p.R338Q and p.R338L was three to five times higher than that of the wild-type FIX at most of the time points examined (P<0.05) (Figure 2A, D, G, J). The expression (FIX:Ag) of FIX p.R338Q was similar to that of FIX p.R338L, but lower than that of wild-type FIX, particularly at a low dose (Figure 2B, E, H, K). The in vivo expression study validated that the FIX p.R338Q mutant had intrinsically higher clotting activity than wild-type FIX, with 4- to 5- fold higher FIX-specific activity as determined in mice given either high or low doses of the vector (Figure 2C, F and I, L, respectively). The FIX clotting activity enhancement brought by p.R338Q was on average 20% less than that brought by p.R338L.
Table 1.
The coagulation profile of the patient.
The amino acid residue Arg338 has a profound effect on the procoagulant activity of FIX and laboratory studies on the artificial mutation FIX p.Arg338Ala showed that replacement of the positively charged arginine by a hydrophobic alanine could increase the FIX activity by 2~3-fold.9 In a pedigree with thrombophilia in Padua (Italy), replacement of the positively charged amino acid residue Arg338 with a nonpolar amino acid residue, leucine, led to an approximately 8-fold increase in FIX clotting activity.1
In our Chinese patient with thrombophilia, we found the long-sought CpG transition-related mutation c.1151G>A (p.Arg338Gln), which we designated FIX Shanghai. Although there have been contradictory reports on the mutation’s impact on clotting activity, previously determined by using an artificially made mutant,11 the early onset and recurrences of DVT in our patient carrying FIX Shanghai evidently substantiate the effect of this mutation on upregulating coagulation activity. The mutation greatly increased the clotting activity of the patient’s plasma FIX, as did the recombinant mutant expressed in HEK293 cells, although on average 20% less than the FIX Padua mutation. Consistent with findings in humans, hemophilia B mice into which the FIX Shanghai mutant was transferred by AAV vectors also presented with increased FIX activity in vivo.
Figure 1.
F9 gene analysis and determination of FIX Shanghai clotting function. (A). A F9 gene variant c.1151G>A was identified in the patient, which predicted a missense mutation in factor IX (FIX) p.Arg338Gln (p.R338Q). (B) The mutation was inherited from the patient’s mother. (C) Subjected to electrophoresis, the FIX mutant p.R338Q expressed in HEK293 cells migrated in the same fashion as wild-type (wt) FIX on a sodium dodecylsulfate polyacrylamide gel. (D) Both recombinant FIX p.R338Q and FIX from the patient’s plasma showed more potent FIX activity, as represented by the increased ratio between FIX:C and FIX:Ag.
The dosage of AAV vectors is directly related to the safety and efficacy of a gene therapy for hemophilia B,12 and it would be ideal to achieve high FIX expression with lower doses of AAV vectors. Discovery of the FIX Padua mutation promoted the development of gene therapy. With high FIX clotting activity, the AAV vector carrying the F9 Padua variant achieved therapeutic levels of FIX in patients without toxic side effects.14 With a similar effect on FIX activity as FIX Padua, the AAV-delivered FIX Shanghai in a hemophilia B mouse model yielded sustained FIX expression, and the FIX clotting activity achieved in the mice by FIX Shanghai was significantly better than that of wild-type FIX and similar to that of FIX Padua. The adoption of a naturally occurring hypercoagulative FIX mutation, FIX Shanghai, is thus an attractive approach for the development of a gene therapy regimen for hemophilia B with high efficacy and fewer safety concerns.
Figure 2.
In vivo evaluation of FIX Shanghai following delivery by adeno-associated virus vectors into hemophilic B mice. Evaluation of codon-optimized hFIXco, hFIXco-R338L and hFIXco-R338Q by recombinant adeno-associated virus (rAAV) delivery in hemophilia B (HB) mice. Each 4- to 8-week old HB mouse was injected with a total amount of 5x1010 vector genomes (low dose) or 4×1011 vector genomes (high dose) via the tail vein (n=5 in each group). Phosphate-buffered saline (PBS) was injected as a negative control (n=3). FIX clotting activity and protein quantity were measured by the activated partial thromboplastin time (APTT) (A, G) and enzyme-linked immunosorbent assay (ELISA) (B, H) using plasma samples collected at the indicated times after rAAV administration. A pooled human plasma was used as the normal standard. Specific activities (C, I) were calculated as APTT/ELISA at the corresponding points. Data are mean ± standard error of mean. Statistical analysis was done with analysis of variance testing using GraphPad Prism 6 (Graphpad Software, Inc. La Jolla, CA, USA). Representative results for A, B, C, G, H and I, at weeks 0, 2, 6 and 24 are shown in D, E, F, J, K and L, respectively. P values less than 0.05 were considered statistically significant (ns: >0.05; *P≤0.05; **P≤0.01; ***P≤0.001).
In conclusion, the new FIX variant identified in the current study, FIX Shanghai (FIX p.Arg338Gln), greatly increased FIX clotting activity and potentiated the risk of thrombosis. Before use in clinical trials, the potential toxicity of this variant should be studied further in animals. However, its high activity also makes it an ideal candidate for the development of novel gene therapy regimens for hemophilia B.
Supplementary Material
Funding Statement
Funding: this work was supported by NSFC 81670130, WW; NSFC 81570115, XW; NSFC 81570114, QD; NSFC 81571792, BD.
References
- 1.Goodeve AC. Hemophilia B: molecular pathogenesis and mutation analysis. J Thromb Haemost. 2015;13(7):1184-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeberl DD, Bottema CD, Buerstedde JM, Sommer SS. Functionally important regions of the factor IX gene have a low rate of polymorphism and a high rate of mutation in the dinucleotide CpG. Am J Hum Genet. 1989;45(3):448-457. [PMC free article] [PubMed] [Google Scholar]
- 3.Vitkup D, Sander C, Church GM. The amino-acid mutational spectrum of human genetic disease. Genome Biol. 2003;4(11):R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green PM, Montandon AJ, Bentley DR, Ljung R, Nilsson IM, Giannelli F. The incidence and distribution of CpG----TpG transitions in the coagulation factor IX gene. A fresh look at CpG mutational hotspots. Nucleic Acids Res. 1990;18(11):3227-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig M, Schwaab R, Eigel A, et al. Identification of a single nucleotide C-to-T transition and five different deletions in patients with severe hemophilia B. Am J Hum Genet. 1989;45(1):115-122. [PMC free article] [PubMed] [Google Scholar]
- 6.Gostout B, Vielhaber E, Ketterling RP, et al. Germline mutations in the factor IX gene: a comparison of the pattern in Caucasians and non-Caucasians. Hum Mol Genet. 1993;2(3):293-298. [DOI] [PubMed] [Google Scholar]
- 7.Bottema CDK, Ketterling RP, Vielhaber E, et al. The pattern of spontaneous germ-line mutation: relative rates of mutation at or near CpG dinucleotides in the factor IX gene. Hum Genet. 1993; 91(5):496-503. [DOI] [PubMed] [Google Scholar]
- 8.Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adenoassociated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107(7):2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J, Jin J, Lollar P, et al. Changing residue 338 in human factor IX from arginine to alanine causes an increase in catalytic activity. J Biol Chem. 1998;273(20):12089-12094. [DOI] [PubMed] [Google Scholar]
- 10.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009; 361(17):1671-1675. [DOI] [PubMed] [Google Scholar]
- 11.Monahan PE, Sun J, Gui T, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther. 2015;26(2):69-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manno CS, Arruda VR, Pierce GF, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342-347. [DOI] [PubMed] [Google Scholar]
- 13.Lozier JN. Factor IX Padua: them that have, give. Blood. 2012; 120(23):4452-4453. [DOI] [PubMed] [Google Scholar]
- 14.George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377(23):2215-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.