Abstract
Background
There is a critical need for carbapenem-sparing therapies for infections caused by extended spectrum β-lactamase (ESBL)-producing Enterobacterales. Enmetazobactam is a novel ESBL inhibitor combined with the cephalosporin cefepime. Treatment outcomes of cefepime-enmetazobactam (FPE) versus piperacillin-tazobactam (PTZ) were assessed in subgroups of patients with ESBL-producing baseline uropathogens (ESBL-BU) in the ALLIUM phase 3 trial of complicated urinary tract infections (cUTI)/acute pyelonephritis (AP).
Methods
1034 cUTI/AP patients randomized 1:1 in a double-blind, multicenter trial received either 2 g cefepime/0.5 g enmetazobactam or 4 g piperacillin/0.5 g tazobactam q8h by 2h infusion for 7 to 14 days. Enterobacterales with MICs ≥1 µg/ml to ceftazidime, ceftriaxone, cefepime, meropenem, or FPE were genotyped for β-lactamases. In addition to the primary analysis (by stratified Newcombe) of overall success (combination of clinical cure and microbiological eradication) in the microbiological modified intent to treat population (mMITT) at test-of-cure (TOC), subgroup analyses were performed on patients with ESBL-BU non-resistant to FPE (MIC≤8 µg/ml) and PTZ (MIC≤64 µg/ml) and a subgroup (mMITT+R) that also included the ESBL-BU resistant to either agent (FPE MIC ≥16 µg/ml or PTZ MIC≥128 µg/ml).
Results
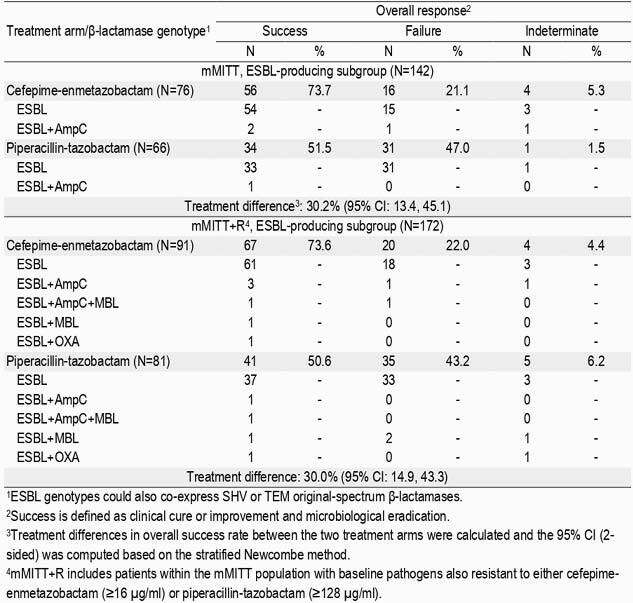
In mMITT, FPE (273/345, 79.1%) demonstrated superiority to PTZ (196/333, 58.9%) at TOC (difference, 21.2%; 95% CI: 14.3, 27.9). The prevalence rate of ESBL-BU was 20.9% (142/678), with 99.3% (141/142) expressing a CTX-M-type (-1, -3, -9, -14, -15, -27, -55, -91, -169) and 3.5% (5/142) co-expressing AmpC (CMY-2/-59). FPE (56/76, 73.7%) demonstrated superiority to PTZ (34/66, 51.5%) in this subgroup at TOC (difference 30.2%; 95% CI: 13.4, 45.1; Table). In mMITT+R, the ESBL-BU prevalence rate was 22.3% (172/771), with 6.4% (11/172) co-expressing AmpC (CMY-2/-4/-59/-99), 4.7% (8/172) co-expressing a metallo-β-lactamase (VIM-1, NDM-1), and 2.3% (4/172) co-expressing OXA-48. Superiority of FPE (67/91, 73.6%) compared to PTZ (41/81, 50.6%) was also observed at TOC despite inclusion of ESBL-BU resistant to either agent (difference 30.0%; 95% CI: 14.9, 43.3).
Table
Conclusion
FPE may represent a new empiric carbapenem-sparing option in settings where ESBL are endemic.
Disclosures
Adam Belley, PHD, Allecra Therapeutics SAS (Consultant) Philip Barth, MD, Allecra Therapeutics SAS (Consultant) Shikhar Kashyap, n/a, Allecra Therapeutics SAS (Consultant)Allecra Therapeutics SAS (Consultant) Omar Lahlou, PhD, Allecra Therapeutics SAS (Employee) Paola Motta, PhD, Allecra Therapeutics SAS (Employee) Patrick Velicitat, MD, Allecra Therapeutics SAS (Employee)


