Abstract
Over the last decade, our understanding of the physiological role of senescent cells has drastically evolved, from merely indicators of cellular stress and ageing to having a central role in regeneration and repair. Increasingly, studies have identified senescent cells and the senescence-associated secretory phenotype (SASP) as being critical in the regenerative process following injury; however, the timing and context at which the senescence programme is activated can lead to distinct outcomes. For example, a transient induction of senescent cells followed by rapid clearance at the early stages following injury promotes repair, while the long-term accumulation of senescent cells impairs tissue function and can lead to organ failure. A key role of the SASP is the recruitment of immune cells to the site of injury and the subsequent elimination of senescent cells. Among these cell types are macrophages, which have well-documented regulatory roles in all stages of regeneration and repair. However, while the role of senescent cells and macrophages in this process is starting to be explored, the specific interactions between these cell types and how these are important in the different stages of injury/reparative response still require further investigation. In this review, we consider the current literature regarding the interaction of these cell types, how their cooperation is important for regeneration and repair, and what questions remain to be answered to advance the field.
Keywords: senescence-associated secretory phenotype, macrophage, regeneration, senescence, ageing, repair
1. Introduction
Tissue repair and regeneration are critical biological processes which occur following injury and are essential for survival. Injury can occur as a result of infection, toxic or mechanical assault, and results in a prominent activation of the immune system and the recruitment of a vast number and type of cells, which infiltrate the damaged area. These consist of natural killer cells, macrophages, neutrophils, B cells, T cells, fibroblasts, epithelial cells and endothelial cells. In a healthy environment such cells work together in a concerted effort to restore tissue function and to limit damage, a process which must be tightly regulated. In many pathological environments, these mechanisms become dysregulated and the recruitment of immune cells can instead initiate, amplify and even sustain tissue injury. This process of healing damaged tissue is known as ‘repair’ and encompasses the two separate processes of regeneration and replacement, where regeneration refers to the process in which new tissue growth restores areas of damaged tissue to their original state while replacement occurs in severely damaged tissue, often in the form of scarring.
While the inflammatory cascade serves to eliminate the noxious stimulus and clear the injured area from dead cells and matrix debris, the healing of injured tissues is dependent on the timely suppression of inflammation, setting the stage for the activation of reparative cells [1]. However, the effectiveness of the reparative response is dependent on the severity and type of injury, the organ affected and species-specific characteristics. While amphibians can regenerate limbs [2] and fish can regenerate myocardium [3], adult mammals fail to regenerate either of these. Furthermore, in adult mammals organs such as the liver retain some regenerative capacity [4], whereas the regeneration of the brain and spinal cord is extremely limited [5]. To add to this complexity, when tissues are exposed to prolonged injury the process of repair can become chronic or dysregulated, leading to pathological processes, including fibrosis or chronic inflammation, which ultimately impact organ function and can result in organ or organism death.
1.1. Cellular senescence
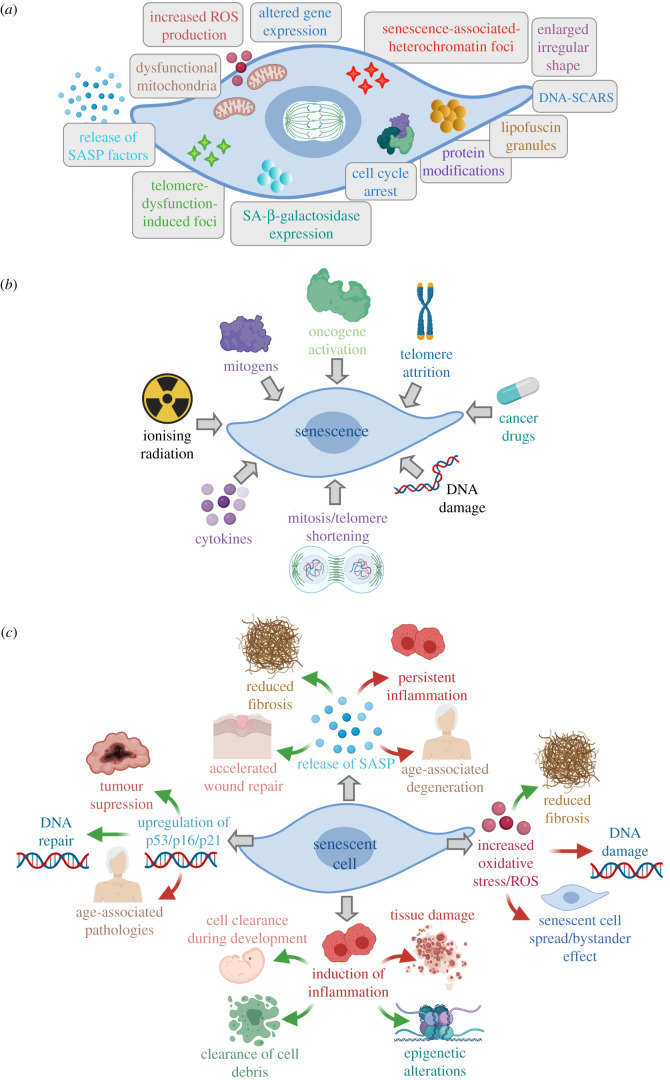
A common outcome of the injury process is cellular senescence, an irreversible but stable form of cell cycle arrest, defined by an altered transcriptome, which occurs in proliferating cells when they have reached the end of their replicative lifespan, or when subjected to stress. Senescent cells are often characterized by an enlarged and flattened shape [6], and exhibit hallmarks of senescence, including DNA and chromatin alterations and gene expression changes [7–11]; mitochondrial dysfunction and the subsequent release of reactive oxygen species (ROS) [12,13]; protein modifications [14] and accumulation of lipofuscin granules [15]; expression of SA-β-galactosidase [16]; and the release of SASP factors [14,17] (figure 1a). Moreover, while often found in injured tissues [27,28], senescent cells can also be present in uninjured organs, especially in organs that have previously experienced damage or disease [29], and particularly in older individuals. Cellular senescence was first discovered in primary cell culture, where cells grown for long periods of time, akin to ageing, reached a state where they were no longer able to replicate [30,31]. Subsequently, cells positive for senescence-associated (SA)-β-galactosidase were observed in aged tissues [16]. For many years following this, senescence was solely viewed as a result of organismal ageing; however, in the last decade, our understanding has dramatically evolved, indicating that cellular senescence can occur in response to a range of stimuli, including cellular damage [18], oxidative stress [32], oncogenic signalling [19], telomere attrition [20], ionizing radiation [21] and some cancer drugs [22] (figure 1b), and is even seen during development [23,24] (figure 1c). Senescence has been reported in numerous cell types during natural ageing, and following injury or disease; including epithelia [33], endothelia [34], immune cells [35], mesenchymal cells [36], bone [37], muscle [38] and adipose tissue [39]. An important role of senescence is therefore to prevent the spread of damage throughout the tissue and, in cancer, acts as a potent barrier against tumorigenesis (reviewed in [40]) (figure 1c). In general, the transient induction of senescence followed by senescent cell elimination promotes tissue remodelling and regeneration [25,26] (figure 1c); however, chronic injury can result in the long-term accumulation of senescent cells, driving persistent inflammation which ultimately impairs tissue function and can contribute to organ failure (figure 1c). For this reason, the fine balance of senescent cells and their presence/clearance is likely to play a pivotal role in tissue repair. In this review, we have concentrated on the literature describing the interplay between senescence in epithelial tissues and immune cells, particularly macrophages.
Figure 1.
The hallmarks, causes and effects of cellular senescence. (a) The key features of a senescent cell, which include an enlarged and irregular/flattened shape [6], DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS) [9] and foci [10], altered gene expression [8] and cell cycle arrest [7], mitochondrial dysfunction and release of reactive oxygen species (ROS) [12,13], protein modifications [14], lipofuscin granules, expression of SA-β-galactosidase [16] and release of SASP factors [14,17]. (b) Cellular senescence can occur in response to cellular or DNA damage [18], oncogene, mitogen and cytokine signalling [19], telomere attrition/shortening [20], ionizing radiation [21] and anti-cancer drugs [22]. (c) Cellular senescence plays a dual role during development [23,24] and throughout tissue repair and regeneration [25,26], where it can promote the clearance of cell debris, reduces fibrosis, elicits epigenetic alterations and acts as a potent barrier against tumorigenesis, while also leading to senescence spread, DNA damage, further tissue injury, and ultimately leading to age-associated tissue deterioration and pathologies. Created with Biorender.com.
1.2. Senescence-associated secretory phenotype
Senescent cells are often characterized by their ability to develop a senescence-associated secretory phenotype (SASP), a pro-inflammatory response which activates and reinforces the senescence phenotype in surrounding cells, modulates fibrosis and promotes regeneration [41] (figure 1c). The SASP consists of a complex mixture of extracellular matrix proteases, growth factors, chemokines and cytokines, which have a profound effect on the tissue microenvironment [42]. Such SASP components can trigger senescence in neighbouring cells in both an autocrine [43,44] and paracrine [41,45] manner, suggesting that senescence creates an inflammatory microenvironment which may lead to the elimination of senescent cells. The secretion of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and interleukin-8 (IL-8) [46], chemokines, such as monocyte chemoattractant proteins (MCPs) and macrophage inflammatory proteins (MIPs) [42], and growth factors, such as transforming growth factor-β (TGFβ) [47], cause inflammation and recruit immune cells to clear senescent cells.
1.3. Macrophages in tissue repair
Among the variety of cell types which orchestrate repair, macrophages have been shown to exhibit critical regulatory activity at all stages of repair and fibrosis. Macrophages are recruited to the site of injury by chemokine gradients and various adhesion molecules, where they carry out their role as scavenger cells that phagocytose cellular debris and invading cells, alongside other apoptotic cells, in response to tissue injury. Importantly, macrophages are key for the clearance of senescent cells following injury [48,49], as well as an important source of chemokines, matrix metalloproteinases (MMPs) and other inflammatory mediators which drive the initial cellular response [50]. Current models suggest that senescence initiates tissue modelling/remodelling by recruiting immune cells through the SASP, where macrophages clear senescent cells, allowing for repopulation by progenitor cells and regeneration of the damaged tissue [25,26,51]. However, in the case of persistent damage or in aged tissues, clearance and regeneration may be compromised due to poor macrophage recruitment, increased senescent cells or even damage to the macrophages themselves. Indeed, if macrophages are depleted in the early stages of repair in a number of organs, the inflammatory response is diminished [52] and leads to less efficient repair and regeneration [53,54].
This review focuses on the recent findings that have advanced our understanding of senescent cells and macrophages in tissue injury, and the importance of the cooperation of these cells as key players in facilitating tissue regeneration and repair.
2. Evidence for the role of senescent cells in tissue injury
Senescence is a state of irreversible proliferative arrest, which cells undergo in response to a variety of detrimental stimuli, and is associated with changes in morphology, lysosomal activity, alternations in chromatin structure (H2Ax expression) and activation of the SASP [55] (figure 1a). Much of our current understanding of senescence has stemmed from studies of either disease or ageing; however, a novel role for senescence in resolving tissue injury has recently emerged. Indeed, senescent cells have been identified in a variety of injured organs, including the liver [28,47], kidney [56,57], heart [58], skeletal muscle [27] and salivary glands [59], and have largely been associated with the loss of tissue function. Nonetheless, the presence of senescent cells has been reported to have both positive and negative effects in their resident organ, depending on their abundance and duration (figure 1c), and provides us with important insights into their physiological function. Indeed, if the function of senescence is the elimination of cells, why do cells not undergo the faster and more direct route of apoptosis, and why were senescent cells selected during evolution? This question has led to the emerging concept that senescent cells play important roles in tissue repair and remodelling, providing a final function before eventually undergoing elimination themselves [26].
2.1. The role of senescent cells in tissue repair
To date, two main approaches have been used to explore the role of senescent cells in repair: genetic depletion strategies, where senescent cells are deleted from the tissue, and through the use of senolytics, where compounds are used to induce senescent cell death.
2.2. Genetic depletion strategies
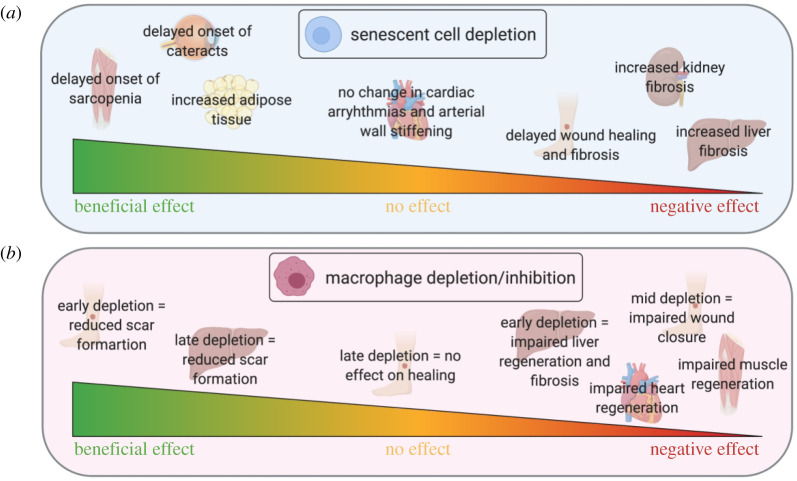
To distinguish the role of senescent versus apoptotic cells in tissue injury, Baker et al. [66] used an inducible ‘senescence-to-apoptosis' progeric mouse model, where transgenic mice express pro-apoptotic proteins under the expression of the p16INK4a promoter. By administering the mice with a ‘chemical switch’, cells expressing the senescence-associated marker p16INK4a were converted into apoptotic cells in vivo. Interestingly, as well as observing a decrease in the number of senescent cells, Baker et al. [66] observed a reversion in a number of age-associated pathologies (figure 2), indicating a role for senescent cells in disrupting tissue homeostasis. However, using a similar but mechanistically different mouse model in cutaneous wound healing studies, it was shown by Demaria et al. [60] that inducing mice to switch from senescence to apoptosis significantly delayed wound healing and caused the wounds to accumulate larger amounts of fibrotic tissue (figure 2). Interestingly, in young mice, a transient burst of P16INK4a+ senescent cells was found to occur during normal wound healing and disappeared following wound closure, indicating an early role for senescent cells in wound recovery and the negative impact of their removal [60]. Similarly, cellular senescence occurs in myofibroblasts in cutaneous wounds during the healing process, which is thought to minimize the extent of fibrosis [61].
Figure 2.
Depletion of macrophages or senescent cells has diverse and opposing effects on organ regeneration. The effects of senescent cell depletion (a) or macrophage depletion or prevention of accumulation (b) are depicted. In many organs, the timing of cell depletion has a crucial role on the regenerative outcome. While senescent cell depletion delays cutaneous wound healing and exacerbates fibrosis [60,61], the effect of deletion of macrophages during wound healing is timing-dependent [54]; similarly while senescent cell depletion leads to liver fibrosis [28,62], depletion of macrophages can lead to both reduced liver scarring and fibrosis, dependent on timing [52]. Moreover, senescent cell removal has no or largely positive effects on muscle and heart [63]; however, macrophage depletion leads to detrimental effects on heart and muscle regeneration [63–65]. Created with Biorender.com.
In support of these observations, contrasting effects of senescent cell function have also been demonstrated in other models of tissue injury. When the liver is injured, hepatic stellate cells become senescent and produce a stable fibrotic scar [28] (figure 2). In vivo, these senescent cells are identified inside the fibrotic lesions; however, mice deficient for p53 and p16INK4A show increased fibrosis in both the liver and kidney [28,62]. Conversely, in a mouse model of oncogenic NRASG12 V, where senescent cells are usually cleared by monocytes and macrophages, immunodeficient mice show reduced clearance, resulting in mature liver tumours [67]. Moreover, the p16-3MR transgenic mouse, a model that contains a p16INK4a promoter which allows the tracing and removal of senescent cells has been used to demonstrate that senescent cell deletion reduces pain in an experimental model of osteoarthritis [68]. Crucially, a mouse model that contains the transgene, INK-ATTAC, which induces apoptosis in p16INK4a-expressing cells, established that senescent cell clearance treatment extended lifespan in both male and female mice, delayed tumorigenesis and attenuated age-related deterioration of several organs, including kidney, heart and fat, without apparent side effects [69].
2.3. Senolytics
The use of senolytic compounds also provides a mechanism to elucidate the role of senescent cells and in particular, the specific timing of depletion. Evidence shows that senolytics can drive the expression of SA-β-gal in cell culture [70]. Moreover, the administration of the senolytic compounds ABT-737 or Dasatinib plus Quercetin (DQ) in vivo induces apoptosis in senescent cells and leads to clearance in mouse skin, lung and the haematopoietic system, and subsequently improves tissue repair [70–73]. Moreover, DQ administration promotes the survival of transplants from aged mice [74].
Taken together, these studies highlight the opposing roles of senescent cells in injury and repair, and the variation in their function as a result of timing, degree and type of injury. Indeed, increasing evidence suggests that cellular senescence is a multi-step, dynamic process, progressing from a transient to a stable state of cell cycle arrest, dictating the outcome [75].
2.4. The role of the senescence-associated secretory phenotype in tissue repair
Furthermore, beyond the direct effect on cell division and clearance, a key mechanism in which senescent cells influence injury is through the SASP. The SASP secretome includes a wide variety of soluble signals capable of influencing tissue inflammation, repair and fibrosis, including IL-1, IL-8, IL-6 and transforming growth factor beta (TGFβ). The SASP is a pro-inflammatory response which activates and reinforces the senescent phenotype in surrounding cells and therefore mediates the spread of senescence throughout the tissue, known as ‘bystander senescence’. Through a variable set of cytokines and chemokines, paracrine senescence is induced and maintained through mechanisms which generate reactive oxygen species (ROS) and the DNA-damage response (DDR) [76]. For example, paracrine senescence has been shown to exacerbate biliary injury and impair regeneration in the liver [47]. Upon the partial ablation of the SASP, through TGFβ inhibition, hepatocyte proliferation is increased, fibrosis is decreased, and overall liver function is improved. Opposingly, in the mouse model of cutaneous skin injury employed by Demaria et al., the authors identified a positive effect of the SASP [60]. Intriguingly, through the secretion of the SASP factor PDGF-AA, the closure of skin wounds was found to be accelerated, by promoting myofibroblast differentiation and granulation tissue formation.
Indeed, when the SASP was first studied, its role in injury and resolution was not well understood, as the components appeared to be mostly pro-inflammatory. It has since been suggested that senescence may impair regeneration through paracrine signalling, leaving neighbouring cells unable to compensate for damage, resulting in enhanced fibrosis and a diminished capacity of the regenerative response [47]. However, it appears that the prominent inflammatory components and detrimental consequences occur largely when the SASP is long-lived and may be beneficial when transient, thus playing a critical role in tissue remodelling at the early stages of injury. In fact, in 2017, Chiche et al. [29] showed that in acute and chronic injury, the release of the SASP factor IL-6 from senescent cells enabled the reprogramming of muscle satellite cells, indicating a role for the SASP in facilitating cellular plasticity and repair. Importantly, this points to a beneficial role of the SASP in promoting the cellular plasticity of stem cell populations during acute muscle injury, as well as in the pathological setting of muscle deterioration [29].
2.5. Cellular senescence in pluripotency
To add to the aforementioned findings, a recent study has demonstrated that the reprogramming factors OCT4, SOX2, KLF4 and cMYC can induce cellular senescence and IL-6 production in vivo, which leads to more efficient reprogramming [77]. Moreover, SASP can promote a pro-regenerative response through the induction of plasticity and stemness in somatic stem/progenitor cells [78]. Ritschka et al. [78] showed that a transient exposure to the SASP in primary mouse keratinocytes caused the increased expression of stem cell markers and regenerative capacity in vivo. However, prolonged exposure to the SASP triggered senescence arrest which countered the regenerative stimuli [78]. It has thus been proposed that in the case of injury, the senescent cell uses the SASP to induce plasticity and stemness in neighbouring cells, enabling the replacement of the senescent cell once it has been cleared and encouraging tissue regeneration [25]. However, it is important to note, when this process is uncontrolled, senescence-associated reprogramming can lead to tumour formation by promoting cancer stemness [79]. By contrast, SASP factors which are secreted by senescent cardiac progenitor cells (CPCs) via paracrine signalling result in the senescence of otherwise healthy CPCs. In this context, the elimination of senescent cells in aged mice or in mice treated with senolytics abrogated the SASP and resulted in the activation of resident CPCs and an increase in the number of proliferating Ki-67 EdU+ cardiomyocytes, thus indicating that the removal of senescent cells may alleviate deterioration following cardiac injury and contribute to the capacity of the heart to regenerate [58]. Moreover, the deletion of the senescence effectors p53 and p16INK4a improves the reprogramming efficiency of human fibroblasts to iPSCs, suggesting, that in this environment, senescence has a negative effect on plasticity [80]. Importantly, these differences in whether senescent cells are beneficial/detrimental to regeneration highlight the importance of the timing in which the senescence program is activated and its changing role during different stages of the injury response.
To date the SASP is known to have important roles in embryonic development, wound healing and tumour growth, indicating that the SASP has more complex physiological roles than we currently understand (reviewed in [81,82]). Taken together these studies demonstrate that senescent cells play important roles in tissue injury and regeneration and can both promote and inhibit tissue repair. Simply, the evidence of the positive effects of senescent cell removal comes from the circumstances where senescent cells accumulate and lead to negative consequences. Conversely, a transient wave of senescent cells appears to play an important role in promoting repair in the early stages of injury. Overall, these findings support the understanding that the senescence programme can be a beneficial regenerative process; however, when it is perturbed, it can play a detrimental role. For example, while acute senescence clearly plays an important role in preventing malignancy and promoting successful tissue repair, the accumulation of chronically senescent cells further contributes to injury, disease and ageing.
3. Role of macrophages in tissue injury
While a large variety of cell types have been shown to play important roles in injury and repair, in recent years, a particular interest in macrophages has developed, due to the contribution of different macrophage populations and their plasticity in the context of injury. Thus, in recent years, there has been a particular focus on the identification of different macrophage states and subsets in many different organ systems and their different roles in injury and repair. Macrophages are crucial for limb regeneration in the salamander [83] and tail fin regeneration in the zebrafish [84] (figure 3). Moreover, macrophages interact intimately with their surroundings integrating cues from invading pathogens, commensal bacteria, as well as tissue-specific functions, rendering macrophages extremely well adapted to their local environment and thus acquiring organ-specific functions [85,86]. The macrophage populations which are found in the many different tissues of the body are termed ‘tissue-resident’ macrophages and are largely derived from the yolk-sac during embryogenesis (reviewed in [87]). As long-lived cells, tissue-resident macrophages are particularly important, due to their witnessing and ‘memorizing’ past and present events in the tissue, which plays an important role in their plasticity. Furthermore, it has been shown that these macrophages are capable of readjusting their behaviours, probably through epigenetic modifications [88,89]. Therefore, tissue-resident macrophages are critical in maintaining tissue homeostasis.
Figure 3.
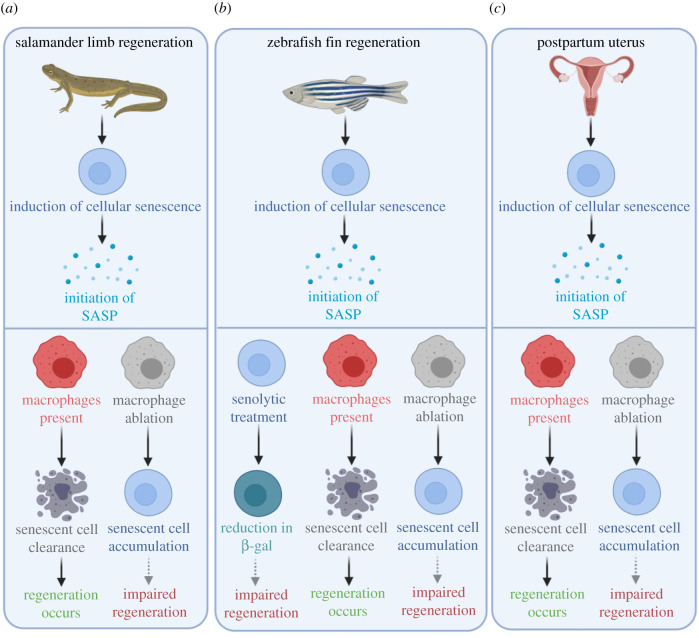
Animal models of regeneration have provided evidence of the interplay between macrophages and senescent cells during tissue regeneration. The induction of cellular senescence leads to the initiation of the senescence-associated secretory phenotype (SASP) during salamander limb regeneration, zebrafish fin regeneration and post-partum uterus regeneration. In the absence of macrophages, senescent cells in the regenerating salamander limb are not cleared, which is a possible reason for impaired regeneration (dashed grey arrow) [48]. The removal of either senescent cells or macrophages during zebrafish regeneration has a deleterious effect on regeneration, hypothesized to be as a result of altering the tightly regulated balance of cell senescence (dashed grey arrows) [125]. The mammalian uterus undergoes extensive remodelling post-partum where senescent cells are normally cleared by macrophages. In the absence of macrophages, senescent cells accumulate in the uterus [126], presumably leading to dysregulated regeneration and function (dashed grey arrow). Created with Biorender.com.
In response to tissue injury, inflammation results in an initial influx of neutrophils, accompanied by monocyte-derived macrophages, which clear cellular debris and coordinate cellular processes to initiate tissue repair. Importantly, this process leads to an overall diluting of the tissue-resident macrophages in the macrophage pool, which is further exacerbated by the proliferation of infiltrating macrophages, which adapt their function to surrounding cues in the local microenvironment. A range of studies have thus identified the specialized roles of monocytes and macrophages and the timing of their activation as critical in the various steps of tissue repair, regeneration and remodelling [52,54].
3.1. Macrophage phenotypes
In the past, macrophages have been broadly separated into two categories: M1 (classically activated) and M2 (alternatively activated), based on their inflammatory and anti-inflammatory/reparative functions, respectively. Nowadays, the binary M1/M2 classification is generally considered an oversimplification of the large variety of macrophage populations that exist in vivo, and to date have been further subdivided, based on their gene expression profiles [90,91]. Nonetheless, pro-inflammatory macrophages are generally associated with the expression of high levels of pro-inflammatory cytokines, an ability to mediate resistance to pathogens, produce reactive nitrogen and oxygen intermediates, and promote Th1 responses [92]. On the other hand, reparative macrophages are characterized by their role in tissue remodelling and repair, regulation of the immune system, scavenging and phagocytic capabilities [93], thus, exerting mainly pro-tumoral and immunoregulatory functions (reviewed in [94]).
Unsurprisingly, the presence of pro-inflammatory macrophages has been shown to sustain tissue-damaging inflammatory responses, and the presence of these cells has been associated with a variety of inflammatory and fibrotic diseases. The role of pro-inflammatory macrophages has been particularly well-studied in models of spinal cord injury, where macrophages have been shown to readily accumulate at the site of injury. In these models, macrophage activation and polarization, depending on changes in the microenvironment, has shown that the sustained recruitment of pro-inflammatory macrophages facilitates axonal dieback and can substantially delay the regenerative response [95], and their death in situ further contributes to tissue damage [96]. Furthermore, the presence of axon growth inhibitors is significantly higher in pro-versus anti-inflammatory macrophages, suggesting that these cells can actively contribute to suppressing regeneration after spinal cord injury [97]. In addition, studies in the liver have also implicated inflammatory macrophages in exacerbating injury, where an increase in inflammatory macrophages is observed in areas of hepatic necrosis [98,99]. This has also been observed during acute kidney injury where inhibition of early, pro-inflammatory macrophages improves renal function [100] (figure 2). However, it is important to note that pro-inflammatory macrophages may also contribute to the processes which lead to recovery. This has been observed in models of skeletal muscle injury, where inhibition of monocyte/macrophage accumulation impairs muscle regeneration [63,64], and cardiac regeneration, where macrophage depletion leads to alterations in myofibroblast infiltration and neovascularization, and subsequent ventricular dilatation and mortality [65] (figure 2). Thus, a fine balance between pro/anti-inflammatory macrophages is probably needed for optimal repair following injury.
3.2. Macrophage depletion and reconstitution studies
Perhaps unsurprisingly, the complete depletion of macrophages has also been found detrimental for tissue repair. Depletion studies in models of tissue injury in the liver have shown that macrophage-depleted mice fail to exert a complete cytokine response, which subsequently compromised liver regeneration [52]. This is likely to be due to the loss of anti-inflammatory pro-regenerative macrophages which play critical roles in promoting tissue repair. These macrophage populations have been partially defined by their production of the anti-inflammatory cytokine IL-10, which functions as an important anti-inflammatory mediator essential for the maintenance of anti-inflammatory activity [101]. Interestingly, in a model of early-onset inflammatory bowel disease, the loss of the IL-10 receptor (IL-10R) resulted in the spontaneous development of colitis [102], indicating that IL-10R signalling in intestinal macrophages is an important factor for controlling intestinal inflammation. Moreover, while cutaneous wound healing is accelerated in mice deficient for IL-10, a result attributed to accelerated re-epithelialization and wound contraction, macrophage infiltration was significantly elevated [103], further implicating IL-10 signalling in inflammation.
Furthermore, a recent paper by Podaru et al. [104] showed that the transplantation of functional ‘reparative macrophages', acquired from bone marrow mononuclear cells, in a mouse myocardial infarction model, resulted in significant improvement in functional recovery. Here, the authors found that transplantation of the reparative macrophages enhanced myocardial tissue repair, by promoting the formation of the vasculature and reducing cardiomyocyte hypertrophy and interstitial fibrosis. Interestingly, the transplantation of such reparative macrophages was also found to increase the number of their host-derived counterparts, which was partly mediated by TGFβ secretion [104]. Moreover, following hepatocyte death during liver injury, the engulfment of debris by macrophages leads to the induction of Wnt3a, which subsequently leads to canonical WNT signalling in nearby hepatic progenitor cells, facilitating their differentiation into hepatocytes and therefore contributing towards the regenerative response [105]. Thus, inflammatory cell-mediated cytokine signalling plays an integral role in regeneration and tissue resolution.
It has recently been shown that small extracellular vesicles (sEVs) derived from M2 bone marrow-derived monocytes (BMDMs) can attenuate spinal cord injury (SCI). sEVs mediate paracrine signalling and are important for regulating cellular function [106]. Here, sEVs from M2 BMDMs were found to protect neurons in SCI mice, by inhibiting the mTOR pathway and enhancing the autophagy ability of neurons, therefore reducing apoptosis in vitro and in vivo. This was found to be due to the transfer of the microRNA miR-421-3p, which regulates the mTOR pathway inside the M2 BMDM-sEVs [107]. This study showed for the first time that M2-derived BMDMs are important in protecting neurons and facilitating recovery following SCI, as well as highlighting that this occurs via the transfer of sEVs. Furthermore, this study thus further elucidates the beneficial roles for M2 macrophages during injury and indicates a mechanism by which they carry out their protective/reparative function.
Interestingly, such anti-inflammatory macrophages have been shown to not only promote tissue repair but also antagonize the function of pro-inflammatory macrophages and fight against their pro-fibrotic capabilities [108,109]. Thus, the interaction/cooperation of macrophages with various cell types, including those involved in the initial phase of inflammation, is of vital importance. It has recently been shown that neutrophils also have a crucial function in liver repair, by promoting the phenotypic conversion of pro-inflammatory Ly6ChiCX3CR1lo monocytes/macrophages to pro-reparative Lyc6loCX3CR1hi macrophages. Furthermore, this conversion was found to be dependent on the expression of reactive oxygen species (ROS) from neutrophils [110,111]. Intriguingly, this study demonstrated the cooperation between neutrophils and macrophages and the importance of their interaction in the resolution of inflammation and tissue repair [111]. This supports the accumulating evidence that monocyte-derived macrophages can undergo both phenotypic and functional transition in order to promote tissue regeneration and healing [112,113].
Finally, macrophage activity during different phases of tissue injury is important for tissue repair. By selectively depleting macrophage populations Duffield et al. [52] showed that macrophages have distinct, opposing roles during injury and repair. Specifically, in a mouse model of Ccl4-induced reversible liver injury, the depletion of macrophages during advanced fibrosis resulted in reduced scarring. However, if macrophages were depleted during the repair period this resulted in the failure of matrix degradation and a persistent activation of the fibrotic response. Importantly, this showed that macrophages perform both injury-inducing and repair-promoting tasks (figure 2), and that functionally distinct subpopulations of macrophages exist within the same tissue that play important roles in different phases of injury/recovery [52]. Moreover, the depletion of macrophages in the early stages of cutaneous wound repair delayed re-epithelialization, leading to reduced scar formation, while depletion in the mid-phase of new tissue formation led to an impaired wound closure. Crucially, depletion in the late stages of repair had no effect on the overall repair response, suggesting that macrophages play different and distinct functions during the phases of skin repair [54,114] (figure 2). Ultimately, studies such as these demonstrate that macrophages exert different and distinct functions at different stages of the repair or regeneration processes; a discovery that is crucial if we are to therapeutically manipulate macrophage function in the future to improve organ regeneration.
4. The interaction between senescent cells and macrophages in tissue regeneration and repair
Senescence is now known to play important roles in many different tissues during murine embryonic development [23,24]. This includes in the mesonephric tubules during mesonephros involution (development of the kidneys and testes), the endolymphatic sac of the inner ear, the apical ectodermal ridge of the limbs, the regressing interdigital webs of the hands and feet, and the closing of the neural tube [23]. Indeed, there is evidence that senescent cells in murine development are surrounded by macrophages at days E13.5–14.5, and that the infiltration of macrophages leads to senescent cell clearance and the promotion of tissue remodelling [23,97]. Importantly, the processes that are observed during development provide us with a unique insight into the mechanisms which drive regeneration and repair following injury in adulthood, and act as a starting point for the manipulation of such processes in vivo.
4.1. Senescent cell surveillance by macrophages
The role of macrophages in clearing senescent cells has been known for more than a decade [115]. Due to the substantial release of cytokines and chemokines by senescent cells via the SASP, it is unsurprising that an increasing number of studies appear to support a macrophage-dependent surveillance mechanism which operates in both normal and regenerating tissues. Indeed, it seems logical that the number of senescent cells must be closely monitored to maintain tissue homeostasis and to mitigate and/or prevent the negative impacts of senescent cell accumulation. The first evidence of the involvement of the immune system in the surveillance of senescent cells came in 2007 from Xue et al. [116], who revealed that the reactivation of p53 in p53-deficient tumours led to complete tumour regression. This was found to be mediated by the upregulation of inflammatory cytokines and the activation of the innate immune response [116]. After this, the role of immune cells, including macrophages, were shown to be important in the removal of senescent cells in models of liver injury, as well as for preventing excessive detrimental fibrosis and in resolving liver fibrosis [117]. Moreover, senescent hepatic stellate cells have been shown to secrete a SASP that attracts macrophages [118]. In 2013, Lujambio et al. showed that p53-expressing stellate cells release IFN-γ and IL-6, which promote resident Kupffer macrophages and infiltrated macrophages polarize towards a tumour-inhibiting M1 state, capable of targeting senescent cells in culture. However, in senescent stellate cells lacking p53, IL-4 was produced, causing macrophage polarization towards the pro-survival M2 phenotype [119]. Thus, this evidence suggests that senescent cells can elicit phenotypic changes in macrophages which can affect their functionality. Interestingly, mesenchymal stem cells (MSCs) also appear to play a regulatory role in shifting local macrophages from a pro-inflammatory to a tissue reparative phenotype [120,121], and in the absence of macrophages, neonates lose their ability to regenerate their myocardia following myocardial infarction [122].
To add to this, senescent cells are also capable of eliciting an adaptive immune response where they are cleared by CD4+ T cells and monocytes/macrophages. This has been shown in the liver, where pre-malignant senescent hepatocytes undergo clearance by CD4+ T cells, which require the presence of monocytes/macrophages, highlighting the importance of immune cell cross-talk in senescent cell clearance [123]. Furthermore, in a model of liver cancer, senescent cell surveillance was shown to require the recruitment and maturation of CCR2+ myeloid cells, while the ablation of CCR2 caused outgrowth of hepatocellular carcinomas [124].
4.2. Senescent cells and macrophage interactions during regeneration
To explore these interactions further, in 2015, Yun et al. [48] showed that macrophages were critical for the clearance of senescent cells, providing the first evidence that senescence-surveillance mechanisms operate during normal regeneration. Here the authors demonstrated that during salamander limb regeneration, there was a significant induction of cellular senescence, indicating its importance as a mechanism for regeneration in normal regenerating tissues. Furthermore, Yun et al. also reported that a strong SASP signature in blastemas coincided with a strong peak in the induction of senescent cells (figure 3), indicating that these could have paracrine effects on regeneration and the chemo-attraction of macrophages [48]. Crucially, macrophages and senescent cells are found in close proximity to each other in regenerating limbs. By contrast, clodronate-mediated deletion of macrophages results in the persistence of senescent cells during limb regeneration [48]. In support of this, reports in other model organisms such as the zebrafish have demonstrated the negative impacts of macrophage ablation during zebrafish fin regeneration [84]. This has been further investigated in a 2020 study by Da Silva-Álvarez et al., who showed that following injury in the zebrafish, senescent cells were present at the site of injury, and that their removal impaired regeneration [125] (figure 3). Moreover, senescent cells that accumulate in the post-partum mammalian uterus are efficiently cleared by macrophages after birth, while macrophage depletion leads to abnormal accumulation of senescent cells [126] (figure 2).
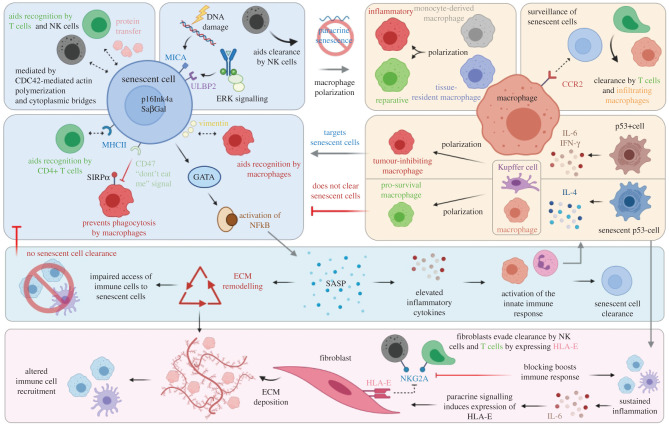
There therefore exists convincing evidence for the role of senescent cells and their interaction with macrophages in tissue injury and repair. SASP promotes macrophage proliferation [127], while P16INK4a+ macrophages accumulate with increasing age and exacerbate SASP [128]. The transcription factor GATA4 is stabilized during cellular senescence, which in turn activates NFκB to facilitate SASP [129] (figure 4). However, to date, questions remain regarding the mechanisms by which senescent cells interact with the immune system, including macrophages, and how this elicits or prevents a reparative response. It has previously been shown that senescent cells express NKG2D ligands MICA and ULBP2 on their cell surface, allowing their recognition and elimination by natural killer (NK) cells. Furthermore, the expression of these ligands has been found to be regulated by the DNA damage response and the ERK signalling pathway as a result of injury [131] (figure 4).
Figure 4.
Senescent cells and macrophages communicate via complex bi-directional signals during tissue remodelling, repair, regeneration and tumorigenesis. Senescent cells are detected through immune surveillance by natural killer (NK) cells, mediated by cell–cell contact, protein transfer, actin polymerization and cytoplasmic bridges, which ultimately aids recognition by NK cells and T cells [130]. Senescent cells express the ligands MICA and ULBP2 on their cell surface in response to DNA damage and ERK signalling, which aids in their clearance by NK cells [131]. In addition, senescent cells often express MHCII and vimentin which allows recognition by T cells and macrophages, respectively [123,132,133]. Conversely, senescent cells can evade detection by expressing the ‘don't eat me’ signal CD47, which impairs phagocytosis by macrophages by binding to the inhibitory receptor SIRPα [111] and GATA4 is stabilized during cellular senescence, which in turn activates NFκB to facilitate SASP [129]. Macrophages exist as tissue-resident cells or are derived from blood-borne monocytes and can be polarized towards a pro-inflammatory or pro-reparative state. Expression of p16Ink4a and SaβGal in macrophages has been implicated in a role for polarization, rather than as a consequence of paracrine senescence [128,134,135]. CCR2 expressed by macrophages is critical for senescent cell surveillance and clearance by T cells and infiltrating macrophages [124]. Under normal physiological conditions, p53+ stellate cells release the pro-inflammatory cytokines, IFN-γ and IL-6, which promote resident Kupffer cells and infiltrating macrophages to polarize towards a pro-inflammatory state, which can target senescent cells. Conversely, in senescent stellate cells lacking p53, IL-4 is produced, resulting in macrophage polarization towards the pro-survival phenotype [119]. Senescence-associated secretory phenotype (SASP) leads to elevated inflammatory cytokines, activation of the immune response and senescent cell clearance. In addition to this classical role, SASP also leads to extracellular remodelling (ECM) which contributes to altered cell recruitment and impaired access of immune cells to senescent cells [136]. The MHC molecule HLA-E, which is expressed by senescent cells and induced by IL-6, interacts with the inhibitory receptor NKG2A, expressed by NK cells and T cells, to evade surveillance, and blocking this interaction improves the immune responses against senescent cells [137], in a manner whereby sustained inflammation also contributes to the persistence of senescent cells. Created with Biorender.com.
4.3. Senescent cells and macrophage interactions with ageing
Another outstanding question is what allows senescent cells to accumulate during ageing/injury and how senescent cells manage to escape being cleared by the immune system. Progress in answering this question has shown that senescent dermal fibroblasts can evade clearance by the immune system by expressing the non-classical MHC molecule HLA-E. HLA-E functions by interacting with inhibitory NKG2 receptors, expressed by NK cells and CD8+ T cells, to inhibit immune responses against senescent cells (figure 4). The authors found that blocking the interaction between HLA-E and the receptor NKG2A boosted immune responses against senescent cells in vitro. Interestingly, they found that the SASP-related cytokine IL-6 induced the expression of HLA-E in non-senescent cells in a paracrine fashion [137]. The upregulation of HLA-E expression by IL-6 thus suggests that sustained inflammation may also contribute to the persistence of senescent cells in tissue, further contributing to the pathogenesis of injury or age-related diseases. This is supported by the fact that IL-6 has been well characterized in senescence [138] and is found in the serum of elderly patients, as well as in various injury models [139,140]. Furthermore, as the authors note, the expansion of CD8+ T cells which are NKG2C+, another isoform of the inhibitory NKG2 receptors, with age may offer an explanation as to why the immune system is less effective in clearing senescent cells in older individuals. Determining whether the abundance/role of CD8+ NKG2C+ T cells is altered during acute and/or chronic injury would therefore be of interest.
Another important component for tissue regeneration is the extracellular matrix (ECM) and its cross-talk with cells in the surrounding microenvironment. Interestingly, the soluble SASP is known to induce ECM remodelling and stiffening, which has previously been shown to alter immune cell recruitment during ageing [136]. In fact, alterations to the ECM as a result of changes in matrix stiffness impair the access of immune cells to senescence-enriched tissues [136]. Furthermore, stromal cells such as fibroblasts are responsible for regulating tissue structure through deposition of the ECM, as well as supporting homeostasis through the secretion of cytokines, chemokines, growth factors and other key signalling proteins [136] (figure 4). We previously discussed the importance of the SASP in the release of soluble factors which influence the surrounding tissue microenvironment and maintain tissue homeostasis. It has been reported that extensive cross-talk exists between senescence-associated stromal populations that are known to accumulate during ageing and an immunosuppressive phenotype.
4.4. The parallels between macrophages and senescent cells
As well as the well-documented phagocytic role of macrophages, it was recently shown, for the first time, that chemotherapy-induced senescent cells (CISCs) are capable of engulfing both neighbouring senescent cells or non-senescent tumour cells in a macrophage-like fashion. It is important to note is that this behaviour was noted after chemotherapy treatment. However, this behaviour is also triggered by the administration of nutlin, which activates p53 without causing genotoxic stress [141]. This fascinating discovery that senescent cells, in the correct environment, can acquire a phagocytic phenotype, is suggestive of a potential survival advantage.
This newly found ability of senescent cells brings to light parallels with macrophages, whereby both macrophages and senescent cells secrete factors that elicit matrix remodelling and immunomodulation [142,143], and express metabolic markers, such as CD38 [144]. Indeed, cannibalism by breast cancer cells has been suggested to play a role in the induction of senescence [141]; thus, it will be important to investigate the role of cannibalism of senescent cells in a range of different contexts, including development, ageing and regeneration/repair. In particular, it will be interesting to determine the role of such cells in senescent cell accumulation, and whether cannibalism may play a role in cell clearance following injury. Furthermore, it will be pivotal to determine what cell types these cannibalistic cells preferentially engulf, and whether there are certain characteristics which lead to their engulfment, in order to determine whether, if such cells exist outside the context of cancer, they are beneficial or detrimental to the process of regeneration and repair.
4.5. The ageing immune system and immunosenescence
As the body ages, the innate immune system gradually declines, a phenomenon now termed immunosenescence [145], resulting in decreased effector immune cell function; and healthy tissue renewal rate decreases dramatically [146]. As a result, the aged tissue microenvironment accumulates senescent cells, such as SASP-associated fibroblasts, and gains the infiltration of immune infiltrates such as immunosuppressive myeloid-derived suppressor cells (MDSCs) and T regulatory cells. MDSCs are a heterogeneous population of cells of myeloid origin which repress T cells via the secretion of arginase 1, TGFβ and reactive oxygen species (ROS), while T regulatory cells have a role in regulating or suppressing other cells in the immune system (reviewed in [147]). It has been suggested that this alteration in tissue microenvironment may encourage the development of pathological conditions such as cancer, and allows for the expansion of cancer cells unabated by the immune system. Indeed, in the event of chronic injury, it is certainly possible that this leniency of the immune system, or impaired immune surveillance, may also have similar effects, for example, by allowing the accumulation of senescent cells. In addition, the immunosenescence of effector T cells, NK cells, macrophages and dendritic cells is known to lead to a dramatic decrease in their cytotoxic activities and infiltration within an aged tumour-promoting microenvironment [148]. This has been supported by the observation that there are systemic increases in immunosuppressive M2 macrophages and N2 neutrophils in elderly people (reviewed in [149]), which may contribute towards an immunosuppressive phenotype. This has been shown in a model of senescence induction, using the fibroblasts accelerate stromal-supported tumorigenesis (FASST) mouse, where the authors observed an accumulation of senescent cells, as well as an increased number of MDSCs and regulatory T cells, which was not observed in a younger tissue microenvironment. Furthermore, this increase in the number of MDSCs and T reg cells in healthy mice was found adjacent to senescent cell populations and was found to primarily be due to the secretion of IL-6 [148]. Moreover, the deletion of perforin (Prf1), an essential component of immune surveillance of senescent cells, leads to the accumulation of senescent cells in the liver [150] and a general increase in the accumulation with advanced age, resulting in chronic inflammation, fibrosis and tissue damage [117]. Thus, this process of transient SASP and senescent cell clearance in young versus aged mice during the natural process of ageing shows very clear similarities with the occurrence of the senescence programme and the cycle of clearance/accumulation of senescent cells in acute and chronic injury, respectively.
A well-characterized mechanism by which senescent and apoptotic cells are regulated by macrophages is via their ‘eat me’ signals. However, senescent cells often express the ‘don't eat me’ signal CD47, a cell surface protein mediated by upregulation of NFκB, which impairs phagocytosis by macrophages by binding to the inhibitory receptor signal regulatory protein alpha (SIRPα) found on the cell surface of macrophages. Conversely, senescent cells also appear to upregulate their expression of MHC class II molecules on their cell surface, which aid in their recognition by CD4+ T cells [123,132]. Furthermore, it has recently been identified that senescent cells also express an oxidized form of vimentin on their cell surface, which eventually gets released into the bloodstream, suggesting that oxidized proteins are capable of being recognized by the humoral innate immune system. This suggests that such proteins form part of a senescence-surveillance mechanism by acting as ‘eat me’ signals, and this process may become impaired with age [133]. Modified vimentin is already known to be expressed on the surface of immune cells including apoptotic neutrophils [151–153] and T cells [154], which eventually leads to elimination by macrophages [155]. In addition, it has been found that radiation-induced proteins found on the surface of apoptotic cells can interact with vimentin found on the surface of neighbouring phagocytes, leading to their removal by macrophages [155]. Understanding the mechanisms which regulate macrophage phagocytic ability and subsequent senescent cell clearance will be vitally important for developing future therapeutic approaches to aid regeneration and improve healthspan.
Senescent cells are also capable of communicating with neighbouring cells through the direct transfer of proteins. In 2015, it was shown by Biran et al. [130] that proteins were transferred to NK and T cells, and that this process was facilitated by immune surveillance of senescent cells by NK cells, resulting in their activation and increased cytotoxicity. The protein transfer was dependent strictly on cell–cell contact and CDC42-regulated actin polymerization, and partially mediated by cytoplasmic bridges. This raises the possibility that senescent cells may use intracellular protein transfer to induce senescence in neighbouring cells or perhaps even to promote survival. Furthermore, it is possible that this process may be important for regeneration, by allowing the cell–cell transfer of molecular mediators between senescent and regenerative or supportive cells. Indeed, the authors found that compared with normal cells, senescent cells preferentially use intercellular protein transfer (IPT) to regulate their own self-elimination by immune cells and communicate with epithelial cells. To support this, a variety of other studies have shown that IPT is used to both initiate and modulate immune responses which ultimately support cell survival [156–158]. Further investigation is required to comprehensively determine the transfer of material between macrophages and other immune cells during senescence and regeneration/repair. The recent publication of a proteomic database of soluble proteins and exosomal cargo SASP factors will undoubtedly lead to a more thorough understanding of cell communication and senescence [14].
While more is becoming known regarding the role of senescent cells and macrophages or the immune system in regeneration and repair, there remain many open questions. For example, it is still unclear whether macrophages themselves become senescent in the context of injury and whether this may contribute to the accumulation of senescent cells. Indeed, while damage to the immune system leads to the failure to clear senescent cells, contributing to their persistence in tissues, such immune failure may be in part due to immunosenescence, leading to immune cell dysfunction. Hall et al. [128] previously reported the observation of p16INK4a and Sa-β-Gal expression in macrophages suggesting that senescent cells could spread senescence to immune cells. However, the authors later reported that the expression of these markers was not due to a spread of senescence, but rather acquired as part of a physiological response to immune stimuli [134]. This is consistent with reports that p16INK4a is involved in macrophage polarization [135]. Thus, whether immune cells become senescent during injury or disease requires further investigation; however, it has been reported that immune cells undergo a progressive decline in function, known as immunosenescence, which has been reported to contribute to senescent cell accumulation [159,160]. Furthermore, if macrophages become senescent, do they still retain their ability to clear target cells, including other senescent cells? If this is the case, it is likely that macrophages contribute to the process of ‘inflammaging’, including immunosenescence, especially if senescent macrophages are found to promote a pro-inflammatory environment. Furthermore, understanding other mechanisms by which macrophages become damaged which cause changes in their function and or/phenotype during injury will also be important when considering therapeutic approaches.
4.6. Senescent cell accumulation and pathology
Other outstanding questions include whether there is a ‘tipping point’ at which senescent cells must accumulate to before the tissue becomes dysfunctional, and whether a similar process occurs at the point where macrophages are unable to clear senescent cells. As previously mentioned, the specific mechanisms by which macrophages interact with senescent cells remains to be elucidated. Understanding the molecular mechanisms which govern these interactions and which regulate macrophage function will be critical for the progression of therapies and the understanding of chronic diseases. In line with the interaction of senescent cells with CD4+ T cells to evade immune surveillance, it would be beneficial to further characterize whether similar mechanisms exist between macrophages and senescent cells. Finally, while various studies have individually indicated the role of different components of the senescence programme in the regenerative response, a comprehensive study linking together these processes remains to be undertaken. While the transient SASP is beneficial for regeneration and sustained SASP signalling promotes senescent cell accumulation, a comprehensive study of the ‘waves’ of the SASP in repair/regeneration, the point at which this process becomes detrimental, and the exact mechanisms which govern this response and how they interact with each other is still lacking.
5. Therapeutic manipulation of macrophages for tissue regeneration/repair
As covered in previous sections, the role of macrophages in maintaining tissue homeostasis, clearing senescent cells and promoting regeneration is beginning to be better established. Due to the innate role of macrophages in these processes, an emerging idea of treating diseases/age-related pathologies related to senescent cell accumulation falls within the realm of manipulating macrophage function. Such ideas include treating Alzheimer's disease [161], myocardial infarction [162], cancer [163] and inflammatory diseases [164] such as rheumatoid arthritis, with macrophage therapy.
5.1. Macrophage engineering approaches
One method of achieving a therapeutic approach is by engineering macrophages to ignore ‘don't eat me’ signals found on the surface of senescent cells. As previously mentioned, the CD47–SIRPα axis allows senescent cells to avoid removal by the immune system; therefore, blocking this axis may be an effective treatment against the accumulation of senescent cells in ageing and injury. Molecules which target this axis have already been developed and include those that target CD47, as well as SIRPα specifically, alongside bispecific targeting agents. The use of these agents has been well characterized in cancer, where anti-CD47 antibodies are currently in clinical trials [111]. Taking into account the role of macrophages in blocking the CD47–SIRPα axis, engineering macrophages to target CD47 may be effective. For example, similar to CAR-T cells, Chimeric Antigen Receptors for Phagocytosis (CAR-Ps) are designed to phagocytose specific targets, and it has been suggested that engineering macrophages to target CD47 may be an effective anti-tumour therapy [111]. Indeed, the use of these macrophages may prove effective in the clearance of chronic senescent cell accumulation following injury. However, there remain concerns over the manipulation of the CD47–SIRPα axis for therapeutic use; including, but not limited to, the consensus that the CD47–SIRPα interactions demonstrated in mouse studies may not completely or as efficiently translate to human [165]; the discovery that the clustering of CD47 can also influence the interaction between CD47 and SIRPα, and that the anti-CD47 non-blocking antibody 2D3 increases CD47 clustering [166,167], and the considerable functions of CD47 that are independent of SIRPα, which instead act upon SIRPγ or Thrombospondin 1 (TSP1), leading to potential off-target effects on T cells [168,169]. Another possibility is to increase the phagocytic capability of macrophages, by increasing ‘eat me’ signals. Defective expression of the ‘eat me’ signal calreticulin, a ligand required for activation of engulfment receptors on phagocytic cells, results in cellular resistance to efferocytosis, and apoptotic cells fail to be cleared by neighbouring macrophages [170]. A proportion of aged and cancerous cells are susceptible to being ‘labelled’ by macrophage-secreted calreticulin and are subsequently cleared from tissue [171]. Thus, the overexpression of calreticulin on senescent cells may provide a manner to increase phagocytosis and clearance from the tissue by macrophages. A third option involves the use of allogenic macrophages or administration of induced pluripotent stem (IPS) cell-derived macrophages from young donors [172]; however, whether young macrophages have better phagocytotic ability is not clear, with some studies reporting reduced ability with age [173], while others report no difference [174]. Moreover, differences appear to be related to the tissue from which they are derived and whether they are tissue resident or infiltrating [175]. Thus, macrophage targeting may be a therapeutic option for the clearance of senescent cells in vivo.
5.2. Senolytics
An alternative way to clear senescent cells is via the use of drugs that kill senescent cells, known as senolytics. It has been shown that the removal of senescent cells using senolytic drugs, such as ABT-263, which work by targeting proteins involved in the apoptosis pathway (such as BCL-xL and BCL-2), have been successful in removing senescent cells in vitro and in vivo [73,176]. In particular, ABT-263, which signals through the anti-apoptotic proteins BCL-xL and BCL-2, has proven effective in eliminating senescent cells in mouse models [73,177] and is currently undergoing phase 1 clinical trials in cancer patients [178]. Moreover, dasatinib plus quercetin (DQ), which not only targets BCL-2 family members, but also HIF-1α, PI3-kinase and p21-related anti-apoptotic pathways [179], has proved efficient at improving physical dysfunction in idiopathic pulmonary fibrosis (IPF) patients [180]. However, there remain limitations with the use of senolytics, largely due to lack of specificity, bioavailability and their route of administration. Indeed, if macrophages themselves are found to become senescent in the context of injury, it may be necessary to combine work on senolytics to include those that can target macrophages as well as other cells. Alternatively, a therapy that activates gene networks to promote a ‘younger’ phenotype in macrophages could provide a realistic option. This is especially important due to the critical role macrophages play in generating a regeneration-permissive environment needed for tissue repair.
As previously mentioned, while senolytics have proven effective in killing senescent cells, they retain broad-spectrum activity, especially considering the heterogeneous nature of the senescence programme. In 2020, Cai et al. [181] developed a new prodrug named SSK1 which is specifically activated by β-galactosidase (β-Gal) activity, believed to be a well-documented marker of senescent cells. The prodrug was shown to eliminate senescent cells, as well as re-establishing low-grade inflammation and restoring some measures of function. This novel drug therefore represents a more selective method of deleting senescent cells in a wide range of cell types and tissues [181]. In addition to reducing the number of senescent cells in general, the prodrug was found to decrease the number of SA-β-Gal positive macrophages in injured lungs and aged livers, which was accompanied by a reduction in inflammation-related cytokines. This suggests that the removal of macrophages themselves may be beneficial. Here it is important to note the previously mentioned study by Hall et al. in which the authors reported that senescent macrophages were evident in young mice, entirely due to their expression of the markers p16INK4a and β-galactosidase [128]. However, the expression of these markers was later described to be markers of macrophage polarization and response [134]. Importantly, this highlights the need for better methods for the identification of senescence in immune cells. Moreover, since senescent cells do not have any one specific universal marker, and due to their dynamic nature, that their markers can alter over time, the specificity of any drug aimed at targeting senescent cells alone is problematic.
5.3. Genetic engineering approaches
As the removal of macrophages themselves tends to lead to severe consequences, an alternative approach is to alter their gene expression in order to induce certain phenotypes. This involves the expression of certain transcription factors, for example IRF5, which is involved in the polarization of macrophages towards an inflammatory phenotype, which in turn prevents healing and promotes inflammation. Thus, manipulating macrophages to express lower levels of IRF5 could be a promising strategy [182]. Furthermore, the delivery of microRNAs or other molecules through methods of delivery such as liposomes may be a promising method for genetic manipulation worthy of further investigation [183,184].
Regarding genetic manipulation, an interesting aspect of senescence that is yet to be thoroughly explored is the regulation of gene expression at the epigenetic level. An interesting idea in regenerative medicine is that pluripotency factors can be expressed in senescent cells, to promote re-entry into the cell cycle and modify gene expression profiles. In addition to this, there has been interest in the use of epigenetic factors to promote reprogramming, as senescent cells display a repressive chromatin configuration, which is thought to stably silence proliferation-promoting genes, while also activating the SASP. In particular, histone modifications such as H3K27me3 have been associated with upregulation of the SASP in senescent cells [185]. Interestingly, in a recent study, it was also shown that the activation of the senescence programme leads to the remodelling of the epigenetic landscape by recruiting BRD4, a transcriptional and epigenetic regulator, which activates newly activated super-enhancers located next to SASP genes [186]. In addition, BRD4 was found to be critical for the SASP and paracrine signalling, and in senescence immune surveillance. Importantly this study revealed how cells can activate immune-modulatory genes required for paracrine immune activation and a tumour-suppressive immune surveillance programme. This may indicate the way in which senescent cells are regulated during homeostasis and may provide targets for regeneration. Indeed, the inhibition of BRD4 disrupts the ability of immune cells to target and eliminate pre-malignant senescent cells in vitro and in vivo [186], suggesting the effectiveness of this approach.
5.4. Epigenetic targeting
To complement macrophage genetic engineering, targeting epigenetic enzymes acting on the chromatin in senescent cells may also be an effective approach to switch on gene regulatory networks associated with a ‘younger morphology’ and may contribute to regulating the SASP. This approach is promising, as through the identification of ‘master regulators’ it allows the regulation of a large number of intricately interlinked genes and gene networks, instead of the manipulation of small subsets. Indeed, senescent cells are known to remodel the epigenetic landscape in order to induce the expression of genes implicated in cellular defence and the inflammatory response, many of which are characterized as part of the SASP [17]. Furthermore, alterations in epigenetic regulation have been associated with immunosenescence, as Menin promotes histone acetylation at the Bach2 locus, thereby suppressing T cell senescence, and subsequently, immunosenescence [187]. Indeed, this approach also relates to macrophages and may be successful in altering macrophage polarization.
5.5. Altering macrophage behaviour
Phagocytosis in macrophages is regulated through activation and inhibition of receptor signals. Activating receptors of macrophages sends a phagocytic signal that induces the ‘eat’ process. Importantly, when targeting senescent cells/macrophages for the purpose of regeneration/repair, it is important to note that an optimal result will probably be achieved through the careful, timely and balanced manipulation of this process. Indeed, the literature has pointed towards an important role of senescent cells in the initiation of the early stages of repair and of macrophages in clearing these cells in the first transient ‘wave’ of repair. It was not until recently that the soluble SASP was identified to have two distinct functional stages, the first stage being a highly anti-inflammatory stage enriched by TGFβ. Thus, the inhibition of this process as demonstrated by previous studies [188] is likely to have negative impacts on repair. However, the activation of macrophages at later points where senescent cells are found to accumulate is likely to be beneficial. Indeed, identifying the time points at which intervention is needed/most effective in different organ systems will be one of the most challenging aspects of developing effective therapies.
6. Conclusion, open questions and perspectives
From the initial thoughts that senescence was merely a natural result of ageing, our understanding of the role of senescence in ageing and human disease has greatly evolved. We are now aware that the senescence programme has more complex roles, notably in tissue regeneration and repair, beyond our current scope of knowledge. Furthermore, the crucial role of macrophages in repair and regeneration has also started to be demonstrated in the last decade, due to their well-reported role during injury. However, to date many questions remain regarding their specific role in the regulation of senescent cells following injury in different organ systems, which is complicated by the highly heterogeneous nature of the senescence programme, which directly influences macrophage function. Thus, to be able to target senescence for therapeutic means, a number of open questions need to be addressed, such as the following. (i) How do senescent cells interact with macrophages, and how does this change in different phases or ‘waves’ of the regenerative response? (ii) How do changes in the microenvironment trigger epigenetic/genomic/phenotypic changes in macrophages? (iii) At what point do senescent cells have to accumulate to before macrophages are unable to clear them, or when do senescent cells start to evade clearance by the immune system? Importantly, answering these questions will better elucidate how senescent cells and macrophages act in concert in the context of injury and repair, and will guide the development of treatments targeting senescent cells for therapeutic means.
Acknowledgements
The authors thank Ella Mercer and Sofia Ferreira Gonzalez for critical reading of the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
S.S.E. researched and wrote the manuscript. E.E. edited the manuscript and created the figures.
Competing interests
We declare we have no competing interests
Funding
S.S.E. is funded by Wellcome Trust grant 108906/Z/15/Z; E.E. is funded by UKRI/MRC grant MR/S005544/1.
References
- 1.Frangogiannis NG. 2017. The Inflammatory Response in Tissue Repair. In Inflammation: from molecular and cellular mechanisms to the clinic (eds Cavaillon JM, Singer M), pp. 1517–1538. Weinheim, Germany: John; Wiley and Sons. [Google Scholar]
- 2.Brockes JP. 1997. Amphibian limb regeneration: rebuilding a complex structure. Science 276, 81–87. ( 10.1126/science.276.5309.81) [DOI] [PubMed] [Google Scholar]
- 3.Poss KD, Wilson LG, Keating MT. 2002. Heart regeneration in zebrafish. Science 298, 2188–2190. ( 10.1126/science.1077857) [DOI] [PubMed] [Google Scholar]
- 4.Michalopoulos GK, DeFrances MC. 1997. Liver regeneration. Science 276, 60–66. ( 10.1126/science.276.5309.60) [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew MV. 2018. Dissecting spinal cord regeneration. Nature 557, 343–350. ( 10.1038/s41586-018-0068-4) [DOI] [PubMed] [Google Scholar]
- 6.Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, Kim KT, Jang IS, Park SC. 2004. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 279, 42 270–42 278. ( 10.1074/jbc.M402352200) [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Zamudio RI, Robinson L, Roux PF, Bischof O. 2017. SnapShot: cellular senescence pathways. Cell 170, 816–816.e811. ( 10.1016/j.cell.2017.07.049) [DOI] [PubMed] [Google Scholar]
- 8.Benhamed M, Herbig U, Ye T, Dejean A, Bischof O. 2012. Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nat. Cell Biol. 14, 266–275. ( 10.1038/ncb2443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodier F, et al. 2011. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 124, 68–81. ( 10.1242/jcs.071340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. 2003. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716. ( 10.1016/s0092-8674(03)00401-x) [DOI] [PubMed] [Google Scholar]
- 11.Hewitt G, et al. 2012. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708 ( 10.1038/ncomms1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplon J, et al. 2013. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112. ( 10.1038/nature12154) [DOI] [PubMed] [Google Scholar]
- 13.Kang HT, Lee HI, Hwang ES. 2006. Nicotinamide extends replicative lifespan of human cells. Aging Cell. 5, 423–436. ( 10.1111/j.1474-9726.2006.00234.x) [DOI] [PubMed] [Google Scholar]
- 14.Basisty N, et al. 2020. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 ( 10.1371/journal.pbio.3000599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgakopoulou EA, et al. 2013. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence: a method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5, 37–50. ( 10.18632/aging.100527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimri GP, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367. ( 10.1073/pnas.92.20.9363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868. ( 10.1371/journal.pbio.0060301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.d'Adda di Fagagna F. 2008. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer. 8, 512–522. ( 10.1038/nrc2440) [DOI] [PubMed] [Google Scholar]
- 19.Courtois-Cox S, Jones SL, Cichowski K. 2008. Many roads lead to oncogene-induced senescence. Oncogene 27, 2801–2809. ( 10.1038/sj.onc.1210950) [DOI] [PubMed] [Google Scholar]
- 20.Bernadotte A, Mikhelson VM, Spivak IM. 2016. Markers of cellular senescence: telomere shortening as a marker of cellular senescence. Aging (Albany NY) 8, 3–11. ( 10.18632/aging.100871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, You L, Xue J, Lu Y. 2018. Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: a mini review. Front. Pharmacol. 9, 522 ( 10.3389/fphar.2018.00522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Kohli J, Demaria M. 2020. Senescent cells in cancer therapy: friends or foes? Trends Cancer 6, 838–857. ( 10.1016/j.trecan.2020.05.004) [DOI] [PubMed] [Google Scholar]
- 23.Muñoz-Espín D, et al. 2013. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118. ( 10.1016/j.cell.2013.10.019) [DOI] [PubMed] [Google Scholar]
- 24.Storer M, et al. 2013. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130. ( 10.1016/j.cell.2013.10.041) [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Espín D, Serrano M. 2014. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496. ( 10.1038/nrm3823) [DOI] [PubMed] [Google Scholar]
- 26.Serrano M. 2014. Senescence helps regeneration. Dev. Cell 31, 671–672. ( 10.1016/j.devcel.2014.12.007) [DOI] [PubMed] [Google Scholar]
- 27.Le Roux I, Konge J, Le Cam L, Flamant P, Tajbakhsh S. 2015. Numb is required to prevent p53-dependent senescence following skeletal muscle injury. Nat. Commun. 6, 8528 ( 10.1038/ncomms9528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. 2008. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667. ( 10.1016/j.cell.2008.06.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiche A, et al. 2017. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20, 407–414.e404. ( 10.1016/j.stem.2016.11.020) [DOI] [PubMed] [Google Scholar]
- 30.Hayflick L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636. ( 10.1016/0014-4827(65)90211-9) [DOI] [PubMed] [Google Scholar]
- 31.Hayflick L, Moorhead PS. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. ( 10.1016/0014-4827(61)90192-6) [DOI] [PubMed] [Google Scholar]
- 32.Burova E, Borodkina A, Shatrova A, Nikolsky N. 2013. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid. Med. Cell Longev. 2013, 474931 ( 10.1155/2013/474931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbadie C, Pluquet O, Pourtier A. 2017. Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses? Cell Mol. Life Sci. 74, 4471–4509. ( 10.1007/s00018-017-2587-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia G, Aroor AR, Jia C, Sowers JR. 2019. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1802–1809. ( 10.1016/j.bbadis.2018.08.008) [DOI] [PubMed] [Google Scholar]
- 35.Bektas A, Schurman SH, Sen R, Ferrucci L. 2017. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 102, 977–988. ( 10.1189/jlb.3RI0716-335R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Ding Y, Liu Z, Liang X. 2020. Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front. Cell Dev. Biol. 8, 258 ( 10.3389/fcell.2020.00258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farr JN, Khosla S. 2019. Cellular senescence in bone. Bone 121, 121–133. ( 10.1016/j.bone.2019.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baar MP, Perdiguero E, Muñoz-Cánoves P, de Keizer PL. 2018. Musculoskeletal senescence: a moving target ready to be eliminated. Curr. Opin. Pharmacol. 40, 147–155. ( 10.1016/j.coph.2018.05.007) [DOI] [PubMed] [Google Scholar]
- 39.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. 2010. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684. ( 10.1111/j.1474-9726.2010.00608.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lecot P, Alimirah F, Desprez PY, Campisi J, Wiley C. 2016. Context-dependent effects of cellular senescence in cancer development. Br. J. Cancer 114, 1180–1184. ( 10.1038/bjc.2016.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta JC, et al. 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990. ( 10.1038/ncb2784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorgoulis V, et al. 2019. Cellular senescence: defining a path forward. Cell 179, 813–827. ( 10.1016/j.cell.2019.10.005) [DOI] [PubMed] [Google Scholar]
- 43.Malaquin N, Martinez A, Rodier F. 2016. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 82, 39–49. ( 10.1016/j.exger.2016.05.010) [DOI] [PubMed] [Google Scholar]
- 44.Acosta JC, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018. ( 10.1016/j.cell.2008.03.038) [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Meljem JM, Apps JR, Fraser HC, Martinez-Barbera JP. 2018. Paracrine roles of cellular senescence in promoting tumourigenesis. Br. J. Cancer 118, 1283–1288. ( 10.1038/s41416-018-0066-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coppé JP, Desprez PY, Krtolica A, Campisi J. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118. ( 10.1146/annurev-pathol-121808-102144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira-Gonzalez S, et al. 2018. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat. Commun. 9, 1020 ( 10.1038/s41467-018-03299-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yun MH, Davaapil H, Brockes JP. 2015. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife 4, e13232 ( 10.7554/eLife.05505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kale A, Sharma A, Stolzing A, Desprez PY, Campisi J. 2020. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 17, 16 ( 10.1186/s12979-020-00187-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491 ( 10.3389/fimmu.2014.00491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prata L, Ovsyannikova IG, Tchkonia T, Kirkland JL. 2018. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin Immunol. 40, 101275 ( 10.1016/j.smim.2019.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. 2005. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65. ( 10.1172/jci22675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang S, et al. 2012. Oval cell response is attenuated by depletion of liver resident macrophages in the 2-AAF/partial hepatectomy rat. PLoS ONE 7, e35180 ( 10.1371/journal.pone.0035180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. 2010. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 184, 3964–3977. ( 10.4049/jimmunol.0903356) [DOI] [PubMed] [Google Scholar]
- 55.Herranz N, Gil J. 2018. Mechanisms and functions of cellular senescence. J. Clin. Invest. 128, 1238–1246. ( 10.1172/jci95148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. 2013. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE 8, e70464 ( 10.1371/journal.pone.0070464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. 2004. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307. ( 10.1172/jci22475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis-McDougall FC, et al. 2019. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931 ( 10.1111/acel.12931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marmary Y, et al. 2016. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer res. 76, 1170–1180. ( 10.1158/0008-5472.can-15-1671) [DOI] [PubMed] [Google Scholar]
- 60.Demaria M, et al. 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 31, 722–733. ( 10.1016/j.devcel.2014.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jun JI, Lau LF. 2010. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2, 627–631. ( 10.18632/aging.100201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin MD. 2010. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 299, F1486–F1495. ( 10.1152/ajprenal.00378.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. 2011. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. Faseb j. 25, 358–369. ( 10.1096/fj.10-171579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Contreras-Shannon V, Ochoa O, Reyes-Reyna SM, Sun D, Michalek JE, Kuziel WA, McManus LM, Shireman PK. 2007. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2-/- mice following ischemic injury. Am. J. Physiol. Cell Physiol. 292, C953–C967. ( 10.1152/ajpcell.00154.2006) [DOI] [PubMed] [Google Scholar]
- 65.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. 2007. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 170, 818–829. ( 10.2353/ajpath.2007.060547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. ( 10.1038/nature10600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yevsa T, Kang TW, Zender L. 2012. Immune surveillance of pre-cancerous senescent hepatocytes limits hepatocellular carcinoma development. Oncoimmunology 1, 398–399. ( 10.4161/onci.19128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeon OH, et al. 2017. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781. ( 10.1038/nm.4324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker DJ, et al. 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. ( 10.1038/nature16932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, et al. 2019. Senolytics (DQ) mitigates radiation ulcers by removing senescent cells. Front. Oncol. 9, 1576 ( 10.3389/fonc.2019.01576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yosef R, et al. 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 ( 10.1038/ncomms11190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lehmann M, et al. 2017. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir J. 50, 1602367 ( 10.1183/13993003.02367-2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang J, et al. 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83. ( 10.1038/nm.4010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iske J, et al. 2020. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 11, 4289 ( 10.1038/s41467-020-18039-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Deursen JM. 2014. The role of senescent cells in ageing. Nature 509, 439–446. ( 10.1038/nature13193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bird TG, et al. 2018. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl. Med. 10, eaan1230. ( 10.1126/scitranslmed.aan1230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mosteiro L, et al. 2016. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445. ( 10.1126/science.aaf4445) [DOI] [PubMed] [Google Scholar]
- 78.Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, Sansom OJ, Zender L, Keyes WM. 2017. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31, 172–183. ( 10.1101/gad.290635.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milanovic M, et al. 2018. Senescence-associated reprogramming promotes cancer stemness. Nature 553, 96–100. ( 10.1038/nature25167) [DOI] [PubMed] [Google Scholar]
- 80.Banito A, et al. 2009. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139. ( 10.1101/gad.1811609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe S, Kawamoto S, Ohtani N, Hara E. 2017. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 108, 563–569. ( 10.1111/cas.13184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh K, Capell BC. 2016. The senescence-associated secretory phenotype: critical effector in skin cancer and aging. J. Invest. Dermatol. 136, 2133–2139. ( 10.1016/j.jid.2016.06.621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Godwin JW, Pinto AR, Rosenthal NA. 2013. Macrophages are required for adult salamander limb regeneration. Proc. Natl Acad. Sci. USA 110, 9415–9420. ( 10.1073/pnas.1300290110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrie TA, Strand NS, Yang CT, Rabinowitz JS, Moon RT. 2014. Macrophages modulate adult zebrafish tail fin regeneration. Development 141, 2581–2591. ( 10.1242/dev.098459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326. ( 10.1016/j.cell.2014.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gosselin D, et al. 2014. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340. ( 10.1016/j.cell.2014.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoeffel G, Ginhoux F. 2015. Ontogeny of tissue-resident macrophages. Front. Immunol. 6, 486 ( 10.3389/fimmu.2015.00486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallner S, et al. 2016. Epigenetic dynamics of monocyte-to-macrophage differentiation. Epigenetics Chromatin 9, 33 ( 10.1186/s13072-016-0079-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stender JD, et al. 2012. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Mol. Cell. 48, 28–38. ( 10.1016/j.molcel.2012.07.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. ( 10.1016/j.it.2004.09.015) [DOI] [PubMed] [Google Scholar]
- 91.Rőszer T. 2015. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015, 816460 ( 10.1155/2015/816460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173. ( 10.4049/jimmunol.164.12.6166) [DOI] [PubMed] [Google Scholar]
- 93.Gensel JC, Zhang B. 2015. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619, 1–11. ( 10.1016/j.brainres.2014.12.045) [DOI] [PubMed] [Google Scholar]
- 94.Sica A, Schioppa T, Mantovani A, Allavena P. 2006. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur. J. Cancer. 42, 717–727. ( 10.1016/j.ejca.2006.01.003) [DOI] [PubMed] [Google Scholar]
- 95.Shechter R, et al. 2009. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6, e1000113 ( 10.1371/journal.pmed.1000113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greenhalgh AD, David S. 2014. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 34, 6316–6322. ( 10.1523/jneurosci.4912-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galtrey CM, Fawcett JW. 2007. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 54, 1–18. ( 10.1016/j.brainresrev.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 98.Antoniades CG, et al. 2012. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 56, 735–746. ( 10.1002/hep.25657) [DOI] [PubMed] [Google Scholar]
- 99.Michalopoulos GK, Bhushan B. 2020. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2, C953–C967. ( 10.1038/s41575-020-0342-4) [DOI] [PubMed] [Google Scholar]
- 100.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. 2011. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326. ( 10.1681/asn.2009060615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shouval DS, et al. 2014. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 40, 706–719. ( 10.1016/j.immuni.2014.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. 1998. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 187, 571–578. ( 10.1084/jem.187.4.571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermöhlen O, Davidson JM, Krieg T, Roers A. 2007. Accelerated wound closure in mice deficient for interleukin-10. Am. J. Pathol. 170, 188–202. ( 10.2353/ajpath.2007.060370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Podaru MN, et al. 2019. Reparative macrophage transplantation for myocardial repair: a refinement of bone marrow mononuclear cell-based therapy. Basic Res. Cardiol. 114, 34 ( 10.1007/s00395-019-0742-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boulter L, et al. 2012. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579. ( 10.1038/nm.2667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borghesan M, et al. 2019. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 27, 3956–3971.e3956. ( 10.1016/j.celrep.2019.05.095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J, et al. 2020. MicroRNA-421-3p-abundant small extracellular vesicles derived from M2 bone marrow-derived macrophages attenuate apoptosis and promote motor function recovery via inhibition of mTOR in spinal cord injury. J. Nanobiotechnol. 18, 72 ( 10.1186/s12951-020-00630-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. 2013. Local arginase 1 activity is required for cutaneous wound healing. J. Invest. Dermatol. 133, 2461–2470. ( 10.1038/jid.2013.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kratochvill F, et al. 2015. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 12, 1902–1914. ( 10.1016/j.celrep.2015.08.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang W, et al. 2019. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 10, 1076 ( 10.1038/s41467-019-09046-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang H, Shao R, Huang H, Wang X, Rong Z, Lin Y. 2019. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPɑ axis. Cancer Med. 8, 4245–4253. ( 10.1002/cam4.2332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramachandran P, et al. 2012. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195. ( 10.1073/pnas.1119964109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dal-Secco D, et al. 2015. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 212, 447–456. ( 10.1084/jem.20141539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding J, Lei L, Liu S, Zhang Y, Yu Z, Su Y, Ma X. 2019. Macrophages are necessary for skin regeneration during tissue expansion. J. Transl. Med. 17, 36 ( 10.1186/s12967-019-1780-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mevorach D, Trahtemberg U, Krispin A, Attalah M, Zazoun J, Tabib A, Grau A, Verbovetski-Reiner I. 2010. What do we mean when we write ‘senescence’, ‘apoptosis’, ‘necrosis’, or ‘clearance of dying cells’? Ann. N Y Acad. Sci. 1209, 1–9. ( 10.1111/j.1749-6632.2010.05774.x) [DOI] [PubMed] [Google Scholar]
- 116.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660. ( 10.1038/nature05529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ovadya Y, et al. 2018. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 ( 10.1038/s41467-018-07825-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Irvine KM, et al. 2014. Senescent human hepatocytes express a unique secretory phenotype and promote macrophage migration. World J. Gastroenterol. 20, 17 851–17 862. ( 10.3748/wjg.v20.i47.17851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lujambio A, et al. 2013. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460. ( 10.1016/j.cell.2013.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ben-Mordechai T, et al. 2013. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 62, 1890–1901. ( 10.1016/j.jacc.2013.07.057) [DOI] [PubMed] [Google Scholar]
- 121.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. 2014. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 46, e70 ( 10.1038/emm.2013.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. 2014. Macrophages are required for neonatal heart regeneration. J Clin Invest. 124, 1382–1392. ( 10.1172/jci72181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang TW, et al. 2011. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551. ( 10.1038/nature10599) [DOI] [PubMed] [Google Scholar]
- 124.Eggert T, et al. 2016. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30, 533–547. ( 10.1016/j.ccell.2016.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Da Silva-Álvarez S, Guerra-Varela J, Sobrido-Cameán D, Quelle A, Barreiro-Iglesias A, Sánchez L, Collado M. 2020. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 19, e13052 ( 10.1111/acel.13052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Egashira M, et al. 2017. F4/80+ macrophages contribute to clearance of senescent cells in the mouse postpartum uterus. Endocrinology 158, 2344–2353. ( 10.1210/en.2016-1886) [DOI] [PubMed] [Google Scholar]
- 127.Covarrubias AJ, et al. 2020. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2, 1265–1283. ( 10.1038/s42255-020-00305-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hall BM, et al. 2016. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 8, 1294–1315. ( 10.18632/aging.100991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang C, et al. 2015. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612 ( 10.1126/science.aaa5612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biran A, Perelmutter M, Gal H, Burton DG, Ovadya Y, Vadai E, Geiger T, Krizhanovsky V. 2015. Senescent cells communicate via intercellular protein transfer. Genes Dev. 29, 791–802. ( 10.1101/gad.259341.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. 2016. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY). 8, 328–344. ( 10.18632/aging.100897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Tuyn J, et al. 2017. Oncogene-expressing senescent melanocytes up-regulate MHC class II, a candidate melanoma suppressor function. J. Invest. Dermatol. 137, 2197–2207. ( 10.1016/j.jid.2017.05.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frescas D, et al. 2017. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl Acad. Sci. USA 114, E1668–e1677. ( 10.1073/pnas.1614661114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hall BM, et al. 2017. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 9, 1867–1884. ( 10.18632/aging.101268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cudejko C, et al. 2011. p16INK4a deficiency promotes IL-4-induced polarization and inhibits proinflammatory signaling in macrophages. Blood 118, 2556–2566. ( 10.1182/blood-2010-10-313106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fane M, Weeraratna AT. 2020. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer. 20, 89–106. ( 10.1038/s41568-019-0222-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pereira BI, et al. 2019. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 10, 2387 ( 10.1038/s41467-019-10335-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. 2008. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031. ( 10.1016/j.cell.2008.03.039) [DOI] [PubMed] [Google Scholar]
- 139.Puzianowska-Kuźnicka M, et al. 2016. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing 13, 21 ( 10.1186/s12979-016-0076-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rodier F, et al. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979. ( 10.1038/ncb1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tonnessen-Murray CA, et al. 2019. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 218, 3827–3844. ( 10.1083/jcb.201904051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghosh D, Mejia Pena C, Quach N, Xuan B, Lee AH, Dawson MR. 2020. Senescent mesenchymal stem cells remodel extracellular matrix driving breast cancer cells to a more-invasive phenotype. J. Cell Sci. 133, jcs232470 ( 10.1242/jcs.232470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y, Chaffee TS, LaRue RS, Huggins DN, Witschen PM, Ibrahim AM, Nelson AC, Machado HL, Schwertfeger KL. 2020. Tissue-resident macrophages promote extracellular matrix homeostasis in the mammary gland stroma of nulliparous mice. Elife 9, e57438 ( 10.7554/eLife.57438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Covarrubias AJ, et al. 2020. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2, 1265–1283. ( 10.1038/s42255-020-00305-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nikolich-Žugich J. 2018. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19. ( 10.1038/s41590-017-0006-x) [DOI] [PubMed] [Google Scholar]
- 146.Tomasetti C, et al. 2019. Cell division rates decrease with age, providing a potential explanation for the age-dependent deceleration in cancer incidence. Proc. Natl Acad. Sci. USA 116, 20 482–20 488. ( 10.1073/pnas.1905722116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. ( 10.1038/nri2506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruhland MK, et al. 2016. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 7, 11762 ( 10.1038/ncomms11762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, Nelson DJ. 2017. Aging and cancer: the role of macrophages and neutrophils. Ageing Res. Rev. 36, 105–116. ( 10.1016/j.arr.2017.03.008) [DOI] [PubMed] [Google Scholar]
- 150.Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. 2013. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 32, 1971–1977. ( 10.1038/onc.2012.206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moisan E, Chiasson S, Girard D. 2007. The intriguing normal acute inflammatory response in mice lacking vimentin. Clin. Exp. Immunol. 150, 158–168. ( 10.1111/j.1365-2249.2007.03460.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moisan E, Girard D. 2006. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc. Biol. 79, 489–498. ( 10.1189/jlb.0405190) [DOI] [PubMed] [Google Scholar]
- 153.Starr AE, Bellac CL, Dufour A, Goebeler V, Overall CM. 2012. Biochemical characterization and N-terminomics analysis of leukolysin, the membrane-type 6 matrix metalloprotease (MMP25): chemokine and vimentin cleavages enhance cell migration and macrophage phagocytic activities. J. Biol. Chem. 287, 13 382–13 395. ( 10.1074/jbc.M111.314179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boilard E, Bourgoin SG, Bernatchez C, Surette ME. 2003. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood 102, 2901–2909. ( 10.1182/blood-2002-12-3702) [DOI] [PubMed] [Google Scholar]
- 155.Ise H, Goto M, Komura K, Akaike T. 2012. Engulfment and clearance of apoptotic cells based on a GlcNAc-binding lectin-like property of surface vimentin. Glycobiology 22, 788–805. ( 10.1093/glycob/cws052) [DOI] [PubMed] [Google Scholar]
- 156.Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. 2006. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc. Natl Acad. Sci. USA 103, 11 258–11 263. ( 10.1073/pnas.0600721103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. 2007. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J. Immunol. 178, 3418–3426. ( 10.4049/jimmunol.178.6.3418) [DOI] [PubMed] [Google Scholar]
- 158.Stern-Ginossar N, et al. 2007. Intercellular transfer of carcinoembryonic antigen from tumor cells to NK cells. J. Immunol. 179, 4424–4434. ( 10.4049/jimmunol.179.7.4424) [DOI] [PubMed] [Google Scholar]
- 159.Aw D, Silva AB, Palmer DB. 2007. Immunosenescence: emerging challenges for an ageing population. Immunology 120, 435–446. ( 10.1111/j.1365-2567.2007.02555.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sidler C, Kovalchuk O, Kovalchuk I. 2017. Epigenetic regulation of cellular senescence and aging. Front. Genet. 8, 138 ( 10.3389/fgene.2017.00138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fani Maleki A, Rivest S. 2019. Innate immune cells: monocytes, monocyte-derived macrophages and microglia as therapeutic targets for Alzheimer's disease and multiple sclerosis. Front. Cell Neurosci. 13, 355 ( 10.3389/fncel.2019.00355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weinberger T, Schulz C. 2015. Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front. Physiol. 6, 107 ( 10.3389/fphys.2015.00107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cassetta L, Pollard JW. 2018. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904. ( 10.1038/nrd.2018.169) [DOI] [PubMed] [Google Scholar]
- 164.Udalova IA, Mantovani A, Feldmann M. 2016. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 12, 472–485. ( 10.1038/nrrheum.2016.91) [DOI] [PubMed] [Google Scholar]
- 165.Kwong LS, Brown MH, Barclay AN, Hatherley D. 2014. Signal-regulatory protein α from the NOD mouse binds human CD47 with an exceptionally high affinity--implications for engraftment of human cells. Immunology 143, 61–67. ( 10.1111/imm.12290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lv Z, et al. 2015. Loss of cell surface CD47 clustering formation and binding avidity to SIRPα facilitate apoptotic cell clearance by macrophages. J. Immunol. 195, 661–671. ( 10.4049/jimmunol.1401719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. 2006. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood 107, 2548–2556. ( 10.1182/blood-2005-04-1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. 2008. Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood 112, 1280–1289. ( 10.1182/blood-2008-01-134429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bergström SE, Bergdahl E, Sundqvist KG. 2013. A cytokine-controlled mechanism for integrated regulation of T-lymphocyte motility, adhesion and activation. Immunology 140, 441–455. ( 10.1111/imm.12154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kojima Y, et al. 2014. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J. Clin. Invest. 124, 1083–1097. ( 10.1172/jci70391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Feng M, et al. 2018. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat. Commun. 9, 3194 ( 10.1038/s41467-018-05211-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ackermann M, Kuhn A, Kunkiel J, Merkert S, Martin U, Moritz T, Lachmann N. 2017. Ex vivo generation of genetically modified macrophages from human induced pluripotent stem cells. Transfus Med. Hemother. 44, 135–142. ( 10.1159/000477129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De La Fuente M. 1985. Changes in the macrophage function with aging. Comp. Biochem. Physiol. A Comp. Physiol. 81, 935–938. ( 10.1016/0300-9629(85)90933-8) [DOI] [PubMed] [Google Scholar]
- 174.Mancuso P, McNish RW, Peters-Golden M, Brock TG. 2001. Evaluation of phagocytosis and arachidonate metabolism by alveolar macrophages and recruited neutrophils from F344xBN rats of different ages. Mech. Ageing Dev. 122, 1899–1913. ( 10.1016/s0047-6374(01)00322-0) [DOI] [PubMed] [Google Scholar]
- 175.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. 2014. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell 13, 699–708. ( 10.1111/acel.12223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharma AK, Roberts RL, Benson RD Jr, Pierce JL, Yu K, Hamrick MW, McGee-Lawrence ME. 2020. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front. Cell Dev. Biol. 8, 354 ( 10.3389/fcell.2020.00354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. 2018. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582. ( 10.1038/s41586-018-0543-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abashev TM, Metzler MA, Wright DM, Sandell LL. 2017. Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium. Dev. Dyn. 246, 135–147. ( 10.1002/dvdy.24476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hickson LJ, et al. 2019. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMed. 47, 446–456. ( 10.1016/j.ebiom.2019.08.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Justice JN, et al. 2019. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMed. 40, 554–563. ( 10.1016/j.ebiom.2018.12.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cai Y, et al. 2020. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 30, 574–589. ( 10.1038/s41422-020-0314-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dalmas E, et al. 2015. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat. Med. 21, 610–618. ( 10.1038/nm.3829) [DOI] [PubMed] [Google Scholar]
- 183.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. 2011. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 17, 64–70. ( 10.1038/nm.2266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. 2011. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl Acad. Sci. USA 108, 1827–1832. ( 10.1073/pnas.1015623108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shah PP, et al. 2013. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799. ( 10.1101/gad.223834.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tasdemir N, et al. 2016. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 6, 612–629. ( 10.1158/2159-8290.Cd-16-0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuwahara M, et al. 2014. The Menin-Bach2 axis is critical for regulating CD4 T-cell senescence and cytokine homeostasis. Nat. Commun. 5, 3555 ( 10.1038/ncomms4555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fafián-Labora JA, O'Loghlen A. 2020. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 30, 628–639. ( 10.1016/j.tcb.2020.05.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.