Abstract
The a disintegrin-like and metalloproteinase with thrombospondin motif (ADAMTS) family comprises 19 proteases that regulate the structure and function of extracellular proteins in the extracellular matrix and blood. The best characterized cardiovascular role is that of ADAMTS-13 in blood. Moderately low ADAMTS-13 levels increase the risk of ischeamic stroke and very low levels (less than 10%) can cause thrombotic thrombocytopenic purpura (TTP). Recombinant ADAMTS-13 is currently in clinical trials for treatment of TTP. Recently, new cardiovascular roles for ADAMTS proteases have been discovered. Several ADAMTS family members are important in the development of blood vessels and the heart, especially the valves. A number of studies have also investigated the potential role of ADAMTS-1, -4 and -5 in cardiovascular disease. They cleave proteoglycans such as versican, which represent major structural components of the arteries. ADAMTS-7 and -8 are attracting considerable interest owing to their implication in atherosclerosis and pulmonary arterial hypertension, respectively. Mutations in the ADAMTS19 gene cause progressive heart valve disease and missense variants in ADAMTS6 are associated with cardiac conduction. In this review, we discuss in detail the evidence for these and other cardiovascular roles of ADAMTS family members, their proteolytic substrates and the potential molecular mechanisms involved.
Keywords: ADAMTS, proteoglycans, atherosclerosis, aortic aneurysms, heart valve, cardiovascular
1. Introduction
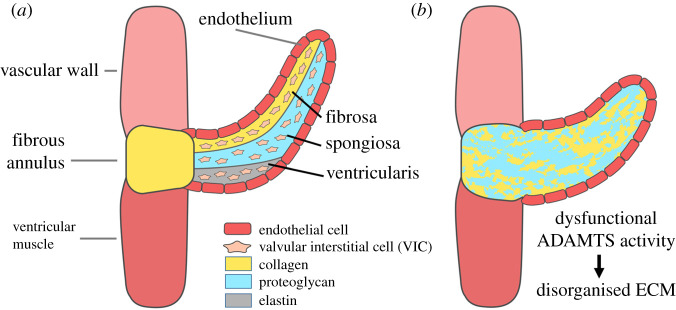
The extracellular matrix (ECM) guides the formation of cardiovascular tissues during embryogenesis and supports it throughout adulthood by providing structural support, guidance of cell behaviour and sequestering of growth factors. In large arteries and blood vessels, collagen and elastic fibres provide essential structural support to prevent rupture. A healthy artery consists of three layers (tunicae): tunica adventitia, media and intima (figure 1). The intima is in contact with the vessel lumen and consists of endothelial cells (ECs) attached to a basal membrane rich in collagen IV, laminin, nidogen and heparan sulfate (HS) proteoglycans (PGs) (syndecan, perlecan). The tunica media is made up of vascular smooth muscle cells (VSMC), which express chondroitin-sulfate (CS)/dermatan-sulfate (DS)-PGs (CSPGs) (such a versican and aggrecan) and elastin. There are fenestrated sheets of elastin called lamellae between which there are collagen fibres, thin layers of PG-rich ECM and VSMCs. Elastin, which is distensible and has a low tensile strength, functions as an elastic reservoir and distributes stress evenly throughout the wall and onto collagen fibres [1]. The adventitia is the outermost layer of a blood vessel and consists of a collagen-rich ECM secreted by fibroblasts that prevents vascular rupture at very high pressures. The adventitia is also a reservoir of stem cells and plays an essential role in regulating the functions of cell populations in the tunica intima and media [2]. Various PGs are present in the different layers. In the tunica media, they contribute to the viscoelastic properties of the vessel wall. This function of PGs is mediated by their glycosaminoglycan (GAG) chains. Owing to their high negative charge, these GAGs attract counter-ions and water into the tissue [3,4]. Moreover, CSPGs such as versican and aggrecan are essential components of cardiac valve leaflets and, in particular, the middle ECM layer, the spongiosa, while elastin and collagens predominate in the ventricularis/atrialis and fibrosa layers, respectively [5,6] (figure 2). The ratios of PGs and the interspersed collagens/elastic fibres provide a means of balance between stiffness and flexibility of the cardiovascular tissues [7]. For this reason, remodelling of the vascular ECM by proteases secreted by both ECs and VSMCs is crucial to establish the mechanical properties of these tissues. Moreover, ECM degradation is required for migration and proliferation of VSMCs, as well as infiltration of inflammatory cells under pathological conditions. Among the proteases involved in this dynamic action, members of the matrix metalloproteinases (MMP) family have been extensively investigated owing to their ability to cleave the elastic ECM components such as collagens (MMP-1, -8, -13, -14) and elastin (MMP-12). In addition, proteases of the related family of a disintegrin and metalloproteinases (ADAMs) exert a fundamental role in the vascular ECM owing to their ability to selectively cleave the ectodomain of membrane proteins (shedding). The role of these metalloproteinase families in cardiovascular disorders has been exhaustively reviewed elsewhere [8,9].
Figure 1.
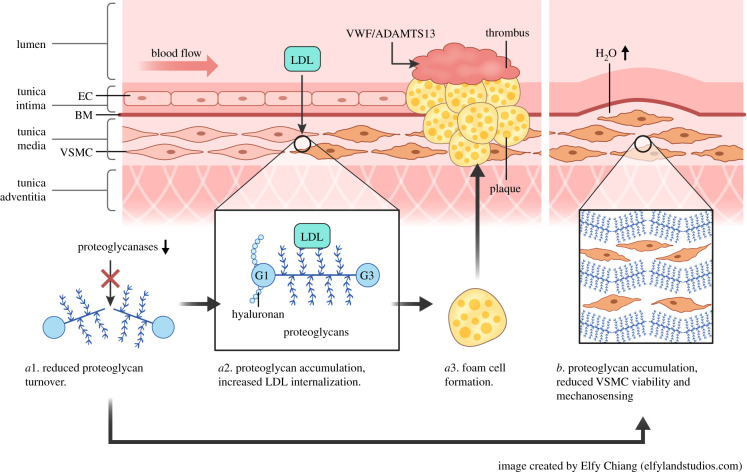
Involvement of ADAMTS proteases in cardiovascular physiology and disease. Atherosclerosis and haemostasis: decreased proteoglycanase activity (a1) is associated with proteoglycan accumulation, increased low-density lipoprotein (LDL) internalization (a2) and foam cell formation (a3), which eventually leads to formation of an atherosclerotic plaque. A thrombus can form on top of a plaque if collagen is exposed to the blood. ADAMTS-13 regulates the capacity of von Willebrand Factor (VWF) to recruit platelets to the site of collagen exposure and initiate thrombus formation. In the aorta, reduced proteoglycanase activity (a1) causes proteoglycan accumulation, increased osmotic pressure, reduced viability of vascular smooth muscle cells (VSMC) and disruption of mechanosensing (b). BM, basal membrane; EC, endothelial cell; VWF, von Willebrand factor.
Figure 2.
Extracellular matrix (ECM) organization in cardiac valves. (a) The ECM composition in a mature valve is shown. The formation of the ventricularis and the fibrosa during development is a complex and tightly regulated molecular process in which ADAMTS proteases play an essential role. (b) Dysfunctional ADAMTS activity can lead to a certain degree of disorganized ECM, altered valve shape and leakage of the valve. Disorganized ECM can present as an abundance of proteoglycans, which can be owing to insufficient proteoglycanase activity or possibly owing to incorrect assembly of the ECM in the ventricularis (e.g. fibrillin-1 microfibrils/elastin). ADAMTS-1, -5 and -9 have been implicated in cardiac valve development by mouse studies and ADAMTS-19 by a human genetic disease.
This review focuses on a closely related metalloprotease family, the a disintegrin-like and metalloproteinase with thrombospondin motif (ADAMTS) and their (patho)physiological role in heart and blood vessels. In humans, the ADAMTS family comprises 19 secreted metalloproteinases as well as 7 ADAMTS-like proteins devoid of catalytic activity [10]. They share a common domain composition consisting of a signal peptide, a prodomain, a metalloproteinase catalytic domain (Mp, absent in the ADAMTS-like proteins), followed by non-catalytic ancillary domains such as a disintegrin-like (Dis) domain, a central thrombospondin-type I motif (TSR), a cysteine-rich (CR) domain, a spacer (Sp) domain, and, with the exception of ADAMTS-4, a various number of TSRs at the C-terminus (table 1). Some members have additional C-terminal domains, such as a mucin domain (present in ADAMTS-7 and ADAMTS-12), a Gon-1 like domain (in ADAMTS-20 and ADAMTS-9) and a PLAC (protease and lacunin) domain (in ADAMTS-2, -3, -6, -10, -12, 14, -16, -17, -18 and -19). Moreover, ADAMTS-13 is unique among ADAMTS proteases since it presents two CUB [complement subcomponent C1r/C1s/embryonic sea urchin protein Uegf (urchin epidermal growth factor)/bone morphogenic protein 1] domains [11,12]. A common feature of the family is the presence of a zinc-binding motif in the Mp domain, containing the consensus sequence HEXXHXXGXXH, in which the three underlined histidine residues coordinate a zinc atom, which together with a glutamate residue, exerts a catalytic role. This motif is followed C-terminally by a methionine residue that constitutes a structural turn (Met-turn) conserved within the metzincin family of metallopeptidases (comprising MMPs, ADAMs, ADAMTSs and astacins) [13].
Table 1.
Summary of the cardiovascular roles, proteolytic functions and domain organization of the ADAMTS family. For the cardiovascular roles and diseases, the numbers in brackets indicate specific ADAMTS family members. CAD, coronary artery disease; PAH, pulmonary arterial hypertension; TAAD, thoracic aortic aneurysms and dissections; TTP, thrombotic thrombocytopenic purpura; VSMC, vascular smooth muscle cells; VWF, von Willebrand factor; WMS, Weill-Marchesani syndrome. The ADAMTS protein domains are abbreviated as follows: S, signal peptide; Pro, prodomain; Mp, metalloproteinase domain; Dis, disintegrin-like domain; CR, cysteine-rich domain; Sp, spacer domain; Muc, mucin-like domain; CUB, complement subcomponent C1r/C1s/embryonic sea urchin protein Uegf (urchin epidermal growth factor)/bone morphogenic protein 1; PL, protease and lacunin domain; GON, gon-1 like domain.
| proteolytic function | family members | domain organisation | cardiovascular role | disease |
|---|---|---|---|---|
| proteoglycanases | ADAMTS-1/15 |  |
regulation of PG turnover, LDL internalisation (5), development of cardiovascular system (1, 5, 9) | atherosclerosis (4) TAAD (1, 4, 5) PAH (8) |
| ADAMTS-4 |  |
|||
| ADAMTS-5/8 |  |
|||
| ADAMTS-9/20 |  |
|||
| procollagen N-propeptidases | ADAMTS-2/3/14 |  |
assembly of collagen fibrils myocardial repair (2) |
|
| unknown | ADAMTS-6/10 |  |
assembly of fibrillin microfibrils (10) | WMS (10) prolonged QRS (6) |
| unknown | ADAMTS-7/12 |  |
VSMC migration and re-endothelialisation (7) | CAD (7) |
| VWF-cleaving protease | ADAMTS-13 |  |
haemostasis | TTP, stroke |
| unknown | ADAMTS-16/18 |  |
regulation of blood pressure (16) | - |
| unknown | ADAMTS-17/19 |  |
assembly of fibrillin microfibrils (17) | WMS (17) heart valve disease (19) |
ADAMTS proteases are generally activated following proteolytic removal of the N-terminal prodomain by subtilisin-type proprotein convertases (e.g. Furin, PCSK6) [14]. With the exception of ADAMTS-13, which circulates in blood, the other ADAMTS family members appear to function in the ECM. The secreted activated enzymes are mainly regulated through inhibition by tissue inhibitors of metalloproteinases (TIMPs) [15] and endocytosis [16].
Distinct ADAMTS subfamilies can be defined on the basis of sequence homology (table 1). They likely evolved through a process of gene duplication resulting in either neo-functionalization or sub-functionalization [17] and some still share a common substrate repertoire.
ADAMTS-1, -4, -5, -8 and -15 are characterized by their ability to cleave PGs and are therefore collectively named ‘proteoglycanases'. Although more distantly related, ADAMTS-9 and -20 also exhibit this distinct proteolytic activity.
ADAMTS-2 is a well-characterized procollagen N-propeptidase that cleaves the N-terminal propeptides of type I, II and III collagen [18]. ADAMTS-3 and ADAMTS-14 show a high degree of homology with ADAMTS-2 and can cleave pro-collagen in vitro. ADAMTS-3 is essential for lymphangiogenesis through activation of vascular endothelial growth factor (VEGF)-C [19,20].
The related pairs ADAMTS7/12, ADAMTS6/10, ADAMTS16/18 and ADAMTS17/19 are not well characterised [21]. ADAMTS10 and ADAMTS17 mutations give rise to Weill-Marchesani syndrome (WMS)-like spectrum and have been functionally associated with the assembly of fibrillin microfibrils [21]. ADAMTS-6 has also been shown to promote fibrillin-1 microfibril formation [22].
ADAMTS-13, by far the best characterized family member, regulates the function of von Willebrand Factor (VWF) in primary haemostasis [23,24].
Whereas non-cardiovascular roles of the different ADAMTS family members have been discussed in recent excellent reviews [10,14], here, we will discuss in detail the involvement of ADAMTS proteases in cardiovascular biology and disease.
2. Proteoglycans and proteoglycanases in cardiovascular physiology and disease
2.1. Proteoglycans
Several ADAMTS family members specifically cleave PGs. Because ‘The biology of a protease is really the biology of its substrates' [14], we will briefly discuss the role of PGs in the cardiovascular system. In blood vessels, PGs are mainly expressed by ECs and VSMCs in the tunica intima and media, respectively, where they regulate the biophysical properties of the ECM [3,19]. Moreover, through their interactions with ECM proteins, growth factors and chemokines, PGs regulate a variety of processes such as cell signalling, proliferation, migration and apoptosis [25]. Increased accumulation of PGs is a feature of atherosclerosis [26,27] and aneurysms [28], as well as hereditary diseases such as pediatric aortic valve disease and adult myxomatous mitral valves [29].
PGs are classed according to the predominant GAG covalently attached to serine residues in their protein core, heparin/heparan sulfate (HS) PGs and chondroitin-sulfate/dermatan sulfate (CS/DS) PGs. CS-GAGs contain D-glucuronic acid and N-acetyl-D-galactosamine, whereas in DS-GAGs, the D-glucuronic acid is epimerized into L-iduronic acid. HS-GAGs contain D-glucuronic acid or L-iduronic acid alternating with N-acetyl-D-glucosamine. GAGs are not only important to generate a Donnan osmotic pressure in the vascular tissue owing to their negative charges [4] but also to mediate lipoprotein uptake from the circulation.
Sub-intimal accumulation of lipid-rich and inflammatory deposits (plaques) in medium and large arteries is a hallmark of atherosclerosis (figure 1a). The enlargement of plaques hampers the normal blood flow, leading to organ ischemia and tissue necrosis. Plaque rupture with subsequent thrombus formation can cause vascular occlusion leading to potentially fatal cardiovascular events such as myocardial infarction and stroke. PGs such as versican and aggrecan also tend to accumulate in thoracic aortic aneurysms and dissections (TAAD) [28,30,31] and during normal ageing of the aorta [32], resulting in an increased osmotic pressure that is disruptive to the ECM (figure 1b). Moreover, PG accumulation can disrupt mechanosensing by VSMCs and create stress-risers in the aortic wall that may predispose to or propagate a dissection [33].
HSPGs such as perlecan and the four transmembrane syndecans are synthesized mainly by ECs in the intima and inhibit pro-atherogenic processes such as lipoprotein retention, infiltration of inflammatory cells, proliferation of VSMCs and thrombosis [34,35]. Since HSPGs are not major ADAMTS substrates, they will not be discussed in detail here. We will instead focus on CSPGs.
CSPGs are mainly expressed by VSMCs in the tunica media [25]. In contrast with HSPGs, CSPGs may initiate atherosclerotic processes by enhancing both deposition and internalization of low-density lipoprotein (LDL) particles that have penetrated into the arterial wall after transcytosis or endothelial dysfunction [27,36] (figure 1a). CSPGs can form high-affinity complexes with LDL particles, which are then internalized more efficiently by VSMCs and infiltrated macrophages than native LDLs [37,38]. Once internalized, LDLs enhance intracellular cholesteryl ester synthesis and subsequent foam formation [39]. The interaction between lipoproteins and CSPGs involves an ionic bond between basic amino acids in apoprotein B (apoB) and negatively charged sulfate groups on the GAGs [40]. Moreover, the longer the CS chains, the higher the affinity for LDL [41]. To support the notion that the direct binding of LDL particles to CSPGs is a key step in atherogenesis, transgenic mice expressing LDL particles where positively charged amino acids in apoB had been replaced with neutral ones exhibited significantly less atherosclerotic lesions than mice expressing wild-type apoB [42]. CSPGs comprise large aggregating PGs such as versican and aggrecan and small leucine-rich PGs (SLRPs) such as biglycan and decorin. Owing to their ability to bind both hyaluronan, the only non-sulfated GAG, and lectins, large aggregating PGs are also called hyalectans [43]. Hyalectans have a similar structure, comprising two globular domains at the N- and C-terminus, named G1 and G3, respectively, and a central GAG domain containing attachment sites for GAG chains. The G1 domain binds to hyaluronan, whereas the G3 domain contains the lectin-binding region (figure 1a).
Versican is the main hyalectan in the vasculature, where it plays a role in developmental and repair processes such as cell adhesion, proliferation and migration, ECM assembly and inflammation [25,26,44]. It is present in 5 isoforms (V0-V4), generated by alternative splicing within the central GAG-rich region. Expression of versican is essential for normal development of heart and blood vessels [45,46].Versican is involved in various aspects of vascular lesion development and is present in atherosclerotic plaques, restenotic lesions, lesions arising during graft repair and aneurysmal lesions [27].Versican levels increase dramatically in atherosclerosis [27,47], suggesting that its accumulation may in part be responsible for increased LDL deposition/internalization in the vessel wall (figure 1a).
Aggrecan, a major CSPG in cartilage, has been recently identified in human aortas [28,30,31,48–53] and, together with versican, it has been shown to accumulate in the aortas of patients with TAAD, resulting in an increased osmotic pressure that is disruptive to the ECM [30] (figure 1b). Here, it is important to note that aggrecan has an order of magnitude more CS than versican [54], and therefore more potential to exert osmotic pressure. In addition to CS, aggrecan also contains keratan sulfate (KS) GAGs (where the D-galactose replaces hexuronic acid) clustered into a KS-rich region [54].
The protein core of SLRPs such as biglycan is small (40–60 kDa) and characterized by the presence of 11–12 leucine-rich tandem repeats and the attachment of 1–2 CS/DS GAG chains [55]. The leucine-rich tandem repeats bind to collagens, thus regulating collagen fibril formation [56–58].
Biglycan is one of the major PGs found in human atherosclerotic lesions [59–62]. Like aggrecan and versican, biglycan binds to LDL particles, although with reduced affinity owing to the lower number of GAG chains [63]. Bgn knockout mice did not show reduced LDL retention in arterial wall, most likely owing to compensation from other CSPGs [64]. However, overexpression of biglycan in mice increased arterial retention of apoB lipoproteins and promoted atherosclerosis [62,65]. Biglycan may also exert an anti-atherosclerotic function. In mice genetically susceptible to develop atherosclerotic lesions (already bearing deletion of either apolipoprotein-E, ApoE, or LDL-receptor, LDLR), abolishing biglycan expression increased macrophage-mediated plaque inflammation [64]. Biglycan is also considered as an early initiator of aortic stenosis lesion, contributing to wall thickening [66]. Furthermore, biglycan has been implicated in the formation of TAAD, since Bgn/LDLR double knockout mice showed increased incidence of TAAD [67] and loss of function mutations in Bgn results in syndromic early-onset TAAD in humans [68]. Bgn knockout mice also showed spontaneous abdominal aortic aneurysms (AAA) [69]. Since biglycan deficiency impairs the formation of collagen fibres in the aortic wall and contributes to the breakdown of elastic fibres [67,70], this will affect the ability of the vessel to sustain tension forces.
2.2. Proteoglycanases
Physiological levels of PGs are regulated by the proteoglycanase activity of several ADAMTS family members. ADAMTS-1, -4, -5, -8, -9, -15 and -20 have been shown to cleave, albeit to a different extent, both versican [71–75] and aggrecan [76]. The only ADAMTS cleavage of versican V1 isoform described to date occurs at the Glu441↓442Ala bond within the βGAG domain [71–75]. The equivalent cleavage sites in the αGAG region in the V0 and V2 isoforms have been identified as Glu1428↓1429Ala [71] and Glu405↓406Gln, respectively [77]. Aggrecan is cleaved by ADAMTS proteases at multiple sites [78], although the cleavage event most detrimental for its function occurs at the Glu392↓Ala393 bond (Uniprot P16112 numbering) in the region between the G1 and G2 domains [79]. Importantly, ADAMTS-mediated cleavage of both aggrecan and versican has been shown to release bioactive fragments. The G1-DPEAAE441 versican V1 cleavage fragment, named versikine, is involved in a variety of biological processes such as immune signalling [80] and apoptosis [81], while the G1-NIVSFE405 V2 cleavage fragment has been identified as a hyaluronan-binding protein previously identified in human brain [77]. A 32-amino acid long aggrecan fragment generated by MMP-mediated cleavage at Asn360↓Phe361 and ADAMTS-mediated cleavage at Glu392↓Ala393 has been shown to interact with toll-like receptor-2 and excite nociceptive neurons in chondrocytes [82,83]. Following ADAMTS-cleavage at Glu392↓Ala393, the 393ARGS neopeptide can diffuse from the ECM into plasma, urine and synovial fluid [84,85]. Among the aforementioned ADAMTS proteases, ADAMTS-4 and -5 show the strongest proteolytic activity against both aggrecan [86,87] and versican [88]; moreover, they have also been shown to cleave the SLRP biglycan at Asn186↓187Cys [86,89]. This proteoglycanase activity exerts important functions in vascular biology and may contribute to cardiovascular diseases such as atherosclerosis and aneurysms, as outlined in the following sections.
2.2.1. ADAMTS-1: friend (in TAAD) or foe (in atherosclerosis)?
ADAMTS-1 was the first member of the family to be described [90]. Early murine knockout models showed the involvement of this enzyme in fertilization and the development of the urogenital system [91–94], but it was soon recognized that ADAMTS-1 plays an important cardiovascular role. The substrate repertoire of ADAMTS-1 appears to extend beyond the CSPGs aggrecan [95] and versican [88]. Reported in vitro substrates include nidogen-1 and -2 [96,97], semaphorin 3C [98], Tissue factor pathway inhibitor (TFPI)-2 [99], insulin growth factor binding protein (IGFBP)-2 [100], syndecan-4 [101] and thrombospondins 1 and 2 [98,102]. ADAMTS-1 versicanase activity in vitro is considerably weaker than that of either ADAMTS-4 or ADAMTS-5 [88] but appears biologically important in certain developmental processes such folliculogenesis [94]. Mouse studies suggest that ADAMTS-1 versicanase activity within the endocardial cushion contributes to valve maturation and myocardial trabeculation [103,104]. However, ADAMTS-1 proteolysis of non-PG substrates or non-proteolytic activities may be partially responsible for these phenotypes.
In the blood vessels, ADAMTS-1, like ADAMTS-4 and -5, is expressed by ECs, VSMCs and invading macrophages [105–108]. ADAMTS-1 expression was upregulated in human atherosclerotic lesions [105] and its versicanase activity may contribute to plaque instability [71,105]. Transgenic mice overexpressing Adamts1 on an ApoE−/− background were investigated by Jönsson-Rylander et al. [107], but measurements of atherosclerotic lesion formation were not reported. However, carotid artery ligation in these mice showed a significant increase in neo-intima formation, suggesting an effect of ADAMTS-1 on VSMC migration/proliferation (table 2). In support of this, two miRNAs targeting ADAMTS1, miR-265b-3p and miR-362–3p, appeared to inhibit VSMC proliferation and migration [123,124].
Table 2.
Adamts knockout mice and overexpression in vivo. AB, aortic banding-induced cardiac hypertrophy; BAV, bicuspid aortic valve; AngII, angiotensin II; PAH, pulmonary arterial hypertension; PPS, pentosan polysulfate; TAAD, thoracic aortic aneurysms and dissections. Genetic manipulation is in mice if not otherwise specified.
| enzyme | human disease modelled | intervention | genetic manipulation | effect | reference |
|---|---|---|---|---|---|
| ADAMTS-1 | arterial injury | carotid artery ligation | transgenic Adamts1 overexpression on Apoe−/− background | enhanced intimal thickening | [107] |
| TAAD | AngII | Adamts1+/− | increased incidence of aneurysm and mortality, hypotension, medial degeneration | [109] | |
| TAAD | AngII | lentivirus-mediated Adamts1 knockout | increased incidence of aneurysm and mortality, hypotension, medial degeneration | [109] | |
| ADAMTS-2 | cardiac hypertrophy | AB | Adamts2−/− | increased cardiac hypertrophy, fibrosis and dysfunction | [110] |
| cardiac hypertrophy | AB | transgenic Adamts2 overexpression | decreased cardiac hypertrophy, fibrosis and dysfunction | [110] | |
| ADAMTS-4 | atherosclerosis | high fat diet | Adamts4−/−; ApoE−/− | increased plaque stability; decreased lipid deposition; decreased macrophage infiltration; decreased versican and aggrecan degradation | [111] |
| TAAD | high fat diet/AngII | Adamts4−/− | reduced incidence of aneurysm and mortality, decreased aortic destruction and versican degradation, decreased macrophage infiltration, decreased VSMC apoptosis | [112] | |
| ADAMTS-5 | TAAD | AngII | Adamts5−/− | reduced blood pressure, increased aortic dilation, accumulation of versican | [50] |
| — | — | Adamts5−/− | aortic and pulmonary valve anomalies, BAV, accumulation of aggrecan and versican | [51,53,113,114] | |
| ADAMTS-6 | — | — | Adamts6−/− | congenital heart defects such as double outlet right ventricle, ventricular hypertrophy, atrial and ventricular septal defects |
[115] |
| ADAMTS-7 | atherosclerosis | high fat diet | Adamts7−/− ApoE−/− | reduced atherosclerotic lesion area | [116] |
| vascular injury | wire injury | Adamts7−/− | reduced neo-intima formation, increased re-endothelialisation | [116,117] | |
| vascular injury | balloon injury | siRNA-mediated Adamts7 knockdown in rats | reduced intimal thickening | [118] | |
| vascular injury | balloon injury | transgenic Adamts7 overexpression in rats | increased intimal thickening | [118] | |
| ADAMTS-8 | PAH | hypoxia-induced PAH | Adamts8ΔSM22α | decreased right ventricular systolic pressure and right ventricular hypertrophy | [119] |
| PAH | hypoxia-induced PAH | Adamts8ΔαMHC | decreased cardiac hypertrophy, fibrosis and right ventricular dysfunction | [119] | |
| ADAMTS-9 | Adamts9+/− | thickened aortic valve leaflets, myxomatous mitral valves, abnormal myocardial projections and interventricular septae, increased adventitial thickness associated with versican accumulation in the aorta | [120] | ||
| ADAMTS-16 | hypertension | — | Adamts16−/− rats | lower systolic blood pressure, decreased arterial stiffness and thickness of the tunica media | [121] |
| ADAMTS-19 | — | — | Adamts19−/− | aortic regurgitation, aortic stenosis, BAV | [122] |
It has also been reported that angiotensin II (Ang II) and other stimuli associated with vascular remodelling induce the expression of ADAMTS-1 in aorta [125]. Since Adamts1 knockout mice have elevated perinatal mortality [92], heterozygous Adamts1+/− mice were tested in a TAAD model [109]. The Adamts1+/− genotype exacerbated aortic aneurisms and lethal aortic dissections induced by treatment with the hypertensive factor Ang II. Administration of Ang II induced TAAD in nearly 80% of the Adamts1+/− mice and lethal aortic dissections in nearly 50% of these mice, compared with nearly 10 and 8% in wild-type mice [109]. This phenotype resembles that of Fbn1C1039G/+, a mouse model of Marfan syndrome (MFS) characterized by a low incidence of aortic dissections and ruptures compared to other MFS mouse models [126]. In these ‘Marfan mice’, the introduced Fbn1 mutation C1039G disrupts the microfibril scaffold, which has complex knock-on effects (including decreased ADAMTS-1 protein levels) owing to the many mechanistic complexities of the fibrillin microfibril niche and its roles in elastic fibre formation, growth factor signalling and VSMC biology [127,128]. However, in human aortic aneurisms, ADAMTS-1 levels are either unchanged or increased [30,129,130].
In conclusion, these findings suggest that ADAMTS-1 may play a detrimental role in the aetiology of atherosclerosis, whereas further studies are required to establish its involvement in the development of aortopathies.
2.2.2. ADAMTS-4, a potential therapeutic target in atherosclerosis and TAAD
ADAMTS-4 cleaves CSPGs such as versican and aggrecan [87,88], brevican [131] and SLRPs such as fibromodulin [86,87] and biglycan [86,89] as well as non-PG substrates such as cartilage oligomeric matrix protein (COMP) [132].
Although ADAMTS-4, like all other ADAMTS family members, with the exception of ADAMTS-13, is predominantly bound to the ECM [86,87], it may diffuse into plasma following cardiovascular damage. Elevated plasma levels of ADAMTS-4 have been consistently found in patients affected by coronary artery disease (CAD) [129,133–135], atherosclerosis [106,136–138] and TAAD [112,129]. Some of these studies associated elevated ADAMTS-4 plasma levels with increased severity of CAD [134,136] and plaque destabilization [137,138]. Increased ADAMTS-4 levels were also found in macrophage-rich areas of human atherosclerotic plaques and unstable coronary plaques [106,138]. Importantly, these findings in humans are in agreement with those obtained from various mouse models (table 2). ADAMTS-4 levels were shown to increase in the atherosclerotic plaques and plasma of ApoE knockout [111,138] and LDLR−/−; ApoB100/100 [99] double knockout mice as atherosclerosis progressed. Moreover, genetic deletion of Adamts4 in an ApoE knockout background produced a milder atherosclerotic phenotype, with increased plaque stability compared to their littermates [111]. ApoE/Adamts4 double knockout mice also showed reduced cleavage of both versican and aggrecan in arteries, with no compensation shown by other proteoglycanases such as ADAMTS-1 and −5. This was associated with reduced lipid deposition and macrophage infiltration but increased VSMC proliferation [111]. Moreover, in the absence of ADAMTS-4, increased macrophage apoptosis and decreased levels of proinflammatory cytokines were observed [111]. These data suggest that the activity of ADAMTS-4 is associated with more unstable atherosclerotic plaques. Therefore, therapeutic inhibition of ADAMTS-4 may be beneficial at late stages of atherosclerotic development.
Deletion of Adamts4 was shown to significantly reduce aortic diameter enlargement, aneurysm formation, dissection and aortic rupture in a mouse model of sporadic TAAD induced by a high-fat diet and AgII infusion [112]. These phenotypes were associated with decreased macrophage infiltration, VSMC apoptosis and versican degradation [112]. Expression of Adamts4 is increased upon Ang II treatment and injection of miR-126a-5p, a miRNA targeting Adamts4, has been recently shown to reduce aortic dilation and versican degradation as well as increase survival in these mice [139]. Severe downregulation of this miRNA may be one of the mechanisms responsible for the observed upregulation of Adamts4 expression in the Ang II model [139].
In vitro, ADAMTS4 knockdown has been shown to reduce macrophage infiltration [129] and VSMC apoptosis [112], two processes that are critical for the development of aortic aneurysms [140], and plaque rapture [141]. In both cases, the versicanase activity of ADAMTS-4 may play a role. Full-length versican is endowed with adhesive properties and its cleavage by ADAMTS proteoglycanases facilitates the migration of immune cells [80,142]. Moreover, versikine, the ADAMTS-generated versican cleavage fragment, can promote apoptosis [81], thus antagonising the anti-apoptotic effect of full-length versican [143,144]. ADAMTS-4 may also be directly involved in apoptosis of VSMCs after translocation to the nucleus and cleavage of poly ADP ribose polymerase-1 (PARP-1), a key molecule in DNA repair and cell survival [112]. Full-length PARP-1 promotes cell survival, whereas cleaved PARP-1 can induce apoptosis [145]. Finally, ADAMTS-4 has been shown to exert pro-apoptotic effects independently of its catalytic activity [146], suggesting that ADAMTS-4 can induce apoptosis through different mechanisms.
Interestingly, ADAMTS-4 expression was increased in the myocardium of rats subjected to hypertension and addition of pentosan polysulfate inhibited both Adamts4 expression and versican cleavage and ameliorated myocardial function [147].
These clinical observations and in vivo data from mouse models of disease point towards multiple roles of ADAMTS-4 in diseased cardiovascular tissues. Whether therapeutic inhibition of ADAMTS-4 could slow the progression of vascular disease in particular warrants further investigation.
2.2.3. ADAMTS-5 regulates cardiovascular proteoglycan levels
ADAMTS-5 has been extensively studied in the context of aggrecan degradation in cartilage and is a validated target for the treatment of osteoarthritis [148]. More recently, a cardiovascular role has emerged for ADAMTS-5, which has been recently reviewed [148] and will be briefly summarized here.
In vitro, ADAMTS-5 is a more potent proteoglycanase than ADAMTS-1 and -4 [87,88] and the absence of ADAMTS-5 in vivo causes accumulation of cardiovascular PGs (table 2). For example, Adamts5 knockout mice showed severe anomalies in the pulmonary valve cusps owing to decreased versican cleavage and subsequent versican and aggrecan accumulation [53,113,114]; similarly, they exhibited dilation of thoracic aorta with accumulation of aggrecan and biglycan [51]. It would be interesting to compare this phenotype with that of knock-in mice expressing ADAMTS cleavage-resistant versican (i.e. mutated at the Glu1428↓1429Ala site), called V1R mice, but unfortunately their cardiovascular phenotype has not been described [149]. While the majority of V1R mice were viable and fertile, some of them die of organ haemorrahage after backcrossing [149].
ADAMTS-5 was found to be markedly reduced in aortas of ApoE knockout mice, which spontaneously developed atherosclerotic lesions, resulting in accumulation of versican and biglycan [150]. Recombinant ADAMTS-5 reduced the LDL-binding ability of biglycan and released LDL particles from human aortic lesions [150], thus suggesting a role for this enzyme in regulating PG-mediated lipoprotein retention (figure 1a). ADAMTS-5 is expressed in both VSMCs [151,152] and macrophages [106], two cell types that also express versican [151,153–155] and TIMP-3 [156–158], the major inhibitor of ADAMTS-4 and -5 [15]. Once a plaque is formed, its stability is associated with high expression levels of TIMP-3 [158,159] and versican [160]. Therefore, atherosclerotic plaque development is impacted by an imbalance between the expression of proteoglycanases, proteoglycanase inhibitors and PGs, where each protein may exert a beneficial/detrimental role at different stages of the process.
In studies of TAAD, the mRNA levels of ADAMTS-5 are found to be decreased [30,161] and this has been recently confirmed at the protein level, both in plasma and in aortic biopsies [152]. Mouse models of TAAD may help to clarify the role of ADAMTS-5 in this disease. In the Ang II model, Adamts5 knockout mice showed increased aortic dilation, suggesting that ADAMTS-5 plays a non-redundant role in maintaining the viscoelastic properties of aortic ECM [51]. Loss of ADAMTS-5 was associated with increased protein levels of versican and TGF-β [50], a crucial player in the development of TAAD [160]. At the same time, low-density lipoprotein receptor-related protein-1 (LRP-1) expression was downregulated [50], a phenomenon that has itself been shown to exacerbate aortic dilation [162]. Remarkably, in this model, an increase in ADAMTS-1 protein levels did not compensate for the absence of ADAMTS-5, since versican cleavage was severely diminished [50]. This may be explained by the higher intrinsic versicanase activity of ADAMTS-5 [88].
Taken together, these data suggest that the proteolytic activity of ADAMTS-5 is essential in regulating the levels of cardiovascular PGs and that disturbances could affect the disease process in atherosclerosis and TAAD. As a consequence, any treatment for osteoarthritis should aim to spare ADAMTS-5 activity in the blood vessels to avoid imbalance in PG levels [148].
2.2.4. ADAMTS-8, a contributor to pulmonary arterial hypertension
The enzymatic properties of ADAMTS-8, including its substrate repertoire, have not been extensively investigated. The only reported substrate is aggrecan, which was cleaved in vitro, but at an extremely high enzyme/substrate ratio [163]. Notwithstanding the homology of ADAMTS-8 with ADAMTS-1, -4 and -5, these data cast doubt on the inclusion of ADAMTS-8 in the proteoglycanase subfamily. ADAMTS8 has been identified as a tumour suppressor gene in several types of cancers [164] and single nucleotide polymorphisms (SNPs) in the ADAMTS8 locus have been associated with hypertension in a genome-wide association study (GWAS) [165]. At the protein level, ADAMTS-8 is highly expressed in the lung and the heart [119,166] and, together with ADAMTS-1, -4 and -5, has been detected within human carotid lesions and advanced coronary atherosclerotic plaques [106,107]. ADAMTS-8 expression was increased in the lungs of patients with pulmonary arterial hypertension (PAH) and in mouse/rat models of PAH [119]. In a model of hypoxia-induced PAH, mice bearing a targeted deletion of Adamts8 in pulmonary arterial smooth muscle cells (Adamts8ΔSM22α) showed decreased right ventricular systolic pressure and right ventricular hypertrophy compared with wild-type mice, suggesting a crucial role for ADAMTS-8 in the development of PAH [119]. Addition of recombinant ADAMTS-8 to pulmonary artery ECs seemed to exert a pro-inflammatory and pro-apoptotic role [119], similar to its effects on nasopharyngeal carcinoma cell lines [167]. These data suggest potential similarities between the function of ADAMTS-8 in PAH and in cancer. Since ADAMTS-8 is also expressed in the heart, a conditional knockout model with cardiomyocyte-specific deletion of Adamts8 (Adamts8ΔαMHC) was generated [119]. These mice showed decreased cardiac hypertrophy, fibrosis and right ventricular dysfunction in response to hypoxia.
Taken together, these results implicate ADAMTS-8 in the development of PAH and potentially other cardiovascular phenotypes, but further studies are necessary to characterize the ADAMTS-8 substrate repertoire, mechanism of action and regulation.
2.2.5. ADAMTS-9 in heart development
Evolutionarily, ADAMTS-9 appears to be the oldest family member, based on its high homology to nematode and fruit fly proteases, named Gon-1 and Adamts-A, respectively [72,168–170]. It is also the largest member of the ADAMTS family, comprising 14 TSR repeats and one Gon-1-like domain at the C-terminus (table 1).
Adamts9 knockout mice did not survive past 7.5 days of gestation for unknown reasons, but possibly owing to an important role of ADAMTS-9 in the formation and function of primary cilia [171,172]. Heterozygous knockout mice showed a variable penetrance of cardiac anomalies involving the myocardium, mitral valves, aortic valves and proximal aorta, associated with excess versican [120], suggesting that this enzyme is involved in development of the heart (table 2).
In a study of gene expression associated with AAA rupture, aortic tissues from emergency repair of ruptured AAA were compared to tissue from elective surgery. This identified a set of 5 fibroblast-expressed genes exclusively upregulated in AAA, including ADAMTS9 [173].
3. ADAMTS-7 in coronary artery disease
ADAMTS-7 is a potential therapeutic target in atherosclerosis and associated diseases such as CAD. The evidence for a detrimental role has accumulated over the past decade and includes 1) reduced atherosclerosis upon ablation of the Adamts7 gene in mice (table 2); 2) GWAS that show an association of ADAMTS7 SNPs with CAD and 3) immunohistochemical detection of ADAMTS-7 in human atherosclerotic plaques.
Knockout of Adamts7 on an atherosclerotic background reduced total atherosclerotic lesion area in the aorta of Adamts7−/−/ApoE−/− mice by 62% (male) and 54% (female) compared to littermate controls. In Adamts7−/−/Ldlr−/− mice, the reductions were 37% and 52%, respectively [116]. These findings suggest that pharmacological inhibition of ADAMTS-7 activity could slow down the progression of atherosclerosis. Adamts7−/− mice also showed an altered response of VSMCs to arterial wire injury [116,117]. They showed greatly reduced neointima formation upon vascular injury, which had been seen previously in rats upon Adamts7 knockdown with siRNA in a balloon injury model [118]. Using the same model, the opposite effect was seen when ADAMTS-7 was overexpressed [118]. These effects of ADAMTS-7 on VSMC in vivo agree with findings in vitro [116–118,174].
GWAS have shown that SNPs in the ADAMTS7 locus are associated with CAD [174]. The SNP that is thought to cause the association is rs3825807, of which the G allele is associated with a reduced risk of CAD. It causes a serine to proline substitution in position 214 of the prodomain of ADAMTS-7. The proline is located near recognition motifs of subtilisin-like proprotein convertases such as furin and PCSK6, which activate ADAMTS-7 by proteolytic removal of the inhibitory prodomain. The proline was shown to hamper prodomain removal, which consequently reduces the activation of ADAMTS-7 [174] and potentially mediates the reduced CAD risk associated with the G allele. VSMCs of the G/G genotype for rs3825807 also migrated less in vitro compared to the A/A genotype.
Immunohistochemistry of human atherosclerotic plaques identified ADAMTS-7 protein [174,175]. In carotid plaques, ADAMTS-7 levels were increased in patients with cerebrovascular symptoms compared to patients without these symptoms and high levels also correlated with increased risk of post-operative cardiovascular events [175].
Several in vitro substrates of ADAMTS-7 have been reported in the literature, but clear links with atherosclerosis and VSMC behaviour have not been established [117,176,177]. The mass spectrometry-based method terminal amine isotopic labelling of substates (TAILS) was used to identify potential ECM substrates, which identified latent TGF-β binding protein 4 (LTBP4) as a substrate [178]. LTBP4 is a component of microfibrils/elastic fibres in the lung and large blood vessels and is also co-expressed with ADAMTS-7 in the heart [179,180]. It binds to several ECM proteins, including fibrillin-1, fibulin-4 and fibulin-5, which are essential for the formation of elastic fibres in large blood vessels [179,181,182]. Proteolysis of LTBP4 by ADAMTS-7 likely affects this process, but this remains to be investigated in vivo. The significance of LTBP4 proteolysis by ADAMTS-7 for atherosclerosis is also currently unclear. However, a recent report showed that the LTBP4 gene was differentially expressed between plaques from symptomatic and asymptomatic patients [183], suggesting LTBP4 may affect the composition of atherosclerotic plaques.
Whereas several other ADAMTS family members are regulated by the endogenous metalloprotease inhibitor TIMP-3, ADAMTS-7 is more susceptible to inhibition by TIMP-4, which inhibited ADAMTS-7 efficiently at low nanomolar concentrations [178]. Both TIMP-4 and ADAMTS-7 have a restricted tissue distribution with particularly abundant expression in adult cardiovascular tissues [116,184].
In summary, ADAMTS-7 is a potential therapeutic target in CAD and related diseases resulting from atherosclerosis, but more research is needed to validate it as a target and allow a better understanding of the molecular mechanisms involved.
4. ADAMTS-13, thrombotic thrombocytopenic purpura and stroke
ADAMTS-13 circulates in blood at a concentration of approximately 6 nM, where it trims newly secreted VWF multimers that would otherwise be too thrombogenic [185] (figure 1a). Very low (less than 10%) ADAMTS-13 activity causes the disease TTP and moderately low ADAMTS-13 activity is associated with ischaemic stroke, which is also a feature of TTP. In TTP, the VWF multimers that are too long spontaneously aggregate platelets in the absence of endothelial damage [186]. TTP is a rare, life-threatening condition that most commonly presents in previously healthy young to middle-aged adults, with an annual incidence of 6 per million in the UK [187,188]. They show severe microangiopathic hemolytic anemia (MAHA), severe thrombocytopenia and end-organ damage. The organs most commonly affected are the heart, brain, kidneys and gastrointestinal tract [189]. The MAHA is the result of the microthombi that occlude the small vessels and damage the red blood cells, whereas the severe thrombocytopenia is caused by consumption of the platelets that are caught up in the microthrombi. The end-organ damage results from the occlusion of the small vessels that oxygenate the organs [188]. In a small minority of TTP patients, ADAMTS-13 activity is low because of inherited mutations in the coding region of the ADAMTS13 gene [189]. The most common form of TTP is immune-mediated TTP (iTTP), involving autoantibodies against ADAMTS-13, which rapidly clear the enzyme from the circulation and inhibit its activity by preventing binding to its substrate VWF [190]. Autoantibodies that target the N-terminal domains up to the Sp domain can be inhibitory, in line with the discovery of several exosites in these domains [190–195]. Treatment of iTTP currently consists of plasma exchange (PEX) to remove pathogenic autoantibodies and provide ADAMTS-13, in conjunction with Rituximab to suppress autoantibody production [186,196]. PEX is critical and lifesaving when patients present at the hospital in an emergency. More recently, PEX is also supplemented with Caplacizumab, a construct consisting of two fused single-domain antibodies that both target the same epitope in the VWF A1 domain and reduce platelet aggregation [197]. Recombinant ADAMTS-13 is currently undergoing a Phase 2 clinical trial, in which it is used to supplement PEX in iTTP patients with the aim to speed up recovery and reduce the use of PEX (ClinicalTrials.gov: NCT03922308). Whereas most antithrombotic agents carry a risk of bleeding, this is unlikely for recombinant ADAMTS-13 because of the unique mechanism by which its activity is regulated. It is not regulated by a physiological inhibitor (e.g. TIMPs, [198]), but by the limited and conditional availability of VWF exosites and scissile bonds. These are normally buried inside the globular VWF A2 domain and only exposed when the A2 domain unfolds upon elevated shear stress in the circulation [199–201]. Ultra low VWF undergoes elevated shear stress when it exits the endothelium and enters the circulation, causing proteolysis by ADAMTS-13, which reduces the size of the multimer and thereby reduces the tensile force exerted upon the molecule, preventing further unfolding and cleavage of VWF A2 domains [199,202]. VWF is the only reported substrate of ADAMTS-13. The specificity of the protease is conferred by exosites in several ADAMTS-13 domains that bind complementary exosites in the VWF A2 domain C-terminal to the scissile bond [193,194,203–205]. In addition, subsites in the Mp domain accommodate VWF side chains either side of the scissile bond and add to the overall specificity of the enzyme [204,206,207].
Whereas ADAMTS-13 activity levels less than 10% can cause TTP, low levels that fall within the normal range (lowest quartile) are associated with increased risk of developing ischemic stroke [208–210]. In patients who present with acute ischemic brain injury, the ratio of VWF antigen levels to ADAMTS-13 activity (VWF:Ag/ADAMTS-13Ac) predicts mortality and is associated with impact on brain function in survivors [211]. Also, in TTP patients who have recovered and are in remission, lower ADAMTS13 activity levels after recovery are associated with stroke [212]. In animal models of ischemic stroke, the administration of recombinant ADAMTS-13 after a stroke appears to be beneficial [213,214].
5. Other ADAMTS family members
5.1. The procollagenase ADAMTS-2 in myocardial repair
ADAMTS-2 is the major enzyme cleaving the N-propeptide from type I procollagen, thus allowing the assembly of collagen trimers into fibrils/fibres [215]. Although this has primarily been studied in the skin, it may also occur in other tissues. For example, the analysis of hearts from cardiomyopathic patients showed upregulation of ADAMTS2, which may reflect the important role of collagen in myocardial repair and scarring. The collagen that is deposited to ‘repair’ myocardial damage requires prodomain removal by ADAMTS-2 for collagen fibres to form. Increased collagen expression is likely to require increased ADAMTS-2 expression. This may also explain the altered Adamts2 expression in mice treated with isoproterenol, which induces cardiac myopathy and hypertrophy [216] (table 2). In Adamts2 null mice, the detrimental effects of pressure overload on the heart were enhanced [110], possibly owing to disturbed repair mechanisms involving collagen.
5.2. ADAMTS-6 in heart development and QRS duration
ADAMTS6 mRNA has been detected in the mouse heart, specifically in the outflow tract, valves, atria and the ventricular myocardium [115]. The physiological function of ADAMTS-6 is not known but in vitro studies suggest that it may be linked to that of fibrillin-1 microfibrils and focal adhesions [22]. Importantly, ADAMTS-6 has been implicated in cardiac biology by a GWAS, which found that two ADAMTS6 missense variants (S90 L and R603 W) were associated with the duration of the QRS interval of the electrocardiogram [115], which reflects cardiac ventricular depolarization. A prolonged QRS is a predictor of mortality both in the general population and in patients affected by cardiovascular disease [217–219]. The missense variants S90 L in the prodomain and R603 W in the first TSP-1-like domain both severely impair protein secretion [115]. Puzzingly, S90 L was associated with longer QRS and R603 W with shorter QRS duration. In people of European descent, approximately 1/500 is heterozygous for the S90 L variant, whereas R603 W is rare in individuals of European ancestry but is common in people of African decent, where approximately 1/70 is heterozygous (https://gnomad.broadinstitute.org/). Both variants may, however, be pathogenic in a homozygous state and/or function as risk factors for cardiac disease in heterozygous form. A cardiac role for ADAMTS-6 is also in line with the fatal congenital heart defects of mice homozygous for a null mutation in Adamts6, which die pre/peri-natally [115]. Their embryonic heart defects comprise the double outlet right ventricle, an atrioventricular septal defect and ventricular hypertrophy. Interestingly, mice hemizygous for the null mutation are viable and do not show structural heart defects but their ventricles express low levels of connexin-43 protein, the main myocardial gap junction protein in mouse and human heart. The reduced levels of connexin-43 appear to have a post-transcriptional cause, as the mRNA levels of the corresponding gene (Gja1) were unaffected [115]. In summary, these findings suggest that ADAMTS-6 may play a role in development of the heart and in regulating gap junction-mediated ventricular depolarization.
5.3. ADAMTS-10 and cardiovascular manifestations of WMS
Mutations in ADAMTS10 cause an autosomal recessive form of WMS [220–222]. WMS is a rare inherited disorder of the connective tissues characterized by short stature, brachydactyly, joint stiffness, broad skull, heart defects and a variety of eye abnormalities [222,223]. Three distinct ADAMTS10 mutations were identified in two consanguineous families and in one sporadic WMS case, including one nonsense mutation and two splice mutations [220]. Among the clinical features of WMS patients bearing ADAMTS10 mutations were aortic and pulmonary stenosis with dysplastic valves and hypertrophic obstructive cardiomyopathy [220]. Weil-Marchesani syndrome is also caused by mutations in fibrillin-1 (FBN1) and LTBP2, implicating ADAMTS-10 in fibrillin-1 microfibril biology [21]. Fibrillin-1 microfibrils are ECM assemblies that are essential for the formation of the elastic fibres in blood vessels, lungs, skin, ligaments and other elastic tissues. They also regulate the bioavailability of growth factors of the TGF-β and bone morphogenetic protein (BMP) superfamily [224]. ADAMTS-10 was subsequently confirmed to regulate fibrillin microfibril function, and to bind fibrillin-1 at two sites that coincide with the fibrillin-1 mutations in WMS [225]. It also co-localizes with fibrillin-1 in tissues and accelerates microfibril assembly in fibroblast cultures [226]. Ocular features in WMS caused by ADAMTS10 mutations may be owing to reduced fibrillin-2 cleavage [227]. Mutations in other genes involved in fibrilin microfibril biology cause the related disorders WMS-like syndrome (ADAMTS17) and geleophysic dysplasia (LTBP3, ADAMTSL2, Fibrillin 1) [228].
5.4. ADAMTS-16, a potential regulator of blood pressure
The function of ADAMTS-16 is currently unknown, with contradictory reports on its possible involvement in male fertility and sex determination [229,230]. So far, the only described substrate is fibronectin [231]. ADAMTS-16 has been identified as a quantitative trait gene (QTG) for blood pressure in humans [232,233] and rats [234]. To investigate the cardiovascular function of ADAMTS-16, Adamts16 knockout rats have been generated [121] (table 2). These rats show lower systolic blood pressure, decreased arterial stiffness and thickness of the tunica media compared with wild-type rats, suggesting an involvement of ADAMTS-16 in regulating haemodynamics [121]. Moreover, Adamts16 knockout rats survived longer, although they exhibited renal anomalies [121]. More data are needed to ascertain a possible role of ADAMTS-16 in blood pressure regulation.
5.5. ADAMTS-19 in progressive heart valve disease
A recent study of early-onset valvular disease identified mutations in ADAMTS19 by whole exome sequencing. Four patients in two consanguineous families carried homozygous mutations in ADAMTS19 causing a large multi exon deletion in one family and a truncated ADAMTS-19 protein in the other family [122]. To confirm causality, Adamts19 knockout mice were generated (table 2). Of the homozygous Adamts19 knockout mice, 38% showed aortic valve regurgitation and/or aortic stenosis at three months of age, confirming an important role of ADAMTS-19 in aortic valve physiology. Expression analysis of lacZ in Adamts19 knockout mice showed strong localized expression of lacZ by valvular interstitial cells in all four valves around E14.5 and expression by these cells until adulthood. Ultrastructural analysis of the ECM suggested alterations in ECM organization, including PG accumulation. The observation of PG accumulation raises the question whether this is secondary to disturbed valve physiology/cellular function or reduced PG turnover by ADAMTS-19.
6. Targeting ADAMTS therapeutically
ADAMTS inhibitors could potentially be used therapeutically to reduce enzyme activity that contributes to or aggravates cardiovascular disease (e.g. for ADAMTS-7). However, it has proven challenging to develop therapeutic metalloprotease inhibitors with sufficient selectivity to prevent side effects caused by cross-inhibition of related metalloproteinases [235]. Selective inhibitors can be either monoclonal antibodies [235] or small molecules, with the latter having the distinct advantage that they can be administered orally. Selective small molecule inhibitors can be designed on the basis of the available tridimensional structure of the target enzyme or identified by high throughput screening (HTS) of large compound libraries. To screen small molecule libraries for their inhibitory potential, purified enzyme and a high throughput activity assay are needed. Typically, high throughput activity assays for proteases involve Förster resonance energy transfer (FRET) technology, where proteolysis of a small peptide generates a fluorescent signal [236]. For ADAMTS-7 we have developed such an activity assay using a small LTBP4 peptide as an efficient substrate and are currently converting this for HTS of small molecule libraries (manuscript in preparation)
On the other hand, where the activity of an ADAMTS family member has proven to be beneficial in a certain pathological context, so-called enhancers or activators may be envisaged. For example, monoclonal antibodies able to increase the catalytic activity of ADAMTS-13 have been reported [237]. Another innovative approach to increase ADAMTS activity may involve interfering with LRP-1-mediated endocytosis. We have recently reported a monoclonal antibody that is able to bind to ADAMTS-5 and block its binding to LRP-1 without interfering with its proteoglycanase activity [238], resulting in accumulation of active ADAMTS-5 in the extracellular milieu. Such an antibody may be used to rescue ADAMTS-5 proteoglycanase activity in mouse models of atherosclerosis and TAAD (table 2).
7. Conclusion
ADAMTS family members fulfil multiple distinct roles in cardiovascular tissues (table 1). Several of them are important for cardiac valve embryogenesis and homeostasis (figure 2), but in general the multitude of physiological processes affected by ADAMTS proteases reflects the many functions of the ECM, such as regulating cell behaviour and sequestering of a wide range of cellular growth factors. Much has been learned about ADAMTS proteases from animal studies, but caution should be exercised when extrapolating mouse data to human disease in the absence of clinical studies. In this regard, a conclusion should be supported by high-quality data collected in vitro, in vivo and ex vivo but so far this goal has been achieved just for few family members. Nevertheless, exciting new findings are published every year, incrementally elucidating the physiological roles of this fascinating family of proteases.
Acknowledgements
Figures 1 and 2 were designed by Elfyland Studios (www.elfylandstudios.com).
Data accessibility
This article has no additional data.
Authors' contributions
S.S. and R.d.G. designed the review content and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by British Heart Foundation grant nos PG/18/15/33566 (S.S) and PG/18/19/33584 (R.d.G.)
References
- 1.Wagenseil JE, Mecham RP. 2009. Vascular extracellular matrix and arterial mechanics . Physiol. Rev. 89, 957–989. ( 10.1152/physrev.00041.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinajero MG, Gotlieb AI. 2020. Recent developments in vascular adventitial pathobiology: the dynamic adventitia as a complex regulator of vascular disease. Am. J. Pathol. 190, 520–534. ( 10.1016/j.ajpath.2019.10.021) [DOI] [PubMed] [Google Scholar]
- 3.Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. 2008. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am. J. Physiol. Heart Circ. Physiol. 294, H1197–H1205. ( 10.1152/ajpheart.01027.2007) [DOI] [PubMed] [Google Scholar]
- 4.Chandran PL, Horkay F. 2012. Aggrecan, an unusual polyelectrolyte: review of solution behavior and physiological implications. Acta Biomater. 8, 3–12. ( 10.1016/j.actbio.2011.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoen FJ. 2008. Evolving concepts of cardiac valve dynamics. Circulation 118, 1864–1880. ( 10.1161/CIRCULATIONAHA.108.805911) [DOI] [PubMed] [Google Scholar]
- 6.Lincoln J, Alfieri CM, Yutzey KE. 2006. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev. Biol. 292, 290–302. ( 10.1016/j.ydbio.2005.12.042) [DOI] [PubMed] [Google Scholar]
- 7.Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. 2007. Transitions in early embryonic atrioventricular valvular functions correspond with changes in cushion biomechanics that are predictable with tissue composition. Circ. Res. 100, 1503–1511. ( 10.1161/CIRCRESAHA.107.148684) [DOI] [PubMed] [Google Scholar]
- 8.Newby AC. 2012. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul. Pharmacol. 56, 232–244. ( 10.1016/j.vph.2012.01.007) [DOI] [PubMed] [Google Scholar]
- 9.van der Vorst EP, Keijbeck AA, de Winther MP, Donners MM.. 2012. A disintegrin and metalloproteases: molecular scissors in angiogenesis, inflammation and atherosclerosis. Atherosclerosis 224, 302–308. ( 10.1016/j.atherosclerosis.2012.04.023) [DOI] [PubMed] [Google Scholar]
- 10.Mead TJ, Apte SS. 2018. ADAMTS proteins in human disorders. Matrix. Biol. 71–72, 225–239. ( 10.1016/j.matbio.2018.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. 2001. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J. Biochem. 130, 475–480. ( 10.1093/oxfordjournals.jbchem.a003009) [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. 2001. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J. Biol. Chem. 276, 41 059–41 063. ( 10.1074/jbc.C100515200) [DOI] [PubMed] [Google Scholar]
- 13.Gomis-Rüth FX, Botelho TO, Bode W. 2012. A standard orientation for metallopeptidases. Biochim. Biophys. Acta 1824, 157–163. ( 10.1016/j.bbapap.2011.04.014) [DOI] [PubMed] [Google Scholar]
- 14.Apte SS. 2020. ADAMTS proteins: concepts, challenges, and prospects. Methods Mol. Biol. 2043, 1–12. ( 10.1007/978-1-4939-9698-8_1) [DOI] [PubMed] [Google Scholar]
- 15.Brew K, Nagase H. 2010. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta 1803, 55–71. ( 10.1016/j.bbamcr.2010.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto K, Murphy G, Troeberg L. 2015. Extracellular regulation of metalloproteinases. Matrix. Biol. 44–46, 255–263. ( 10.1016/j.matbio.2015.02.007) [DOI] [PubMed] [Google Scholar]
- 17.Huxley-Jones J, Apte SS, Robertson DL, Boot-Handford RP. 2005. The characterisation of six ADAMTS proteases in the basal chordate Ciona intestinalis provides new insights into the vertebrate ADAMTS family. Int. J. Biochem. Cell Biol. 37, 1838–1845. ( 10.1016/j.biocel.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 18.Colige A, et al. 1999. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am. J. Hum. Genet. 65, 308–317. ( 10.1086/302504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouillard P, Dupont L, Helaers R, Coulie R, Tiller GE, Peeden J, Colige A, Vikkula M. 2017. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia–lymphedema syndrome 3. Hum. Mol. Gen. 26, 4095–4104. ( 10.1093/hmg/ddx297) [DOI] [PubMed] [Google Scholar]
- 20.Jeltsch M, et al. 2014. CCBE1 enhances lymphangiogenesis via a disintegrin and metalloprotease with thrombospondin motifs-3–mediated vascular endothelial growth factor-C activation. Circulation 129, 1962–1971. ( 10.1161/CIRCULATIONAHA.113.002779) [DOI] [PubMed] [Google Scholar]
- 21.Karoulias SZ, Taye N, Stanley S, Hubmacher D. 2020. The ADAMTS/fibrillin connection: insights into the biological functions of ADAMTS10 and ADAMTS17 and their respective sister proteases. Biomolecules 10, E596 ( 10.3390/biom10040596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cain SA, Mularczyk EJ, Singh M, Massam-Wu T, Kielty CM. 2016. ADAMTS-10 and -6 differentially regulate cell–cell junctions and focal adhesions. Sci. Rep. 6 35956 ( 10.1038/srep35956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman TS, Ruggeri ZM. 1987. von Willebrand disease. Hum. Pathol. 18, 140–152. ( 10.1016/s0046-8177(87)80332-5 [DOI] [PubMed] [Google Scholar]
- 24.Furlan M, Robles R, Lämmle B. 1996. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 87, 4223–4234. ( 10.1182/blood.V87.10.4223.bloodjournal87104223) [DOI] [PubMed] [Google Scholar]
- 25.Wight TN. 2018. A role for proteoglycans in vascular disease. Matrix. Biol. 71–72, 396-420. ( 10.1016/j.matbio.2018.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wight TN. 2017. Provisional matrix: a role for versican and hyaluronan. Matrix. Biol. 60–61, 38–56. ( 10.1016/j.matbio.2016.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wight TN, Merrilees MJ. 2004. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ. Res. 94, 1158–1167. ( 10.1161/01.RES.0000126921.29919.51) [DOI] [PubMed] [Google Scholar]
- 28.Koch CD, Lee CM, Apte SS. 2020. Aggrecan in cardiovascular development and disease. J. Histochem. Cytochem. 68 ( 10.1369/0022155420952902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. 2006. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 98, 1431–1438. ( 10.1161/01.RES.0000224114.65109.4e) [DOI] [PubMed] [Google Scholar]
- 30.Cikach FS, et al. 2018. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight 3, e97167 ( 10.1172/jci.insight.97167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin X, et al. 2019. Glycoproteomic analysis of the aortic extracellular matrix in Marfan patients. Arterioscler. Thromb. Vasc. Biol. 39, 1859–1873. ( 10.1161/ATVBAHA.118.312175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlatmann TJ, Becker AE. 1977. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am. J. Cardiol. 39, 13–20. ( 10.1016/s0002-9149(77)80004-0) [DOI] [PubMed] [Google Scholar]
- 33.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. 2014. Cell biology. Dysfunctional mechanosensing in aneurysms. Science 344, 477–479. ( 10.1126/science.1253026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillarisetti S. 2000. Lipoprotein modulation of subendothelial heparan sulfate proteoglycans (perlecan) and atherogenicity. Trends Cardiovasc. Med. 10, 60–65. ( 10.1016/s1050-1738(00)00048-7) [DOI] [PubMed] [Google Scholar]
- 35.Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM. 2014. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix. Biol. 35, 112–122. ( 10.1016/j.matbio.2014.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraehling JR, et al. 2016. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat. Commun. 7, 13516 ( 10.1038/ncomms13516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurt-Camejo E, Camejo G, Rosengren B, López F, Ahlström C, Fager G, Bondjers G. 1992. Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler. Thromb. 12, 569–583. ( 10.1161/01.atv.12.5.569) [DOI] [PubMed] [Google Scholar]
- 38.Llorente-Cortés V, Otero-Viñas M, Hurt-Camejo E, Martínez-González J, Badimon L. 2002. Human coronary smooth muscle cells internalize versican-modified LDL through LDL receptor related protein and LDL receptors. Arterioscler. Thromb. Vasc. Biol. 22, 387–393. ( 10.1161/hq0302.105367) [DOI] [PubMed] [Google Scholar]
- 39.Vijayagopal P, Figueroa JE, Fontenot JD, Glancy DL. 1996. Isolation and characterization of a proteoglycan variant from human aorta exhibiting a marked affinity for low density lipoprotein and demonstration of its enhanced expression in atherosclerotic plaques. Atherosclerosis 127, 195–203. ( 10.1016/s0021-9150(96)05954-0) [DOI] [PubMed] [Google Scholar]
- 40.Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. 1998. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J. Clin. Invest. 101, 2658–2664. ( 10.1172/JCI2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camejo G, Fager G, Rosengren B, Hurt-Camejo E, Bondjers G. 1993. Binding of low density lipoproteins by proteoglycans synthesized by proliferating and quiescent human arterial smooth muscle cells. J. Biol. Chem. 268, 14 131–14 137. [PubMed] [Google Scholar]
- 42.Skalén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, Borén J. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754. ( 10.1038/nature00804) [DOI] [PubMed] [Google Scholar]
- 43.Iozzo RV. 1998. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652. ( 10.1146/annurev.biochem.67.1.609) [DOI] [PubMed] [Google Scholar]
- 44.Wight TN, Kang I, Merrilees MJ. 2014. Versican and the control of inflammation. Matrix. Biol. 35, 152–161. ( 10.1016/j.matbio.2014.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. 1998. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev. Biol. 202, 56–66. ( 10.1006/dbio.1998.9001) [DOI] [PubMed] [Google Scholar]
- 46.Henderson DJ, Copp AJ. 1998. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ. Res. 83, 523–532. ( 10.1161/01.res.83.5.523) [DOI] [PubMed] [Google Scholar]
- 47.Merrilees MJ, Beaumont B, Scott LJ. 2001. Comparison of deposits of versican, biglycan and decorin in saphenous vein and internal thoracic, radial and coronary arteries: correlation to patency. Coron. Artery Dis. 12, 7–16. ( 10.1097/00019501-200102000-00002) [DOI] [PubMed] [Google Scholar]
- 48.Talusan P, Bedri S, Yang S, Kattapuram T, Silva N, Roughley PJ, Stone JR. 2005. Analysis of intimal proteoglycans in atherosclerosis-prone and atherosclerosis-resistant human arteries by mass spectrometry. Mol. Cell. Proteomics. 4, 1350–1357. ( 10.1074/mcp.M500088-MCP200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. 2010. Proteomics characterization of extracellular space components in the human aorta. Mol. Cell. Proteomics 9, 2048–2062. ( 10.1074/mcp.M110.001693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fava M, et al. 2018. Role of ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler. Thromb. Vasc. Biol. 38, 1537–1548. ( 10.1161/ATVBAHA.117.310562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suna G, et al. 2018. Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation 137, 166–183. ( 10.1161/CIRCULATIONAHA.116.023381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasmin MRA, et al. 2018. The matrix proteins aggrecan and fibulin-1 play a key role in determining aortic stiffness. Sci. Rep. 8, 8550 ( 10.1038/s41598-018-25851-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupuis LE, Nelson EL, Hozik B, Porto SC, Rogers-DeCotes A, Fosang A, Kern CB. 2019. Adamts5−/− mice exhibit altered Aggrecan proteolytic profiles that correlate with ascending aortic anomalies. Arterioscler. Thromb. Vasc. Biol. 39, 2067–2081 ( 10.1161/ATVBAHA.119.313077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. 2002. Structure and function of aggrecan. Cell Res. 12,19–32. ( 10.1038/sj.cr.7290106) [DOI] [PubMed] [Google Scholar]
- 55.Seidler DG. 2012. The galactosaminoglycan-containing decorin and its impact on diseases. Curr. Opin Struct. Biol. 22, 578–582. ( 10.1016/j.sbi.2012.07.012) [DOI] [PubMed] [Google Scholar]
- 56.Hedbom E, Heinegård D. 1993. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J. Biol. Chem. 268, 27 307–27 312. [PubMed] [Google Scholar]
- 57.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. 2002. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 16, 673–680. ( 10.1096/fj.01-0848com) [DOI] [PubMed] [Google Scholar]
- 58.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. 2006. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 7, 2388–2393. ( 10.1021/bm0603746) [DOI] [PubMed] [Google Scholar]
- 59.O'Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, Wight TN, Chait A. 1998. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation 98, 519–527. ( 10.1161/01.cir.98.6.519) [DOI] [PubMed] [Google Scholar]
- 60.Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. 2001. Biglycan, a vascular proteoglycan, binds differently to HDL2 and HDL3: role of ApoE. Arterioscler. Thromb. Vasc. Biol. 21, 129–135. ( 10.1161/01.atv.21.1.129) [DOI] [PubMed] [Google Scholar]
- 61.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. 2007. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 27, 1159–1165. ( 10.1161/ATVBAHA.106.134080) [DOI] [PubMed] [Google Scholar]
- 62.Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. 2008. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J. Lipid Res. 49, 521–530. ( 10.1194/jlr.M700329-JLR200) [DOI] [PubMed] [Google Scholar]
- 63.Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. 1999. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J. Biol. Chem. 274, 34 629–34 636. ( 10.1074/jbc.274.49.34629) [DOI] [PubMed] [Google Scholar]
- 64.Grandoch M, et al. 2016. Loss of biglycan enhances thrombin generation in apolipoprotein E-deficient mice: implications for inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 36, e41–e50. ( 10.1161/ATVBAHA.115.306973) [DOI] [PubMed] [Google Scholar]
- 65.Thompson JC, Tang T, Wilson PG, Yoder MH, Tannock LR. 2014. Increased atherosclerosis in mice with increased vascular biglycan content. Atherosclerosis 235, 71–75. ( 10.1016/j.atherosclerosis.2014.03.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakashima Y, Wight TN, Sueishi K. 2008. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc. Res. 79, 14–23. ( 10.1093/cvr/cvn099) [DOI] [PubMed] [Google Scholar]
- 67.Tang T, Thompson JC, Wilson PG, Yoder MH, Müeller J, Fischer JW, Williams KJ, Tannock LR. 2014. Biglycan deficiency: increased aortic aneurysm formation and lack of atheroprotection. J. Mol. Cell. Cardiol. 75, 174–180. ( 10.1016/j.yjmcc.2014.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meester JAN, et al. 2017. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet. Med. 19, 386–395. ( 10.1038/gim.2016.126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. 2007. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation 115, 2731–2738. ( 10.1161/CIRCULATIONAHA.106.653980) [DOI] [PubMed] [Google Scholar]
- 70.Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, Michel JB, Vranckx R.. 2009. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J. Pathol. 218, 131–142. ( 10.1002/path.2516) [DOI] [PubMed] [Google Scholar]
- 71.Sandy JD, et al. 2001. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J. Biol. Chem. 276, 13 372–13 378. ( 10.1074/jbc.M009737200) [DOI] [PubMed] [Google Scholar]
- 72.Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. 2003. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J. Biol. Chem. 278, 9503–9513. ( 10.1074/jbc.M211009200) [DOI] [PubMed] [Google Scholar]
- 73.Longpré JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. 2009. Characterization of proADAMTS5 processing by proprotein convertases. Int. J. Biochem. Cell Biol. 41, 1116–1126. ( 10.1016/j.biocel.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 74.Dancevic CM, Fraser FW, Smith AD, Stupka N, Ward AC, McCulloch DR. 2013. Biosynthesis and expression of a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats-15: a novel versican-cleaving proteoglycanase. J. Biol. Chem. 288, 37 267–37 276. ( 10.1074/jbc.M112.418624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Foulcer SJ, Nelson CM, Quintero MV, Kuberan B, Larkin J, Dours-Zimmermann MT, Zimmermann DR, Apte SS. 2014. Determinants of versican-V1 proteoglycan processing by the metalloproteinase ADAMTS5. J. Biol. Chem. 289, 27 859–27 873. ( 10.1074/jbc.M114.573287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tortorella MD, Malfait AM. 2008. Will the real aggrecanase(s) step up: evaluating the criteria that define aggrecanase activity in osteoarthritis. Curr. Pharm. Biotechnol. 9, 16–23. ( 10.2174/138920108783497622) [DOI] [PubMed] [Google Scholar]
- 77.Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. 2004. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem. J. 377, 787–795. ( 10.1042/BJ20030896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santamaria S, Yamamoto K. 2020. Analysis of aggrecanase activity using neoepitope antibodies. Methods Mol. Biol. 2043, 125–136. ( 10.1007/978-1-4939-9698-8_11) [DOI] [PubMed] [Google Scholar]
- 79.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. 1992. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J. Clin. Invest. 89, 1512–1516. ( 10.1172/JCI115742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hope C, et al. 2016. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood 128, 680–685. ( 10.1182/blood-2016-03-705780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCulloch DR, et al. 2009. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell. 17, 687–698. ( 10.1016/j.devcel.2009.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, Fosang AJ. 2015. Bioactivity in an aggrecan 32-mer fragment is mediated via toll-like receptor 2. Arthritis Rheumatol. 67, 1240–1249. ( 10.1002/art.39063) [DOI] [PubMed] [Google Scholar]
- 83.Miller RE, Ishihara S, Tran PB, Golub SB, Last K, Miller RJ, Fosang AJ, Malfait AM. 2018. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 3, e95704 ( 10.1172/jci.insight.95704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dufield DR, Nemirovskiy OV, Jennings MG, Tortorella MD, Malfait AM, Mathews WR. 2010. An immunoaffinity liquid chromatography–tandem mass spectrometry assay for detection of endogenous aggrecan fragments in biological fluids: use as a biomarker for aggrecanase activity and cartilage degradation. Anal. Biochem. 406, 113–123. ( 10.1016/j.ab.2010.06.044) [DOI] [PubMed] [Google Scholar]
- 85.Germaschewski FM, et al. 2014. Quantitation oF ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthritis Cartilage 22, 690–697. ( 10.1016/j.joca.2014.02.930) [DOI] [PubMed] [Google Scholar]
- 86.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thøgersen IB, Hughes C, Caterson B, Nagase H. 2007. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J. Biol. Chem. 282, 18 294–18 306. ( 10.1074/jbc.M701523200) [DOI] [PubMed] [Google Scholar]
- 87.Fushimi K, Troeberg L, Nakamura H, Lim NH, Nagase H. 2008. functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity. J. Biol. Chem. 283, 6706–6716. ( 10.1074/jbc.M708647200) [DOI] [PubMed] [Google Scholar]
- 88.Santamaria S, Yamamoto K, Teraz-Orosz A, Koch C, Apte SS, de Groot R, Lane DA, Ahnström J.. 2019. Exosites in hypervariable loops of ADAMTS spacer domains control substrate recognition and proteolysis. Sci. Rep. 9, 10 914 ( 10.1038/s41598-019-47494-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. 2006. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage 14, 1147–1154. ( 10.1016/j.joca.2006.05.014) [DOI] [PubMed] [Google Scholar]
- 90.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. 1997. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J. Biol. Chem. 272, 556–562. ( 10.1074/jbc.272.1.556) [DOI] [PubMed] [Google Scholar]
- 91.Shindo T, et al. 2000. ADAMTS1: a metalloproteinase–disintegrin essential for normal growth, fertility, and organ morphology and function. J. Clin. Invest. 105, 1345–1352. ( 10.1172/JCI8635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. 2004. Adamts-1 is essential for the development and function of the urogenital system. Biol. Reprod. 70, 1096–1105. ( 10.1095/biolreprod.103.023911) [DOI] [PubMed] [Google Scholar]
- 93.Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. 2006. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev. Biol. 300, 699–709. ( 10.1016/j.ydbio.2006.10.012) [DOI] [PubMed] [Google Scholar]
- 94.Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, Russell DL. 2010. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 83, 549–557. ( 10.1095/biolreprod.110.084434) [DOI] [PubMed] [Google Scholar]
- 95.Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. 2000. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 478, 241–245. ( 10.1016/s0014-5793(00)01854-8) [DOI] [PubMed] [Google Scholar]
- 96.Canals F, Colomé N, Ferrer C, Plaza-Calonge Mdel C, Rodríguez- Manzaneque JC. 2006. Identification of substrates of the extracellular protease ADAMTS1 by DIGE proteomic analysis. Proteomics 6, S28–S35. ( 10.1002/pmic.200500446) [DOI] [PubMed] [Google Scholar]
- 97.Martino-Echarri E, et al. 2013. Contribution of ADAMTS1 as a tumor suppressor gene in human breast carcinoma. Linking its tumor inhibitory properties to its proteolytic activity on nidogen-1 and nidogen-2. Int. J. Cancer 133, 2315–2324. ( 10.1002/ijc.28271) [DOI] [PubMed] [Google Scholar]
- 98.Esselens C, Malapeira J, Colomé N, Casal C, Rodríguez-Manzaneque JC, Canals F, Arribas J. 2010. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J. Biol. Chem. 285, 2463–2473. ( 10.1074/jbc.M109.055129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torres-Collado AX, Kisiel W, Iruela-Arispe ML, Rodríguez-Manzaneque JC. 2006. ADAMTS1 interacts with, cleaves, and modifies the extracellular location of the matrix inhibitor tissue factor pathway inhibitor-2. J. Biol. Chem. 281, 17 827–17 837. ( 10.1074/jbc.M513465200) [DOI] [PubMed] [Google Scholar]
- 100.Martino-Echarri E, et al. 2014. Relevance of IGFBP2 proteolysis in glioma and contribution of the extracellular protease ADAMTS1. Oncotarget 5, 4295–4304. ( 10.18632/oncotarget.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodríguez-Manzaneque JC, Carpizo D, Plaza-Calonge MD, Torres-Collado AX, Thai SN, Simons M, Horowitz A, Iruela-Arispe ML. 2008. Cleavage of syndecan-4 by ADAMTS1 provokes defects in adhesion. Int. J. Biochem. Cell Biol. 41, 800–810. ( 10.1016/j.biocel.2008.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. 2006. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 25, 5270–5283. ( 10.1038/sj.emboj.7601400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe ML, Argraves WS. 2006. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev. Dyn. 235, 2238–2247. ( 10.1002/dvdy.20838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stankunas K, et al. 2008. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev. Cell 14, 298–311. ( 10.1016/j.devcel.2007.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pelisek J, Deutsch L, Ansel A, Pongratz J, Stadlbauer T, Gebhard H, Matevossian E, Eckstein HH. 2017. Expression of a metalloproteinase family of ADAMTS in human vulnerable carotid lesions. J. Cardiovasc. Med. (Hagerstown) . 18, 10–18. ( 10.2459/JCM.0000000000000254) [DOI] [PubMed] [Google Scholar]
- 106.Wågsäter D, Bjork H, Zhu C, Björkegren J, Valen G, Hamsten A, Eriksson P. 2008. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis 196, 514–522. ( 10.1016/j.atherosclerosis.2007.05.018) [DOI] [PubMed] [Google Scholar]
- 107.Jönsson-Rylander A, et al. 2005. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of Versican. Arterioscler. Thromb. Vasc. Biol. 25, 180–185. ( 10.1161/01.ATV.0000150045.27127.37) [DOI] [PubMed] [Google Scholar]
- 108.Ashlin TG, Kwan AP, Ramji DP. 2013. Regulation of ADAMTS-1, -4 and -5 expression in human macrophages: differential regulation by key cytokines implicated in atherosclerosis and novel synergism between TL1A and IL-17. Cytokine 64, 234–242. ( 10.1016/j.cyto.2013.06.315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oller J, et al. 2017. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 23, 200–212. ( 10.1038/nm.4266) [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Chen W, Zhang J, Khan A, Li L, Huang F, Qiu Z, Wang L, Chen X. 2017. Critical role of ADAMTS2 (A disintegrin and metalloproteinase with thrombospondin motifs 2) in cardiac hypertrophy induced by pressure overload. Hypertension 69, 1060–1069. ( 10.1161/HYPERTENSIONAHA.116.08581) [DOI] [PubMed] [Google Scholar]
- 111.Kumar S, et al. 2016. Loss of ADAMTS4 reduces high fat diet-induced atherosclerosis and enhances plaque stability in ApoE−/− mice. Sci. Rep. 6, 31130 ( 10.1038/srep31130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren P, et al. 2017. Critical role of ADAMTS-4 in the development of sporadic aortic aneurysm and dissection in mice. Sci. Rep. 7, 12351 ( 10.1038/s41598-017-12248-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dupuis LE, et al. 2011. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev. Biol. 357, 152–164. ( 10.1016/j.ydbio.2011.06.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dupuis LE, Osinska H, Weinstein MB, Hinton RB, Kern CB. 2013. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J. Mol. Cell. Cardiol. 60, 50–59. ( 10.1016/j.yjmcc.2013.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prins BP, et al. 2018. Exome-chip meta-analysis identifies novel loci associated with cardiac conduction, including ADAMTS6. Genome Biol. 19, 87 ( 10.1186/s13059-018-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bauer RC, et al. 2015. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation 131, 1202–1213. ( 10.1161/circulationaha.114.012669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kessler T, et al. 2015. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation 131, 1191–1201. ( 10.1161/circulationaha.114.014072) [DOI] [PubMed] [Google Scholar]
- 118.Wang L, et al. 2009. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ. Res. 104, 688–698. ( 10.1161/CIRCRESAHA.108.188425) [DOI] [PubMed] [Google Scholar]
- 119.Omura J, et al. 2019. ADAMTS8 promotes the development of pulmonary arterial hypertension and right ventricular failure: a possible novel therapeutic target. Circ. Res. 125, 884–906. ( 10.1161/CIRCRESAHA.119.315398) [DOI] [PubMed] [Google Scholar]
- 120.Kern CB, et al. 2010. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix. Biol. 29, 304–316. ( 10.1016/j.matbio.2010.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gopalakrishnan K, et al. 2012. Targeted disruption of Adamts16 gene in a rat genetic model of hypertension. Proc. Natl Acad. Sci. USA. 109, 20 555–20 559. ( 10.1073/pnas.1211290109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wünnemann F, Ta-Shma A, Preuss C et al. . 2020. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 52, 40–47. ( 10.1038/s41588-019-0536-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M, Liu Q, Lei J, Wang X, Chen X, Ding Y. 2017a. MiR-362–3p inhibits the proliferation and migration of vascular smooth muscle cells in atherosclerosis by targeting ADAMTS1. Biochem. Biophys. Res. Commun. 493, 270–276. ( 10.1016/j.bbrc.2017.09.031) [DOI] [PubMed] [Google Scholar]
- 124.Qu Y, Zhang N. 2018. miR-365b-3p inhibits the cell proliferation and migration of human coronary artery smooth muscle cells by directly targeting ADAMTS1 in coronary atherosclerosis. Exp. Ther. Med. 16, 4239–4245. ( 10.3892/etm.2018.6720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oller J., et al. 2015. C/EBP-β and nuclear factor of activated T cells differentially regulate Adamts1 induction by stimuli associated with vascular remodeling. Mol. Cell. Biol. 35, 3409–3422. ( 10.1128/MCB.00494-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chung AWY, Yang HHC, Breemen van Breemen C. 2007. Imbalanced synthesis of cyclooxygenase-derived thromboxane A2 and prostacyclin compromises vasomotor function of the thoracic aorta in Marfan syndrome. Br. J. Pharmacol. 152, 305–312. ( 10.1038/sj.bjp.0707391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sengle G, Sakai LY. 2015. The fibrillin microfibril scaffold: a niche for growth factors and mechanosensation? Matrix. Biol. 47, 3–12. ( 10.1016/j.matbio.2015.05.002) [DOI] [PubMed] [Google Scholar]
- 128.Godwin ARF, Singh M, Lockhart-Cairns MP, Alanazi YF, Cain SA, Baldock C. 2019. The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly. Matrix Biol. 84, 17–30. ( 10.1016/j.matbio.2019.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren P, Zhang L, Xu G, Palmero LC, Albini PT, Coselli JS, Shen YH, LeMaire SA. 2013. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 95, 570–577. ( 10.1016/j.athoracsur.2012.10.084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Güneş MF, et al. 2016. The investigation of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 1, 5 and 16 in thoracic aortic aneurysms and dissections. Clin. Lab. 62, 425–433. ( 10.7754/Clin.Lab.2015.150730) [DOI] [PubMed] [Google Scholar]
- 131.Matthews R, Gary S, Zerillo C, Pratta M, Solomon K, Arner E, Hockfield S. 2000. Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J. Biol. Chem. 275, 22 695–22 703. ( 10.1074/jbc.M909764199) [DOI] [PubMed] [Google Scholar]
- 132.Dickinson SC, Vankemmelbeke MN, Buttle DJ, Rosenberg K, Heinegård D, Hollander AP. 2003. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 22, 267–278. ( 10.1016/S0945-053X(03)00034-9) [DOI] [PubMed] [Google Scholar]
- 133.Chen L, Yang L, Zha Y, Cui L. 2011. Association of serum a disintegrin and metalloproteinase with thrombospodin motif 4 levels with the presence and severity of coronary artery disease. Coron. Artery Dis. 22, 570–576. ( 10.1097/MCA.0b013e32834c7565) [DOI] [PubMed] [Google Scholar]
- 134.Uluçay S, Çam FS, Batır MB, Sütçü R, Bayturan Ö, Demircan K. 2015. A novel association between TGFb1 and ADAMTS4 in coronary artery disease: a new potential mechanism in the progression of atherosclerosis and diabetes. Anatol. J. Cardiol. 15, 823–829. ( 10.5152/akd.2014.5762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zha Y, Chen Y, Xu F, Li T, Zhao C, Cui L. 2010. ADAMTS4 level in patients with stable coronary artery disease and acute coronary syndromes. Biomed. Pharmacother 64, 160–164. ( 10.1016/j.biopha.2009.09.012) [DOI] [PubMed] [Google Scholar]
- 136.Zha Y, Chen Y, Xu F, Zhang J, Li T, Zhao C, Cui L. 2010a. Elevated level of ADAMTS4 in plasma and peripheral monocytes from patients with acute coronary syndrome. Clin. Res. Cardiol. 99, 781–786. ( 10.1007/s00392-010-0183-1) [DOI] [PubMed] [Google Scholar]
- 137.Dong H, Du T, Premaratne S, Zhao CX, Tian Q, Li Y, Yan S, Zhang WW.. 2018. Relationship between ADAMTS4 and carotid atherosclerotic plaque vulnerability in humans. J. Vasc. Surg. 67, 1120–1126. ( 10.1016/j.jvs.2017.08.075) [DOI] [PubMed] [Google Scholar]
- 138.Chen YC, et al. 2013. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ. Res. 113, 252–265. ( 10.1161/CIRCRESAHA.113.301562) [DOI] [PubMed] [Google Scholar]
- 139.Li L, Ma W, Pan S, Li Y, Wang H, Wang B, Khalil RA. 2020. MiR-126a-5p limits the formation of abdominal aortic aneurysm in mice and decreases ADAMTS-4 expression. J. Cell. Mol. Med. 24, 7896–7906. ( 10.1111/jcmm.15422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu B, Granville DJ, Golledge J, Kassiri Z. 2020. Pathogenic mechanisms and the potential of drug therapies for aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 318, H652-H670. ( 10.1152/ajpheart.00621.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY. 2018. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 114, 622–634. ( 10.1093/cvr/cvy007) [DOI] [PubMed] [Google Scholar]
- 142.McMahon M, Ye S, Izzard L, Dlugolenski D, Tripp RA, Bean AG, McCulloch DR, Stambas J. 2016. adamts5 is a critical regulator of virus-specific T cell immunity. PLoS Biol. 14, e1002580 ( 10.1371/journal.pbio.1002580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kenagy RD, Min SK, Clowes AW, Sandy JD. 2009. Cell death-associated ADAMTS4 and versican degradation in vascular tissue. J. Histochem. Cytochem. 57, 889–897. ( 10.1369/jhc.2009.953901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Evanko SP, Angello JC, Wight TN. 1999. Formation of hyaluronan and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 1004–1013. ( 10.1161/01.atv.19.4.1004) [DOI] [PubMed] [Google Scholar]
- 145.D'Amours D, Sallmann FR, Dixit VM, Poirier GG. 2001. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J. Cell Sci. 114, 3771–3778. [DOI] [PubMed] [Google Scholar]
- 146.Rao N, Ke Z, Liu H, Ho CJ, Kumar S, Xiang W, Zhu Y, Ge R. 2013. ADAMTS4 and its proteolytic fragments differentially affect melanoma growth and angiogenesis in mice. Int. J. Cancer 133, 294–306. ( 10.1002/ijc.28037) [DOI] [PubMed] [Google Scholar]
- 147.Vistnes M, Aronsen JM, Lunde IG, Sjaastad I, Carlson CR, Christensen G. 2014. Pentosan polysulfate decreases myocardial expression of the extracellular matrix enzyme ADAMTS4 and improves cardiac function in vivo in rats subjected to pressure overload by aortic banding. PLoS ONE 9, e89621 ( 10.1371/journal.pone.0089621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Santamaria S. 2020. ADAMTS-5: a difficult teenager turning 20. Int. J. Exp. Pathol. 101, 4–20. ( 10.1111/iep.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Islam S, et al. 2020. Accumulation of versican facilitates wound healing: implication of its initial ADAMTS-cleavage site. Matrix Biol. 87, 77–93. ( 10.1016/j.matbio.2019.10.006) [DOI] [PubMed] [Google Scholar]
- 150.Didangelos A, Mayr U, Monaco C, Mayr M. 2012. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J. Biol. Chem. 287, 19 341–19 345. ( 10.1074/jbc.C112.350785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McCulloch DR, Le Goff C, Bhatt S, Dixon LJ, Sandy JD, Apte SS.. 2009. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr. Patterns. 9, 314–323. ( 10.1016/j.gep.2009.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zeng T, et al. 2020. ADAMTS-5 decreases in aortas and plasma from aortic dissection patients and alleviates angiotensin II-induced smooth muscle-cell apoptosis. Front. Cardiovasc. Med. 7, 136 ( 10.3389/fcvm.2020.00136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chang MY, Chan CK, Braun KR, Green PS, O'Brien KD, Chait A, Day AJ, Wight TN. 2012. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 287, 14 122–14 135. ( 10.1074/jbc.M111.324988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. 2014. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 34, 1–12. ( 10.1016/j.matbio.2014.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang MY, et al. 2017. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am. J. Phys. Lung Cell. Mol. Phys. 313, L1069–L1086. ( 10.1152/ajplung.00353.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fabunmi RP, Sukhova GK, Sugiyama S, Libby P. 1998. Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells. Circ. Res. 83, 270–278. ( 10.1161/01.res.83.3.270) [DOI] [PubMed] [Google Scholar]
- 157.Johnson JL, Sala-Newby GB, Ismail Y, Aguilera CM, Newby AC. 2008. Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages. Arterioscler. Thromb. Vasc. Biol. 28, 1647–1653. ( 10.1161/ATVBAHA.108.170548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johnson JL, Jenkins NP, Huang WC, Di Gregoli K, Sala-Newby GB, Scholtes VP, Moll FL, Pasterkamp G, Newby AC.. 2014. Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediat. Inflamm. 2014, 276457 ( 10.1155/2014/276457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Di Gregoli K, Mohamad Anuar NN, Bianco R, White SJ, Newby AC, George SJ, Johnson JL.. 2017. MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ. Res. 120, 49–65. ( 10.1161/CIRCRESAHA.116.309321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jones JA, Spinale FG, Ikonomidis JS. 2009. Transforming growth factor-β signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J. Vasc. Res. 46, 119–137. ( 10.1159/000151766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kimura N, et al. 2017. Gene expression profiling of acute type A aortic dissection combined with in vitro assessment. Eur. J. Cardiothorac. Surg. 52, 810–817. ( 10.1093/ejcts/ezx095) [DOI] [PubMed] [Google Scholar]
- 162.Davis FM, et al. 2015. Smooth muscle cell deletion of low-density lipoprotein receptor-related protein 1 augments angiotensin II-induced superior mesenteric arterial and ascending aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 35, 155–162. ( 10.1161/ATVBAHA.114.304683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Collins-Racie LA, et al. 2004. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix. Biol. 23, 219–230. ( 10.1016/j.matbio.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 164.Binder MJ, McCoombe S, Williams ED, McCulloch DR, Ward AC. 2017. The extracellular matrix in cancer progression: role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Lett. 385, 55–64. ( 10.1016/j.canlet.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 165.Wain LV, et al. 2011. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 43, 1005–1011. ( 10.1038/ng.922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vázquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. 1999. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J. Biol. Chem. 274, 23 349–23 357. ( 10.1074/jbc.274.33.23349) [DOI] [PubMed] [Google Scholar]
- 167.Choi GC, et al. 2014. The metalloprotease ADAMTS8 displays antirumor properties through antagonizing EGFR-MEK-ERK signaling and is silenced in cancinomas by CpG methylation. Mol. Cancer Res. 12, 228–238. ( 10.1158/1541-7786.MCR-13-0195) [DOI] [PubMed] [Google Scholar]
- 168.Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. 1999. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev. Biol. 216, 382–393. ( 10.1006/dbio.1999.9491) [DOI] [PubMed] [Google Scholar]
- 169.Clark ME, Kelner GS, Turbeville LA, Boyer A, Arden KC, Maki RA. 2000. ADAMTS9, a novel member of the ADAM-TS/metallospondin gene family. Genomics 67, 343–350. ( 10.1006/geno.2000.6246) [DOI] [PubMed] [Google Scholar]
- 170.Ismat A, Cheshire AM, Andrew DJ. 2013. The secreted AdamTS-A metalloprotease is required for collective cell migration. Development 140, 1981–1993 ( 10.1242/dev.087908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choi YJ, et al. 2019. Mutations of ADAMTS9 cause nephronophthisis-related ciliopathy. Am. J. Hum. Genet. 104, 45–54. ( 10.1016/j.ajhg.2018.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nandadasa S, et al. 2019. Secreted metalloproteases ADAMTS9 and ADAMTS20 have a non-canonical role in ciliary vesicle growth during ciliogenesis. Nat. Commun. 10, 953 ( 10.1038/s41467-019-08520-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gäbel G, et al. 2017. Molecular fingerprint for terminal abdominal aortic aneurysm disease. J. Am. Heart Assoc. 6, e006798 ( 10.1161/JAHA.117.006798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pu X, et al. 2013. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am. J. Hum. Genet. 92, 366–374. ( 10.1016/j.ajhg.2013.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bengtsson E, et al. 2017. ADAMTS-7 is associated with a high-risk plaque phenotype in human atherosclerosis. Sci. Rep. 7, 3753 ( 10.1038/s41598-017-03573-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pi L, et al. 2015. A disintegrin and metalloprotease with thrombospondin type I motif 7: a new protease for connective tissue growth factor in hepatic progenitor/oval cell niche. Am. J. Pathol. 185, 1552–1563. ( 10.1016/j.ajpath.2015.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Santamaria S, Crawley JTB, Yamamoto K, Ahnstrom J, de Groot R.. 2017. A comparison of COMP (TSP5) proteolysis by ADAMTS7 and ADAMTS4. Int. J. Exp. Biol. 98, A3–A4. [Google Scholar]
- 178.Colige A, Monseur C, Crawley JT B, Santamaria S, de Groot R.. 2019. Proteomic discovery of substrates of the cardiovascular protease ADAMTS7. J. Biol. Chem. 294, 8037–8045. ( 10.1074/jbc.RA119.007492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bultmann-Mellin I, Essers J, van Heijingen PM, von Melchner H, Sengle G, Sterner-Kock A.. 2016. Function of Ltbp-4 L and fibulin-4 in survival and elastogenesis in mice. Dis. Model Mech. 9, 1367–1374. ( 10.1242/dmm.026005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sterner-Kock A, Thorey IS, Koli K et al. . 2002. Disruption of the gene encoding the latent transforming growth factor-β binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 16, 2264–2273. ( 10.1101/gad.229102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kumra H, Nelea V, Hakami H et al. . 2019. Fibulin-4 exerts a dual role in LTBP-4 L-mediated matrix assembly and function. Proc. Natl Acad. Sci. USA 116, 20 428–20 437. ( 10.1073/pnas.1901048116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Noda K, Dabovic B, Takagi K et al. . 2013. Latent TGF-β binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc. Natl Acad. Sci. USA 110, 2852–2857. ( 10.1073/pnas.1215779110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Langley SR, Willeit K, Didangelos A et al. . 2017. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J. Clin. Invest. 127, 1546–1560. ( 10.1172/JCI86924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koskivirta I, et al. 2006. Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in inflammatory processes of human cardiovascular pathology. Histochem. Cell Biol. 126, 335–342. ( 10.1007/s00418-006-0163-8) [DOI] [PubMed] [Google Scholar]
- 185.Feys HB, Liu F, Dong N, Pareyn I, Vauterin S, Vandeputte N, Noppe W, Ruan C, Deckmyn H, Vanhoorelbeke K. 2006. ADAMTS-13 plasma level determination uncovers antigen absence in acquired thrombotic thrombocytopenic purpura and ethnic differences. J. Thromb. Haemost. 4, 955–962. ( 10.1111/j.1538-7836.2006.01833.x) [DOI] [PubMed] [Google Scholar]
- 186.Scully M, et al. 2017. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 15, 312–322. ( 10.1111/jth.13571) [DOI] [PubMed] [Google Scholar]
- 187.Scully M, Yarranton H, Liesner R, Cavenagh J, Hunt B, Benjamin S, Bevan D, Mackie I, Machin S. 2008. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br. J. Haematol. 142, 819–826. ( 10.1111/j.1365-2141.2008.07276.x) [DOI] [PubMed] [Google Scholar]
- 188.Scully MA, Cataland SR. 2020. Fast facts: thrombotic thrombocytopenic purpura: prompt action saves lives. Basel, Switzerland: Karger Medical and Scientific Publishers. [Google Scholar]
- 189.Alwan F, et al. 2019. Characterization and treatment of congenital thrombotic thrombocytopenic purpura. Blood 133, 1644–1651. ( 10.1182/blood-2018-11-884700) [DOI] [PubMed] [Google Scholar]
- 190.Thomas MR, de Groot R, Scully MA, Crawley JT.. 2015. Pathogenicity of anti-ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. EBioMedicine 2, 942–952. ( 10.1016/j.ebiom.2015.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pos W, Luken BM, Kremer Hovinga JA, Turenhout EA, Scheiflinger F, Dong JF, Fijnheer R, Voorberg J. 2009. VH1–69 germline encoded antibodies directed towards ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 7, 421–428. ( 10.1111/j.1538-7836.2008.03250.x) [DOI] [PubMed] [Google Scholar]
- 192.Verbij FC, Fijnheer R, Voorberg J, Sorvillo N. 2014. Acquired TTP: ADAMTS13 meets the immune system. Blood Rev. 28, 227–234. ( 10.1016/j.blre.2014.07.004) [DOI] [PubMed] [Google Scholar]
- 193.de Groot R, Bardhan A, Ramroop N, Lane DA, Crawley JT.. 2009. Essential role of the disintegrin-like domain in ADAMTS13 function. Blood 113, 5609–5616. ( 10.1182/blood-2008-11-187914) [DOI] [PubMed] [Google Scholar]
- 194.de Groot R, Lane DA, Crawley JT.. 2015. The role of the ADAMTS13 cysteine-rich domain in VWF binding and proteolysis. Blood 125, 1968–1975. ( 10.1182/blood-2014-08-594556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gao W, Anderson PJ, Sadler JE. 2008. Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood 112, 1713–1719. ( 10.1182/blood-2008-04-148759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Westwood JP, et al. 2017. Rituximab prophylaxis to prevent thrombotic thrombocytopenic purpura relapse: outcome and evaluation of dosing regimens. Blood Adv. 1, 1159–1166. ( 10.1182/bloodadvances.2017008268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scully M, et al. 2019. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N. Engl. J. Med. 380, 335–346. ( 10.1056/NEJMoa1806311) [DOI] [PubMed] [Google Scholar]
- 198.Guo C, Tsigkou A, Lee MH. 2016. ADAMTS13 and 15 are not regulated by the full length and N-terminal domain forms of TIMP-1, -2, -3 and -4. Biomed. Rep. 4, 73–78. ( 10.3892/br.2015.535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. 2009. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science 324, 1330–1334. ( 10.1126/science.1170905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dong JF, et al. 2002. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 100, 4033–4039. ( 10.1182/blood-2002-05-1401) [DOI] [PubMed] [Google Scholar]
- 201.Jakobi AJ, Mashaghi A, Tans SJ, Huizinga EG. 2011. Calcium modulates force sensing by the von Willebrand factor A2 domain. Nat. Commun. 2, 385 ( 10.1038/ncomms1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. 2013. Unwinding the von Willebrand factor strings puzzle. Blood 121, 270–277. ( 10.1182/blood-2012-07-442285) [DOI] [PubMed] [Google Scholar]
- 203.Pos W, Crawley JT, Fijnheer R, Voorberg J, Lane DA, Luken B. M. 2010. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood 115, 1640–1649. ( 10.1182/blood-2009-06-229203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kretz CA, Dai M, Soylemez O et al. . 2015. Massively parallel enzyme kinetics reveals the substrate recognition landscape of the metalloprotease ADAMTS13. Proc. Natl Acad. Sci. USA 112, 9328–9333. ( 10.1073/pnas.1511328112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Petri A, Kim HJ, Xu Y, de Groot R, Li C, Vandenbulcke A, Vanhoorelbeke K, Emsley J, Crawley JTB. 2019. Crystal structure and substrate-induced activation of ADAMTS13. Nat. Commun. 10, 3781 ( 10.1038/s41467-019-11474-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.de Groot R, Lane DA, Crawley JT.. 2010. The ADAMTS13 metalloprotease domain: roles of subsites in enzyme activity and specificity. Blood 116, 3064–3072. ( 10.1182/blood-2009-12-258780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xiang Y, de Groot R, Crawley JT, Lane DA.. 2011. Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). Proc. Natl Acad. Sci. USA 108, 11 602–11 607. ( 10.1073/pnas.1018559108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Andersson HM, Siegerink B, Luken BM, Crawley JTB, Algra A, Lane DA, Rosendaal F R. 2012. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood 119, 1555–1560. ( 10.1182/blood-2011-09-380618) [DOI] [PubMed] [Google Scholar]
- 209.Lambers M, et al. 2013. Role of reduced ADAMTS13 in arterial ischemic stroke: a pediatric cohort study. Ann. Neurol. 73, 58–64. ( 10.1002/ana.23735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sonneveld MA, et al. 2015. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood 126, 2739–2746. ( 10.1182/blood-2015-05-643338) [DOI] [PubMed] [Google Scholar]
- 211.Taylor A, Vendramin C, Singh D, Brown MM, Scully M. 2020. von Willebrand factor/ADAMTS13 ratio at presentation of acute ischemic brain injury is predictive of outcome. Blood Adv. 4, 398–407. ( 10.1182/bloodadvances.2019000979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Upreti H, et al. 2019. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood 134, 1037–1045. ( 10.1182/blood.2019001056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. 2009. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood 114, 3329–3334. ( 10.1182/blood-2009-03-213264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Denorme F, et al. 2016. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood 127, 2337–2345. ( 10.1182/blood-2015-08-662650) [DOI] [PubMed] [Google Scholar]
- 215.Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapière CM. 1997. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zincmetalloproteinases with binding sites for cells and other matrix components. Proc. Natl Acad. Sci. USA 94, 2374–2379. ( 10.1073/pnas.94.6.2374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rau CD, et al. 2017. Systems genetics approach identifies gene pathways and Adamts2 as drivers of isoproterenol-induced cardiac hypertrophy and cardiomyopathy in mice. Cell Syst. 4, 121–128. ( 10.1016/j.cels.2016.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dhingra R, et al. 2006. Electrocardiographic QRS duration and the risk of congestive heart failure: the Framingham heart study. Hypertension 47, 861–867. ( 10.1161/01.HYP.0000217141.20163.23) [DOI] [PubMed] [Google Scholar]
- 218.Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. 2011. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ. Arrhythm Electrophysiol. 4, 704–710. ( 10.1161/CIRCEP.111.963561) [DOI] [PubMed] [Google Scholar]
- 219.Kashani A, Barold SS. 2005. Significance of QRS complex duration in patients with heart failure. J. Am. Coll. Cardiol. 46, 2183–2192. ( 10.1016/j.jacc.2005.01.071) [DOI] [PubMed] [Google Scholar]
- 220.Dagoneau N, et al. 2004. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 75, 801–806. ( 10.1086/425231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Morales J, et al. 2009. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 85, 558–568. ( 10.1016/j.ajhg.2009.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kojuri J, Razeghinejad MR, Aslani A. 2007. Cardiac findings in Weill-Marchesani syndrome. Am. J. Med. Genet. Part A 143, 2062–2064. ( 10.1002/ajmg.a.31861) [DOI] [PubMed] [Google Scholar]
- 223.Mularczyk EJ, et al. 2018. ADAMTS10-mediated tissue disruption in Weill-Marchesani syndrome. Hum. Mol. Genet. 27, 3675–3687. ( 10.1093/hmg/ddy276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thomson J, Singh M, Eckersley A, Cain SA, Sherratt MJ, Baldock C. 2019. Fibrillin microfibrils and elastic fibre proteins: functional interactions and extracellular regulation of growth factors. Semin. Cell Dev. Biol. 89, 109–117. ( 10.1016/j.semcdb.2018.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kutz WE, Wang LW, Bader HL, Majors AK, Iwata K, Traboulsi EI, Sakai LY, Keene DR, Apte SS. 2011. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 286, 17156–17 167. ( 10.1074/jbc.M111.231571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sengle G, et al. 2012. Microenvironmental regulation by fibrillin-1. PLoS Genet. 8, e1002425 ( 10.1371/journal.pgen.1002425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang LW, Kutz WE, Mead TJ, Beene LC, Singh S, Jenkins MW, Reinhardt DP, Apte SS. 2019. Adamts10 inactivation in mice leads to persistence of ocular microfibrils subsequent to reduced fibrillin-2 cleavage. Matrix. Biol. 77, 117–128. ( 10.1016/j.matbio.2018.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stanley S, Balic Z, Hubmacher D. 2020. Acromelic dysplasias: how rare musculoskeletal disorders reveal biological functions of extracellular matrix proteins. Ann. N. Y. Acad. Sci. Sep. 2 ( 10.1111/nyas.14465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Abdul-Majeed S, Mell B, Nauli S. M., Joe B (2014) Cryptorchidism and infertility in rats with targeted disruption of the Adamts16 locus. PLoS ONE 9, e100967 ( 10.1371/journal.pone.0100967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Livermore C, Warr N, Chalon N, Siggers P, Mianné J, Codner G, Teboul L, Wells S, Greenfield A. 2019. Male mice lacking ADAMTS-16 are fertile but exhibit testes of reduced weight. Sci. Rep. 9, 17195 ( 10.1038/s41598-019-53900-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schnellmann R, Sack R, Hess D, Annis DS, Mosher DF, Apte SS, Chiquet-Ehrismann R. 2018. A selective extracellular matrix proteomics approach identifies fibronectin proteolysis by a disintegrin-like and metalloprotease domain with thrombospondin Type 1 motifs (ADAMTS16) and its impact on spheroid morphogenesis. Mol. Cell. Proteomics. 17, 1410–1425. ( 10.1074/mcp.RA118.000676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rice T, Rankinen T, Province MA, Chagnon YC, Pérusse L, Borecki IB, Bouchard C, Rao DC. 2000. Genome-wide linkage analysis of systolic and diastolic blood pressure: the Québec Family study. Circulation 102, 1956–1963. ( 10.1161/01.cir.102.16.1956) [DOI] [PubMed] [Google Scholar]
- 233.Joe B, et al. 2009. Positional identification of variants of Adamts16 linked to inherited hypertension. Hum. Mol. Genet. 18, 2825–2838. ( 10.1093/hmg/ddp218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Joe B, Garrett MR, Dene H, Rapp JP. 2003. Substitution mapping of a blood pressure quantitative trait locus to a 2.73 Mb region on rat chromosome 1. J. Hypertens. 21, 2077–2084. ( 10.1097/00004872-200311000-00017) [DOI] [PubMed] [Google Scholar]
- 235.Santamaria S, de Groot R.. 2019. Monoclonal antibodies against metzincin targets. Br. J. Pharmacol. 176, 52–66. ( 10.1111/bph.14186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Santamaria S, Nagase H. 2018. Measurement of protease activities using fluorogenic substrates. Methods Mol. Biol. 1731, 107–122. ( 10.1007/978-1-4939-7595-2_11) [DOI] [PubMed] [Google Scholar]
- 237.Schelpe AS, Petri A, Roose E, Pareyn I, Deckmyn H, De Meyer SF, Crawley JTB, Vanhoorelbeke K.. 2020. Antibodies that conformationally activate ADAMTS13 allosterically enhance metalloprotease domain function. Blood Adv. 4, 1072–1080. ( 10.1182/bloodadvances.2019001375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Santamaria S, Fedorov O, McCafferty J, Murphy G, Dudhia J, Nagase H, Yamamoto K. 2017. Development of a monoclonal anti-ADAMTS-5 antibody that specifically blocks the interaction with LRP1. MAbs 9, 595–602. ( 10.1080/19420862.2017.1304341) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.