Significance
Stem xylem uptake and transport of plant source water is widely considered a critical component of the global water cycle. The deuterium signature of cryogenically extracted plant stem water (δ2Hstem_CVD) provides a useful means for studying water fluxes, but recent studies have shown uncertainty as to whether δ2Hstem_CVD is a faithful reflection of true source water isotopic signal (δ2Hsource). Here we confirm the common presence of significant deviations in δ2Hstem_CVD from δ2Hsource in various plant species and demonstrate that this phenomenon is not caused by deuterium fractionation during root water uptake as traditionally thought, but rather is rooted in a cryogenic extraction-associated methodological artifact. Our findings may have wide-ranging implications for isotope-based ecohydrological and climate change studies.
Keywords: cryogenic vacuum distillation, deuterium isotope, source water, plant water uptake, ecohydrological separation
Abstract
The hydrogen isotope ratio of water cryogenically extracted from plant stem samples (δ2Hstem_CVD) is routinely used to aid isotope applications that span hydrological, ecological, and paleoclimatological research. However, an increasing number of studies have shown that a key assumption of these applications—that δ2Hstem_CVD is equal to the δ2H of plant source water (δ2Hsource)—is not necessarily met in plants from various habitats. To examine this assumption, we purposedly designed an experimental system to allow independent measurements of δ2Hstem_CVD, δ2Hsource, and δ2H of water transported in xylem conduits (δ2Hxylem) under controlled conditions. Our measurements performed on nine woody plant species from diverse habitats revealed a consistent and significant depletion in δ2Hstem_CVD compared with both δ2Hsource and δ2Hxylem. Meanwhile, no significant discrepancy was observed between δ2Hsource and δ2Hxylem in any of the plants investigated. These results cast significant doubt on the long-standing view that deuterium fractionation occurs during root water uptake and, alternatively, suggest that measurement bias inherent in the cryogenic extraction method is the root cause of δ2Hstem_CVD depletion. We used a rehydration experiment to show that the stem water cryogenic extraction error could originate from a dynamic exchange between organically bound deuterium and liquid water during water extraction. In light of our finding, we suggest caution when partitioning plant water sources and reconstructing past climates using hydrogen isotopes, and carefully propose that the paradigm-shifting phenomenon of ecohydrological separation (“two water worlds”) is underpinned by an extraction artifact.
The analysis of the stable isotope ratios of plant source water (δsource) is a powerful tool enabling the elucidation of a range of plant physiological, ecological, and hydrological processes from scales ranging from individual plants to the planet. δsource provides a foundation on which to form isotope signals of transpired water vapor and plant-derived biomarkers (i.e., cellulose and lipids) and thus is of high relevance to studies of terrestrial water fluxes (1, 2) and paleoclimate reconstructions (3, 4). δsource also contains information on the spatial and temporal origins of water used by plants and so is commonly used for investigating plant water uptake patterns under natural conditions (5, 6). Moreover, dual-isotope (δ2H and δ18O) analysis of δsource was critical in formulating the paradigm-shifting “two water worlds” (TWW) hypothesis, whereby ecohydrological separation exists between plant-accessible soil water pools and those recharging streams and groundwater (7, 8).
Elucidation of the foregoing processes rest on the assumption that water extracted from plant stems is isotopically identical to water taken up by plant roots. Plant stem water is typically extracted with the cryogenic-vacuum distillation technique; δ generated with this method is hereinafter referred as δstem_CVD (9). For δstem_CVD to be an accurate indicator of δsource (i.e., δstem_CVD = δsource), two prerequisites must be met: 1) isotope change does not occur during root uptake and/or xylem transport of the source water (prerequisite I) and 2) stem water cryogenic extraction is a robust approach toward isotope recovery of xylem water (prerequisite II). The “δstem_CVD = δsource” assumption is generally valid for oxygen isotopes of water, but numerous studies have used hydrogen isotopes to assess source water, and here this assumption has faced scrutiny, as multiple studies have reported significant depletion in δ2Hstem_CVD compared with δ2Hsource in plants from various habitats (10–18).
A frequently invoked explanation for the observed δ2Hstem_CVD depletion is a violation of prerequisite I, as it is believed that symplastic uptake of source water into the root xylem can give rise to hydrogen isotope fractionation (10, 11, 13, 19). The available evidence (10, 11) in support of such an explanation is largely peripheral, because direct, unambiguous confirmation of water uptake/transport-related fractionation would require a comparison of deuterium in source water and water transported within xylem conduits (δ2Hxylem). However, this type of comparison is difficult owing to the technical challenges in obtaining targeted measurements of δ2Hxylem in most plants. Intriguingly, in a field-grown riparian tree species (Populus euphratica) in which δ2Hxylem measurement was made possible with the aid of a syringe-aided xylem sap bleeding technique, no significant difference was observed between δ2Hxylem and δ2Hsource (12). This led to the suggestion that, at least for the investigated species, δ2Hstem_CVD depletion arises not from a violation of prerequisite I, but rather from a violation of prerequisite II. The violation of prerequisite II has been deemed possible (12, 17) based on the argument that hydrogen isotope heterogeneity could be present within the bulk stem water (i.e., the outside xylem water may carry a metabolism-induced, more-depleted δ2H signature compared with the xylem water), potentially causing the stem water extraction technique to artifactually underestimate δ2Hxylem.
Given the controvertible state of knowledge regarding the mechanism driving δ2Hstem_CVD depletion, it is imperative for us to build a better and more comprehensive understanding of the isotopic relationships among cryogenic extracted bulk stem water, source water, and xylem water in different plants, so as to put the application of the stem water cryogenic extraction technique in diverse fields on firmer ground. In this context, it should be pointed out that the xylem water direct sampling technique (12) is applicable only to a few riparian tree species. Recently, a new method relying on laser-enabled isotope measurement of water vapor in equilibrium with xylem water has demonstrated potential for in situ continuous monitoring of xylem isotope signatures in trees (20, 21); however, the method needs further development before it becomes broadly applicable to different plant types. Thus, a more generally applicable method is needed for determining xylem water signature across diverse plant types.
Toward this goal, and capitalizing on the well-recognized mass balance-dictated principle that the isotopic composition of steady-state (SS) plant transpiration is identical to that of the xylem water supplying the plant canopy, we custom-designed a measurement system to enable independent quantification of xylem water isotope composition through isotope measurement of SS plant transpiration. This measurement system conferred the ability to compare values of δstem_CVD, δsource, and δxylem across a number of plant species of varying native habitats. The data allowed us to confirm the common presence of δ2Hstem_CVD depletion across all plant types measured, and also to demonstrate that this phenomenon is caused by cryogenic extraction-associated artifact and not by water uptake/transport-related fractionation. We also performed a rehydration experiment to illustrate that the extraction artifact is unrelated to within-stem isotope heterogeneity as has been recently suggested, but rather is more likely linked to a deuterium-exchange process that occurs dynamically during cryogenic extraction. Using the TWW hypothesis as an example, we further discuss the ramifications for ecological/hydrological queries that rely on accurate isotopic information on plant source/xylem water.
Results and Discussion
Measurements of δxylem, δstem_CVD, and δsource were performed in pot-grown seedlings of nine plant species native to a variety of habitats (i.e., saline, xeric, and mesic). Specifically, δxylem was determined through isotopic measurement of plant transpiration at SS, and δstem_CVD and δsource were determined through isotopic analysis of cryogenically extracted stem water and soil water, respectively. Note that the main focus of the present study was on the cryogenic extraction technique as applied to plant stem samples, and that we consider cryogenic extraction of soil water a valid method for recovering δsource, as we discuss below. Complete details are provided in Materials and Methods and SI Appendix.
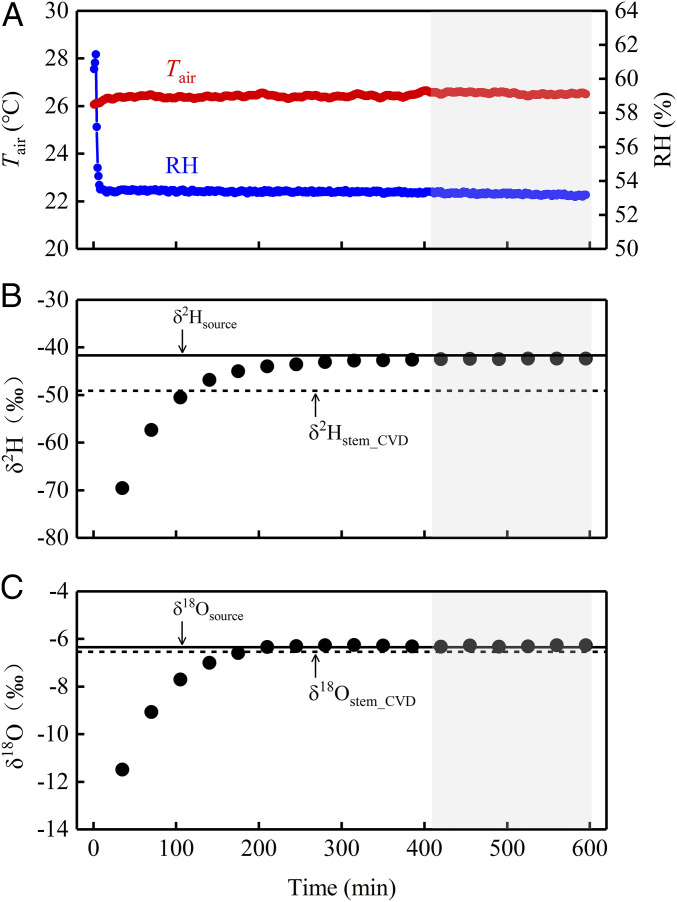
Fig. 1A exemplifies the ability of our experimental system to provide a stable within-chamber environmental condition (i.e., stability in both air temperature and relative humidity [RH]) throughout the transpiration isotope monitoring period, during which the plant canopy was enclosed in a whole-plant flow-through chamber coupled to a water vapor laser spectrometer. Because environmental stability was ensured (SI Appendix), oxygen and hydrogen isotope ratios of plant transpiration exhibited variations with time following a typical exponential trajectory toward the SS, and, more importantly, these variables were able to persist at SS once it was reached, as clearly shown in Fig. 1 B and C. The capacity for SS maintenance as conferred by our measurement system constituted an indispensable prerequisite for unbiased determination of isotope composition of SS transpiration (and consequently of xylem water) using the laser isotope instrument.
Fig. 1.
Example time series of within-chamber air temperature (Tair) and RH (A), δ2H (B), and δ18O (C) of transpired water vapor for an individual plant (T. chinensis) during the period of enclosure within a whole-canopy chamber for online, continuous transpiration isotope measurements. The shaded region denotes the period of ISS. The solid and dashed horizontal lines in B and C indicate isotope ratios of cryogenically extracted soil (or source water) and stem water, respectively.
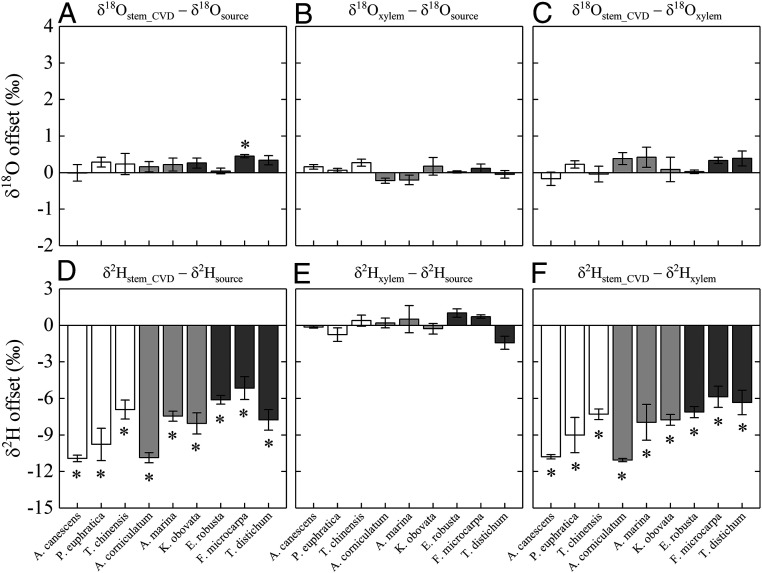
With respect to oxygen isotopes (Fig. 2 A and B), we found that offsets of both extracted stem water and xylem water from the source water signal were consistently small in magnitude and mostly not statistically different from zero (across-species range: −0.01‰ to 0.45‰ and −0.22‰ to 0.28‰ for deviations of δ18Ostem_CVD and δ18Oxylem from δ18Osource, respectively). The observed nil or minor oxygen isotope offsets are in line with previous studies that showed a sufficient degree of accuracy with which to use stem water cryogenic extraction for inferring δ18Osource or δ18Oxylem in various plants (10, 17, 22–24).
Fig. 2.
Hydrogen and oxygen isotope offsets between source water, xylem water, and cryogenically extracted stem water in the nine plant species. A, B, and C refer to oxygen isotope offsets between cryogenically extracted stem water and source water, xylem water and source water, and cryogenically extracted stem water and source water, respectively. D, E, and F refer to hydrogen isotope offsets between cryogenically extracted stem water and source water, xylem water and source water, and cryogenically extracted stem water and source water, respectively. Presence of asterisk above or below a bar indicates that the value is significantly different from zero. White, light-gray, and dark-gray bars indicate species from xeric, saline, and mesic habitats, respectively.
With respect to deuterium isotopes, however, all nine species displayed considerable isotopic offsets between extracted stem water and source water (Fig. 2D). Values of such offsets were always significantly below zero irrespective of species identity/habitat type (across-species range: −10.9‰ to −5.2‰; mean: −8.1‰). According to the results of a nested ANOVA, these values differed significantly among species (P < 0.05) but not among habitats (P = 0.136). These results contribute to the growing body of literature reporting a tendency for cryogenically extracted stem water to deviate negatively from source water in δ2H in plants from both “extreme” (i.e., saline or xeric) and “nonextreme” (i.e., mesic) environments (10, 11, 13, 16), reinforcing the notion that δ2Hstem_CVD depletion is a common phenomenon not necessarily restricted to a particular habitat type. The observed δ2Hstem_CVD–δ2Hsource offsets notwithstanding, further comparison of deuterium signals revealed no isotopic separation between xylem water and source water, as is clearly visible in Fig. 2E, where all species display only minor deviations of δ2Hxylem from δ2Hsource that were not statistically different from zero. Notably, such pervasive exhibition of a similarity between δ2Hxylem and δ2Hsource despite the δ2Hstem_CVD–δ2Hsource discrepancy not only agrees with, but also extends beyond the previous findings (12) shown in a single species (note that this same species was also included in the present study), although the more generally applicable method that we used for xylem water isotope measurement was distinctively different from that used previously (12).
Based on the consistently observed deuterium matching between xylem water and source water, we can reasonably conclude that plants generally do not fractionate against 2H during source water uptake/transport, irrespective of habitat type. Importantly, this conclusion is in direct contrast to the long-held view that water uptake-associated fractionation is pervasive at least in certain plant types (e.g., mangroves, woody xerophytes). Such a contrast highlights the critical importance of obtaining unbiased xylem water signatures for evaluating isotope effect (or lack thereof) along the pathway of water movement into and through the xylem.
Due to an inability to measure δ2Hxylem explicitly, previous studies that came to the “deuterium fractionation” conclusion had to assume a priori that δ2Hstem_CVD faithfully reflects δ2Hxylem when interpreting an observed discrepancy between δ2Hstem_CVD and δ2Hsource. However, the “δ2Hstem_CVD = δ2Hxylem” assumption is incorrect, as demonstrated by our observation that deuterium deviations in cryogenically extracted stem water from xylem water were consistently and significantly below zero in all species (Fig. 2F), which hints at a systematic tendency for the stem water cryogenic extraction technique to bias δ2Hxylem estimates.
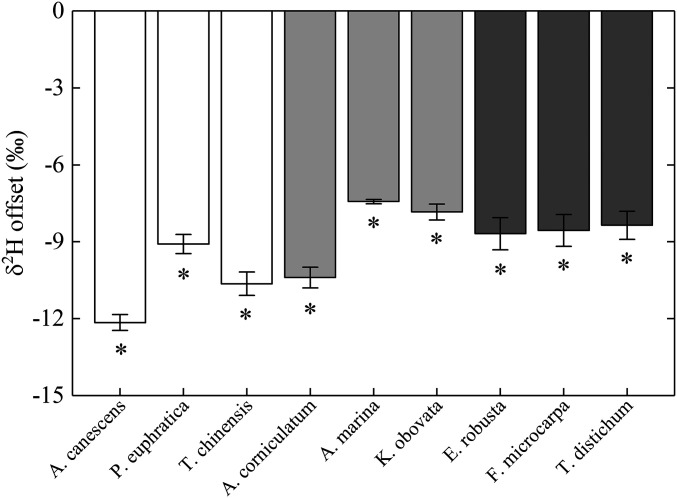
To further explore possibility of isotopic heterogeneity in stem water—that the deuterium offset is a result of extracted water being a combination of a deuterium-depleted outside-xylem water pool (i.e., water within metabolically active parenchyma cells) and more enriched xylem water (12)—we performed a rehydration experiment. For this, cryogenically extracted dry stem samples were immersed into water of known isotope compositions for 24 h. Because the reference water was sufficiently large in volume and the immersion time was sufficiently long, we would expect our rehydration procedure to result in a complete isotope labeling of the bulk stem water by the reference signal. This in turn means that any preexistent isotope heterogeneity within the stem should be effectively eliminated after rehydration. However, despite this expectancy, we found that δ2H depletion persisted between water extracted from the rehydrated samples and the reference water (Fig. 3), with the magnitude of depletion (across-species range: −12.2‰ to −7.4‰, mean: −9.2‰) similar to that of the deuterium difference between the extracted stem water and xylem water from the SS transpiration experiment. This result, combined with the current lack of any direct, empirical evidence to support the existence of an isotopically depleted outside-xylem water pool within the plant stem, led us to rule out “within-stem isotope heterogeneity” as the root cause of the observed δ2Hstem_CVD–δ2Hxylem offsets.
Fig. 3.
Hydrogen isotope offset between water extracted from the rehydrated stem samples from the reference water. The presence of an asterisk above or below a bar indicates that the value is significantly different from zero. White, light-gray, and dark-gray bars indicate species from xeric, saline, and mesic habitats, respectively.
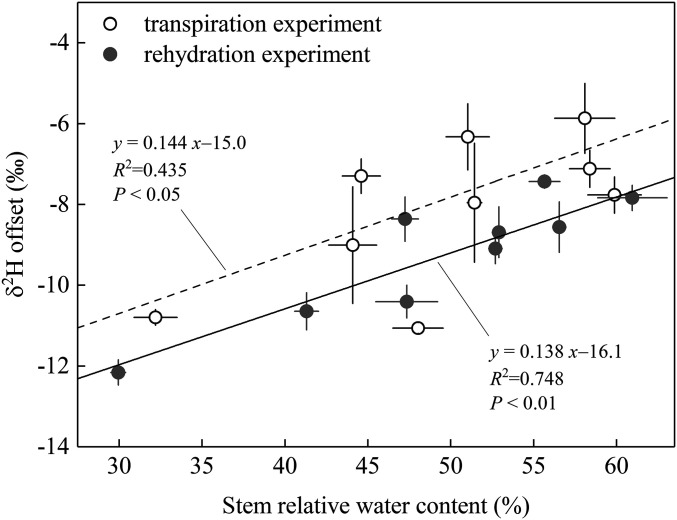
Alternatively, we suggest that the incapability for δ2Hstem_CVD to accurately reflect δ2Hxylem may be related to the fact that an appreciable portion of stem organic H (i.e., up to 30% in cellulose) (25–28) is exchangeable with environmental water. In the presence of deuterium exchange, we would expect cryogenic extraction to increase the δ2H value of stem organic matter as a result of progressive enrichment of the substrate water with which stem organic matter may dynamically exchange (i.e., stem residual water) during water distillation. Given the resultant deuterium enrichment in stem organic matter, isotope mass balance predicts that the bulk stem water collected on completion of the extraction must become deuterium-depleted, consistent with our observed direction of change in δ2Hstem_CVD from δ2Hxylem. Furthermore, from the same mass balance considerations, we anticipate that deuterium exchange-caused δ2Hstem_CVD depletion should be more pronounced when stem water content is low compared to when it is high (i.e., the effect should be more damped when water abundance is high). This possibility is also supported by our data showing a significantly positive across-species correlation of stem relative water content with the δ2Hstem_CVD–δ2Hxylem discrepancy in the SS transpiration experiment, and also similarly with the δ2Hstem_CVD–δ2Hreference discrepancy in the rehydration experiment (Fig. 4). Fig. 4 further implies that stem relative water content may serve as a predictor of the extent of the measurement bias associated with cryogenic extraction. Therefore, to effectively correct for cryogenic method-associated artifact in δ2Hxylem estimation, we recommend that stem relative water content should be always determined when performing stem water cryogenic extraction.
Fig. 4.
Relationships of stem relative water content to δ2Hstem_CVD − δ2Hxylem offset in the ISS transpiration experiment (open dots) and to δ2Hstem_CVD - δ2Hreference offset in the rehydration experiment (closed dots). No significant difference was detected between the two fitted lines (P > 0.05 for comparisons in both slope and intercept).
Central to the mechanism proposed above is the requirement that stem samples subjected to water extraction be exposed to an environment in which the δ2H of the surrounding water is dynamically changing, such as from progressive enrichment during water evaporation. It is reasonable to infer that a deuterium-exchange–related artifact may be similarly present in other methods that also involve water removal through evaporation, such as azeotropic distillation (29) and microwave extraction (30), but it is unlikely to be a significant source of error for methods that extract liquid water directly, such as mechanical squeezing (31) and centrifugation (32). The recently developed equilibration-based measurement methods (33–35) are also expected to be little influenced by this type of artifact, because these techniques eliminate the water extraction step completely.
Aside from its effect on stem samples, deuterium exchange could also exert an influence on water extraction from soil samples with high organic content. This effect is expected to be minimal in our study, however, because we used sandy soils for plant growth, which typically contain only a minor portion of organic matter in their dry matter and hence provide a limited opportunity for H exchange. Indeed, in a separate rehydration test, we found no significant difference in deuterium between water extracted from fully rehydrated sandy soils (δ2H, −45.4 ± 0.10‰; δ18O, −6.93 ± 0.01‰) and the reference water (δ2H, −45.0 ± 0.07‰; δ18O, −7.11 ± 0.03‰). This agrees with the available evidence in the literature indicating that sandy soils are generally not prone to extraction artifacts (23, 36–38), justifying the use of the soil water cryogenic extraction approach for determining source water signals in the present study. Nevertheless, recent studies have uncovered complex mechanisms that could cause cryogenic extraction to yield biased isotope values for certain soil types under certain conditions (39–44). More research is clearly needed to fully understand how the extraction method influences isotopic recovery from these soil types, especially in context of realistic drying and rewetting cycles as would occur in nature (43).
Implications for Isotope-Based Ecological/Hydrological Research.
While the erroneous δ2Hstem_CVD = δ2Hxylem assumption impacts many previous studies using stable isotopes of water, it is perhaps the conclusion of ecohydrological separation, or the TWW hypothesis, on which the impact is the greatest. Thus far, most of the published work claiming support for TWW have relied on evidence of “isotopic separation,” which when viewed in a δ2H-δ18O dual space is equivalent to a pattern of plant xylem water (typically assessed by cryogenically extracted stem water) plotting together with the soil water along an evaporation line but below the local meteoric water line (LMWL) on which precipitation, stream flow, and groundwater typically fall (7, 45, 46). However, there is cause to question whether, and if so, to what extent, the published “isotopic separation” patterns would still hold on correction of the extraction-caused underestimation in δ2Hxylem, as any attempt toward such a correction would inevitably shift the xylem water line up in the dual isotope space, resulting in a closer proximity to (or even an overlap with) the LMWL and a pattern inconsistent with the TWW concept.
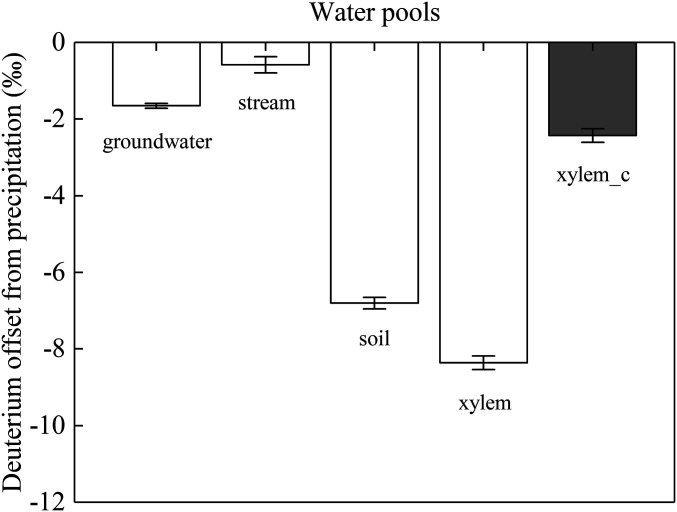
To demonstrate the relevance of our work to the TWW hypothesis, we reanalyzed data from a recent global-scale study that examined “isotopic separation” through analysis of deuterium offsets of various ecosystem water pools from local precipitation (8). In that study, worldwide occurrence of ecohydrological separation (or TWW) was concluded based largely on the observation that the globally averaged xylem water deuterium offset from the local precipitation was in close proximity to its soil water counterpart but considerably and negatively deviated from those of groundwater and stream water. However, when we reanalyzed these data by taking the extraction-caused δ2Hxylem bias into account (details in SI Appendix), we arrived at a different pattern in which the recalculated global average of precipitation offset of xylem water is much closer to the offsets of groundwater and stream water than to the offset of soil water (Fig. 5). Therefore, the extraction error-corrected result tends to nullify support for ecohydrological separation as a globally widespread phenomenon and lends support to the traditional perspective that plant-accessible water pools are for the most part well connected to those recharging streams and groundwater (47, 48).
Fig. 5.
Globally averaged precipitation offsets of groundwater (groundwater as labeled in the figure), stream water (stream), soil water (soil), and xylem water with (xylem_c) and without (xylem) taking cryogenic extraction-caused bias into consideration. The values presented were calculated from an extensive global compilation of data (8). SI Appendix, Eq. S3 was used to convert xylem to xylem_c. More details are provided in SI Appendix.
The foregoing example was intended to illustrate the need for measurement artifact correction in a particular research area; nonetheless, accounting for extraction-generated deuterium bias could be expected to benefit many other isotope applications to which stem water cryogenic extraction is routinely applied, including, but not limited to, plant water source apportionment, evapotranspiration partitioning, isotope modeling of leaf water and plant organic matter (i.e., leaf wax), and its associated application to climate reconstructions. In the present study, we carefully quantified deuterium offsets in a range of plant species and found a linear correlation between stem relative water content and δ2Hstem_CVD–δ2Hxylem offset. These results not only demonstrate a critical “missing piece” in cryogenic extraction-based isotope studies, but also can serve as a basis to guide future efforts to obtain correct source/xylem water deuterium information from cryogenic extraction-generated “raw” data. As such, the results of our study have important implications with respect to interpretation of ecological and hydrological processes in a wide range of isotope applications.
Materials and Methods
Plant Species and Growth Conditions.
Here 2- to 4-y-old saplings of nine plant species—including three mangrove species from saline habitats (Aegiceras corniculatum, Avicennia marina, and Kandelia obovata), one shrub and two tree species native to arid areas (Atriplex canescens, P. euphratica, and Tamarix chinensis) and three tree species from mesic habitats (Eucalyptus robusta, Ficus microcarpa, and Taxodium distichum)—were grown individually in 5-L sandy-soil-filled, free-draining pots in a controlled-environment room, under day/night temperature of 25/20 °C, RH of 70%, 16 h of daylight, and photosynthetically active radiation (PAR) of ∼700 μmol m−2 s−1. All non-mangrove species were irrigated daily to saturation with water of known isotope composition. Hoagland nutrient solution prepared with the same water was also applied once weekly. For the mangrove plants, the pots were subirrigated by placement into 8-L plastic containers filled with 20 ppt saline water. Several measures as detailed in SI Appendix were taken to guarantee stability in salinity and isotope ratios of the water within the container over the growth period. Tinfoil was used to cover the soil surface in each pot and to wrap around all of the stem, twig, and petiole portions of each individual plant, so as to restrict the evaporative enrichment of soil/stem/petiole water.
Isotope Measurement of Plant Transpiration at SS.
After 4 to 6 wk of treatment with constant source water, plants were moved to an air-conditioned room for online monitoring of oxygen and hydrogen isotope ratios of canopy transpiration (δT). The measurement system consisted of three whole-plant through-flow chambers coupled to a water vapor isotope ratio infrared spectrometer (IWA-45-EP; Los Gatos Research [LGR]), an RH stabilizer, and several other components, as detailed in SI Appendix, Fig. S1.
Three individual plants from a single species were used on each measurement day. To measure δT, the entire canopy portion of each plant was enclosed into a whole-plant chamber, with illumination (∼700 μmol m−2 s−1 PAR at the canopy level) provided by an LED lamp placed ∼20 cm above the chamber. A mixing fan installed inside the chamber served to mix the chamber air as well as to facilitate aerodynamic coupling between the leaves and the air. The measurement room was maintained at a constant temperature of 26 ± 0.5 °C; as a result, within-chamber air temperature also remained steady, as confirmed by measurements recorded from a temperature sensor installed inside each chamber. The air stream entering each chamber was completely dried (details in SI Appendix), so that water vapor of the air exiting the chamber was derived entirely from plant transpiration. The exiting airflow was sent via an LGR multiport inlet unit (MIU) to a laser spectrometer (IWA-45-EP) for online monitoring of transpiration isotope signals. The MIU was programmed to allow sequential sampling from each chamber at 10-min intervals.
A unique feature of our measurement system is its ability to actively control RH inside the chamber at a stable level throughout the measurement period (details in SI Appendix). This, combined with ensured stability in within-chamber air temperature (Ta), provided an ideal environment for plant transpiration to approach isotonic SS (ISS) through a typical exponential trajectory, as well as to subsequently maintain it at the ISS once attained. For each individual plant, we determined that ISS transpiration was reached according to the criterion that the measured δT values (both hydrogen and oxygen isotopes) had flattened off and thus remained invariable with time for at least 90 min, as confirmed by the slope of the regressed relationship of δT with time during the last 90 min of measurement being not statistically different from zero. Oxygen and hydrogen isotope ratios of the ISS transpiration (δT_ISS) were then determined using data averaged across the considered ISS period.
Stem and Soil Sample Collection.
The online isotope monitoring was terminated on confirmation of the ISS. Stem samples were then collected from the main stem part (with the tinfoil cover, bark, and phloem all removed) of the plant located beneath the chamber body. Soil samples were collected from a depth corresponding to the midportion in the pot. A preliminary test revealed no significant oxygen or hydrogen difference in cryogenically extracted soil water from soil samples collected at upper, middle, and lower portions in the pot (SI Appendix, Table S1). All samples were sealed into 15-mL vials and stored at −20 °C until water extraction by cryogenic vacuum distillation at 100 °C as described previously (49). A detailed description of the cryogenic vacuum line, along with the procedure to verify complete water extraction, are provided in SI Appendix.
Rehydration Experiment.
Before the rehydration experiment, cryogenically extracted stem samples were oven-dried at 100 °C for 12 h to eliminate any residual moisture that might have accumulated during sample translocation and storage. The dried stem samples of each species were then immersed in a 50-mL container filled with water of known isotope ratios (i.e., the reference water) for 24 h at 25 °C. After rehydration, samples were immediately dried of surface water and stored in sealed vials at −20 °C, followed by another round of cryogenic extraction of water. Note that for the cryogenic extraction procedures in both the ISS transpiration and rehydration experiments, samples were always weighed before and after extraction.
Isotope Calibration and Analysis.
Calibration of the transpiration isotope measurements from the LGR laser spectrometer was performed at the end of each day of measurements, following a procedure involving water standards and a LGR water vapor isotope standard source (WVISS), as detailed previously (50). Stem and soil water samples were analyzed on the same LGR analyzer operating in liquid mode. Each sample was analyzed six times (with the last four injections used for calculations) alongside a set of the three LGR standards (LGR3E, LGR4E, and LGR5E). The acquired isotope data were checked for spectral contamination using the manufacturer’s postprocessing software (LWIA Post-Analysis Software; LGR). In the present dataset, no spectral contamination signals were identified in any of the samples analyzed (details in SI Appendix). As a further check of LGR performance, we conducted a separate measurement on a subset (∼30%) of the extracted water samples using a high-temperature TC/EA (high temperature conversion elemental analyzer) coupled to an isotope ratio mass spectrometer (Delta V Advantage; Thermo Fisher Scientific), and found that the measured δ18O and δ2H values were not statistically different from those measured by LGR (SI Appendix, Fig. S2). Isotope ratios were expressed in the per mil (‰) notation relative to V-SMOW (Vienna Standard Mean Ocean Water). The measurement precision of the LGR instrument was <0.2‰ for both δ2H and δ18O.
Supplementary Material
Acknowledgments
We thank Jian Sun, Li Zhong, Wei Wen, Wen Lin, and Ke Zhang for their help with the laboratory work. This work was financially supported by the National Natural Science Foundation of China (grants 31770435, 41806103, 41773032, and 41973072), China Postdoctoral Science Foundation (2018M633111), Shenzhen Peacock Innovative Research Team Program (KQTD2017032715165926), and Shenzhen Basic Research Program of Science and Technology (JCYJ20180305123818782).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014422117/-/DCSupplemental.
Data Availability.
All study data are included in the main text and SI Appendix.
References
- 1.Williams D. G., et al. , Evapotranspiration components determined by stable isotope, sap flow and eddy covariance techniques. Agric. For. Meteorol. 125, 241–258 (2004). [Google Scholar]
- 2.Jasechko S., et al. , Terrestrial water fluxes dominated by transpiration. Nature 496, 347–350 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Roden J. S., Lin G., Ehleringer J. R., A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose. Geochim. Cosmochim. Acta 64, 21–35 (2000). [Google Scholar]
- 4.Schefuss E., Schouten S., Schneider R. R., Climatic controls on central African hydrology during the past 20,000 years. Nature 437, 1003–1006 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Dawson T. E., Ehleringer J. R., Streamside trees that do not use stream water. Nature 350, 335–337 (1991). [Google Scholar]
- 6.Brinkmann N., Eugster W., Buchmann N., Kahmen A., Species-specific differences in water uptake depth of mature temperate trees vary with water availability in the soil. Plant Biol. 21, 71–81 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Brooks J. R., Barnard H. R., Coulombe R., McDonnell J. J., Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 3, 100–104 (2010). [Google Scholar]
- 8.Evaristo J., Jasechko S., McDonnell J. J., Global separation of plant transpiration from groundwater and streamflow. Nature 525, 91–94 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Ehleringer J. R., Phillips S. L., Schuster W. S. F., Sandquist D. R., Differential utilization of summer rains by desert plants. Oecologia 88, 430–434 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Lin G., da Sternberg L. S. L., “Hydrogen isotopic fractionation by plant roots during water uptake in coastal wetland plants” in Stable Isotopes and Plant Carbon-Water Relations, Ehleringer J. R., Hall A. E., Farquhar G. D., Eds. (Academic Press, 1993), pp. 497–510. [Google Scholar]
- 11.Ellsworth P. Z., Williams D. G., Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil 291, 93–107 (2007). [Google Scholar]
- 12.Zhao L., et al. , Significant difference in hydrogen isotope composition between xylem and tissue water in Populus euphratica. Plant Cell Environ. 39, 1848–1857 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Evaristo J., McDonnell J. J., Clemens J., Plant source water apportionment using stable isotopes: A comparison of simple linear, two‐compartment mixing model approaches. Hydrol. Processes 31, 3750–3758 (2017). [Google Scholar]
- 14.Wang J., Fu B., Lu N., Zhang L., Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid Loess Plateau. Sci. Total Environ. 609, 27–37 (2017). [DOI] [PubMed] [Google Scholar]
- 15.De Deurwaerder H., et al. , Liana and tree below-ground water competition—evidence for water resource partitioning during the dry season. Tree Physiol. 38, 1071–1083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbeta A., et al. , Unexplained hydrogen isotope offsets complicate the identification and quantification of tree water sources in a riparian forest. Hydrol. Earth Syst. Sci. 23, 2129–2146 (2019). [Google Scholar]
- 17.Barbeta A., et al. , An explanation for the isotopic offset between soil and stem water in a temperate tree species. New Phytol. 227, 766–779 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Poca M., et al. , Isotope fractionation during root water uptake by Acacia caven is enhanced by arbuscular mycorrhizas. Plant Soil 441, 485–497 (2019). [Google Scholar]
- 19.Mamonov A. B., Coalson R. D., Zeidel M. L., Mathai J. C., Water and deuterium oxide permeability through aquaporin 1: MD predictions and experimental verification. J. Gen. Physiol. 130, 111–116 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkmann T. H. M., Kühnhammer K., Herbstritt B., Gessler A., Weiler M., A method for in situ monitoring of the isotope composition of tree xylem water using laser spectroscopy. Plant Cell Environ. 39, 2055–2063 (2016a). [DOI] [PubMed] [Google Scholar]
- 21.Marshall J. D., Cuntz M., Beyer M., Dubbert M., Kuehnhammer K., Borehole equilibration: Testing a new method to monitor the isotopic composition of tree xylem water in situ. Front Plant Sci 11, 358 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West A. G., Patrickson S. J., Ehleringer J. R., Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun. Mass Spectrom. 20, 1317–1321 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Koeniger P., Marshall J. D., Link T., Mulch A., An inexpensive, fast, and reliable method for vacuum extraction of soil and plant water for stable isotope analyses by mass spectrometry. Rapid Commun. Mass Spectrom. 25, 3041–3048 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Rothfuss Y., Javaux M., Isotopic approaches to quantify root water uptake: A review and comparison of methods. Biogeosciences 14, 2199–2224 (2017). [Google Scholar]
- 25.Epstein S., Yapp C. J., Hall J. H., The determination of the D/H ratio of non-exchangeable hydrogen in cellulose extracted from aquatic and land plants. Earth Planet. Sci. Lett. 30, 241–251 (1976). [Google Scholar]
- 26.Schimmelmann A., Determination of the concentration and stable isotopic composition of nonexchangeable hydrogen in organic matter. Anal. Chem. 63, 2456–2459 (1991). [Google Scholar]
- 27.Feng X., Krishnamurthy R. V., Epstein S., Determination of D/H ratios of nonexchangeable hydrogen in cellulose: A method based on the cellulose-water exchange reaction. Geochim. Cosmochim. Acta 57, 4249–4256 (1993). [Google Scholar]
- 28.Filot M. S., Leuenberger M., Pazdur A., Boettger T., Rapid online equilibration method to determine the D/H ratios of non-exchangeable hydrogen in cellulose. Rapid Commun. Mass Spectrom. 20, 3337–3344 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Revesz K., Woods P. H., A method to extract soil water for isotopic analysis. J. Hydrol. (Amst.) 115, 397–406 (1990). [Google Scholar]
- 30.Munksgaard N. C., Cheesman A. W., Wurster C. M., Cernusak L. A., Bird M. I., Microwave extraction-isotope ratio infrared spectroscopy (ME-IRIS): A novel technique for rapid extraction and in-line analysis of δ18O and δ2H values of water in plants, soils and insects. Rapid Commun. Mass Spectrom. 28, 2151–2161 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Böttcher G., Brumsack H. J., Heinrichs H., Pohlmann M., A new high-pressure squeezing technique for pore fluid extraction from terrestrial soils. Water Air Soil Pollut. 94, 289–296 (1997). [Google Scholar]
- 32.Peters L. I., Yakir D., A direct and rapid leaf water extraction method for isotopic analysis. Rapid Commun. Mass Spectrom. 22, 2929–2936 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Wassenaar L. I., Hendry M. J., Chostner V. L., Lis G. P., High-resolution pore water δ2H and δ18O measurements by H2O(liquid)-H2O(vapor) equilibration laser spectroscopy. Environ. Sci. Technol. 42, 9262–9267 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Rothfuss Y., Vereecken H., Brüggemann N., Monitoring water stable isotopic composition in soils using gas-permeable tubing and infrared laser absorption spectroscopy. Water Resour. Res. 49, 3747–3755 (2013). [Google Scholar]
- 35.Volkmann T. H. M., Haberer K., Gessler A., Weiler M., High-resolution isotope measurements resolve rapid ecohydrological dynamics at the soil-plant interface. New Phytol. 210, 839–849 (2016b). [DOI] [PubMed] [Google Scholar]
- 36.Walker G. R., Woods P., Allison G. B., Interlaboratory comparison of methods to determine the stable isotope composition of soil water. Chem. Geol. 111, 297–306 (1994). [Google Scholar]
- 37.Araguás-Araguása L., Rozanskia K., Gonfiantinia R., Louvat D., Isotope effects accompanying vacuum extraction of soil water for stable isotope analyses. J. Hydrol. (Amst.) 168, 159–171 (1995). [Google Scholar]
- 38.Orlowski N., Breuer L., McDonnell J. J., Critical issues with cryogenic extraction of soil water for stable isotope analysis. Ecohydrol. 9, 3–10 (2016a). [Google Scholar]
- 39.Meißner M., Kohler M., Schwendenmann L., Holscher D., Dyckmans J., Soil water uptake by trees using water stable isotopes (δ2H and δ18O) - a method test regarding soil moisture, texture and carbonate. Plant Soil 376, 327–335 (2014). [Google Scholar]
- 40.Orlowski N., Pratt D. L., McDonnell J. J., Intercomparison of soil pore water extraction methods for stable isotope analysis. Hydrol. Processes 30, 3434–3449 (2016b). [Google Scholar]
- 41.Orlowski N., et al. , Inter-laboratory comparison of cryogenic water extraction systems for stable isotope analysis of soil water. Hydrol. Earth Syst. Sci. 22, 3619–3637 (2018). [Google Scholar]
- 42.Gaj M., Kaufhold S., McDonnell J. J., Potential limitation of cryogenic vacuum extractions and spiked experiments. Rapid Commun. Mass Spectrom. 31, 821–823 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Newberry S. L., Prechsl U. E., Pace M., Kahmen A., Tightly bound soil water introduces isotopic memory effects on mobile and extractable soil water pools. Isotopes Environ. Health Stud. 53, 368–381 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Thielemann L., Gerjets R., Dyckmans J., Effects of soil-bound water exchange on the recovery of spike water by cryogenic water extraction. Rapid Commun. Mass Spectrom. 33, 405–410 (2019). [DOI] [PubMed] [Google Scholar]
- 45.McDonnell J. J., The two water worlds hypothesis: Ecohydrological separation of water between streams and trees? Wiley Interdisciplinary Reviews: Water 1, 323–329 (2014). [Google Scholar]
- 46.Berry Z. C., et al. , The two water worlds hypothesis: Addressing multiple working hypotheses and proposing a way forward. Ecohydrol. 11, e1843 (2018). [Google Scholar]
- 47.Hewlett J. D., Hibbert A. R., “Factors affecting the response of small watersheds to precipitation in humid areas” in Forest Hydrology, Sopper W. E., Lull H. W., Eds. (Pergamon Press, 1967), pp. 275–291. [Google Scholar]
- 48.Dubbert M., Caldeira M. C., Dubbert D., Werner C., A pool-weighted perspective on the two-water-worlds hypothesis. New Phytol. 222, 1271–1283 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Ehleringer J. R., Roden J. S., Dawson T. E., “Assessing ecosystem-level water relations through stable isotope ratio analysis” in Methods in Ecosystem Science, Sala O., Jackson R., Mooney H., Eds. (Academic Press, 2000), pp. 181–198. [Google Scholar]
- 50.Song X., Loucos K. E., Simonin K. A., Farquhar G. D., Barbour M. M., Measurements of transpiration isotopologues and leaf water to assess enrichment models in cotton. New Phytol. 206, 637–646 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.