Abstract
Mosquitoes are a widely diverse group of organisms, comprising ∼3,500 species that live in an enormous range of habitats. Some species are vectors of diseases that afflict hundreds of millions of people each year. Although understanding of mosquito olfaction has progressed dramatically in recent years, mosquito taste remains greatly understudied. Since taste is essential to feeding, egg laying, and mating decisions in insects, improved understanding of taste in mosquitoes could provide new mechanistic insight into many aspects of their behavior. We provide a guide to current knowledge in the field, and we suggest a wealth of opportunities for research that are now enabled by recent scientific and technological advances. We also propose means by which taste might be exploited in new strategies for mosquito control, which may be urgently needed as the geographical ranges of vector species increase with climate change.
Keywords: mosquito, vector biology, taste
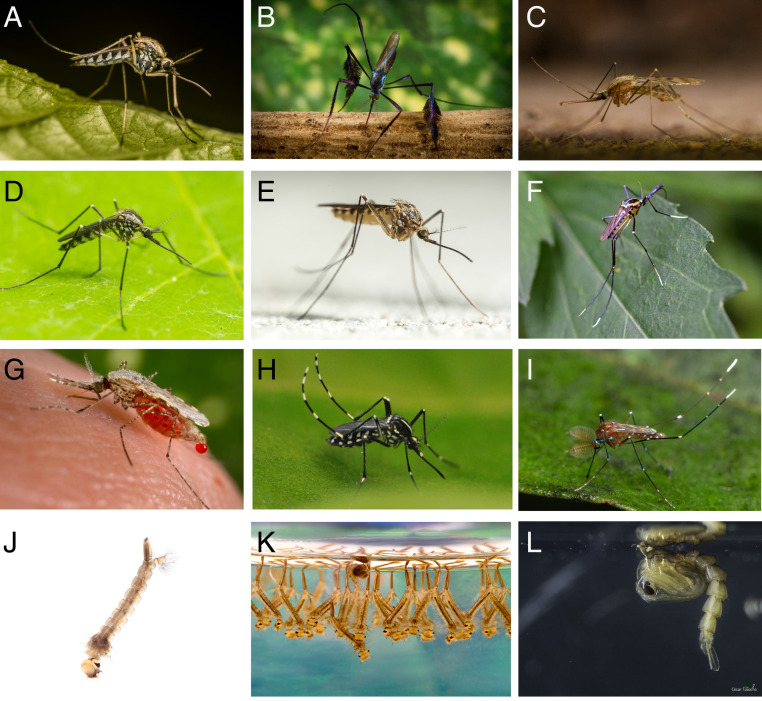
Mosquitoes are remarkably diverse in terms of their morphology, the environments that they inhabit, the hosts upon which they feed, and the behaviors that they exhibit (Fig. 1). Mosquitoes have been on Earth for over 200 million years and comprise ∼3,500 species. They reside on six continents and in a wide range of habitats—in marshes, forests, deserts, Arctic regions, and urban centers.
Fig. 1.
Mosquitoes. (A) Aedes sticticus; (B) Sabethes cyaneus; (C) Anopheles maculipennis; (D) Toxorhynchites speciosus; (E) Culiseta glaphyroptera; (F) Toxorhynchites rutilus; (G) Anopheles stephensi; (H) Aedes albopictus; (I) Uranotaenia sapphirina; (J) Aedes larva; (K) Culex larvae; and (L) Aedes aegypti pupa. Image credits: (A, C, D, E, and J) Anders Lindström (photographer); (B, G, and K) Centers for Disease Control and Prevention/James Gathany; (F) Ellen Honeycutt (photographer); (H) Ary Faraji (photographer); and (I and L) César Favacho (photographer).
Mosquitoes have an enormous ecological impact as pollinators, food sources, and vectors of pathogens that afflict wildlife. Males and females of most species feed on nectar and other plant juices, pollinating host plants. Mosquitoes also serve as food sources to a variety of predators, including birds, frogs, spiders, and lizards. Females of most species bite animals and draw blood, which provides nutrients essential for reproduction. Because of their need for blood, certain mosquitoes are vectors of pathogens causing animal diseases and have thereby had major impacts on species abundance and biodiversity. These mosquitoes have decimated a number of North American bird populations by transmitting West Nile virus, and they have driven certain native Hawaiian songbirds extinct via avian malaria (1–3). Mosquitoes also transmit pathogens that afflict livestock such as cows, sheep, and horses.
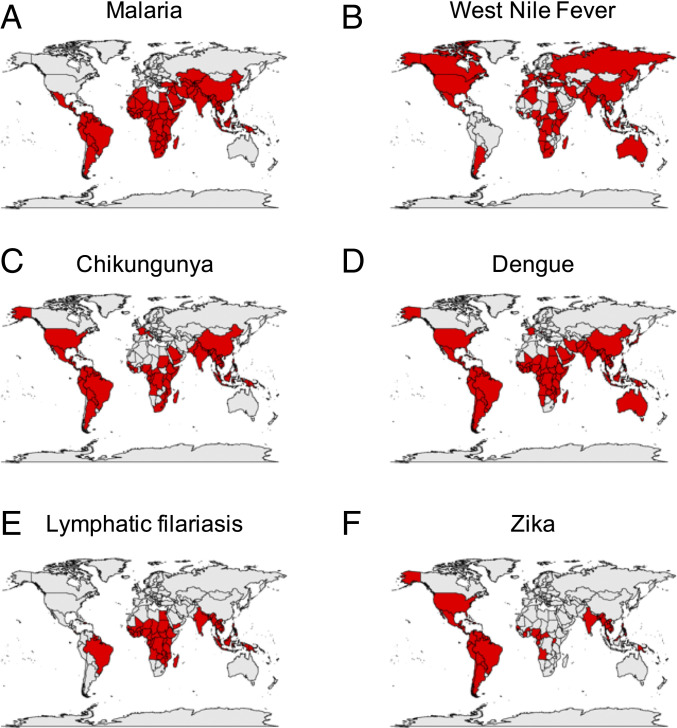
Mosquitoes are also vectors of pathogens causing human diseases. Although only a small fraction of mosquito species is anthropophilic and bites humans, they have an enormous impact on global health (Fig. 2). These species collectively spread diseases to hundreds of millions of people and kill nearly a million each year. These diseases include malaria, dengue fever, yellow fever, Zika fever, West Nile fever, and chikungunya. Because of the toll they take on human life, mosquitoes are often said to be the deadliest animals on Earth. Interest in mosquito vectors of disease pathogens is increasing as their geographic ranges expand due to climate change.
Fig. 2.
Global distribution of mosquito-borne diseases. World maps indicating prevalence of diseases caused by mosquito-borne pathogens. Red indicates countries with recent or current reports of disease presence. (A) Malaria. Data from ref. 108. (B) West Nile fever. Data from ref. 109. (C) Chikungunya. Data from ref. 110. (D) Dengue. Data from ref. 110. (E) Lymphatic filariasis. Data from ref. 108. (F) Zika. Data from ref. 108.
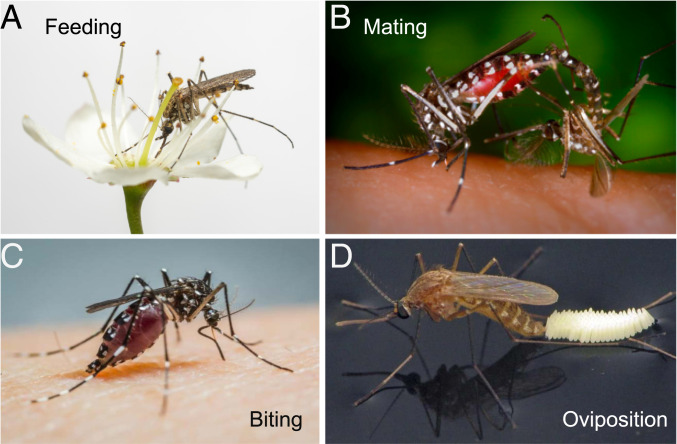
Mosquitoes have evolved sophisticated chemosensory systems to detect and identify chemical cues in their environments, including cues of the hosts they bite. Our understanding of mosquito olfaction has advanced a great deal in recent years (4–13), but mosquito taste remains greatly understudied. Taste is essential to feeding, mating, biting, and egg-laying decisions in insects (Fig. 3), and mosquitoes have evolved elaborate taste organs on their mouthparts and legs and internally. The molecular, cellular, and circuit mechanisms by which these organs signal taste cues remain a largely unexplored frontier. Progress in understanding mosquito taste could yield insight into many aspects of their diverse behaviors and could provide new means of controlling both mosquitoes and the diseases they spread.
Fig. 3.
Mosquito behaviors. (A) Aedes cantans feeding on nectar. Image credit: Anders Lindström (photographer). (B) Aedes albopictus mating. Image credit: Centers for Disease Control and Prevention/James Gathany. (C) Aedes albopictus biting. Image credit: Centers for Disease Control and Prevention/Pablo Cabrera. (D) Culex quinquefasciatus ovipositing. Image credit: Sean McCann (photographer).
Taste Organs
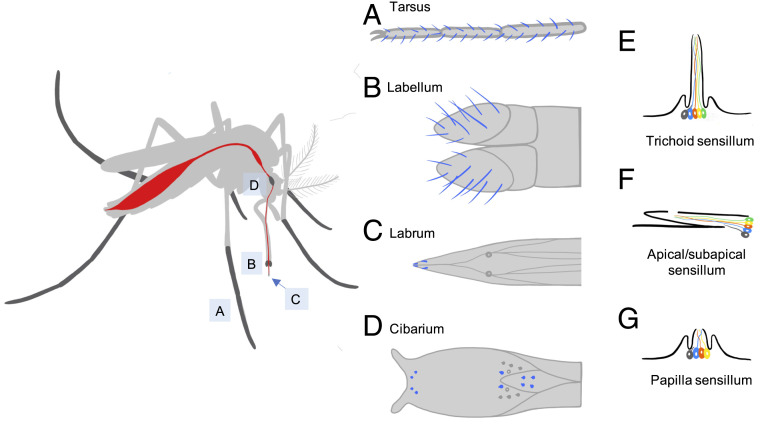
Mosquito taste organs include the tarsal segments of the legs, the labellum and labrum of the mouthparts, and the cibarium, an internal organ (Fig. 4 A–D). The tarsi are the first taste organs to make contact with a potential food source, blood source, or oviposition site. The labellum lies at the distal tip of one of the mouthparts. The labrum, a distinct mouthpart, is shaped like a needle and serves as a conduit through which blood or food is ingested. Once ingested, the blood or food passes the cibarium on their transit to the midgut or crop, respectively.
Fig. 4.
Anatomy of mosquito taste organs and sensilla. (Left) Illustration of mosquito with taste organs highlighted in dark gray. (A–D) Taste sensilla are depicted in blue: (A) tarsus (third to fifth segments), (B) labellum, (C) apical/subapical portion of labrum, and (D) cibarium. (E–G) Morphology of taste sensilla containing several gustatory receptor neurons (in color) and one mechanosensory neuron (in dark gray): (E) trichoid sensillum, (F) apical/subapical sensillum, and (G) papilla sensillum.
These taste organs all contain sensory hairs called sensilla, which house gustatory receptor neurons. The sensilla fall into different morphological types. Trichoid sensilla extend outward from the surface of the tarsi and the labellum and typically have a single pore at the distal end of a blunt tip (Fig. 4E). Other sensilla lie at an acute angle to the apical and subapical surfaces of the labrum; they are commonly referred to as apical and subapical sensilla (Fig. 4F). Papilla sensilla are small and found on the cibarium (Fig. 4G).
Tarsi contain more trichoid sensilla on the forelegs and midlegs than the hindlegs in some species (14–16). Correspondingly, the forelegs and midlegs make more contact with substrates than do hindlegs. Interestingly, hindlegs are often suspended in air and move while the animal is on a surface, suggesting the possibility they may have additional sensory functions other than taste.
Sexual dimorphism is observed at many levels in the mosquito taste system. Among organs, the labrum is sharper in females, which use it to pierce skin. The presence or number of some types of taste sensilla is sexually dimorphic. For example, in the vector species Aedes aegypti, there are more tarsal sensilla in females than males, suggesting the possibility of a host-recognition function for the supernumerary sensilla in females (14, 16). In blood-feeding species, the apical and subapical sensilla of the labrum and the ventral papilla sensilla of the cibarium are present only in females. These sensilla are absent in both males and females of nonblood-feeding species, suggesting a role in blood sensing (17–21).
Other potential taste organs include the wing margin, the pharynx, and the ovipositor. These organs have been implicated in taste in Drosophila or other dipterans. Their roles in taste detection and in the behavior of mosquitoes present interesting directions for future research.
Taste Neurons
Taste sensilla house small numbers of gustatory neurons. Trichoid sensilla on the tarsi typically house up to five taste neurons; labellar trichoid sensilla and labral sensilla typically house up to four, and cibarial sensilla contain up to three (14, 15, 19, 22). The cell bodies of these neurons project dendrites toward the pore through which tastants enter (Fig. 4 E–G).
The sensitivities of taste neurons can be investigated by electrophysiological recording. An electrode containing a tastant solution is placed in contact with the tip of the sensillum (Fig. 5). The physiological responses elicited by the tastant are then measured by recording the amplitudes and frequencies of action potentials. Different neurons within the sensillum, often distinguishable by their different action potential amplitudes, respond to different tastants (23–28).
Fig. 5.
Electrophysiological recordings of taste sensilla. (A) Schematic of single-sensillum electrophysiological recording. An electrode containing a tastant solution is placed over the tip of a sensillum. The tastant enters the sensillum via a pore at the tip of the sensillum and activates taste neurons within. (B) Physiological recordings from Aedes albopictus labellar sensilla in response to sucrose (Top), berberine chloride (Middle; bitter compound), or control diluent tricholine citrate (Bottom).
Neurons in the labellar sensilla respond to sugars, bitter compounds, salts, and amino acids (22–28). Recording from tarsal sensilla has been limited, but responses to sucrose, salt, and amino acids have been reported (22, 29). In a sensillum of the labrum, one neuron responded to salt, while another responded physiologically to adenosine triphosphate (ATP) (22, 30, 31). Interestingly, ATP induces engorgement (i.e., ingestion of a large volume), a response usually induced by blood but not by nectar (32).
Taste Receptors
Mosquito genomes contain a wide variety of candidate taste receptor genes, including members of the Gr (Gustatory receptor), IR (Ionotropic receptor), Trp (Transient receptor potential), and Ppk (Pickpocket) families (Fig. 6). There are large numbers of some of these genes. For example, there are 90 Gr genes in the malaria vector Anopheles gambiae; 107 in the Zika, dengue, and yellow fever vector Ae. aegypti; and 126 in Culex quinquefasciatus, which transmits Zika and West Nile viruses (33–35).
Fig. 6.
Classes of taste receptors. Several classes of taste receptors are shown: Grs, IRs, TRPs, and Ppks, which are members of the degenerin/epithelial sodium channel family. Adapted from ref. 111, with permission from Elsevier.
Expression of some of these genes shows organ specificity. Some Gr genes are expressed in the labellum but not the tarsi, whereas others are expressed in the tarsi but not the labellum (36). These differences invite questions about the roles of individual taste receptors and suggest different roles for these organs in taste. Intriguing questions about function are also posed by the differential expression of taste receptors in mosquitoes of different sex, developmental stage, or physiological state (e.g., after mating or a blood meal) (36–40).
Roles of the Taste System
Taste input modulates many behaviors in insects. Studies in mosquitoes have revealed roles for taste in driving or modulating several behaviors that are likely to contribute to survival and disease spread. These behaviors include feeding, biting, mating, and oviposition (Fig. 3).
Feeding.
Feeding decisions are made after evaluating the benefits and risks of a potential food source. Taste provides a mechanism for evaluating the content of potential food sources. It allows detection of both nutritive and toxic compounds.
Adult mosquitoes feed on sugary substrates including plant nectar, honey dew, and plant sap. Nectar meals generally contain high concentrations of sugars. When mosquito taste organs are stimulated with sugars, the animal responds with proboscis movements and pharyngeal pumping, which result in ingestion (41–43). Behavioral responses depend on the identity of the sugar molecule (e.g., sucrose) as well as on the dose.
By contrast, bitter compounds and high concentrations of salts elicit aversive responses. Ammonium chloride or high concentrations of salts evoke rejection responses, such as proboscis withdrawal, and inhibit feeding behavior (23, 43–46). These responses may have evolved in part to prevent ingestion of harmful food sources.
The biting behavior that precedes blood feeding is also influenced by taste cues. N,N-Diethyl-meta-toluamide (DEET), a repellent that is a bitter tastant as well as an odorant, suppresses biting in part through taste organs (47). We note also a classical behavioral experiment with mosquitoes that had undergone surgical ablation of their antennae, which are the primary, although not the only, olfactory organs of the mosquito. These antennaless mosquitoes showed biting behavior upon contact with human skin, indicating that biting behavior is not driven exclusively by chemosensory input through the antenna (48).
Mating.
Identification of a mating partner of the same species and subsequent mating behaviors are driven by taste detection of nonvolatile pheromones in many insects. In a number of mosquito species, males have enlarged claws that they use to grasp females. In so doing, male legs make contact with females and may detect nonvolatile pheromones that induce male behavioral responses, including excitatory behavior and mating attempts (49–53).
The context of male–female contact varies dramatically among the many species of mosquitoes. In some species, an isolated male and an isolated female make contact and mate. In other species, the males form a flying swarm into which a female flies and mates with a male. Males of Opifex fuscus and Deinocerites cancer perform a behavior called “pupal attendance”: an adult male contacts a female pupa with its tarsi, grabs it, and awaits her eclosion, upon which the male attempts to mate (52, 54). In some instances, males attempt to mate with recently discarded pupal cases (52). Little is known about the nonvolatile pheromones that are detected and their roles in driving mosquito mating behaviors.
Oviposition.
The selection of a suitable site to lay eggs is critical to the survival of a mosquito species. After hatching from eggs, larvae and pupae develop at the oviposition site, so the presence of nutrition and the absence of predators are essential. Oviposition behavior of adult female mosquitoes varies across species. Culex species lay eggs directly on the surface of water, Anopheline species tend to hover over the surface of water and drop eggs onto it from the air, and Aedine species lay eggs on surfaces adjacent to water, often right above the waterline. Despite these differences, females of diverse species contact the substrate with taste organs before oviposition, consistent with a role for taste cues in oviposition site selection (55–57). We note that oviposition is also influenced by olfactory cues, and some cues may operate via both olfaction and taste.
What taste cues stimulate or deter oviposition? Salinity is one factor. Many species prefer to lay eggs in water with low salt concentrations (56, 58, 59). Other species are less discriminating and lay eggs in salt marshes or marine rock pools with much higher salt concentrations (60, 61). The ability to discriminate oviposition site salinity affects offspring fitness and survival and requires taste organs (56, 58, 59, 62).
Most mosquito larvae eat various plant materials and microorganisms. Correspondingly, a variety of chemical cues from plant and microbial sources stimulates oviposition through either taste, olfaction, or both (63). By contrast, cues associated with natural predators such as fish, dragonflies, and other insect predators deter oviposition (63–66).
Chemical cues from eggs, larvae, and pupae are a particularly intriguing factor. Many species such as Ae. aegypti, Aedes albopictus, Cx. quinquefasciatus, and Ochlerotatus australis prefer to oviposit in water with conspecific larvae and pupae or cues associated with them (63, 67–70). Some Culex species also prefer to oviposit in water containing a cue from conspecific egg rafts, Mosquito Oviposition Pheromone (71–73). Oviposition site attraction has been shown to be mediated at least in part by the olfactory system, but a role for taste has not yet been explored. In contrast to this preference for conspecific cues, adult Toxorhynchites females prefer to lay eggs in water containing cues of heterospecific larvae, which Toxorhynchites larvae eat (74).
The Larval Taste System
Not only do chemical cues of larval food sources stimulate oviposition by adult females, but they also stimulate feeding in larvae. Larvae feed on microorganisms, organic detritus, and even on live larvae of other mosquito species and carcasses of their own species (75). Both the rate and duration of feeding are increased by mixtures of nutrients such as yeast extract or nucleotides (75–79). Mixtures are more effective than individual nutrients in stimulating feeding and larval aggregation (75, 79). Some bitter compounds such as DEET and quinine elicit aversive or turning behaviors in larvae (80, 81).
Larvae have an antenna that contains a sensillum with the anatomical characteristics of a taste sensillum. It contains a pore at the tip and houses several neurons, each with dendrites that extend to the tip (82–84). There are also chemosensory sensilla on the larval maxillary palp and internally (15, 85). Multiple IR genes are expressed in the larval antenna, and one has been implicated in DEET response (80). The molecular basis of larval taste awaits exploration.
Opportunities for Future Directions
Mosquito taste offers a great variety of problems to solve and excellent opportunities to solve them. Questions lie at every level of biological organization, from molecular biology to ecology. Mosquitoes have had hundreds of millions of years to evolve sensitive and discriminating taste organs, but little is known about the receptors, neurons, and circuits that underlie mosquito taste. A particularly intriguing dimension is added by the large number of diverse mosquito species, each likely to have a taste system adapted to its own special needs.
Taste Cues.
The first and most basic question is what tastants mosquitoes detect. The number of taste compounds that have been tested in physiological or behavioral assays is relatively small. Yet, Drosophila and other insects have been found to respond to an immense variety of tastants. These compounds extend far beyond common sugars, salts, and amino acids to include nucleic acids, polyamines, organic acids, and a vast panoply of structurally diverse bitter compounds (86, 87).
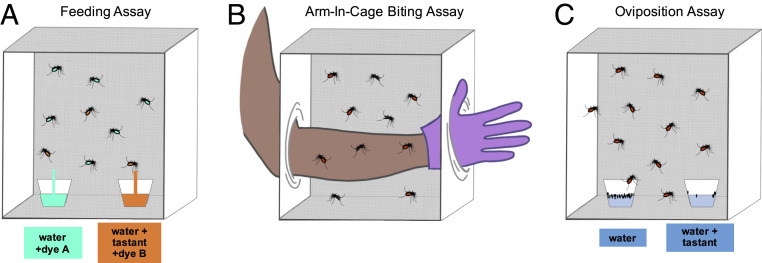
Taste compounds found on human and animal hosts are of particular interest. It seems plausible that taste cues could provide a checkpoint to confirm the suitability of a host for biting. Taste cues could also help guide the precise location of a site at which to bite. A great deal of chemical information is available to the mosquito on human and animal skin, including compounds synthesized by the hosts and by the microbes that inhabit their surfaces. It seems likely that the mosquito taste system uses this information. Salient compounds can be identified by behavioral testing in feeding, biting, or oviposition paradigms (Fig. 7). Alternatively, compounds can be tested in physiological assays (Fig. 5).
Fig. 7.
Behavioral assays measuring taste-driven behaviors. (A) Feeding assay measures feeding preference by presenting mosquitoes with a choice of two feeding sites: one containing water and the other containing water with tastant. Each choice contains different fluorescent dyes used to visualize feeding choice. (B) Arm-in-cage biting assay measures percentage of female mosquitoes biting human arm treated with either solvent or tastant. (C) In an oviposition assay, blood-fed and egg-carrying female mosquitoes are presented with a choice of two oviposition sites: one containing water alone and the other containing water with tastant. Oviposition is measured as percentage of eggs laid in each site.
The ∼3,500 mosquito species collectively live in immensely diverse habitats such as Alpine meadows, tropical rainforests, and arid plateaus. They feed on different plants and different hosts that produce a wide variety of taste cues. It will be interesting to determine which taste responses have been conserved in evolution and which have evolved to serve the needs of individual species.
Taste Coding.
What are the principles by which mosquitoes encode tastants? A great deal of insight can be obtained in straightforward fashion via electrophysiology. Available data are currently sparse: they are from few tastants, few neurons, and few species. Moreover, most sensilla that have been analyzed were not identified by name or position.
Recordings with relatively small panels of tastants should indicate how many distinct functional types of taste sensilla are in each organ. Testing with a modest number of tastants should reveal whether individual taste neurons of a particular class (e.g., bitter-sensing neurons) are narrowly tuned to a small fraction of bitter compounds or broadly tuned to many. Such studies should also show whether there are distinct classes of bitter neurons. If different bitter compounds elicit different responses from multiple bitter-sensing neurons, this organization would provide the basis of a combinatorial code of bitter taste. Such organization would endow the system with the capacity to discriminate among bitter compounds.
Testing different doses of tastants should illuminate how taste intensity is encoded. For example, different neurons may be specialized to report the concentration of a tastant over different concentration ranges. The intensity of some compounds, such as salt, might thereby be assessed more precisely than if it were represented by the activity of a single class of neuron.
The function of sex-specific sensilla is particularly interesting. For example, there are more tarsal sensilla in females than males in Ae. aegypti (14, 16). It will be interesting to determine if there are female-specific tarsal sensilla in these vector species that detect host tastants; alternatively, they might be specialized for identifying suitable oviposition sites. Similarly, sensilla unique to blood-feeding species may detect host cues or blood.
Taken together, this analysis should illuminate the neural basis of taste coding. It may reveal how the coding properties of the tarsi and labellum differ, which may provide insight into the functions of these organs in influencing mosquito behavior. The feasibility of elucidating principles of neural coding by taste organs via electrophysiology has been demonstrated in Drosophila (88–90).
At the molecular level, recent technological and intellectual advances provide new and exciting opportunities. Candidate taste receptors in several mosquito species have been identified, including over a hundred Grs and/or IRs for some species, and await functional analysis (33, 34, 80, 91–94). The molecular basis of taste coding is now accessible for genetic analysis via CRISPR-Cas9 gene editing technology. For example, if a species shows female-specific expression of a Gr gene in the labellum, one can mutate the gene and determine if it is required for responses to particular host or oviposition cues. There is now ample precedent for genetic analysis of sensory receptors in Ae. aegypti and An. gambiae (62, 95–97).
The expression of taste receptors can be analyzed at high resolution by using the promoters of receptor genes to drive reporter constructs. Gene editing also allows the use of genetically encoded Ca2+ or voltage indicators (9, 62, 98–100). Such indicators permit functional analysis of taste neurons such as those of the cibarium, which are less accessible to electrophysiological recording than those of the tarsi or labellum on account of their internal location. Similarly, the activity of higher-order neurons in taste circuits can be monitored using such indicators.
Taste Behaviors.
Taste behavior can now be investigated at the molecular and cellular levels. Analysis of taste receptor mutants may identify not only ligands of the receptors but also their roles in driving feeding, biting, or oviposition behavior. Just as promoters of receptor genes may drive reporters, they may also drive effectors that silence or activate defined taste neurons. In this manner, one can identify and characterize neurons that stimulate or deter critical mosquito behaviors.
Mating behavior is particularly understudied in mosquitoes. Analysis of the taste system could identify pheromones and neurons that influence either male or female behavior. It will be interesting to determine whether taste plays a role in preventing interspecies mating. Understanding mechanisms that mosquitoes use to recognize each other could also have applications to mosquito control.
Taste circuits can also be investigated by expressing genetically encoded Ca2+ indicators or voltage indicators in higher-order neurons (9, 62, 98–100). While genetic manipulation of mosquitoes is less convenient than that of Drosophila, a variety of genetic tools used in Drosophila can in principle now be used in the mosquito. One question of particular interest is how taste cues from a host are processed and integrated with other host cues in the central nervous system to drive behaviors such as biting.
Another intriguing question is how taste reception and perception are influenced by the internal state of the mosquito. After a female mosquito bites, the need for a host is supplanted by the need for an oviposition site. Are there molecular or cellular changes in the physiology of individual taste neurons? Are there changes in the activity of higher-order neurons in the taste circuit?
In the long term, comparative studies could illuminate the mechanisms by which mosquito behaviors have evolved. For example, what molecular and cellular differences are there between the taste systems of mosquitoes that feed on animal hosts and those that feed exclusively on nectar? A related problem of interest is how the taste systems of mosquitoes that feed on humans differ from those that do not. Identifying differences between anthropophilic mosquitoes and zoophilic mosquitoes could suggest new targets useful in vector control.
Disease Control.
Mosquitoes spread a multitude of diseases, including malaria, dengue, yellow fever, chikungunya, and Zika. As climate change expands the range of mosquito vectors, so too it will extend their devastating impact on health. New means of mosquito control will be sought with increasing urgency. Taste might be exploited in a variety of ways to manipulate mosquito behavior and prevent the spread of disease. Thus, new insight into basic mechanisms of taste may be invaluable. Moreover, prospects for translating discoveries into practical applications should improve rapidly with the accelerating development of technology for studying and manipulating mosquitoes.
Screening of tastant libraries may identify several kinds of compounds useful in mosquito control. Aversive compounds that act via taste could be useful when applied to skin surfaces or even clothing. Such aversive compounds could act in several ways. They could conceivably reduce the time a mosquito spends on a skin surface, the likelihood that a biting event is initiated, or the duration of a biting event. Tastants that activate bitter-sensing neurons may be particularly interesting to test in biting assays. In addition to compounds that act exclusively via taste, it may be possible to identify compounds like DEET that deter mosquitoes via taste as well as olfaction (47, 99, 101).
Taste compounds that activate feeding behaviors could also be useful. If applied to insecticide-treated bed nets, they may stimulate greater ingestion of insecticides and thus, increase lethality. Likewise, taste compounds that stimulate feeding could be added to attractive toxic sugar baits, which are commonly used in pest control.
Tastants that stimulate oviposition behavior could be deployed in oviposition traps that kill adult females or their progeny. There is precedent for the use of lethal oviposition traps in the control of Ae. aegypti, and the attractiveness of the traps is considered a critical parameter in their effectiveness (102). Accordingly, it seems plausible that the presence of positive oviposition cues could enhance the effectiveness of such traps and the success of this form of control.
In addition to tastants that activate aversion, feeding, or oviposition responses, another kind of compound could also be useful: molecules that inhibit or mask taste responses. A wide variety of compounds inhibits the activities of insect olfactory receptor neurons (99, 101, 103, 104), and it seems plausible that various compounds might inhibit taste neurons. Other compounds might act by masking the stimulatory effects of other tastants. Compounds that inhibit or mask taste responses might be used, for example, to inhibit biting behaviors elicited by taste cues on the skin.
Another interesting prospect for future research is the potential of nonvolatile pheromones for mosquito control. Pheromones, both volatile and nonvolatile, drive a variety of insect behaviors (105, 106). A wide variety of control strategies based on the deployment of pheromones has been used successfully to control agricultural pests. There are interactions between the responses elicited by pheromonal signals and those elicited by food sources (107). Further research is needed to determine whether nonvolatile mosquito pheromones, of negative or positive valence, might reduce the duration of landing on a surface or reduce the frequency of biting events.
If genetic modification of mosquitoes via gene drive or other systems becomes common practice, then the taste system may conceivably offer useful targets that could be manipulated. For example, if individual taste receptors influence the likelihood of biting a human vs. an animal host, then manipulation of genes encoding such receptors in mosquito populations might redirect biting from humans to animal hosts while avoiding strong selective pressure against the genetic modification. Analysis of Grs that are specific to females of anthropophilic species could identify interesting candidates for modification. Receptors that detect human host cues could be especially worthy of investigation. If genetic modification proves difficult, another option is to screen for compounds that manipulate such receptors.
In the long term, understanding of taste at the circuit level may allow additional vector control strategies. For example, one might envision the conditional activation of circuits that deter feeding or the inactivation of circuits that promote biting. In principle, such circuits could be manipulated chemically and/or genetically. Further research into the mechanisms of mosquito taste will allow assessment of the feasibility of such possibilities.
In summary, a great variety of interesting problems awaits investigation in the taste system of mosquitoes. Studies of this system may provide new insight into the biology of an intriguing and highly diverse group of insects. The results of such studies could also have applications to a global health challenge of enormous dimension.
Acknowledgments
We thank Zina Berman for support; Dr. Joseph Fauver for help with generating the maps in Fig. 2; and Anders Lindström, James Gathany, Graham Winterflood, Ellen Honeycutt, William Collins, Ary Faraji, César Favacho, Pablo Cabrera, and Sean McCann for the photos. This work was supported by NIH Grants F32DC019250 (to L.S.B.), R01 DC02174 (to J.R.C.), R01 DC04729 (to J.R.C.), and R01 DC11697 (to J.R.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data Availability.
There are no data underlying this work.
References
- 1.LaDeau S. L., Kilpatrick A. M., Marra P. P., West Nile virus emergence and large-scale declines of North American bird populations. Nature 447, 710–713 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Van Riper C. III, Van Riper S. G., Goff M. L., Laird M., The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 56, 327–344 (1986). [Google Scholar]
- 3.Warner R. E., The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70, 101–120 (1968). [Google Scholar]
- 4.Carey A. F., Wang G., Su C. Y., Zwiebel L. J., Carlson J. R., Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallem E. A., Nicole Fox A., Zwiebel L. J., Carlson J. R., Olfaction: Mosquito receptor for human-sweat odorant. Nature 427, 212–213 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Lu T., et al. , Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533–1544 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G., Carey A. F., Carlson J. R., Zwiebel L. J., Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 107, 4418–4423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinauger C., et al. , Modulation of host learning in Aedes aegypti mosquitoes. Curr. Biol. 28, 333–344.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinauger C., et al. , Visual-olfactory integration in the human disease vector mosquito Aedes aegypti. Curr. Biol. 29, 2509–2516 e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu P., Zeng F., Bedoukian R. H., Leal W. S., DEET and other repellents are inhibitors of mosquito odorant receptors for oviposition attractants. Insect Biochem. Mol. Biol. 113, 103224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride C. S., et al. , Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222–227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riabinina O., et al. , Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun. 7, 13010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon Y., et al. , Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 20, 1672–1678 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIver S., Siemicki R., Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae). J. Morphol. 155, 137–155 (1978). [DOI] [PubMed] [Google Scholar]
- 15.McIver S. B., Sensilla mosquitoes (Diptera: Culicidae). J. Med. Entomol. 19, 489–535 (1982). [DOI] [PubMed] [Google Scholar]
- 16.Super E. H., Sensory hairs with permeable tips on the tarsi of the yellow-fever mosquito, Aedes aegypti. Ann. Entomol. Soc. Am. 55, 531–535 (1962). [Google Scholar]
- 17.Lee R. M. K. W., Craig D. A., Cibarial sensilla and armature in mosquito adults (Diptera: Culicidae). Can. J. Zool. 61, 633–646 (1983). [Google Scholar]
- 18.Lee R. M. K. W., Structure and function of the fascicular stylets, and the labral and Cibarial sense organs of male and female Aedes aegypti (L.) (Diptera, Culicidae). Quaest. Entomol. 10, 187–216 (1974). [Google Scholar]
- 19.McIver S., Siemicki R., Innervation of cibarial sensilla of Aedes aegypti (L.)(Diptera: Culicidae). Int. J. Insect Morphol. Embryol. 10, 355–357 (1981). [Google Scholar]
- 20.Uchida K., Cibarial sensilla and pharyngeal valves in Aedes albopictus (Skuse) and Culex pipiens pallens Coquillett (Diptera: Culicidae). Int. J. Insect Morphol. Embryol. 8, 159–167 (1979). [Google Scholar]
- 21.Lee R. M. K. W., Davies D. M., Cibarial sensilla of Toxorhynchites mosquitoes (Diptera: Culicidae). Int. J. Insect Morphol. Embryol. 7, 189–194 (1978). [Google Scholar]
- 22.Pappas L. G., Larsen J. R., Gustatory hairs on the mosquito, Culiseta inornata. J. Exp. Zool. 196, 351–360 (1976). [DOI] [PubMed] [Google Scholar]
- 23.Owen W. B., Larsen J. R., Pappas L. G., Functional units in the labellar chemosensory hairs of the mosquito Culiseta inornata (Williston). J. Exp. Zool. 188, 235–247 (1974). [DOI] [PubMed] [Google Scholar]
- 24.Kessler S., Vlimant M., Guerin P. M., The sugar meal of the African malaria mosquito Anopheles gambiae and how deterrent compounds interfere with it: A behavioural and neurophysiological study. J. Exp. Biol. 216, 1292–1306 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Kessler S., Vlimant M., Guerin P. M., Sugar-sensitive neurone responses and sugar feeding preferences influence lifespan and biting behaviours of the Afrotropical malaria mosquito, Anopheles gambiae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 201, 317–329 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Sparks J. T., Dickens J. C., Bitter-sensitive gustatory receptor neuron responds to chemically diverse insect repellents in the common malaria mosquito Anopheles quadrimaculatus. Naturwissenschaften 103, 39 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Sparks J. T., Dickens J. C., Electrophysiological responses of gustatory receptor neurons on the labella of the common malaria mosquito, Anopheles quadrimaculatus (Diptera: Culicidae). J. Med. Entomol. 53, 1148–1155 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Sanford J. L., Shields V. D., Dickens J. C., Gustatory receptor neuron responds to DEET and other insect repellents in the yellow-fever mosquito, Aedes aegypti. Naturwissenschaften 100, 269–273 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Elizarov I. U., Sinitsina E. E., Contact chemoreceptors in Aedes aegypti L. Zoologicheskii zhurnal 577–584 (1974). [Google Scholar]
- 30.Werner-Reiss U., Galun R., Crnjar R., Liscia A., Sensitivity of the mosquito Aedes aegypti (Culicidae) labral apical chemoreceptors to phagostimulants. J. Insect Physiol. 45, 629–636 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Liscia A., et al. , Electrophysiological responses of labral apical chemoreceptors to adenine nucleotides in Culex pipiens. J. Insect Physiol. 39, 261–265 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Galun R., Avi-Dor Y., Bar-Zeev M., Feeding response in Aedes aegypti: Stimulation by adenosine triphosphate. Science 142, 1674–1675 (1963). [DOI] [PubMed] [Google Scholar]
- 33.Kent L. B., Walden K. K., Robertson H. M., The Gr family of candidate gustatory and olfactory receptors in the yellow-fever mosquito Aedes aegypti. Chem. Senses 33, 79–93 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Matthews B. J., et al. , Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563, 501–507 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arensburger P., et al. , Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330, 86–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks J. T., Vinyard B. T., Dickens J. C., Gustatory receptor expression in the labella and tarsi of Aedes aegypti. Insect Biochem. Mol. Biol. 43, 1161–1171 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Alonso D. P., et al. , Gene expression profile of Aedes aegypti females in courtship and mating. Sci. Rep. 9, 15492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparks J. T., Bohbot J. D., Dickens J. C., The genetics of chemoreception in the labella and tarsi of Aedes aegypti. Insect Biochem. Mol. Biol. 48, 8–16 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Matthews B. J., McBride C. S., DeGennaro M., Despo O., Vosshall L. B., The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics 17, 32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitts R. J., Rinker D. C., Jones P. L., Rokas A., Zwiebel L. J., Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics 12, 271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen W. B., The contact chemoreceptor organs of the mosquito and their function in feeding behaviour. J. Insect Physiol. 9, 73–87 (1963). [Google Scholar]
- 42.Feir D., Lengy J. I., Owen W. B., Contact chemoreception in the mosquito, Culiseta inornata (Williston); sensitivity of the tarsi and labella to sucrose and glucose. J. Insect Physiol. 6, 13–20 (1961). [Google Scholar]
- 43.Frings H., Hamrum C. L., The contact chemoreceptors of adult yellow fever mosquitoes, Aedes aegypti. J. N. Y. Ent. Soc. 58, 133–142 (1950). [Google Scholar]
- 44.Owen W. B., Behavioral studies of inhibition and integration in the mosquito Culiseta inornata (Williston). J. Exp. Zool. 166, 301–305 (1967). [DOI] [PubMed] [Google Scholar]
- 45.Salama H. S., The function of mosquito taste receptors. J. Insect Physiol. 12, 1051–1060 (1966). [DOI] [PubMed] [Google Scholar]
- 46.Ignell R., Okawa S., Englund J., Hill S. R., Assessment of diet choice by the yellow fever mosquito Aedes aegypti. Phys. Ent. 35, 274–286 (2010). [Google Scholar]
- 47.Dennis E. J., Goldman O. V., Vosshall L. B., Aedes aegypti mosquitoes use their legs to sense DEET on contact. Curr. Biol. 29, 1551–1556.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth L. M., Loci of sensory end-organs used by mosquitoes (Aedes aegypti (L.) and Anopheles quadrimaculatus Say) in receiving host stimuli. Ann. Entomol. Soc. Am. 44, 59–74 (1951). [Google Scholar]
- 49.Lang J. T., Foster W. A., Is there a female sex pheromone in the mosquito Culiseta inornata? Environ. Entomol. 5, 1109–1115 (1976). [Google Scholar]
- 50.Lang J. T., Contact sex pheromone in the mosquito Culiseta inornata (Diptera: Culicidae). J. Med. Entomol. 14, 448–454 (1977). [DOI] [PubMed] [Google Scholar]
- 51.Nijhout H. F., Craig G. B. Jr, Reproductive isolation in Stegomyia mosquitoes. III. Evidence for a sexual pheromone. Entomol. Exp. Appl. 14, 399–412 (1971). [Google Scholar]
- 52.Provost M. W., Haeger J. S., Mating and pupal attendance in Deinocerites cancer and comparisons with Opifex fuscus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 60, 565–574 (1967). [Google Scholar]
- 53.Kim S., Trocke S., Sim C., Comparative studies of stenogamous behaviour in the mosquito Culex pipiens complex. Med. Vet. Entomol. 32, 427–435 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Conner W. E., Itagaki H., Pupal attendance in the crabhole mosquito Deinocerites cancer: The effects of pupal sex and age. Phys. Ent. 9, 263–267 (1984). [Google Scholar]
- 55.Kennedy J. S., On water-finding and oviposition by captive mosquitoes. Bull. Entomol. Res. 32, 279–301 (1942). [Google Scholar]
- 56.Wallis R. C., A study of oviposition activity of mosquitoes. Am. J. Hyg. 60, 135–168 (1954). [DOI] [PubMed] [Google Scholar]
- 57.Bentley M. D., Day J. F., Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 34, 401–421 (1989). [DOI] [PubMed] [Google Scholar]
- 58.Kligler I. J., Theodor O., Effect of salt concentration and reaction on the development of Anopheles larvae. Bull. Entomol. Res. 16, 45–49 (1925). [Google Scholar]
- 59.Hudson B. N. A., The behaviour of the female mosquito in selecting water for oviposition. J. Exp. Biol. 33, 478–492 (1956). [Google Scholar]
- 60.Trimble R. M., Wellington W. G., Effects of salinity on site selection by ovipositing Aedes togoi (Diptera: Culicidae). Can. J. Zool. 57, 593–596 (1979). [Google Scholar]
- 61.McGaughey W. H., Knight K. L., Preoviposition activity of the black salt-marsh mosquito, Aedes taeniorhynchus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 60, 107–115 (1967). [DOI] [PubMed] [Google Scholar]
- 62.Matthews B. J., Younger M. A., Vosshall L. B., The ion channel ppk301 controls freshwater egg-laying in the mosquito Aedes aegypti. eLife 8, e43963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afify A., Galizia C. G., Chemosensory cues for mosquito oviposition site selection. J. Med. Entomol. 52, 120–130 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Zuharah W. F., Fadzly N., Wei W. O., Hashim Z. H., Oviposition habitat selection of Dengue vectors, Aedes aegypti and Aedes albopictus in response to fish predator. Trop. Life Sci. Res. 27, 117–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaustein L., Kiflawi M., Eitam A., Mangel M., Cohen J. E., Oviposition habitat selection in response to risk of predation in temporary pools: Mode of detection and consistency across experimental venue. Oecologia 138, 300–305 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Eitam A., Blaustein L., Oviposition habitat selection by mosquitoes in response to predator (Notonecta maculata) density. Phys. Ent. 29, 188–191 (2004). [Google Scholar]
- 67.Wasserberg G., Bailes N., Davis C., Yeoman K., Hump-shaped density-dependent regulation of mosquito oviposition site-selection by conspecific immature stages: Theory, field test with Aedes albopictus, and a meta-analysis. PLoS One 9, e92658 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mokany A., Shine R., Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Austral Ecol. 28, 33–37 (2003). [Google Scholar]
- 69.Soman R. S., Reuben R., Studies on the preference shown by ovipositing females of Aedes aegypti for water containing immature stages of the same species. J. Med. Entomol. 7, 485–489 (1970). [DOI] [PubMed] [Google Scholar]
- 70.Wong J., Stoddard S. T., Astete H., Morrison A. C., Scott T. W., Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl. Trop. Dis. 5, e1015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laurence B. R., Pickett J. A., Erythro-6-acetoxy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone. J. Chem. Soc. Chem. Comm. 1982, 59–60 (1982). [Google Scholar]
- 72.Sullivan G. A., Liu C., Syed Z., Oviposition signals and their neuroethological correlates in the Culex pipiens complex. Infect. Genet. Evol. 28, 735–743 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Pickett J. A., et al. , Chemical ecology of animal and human pathogen vectors in a changing global climate. J. Chem. Ecol. 36, 113–121 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Trimble R. M., Laboratory observations on oviposition by the predaceous tree-hole mosquito, Toxorhynchites rutilus septentrionalis (Diptera: Culicidae). Can. J. Zool. 57, 1104–1108 (1979). [Google Scholar]
- 75.Merritt R. W., Dadd R. H., Walker E. D., Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37, 349–376 (1992). [DOI] [PubMed] [Google Scholar]
- 76.Dadd R. H., Comparison of rates of ingestion of particulate solids by Culex pipiens larvae: Phagostimulant effect of water‐soluble yeast extract. Entomol. Exp. Appl. 13, 407–419 (1970). [Google Scholar]
- 77.Aly C., Mulla S., Orientation and ingestion rates of larval Anopheles albimanus in response to floating particles. Entomol. Exp. Appl. 42, 83–90 (1986). [Google Scholar]
- 78.Dadd R. H., Kleinjan J. E., Phagostimulation of larval Culex pipiens L. by nucleic acid nucleotides, nucleosides and bases. Phys. Ent. 10, 37–44 (1985). [Google Scholar]
- 79.Dadd R. H., Kleinjan J. E., Merrill L. D., Phagostimulant effects of simple nutrients on larval Culex pipiens (Diptera: Culicidae). Ann. Entomol. Soc. Am. 75, 605–612 (1982). [Google Scholar]
- 80.Liu C., et al. , Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 8, e1000467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lutz E. K., Grewal T. S., Riffell J. A., Computational and experimental insights into the chemosensory navigation of Aedes aegypti mosquito larvae. Proc. Biol. Sci. 286, 20191495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zacharuk R. Y., Yin L. R., Blue S. G., Fine structure of the antenna and its sensory cone in larvae of Aedes aegypti (L.). J. Morphol. 135, 273–297 (1971). [DOI] [PubMed] [Google Scholar]
- 83.Jez D. H., McIver S. B., Fine structure of antennal sensilla of larval Toxorhynchites brevipalpis Theobald (Diptera: Culicidae). Int. J. Insect Morphol. Embryol. 9, 147–159 (1980). [Google Scholar]
- 84.Xia Y., et al. , The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl. Acad. Sci. U.S.A. 105, 6433–6438 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McIver S., Siemicki R., Fine structure of maxillary sensilla of larval Toxorhynchites brevipalpis (Diptera: Culicidae) with comments on the role of sensilla in behavior. J. Morphol. 171, 293–303 (1982). [DOI] [PubMed] [Google Scholar]
- 86.Liman E. R., Zhang Y. V., Montell C., Peripheral coding of taste. Neuron 81, 984–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y. D., Dahanukar A., Recent advances in the genetic basis of taste detection in Drosophila. Cell. Mol. Life Sci. 77, 1087–1101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ling F., Dahanukar A., Weiss L. A., Kwon J. Y., Carlson J. R., The molecular and cellular basis of taste coding in the legs of Drosophila. J. Neurosci. 34, 7148–7164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weiss L. A., Dahanukar A., Kwon J. Y., Banerjee D., Carlson J. R., The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y. V., Ni J., Montell C., The molecular basis for attractive salt-taste coding in Drosophila. Science 340, 1334–1338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Croset V., et al. , Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6, e1001064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Q., Man Y., Li J., Pei D., Wu W., Olfactory ionotropic receptors in mosquito Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 54, 1229–1235 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Hill C. A., et al. , G protein-coupled receptors in Anopheles gambiae. Science 298, 176–178 (2002). [DOI] [PubMed] [Google Scholar]
- 94.Lombardo F., et al. , Deciphering the olfactory repertoire of the tiger mosquito Aedes albopictus. BMC Genomics 18, 770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greppi C., et al. , Mosquito heat seeking is driven by an ancestral cooling receptor. Science 367, 681–684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeGennaro M., et al. , Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raji J. I., et al. , Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 29, 1253–1262.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melo N., et al. , Geosmin attracts Aedes aegypti mosquitoes to oviposition sites. Curr. Biol. 30, 127–134.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Afify A., Betz J. F., Riabinina O., Lahondere C., Potter C. J., Commonly used insect repellents hide human odors from Anopheles mosquitoes. Curr. Biol. 29, 3669–3680.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bui M., et al. , Live calcium imaging of Aedes aegypti neuronal tissues reveals differential importance of chemosensory systems for life-history-specific foraging strategies. BMC Neurosci. 20, 27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Syed Z., Leal W. S., Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. U.S.A. 105, 13598–13603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson B. J., Ritchie S. A., Fonseca D. M., The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects 8, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hallem E. A., Carlson J. R., Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Su C. Y., Martelli C., Emonet T., Carlson J. R., Temporal coding of odor mixtures in an olfactory receptor neuron. Proc. Natl. Acad. Sci. U.S.A. 108, 5075–5080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wyatt T. D., Pheromones and Animal Behaviour: Communication by Smell and Taste (Cambridge University Press, 2003). [Google Scholar]
- 106.Shorey H. H., Behavioral responses to insect pheromones. Annu. Rev. Entomol. 18, 349–380 (1973). [DOI] [PubMed] [Google Scholar]
- 107.Reddy G. V., Guerrero A., Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 9, 253–261 (2004). [DOI] [PubMed] [Google Scholar]
- 108.World Health Organization , World Health Organization (2020). https://www.who.int/. Accessed 24 June 2020.
- 109.Chancey C., Grinev A., Volkova E., Rios M., The global ecology and epidemiology of West Nile virus. BioMed Res. Int. 2015, 376230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Centers for Disease Control and Prevention , Centers for Disease Control and Prevention (2020). https://www.cdc.gov/. Accessed 24 June 2020.
- 111.Joseph R. M., Carlson J. R., Drosophila chemoreceptors: A molecular interface between the chemical world and the brain. Trends Genet. 31, 683–695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.