Significance
There is currently great concern about the ability of organisms to adapt to warmer environments. During heat waves, upper thermal tolerance is often critical for survival, but it is largely unknown how rapidly tolerance can evolve, especially in vertebrates. We artificially selected on upper thermal tolerance in a tropical fish to see whether and how quickly thermal tolerance evolves, and how warm acclimation prior to a thermal challenge alters this evolutionary process. Upper thermal tolerance evolved but at a slow rate toward higher temperature. Furthermore, acclimation capacity decreased in the lines selected for higher thermal tolerance. These results suggest that tropical fishes will struggle to adapt in pace with the current climate warming.
Keywords: CTmax, artificial selection, asymmetrical response, global warming, teleost
Abstract
Climate change is increasing global temperatures and intensifying the frequency and severity of extreme heat waves. How organisms will cope with these changes depends on their inherent thermal tolerance, acclimation capacity, and ability for evolutionary adaptation. Yet, the potential for adaptation of upper thermal tolerance in vertebrates is largely unknown. We artificially selected offspring from wild-caught zebrafish (Danio rerio) to increase (Up-selected) or decrease (Down-selected) upper thermal tolerance over six generations. Selection to increase upper thermal tolerance was also performed on warm-acclimated fish to test whether plasticity in the form of inducible warm tolerance also evolved. Upper thermal tolerance responded to selection in the predicted directions. However, compared to the control lines, the response was stronger in the Down-selected than in the Up-selected lines in which evolution toward higher upper thermal tolerance was slow (0.04 ± 0.008 °C per generation). Furthermore, the scope for plasticity resulting from warm acclimation decreased in the Up-selected lines. These results suggest the existence of a hard limit in upper thermal tolerance. Considering the rate at which global temperatures are increasing, the observed rates of adaptation and the possible hard limit in upper thermal tolerance suggest a low potential for evolutionary rescue in tropical fish living at the edge of their thermal limits.
Globally, both mean and extreme environmental temperatures are increasing due to climate change with mean temperatures predicted to increase by 0.3–4.8 °C by the end of the century (1, 2). Aquatic ectotherms are particularly vulnerable to rising temperatures as their body temperature closely tracks the environmental temperature (3). These organisms can avoid thermal stress by migrating to cooler waters, acclimating, and/or adapting genetically (4–6). For species with a limited dispersal ability (e.g., species from shallow freshwater habitats; ref. 7), acclimation and evolutionary adaptation are the only possible strategies. Furthermore, for ectotherms living at the edge of their upper thermal limits, an increase in extreme temperatures may generate temperature peaks that exceed physiological limits and cause high mortality (5, 8–10). Although this is expected to cause strong selection toward higher upper thermal tolerance, it is largely unknown, particularly within vertebrates, whether and at what rate organisms may adapt by evolving their thermal limits (11–14). These are important issues because constrained or limited evolvability (15) of upper thermal tolerance could lead to population extinctions as climate change increases the severity of heat waves.
Ectotherms can also increase their thermal limits through physiological and biochemical adjustments, in a process known as thermal acclimation when they are exposed to elevated temperatures for a period of time (16, 17). Thermal acclimation, sometimes called thermal compensation, is here used interchangeably with the term physiological plasticity as outlined by Seebacher et al. (18). In the wild, individuals may experience days or weeks of warmer temperatures prior to a thermal extreme. Through physiological plasticity, the severity of an ensuing thermal extreme may be reduced, thus increasing the chance for survival (19). Furthermore, in some cases, adaptation can be accelerated by plasticity (20–22). This requires that the physiological mechanisms responsible for acclimation are also (at least partly) involved in the acute response; that is, that there is a positive genetic correlation between physiological plasticity and (acute) upper thermal tolerance. It is therefore crucial to quantify the evolutionary potential of upper thermal tolerance of fish populations threatened by climate change (23, 24) and to understand the link between the evolutionary response of upper thermal tolerance and physiological plasticity.
Previously detected evolution of upper thermal tolerance generally points toward a slow process (12, 13, 25–31). However, estimates of the evolutionary potential in upper thermal tolerance mostly come from studies on Drosophila (12, 25, 27, 32), and empirical evidence in aquatic ectotherms and specifically vertebrates is limited. The few studies that have been performed on fish show disparate responses to selection on heat tolerance even within the same species. Baer and Travis (33) detected no response to selection yet Doyle et al. (34) and Klerks et al. (28) detected selection responses with heritabilities of 0.2 in killifish (Heterandria formosa). Despite the typical asymmetry of thermal performance curves (3, 35), studies in vertebrates are limited to unidirectional estimates of evolutionary potential (28, 31, 33) or do not account for the direction of evolution when estimating heritability in upper thermal tolerance from breeding designs (36, 37). Furthermore, while several studies have found that populations with different thermal histories have evolved different levels of heat tolerance (29–31), we still lack a good understanding of how physiological plasticity within a generation, in response to a short heat exposure, interacts with genetic changes during evolution of thermal tolerance.
To investigate possible asymmetry in the evolutionary potential of upper thermal tolerance in a vertebrate species, we artificially selected offspring of wild-caught zebrafish (Danio rerio) to increase and decrease upper thermal tolerance for six generations. Furthermore, to disentangle the contribution of acclimation from the genetic response to increase upper thermal tolerance, we selected two lines that were exposed to a period of warm acclimation prior to a thermal challenge. The size (>20,000 phenotyped fish) and duration (six generations) of this study are unique in a vertebrate species for a climate change-relevant selection experiment, and the results provide critical and robust information on how tropical fish may adapt to a changing climate.
Being a freshwater and tropical species, zebrafish are likely to be especially vulnerable to climate change (7, 38). In the wild, zebrafish can already be found living only a few degrees below their thermal limits (17, 39) and live in shallow streams and pools (40) that have the potential to rapidly warm during heat waves. Zebrafish therefore represent a species living at the edge of its thermal limit in which rapid adaptation of thermal tolerance would be particularly beneficial for its survival. Wild-caught zebrafish originating from different sites in West Bengal, India (17, 40), were used to maximize the genetic diversity of the parental population. These wild-caught zebrafish (n = 2,265) served as parents of the starting F0 generation (n = 1,800) on which we selected upper thermal tolerance for six generations. Upper thermal tolerance was measured as the critical thermal maximum (CTmax), a commonly used measure of an organism’s acute upper thermal tolerance (16, 41). CTmax is defined as the temperature at which an individual loses equilibrium (i.e., uncontrolled and disorganized swimming in zebrafish; ref. 42) during thermal ramping. Measuring CTmax is rapid, repeatable, and does not appear to harm zebrafish (42). CTmax is ecologically relevant because it is highly correlated with both tolerance to slow warming (43) and to the upper temperature range boundaries of wild aquatic ectotherms (9).
Our selection experiment consisted of four treatment groups (Up-selected, Down-selected, Acclimated Up-selected, and Control) with two replicate lines in each treatment. We established these lines by selecting fish on their CTmax in the F0 generation with each line consisting of 150 individuals (see Methods for further details of F0 generation). The offspring of those fish formed the F1 generation that consisted of 450 offspring in each line. At each generation, the Up, Down, and Control lines were all held at optimal temperature (28 °C) (39), whereas the Acclimated Up-selected lines were acclimated to a supraoptimal temperature (32 °C) for 2 wk prior to selection (17). From the F1 to F6 generations, we measured CTmax for all 450 fish in each line and selected the 33% with the highest CTmax in the Up-selected and in the Acclimated Up-selected lines, and the 33% with the lowest CTmax in the Down-selected lines. In the Control lines, 150 fish were randomly selected, measured, and retained. Thus, CTmax was measured on a total of 3,000 fish per generation and 150 individuals remained in each of the eight lines after selection, forming the parents for the next generation. The nonselected lines (Control) represented a control for the Up-selected and Down-selected lines, while the Up-selected lines represented a control for the Acclimated Up-selected lines, because these two treatments solely differed by the acclimation period to which the latter were exposed before selection. Thus, differences in CTmax between Up-selected and Acclimated Up-selected lines represent the contribution of physiological plasticity to upper thermal tolerance. If the difference between these two treatments increases during selection, it would suggest that plasticity increases during adaptation to higher CTmax (i.e., the slope the reaction norm describing the relationship between CTmax and acclimation temperature would become steeper).
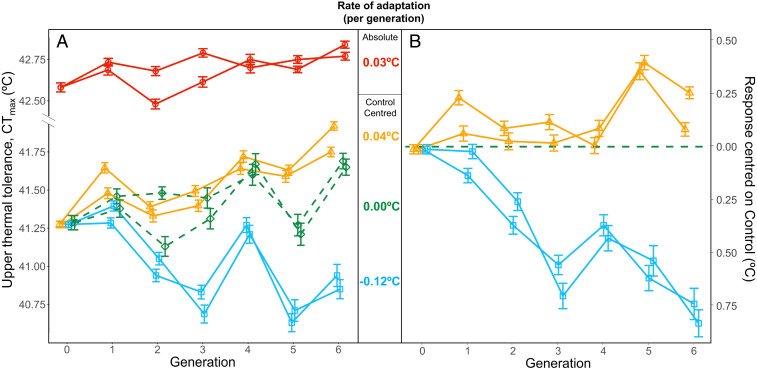
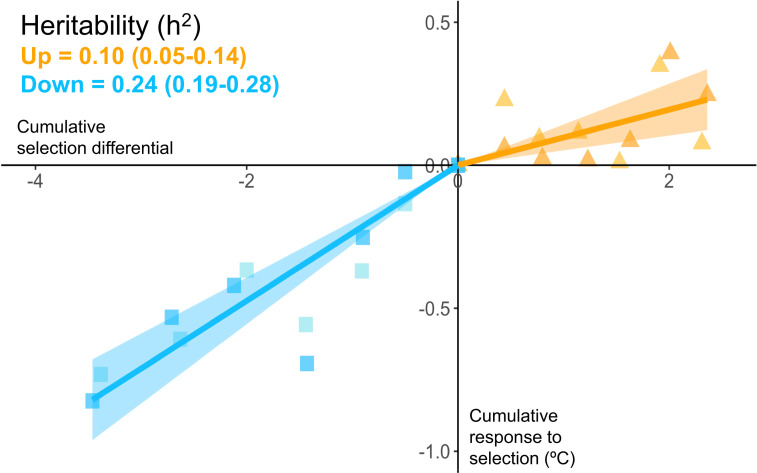
After six generations of selection, upper thermal tolerance had evolved in both the Up-selected and the Down-selected lines (Fig. 1). In the Up-selected lines, upper thermal tolerance increased by 0.22 ± 0.05 °C (x̄ ± 1 SE) compared to the Control lines whereas the Down-selected lines displayed a mean upper thermal tolerance 0.74 ± 0.05 °C lower than the Control (Fig. 1B; estimates for replicated lines combined). The asymmetry in the response to selection was confirmed by the estimated realized heritability, which was more than twice as high in the Down-selected lines (h2 = 0.24; 95% CI: 0.19–0.28) than in the Up-selected lines (h2 = 0.10; 95% CI: 0.05–0.14; Fig. 2).
Fig. 1.
Upper thermal tolerance (CTmax) of wild-caught zebrafish over six episodes of selection. Duplicated lines were selected for increased (Up-selected, orange lines and triangles) and decreased (Down-selected, blue lines and squares) upper thermal tolerance. In addition, we had two Control lines (green dashed lines and diamonds). The Up, Down, and Control lines were all acclimated to a temperature of 28 °C. In addition, two lines were selected for increased upper thermal tolerance after 2 wk of warm acclimation at 32 °C (Acclimated Up-selected, red lines and circles). At each generation, the mean and 95% CIs of each line are shown (n ∼ 450 individuals per line). (A) Absolute upper thermal tolerance values. (B) The response to selection in the Up and Down lines centered on the Control lines (dashed green line). Difference between Up-selected and Acclimated-Up lines are shown in Fig. 3. The rate of adaptation (°C per generation) is reported for each treatment using estimates obtained from linear mixed effects models using the Control-centered response in the Up-selected and Down-selected lines and the absolute response for the Acclimated-Up lines (SE = ±0.01 °C in all lines).
Fig. 2.
Realized heritability (h2) of upper thermal tolerance (CTmax) in wild-caught zebrafish. The realized heritability was estimated for each treatment as the slope of the regression of the cumulative response to selection on the cumulative selection differential using mixed effect models passing through the origin with replicate as a random effect. Slopes are presented with their 95% CIs (shaded area) for the Down-selected lines (blue) and Up-selected lines (orange). Data points represent the mean of each replicate line (n ∼ 450) over six generations of selection. Average selection differentials are 0.57 (Down) and 0.39 (Up), respectively, see SI Appendix, Table S1 for more information.
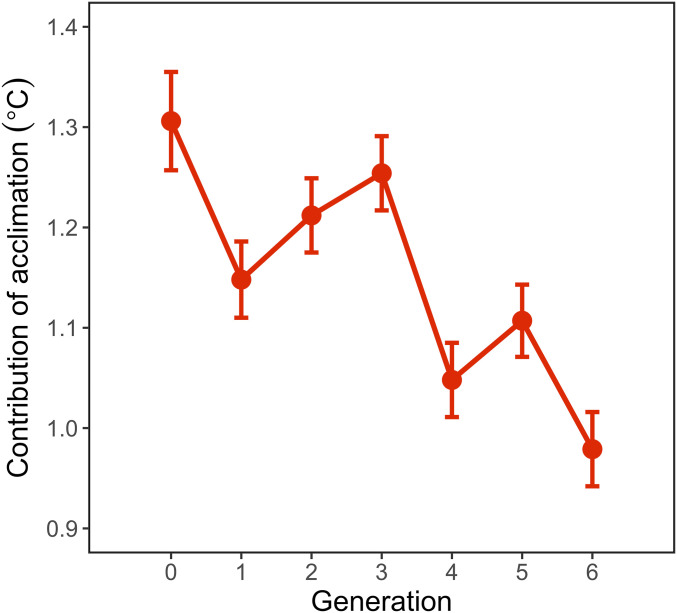
At the start of the experiment (F0), warm acclimation (32 °C) increased thermal tolerance by 1.31 ± 0.05 °C (difference in CTmax between the Up-selected and Acclimated Up-selected lines in Figs. 1A and 3), which translates to a 0.3 °C change in CTmax per 1 °C of warming. In the last generation, the effect of acclimation had decreased by 25%, with the Acclimated-Up lines having an average CTmax 0.98 ± 0.04 °C higher than the Up lines (Fig. 3). This suggests that, despite a slight increase in CTmax in the Acclimated Up-selected lines during selection, the contribution of plasticity decreased over the course of the experiment.
Fig. 3.
Contribution of acclimation to the upper thermal tolerance in the Acclimated-Up selected lines at each generation of selection. The contribution of acclimation was estimated as the difference between the Up and Acclimated-Up selected lines. Points and error bars represent the estimates (±SE) from a linear mixed effects model with CTmax as the response variable; Treatment (factor with two levels: Up and Acclimated Up), Generation (factor with seven levels), and their interaction as the predictor variables; and replicate line as a random factor.
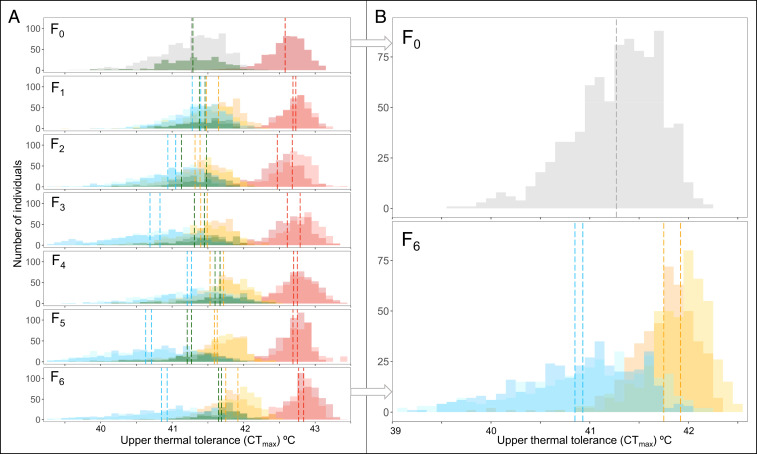
During the experiment, the phenotypic variation of CTmax that was left-skewed at F0 increased in the Down-selected lines and decreased in the Up-selected lines (Fig. 4). At the F6 generation, phenotypic variance was four times lower in the Up-selected lines (0.09 ± 0.01 and 0.12 ± 0.02 °C2; variance presented for each replicate line separately and SE obtained by nonparametric bootstrapping) than in the Down-selected lines (0.41 ± 0.03 and 0.50 ± 0.04 °C2), which had doubled since the start of the experiment (F0: 0.20 ± 0.01 °C2, see SI Appendix, Fig. S1). In the Acclimated Up-selected lines, the phenotypic variance that was already much lower than the Control at the F0 also decreased and reached 0.06 ± 0.01 °C2 and 0.07 ± 0.01 °C2 for the two replicates at the last generation (SI Appendix, Fig. S1).
Fig. 4.
Distribution of upper thermal tolerance (CTmax) in selected lines. (A) Distribution for each line at each generation (F0 to F6). In the F0 generation, histograms show the preselection distribution in gray for the nonacclimated fish, in dark green for the Control lines, and in red for the Acclimated-Up fish. In all subsequent generations the Down-selected lines are in blue, the Up-selected lines in yellow, the Control lines in dark green, and Acclimated-up lines in red. All treatments use two shades, one for each replicate line. Dashed lines represent the mean CTmax for each line (n ∼ 450 individuals). (B) Distribution of upper thermal tolerance at the start (F0, in gray) and the end (F6, in blue and yellow) of the experiment for the Up-selected and Down-selected lines. The dashed gray line represents the mean of the Up-selected and Down-selected lines in the F0 generation preselection (n ∼ 900 individuals). Dashed blue and yellow lines represent the mean CTmax for Up and Down-selected lines for the F6 generation (n ∼ 450 individuals).
Together with the asymmetrical response to selection and the lower response of the Acclimated Up-selected lines, these changes in phenotypic variance suggest the existence of a hard-upper limit for thermal tolerance (e.g., major protein denaturation (44), similar to the “concrete ceiling” for physiological responses to warming (14)). Such a hard-upper limit is expected to generate a nonlinear mapping of the genetic and environmental effects on the phenotypic expression of CTmax. This nonlinearity will affect the phenotypic variance of CTmax when mean CTmax approaches its upper limit (SI Appendix, Fig. S2A). For example, with directional selection toward higher CTmax, genetic changes in upper thermal tolerance will translate into progressively smaller phenotypic changes. Similarly, warm acclimation that shifts CTmax upwards will also decrease phenotypic variation in CTmax (see differences in phenotypic variance between control and Acclimated lines at the F0). This hard ceiling can also explain why an evolutionary increase in CTmax reduces the magnitude of physiological plasticity in CTmax achieved after a period of acclimation (Fig. 3 and see SI Appendix, Fig. S2B). If the sum of the genetic and plastic contributions to CTmax cannot exceed a ceiling value, this should generate a zero-sum gain between the genetic and plastic determinants of thermal tolerance. An increase in the genetic contribution to CTmax via selection should thus decrease the contribution of plasticity. Selection for a higher CTmax should therefore negatively affect the slope of the reaction norm of thermal acclimation because acclimation will increase CTmax more strongly at low than high acclimation temperature (SI Appendix, Fig. S2B).
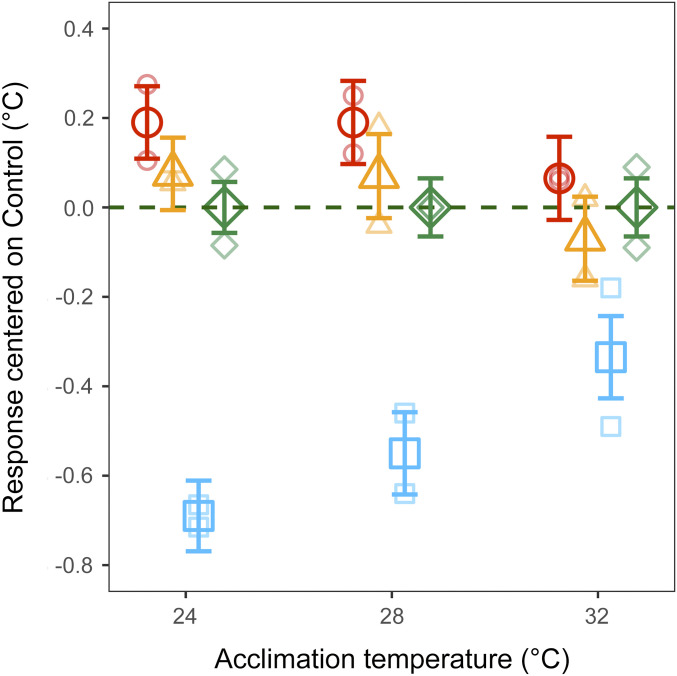
To test this hypothesis, we measured CTmax in all selected lines at the final generation (F6) after acclimation to 24, 28, and 32 °C. At all three acclimation temperatures, the Acclimated-Up lines did not differ from the Up-selected lines (average difference 0.14 ± 0.08 °C; 0.12 ± 0.09 °C; 0.14 ± 0.09 °C; at 24, 28, and 32 °C respectively; Fig. 5). This suggests that warm acclimation prior to selection did not affect the response to selection. However, considering the within-treatment differences in CTmax between fish acclimated to 28 and 32 °C, we show that the gain in CTmax due to acclimation decreases in both the Up and Acclimated-Up treatments compared to the Control and Down treatments (SI Appendix, Fig. S3). This confirms a loss of thermal plasticity in both Up-selected treatments (Up and Acclimated-Up) at higher acclimation temperatures. Notably, the loss of thermal plasticity is not evident in fish acclimated to 24 and 28 °C, possibly because at these temperatures CTmax remains further away from its hard upper limit.
Fig. 5.
Upper thermal tolerance (CTmax) of the selected lines measured at the last generation (F6) after acclimation at 24, 28, and 32 °C. The response is calculated as the mean difference in upper thermal tolerance (CTmax) relative to the Control lines. Large points and whiskers represent mean ±1 SE for each treatment (n = 120 individuals): Up-selected (orange triangles), Down-selected (blue squares), Acclimated Up-selected (red circles), and Control (green diamonds). Smaller translucent points represent means of each replicate line (n = 60 individuals). See SI Appendix, Fig. S3 for absolute CTmax values and model estimates.
Acclimated Up-selected lines are perhaps the most ecologically relevant in our selection experiment. In the wild, natural selection on upper thermal tolerance may not result from increasing mean temperatures but through rapid heating events such as heat waves (45). During heat waves, temperature may rise for days before reaching critical temperatures. This gives individuals the possibility to acclimate and increase their upper thermal tolerance prior to peak temperatures. Our results show that while warm acclimation allowed individuals to increase their upper thermal tolerance, it did not increase the magnitude or the rate of adaptation of upper thermal tolerance.
For the past two decades it has been recognized that rapid evolution, at ecological timescales, occurs and may represent an essential mechanism for the persistence of populations in rapidly changing environments (24, 46, 47). Yet, in the absence of an explicit reference, rates of evolution are often difficult to categorize as slow or rapid (48). For traits related to thermal tolerance or thermal performance, this issue is complicated by the fact that the scale on which traits are measured (temperature in °C) cannot meaningfully be transformed to a proportional scale. This prevents us from comparing rates of evolution between traits related to temperature with other traits measured on different scales (49, 50). However, for thermal tolerance, the rate of increase in ambient temperature predicted over the next century represents a particularly meaningful standard against which the rate of evolution observed in our study can be compared.
In India and surrounding countries where zebrafish are native, heat waves are predicted to increase in frequency, intensity, and duration, and maximum air temperatures in some regions are predicted to exceed 44 °C in all future climate scenarios (51). Air temperature is a good predictor of water temperature in shallow ponds and streams where wild zebrafish are found (17, 40, 52, 53). Thus, strong directional selection on the thermal limits of zebrafish is very likely to occur in the wild. At first sight, changes in the upper thermal tolerance observed in our study (0.04 °C per generation) as well as the heritability estimates (Down-selected: h2 = 0.24, Up-selected: h2 = 0.10) similar to those obtained in fruit flies (Drosophila melanogaster) selected for acute upper thermal tolerance (Down-selected: h2 = 0.19, Up-selected: h2 = 0.12; ref. 12), suggest that zebrafish may just be able to keep pace with climate change and acutely tolerate temperatures of 44 °C predicted by the end of the century. However, several cautions make such an optimistic prediction unlikely.
First, such an extrapolation assumes a generation time of 1 y, which is likely for zebrafish but unrealistic for many other fish species. Second, such a rate of evolution is associated with a thermal culling of two-thirds of the population at each generation, a strength of selection that may be impossible to sustain in natural populations exposed to other selection pressures such as predation or harvesting. Third, the heritability and rate of adaptation toward higher upper thermal tolerance observed here may be considered as upper estimates because of the potentially high genetic variance harbored by our parental population where samples from several sites were mixed. While mixing of zebrafish populations often occurs in the wild during monsoon flooding (54, 55), there are likely to be some isolated populations that may have a lower genetic diversity and adaptation potential than our starting population. Finally, and most importantly, the reduced phenotypic variance and decreased acclimation capacity with increasing CTmax observed in our study suggest the existence of a hard-upper limit to thermal tolerance that will lead to an evolutionary plateau similar to those reached in Drosophila selected for increased heat resistance over many generations (12, 56). Overall, the rate of evolution observed in our study is likely higher than what will occur in the wild and, based on this, it seems unlikely that zebrafish, or potentially other tropical fish species, will be able to acutely tolerate temperatures predicted by the end of the century. It is possible that other fish species, especially those living in cooler waters and with wider thermal safety margins, will display higher rates of adaptation than the ones we observed here, and more studies of this kind in a range of species are needed to determine whether slow adaptation of upper thermal tolerance is a general phenomenon.
Transgenerational plasticity (e.g., epigenetics) has been suggested to modulate physiological thermal tolerance (57). However, the progressive changes in CTmax observed across generations in our study indicate that these changes were primarily due to genetic changes because effects of transgenerational plasticity are not expected to accumulate across generations. Therefore, the effects of transgenerational plasticity in the adaptation of upper thermal tolerance may be insufficient to mitigate impacts of climate change on zebrafish, yet the potential contribution of transgenerational plasticity is still an open question.
By phenotyping more than 20,000 fish over six generations of selection, we show that evolution of upper thermal tolerance is possible in a vertebrate over short evolutionary time. However, the evolutionary potential for increased upper thermal tolerance is low due to the slow rate of adaptation compared to climate warming, as well as the diminishing effect of acclimation as adaptation progresses. Our results thus suggest that fish populations, especially warm water species living close to their thermal limits, may struggle to adapt with the rate at which water temperatures are increasing.
Methods
Animal Collection.
Based on the high fecundity, relatively short generation times, high survival after thermal challenges, and extensive background knowledge of their thermal biology, we chose the tropical zebrafish for this selection experiment. To ensure high genetic diversity in the parental stock, we used wild zebrafish collected by local fishermen from multiple sites in West Bengal, India, from August to September 2016 (n = 5,000). The fish were caught using small seine and hand nets, and the sampling locations were largely the same as those presented in refs. 17 and 40. The fish were imported by a commercial fish importer (Imazo AB) and held in quarantine for 3 wk in Sweden. During that time, some fish were not feeding for unknown reasons and these were removed from the population. The fish were then transported to the laboratory aquarium facility at the Norwegian University of Science and Technology (NTNU), Trondheim, Norway, in November 2016 (parental stock, n = 2,265). During this final transport phase, mortality was very low (<5 individuals) (17, 40). The experiments were approved by the Norwegian Food Safety Authority (permit number 8578).
Breeding Design and Fish Husbandry.
The parental generation (F0, n = 1,800) was produced by random mating of the wild caught fish and represents the first captive generation. This was done to allow genetic mixing of the different populations and minimize variation due to environmental conditions among selected fish. To facilitate breeding, the water temperature was dropped from 28 °C to 26 °C a week before breeding commenced. This was done to imitate monsoon rains, which are thought to trigger breeding in the wild. Contrary to laboratory strain (58), wild zebrafish appeared unable to breed if only one male and one female were paired. To maximize the number of spawning individuals contributing to the next generation, we randomly placed three mature males and three mature females in 2.5-L aquaria with a spawning box that contained a mesh to prevent cannibalism of the eggs and a plastic plant for shelter. The fish were left in the spawning boxes overnight, and the boxes were checked for eggs the following morning. If fish did not spawn the first time, they underwent extra spawning trials, each time with a different group of fish to increase the number of parents. Between 118 (56 females, 62 males) and 366 fish (180 females, 186 males) from the parental stock contributed to the F0 generation, and in the subsequent generations, a minimum of 23 male and 17 female fish and a maximum of 68 male and 51 female fish contributed to produce the next generation in each line (see SI Appendix, Table S3 for exact numbers).
At 2 d postfertilization (dpf), the fertilized eggs were pipetted out of the spawning boxes into new boxes filled with ∼2 cm (0.5 L) of fresh water. Once the majority of eggs hatched into free-swimming larvae (4 dpf), the boxes were filled with water and aerated. Feeding commenced after 5 dpf. The larvae were fed zebrafeed (Sparos) ad libitum once a day. At 7–10 dpf, the larvae were transferred to larger 63-L aquaria with internal filtering and a flow-through system replacing 40% of the water volume daily. A maximum of 300 larvae were added to each aquarium, and each replicate had larvae spread out over a minimum of two tanks randomly distributed in the fish laboratory. Temperature was maintained at 26 ± 0.5 °C, and the water was well aerated. Larvae were fed three times a day, altering zebrafeed (Sparos), GEMMA Micro (Skretting) and live Artemia sp. to ensure a fully nutritional diet. As the fish grew, the larvae feed was replaced with ground, followed by whole, dry flakes (TetraPro, ad libitum twice a day) and live Artemia sp. once per day. Water temperature was increased to 28 °C when the fish were ∼3 wk old.
CTmax Test.
Upper thermal tolerance was assessed using the CTmax test. This commonly used, quick, and repeatable method measures the acute upper thermal tolerance of an organism. A heating rate of 0.3 °C·min−1 was chosen in accordance with Lutterschmidt and Hutchison (41). We have previously shown that this heating rate causes a lag of less than 0.2 °C between the ambient water temperature and the deep dorsal muscle in zebrafish (42).
The CTmax test used is described in full in Morgan et al. (42). In short, a heating tank (25 × 22 × 18 cm) containing a water pump (Eheim Universal 300) attached to a custom-made heating case consisting of a 300 W coil heater, was filled with 11 L of 28 °C water. We placed 10–15 fish in the fish compartment that was separated from the heater by a mesh. This setup ensured a homogenous temperature in the entire arena (±0.1 °C), while minimizing the water current within the tank. Temperature was continuously monitored in the fish compartment using high precision thermometers to ±0.1 °C (testo-112, Testo) and regularly verified against an ultra-high precision and accuracy thermometer (testo-735–2, Testo). Loss of equilibrium, defined as uncontrolled and disorganized swimming for at least 3 s, was chosen as the CTmax endpoint. Once loss of equilibrium occurred in an individual, the water temperature was recorded to ±0.1 °C, and the fish was immediately transferred into an individual tank with 28 °C water for recovery. Once all of the fish in the trial had reached their CTmax and recovered, they were moved into temperature bins (7-L aquaria) corresponding to their CTmax (0.1 °C precision), allowing all fish with the same CTmax to be stored together. The water in the CTmax box was replaced, and 10–15 new fish were added to the compartment and a new trial commenced. Four CTmax boxes were run simultaneously, allowing up to 450 fish to be tested per day. Fish were held in their CTmax temperature bins for 12–24 h after the trial and before being selected. During this time, mortality was recorded and was low overall (<2% across all lines at 28 °C). Our definition of CTmax states that the fish have to recover and survive after the test in order to be selected. Consequently, fish that died posttest did not fulfill the criteria and were removed from the experiment. This means that the average CTmax for each line, selection differentials, and responses to selection were all calculated on the CTmax distribution of surviving fish.
Selection on Upper Thermal Tolerance.
Selection for CTmax occurred on juvenile fish approximately 6 wk old (weight ∼ 0.10–0.30 g; length ∼ 18–23 mm). This was considered an appropriate age to reduce any potentially detrimental effect of a heat shock on both the fish and their gonads but still perform selection on sufficiently developed individuals.
Temperatures were monitored and adjusted daily in every aquarium for 2 wk prior to selection, using a high precision thermometer. This ensured a temperature at ± 0.2 °C of the desired acclimation temperature.
In the F0 generation, all fish were tested for CTmax in September 2017, with 1,200 fish kept at their optimal temperature of 28 °C, and the remaining 600 fish acclimated to 34 °C for 2 wk prior to selection (Acclimated Up lines). For the fish kept at 28 °C, 300 fish were randomly selected prior to CTmax to form the Control lines and their CTmax was subsequently recorded. After the CTmax trial, these fish were split into two replicate Control lines of 150 fish each, ensuring that each line had the same CTmax distribution. For the remaining 900 fish at 28 °C, we measured their CTmax and selected the 300 fish with the highest CTmax to form the Up-selected lines, and the 300 with the lowest CTmax to form the Down-selected lines. Both groups were split into two replicate lines of 150 fish, with CTmax evenly distributed between each line. Maintaining duplicates of each treatment allowed us to partly account for any effects of genetic drift on the phenotypic changes. The intermediate 300 fish were immediately euthanized using MS-222. The 150 fish in each of the six replicate lines (Control × 2, Up-selected × 2, and Down-selected × 2) were then transferred back into 63-L aquaria with each line split between two tanks of 75 fish each. Once the fish reached maturity (∼3 mo), they were bred within their lines to produce the F1 generation. To ensure 450 surviving juveniles, ∼1,000 larvae were produced per line, using the protocol outlined above. If more than 450 fish survived, we chose fish in a way to maximize the genetic diversity by weighing the number of fish selected from each tank according to the number of parents that contributed to it, and less weight was given to the tank if the parents had spawned previously. Care was also taken to ensure that the chosen fish were not always the first or last fish caught from the tank.
From the F1, each of the six lines comprised 450 fish, and we selected 33% of them (150) to produce the next generation. Selected fish were the ones with the highest or lowest CTmax, in the Up- and Down-selected lines, respectively, while 150 fish were randomly selected and then tested for CTmax in each of the Control lines. The order in which the lines were tested for CTmax was randomized at each generation to ensure that no line was consistently tested first or last. The observers performing the CTmax trial were not aware of the treatment to which the fish belonged to minimize observer bias. In addition, due to the high throughput and long-term nature of the selection experiment, more than 20 experimenters were involved, limiting possible observer bias. The selection protocol continued until June 2019 when the CTmax of the F6 generation were measured.
Acclimated-Up Lines.
In addition to the six lines described above that were acclimated to zebrafish’s optimal temperature of 28 °C, we maintained two upward selected lines with fish acclimated to a higher temperature for 2 wk prior to CTmax trial. In these lines, the water temperature was increased by 2 °C per day using titanium heaters (TH-100, Aqua Medic) controlled by thermostats (ITC-306T, Inkbird), until the final acclimation temperature was reached. In the F0 generation, 600 fish were acclimated to 34 °C and the 300 with the highest CTmax were selected and split into two replicate lines of 150 fish. In subsequent generations, 450 fish were tested from each line after acclimation and the 150 fish with the highest CTmax were selected to propagate the next generation. The selection protocol was the same as for the Up-selected lines described above, except that the starting temperature in the CTmax trial was 34 °C. After the CTmax trial, the fish were reacclimated to 28 °C and maintained under the same conditions as the other selected lines (e.g., the temperature was also reduced to 26 °C 1 wk prior to mating in these lines). The acclimation temperature in these Acclimated-Up lines was reduced to 32 °C in generations F4 to F6 to reduce mortality after CTmax trials. In the final F6 generation, the duration of a CTmax trial was on average 10 min shorter in the Acclimated-Up lines (36 ± 0.03 min) than in the Up lines (46 ± 0.05 min), 9 min shorter than the Control lines (45 ± 0.09 min), and 7 min shorter than the Down lines (43 ± 0.12 min).
Assessing Thermal Reaction Norm.
In the final generation (F6), the thermal reaction norm was investigated in all eight lines using fish produced separately from the main experiment but using the same F5 parental fish. The larvae were held and grown for 6 wk (late juvenile stage) under the same conditions as in the main experiment, at which point they were transferred to new 63-L aquaria and the experiment commenced. Each line was split into three aquaria with 60 fish per aquaria, giving a total of 24 aquaria and 1,440 fish. Starting with a water temperature of 28 °C in all tanks, one tank per line was acclimated to one of three acclimation temperatures: 24, 28, and 32 °C by increasing or decreasing water temperature by 2 °C per day using titanium heaters (TH-100, Aqua Medic) controlled by thermostats (ITC-306T, Inkbird), until the final acclimation temperature was reached.
Thermocouples (type K, 10 m, Pico Technology) connected to universal serial bus (USB) port thermocouple data loggers (TC-08, Pico Technology) were installed in each tank for real-time monitoring of the temperatures throughout the experiment and adjusted daily to ensure the tanks were within 0.2 °C of their target temperature. After 2 wk of acclimation, CTmax was measured in all fish over 3 d using the same protocol as described above with the start temperature in the CTmax trial corresponding to the acclimation temperature. Observer bias was reduced by the observers being unaware which treatment the fish were from when conducting the CTmax trials. Fish were euthanized immediately after this test and a subsample (n = 20 per line) were measured for standard length (to the nearest 1 mm) and weight (to the nearest 0.01 g).
Statistical Analysis.
Response to selection.
We first estimated the response to selection and the realized heritability of CTmax for the Up-selected and Down-selected lines using data centered on the mean of the two Control (randomly selected) lines at each generation. This method removes undesirable variation of CTmax values due to uncontrolled variation in the laboratory environment between generations as well as the nonspecific upward drift detected in the Control lines.
Realized heritabilities were estimated in the Up-selected and Down-selected lines using the breeder’s equation (59):
| [1] |
where R is the response to selection, h2 is the narrow sense heritability, and S represents the selection differential. The response to selection was calculated as the difference in the population mean CTmax between two generations, while the selection differential was calculated as the difference in the mean CTmax between the total population and the selected individuals. Realized heritability was estimated as the slope of the regression of the cumulated response to selection on the cumulated selection differential with an intercept fixed to zero (60). We used linear mixed effect models (lme) separately for each treatment, where replicated line was a random effect. The SEs used were obtained from these models, i.e., models performed on the line means (see SI Appendix for more details).
The breeder’s equation assumes that the parent-offspring regression is linear. This may not be the case here as suggested by the skewed distribution of the CTmax. However, in absence of a clear understanding of physiological mechanisms affecting CTmax in both directions (i.e., to increase and decrease CTmax) (61–63), it is difficult to transform the data in a sensible way that would preserve a meaningful interpretation of the results. Therefore, \we decided to analyze the data on the original scale without transformation.
Differences among selected lines.
We estimated the per-generation response to selection of upper thermal tolerance in the selected lines using linear mixed effect models (lme) where the response variable was the individual CTmax values; the predictor variables were Generation, Treatment (Up-selected, Down-selected, and Control), and their interaction; and replicate line was a random factor. In addition, using the same structure, we ran one model for the Acclimated-Up lines where data were centered on the mean value of CTmax for F0. We also ran a model including only Up-selected and Down-selected lines, where CTmax values were centered on the mean value of the Control lines at each generation, and the intercept of the regression was fixed to zero. The individual response of the Control lines was also included in the second model to account for the variance in the Control lines.
Acclimated lines.
To account for the difference in acclimation temperature between generations 0–3 and 4–6, all individual CTmax values in the Acclimated-Up lines were adjusted down by 0.3 °C in the F0 to F3 generations. This adjustment was chosen based on previous CTmax data of wild-caught zebrafish that were acclimated to both 32 and 34 °C, which showed that these two acclimation temperatures gives a difference in CTmax of precisely 0.29 °C (17).
We tested the contribution of acclimation to upper thermal tolerance at each generation by comparing the Up and Acclimated-Up lines. To do this, we used a linear mixed effect model where the response variable was the individual CTmax values; the predictor variables were Generation, Treatment, (Up-selected and Acclimated Up-selected) and their interaction; and replicate line was a random factor. In this model, parameter estimates for Treatment at each generation represent the contribution of acclimation to the CTmax recorded in the Acclimated-Up lines.
Thermal reaction norm.
A linear mixed effect model (lme) was used to test whether selection on upper thermal tolerance at one temperature (28 °C for the Up-selected and Down-selected lines; 32 °C in Acclimated Up-selected lines) affected the upper thermal tolerance at other acclimation temperatures (24, 28, and 32 °C). Upper thermal tolerance was centered on the mean of the Control lines at each temperature. Acclimation temperature, selection treatment, and their interaction were included as explanatory variables. Replicated lines were included as a random effect.
Supplementary Material
Acknowledgments
We thank Miriam Dørum, Astrid Raunsgard, Eline Rypdal, Mari Fjelldal, Marga Villalonga Roca de Togores, Mireia Silvestre, Laura Hildesheim, Kristine Bertheussen, Inger Larsen Lyngstad, Karoline Hansen Skåra, Anders Meyer, Andrine Vedal, Hedda Stabell, and everyone else who contributed to the selection experiment and helped reproduce the fish each generation. We thank Helge Bjerck for discussions on plasticity. We also thank Kerstin Johannesson and the University of Gothenburg The Linnaeus Centre for Marine Evolutionary Biology (CEMEB) group for fruitful discussions on climate change adaptation. We would also like to thank the reviewers for their constructive comments that helped strengthen the paper. This work was supported by the Research Council of Norway (Norges Forskningsråd Grant 62942) (to F.J.), the Department of Biology, NTNU, and the Outstanding Academic Fellows Programme (OAFP) grant, NTNU. C.P. and H.J. were supported by Research Council of Norway Centres of Excellence funding scheme Grant 223257. C.P. was hosted by the Center of Advanced Study in Oslo during the writing of this paper.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011419117/-/DCSupplemental.
Data Availability.
All data and code are freely available on Figshare at: https://figshare.com/articles/dataset/Dataset_and_R_script_for_Low_potential_for_evolutionary_rescue_from_climate_change_in_a_tropical_fish_/12847541/1 (64).
References
- 1.IPCC , Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (Cambridge University Press, Cambridge, UK and New York, 2013), 10.1017/CBO9781107415324. [DOI] [Google Scholar]
- 2.Meehl G. A., Tebaldi C., More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Angilletta M., Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford University Press, 2009). [Google Scholar]
- 4.Gunderson A. R., Stillman J. H., Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. Biol. Sci. 282, 20150401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huey R. B., et al. , Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1665–1679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams S. E., Shoo L. P., Isaac J. L., Hoffmann A. A., Langham G., Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, 2621–2626 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward G., Perkins D. M., Brown L. E., Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2093–2106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutsch C. A., et al. , Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. U.S.A. 105, 6668–6672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunday J. M., Bates A. E., Dulvy N. K., Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2, 686–690 (2012). [Google Scholar]
- 10.Genin A., Levy L., Sharon G., Raitsos D. E., Diamant A., Rapid onsets of warming events trigger mass mortality of coral reef fish. Proc. Natl. Acad. Sci. USA 117, 25378–25385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw W. E., Holzapfel C. M., Climate change. Evolutionary response to rapid climate change. Science 312, 1477–1478 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist G. W., Huey R. B., The direct response of Drosophila melanogaster to selection on knockdown temperature. Heredity 83, 15–29 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann A. A., Chown S. L., Clusella‐Trullas S., Upper thermal limits in terrestrial ectotherms: How constrained are they? Funct. Ecol. 27, 934–949 (2013). [Google Scholar]
- 14.Sandblom E., et al. , Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat. Commun. 7, 11447 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houle D., Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker C. D., Genoway R. G., Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ. Biol. Fishes 4, 245 (1979). [Google Scholar]
- 17.Morgan R., et al. , Are model organisms representative for climate change research? Testing thermal tolerance in wild and laboratory zebrafish populations. Conserv. Physiol. 7, coz036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seebacher F., White C. R., Franklin C. E., Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Chang. 5, 61–66 (2015). [Google Scholar]
- 19.Kovach-Orr C., Fussmann G. F., Evolutionary and plastic rescue in multitrophic model communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevin L.-M., Lande R., When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Lande R., Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 (2009). [DOI] [PubMed] [Google Scholar]
- 22.West-Eberhard M. J., Developmental Plasticity and Evolution (Oxford University Press, 2003). [Google Scholar]
- 23.Bell G., Evolutionary rescue and the limits of adaptation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120080 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson S. M., Cunningham C. J., Westley P. A. H., Evolutionary rescue in a changing world. Trends Ecol. Evol. 29, 521–530 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Mitchell K. A., Hoffmann A. A., Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 24, 694–700 (2016). [Google Scholar]
- 26.Diamond S. E., Chick L., Perez A., Strickler S. A., Martin R. A., Rapid evolution of ant thermal tolerance across an urban-rural temperature cline. Biol. J. Linn. Soc. Lond. 121, 248–257 (2017). [Google Scholar]
- 27.McColl G., Hoffmann A. A., McKechnie S. W., Response of two heat shock genes to selection for knockdown heat resistance in Drosophila melanogaster. Genetics 143, 1615–1627 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klerks P. L., Athrey G. N., Leberg P. L., Response to selection for increased heat tolerance in a small fish species, with the response decreased by a population bottleneck. Front. Ecol. Evol. 7, 270 (2019). [Google Scholar]
- 29.Geerts A. N., et al. , Rapid evolution of thermal tolerance in the water flea Daphnia. Nat. Clim. Chang. 5, 665–668 (2015). [Google Scholar]
- 30.Brans K. I., et al. , The heat is on: Genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob. Change Biol. 23, 5218–5227 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Meffe G. K., Weeks S. C., Mulvey M., Kandl K. L., Genetic differences in thermal tolerance of eastern mosqyitofish (Gambusia holbrooki; Poeciliidae) from ambient and thermal ponds. Can. J. Fish. Aquat. Sci. 52, 2704–2711 (1995). [Google Scholar]
- 32.Krebs R. A., Loeschcke V., Estimating heritability in a threshold trait: Heat-shock tolerance in Drosophila buzzatii. Heredity 79, 252–259 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Baer C. F., Travis J., Direct and correlated responses to artificial selection on acute thermal stress tolerance in a livebearing fish. Evolution 54, 238–244 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Doyle C. M., Leberg P. L., Klerks P. L., Heritability of heat tolerance in a small livebearing fish, Heterandria formosa. Ecotoxicology 20, 535–542 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Schulte P. M., Healy T. M., Fangue N. A., Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Perry G. M. L., Martyniuk C. M., Ferguson M. M., Danzmann R. G., Genetic parameters for upper thermal tolerance and growth-related traits in rainbow trout (Oncorhynchus mykiss). Aquaculture 250, 120–128 (2005). [Google Scholar]
- 37.Zhang T., et al. , Genetic parameter estimation for juvenile growth and upper thermal tolerance in turbot (Scophthalmus maximus Linnaeus). Acta Oceanol. Sin. 33, 106–110 (2014). [Google Scholar]
- 38.Carpenter S. R., Fisher S. G., Grimm N. B., Kitchell J. F., Global change and freshwater ecosystems. Annu. Rev. Ecol. Syst. 23, 119–139 (1992). [Google Scholar]
- 39.Morgan R., “Physiological plasticity and evolution of thermal performance in zebrafish,” PhD thesis, Norwegain University of Science and Technology, Trondheim, Norway (2020).
- 40.Sundin J., et al. , On the observation of Wild Zebrafish (Danio rerio) in India. Zebrafish 16, 546–553 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Lutterschmidt W. I., Hutchison V. H., The critical thermal maximum: History and critique. Can. J. Zool. 75, 1561–1574 (1997). [Google Scholar]
- 42.Morgan R., Finnøen M. H., Jutfelt F., CT max is repeatable and doesn’t reduce growth in zebrafish. Sci. Rep. 8, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Åsheim E., Andreassen A., Morgan R., Jutfelt F., Rapid-warming tolerance correlates with tolerance to slow warming but not growth at non-optimal temperatures in zebrafish. J. Exp. Biol., 10.1242/jeb.229195 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Somero G. N., Adaptation of enzymes to temperature: Searching for basic “strategies”. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 139, 321–333 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Wegner K. M., Kalbe M., Milinski M., Reusch T. B., Mortality selection during the 2003 European heat wave in three-spined sticklebacks: Effects of parasites and MHC genotype. BMC Evol. Biol. 8, 124 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomulkiewicz R., Holt R. D., When does evolution by natural selection prevent extinction? Evolution 49, 201–207 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Bell G., Evolutionary rescue. Annu. Rev. Ecol. Evol. Syst. 48, 605–627 (2017). [Google Scholar]
- 48.Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A., Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 (2005). [Google Scholar]
- 49.Hansen T. F., Pélabon C., Houle D., Heritability is not evolvability. Evol. Biol. 38, 258 (2011). [Google Scholar]
- 50.Pélabon C., Hilde C. H., Einum S., Gamelon M., On the use of the coefficient of variation to quantify and compare trait variation. Evol. Lett. 4, 180–188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murari K. K., Ghosh S., Patwardhan A., Daly E., Salvi K., Intensification of future severe heat waves in India and their effect on heat stress and mortality. Reg. Environ. Change 15, 569–579 (2015). [Google Scholar]
- 52.Kaushal S. S., et al. , Rising stream and river temperatures in the United States. Front. Ecol. Environ. 8, 461–466 (2010). [Google Scholar]
- 53.Webb B. W., Nobilis F., Long-term changes in river temperature and the influence of climatic and hydrological factors. Hydrol. Sci. J. 52, 74–85 (2007). [Google Scholar]
- 54.Whiteley A. R., et al. , Population genomics of wild and laboratory zebrafish (Danio rerio). Mol. Ecol. 20, 4259–4276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gratton P., et al. , Allozyme and microsatellite genetic variation in natural samples of zebrafish, Danio rerio. J. Zool. Syst. Evol. Res. 42, 54–62 (2004). [Google Scholar]
- 56.Hangartner S., Hoffmann A. A., Evolutionary potential of multiple measures of upper thermal tolerance in Drosophila melanogaster. Funct. Ecol. 30, 442–452 (2016). [Google Scholar]
- 57.Massamba-N’Siala G., Prevedelli D., Simonini R., Trans-generational plasticity in physiological thermal tolerance is modulated by maternal pre-reproductive environment in the polychaete Ophryotrocha labronica. J. Exp. Biol. 217, 2004–2012 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Hutter S., Penn D. J., Magee S., Zala S. M., Reproductive behaviour of wild zebrafish (Danio rerio) in large tanks. Behaviour 147, 641–660 (2010). [Google Scholar]
- 59.Lush J. L., Animal Breeding Plans (Iowa State College Press, 1937). [Google Scholar]
- 60.Walsh B., Lynch M., Evolution and Selection of Quantitative Traits (Oxford University Press, 2018). [Google Scholar]
- 61.Clark T. D., Sandblom E., Jutfelt F., Aerobic scope measurements of fishes in an era of climate change: Respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Jutfelt F., et al. , Oxygen- and capacity-limited thermal tolerance: Blurring ecology and physiology. J. Exp. Biol. 221, jeb169615 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Jutfelt F., et al. , Brain cooling marginally increases acute upper thermal tolerance in Atlantic cod. J. Exp. Biol. 222, jeb208249 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Morgan R., Finnøen M. H., Jensen H., Pélabon C., Jutfelt F., Dataset and R script for “Low potential for evolutionary rescue from climate change in a tropical fish.” Figshare. https://figshare.com/articles/dataset/Dataset_and_R_script_for_Low_potential_for_evolutionary_rescue_from_climate_change_in_a_tropical_fish_/12847541. Deposited 18 September 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code are freely available on Figshare at: https://figshare.com/articles/dataset/Dataset_and_R_script_for_Low_potential_for_evolutionary_rescue_from_climate_change_in_a_tropical_fish_/12847541/1 (64).