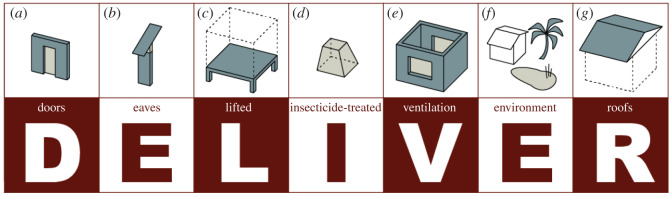
Abstract
In sub-Saharan Africa, most transmission of mosquito-transmitted diseases, such as malaria or dengue, occurs within or around houses. Preventing mosquito house entry and reducing mosquito production around the home would help reduce the transmission of these diseases. Based on recent research, we make key recommendations for reducing the threat of mosquito-transmitted diseases through changes to the built environment. The mnemonic, DELIVER, recommends the following best practices: (i) Doors should be screened, self-closing and without surrounding gaps; (ii) Eaves, the space between the wall and roof, should be closed or screened; (iii) houses should be Lifted above the ground; (iv) Insecticide-treated nets should be used when sleeping in houses at night; (v) houses should be Ventilated, with at least two large-screened windows to facilitate airflow; (vi) Environmental management should be conducted regularly inside and around the home; and (vii) Roofs should be solid, rather than thatch. DELIVER is a package of interventions to be used in combination for maximum impact. Simple changes to the built environment will reduce exposure to mosquito-transmitted diseases and help keep regions free from these diseases after elimination.
This article is part of the theme issue ‘Novel control strategies for mosquito-borne diseases'.
Keywords: malaria, dengue, housing, built environment, sub-Saharan Africa
1. Introduction
Over 80% of the world's population is threatened by at least one disease transmitted by insects or ticks, with 50% threatened by two or more [1]. These diseases represent 17% of the global burden of infectious diseases and kill over 700 000 people each year [2], with much of the impact occurring among the poorest of the poor in sub-Saharan Africa. In this region, our two greatest concerns are the mosquito-transmitted diseases: malaria, which is a parasitic disease transmitted by Anopheles mosquitoes, and Aedes-transmitted diseases, that include dengue, yellow fever, Zika and chikungunya, which are transmitted by the world's most efficient transmitter of viruses, Aedes aegypti.
Since the turn of the millennium, a concerted campaign of malaria control has halved the proportion of those infected with malaria parasites in Africa [3]. Malaria, however, continues to be a major drain on the health and economy of Africans, as 93% of the global malaria burden occurs in sub-Saharan Africa [4]. In 2018, there were still 213 million cases of malaria and 380 000 malaria-associated deaths in sub-Saharan Africa, and it is becoming clear that our current arsenal of weapons that include insecticide-treated bednets (ITNs), indoor residual spraying and prompt and effective treatment with antimalarials are insufficient to achieve malaria elimination from the region. Among the many health benefits, improved housing can be an additional tool to help achieve malaria control and elimination and reduce the opportunities for re-emergence after elimination.
As malaria has declined in the region, dengue is rising in importance, predominantly affecting urban populations [5], unlike malaria, which is essentially rural or restricted to the greener parts of African towns and cities. This is due to the differing habitat preferences of the different mosquito species. Dengue is the fastest increasing infectious disease in the world, and the number of estimated global cases has risen sharply from 8.3 million reported cases in 1990 to 58.4 million in 2013 [6] due to rapid urbanization, increasing movement of goods and, to a lesser extent, climate change. Today, the World Health Organization (WHO) estimate that there are 390 million cases of dengue globally each year (who.int). The true extent of dengue in sub-Saharan Africa is unknown due to weak surveillance and inadequate diagnosis. From 1960 to 2010, 22 countries reported dengue outbreaks in the region [7] and, more recently, there have been outbreaks reported in Benin, Cote d'Ivoire, Senegal and Tanzania (who.int, 14 November 2019), countries previously not reporting dengue infections. It is clear that dengue is increasing its range and outbreaks are becoming more severe in sub-Saharan Africa.
Other viruses transmitted by Ae. aegypti are also common in sub-Saharan Africa. A major epidemic of yellow fever occurred in Angola and the Democratic Republic of the Congo (DRC) from December 2015 to November 2016, resulting in 7334 suspected cases and 393 deaths [8]. A follow-up study to a yellow fever outbreak which took place in Ethiopia from 2012 to 2014, showed that in 2017 entomological risk indices classified most sites as ‘high risk’ for future outbreaks under current WHO criteria [9]. In 2018, Zika was circulating in Angola, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic, Côte d'Ivoire, Gabon, Guinea Bissau, Nigeria, Senegal and Uganda [10]. Recent outbreaks of chikungunya have been reported in the DRC with 6149 suspected cases between January and April 2019 [11]. Other outbreaks of chikungunya (Makonde for ‘bone crushing pain’) were reported by WHO in Sudan and Kenya in 2018. All these outbreaks are likely to be underestimated, due to the limited detection capacity of the existing surveillance systems in most countries in sub-Saharan Africa.
Improvements to the built environment offer new opportunities for control of both malaria and Aedes-transmitted viruses, and these interventions are timely for two principal reasons. First, Africa's population is expanding rapidly. Between 2019 and 2050, the United Nations forecast that the population of sub-Saharan Africa will double, from 1.07 billion people to 2.1 billion [12]. Millions of new homes have to be built to accommodate the growing population and replace many existing dwellings. Second, the economy of Africa has strengthened in recent years. The International Monetary Fund forecast that in 2020 the region will have the second-fastest growth rate in the world [13], providing the economic catalyst for building better houses. A recent analysis of housing in sub-Saharan Africa provided evidence that improvement of the housing stock is underway. From 2000 to 2015, the percentage of good housing (with improved water and sanitation, sufficient living area and constructed from durable material) in the region doubled from 11% to 23% [14]. These improvements in housing may have contributed to a reduction in malaria since a recent systematic review of over 15 000 publications showed that residents of modern housing had 47% lower odds of malaria infection and 45–65% fewer malaria cases than those in traditional homes [15]. Additional support comes from an analysis of 29 national health and demographic surveys of 28 400 children in sub-Saharan Africa from 2008 to 2015 [16]. Across all surveys, there was a 9–14% reduction in malaria infection in children living in modern housing compared to traditional ones, after adjusting for household wealth and other factors. Considering that 213 million malaria cases are still occurring annually in sub-Saharan Africa such benefits could result in between 19 and 30 million malaria episodes avoided. There has never been a better time to influence the design of houses and the built environment to help reduce the transmission of mosquito-transmitted diseases.
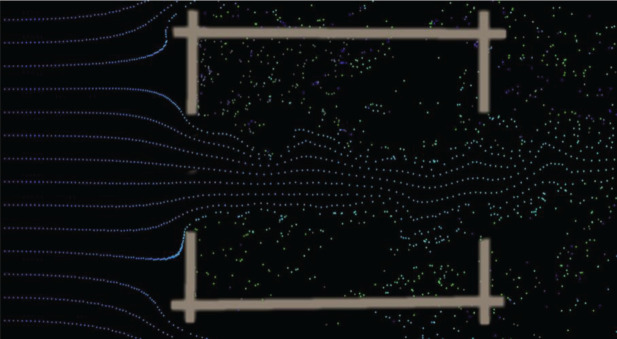
Most of us think of our home as a place of sanctuary, yet for mosquito-transmitted diseases, like malaria and Aedes-transmitted diseases, the opposite is true for many traditional households. In sub-Saharan Africa, up to 90% of malaria transmission occurs indoors at night [17,18], while for Ae. aegypti, transmission occurs in and around buildings during the day. For both diseases, the nidus of infection is in or outside buildings. Since much of the biting, and hence disease transmission, occurs indoors, it is important to understand that the risk of being bitten depends on both the extent and position of entry points into a building, as well as the quantity and shape of host odours emanating from a house at night [19]. It is the cues in host volatiles, notably carbon dioxide, that mosquitoes use to locate an individual (figure 1). The number of mosquitoes which enter a building is dependent on the number of occupants in a dwelling, the indoor climate, geometry and materials used for building a house and the extent of any openings [20–22]. We illustrate that in a poorly ventilated and hot metal-roofed house (figure 1a), a plume of carbon dioxide produced by a sleeping person disperses out of the open eaves, while an occupied house that is well-ventilated and cooler (figure 1b) produces less carbon dioxide, attracting fewer mosquitoes indoors.
Figure 1.
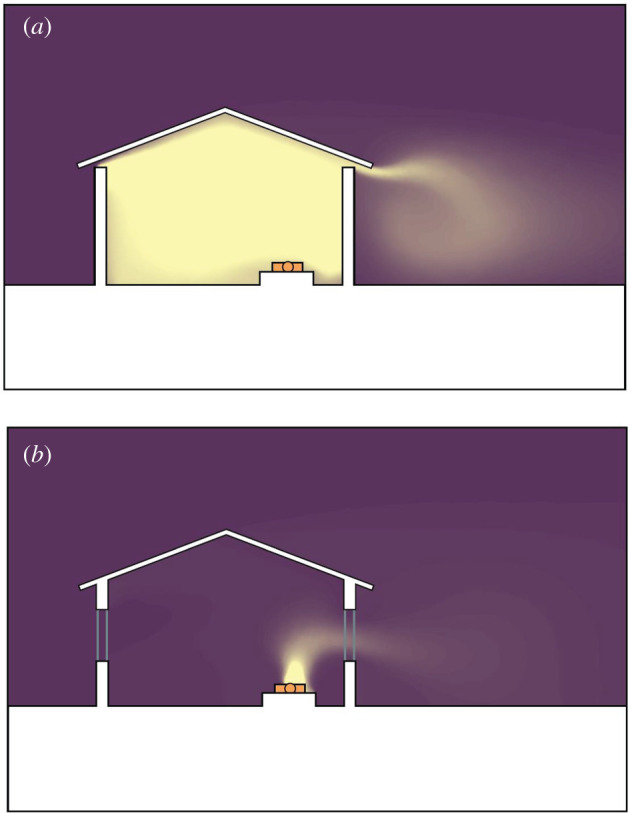
Models of carbon dioxide (yellow) dispersion from a sleeping person (orange) in: (a) a metal-roofed mud walled house with open eaves and (b) a similarly constructed house, but with closed eaves and two windows to improve ventilation. Simulations produced using Ansys Fluent v19 (https://www.ansys.com).
2. DELIVER
This manuscript is written for experts in vector-borne diseases and those in the built environment (Box 1). It provides recommendations for constructing mosquito-proof houses and keeping neighbourhoods free of those species of mosquitoes that transmit malaria in sub-Saharan Africa and Aedes-borne diseases through their bite. Our recommendations are crystallized in the mnemonic, DELIVER (figure 2), and here we provide evidence supporting each one.
Box 1. Essential entomology.
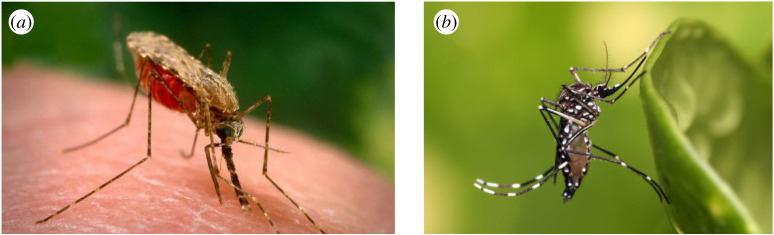
(a), Anopheles gambiae, the main transmitter of malaria in Africa, and (b), Aedes aegypti, the world's principal transmitter of viruses including dengue, yellow fever, Zika and chikungunya.
Experts on mosquito-transmitted diseases can miss out this section, but for those new to the subject, please read on. In sub-Saharan Africa, the predominant transmitter of malaria is the mosquito Anopheles gambiae. It is actually a complex of species that look similar, but each has markedly different behaviours and ecology. For our purpose, there are three species that are important: An. gambiae sensu stricto (in the strict sense), An. coluzzii and An. arabiensis. Both An. gambiae s.s. and An. coluzzii are extraordinarily adapted for feeding on people indoors, while An. arabiensis is less choosy in its feeding preference, feeding on humans and large domestic animals, and will feed indoors and outdoors. The aquatic habitats of the malaria transmitters are generally man-made and wide ranging, including small ponds, drainage ditches, rice fields and foot and tyre prints [23]. A fourth species, An. funestus, is also important and has a propensity for flooded denser vegetation, such as overgrown ditches. For all four mosquito species, the aquatic habitats are rural in character, but can also be found readily on the edges of African towns and cities, and where greenery enters urban areas. These mosquitoes predominantly feed indoors at night.
Aedes aegypti is the world's most efficient mosquito transmitter of viruses. It lays its eggs in containers and thrives in small amounts of water that occur in myriads of places including old tyres, blocked guttering, water containers, underground concrete structures or in a plethora of plastic waste that accumulates in the urban environment. Where there is high-density housing, few adult Ae. aegypti will fly further than 50 m from where they emerged, unless aquatic habitats are rare. This species predominantly bites in and around buildings during the day.
Figure 2.
DELIVER at a glance. The mnemonic, DELIVER, recommends the following best practices: (a) Doors should be screened, self-closing and without surrounding gaps; (b) Eaves, the space between the wall and roof, should be closed or screened; (c) houses should be Lifted above the ground; (d) Insecticide-treated nets should be used when sleeping in houses at night; (e) houses should be Ventilated, with at least two large-screened windows to facilitate airflow; (f) Environmental management should be conducted regularly inside and around the home; and (g) Roofs should be solid, rather than thatch.
(a). Doors
In rural Africa, most doors do not fit tightly. While this allows good air flow, it also allows mosquitoes to readily enter buildings. Studies using experimental houses (Box 2) in The Gambia showed that appreciable numbers of mosquitoes enter houses where the only openings in the building are narrow horizontal slits (to simulate poorly fitting doors) above and below a front and back door and that An. gambiae can enter a house through the gap at the top of doors as readily as those on the bottom [25]. Similar findings were made by Snow in The Gambia who found that An. gambiae entered small slits in experimental huts at either ground level or eaves level (i.e. 1.72 m) [26]. In marked contrast, far fewer culicine mosquitoes (which do not transmit malaria) enter a house where the only entry point is a gap at the top of the door (Culex spp. = 60%, 95% confidence intervals, CI = 44–76%, Mansonia spp. = 31%, 95%CI = 10–46%), compared to houses with gaps at the top and bottom of the door. Clearly, all gaps in a house need to be closed or screened to prevent mosquito entry.
Box 2. Experimental houses for assessing how mosquitoes enter houses.

Experimental houses in The Gambia. Experimental houses are a powerful tool for studying how mosquitoes enter homes. These houses, of a similar design to local homes, are built along a straight line, 10 m apart, each house differing in design to the reference house [21,24]. At night, one or two volunteers sleep under an insecticide-treated net in each house, the current established best practice for malaria control. This way, the volunteers are protected from malaria, but the odours emanating from the houses attract mosquitoes indoors. Mosquitoes entering the house are captured in a trap with a light on it placed close to the bed of the volunteers. The following morning, mosquitoes collected in each trap are identified and counted. Repeat nightly collections are made for a set number of nights, usually four or five, and then each house typology is rotated, so that at the end of the experiment, each typology has been tested in each house position, for a similar number of nights. It is important to rotate house typologies between positions so that the effect of the typology, which is what one is interested in, can be separated from the effect of geographical position. If this was not done, one could not be certain whether any effect was due to the house typology or its position, e.g. if it was close to or far away from a source of mosquitoes, such as a large area of irrigated rice. Volunteers are also rotated between experimental houses nightly, since some individuals are more attractive to mosquitoes than others. An example of how houses are rotated using a Latin square is shown below (table 1).
Table 1.
Schedule for testing interventions, where A, B, C, D and E are different house typologies.
| block | weekly house position |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1 | A | C | B | D | E |
| 2 | B | E | A | C | D |
| 3 | C | D | E | B | A |
| 4 | D | A | C | E | B |
| 5 | E | B | D | A | C |
The WHO's 1982 manual on environmental management [27] and the 1997 guide to vector control [28] provide guidance on constructing a screened door, based on an original design from 1947 [29]. This door was used in a randomized controlled trial in The Gambia, where 200 houses were randomly allocated screened doors and had their eaves closed with mud (see next section for more on eaves) and 100 houses left with their original doors and open eaves [30]. The trial demonstrated that screened doors and closed eaves were associated with a 59% reduction in malaria mosquito numbers and, more importantly, a 47% (95% CI = 3–71%) reduction in the risk of children being anaemic, compared with traditional houses. Lacking red blood cells (anaemia) is associated with chronic malaria infections and is a major cause of mortality in children under 2 years old, so theoretically if screening was scaled-up, it could have the potential to reduce malaria deaths.
Recently, wood has become an expensive commodity in Africa, and doors constructed from steel, PVC or aluminium can be cheaper than wooden doors. A recent trial of screened steel doors in The Gambia, however, found that many of the screens embedded in the doors were easily damaged, and hence no longer protected people from malaria mosquitoes (M. Pinder 2017, unpublished data). For this reason, new prototype screened doors, more robust and made entirely of metal [31], were designed to allow air to pass through 1.61 mm2 diameter holes in the door, hence allowing ventilation in the house, but preventing access to mosquitoes. Four types of prototype screened doors were randomly allocated to houses in a Gambian village and compared with traditional doors. All types of screened doors reduced the number of mosquitoes entering houses by 59–77% compared with houses without the new doors.
One important feature of the new screened doors was that they had springs in the hinges which made the doors self-closing, to maximize protection through the night. Keeping the doors closed at night in hot environments is, however, not without problems. Some house owners prefer to keep the doors open until late in the night to allow the house to cool from the heat of the day and for people to readily enter houses in the evening. Data loggers fixed to the front and back doors in village houses showed that the period between sunset and midnight is particularly busy, with people moving in and out of houses [31]. In some households people also thought that keeping the doors open allowed ‘good luck’ to enter. It is also true for many societies that shutting or locking the house might indicate that householders have something to hide or seem unwelcoming to other community members. This, however, is not normally an issue where people leave their homes to work in the fields during the day or in many urban situations where the fear of thieves means that most houses are well secured throughout the day and night.
(b). Eaves
The main entrance for An. gambiae into traditional houses is through the open eaves, the gap between the top of the wall and the over-hanging roof (figure 1), where mosquitoes approach the house at eaves level [32]. Observational studies from The Gambia [20,33] and Uganda [34] showed that houses with open eaves had more malaria mosquitoes than those with closed eaves, and that there was less malaria in houses with closed eaves than open ones [34,35]. Stronger evidence comes from four experimental studies. The first was a cross-over study in The Gambia using 12 thatched-roofed village houses with open eaves. In the first period, six houses were randomly selected, and their eaves closed. Mosquito collections were made in all study houses eight times in four weeks. In the second period, the intervention was swopped over, such that the six houses with open eaves were closed and the six that were closed, opened, and mosquito collections made as before. Over the study period, closing the eaves was associated with 65% fewer malaria mosquitoes, but no reduction in culicine mosquitoes. In a recent study using experimental houses (Box 2), closing the eaves of thatched houses resulted in 94% (95% CI = 89–97%) fewer malaria mosquitoes and 43% (95% CI = 23–58%) fewer culicine mosquitoes than with open eaves. The third experiment was done using experimental huts in Tanzania [36], where screening the eaves reduced entry of malaria mosquitoes. A fourth experiment using village houses in Malawi again demonstrated that closing the eaves reduced the entry of malaria mosquitoes, but not culicine mosquitoes [22]. These findings are consistent with those of Bill Snow in The Gambia who found that An. gambiae and Mansonia spp. were only slightly affected by the wall height, while more mosquitoes that usually live outside human dwellings, including Aedes spp., An. pharoensis, Culex poicilipes and Cx. thalassius, showed a marked exclusion with increasing wall height from 0 to 1.72 m [26]. Closing the eaves of a traditional rural house is a cheap and simple method for reducing the house entry of malaria mosquitoes. Health promotion by the National Malaria Control Programme in The Gambia has resulted in far fewer houses with open eaves houses in the rural areas of the Upper River Region than in the past (E. Jatta 2015, personal communication). The problem is though, that closing the eaves reduces ventilation and can make the house hotter by 0.5°C between 19.00 and 23.30 h, when most people are going to bed [21], thereby decreasing the likelihood of bednet use. On the other hand, screening the eaves would have less of an impact on ventilation reduction.
The presence of ceilings has an effect similar to closing the eaves. Ceilings reduce the entry of An. gambiae into homes, since if they enter the house through open eaves, they cannot enter the rooms below through an intact ceiling [24,30].
(c). Lifted
Between 450 and 420 BC, Herodotus described Egyptian fishermen living near marshes sleeping on raised platforms to escape the bites of mosquitoes [37]. And, at the turn of the nineteenth century, it was recommended that houses near Rome should be raised off the ground, and two-storey buildings built with the bedroom on the top floor to avoid mosquito bites [38]. Studies of mosquito behaviour over the past 50 years suggest that both historical anecdotes describe adaptations to avoid mosquito bites. In the 1970s, a series of pioneering studies exploring the height at which mosquitoes fly above the ground were carried out using suction traps placed at different heights on scaffolding towers placed over different terrain in The Gambia [39–41]. Although some culicine mosquitoes are frequently found at all flight levels up to 8 m, most mosquito species fly close to the ground, with 80% of the flying population found below 1 m in height [40]. This work suggested to Gillies and Wilkes that placing circular mosquito-proof fences around people alone or inhabited buildings might be protective [42]. Their findings, however, showed that mosquitoes flew over the 6 m high fences and provided little, if any protection. Nonetheless, recent studies in The Gambia showed that if there is no fencing guiding the mosquitoes upwards, raising houses above the ground would reduce mosquito house entry and hence disease transmission (M. Carrasco-Tenezaca 2019, unpublished data). There is also evidence from a pilot study in Tanzania that fewer malaria mosquitoes enter bedrooms on the second storey, compared with those on the first storey [43].
The principal malaria mosquitoes in sub-Saharan Africa all prefer to feed close to ground level [44]. Even sitting with one's feet raised off the ground reduces mosquito biting by 32% (95% CI = 9–48%) compared with sitting with the feet planted on the floor [45]. Similar conclusions have been reached from studies conducted in Papua New Guinea, which demonstrated that raised platforms were protective [46]. We have little information on the house-entering habits of Ae. aegypti, but it is often quoted that Ae. aegypti can be found in the top storeys of high-rise flats. Presumably, in these cases, adult mosquitoes fly up the stairs to higher floors, where small populations become established living in water-storage containers, air conditioning units or other types of domestic water. In conclusion, there is compelling evidence that raising a house off the ground will reduce mosquito house entry, and even closing the area beneath the building with permeable or impermeable walls is likely to be protective [43]. One simple way to raise a house off the ground is to use steel or concrete supports, or one could simply move the bedroom to the second storey [43]. Homes are commonly raised across much of South-East Asia today [47], and we similarly recommend this for African housing, especially rural housing which is currently built at ground level.
(d). Insecticide-treated bednets
ITNs have had a major impact in reducing malaria transmission and are considered a key tool for malaria control. Since the turn of the century, there has been massive deployment of ITNs across sub-Saharan Africa and between 2000 and 2015 the malaria infection prevalence halved and the incidence of clinical episodes of malaria declined by 40% in Africa [3]. ITNs were by far the most important malaria control intervention and are thought to account for 62% of cases averted. A recent systematic review and meta-analysis of 23 intervention trials, enrolling more than 275 000 adults and children showed that ITNs were associated with a 42% reduction (95% CI = 22–56%) in malaria compared with untreated nets [48]. Despite the concern about increasing resistance of malaria mosquitoes to the insecticides used to treat bednets [49], the lack of durability and coverage of the nets, it is clear that ITNs are still effective weapons against malaria in sub-Saharan Africa and remain the mainstay malaria prevention tool. Surprisingly, since Ae. aegypti is predominantly a day-time feeder, ITNs can also be an effective tool against this mosquito as evidenced by a study in Haiti that showed this intervention caused an immediate reduction in mosquitoes after deployment [50], presumably because they contact the treated netting when flying indoors. ITN use should be strongly encouraged for malaria control in sub-Saharan Africa, but their use for the control of Aedes-transmitted diseases is uncertain.
Although we strongly recommend the use of ITNs, indoor residual spraying (IRS) has also been shown to be protective against malaria [51] and dengue [52,53]. Other insecticide-based household interventions that are at various stages of development and testing include eave tubes [54], window screens and eave baffles [55], and insecticide-treated eave ribbons [56]. In the future, the insecticide-based tools may differ markedly from those used today, with interventions targeted at different typologies of housing.
(e). Ventilation (and screening)
One of the biggest problems facing traditional houses in sub-Saharan Africa is that they are too hot [47]. Houses constructed from mud or cement blocks will heat up during the day and radiate heat at night, making the houses hot and stuffy at night. A hot bedroom at night is not conducive for sleeping under a bednet and is the primary reason that people will not use a net at night [57]. Thus, providing ventilation to cool the building at night should help increase bednet use and hence increase protection against malaria. Improved ventilation is also important for reducing indoor air pollution, thereby reducing respiratory illness [58]. A house can be cooled by raising the building above the ground, using permeable materials for the walls of the house and, most importantly introducing large-screened windows on opposite sides of the house (figure 3), or at least on different sides. Cross ventilation is also improved if there are no internal walls or other obstructions between the windows.
Figure 3.
Importance of windows on opposite walls to encourage airflow through the building.
Unfortunately open windows need to be screened from mosquitoes, and this will typically halve the airflow across the room [43] and reduce it further if curtains are used. To maximize airflow, both windows and doors should be large, with screening to restrict mosquito house entry. Screening is common in some African cities, such as Dar es Salaam, where 83% of homes surveyed had screened windows and 49% had ceilings in 2007 [59]. In Dar es Salaam, a rise in mosquito screening among local residents was associated with fewer An. arabiensis and An. funestus and less malaria [60]. Interestingly, in this study, the increase in screening was through commercial channels, without health promotion or subsidies. In Ethiopia, screening doors and windows reduced numbers of An. arabiensis indoors by 48% and malaria episodes by 61% (95% CI = 20–80%) [61]. Well-screened houses can offer additional benefits especially if there are many people sharing a bednet or where there are no bednets. Screening is an equitable intervention since it protects everyone in the house and, in the future, may provide additional protection against malaria if treated with insecticide [55].
A high prevalence of untreated house screening, however, may, like repellents, increase the risk of mosquito bites in neighbouring unscreened houses [62]. It is therefore important for coverage of screening to be as high as possible. Additionally, adding insecticide to the screens could improve protection in screened houses and any unscreened neighbouring houses by reducing mosquito survival. In Vietnam, a study of insecticide-treated screening reduced both the numbers of Ae. aegypti and, most importantly, provided 81% protection against dengue (95% CI = 53–92%) [63,64]. In Mexico, insecticide-treated screening resulted in 60% (95 CI = 30–77%) fewer Ae. aegypti 12 months after deployment than areas without the intervention and protection lasted up to 2 years [65–67]. Novel forms of three-dimensional untreated screening could also serve as mosquito traps, thereby reducing both house entry and the overall mosquito population size [68]. Keeping a house well ventilated and screened would help keep houses cool at night and mosquito free.
(f). Environmental management
Malaria and Aedes-transmitted diseases are consequences of surface water, since without water there would be no mosquitoes. For malaria mosquitoes, it is the larger semi-permanent and permanent water bodies, often human made, that are the main aquatic habitats [23]. Where possible, drainage and filling are effective for reducing the aquatic habitats of An. gambiae and are particularly important in urban settings [69]. In many situations, human-created habitats such as drains, concrete pits used as water sources for mixing concrete and road construction create water pooling and should be dealt with. In Khartoum, environmental management was the principal method for reducing malaria in the city [70] and was the backbone of malaria control in the Copper Belt in Zambia [71]. Although bush clearance around homes is unlikely to reduce malaria mosquito abundance [72], it can certainly help improve ventilation inside the house.
For Ae. aegypti, the peri-domestic environment is of primary importance as a source of aquatic habitats. A plethora of small containers become a potential habitat for the immature stages of this mosquito: including discarded plastic waste, old tyres, blocked guttering, water-storage containers, [73] and underground cisterns [74]. Accumulating solid waste is a common sight in many African towns and cities. In the Nigeria delta region, only 20% of the 40 000 metric tons of waste produced daily is collected [75]. After rainfall, much of this discarded waste provides a rich source of aquatic habitats for Ae. aegypti. Of more immediate importance is the issue of providing water to rapidly growing cities, particularly with climate change impacting on water scarcity [76]. In 2018, Cape Town, home to some 4 million people, was close to fully depleting its water source, while in 2019, Chennai, India's sixth largest city with 4.6 million people, suffered severe water shortages. In Chennai tap water stopped running, so people had to collect water in containers, providing an abundance of new mosquito habitats. In such situations, water-storage containers should be covered and scrubbed weekly to remove any mosquito eggs [77]. In the long-term, cities need to plan for adequate and reliable piped water at affordable prices to reduce the potential for Ae. aegypti [78,79] and other mosquito species, such as Anopheles stephensi. The introduction of An. stephensi into sub-Saharan Africa is a new and major concern since it is an important transmitter of urban malaria in India and, like Ae. aegypti, is also a container-breeder. Worryingly, it has recently invaded parts of northern Africa and has been identified in Djibouti, Ethiopia and Sudan [80]. If not controlled, it is likely to spread to other towns and cities in the Horn of Africa. Environmental management and effective surveillance for the control of malaria and Aedes-transmitted diseases should be the foundation for disease control, particularly in towns and cities. Moreover, efficient solid waste management and a reliable supply of piped water are desirable for all communities and will have multiple benefits beyond mosquito control.
(g). Roofs
Traditional thatched-roofs, once common in much of rural Africa, are now on the decline, being replaced gradually by roofs made from solid materials, such as metal or tile [14]. For example, in Tororo district, eastern Uganda, the prevalence of thatched-roofed homes decreased from 40% in 2013 to 23% in 2016 [81]. In 2011 in Rwanda, as part of a government-run programme, thatched-roof houses were replaced with metal-roofed houses. This programme, however, has been criticized since it was alleged that force was used to remove the thatch from houses (https://www.survivalinternational.org/news/7154).
Metal-roofed houses are hotter than traditional thatched-roof houses, particularly when the eaves are closed [21]. This heating effect has potentially both positive and negative impacts on health. The benefit is that in hot parts of sub-Saharan Africa, the extremely high temperatures created by metal roofs and high thermal mass walls during the late afternoon are often lethal to mosquitoes sheltering indoors during the day. The high temperatures may reduce the survival of malaria mosquitoes to such an extent that few survive long enough to become infective with malaria parasites [82]. The disadvantages also result from the heating effect of metal roofs. In hot countries, the high indoor temperatures experienced during the day may be too uncomfortable for many people, who will stay outdoors, potentially exposing them to Aedes bites. Night-time temperatures are also hotter in metal-roofed houses, unless ventilated, than traditional thatched houses [21], and this is likely to increase the time spent by people outside the house, reduce the number of people sleeping under a bednet at night [57] and may even contribute to increased growth faltering in young children living in hot houses [83].
An observational study in Burkina Faso found that children living in mud-roofed houses were nearly at three times greater risk of malaria infection than those living in metal-roofed houses [84]. However, caution is needed in interpreting the findings from observational studies since protection may not be related to the presence of a metal roof, but to the presence of closed eaves or confounded by socioeconomic status. In these correlational studies a metal roof, or more simply, a good home, may be a marker for a higher socioeconomic status, and other features of being wealthy may be protective, such as the ability to purchase antimalarials or use a bednet. Even adjusting for socioeconomic status in a risk model, one cannot exclude the possibility of residual confounding—that good housing is a measure of wealth. However, despite these caveats, exploring cheap, durable and clean alternatives to thatch roofs is recommended in sub-Saharan Africa to reduce disease transmission, as long as the house can remain well ventilated to keep the interior cool. Further research is needed to identify the best solid roof types for disease control.
3. Facilitators
The recommendations comprising the DELIVER mnemonic are intended to be implemented as an integrated package addressing multiple hazards in the transition to healthier homes. Nonetheless, certain recommendations may be more appropriate depending on each particular situation. To roll out such innovations important enablers or ‘facilitators’ are needed.
(a). Political leadership
Reducing the risk of mosquito-transmitted diseases by changes to the built environment on a large scale requires that we do things differently; this is not something the health sector can do alone. Multi-sectoral action against mosquito-transmitted diseases is needed to control diseases more sustainably and at a lower cost than traditional mosquito control interventions and necessitates the health sector working with those in the built environment, including those responsible for housing, infrastructure, parks, waste management and water (figure 4). One framework to do this is through established committees developing resilience plans for towns and cities. We propose that the United Nations' Housing Unit should incorporate these messages into their international guidelines for building healthy houses and communities for the future. Where inter-sectoral committees are already established and planning of resilience activities is in hand, this may be the most appropriate institutional structure for developing these action plans further. For those at an earlier stage, setting up cross-sectoral working groups should be the first step. Success relies on strong leadership, effective governance structures, political commitment and the necessary financial resources to make the changes. Two recent examples of success come from Khartoum city in Sudan [70] and Singapore [85]. City mayors are in a powerful position to take multi-sectoral action against mosquito-transmitted diseases by forging links between sectors and, most importantly, providing the financial support needed.
Figure 4.
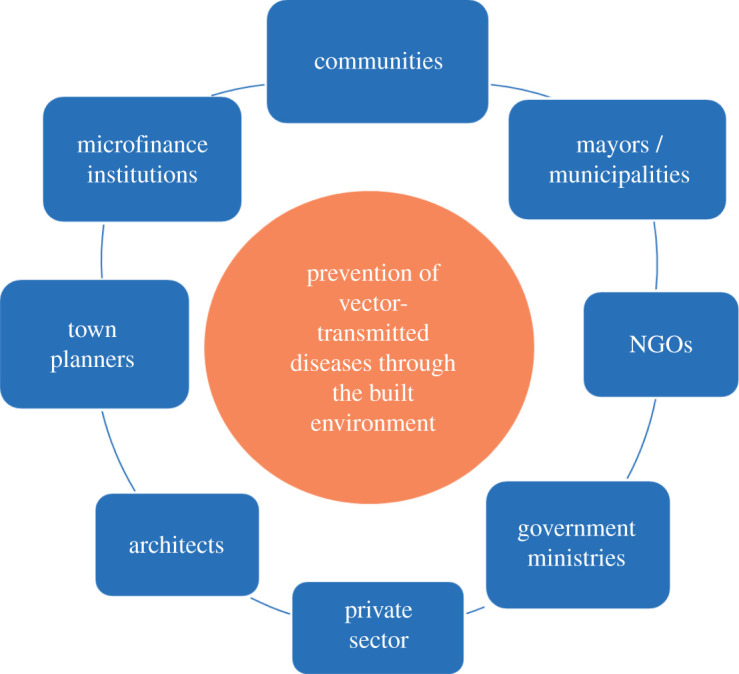
Framework for inter-sectoral action. An example of those bodies that could be brought together to make towns and cities resilient against the threat of mosquito-transmitted diseases.
(b). Private sector involvement
Engagement of the private sector can help to reduce mosquito habitats in the environment. In Kenya, for example, the growth of solar panels can provide power for fans, improving ventilation in a building. In Brazil a public–private partnership between the Ministry of Environment and Reciclanip (http://www.reciclanip.org.br/), an organization linked to the National Association of the Tyre Industry, collects and recycles scrap tyres that provide an ideal habitat for Aedes mosquitoes. Tyres are recycled into a range of useful materials including flooring for sports facilities, added to asphalt or used as an alternative fuel in the cement industry replacing fossil fuels. The partnership is a win-win since the recycling industry boosts the economy by employing large numbers of people. In Lagos, recyclable waste is collected using a fleet of cargo bikes, motorized tricycles, vans and trucks (http://wecyclers.com/about). The collected material is exchanged for food and household items and then converted to tissue paper, stuffing for mattresses, plastic furniture, aluminium sheets and nylon bags. Such innovative solutions offer exciting ways for removing waste, reducing mosquito habitats and creating a new industry.
(c). Community participation
Extensive changes to the built environment can only be successful with the partnership of local communities. Thus, the possibilities for change need to be discussed with local residents and decisions made with their support. A good example of such a process was the Camino Verde (Green way) campaign in Nicaragua and Mexico [86]. Here pesticide-free interventions (emptying, brushing/scrubbing water containers or covering them) identified and mediated through community mobilization reduced the abundance of Ae. aegypti and increased the effectiveness of government programmes to control dengue. Similarly, effective community-led interventions have been successful in Cuba [87], Indonesia [88], Mexico [89] and Puerto Rico [90]. This co-production in policy-making, governance and research towards equitable and sustainable cities is gaining traction (ARISE and https://www.mistraurbanfutures.org/en) and is seen as a way of working together to improve health and of creating user-led, people-centred healthcare services [91].
The DELIVER recommendations can be implemented while preserving vernacular building styles and skills—rather than promoting a homogeneous house across all cultures and geographies. It is important, therefore, that community participation should include local architecture, engineering and design schools [90].
(d). Education
In East Africa, city engineers were once imbedded into malaria control programmes, to help remove surface water through drainage and filling [92]. Today, this no longer happens, and few engineers are taught about the critical importance of public health engineering and politicians have failed to emphasize the value of public health considerations in construction and urban planning. This needs to change, and in disease-endemic countries teaching about diseases caused by mosquitoes and methods for their control needs to be incorporated into curricula from primary, secondary to tertiary training bodies, especially those dealing with the built environment. With education comes innovation and the creation of new methods for controlling these diseases. The use of social media offers exciting opportunities for spreading knowledge and good practice. Healthy, ‘show homes’ are also a novel way to share good practice, as illustrated by entirely new ‘healthy’ house designs in the Magoda project carried out in Tanzania [43].
4. Global policy supporting ‘building out’ mosquito-transmitted diseases
Malaria and Aedes-transmitted diseases are environmental diseases: the risk of each is influenced by local environmental factors. Over recent years there has been a growing appreciation that action to improve people's health needs to also improve their environment. Such an approach recognizes that health can no longer be the sole responsibility of the health sector and calls for greater cross-sectoral collaboration. Global policy provides a key opportunity for acknowledging and addressing such challenges.
The United Nations’ Sustainable Development Goals (SDGs) explicitly acknowledges the importance of cross-sectoral collaboration. The SDGs most closely linked to addressing mosquito-transmitted diseases are Goal 3: Good health and well-being, Goal 11: Sustainable cities and communities, and Goal 17: Partnerships to achieve the goals. How the principal SDGs drive the United Nation's organizations to produce a recommendation on health, housing and resilience is illustrated in figure 5. Here, these multi-sectoral approaches based on the UN's SDGs need to be combined to help communities become resilient to the threat of mosquito-transmitted diseases.
Figure 5.
How the United Nations' Sustainable Development Goals (SDGs) stimulate UN organizations to produce international guidelines on health, the built environment and resilience. Our plea is for integration across these three organizations to reduce the threat from mosquito-transmitted diseases. Note that only the principal SDGs are shown.
For health professionals specifically, the United Nations Development Programme (UNDP) and the Roll Back Malaria Partnership (RBM) published key documents on the importance of multi-sectoral action for malaria control [93] and later RBM, UNDP and UN-Habitat produced the first policy document supporting housing interventions for malaria control, called the Housing and Malaria Consensus Statement [94]. Importantly, WHO's strategy for the control of mosquito-transmitted diseases, the Global Vector Control Response 2017–30 [2], strongly advocates the need for multi-sectoral action, especially in respect to malaria and Aedes-transmitted diseases.
For built environment professionals, the United Nations' New Urban Agenda (NUA) [95] provides global guidelines for achieving sustainable urban development. The NUA explicitly recognizes urban centres as exhibiting characteristics that make them and their inhabitants particularly vulnerable to climate change and other natural and human-made hazards, including mosquito-transmitted diseases (like malaria and Aedes-transmitted diseases) [96,97].
More specifically, for actors working on improving the disaster resilience of cities to support sustainable urban development, there is the United Nations' Office for Disaster Risk Reductions (UNDRR, formerly UNISDR) Sendai Framework for Disaster Risk Reduction 2015–2030 [98]. The framework more directly relates to the built environment through the ‘Ten Essentials for Making Cities Resilient’ operating framework delivered through UNDRR's ‘Making Cities Resilient’ campaign (unisdr.org/campaign/resilientcities). While it does refer to health as a key element, there is no reference to mosquito-transmitted diseases in the Sendai framework and by extension the essentials or campaign. This is a missed opportunity, since malaria and Aedes-transmitted diseases are an engineering problem, not just a health problem.
Another key opportunity relates to the WHO Housing and Health Guidelines (HHGL) [99] that were published in December 2018. The HHGL provide practical recommendations to reduce the health burden due to unsafe and substandard housing conditions in order to inform housing policies and regulations at the national, regional and local level. Unfortunately, the first edition of the HHGL does not cover housing interventions that protect people from mosquito-transmitted diseases. However, it is the intention of the WHO to rectify this omission in the next edition.
5. Conclusion
DELIVER describes the packet of interventions that when used together will be effective in the fight against mosquito-transmitted diseases. The selection of interventions is based on the best evidence we have to date, but new evidence may bring improvements and/or modifications and new technologies are continually emerging. It is important that such innovations are rigorously assessed before including them in our current arsenal of interventions [100]. It is also important to realize that research on mosquito-transmitted diseases in the built environment is a new and emerging field. A recent international workshop at the UN-Habitat headquarters in Nairobi identified a number of important research gaps that need filling [101], including: (i) evidence of the health benefits of changing the built environment; (ii) understanding how mosquitoes, particularly Ae. aegypti, enter buildings; (iii) novel methods for reducing mosquito house entry; (iv) sustainable approaches for reducing mosquito habitats; (v) case studies of micro-financing for healthy homes; and (vi) methods for increasing scale-up.
DELIVER will contribute positively to several SDG goals, including goal 3 (good health and well-being), goal 6 (clean water and sanitation), goal 9 (industry, innovation and infrastructure), goal 11 (sustainable cities and communities), goal 13 (climate action) and goal 17 (partnerships to achieve the goal) while reducing the amount of insecticides used. Many of the suggestions made in this manuscript are not specifically directed at solely shrinking the number of cases of mosquito-transmitted diseases. For example, providing reliable piped water, effective waste management and drainage and having a quiet night's sleep undisturbed from biting mosquitoes is something very few of us reading this article would disagree with: it is development. But the threat of these diseases provides us with an impetus to direct our efforts at those areas most in need and provides urgency for action. Identifying areas at high risk of infection can be challenging as it depends upon a complex interaction of urban ecology and climate [102]. In addition, improvements in housing are likely to lead to other improvements in health, including reduced incidence of diarrhoea, anaemia and undernutrition [103]. Such changes will provide many collateral benefits including reducing the use of insecticides and the threat of flooding and helping to decrease plastic waste in our communities. Mosquito control can lead the way to more sustainable control of mosquito-transmitted diseases, particularly if the community and other stakeholders are engaged in the design and implementation of solutions. Building resilience against mosquito-transmitted diseases is very much part of a green revolution, providing safe and resilient communities for the future. Importantly, we need to build out mosquito-transmitted diseases from our communities, not build them in.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the conception and design of the manuscript; S.W.L. drafted the article and all other authors revised it critically and gave final approval of the version to be published.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by UK's Global Challenges Research Fund (BB/R00532X/1) and supported by the Biotechnology and Biological Sciences Research Council and Medical Research Council.
References
- 1.Golding N, et al. 2015. Integrating vector control across diseases. BMC Med. 13, 249 ( 10.1186/s12916-015-0491-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. 2017. Global vector control response 2017–2030. Geneva, Switzerland: WHO; See https://www.who.int/vector-control/publications/global-control-response/en/. [Google Scholar]
- 3.Bhatt S, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. ( 10.1038/nature15535). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2019. World malaria report. Geneva, Switzerland: WHO; See https://www.who.int/publications-detail/world-malaria-report-2019. [Google Scholar]
- 5.Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, Pinto J, Lambrechts L, McCall PJ. 2018. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int. J. Environ. Res. Publ. Hlth 15, 2 ( 10.3390/ijerph15020220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanaway JD, et al. 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 16, 712–723. ( 10.1016/S1473-3099(16)00026-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. 2011. Dengue virus infection in Africa. Emerg. Infect. Dis. 17, 1349–1354. ( 10.3201/eid1708.101515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. 2016. Yellow fever situation report 28 October 2016. Geneva, Switzerland: WHO; See https://www.who.int/emergencies/yellow-fever/situation-reports/28-october-2016/en/. [Google Scholar]
- 9.Mulchandani R, Massebo F, Bocho F, Jeffries CL, Walker T, Messenger LA. 2019. A community-level investigation following a yellow fever virus outbreak in South Omo Zone, South-West Ethiopia. PeerJ. 7, e6466 ( 10.7717/peerj.6466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. 2018. Zika virus (ZIKV) classification table. Data as of 15 February 2018. Geneva, Switzerland: WHO; See https://apps.who.int/iris/bitstream/handle/10665/260419/zika-classification-15Feb18-eng.pdf;jsessionid=25B269935925E716FD7AAFF73612E703?sequence=1. [Google Scholar]
- 11.WHO. 2019. Chikungunya - Congo. Geneva, Switzerland: WHO; See https://www.who.int/csr/don/01-may-2019-chikungunya-congo/en/. [Google Scholar]
- 12.UN. 2019. 2019 revision of world population prospects. New York, NY: UN: See https://population.un.org/wpp/. [Google Scholar]
- 13.McKinsey Global Institute. 2016. Lions on the move II: realizing the potential of Africa's economies. Brussels, Belgium: McKinsey & Co; See https://www.mckinsey.com/~/media/McKinsey/Featured%20Insights/Middle%20East%20and%20Africa/Realizing%20the%20potential%20of%20Africas%20economies/MGI-Lions-on-the-Move-2-Executive-summary-September-2016v2.ashx. [Google Scholar]
- 14.Tusting LS, et al. 2019. Mapping changes in housing in sub-Saharan Africa from 2000 to 2015. Nature 568, 391–394. ( 10.1038/s41586-019-1050-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling R, Lindsay SW. 2015. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malaria J. 14, e209 ( 10.1186/s12936-015-0724-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, Gething PW. 2017. Housing improvements and malaria risk in sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med. 14, e1002234 ( 10.1371/journal.pmed.1002234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huho B, et al. 2013. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int. J. Epidemiol. 42, 235–247. ( 10.1093/Ije/Dys214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherrard-Smith E, et al. 2019. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl Acad. Sci. 116, 15 086–15 095. ( 10.1073/pnas.1820646116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies MT. 1980. The role of carbon dioxide in host finding by mosquitoes (Diptera: Culicidae): a review. Bull. Entomol. Res. 70, 525–532. ( 10.1017/S0007485300007811) [DOI] [Google Scholar]
- 20.Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, Lindsay SW. 2008. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malaria J. 7, e2 ( 10.1186/1475-2875-7-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jatta E, et al. 2018. How house design affects malaria mosquito density, temperature, and relative humidity: an experimental study in The Gambia. Lancet Planet. Hlth 2 e498–e508. ( 10.1016/S2542-5196(18)30234-1) [DOI] [PubMed] [Google Scholar]
- 22.Mburu MM, Juurlink M, Spitzen J, Moraga P, Hiscox A, Mzilahowa T, Takken W, McCann RS. 2018. Impact of partially and fully closed eaves on house entry rates by mosquitoes. Parasit. Vectors 11, 383 ( 10.1186/s13071-018-2977-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillinger U, Lindsay SW. 2011. Larval source management for malaria control in Africa: myths and reality. Malar. J. 10, 353 ( 10.1186/1475-2875-10-353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GE, Emerson PM. 2003. Changes in house design reduce exposure to malaria mosquitoes. Trop. Med. Int. Health 8, 512–517. ( 10.1046/j.1365-3156.2003.01059.x) [DOI] [PubMed] [Google Scholar]
- 25.Jatta E. 2018. The impact of different house designs on mosquito house entry in rural Gambia. Durham, UK: Durham University; See http://etheses.dur.ac.uk/12865/. [Google Scholar]
- 26.Snow WF. 1987. Studies of the house-entering habits of mosquitoes in The Gambia, West Africa: experiments with prefabricated huts with varied wall apertures. Med. Vet. Entomol. 1, 9–21. ( 10.1111/j.1365-2915.1987.tb00318.x) [DOI] [PubMed] [Google Scholar]
- 27.WHO. 1982. Manual on environmental management for mosquito control, with special emphasis on malaria vectors. WHO Offset Publication no. 66. Geneva, Switzerland: World Health Organisation; See https://apps.who.int/iris/handle/10665/37329. [PubMed] [Google Scholar]
- 28.Rozendaal J. 1997. Vector control: methods for use by individuals and communities. Geneva, Switzerland: WHO; See https://apps.who.int/iris/handle/10665/41968. [Google Scholar]
- 29.Tennessee Valley Authority. 1947. Malaria control on impounded waters. Washington, DC: United States Public Health Service. [Google Scholar]
- 30.Kirby MJ, Ameh D, Bottomley C, Green C, Jawara M, Milligan PJ, Snell PC, Conway DJ, Lindsay SW. 2009. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet 374, 998–1009. ( 10.1016/S0140-6736(09)60871-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jawara M, et al. 2018. New prototype screened doors and windows for excluding mosquitoes from houses: a pilot study in rural Gambia. Am. J. Trop. Med. Hyg. 99, 1475–1484. ( 10.4269/ajtmh.18-0660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzen J, Koelewijn T, Mukabana WR, Takken W. 2016. Visualization of house-entry behaviour of malaria mosquitoes. Malar. J. 15, 233 ( 10.1186/s12936-016-1293-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsay SW, Snow RW. 1988. The trouble with eaves; house entry by vectors of malaria. Trans. R. Soc. Trop. Med. Hyg. 82, 645–646. ( 10.1016/0035-9203(88)90546-9) [DOI] [PubMed] [Google Scholar]
- 34.Wanzirah H, et al. 2015. Mind the gap: house structure and the risk of malaria in Uganda. PLoS ONE 10 e0117396 ( 10.1371/journal.pone.0117396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsay SW, Emerson PM, Charlwood JD. 2002. Reducing malaria by mosquito-proofing houses. Trends Parasitol. 18, 510–514. ( 10.1016/s1471-4922(02)02382-6) [DOI] [PubMed] [Google Scholar]
- 36.Ogoma SB, Lweitoijera DW, Ngonyani H, Furer B, Russell TL, Mukabana WR, Killeen GF, Moore SJ. 2010. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PloS Neglect. Trop. Dis. 4, e773 ( 10.1371/journal.pntd.0000773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herodotus SAD. 1996. Herodotus. The histories. London, UK: Penguin. [Google Scholar]
- 38.Celli A. 1900. Notes on the new researches on the propagation of malaria in relation to engineering and agriculture. J. Sanit. Inst. 21, 617–628. ( 10.1177/146642400002100405) [DOI] [Google Scholar]
- 39.Snow WF. 1975. The vertical distribution of flying mosquitoes (Diptera, Culicidae) in West African savanna. Bull. Entomol. Res 65, 269–277. ( 10.1017/S0007485300005952) [DOI] [Google Scholar]
- 40.Snow WF. 1979. The vertical distribution of flying mosquitoes (Diptera: Culicidae) near an area of irrigated rice-fields in The Gambia. Bull. Entomol. Res. 69, 561–571. ( 10.1017/S0007485300020113) [DOI] [Google Scholar]
- 41.Gillies MT, Wilkes TJ. 1976. The vertical distribution of some West African mosquitoes (Diptera, Culicidae) over open farmland in a freshwater area of The Gambia. Bull. Entomol. Res. 66, 5–15. ( 10.1017/S0007485300006441) [DOI] [Google Scholar]
- 42.Gillies MT, Wilkes TJ. 1978. The effect of high fences on the dispersal of some West African mosquitoes (Diptera: Culicidae). Bull. Entomol. Res. 68, 401–408. ( 10.1017/S000748530000938X) [DOI] [Google Scholar]
- 43.von Seidlein L, et al. 2017. Affordable house designs to improve health in rural Africa: a field study from northeastern Tanzania. Lancet Planet. Hlth 1, e188–e199. ( 10.1016/S2542-5196(17)30078-5) [DOI] [PubMed] [Google Scholar]
- 44.Braack L, et al. 2015. Biting behaviour of African malaria vectors: 1. where do the main vector species bite on the human body? Parasit. Vectors 8, 76 ( 10.1186/s13071-015-0677-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsay SW, Janneh LM. 1989. Preliminary field trials of personal protection against mosquitoes in The Gambia using deet or permethrin in soap, compared with other methods. Med. Vet. Entomol. 3, 97–100. ( 10.1111/j.1365-2915.1989.tb00481.x) [DOI] [PubMed] [Google Scholar]
- 46.Charlwood JD, Paru R, Dagoro H. 1984. Raised platforms reduce mosquito bites. Trans. R. Soc. Trop. Med. Hyg. 78, 141–142. ( 10.1016/0035-9203(84)90204-9) [DOI] [PubMed] [Google Scholar]
- 47.Knudsen J, Von Seidlein L. 2014. Healthy homes in tropical zones: improving rural housing in Asia and Africa. Stuttgart, Germany: Axel Menges. [Google Scholar]
- 48.Pryce J, Richardson M, Lengeler C. 2018. Insecticide-treated nets for preventing malaria. Cochrane Database Syst. Rev. 11, CD000363 ( 10.1002/14651858.CD000363.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranson H, Lissenden N. 2016. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196. ( 10.1016/j.pt.2015.11.010) [DOI] [PubMed] [Google Scholar]
- 50.Lenhart A, Orelus N, Maskill R, Alexander N, Streit T, McCall PJ. 2008. Insecticide-treated bednets to control dengue vectors: preliminary evidence from a controlled trial in Haiti. Trop. Med. Int. Health 13, 56–67. ( 10.1111/j.1365-3156.2007.01966.x) [DOI] [PubMed] [Google Scholar]
- 51.Pluess B, Tanser FC, Lengeler C, Sharp BL. 2010. Indoor residual spraying for preventing malaria. Cochrane Database Syst. Rev. 4, CD006657 ( 10.1002/14651858.CD006657.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vazquez-Prokopec G, Montgomery B, Horne P, Clennon J, Ritchie S. 2017. Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Science Adv. 3, e1602024 ( 10.1126/sciadv.1602024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paredes-Esquivel C, Lenhart A, del Rio R, Leza MM, Estrugo M, Chalco E, Casanova W, Miranda MA. 2016. The impact of indoor residual spraying of deltamethrin on dengue vector populations in the Peruvian Amazon. Acta Trop. 154, 139–144. ( 10.1016/j.actatropica.2015.10.020) [DOI] [PubMed] [Google Scholar]
- 54.Sternberg ED, Ng'habi KR, Lyimo IN, Kessy ST, Farenhorst M, Thomas MB, Knols BG, Mnyone LL. 2016. Eave tubes for malaria control in Africa: initial development and semi-field evaluations in Tanzania. Malar. J. 15, 447 ( 10.1186/s12936-016-1499-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Killeen GF, Masalu JP, Chinula D, Fotakis EA, Kavishe DR, Malone D, Okumu F. 2017. Control of malaria vector mosquitoes by insecticide-treated combinations of window screens and eave baffles. Emerg. Infect. Dis. 23, 782–789. ( 10.3201/eid2305.160662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. 2018. Eave ribbons treated with the spatial repellent, transfluthrin, can effectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malar. J. 17, 368 ( 10.1186/s12936-018-2520-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pulford J, Hetzel MW, Bryant M, Siba PM, Mueller I. 2011. Reported reasons for not using a mosquito net when one is available: a review of the published literature. Malar. J. 10, 1–10. ( 10.1186/1475-2875-10-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchner H, Rehfuess EA. 2015. Cooking and season as risk factors for acute lower respiratory infections in African children: a cross-sectional multi-country analysis. PLoS ONE 10, e0128933 ( 10.1371/journal.pone.0128933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogoma SB, Kannady K, Sikulu M, Chaki PP, Govella NJ, Mukabana WR, Killeen GF. 2009. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malar. J. 8, e773 ( 10.1186/1475-2875-8-221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Killeen GF, Govella NJ, Mlacha YP, Chaki PP. 2019. Suppression of malaria vector densities and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. Lancet Plan. Hlth 3, e132–e143. ( 10.1016/S2542-5196(19)30035-X) [DOI] [PubMed] [Google Scholar]
- 61.Getawen SK, Ashine T, Massebo F, Woldeyes D, Lindtjorn B. 2018. Exploring the impact of house screening intervention on entomological indices and incidence of malaria in Arba Minch town, southwest Ethiopia: a randomized control trial. Acta Trop. 181, 84–94. ( 10.1016/j.actatropica.2018.02.009) [DOI] [PubMed] [Google Scholar]
- 62.Maia M, Sangoro P, Thele M, Turner E, Moore S. 2012. Do topical repellents divert mosquitoes within a community? Malaria J. 11, S67–S68. ( 10.1371/journal.pone.0084875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Igarashi A. 1997. Impact of dengue virus infection and its control. FEMS Immunol. Med. Microbiol. 18, 291–300. ( 10.1111/j.1574-695X.1997.tb01058.x) [DOI] [PubMed] [Google Scholar]
- 64.Nguyen HT, Tien NC, Ninh TU, Hoa NT. 1996. The effect of Olyset net screen to control the vector of dengue fever in Viet Nam. Dengue Bull. 20, 87–92. [Google Scholar]
- 65.Manrique-Saide P, et al. 2015. Use of insecticide-treated house screens to reduce infestations of dengue virus vectors, Mexico. Emerg. Infect. Dis. 21, 308–311. ( 10.3201/eid2102.140533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Che-Mendoza A, et al. 2015. Long-lasting insecticide-treated house screens and targeted treatment of productive breeding-sites for dengue vector control in Acapulco, Mexico. Trans. R. Soc. Trop. Med. Hyg. 109, 106–115. ( 10.1093/trstmh/tru189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Che-Mendoza A, et al. 2018. House screening with insecticide-treated netting provides sustained reductions in domestic populations of Aedes aegypti in Merida, Mexico. PloS Neglect. Trop. Dis. 12, 17 ( 10.1371/journal.pntd.0006283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khattab A, Jylha K, Hakala T, Aalto M, Malima R, Kisinza W, Honkala M, Nousiainen P, Meri S. 2017. 3D mosquito screens to create window double screen traps for mosquito control. Parasit. Vectors 10, 12 ( 10.1186/s13071-017-2322-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castro MC, et al. 2010. The importance of drains for the larval development of lymphatic filariasis and malaria vectors in Dar es Salaam, United Republic of Tanzania. PloS Neglect. Trop. Dis. 4, e693 ( 10.1371/journal.pntd.0000693). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO. 2016. A toolkit for integrated vector management in sub-saharan Africa. Geneva, Switzerland: WHO; See https://www.who.int/neglected_diseases/resources/9789241549653/en/. [Google Scholar]
- 71.Watson M. 1953. African highway: the battle for health in Central Africa. London, UK: John Murray. [Google Scholar]
- 72.Opiyo P, Mukabana WR, Kiche I, Mathenge E, Killeen GF, Fillinger U. 2007. An exploratory study of community factors relevant for participatory malaria control on Rusinga Island, western Kenya. Malar. J. 6, 48 ( 10.1186/1475-2875-6-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindsay SW, Wilson A, Golding N, Scott TW, Takken W. 2017. Improving the built environment in urban areas to control Aedes aegypti-borne diseases. Bull. World Hlth Organ. 95, 607–608. ( 10.2471/BLT.16.189688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.WHO. 2020. Managing the links between water storage and dengue vectors in Barbados. Geneva, Switzerland: WHO. See https://www.who.int/globalchange/resources/vulnerability_adaptation/case_studies/barbados/en/. [Google Scholar]
- 75.Numbere AO. 2019. Municipal solid waste disposal in mangrove forest: environmental implication and management strategies in the Niger Delta, Nigeria. In Municipal solid waste management (ed. Saleh HE.). Cairo, Egypt: Egyptian Atomic Energy Authority. [Google Scholar]
- 76.Padmanabha H, Soto E, Mosquera M, Lord CC, Lounibos LP. 2010. Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. Ecohealth 7, 78–90. ( 10.1007/s10393-010-0301-6) [DOI] [PubMed] [Google Scholar]
- 77.Phuanukoonnon S, Mueller I, Bryan JH. 2005. Effectiveness of dengue control practices in household water containers in Northeast Thailand. Trop. Med. Int. Hlth 10, 755–763. ( 10.1111/j.1365-3156.2005.01452.x) [DOI] [PubMed] [Google Scholar]
- 78.Tsuzuki A, Trang H, Luu L, Tsunoda T, Takagi M. 2009. Effect of water supply system installation on distribution of water storage containers and abundance of Aedes aegypti immatures in urban premises of Ho Chi Minh City, Viet Nam. WHO IRIS. See https://apps.who.int/iris/handle/10665/170730.
- 79.Schmidt WP, et al. 2011. Population density, water supply, and the risk of dengue fever in Vietnam: cohort study and spatial analysis. PLoS Med. 8, 9 ( 10.1371/journal.pmed.1001082). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO. 2019. Vector alert: Anopheles stephensi invasion and spread. Geneva, Switzerland: WHO; See https://www.who.int/news-room/detail/26-08-2019-vector-alert-anopheles-stephensi-invasion-and-spread. [Google Scholar]
- 81.Rek JC, et al. 2018. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet. Hlth 2, e83–e94. ( 10.1016/S2542-5196(18)30010-X). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindsay SW, et al. 2019. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa. Nature Sci. Rep. 9, 7770 ( 10.1038/s41598-019-43816-0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tusting LS, Bradley J, Bhatt S, Gibson HS, Weiss DJ, Shenton FC, Lindsay SW. 2020. Environmental temperature and growth faltering in African children: a cross-sectional study. Lancet Planet. Hlth 4, e116–e123. ( 10.1016/S2542-5196(20)30037-1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye Y, Hoshen M, Louis V, Seraphin S, Traore I, Sauerborn R. 2006. Housing conditions and Plasmodium falciparum infection: protective effect of iron-sheet roofed houses. Malar. J. 5, 8 ( 10.1186/1475-2875-5-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sim S, Ng LC, Lindsay SW, Wilson AL. 2020. A greener vision for vector control: the example of the Singapore dengue control programme. PLoS Negl. Trop. Dis. 14, e0008428 ( 10.1371/journal.pntd.0008428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andersson N, et al. 2015. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. Br. Med. J. 351, h3267 ( 10.1136/bmj.h3267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez L, Perez D, Perez T, Sosa T, Cruz G, Kouri G, Boelaert M, Van der Stuyft P.. 2005. Intersectoral coordination in Aedes aegypti control. A pilot project in Havana City, Cuba. Trop. Med. Int. Hlth 10, 82–91. ( 10.1111/j.1365-3156.2004.01347.x) [DOI] [PubMed] [Google Scholar]
- 88.Suroso H, Suroso T. 1990. Aedes aegypti control through source reduction by community efforts in Pekalongan, Indonesia. Mosquito Borne Dis. Bull. 7, 59–62. [Google Scholar]
- 89.Barrera-Perez MA, et al. 2015. Control of Aedes aegypti breeding sites with the program Recicla por tu bienestar in Merida, Mexico. Salud. Publica Mex. 57, 201–210. ( 10.21149/spm.v57i3.7556) [DOI] [PubMed] [Google Scholar]
- 90.Winch PJ, Leontsini E, Rigau-Perez JG, Ruiz-Perez M, Clark GG, Gubler DJ. 2002. Community-based dengue prevention programs in Puerto Rico: impact on knowledge, behavior, and residential mosquito infestation. Am. J. Trop. Med. Hyg. 67, 363–370. ( 10.4269/ajtmh.2002.67.363) [DOI] [PubMed] [Google Scholar]
- 91.Filipe A, Renedo A, Marston C. 2017. The co-production of what? Knowledge, values, and social relations in health care. PLoS Biol. 15, e2001403 ( 10.1371/journal.pbio.2001403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, Lindsay SW. 2020. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831 ( 10.1371/journal.pntd.0007831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.RBM/UNDP. 2013. Multisectoral action framework for malaria. Geneva, Switzerland: UNDP and RBM; See https://www.undp.org/content/undp/en/home/librarypage/hiv-aids/multisectoral-action-framework-for-malaria-html. [Google Scholar]
- 94.RBM/UNDP/UN-Habitat. 2015. Housing and malaria consensus statement. Geneva, Switzerland: RBM/UNDP/UN-Habitat; See https://malariaworld.org/sites/default/files/RBM%20VCWG%20Housing%20and%20Malaria%20Consensus%20Statement_final.pdf. [Google Scholar]
- 95.UN. 2017. New urban agenda. Habitat III Quito 17-20 October 2016. United Nations conference on housing and sustainable urban development. New York, NY: UN; See http://habitat3.org/the-new-urban-agenda/. [Google Scholar]
- 96.Li R, Xu L, Bjornstad ON, Liu K, Song T, Chen A, Xu B, Liu Q, Stenseth NC. 2019. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proc. Natl Acad. Sci. USA 116, 3624–3629. ( 10.1073/pnas.1806094116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.IPCC. 2014. Climate change 2014. Impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 98.UNISDR. 2016. Fact sheet: health in the context of the Sendai framework for disaster risk reduction. Geneva, Switzerland: UNISDR; See https://www.unisdr.org/files/globalplatform/59224c164c630Health_in_Sendai_Framework_for_DRR-Factsheet.pdf. [Google Scholar]
- 99.WHO. 2018. Housing and health guidelines. Geneva, Switzerland: WHO; See https://www.who.int/sustainable-development/publications/housing-health-guidelines/en/. [Google Scholar]
- 100.Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, Lindsay SW. 2015. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 31, 380–390. ( 10.1016/j.pt.2015.04.015) [DOI] [PubMed] [Google Scholar]
- 101.Shenton FC, et al. 2019. Research agenda for preventing mosquito-transmitted diseases through improving the built environment in sub-Saharan Africa. Cities Hlth, online. ( 10.1080/23748834.2019.1684771) [DOI] [Google Scholar]
- 102.Little E, Biehler D, Leisnham PT, Jordan R, Wilson S, LaDeau SL. 2017. Socio-ecological mechanisms supporting high densities of Aedes albopictus (Diptera: Culicidae) in Baltimore, MD. J. Med. Entomol. 54, 1183–1192. ( 10.1093/jme/tjx103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tusting LS, Gething PW, Gibson HS, Greenwood B, Knudsen J, Lindsay SW, Bhatt S. 2020. Housing and child health in sub-Saharan Africa: a cross-sectional analysis. PLoS Med. 17, e1003055 ( 10.1371/journal.pmed.1003055) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.