Abstract
The scientific community recognizes that molecular xenomonitoring (MX) can allow infected mosquitoes to serve as a proxy for human infection in vector-borne disease surveillance, but developing reliable MX systems for programmatic use has been challenging. The primary aim of this article is to examine the available evidence to recommend how MX can best be used for various purposes. Although much of the literature published within the last 20 years focuses on using MX for lymphatic filariasis elimination, a growing body of evidence supports its use in early warning systems for emerging infectious diseases (EIDs). An MX system design must consider the goal and target (e.g. diseases targeted for elimination versus EIDs), mosquito and pathogen characteristics, and context (e.g. setting and health system). MX is currently used as a ‘supplement’ to human surveillance and will not be considered as a ‘replacement’ until the correlation between pathogen-infection rates in human and mosquito populations is better understood. Establishing such relationships may not be feasible in elimination scenarios, due to increasingly dwindling human infection prevalence after successful control, but may still be possible for EIDs and in integrated disease surveillance systems.
This article is part of the theme issue ‘Novel control strategies for mosquito-borne diseases'.
Keywords: molecular xenomonitoring, surveillance, disease elimination, emerging infectious diseases, mosquito, lymphatic filariasis
1. Introduction
Xenomonitoring, defined broadly as the detection of human pathogens in arthropod vectors, has been practised by medical entomologists to estimate the risk of human exposure to transmission of different vector-borne diseases (VBDs) for many decades. For example, the Onchocerciasis Control Programme in West Africa began using the annual transmission potential (total number of infective larvae divided by number of blackflies dissected multiplied by number of bites/person/year) in the late 1970s; their strategy was later adapted for use in lymphatic filariasis (LF) surveillance. Similarly, the malaria surveillance community has used the entomological inoculation rate (a product of the human biting rate and the proportion of vector mosquitoes that are infective per unit time) for decades. Scientists have long believed that the ability to monitor vectors as a proxy for human infection could be extremely useful, particularly given the numerous challenges (e.g. logistical, cost, ethical) related to pathogen monitoring in human populations. However, developing accurate, reliable, responsive and cost-effective xenomonitoring systems has been extremely complex, given the vector, pathogen and human factors involved.
Until relatively recently, the terms ‘xenomonitoring’ and ‘xenosurveillance’ were, confusingly, used synonymously and defined by the World Health Organization (WHO) as a ‘method in which the infection rate in the mosquito population is used to determine whether transmission is still occurring in the human population’ [1]. Now, ‘xenosurveillance’ (XS) is generally used when pathogens are screened in bloodmeals of mosquitoes where no replication occurs, and defined as ‘a surveillance technique that makes use of the haematophagous behaviour of some arthropods to survey vertebrates for the presence of pathogens’ [2,3].
Molecular xenomonitoring (MX), the detection of pathogen DNA/RNA in a vector as a proxy of human infection, was developed after significant advances in laboratory methods over the past two decades and has the ability to transform VBD surveillance. The focus of the present article is to review where MX for mosquito-borne diseases has been performed in order to recommend if, when, where and how MX can best be used for maximum public health and VBD surveillance benefit. Although historically the term MX has primarily been associated with LF, there is great potential for MX in the surveillance of multiple mosquito-borne diseases, such as malaria and arboviral diseases (e.g. dengue, DENV; chikungunya, CHIKV; and Zika, ZIKV) as well as for other VBDs not vectored by mosquitoes.
This article details the challenges involved in MX, describes its evolution as a field, including using LF as a case study, and provides examples of MX for other pathogens and diseases. It examines MX in elimination and control contexts, as well as for detecting emerging infectious diseases (EIDs). Finally, it explores scientific, practical, logistical, political and economic considerations for MX systems and offers recommendations for future research, development and scaling-up for programmatic use. It also encourages using consistent terminology, sharing researcher experiences across VBDs/EIDs and finding common ground in order to facilitate the development of MX frameworks for integrated disease surveillance.
2. Molecular xenomonitoring for lymphatic filariasis
(a). Historical development of molecular xenomonitoring for lymphatic filariasis elimination, 2000–2012
In 2000, the WHO launched the world's largest public–private partnership and disease elimination programme using mass drug administration (MDA): the Global Programme to Eliminate Lymphatic Filariasis (GPELF) [4]. The national programmes for the elimination of LF (NPELFs) of 83 ministries of health, supported by private, non- and extra-governmental partners, pledged to eliminate the world's leading cause of disability due to infectious disease, and the second leading cause of long-term disability [5]. Even then, the GPELF realized that if it succeeded in reducing infection and disease, it would need robust methods to detect if transmission was truly interrupted and continually monitor to prevent recrudescence. The development of the immunochromatographic test (ICT) to detect parasite antigen was extremely important for ease of use, rapidity of results and ability to test at any time of day thereby averting issues with nocturnal periodicity of the principal LF parasite. Still, the GPELF knew there would come a point towards the ‘endgame’ when supplementary measures would be needed to ensure that LF was truly eliminated. This ignited an interest to use vector, not just human, endpoints for monitoring LF elimination.
Shortly after GPELF inception, scientists developed a protocol for polymerase chain reaction (PCR) to detect Wuchereria bancrofti DNA in mosquitoes [6]. They recommended using blood-fed mosquitoes to screen for infection and validating positive results against human blood surveys, usually ICT of circulating filarial antigen (CFA) or night-drawn thick blood films (TBFs) of parasitic microfilariae (mf) [7]. Before this, a standardized PCR protocol for detecting W. bancrofti, Brugia malayi or B. timori DNA in mosquitoes had not been finalized nor evaluated for GPELF xenomonitoring in parallel with human CFA or mf prevalence [7]. While this seminal laboratory-based study provided the first molecular tools and protocols for MX, it did not offer a direct assessment of field-caught mosquitoes. However, it helped give rise to the concept that adding MX of mosquitoes to LF surveillance could be a more sensitive approach than solely relying upon diagnostic techniques focused on human infection.
Almost concurrently, researchers in Trinidad, West Indies, developed a field protocol for collecting resting, blood-fed Culex quinquefasciatus mosquitoes in households for subsequent screening for W. bancrofti DNA [8]. This consisted of five steps: (1) household notification and consent, (2) residential mapping (to enable spatial and temporal monitoring), (3) equipment preparation (e.g. U.S. Centers for Disease Control and Prevention—CDC—backpack aspirators, mouth aspirators and hand nets), (4) mosquito collection (early morning to obtain greater than 3000 freshly blood-fed females), and (5) laboratory processing and storage (at 4°C for dried, pooled blood-fed females) until PCR analysis using Poolscreen software to calculate mosquito infection prevalence [8]. Although this formative field-based study did not include PCR testing, it provided a helpful field protocol for mosquito collection and storage that could be applied globally provided that vector and parasite dynamics in different epidemiological settings were taken into account.
The earliest comprehensive study of MX, combining field collection and molecular screening, demonstrated clearance of W. bancrofti DNA in Cx. pipiens after five annual rounds of MDA via the Egyptian NPELF [9]. While the authors concluded that MX was a powerful tool for measuring MDA impact, they highlighted limitations of the labour-intensive and sometimes inefficient methods used. First, manual aspiration yielded low numbers of blood-fed or gravid female mosquitoes for PCR analysis. Second, the PCR method tested was suboptimal, so the development of a real-time PCR with better sensitivity and higher throughput was recommended as a research priority. Data analysis and interpretation was also a major challenge. Later, NPELF researchers comparing gravid traps (GTs) with manual aspirators with the aim of yielding more mosquitoes to detect infection found that GTs caught six times more mosquitoes, and more infected mosquitoes, per collection than aspirators [10]. They developed a field protocol using 50 GTs placed for two nights in 44 of 183 formerly endemic localities that had undergone five rounds of MDA. Next, they delineated provisional elimination criteria: CFA rate less than 2%, mf rate (by 50 μl TBF) less than 0.5%, antibody rate in first-year primary school children less than 2% and mosquito infection rate by Poolscreen less than 0.25% [11]. After testing 200 pools of 15 gravid Cx. pipiens females/pool/village by ligase detection reaction-qPCR, Poolscreen infection rates ranged from 0.03% to 0.37% and CFA rates ranged from 0.2% to 3.6%, but only one mf carrier was identified in the village with the highest CFA rate. They concluded that CFA testing using ICT and MX is more sensitive than mf [11].
Before the first decade of MX for LF concluded, studies on comparisons of microscopy versus PCR for estimating prevalence rates [12], the distribution of filarial parasites and their DNA in mosquitoes [13], and the development of multiplex qPCR assays for W. bancrofti and/or B. malayi DNA in different vectors [14,15] demonstrated how PCR provided a sensitive, high-throughput method for routine surveillance. In 2009, researchers published a review on available tools for quantifying mosquito infection and provided evidence on potential thresholds of infected mosquitoes to indicate interruption of LF transmission [16]. Using modelling, the authors concluded that accurate, high-throughput mosquito screening could detect low infection thresholds (0.65% for any stage and 0.085% for L3 larval infection) if 450–3500 mosquitoes were examined [16]. This led to additional research on simple DNA extraction methods, including a Tris-EDTA boiling method, for more cost-effective, high-throughput screening of different human (W. bancrofti, B. malayi, B. pahangi) and animal (Dirofilaria immitis) filarial DNA in mosquitoes (Cx. quinquefasciatus, Aedes aegypti and Anopheles stephensi) [17,18], which were later tested in the field [19].
By 2013, the WHO proposed that MX, in combination with human-focused LF endpoints, could play a role in post-MDA surveillance. Transmission Assessment Surveys (TAS) were developed to determine whether MDA reduced LF prevalence (measured via CFA or mf) in primary school children to levels equal or below critical cut-off thresholds for specific vector species: for Bancroftian LF this was defined as less than 2% (if vectored by Culex or Anopheles mosquitoes) or less than 1% (if vectored by Aedes mosquitoes) and less than 2% for Brugian LF [1]. If TAS results exceed these levels, then WHO recommend that MDA should be continued/resumed. A multi-centre TAS supported the use of complementary tools such as MX for longer-term post-MDA surveillance, so MX was included in subsequent WHO GPELF guidelines [20]. However, it must be reinforced that from development, inclusion in TAS and to present day, MX has never been recommended as a replacement for human-focused LF surveillance methods (e.g. ICT, TBF) but has only been recommended as a supplement to enhanced LF surveillance. This is likely due to current MX limitations, including that a standard protocol for sampling mosquitoes at sentinel sites representative of human infection heterogeneity is still not available and that collecting several thousand mosquitoes (as are required to detect low infection rates, with even larger numbers for infective rates) may exceed logistical constraints and technical capacity [1].
(b). Molecular xenomonitoring in lymphatic filariasis elimination programmatic use, 2013–2020
A fairly recent review suggested that MX for LF surveillance would benefit from technological advances and standardization, identified operational gaps and proposed MX prospects for the future [21]. Since then, an escalation in MX research for LF elimination programmatic use has followed and is described below.
In the past decade, MX has been tested in all six WHO regions, covering a variety of LF-endemic settings with different vector–parasite dynamics. The principal field studies in the five WHO regions where human LF is endemic are summarized in table 1; field studies performed in Europe, which focus on filarial parasites of veterinary importance, are not included in this article. From table 1, it is evident that MX has been used for different purposes, most importantly as a tool not only for monitoring MDA impact, defining elimination endpoints and mapping LF occurrence but also for comparing different methods for mosquito sampling and/or filarial DNA or RNA detection (table 1).
Table 1.
Principal field studies that have employed molecular xenomonitoring in different LF-endemic WHO regions.
| WHO region & endemic countries | setting/s | purpose/s | mosquito collection methods (MCMs) | mosquito species | parasite/s | mosquito pools/infection | source |
|---|---|---|---|---|---|---|---|
| Africa (AFRO) | |||||||
| Ghana | rural, two communities | comparing diagnostic tools for assessing interruption of LF | GTs (outside houses for Culex) and pyrethrum knock-down collection (inside houses for Anopheles) |
Culex spp., Anopheles spp. |
W. bancrofti |
Culex spp: 1/264 pools (4099 females in total) tested positive for W. bancrofti DNA by PCR; Anopheles spp: 13/30 pools (401 females in total) tested positive for W. bancrofti DNA by PCR |
[22] |
| Tanzania | rural, two communities: Masaika (end of rainy season); Vyeru, Tanga Region (rainy season) |
comparing MCMs | CDC LTs (inside houses) and CDC GTs (outside houses) |
Cx. quinquefasciatus
|
W. bancrofti | masaika (individually tested)—2/150 females collected using GTs tested positive (1.3%) by PCR; Vyeru (25 females/pool)—15/110 pools collected from LTs tested positive (0.6%) by PCR; 1/115 pools collected using GTs tested positive (0.03%) by PCR | [23] |
| Togo | rural, 37 villages in three districts in one of four evaluation units (EUs) | post-MDA surveillance | pyrethroid spray catches inside houses (PSC) versus exit trap collection (ETC) over 5 months |
Anopheles spp., Cx. quinquefasciatus |
W. bancrofti | pools of 25 or less females; LAMP used to detect W. bancrofti DNA; positive pools confirmed by PCR; Anopheles spp: 0/629 pools (9191 females in total including 6992 An. gambiae) tested positive |
[24] |
| Americas (AMR/PAH) | |||||||
| Brazil | urban, Olinda (RMR) | comparing MCMs | CDC LTs (inside houses) and large battery-powered aspirators (inside houses) | Cx. quinquefasciatus | W. bancrofti | 0/182 pools (1556 mosquitoes) tested positive by PCR | [25] |
| Eastern Mediterranean (EMRO) | |||||||
| Egypt | rural, two communities (150–200 houses per year sampled in each study area): Giza villages (high LF prevalence) and Qalubiya villages (low LF prevalence) | post-MDA surveillance | manual aspiration (inside houses) |
Cx. pipiens
|
W. bancrofti | pools of >25 blood-fed or gravid females; Poolscreen infection rates decreased following MDA: 3.07% pre-MDA to 0.19% post-MDA (Giz) and 4.37% pre-MDA to 0.00% post-MDA (Qal) after five annual rounds | [9] |
| Egypt | rural, seven districts | comparing single PCRs and multiplex PCRs | battery-powered aspiration (inside houses) |
Culex spp., Anopheles spp., Aedes spp. |
W. bancrofti, D. immitis, Dirofilaria repens |
100 pools of 25 females/pool tested using single PCRs and mulitplex PCRs for the 3 filarial parasites; the overall estimated rate of infection using Poolscreen was 0.6%; the multiplex PCR had a higher sensitivity (100%) compared with single PCR, and 98% specificity | [19] |
| Egypt | rural, two villages: Samalay and Kafr El-Tarainah after 13th round of MDA | post-MDA surveillance | 25 GTs (outside houses) for 6 successive nights | Cx. pipiens | W. bancrofti | pools of 25 mosquitoes were tested by a real-time PCR: 152 pools for Samalay, 167 pools for Kafr El-Tarainah; all pools were negative; all primary school children were negative by ICT | [26] |
| Southeast Asia (SEARO) | |||||||
| Sri Lanka | rural, 19 PHI areas in eight LF-endemic districts, plus two in Colombo, 6 years after MDA (conducted 2002–2006) | post-MDA surveillance | CDC GTs placed in each quadrant of each PHI for 1–4 days | Cx. quinquefasciatus | W. bancrofti | almost 3900 pools (20 females/pool) in 19 PHIs tested for filarial DNA by qPCR; filarial rates exceeded the 0.25% target in 10/19 PHIs and were >1% in Galle | [27] |
| Sri Lanka | two Galle EUs, one coastal/high risk and one inland/low risk, 8 years after last round of MDA in 2006 | post-MDA surveillance comparing number of trap sites required/scale |
CDC GTs placed in 300 trap locations per EU |
Cx. quinquefasciatus
|
W. bancrofti | qPCR results: 28 000 females collected and tested in 1208 pools (1–25 females/pool, 89.5% contained 25 females); 92/625 positive (=0.63% Poolscreen filarial DNA rate) in high-risk EU; 8/583 positive (=0.0.6% Poolscreen filarial DNA rate) in low-risk EU; filarial DNA rates do not differ whether 75, 150 or 300 trap sites are tested | [28] |
| Sri Lanka | six of the 19 PHI areas previously examined [27] | post-MDA surveillance | CDC GTs placed in each quadrant of each PHI (50 trapping locations/PHI) | Cx. quinquefasciatus | W. bancrofti | four pools of 20 fed, gravid or semigravid mosquitoes were tested/PHI; filarial DNA was detected in all six PHI areas, and exceeded the target in three: two in Galle District (1.17% in Ambalangoda and 1.23 in Unawatuna), and one in Matara District (1.09% in Weligama) | [29] |
| Sri Lanka | hotspot with persistent LF (Balapitya PHI area in Galle EU) | post-MDA surveillance | in 2015 trapping was as previously described [29]; in 2016, CDC GTs placed in each quadrant: eight trapping locations in Balapitya PHI and 14 in coastal Galle EU |
Cx. quinquefasciatus | W. bancrofti | two pools of 25 fed, gravid or semigravid mosquitoes were tested/PHI; filarial DNA was detected in all five Public Health Midwife areas within Balapitia PHI; mean prevalence rates were lower in 2016 compared with 2015 but were 3.0% overall | [30] |
| India | one hotspot (≥1% community microfilaria prevalence) and PHC area in Ammapettai, Thanjavur District, Tamil Nadu | post-MDA surveillance comparing number of trap sites required/scale |
CDC GTs placed at 204 households (HHs) for the hotspot, and 231 HHs in the PHC in 2010 and 2012 | Cx. quinquefasciatus | W. bancrofti | qPCR results: 231 pools of over 5000 mosquitoes (around 23 females/pool) were tested for each sample taken each year; the hotspot pool rates and estimated LF infection rates were higher in the hotspot than the PHC area, e.g. W. bancrofti DNA prevalence in hotspot versus PHC area was 1.1% versus 0.9% and 0.3% versus 0.2% in 2010 and 2012, respectively | [31] |
| India | surveyed nine districts with LF hotspots pre-MDA in two states: Maharashtra and Karnataka | post-MDA surveillance | CDC LTs on porches of HH | not specified in publication | W. bancrofti | PCR: unspecified number of pools (10 mosquitoes/pool); 6/9 districts had pools positive for Ssp1 gene specific to L3 (infective mosquitoes) | [32] |
| Bangladesh | rural, two districts (30 villages/EU): Panchagarh (previously endemic, stopped MDA) and Gaibandha (non-endemic) | assessing interruption of LF following 12 rounds of MDA | CDC GTs placed 180 trap sites (HH courtyards)/district | Cx. quinquefasciatus | W. bancrofti | PCR results: 0/594 pools (1–25 females, mean 16.9) tested positive for W. bancrofti DNA | [33] |
| Western Pacific (WPRO) | |||||||
| American Samoa | rural, three coastal villages of Tutuila island | comparing microscopy and PCR for parasite detection | 10 BG Sentinel traps baited with BG Lure placed on ground locations in each village for 4 consecutive days; repeated to give a total of 8 trap days/village | Aedes spp. | W. bancrofti | compared microscopy (hemalum staining and dissection) with PCR (average pool size=4.9 for Aedes polynesiensis, 1.92 for Ae. aegypti and 2.10 for Ae. upolensis); overall prevalence of W. bancrofti in Aedes polynesiensis was 0.16% by microscopy, and 0.69% by PCR (Poolscreen rate) | [12] |
| American Samoa | rural, multiple coastal villages in five islands of Tutuila, Aunu'u, Ofu, Olosega and Ta'u | comparing diagnostic tools for assessing interruption of LF following 10 rounds of MDA | usually, 10 BG Sentinel traps baited with BG Lure placed on ground locations for 24–48 h throughout each village |
Aedes spp., Cx. quinquefasciatus |
W. bancrofti | females placed in pools of ≤20 prior to PCR/Poolscreen; Aedes polynesiensis most abundant (15 215 females: 42/1250 pools positive (0.28% prevalence); Cx. quinquefasciatus (4413 females) had a prevalence rate of 0.11% (5/584 positive pools); Ae. aegypti (887 females) had the highest prevalence (0.92%; 8/360 pools) | [34] |
| Samoa | rural, Fsitoo-Tai village on coast of Upolu island | comparing MCMs | compared BG Sentinel traps, HBCs and UV LED CDC LTs |
Ae. polynesiensis. Ae. (Finlaya) spp., Ae. aegypti. Cx. quinquefasciatus |
W. bancrofti | Ae. polynesiensis: 57 pools (2251 females) were analysed by PCR and four were positive for LF (4.7% prevalence); Ae. (Finlaya) spp.: 17 pools (153 females) were analysed by PCR and one was positive for LF (0.67% prevalence) | [35] |
| Malaysia | rural, 21 endemic implementation units (red IUs) and six non-endemic (green IUs) | investigate evidence of local transmission | human-landing catches and CDC LTs |
Culex spp., Anopheles spp., Aedes spp., Armigeres spp., Coquillettidia spp., Mansonia spp. |
W. bancrofti,
B. malayi |
0/668 pools (4738 mosquitoes) tested by PCR were positive for infection | [36] |
| Papua New Guinea | rural, three villages in Drekkier District (two high, one moderate transmission zones) | pre-MDA (2014) and post-MDA (2015) surveillance | human-landing catches | not specified in publication | W. bancrofti | 293 samples were collected in 2014, and 448 samples were collected in 2015; DNA was run using conventional PCR and gel electrophoresis; mosquito infection rates decreased in both high (10.8% to 2.6%) and moderate (16.3% to 1.6%) transmission zones | [37] |
(i). Molecular xenomonitoring for Cx. quinquefasciatus-vectored lymphatic filariasis
In Tanzania, MX was used within research contexts albeit with important insights for programmatic use. A mosquito collection method (MCM) comparison demonstrated that CDC GTs collected the highest number of Cx. quinquefasciatus females, and the highest proportion of gravid females, compared with three other types of GTs [38]. However, in a subsequent comparison of CDC GTs versus CDC light traps (LTs), CDC LTs collected more mosquitoes with W. bancrofti DNA [23]. Since GTs were placed outdoors they should have trapped mosquitoes exiting multiple houses after having bitten many different people; thus MX of GT-collected PCR-positive pools was likely representative of community LF prevalence [23].
In Sri Lanka, the NPELF conducted MX using CDC GTs for post-MDA surveillance at varying periods to verify progress towards elimination. Six years after MDA cessation, TAS for filarial antigenemia (via ICT) in primary school children and MX were performed in eight LF-endemic districts [27]. The filarial DNA rates in mosquitoes exceeded the WHO-mandated 0.25% target in 10 of the 19 Public Health Inspector (PHI) areas, and authors concluded that MX was more sensitive than TAS for detecting low levels of LF persistence, but the two methods were complementary [27]. Next, revised endpoint targets for NPELFs taking upper confidence intervals of fixed-point measurement into consideration were suggested as follows: CFA less than 2%; antibody prevalence in primary school children less than 5%; and filarial DNA prevalence in gravid, semigravid or blood-fed Culex mosquitoes less than 1% [27]. Subsequently, MX was conducted in two elimination units (EUs) in Galle District, where mosquito prevalence rates were 0.65% and 0.06% in the high- and low-risk EUs, respectively [28]. Comprehensive post-MDA surveillance included assessing community CFA and mf prevalence, CFA and anti-filarial antibody in 6–8-year-old school children and MX in 6 of 19 PHI areas [29]. NPELF authors suggested that MX may be a better predictor of LF persistence because human diagnostic tools rely on human compliance, and it was likely that infected people who were non-complaint with MDA were unlikely to participate in community surveys [29]. In 2016, the WHO certified that Sri Lanka had eliminated LF as a public health problem, but recommended continued surveillance and intervention in areas with persistent foci to clear residual infections [30]. After MDA in 2014 and 2015 and MX surveys in 2015 and 2016, community surveys detected infection (measured via CFA and mf) in humans, particularly aged over 40 years and male, and mosquitoes (W. bancrofti DNA). Since school-based TAS would miss infection in older age groups, additional interventions and surveillance tools, including MX, were recommended in transmission hotspots [30]. Subsequently, adult-TAS was performed in two EUs in Galle District and was found to be more sensitive than school-TAS; thus, adult-TAS together with MX was recommended for detecting residual LF infections [39].
In India, similarly, a study in Primary Health Centre (PHC) areas of Tamil Nadu found higher mosquito pool positivity in a hotspot than in the overall PHC area, prompting authors to suggest that MX can complement TAS, particularly in areas that have either passed TAS twice or passed the first TAS, but in subsequent surveys found antigen positivity rates close to the critical thresholds [31]. A separate study of nine previously LF-endemic districts with hotspot sites in the states of Maharashtra and Karnataka incorporated MX into post-MDA surveillance [32]. Night-drawn TBFs detected mf in 51/916 (5.56%) people, including new LF cases that had not been previously recorded, and PCR using specific Ssp1 primers detected the L3 gene in infective mosquitoes in 6/9 districts, indicating active transmission [32]. Most recently, a standard protocol using MX to complement TAS for post-MDA surveillance was validated in Tamil Nadu [40].
In Bangladesh, MX was used to complement identifying active LF transmission areas and determining whether W. bancrofti infection rates in mosquitoes were below the 0.25% threshold from one previously endemic and one non-endemic district after 12 rounds of MDA [33]. While none of the 594 pools tested positive for W. bancrofti DNA, in accordance with TAS in the previously endemic district, authors raised concerns about the feasibility of obtaining sufficiently large numbers of mosquitoes to estimate positivity thresholds of less than 0.25% [33]. Despite using the same number of trap sites recommended by a previous Sri Lankan study [28], authors could only collect and analyse just over 10 000 females of a 13 500 target [33].
Until this point, MX studies were mainly restricted to rural sites. However, a field trial was conducted in two sites of the Recife Metropolitan Region (RMR), Brazil, to optimize an MX sampling strategy for collecting adult females for Brazil's NPELF [25]. It compared two operationally feasible tools, large handheld battery-operated aspirators (Horst armadilhas) and CDC LTs, to determine which method was better at capturing female mosquitoes and performed a mosquito mark-release-recapture study to determine how far mosquitoes dispersed [25]. Aspirators were significantly better at collecting blood-fed, semigravid and gravid Cx. quinquefasciatus, with more than 10 000 Cx. quinquefasciatus and 910 Ae. aegypti females captured in mornings over one month. No W. bancrofti DNA was detected by PCR in 182 pools of Cx. quinquefasciatus, which was consistent with the result that no antigen was found among study area residents who underwent concurrent ICT screening. The finding that the furthest distance Cx. quinquefasciatus was recaptured was at 85 m helped inform future grid-based MX surveillance systems [25].
(ii). Molecular xenomonitoring for Anopheles spp.-vectored lymphatic filariasis
In Ghana, an MX study captured Anopheles mosquitoes, the principal local LF vectors, using pyrethrum spray collections (PSC) and Culex mosquitoes using GTs [22]. The trial was conducted in six countries to compare the effectiveness of seven diagnostic tests for detecting evidence of human W. bancrofti infection where infection prevalence was likely to be low following successive MDA rounds [41]. In Ghana, ICT was found to be the second most sensitive human diagnostic test and the most conducive for use in the field; authors also recommended incorporating MX into routine LF surveillance, particularly where mf prevalence is lower than 1%, to help decide when to stop MDA because low or no filarial infection in mosquitoes indicates transmission may have been interrupted [22]. Given that people are becoming more reluctant to provide blood and urine samples in LF elimination programmes, particularly after successive screening and as evident disease declines, the possibility that MX could prove to be a better non-invasive tool than human diagnostic tests for defining LF elimination endpoints is an important development [22].
In Togo, an MX study captured An. gambiae, the predominant local LF vector, using PSC and exit traps to investigate whether the absence of filarial infection in mosquitoes may indicate interruption of LF transmission [24]. Previously, MDA concluded in 2009 and subsequent human-focused surveillance of parasite (nocturnal TBFs) and antigen (Og4C3) was conducted during and after MDA (2006–2015). Two post-MDA TAS were conducted between 2012 and 2015, and MX revealed that none of the 9191 mosquito pools tested positive for W. bancrofti, confirming LF interruption [24]. Valuably, the NPELF MX strategy demonstrated that community volunteers, rather than entomologists, could be trained for mosquito collections. After detecting no human and mosquito infection several years post-MDA, Togo became the first country in sub-Saharan Africa to receive WHO certification of LF elimination.
(iii). Molecular xenomonitoring for Aedes spp.-vectored lymphatic filariasis
In American Samoa, MX studies used BG Sentinel (BGS) traps baited with BG Lure (Biogents AG, Regensburg, Germany) to preferentially collect host-seeking Aedes females, including Ae. polynesiensis, the most abundant local vector of W. bancrofti, to map LF infection [34]. Authors reiterated important points about MX interpretation: a PCR method that does not specifically target the infective L3 stage cannot provide a direct measurement of ongoing transmission, only the occurrence of parasite DNA, and parasite DNA can be found in any blood-fed arthropod (vector and non-vector species) [34]. They concluded that MX will only have programmatic value for decision making if better MCMs are developed, and the relationship between human and mosquito infection is fully understood. Another field trial, similar to the Ghana study [22] above, supported MX for post-MDA surveillance by demonstrating significant relationships between high PCR-positive mosquito prevalence and human seropositivity (Og4C3 Ag and Wb123 Ab but not BM14), including that no PCR-positive pools were found in areas where there were no seropositive people [42,43].
In Papua New Guinea, an MX study used human-landing catches to collect mosquitoes to compare pre- and post-MDA mosquito infection rates in two villages considered as high transmission zones, and one village considered a moderate transmission zone [37]. Following one round of MDA, mosquito infection rates decreased significantly in both high and moderate zones corresponding with decreased human infection rates as estimated by light microscopy [37].
The above-presented work is broadly representative of the overall body of literature on MX. It reflects the intense focus of the LF community, largely motivated by the GPELF and its elimination framework, as well as the desire to have a vector-driven elimination metric analogous and supplementary to TAS. Further, unlike the research communities of other pathogens and diseases, the LF community has opted to use consistent language (i.e. MX), which has helped coalesce movement towards developing unified MX tools, protocols and research as well as programmatically deployable methodologies.
3. Molecular xenomonitoring for active and passive surveillance of other mosquito-borne diseases, multiple pathogens and integrated disease surveillance
(a). Active surveillance and sympatric mosquito vectors
In addition to diseases under elimination, there is great potential to use MX to screen for emerging infections (e.g. ZIKV and CHIKV) including those that cause explosive epidemics (e.g. DENV and yellow fever virus, YFV). Further, since different species of mosquitoes with overlapping distributions may vector one or more pathogens, such as Anopheles spp. transmitting LF and malaria parasites [44] or Aedes ssp. transmitting LF parasites and DENV, MX can be used to indicate whether transmission of multiple pathogens may be occurring in a particular area. Additionally, while most MX programmes focus on one or two principal diseases or pathogens (which often means they will target one or two principal putative vectors), it is often the case that non-target mosquito species will be incidental captures, or ‘by-products', of the primary vector(s) targeted in mosquito collections. As these additional vectors are collected, they present tremendous opportunities for complementary screening in enhanced integrated surveillance systems incorporating EIDs, thereby maximizing use of resources.
For example, while the aim of an MX study in Bangladesh was to collect vectors of W. bancrofti for LF surveillance, potential vectors of malaria (An. barbirostris, An. vagus and An. umbrosus) and Japanese encephalitis virus (Cx. gelidus, Cx. pseudovishnui, Cx. vishnui and Cx. tritaeniorhynchus) were also captured [33]. Similarly, an MX study in the RMR, Brazil, initially aimed to collect Cx. quinquefasciatus for W. bancrofti surveillance, but also planned to test the by-product Ae. aegypti for DENV (the only arbovirus known to be circulating locally at the time of MX commencement in early 2015) so mosquitoes were stored at −80°C for subsequent RNA virus analysis [25]. Since the site was the epicentre of the 2015 ZIKV epidemic at a time when the vector had not been incriminated, and concurrent microcephaly cases were detected in the MX collection area, a subsample of 1556 Cx. quinquefasciatus (roughly 10% captured) and all 939 Ae. aegypti were pooled in up to 10 mosquitoes/house/week and screened for ZIKV RNA via real-time PCR. The Ae. aegypti pools were also screened for DENV and CHIKV RNA, given that the RMR typically had explosive DENV outbreaks and was experiencing its first CHIKV epidemic successive but seemingly concurrent to that of ZIKV. None of the 156 Ae. aegypti and 182 Cx. quinquefasciatus pools tested were positive for arboviral RNA [45].
(b). Passive surveillance, mosquito non-vectors and non-mosquito vectors
The idea that mosquitoes may be perceived as ‘biological syringes’ [46] gave rise to research investigating the potential role of MX as a passive surveillance tool for indicating whether a given pathogen is circulating in the human population. This involves screening mosquitoes that have recently blood-fed on humans for pathogens that are not necessarily vectored by the mosquitoes sampled. This approach can be used to screen for animal as well human pathogens in a One Health context [47,48].
In two rural villages in northern Liberia, blood-fed An. gambiae were collected indoors, using aspirators, and stored on 96-well format Flinders Technology Associates (Whatman FTA®) classic cards (FTA cards) cards at −80°C prior to RNA extraction. Real-time PCR detected Epstein–Barr virus and canine distemper virus. Using mosquito rather than human samples could be used to detect and predict outbreaks earlier for better management [3]. A subsequent laboratory-based study demonstrated that RNA from Trypanosoma brucei gambiense, Bacillus anthracis, Middle East Respiratory Syndrome Coronavirus and ZIKV could be detected from An. gambiae mosquitoes in the laboratory up to 24 h post blood-feeding of clinically relevant pathogen levels [2].
In a peri-urban area of Manaus, Brazilian Amazon, blood-fed non-vector culicine mosquitoes and Anopheles vectors were collected indoors using either large, battery-powered aspirators or BGS traps baited with BG lure for Plasmodium DNA screening [49]. Indeed, the vast majority of mosquitoes collected were Culex spp. (96.6%), with Cx. quinquefasciatus the dominant species. BGS traps captured significantly more Cx. quinquefasciatus than aspirators and significantly more blood-fed females, despite aspirators capturing proportionally more blood-fed females. There was no significant difference between Plasmodium DNA positivity rates from BGS traps and aspirators (2.7% versus 3.2%, respectively). Given the spatial correlation between Plasmodium DNA positivity rates in culicine mosquitoes and Plasmodium infection rates in humans, XS was recommended as a complementary strategy for estimating malaria prevalence [49].
A proof-of-concept high-throughput method for collecting excreta/faeces from large numbers of mosquitoes in a superhydrophobic cone, then screening samples for filarial and malarial DNA, was developed [50] and tested in two rural communities of Ghana [51]. Mosquitoes (greater than 95% Anophleles spp., mostly An. gambiae s.l.) were collected indoors by aspirators and outdoors by box GTs and BGS traps. The new method was compared with traditional MX, using insect carcasses, with mosquito samples screened for W. bancrofti, P. falciparum and Mansonella perstans DNA despite Culicoides midges (not mosquitoes) being vectors of M. perstans [51]. Human blood samples, finger prick Filariasis Test Strip and ICT to detect CFA and night-drawn venous blood for the acetic acid fixation and counting chamber method to detect mf were also examined for all three pathogens. Both the new method and traditional MX detected all pathogens and were considered highly sensitive when compared to human-based antigen and mf diagnostic methods [51].
(c). Integrated disease surveillance
Enhanced vector surveillance and monitoring is one of the four pillars identified by the WHO's 2017–2030 Global Vector Control Response, but must be integrated with health information systems and include targets for protection of at-risk populations [52]. In practice, vector surveillance is often a weak component of national VBD control programmes, and clear guidelines with entomological indicators are required to strengthen capacity. Moreover, appropriate vector control cannot be designed, let alone deployed and evaluated correctly, without an understanding of the spatio-temporal infection dynamics that can only be defined from a fully operational VBD surveillance system.
The potential of integrated MX systems for the surveillance of multiple VBDs has not been fully explored. This is especially important given that multiple VBDs often exist in the same space and time, and that a routinely functional MX system that has been developed for one VBD can easily be leveraged for other/multiple VBDs if the appropriate factors are considered. Thus, the inability to consider designing systems for more than one vector and pathogen combination at a time could lead to huge missed opportunities. It is promising that some surveillance systems, such as ArboNET coordinated by the CDC in the US have built upon surveillance systems created for one pathogen (e.g. West Nile virus) and now also incorporate monitoring for others (e.g. ZIKV and Eastern equine encephalitis) [53].
4. Considerations for molecular xenomonitoring system development
In disease elimination contexts, many national and global programmes have successfully reduced the prevalence of several VBDs. However, there still exist enormous challenges to have appropriate, sensitive and cost-effective surveillance systems in place in order to measure whether programmes have met their target elimination thresholds, which in turn allow them to sustain success and critically guard against recrudescence. As human infection prevalence declines, the number of human volunteers needed for pathogen screening rises, so active case surveillance is no longer cost-effective. In such situations, vector-based pathogen surveillance via MX may serve as a useful alternative to monitoring human infection and thereby residual transmission. Moreover, MX may improve the evaluation of interventions such as MDA and integrated vector management by serving as a proxy for predicting impacts on clinical outcomes when cluster-randomized trials are no longer a feasible option. This means that MX could prove not only extremely useful, but also cost-effective.
In EID and early warning contexts, MX could aid in several ways by supplementing or in some cases pre-empting detection via existing surveillance systems (e.g. routine passive surveillance of suspected and laboratory-confirmed pathogens). For instance, in early warning contexts, MX systems that have been built for multiple vectors and pathogens may be able to provide the first information on new pathogens that are entering a given area; this is especially relevant if such systems use routine screening (e.g. via virus panels) for various likely novel pathogens that may have been reported elsewhere in relatively near regions. In terms of hotspot detection or situations where better data are needed, MX systems may be able to provide more information, including more refined and consistent estimates of spatio-temporal transmission dynamics. Routine surveillance of immature stages such as house, container and Breteau indices could use the vast human capacity and resources already being exerted and incorporate collection and rearing to adult stage stages for screening of transovarial transmitted arboviruses if the levels of transovarial transmission justify these efforts and the laboratories have the required levels of security [ 54,55]. By leveraging routine surveillance resources, MX could provide extremely cost-effective for ongoing EID surveillance.
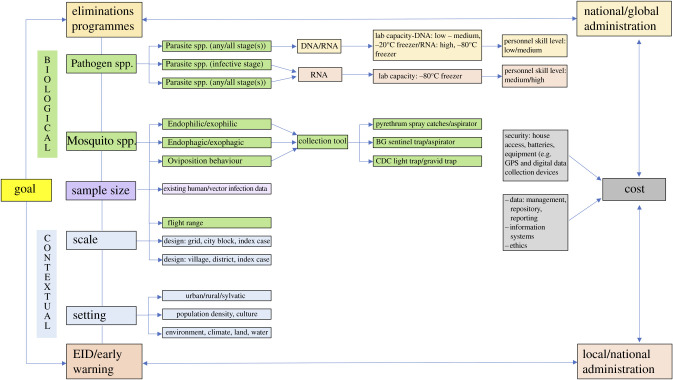
Given that MX can enhance surveillance systems for multiple diseases under elimination (e.g. LF, malaria) as well as contribute to early warning systems of newly emerging and cyclical infections (e.g. arboviruses), factors that require consideration when designing MX systems are discussed below. Broadly, these relate to the overall goal and target of the MX system defined by the programme, biological factors (e.g. the mosquito and pathogen under surveillance) and the context (e.g. setting, human population) and are represented in figure 1.
Figure 1.
Considerations for MX system development.
(a). Goal: diseases that are under elimination, emerging or both
Prior to MX system development, it is advantageous to take an assessment of the local vector/pathogen landscape and decide if the MX system will target one or multiple vector/pathogens. An MX system designed for disease elimination could have very different constraints than one for detecting EIDs. For instance, in the former, there may be a necessity to adhere to global elimination programme design, endpoints and outputs, while in the latter MX systems may vary greatly based on local priorities and politics around EIDs. Although it may be possible to design flexible MX systems capable of shifting priorities and resources according to demand, at minimum MX systems require significant upstream planning to ensure robust data over time and appropriate curation of data and samples (e.g. a biorepository).
(b). Biological factors
(i). Vector and pathogen characteristics
One of the most important challenges in MX system development relates to the target vector(s) and pathogen(s). Species within each of the three principal genera of mosquitoes vectoring human diseases, Culex, Aedes and Anopheles, differ in their behaviours (host-seeking, resting and oviposition) and preferred habitats (domestic, peri-domestic or sylvatic), which influence the ideal tool and method of collection. Their flight ranges influence how far to space geographically linked sentinel surveillance sites (which may be affected by their infection status, the type of pathogen and pathogen load). MX systems for chronic parasitic infections (e.g. LF) that persist over years in human and vector populations may be more flexible in terms of temporal collection than those for the more typically explosive epidemic viruses. Since many arboviruses circulate in discrete seasonal patterns, MX systems must consider seasonal characteristics and be particularly sensitive to new introductions (e.g. new serotypes, viruses).
(ii). Mosquito collection tools and methods
Table 1 illustrates different mosquito collection methods used in previous MX systems. It is well established that each preferentially selects mosquitoes displaying different types of behaviour and yields vary between species and trap placement/collection site. Furthermore, utilization often depends on local preferences of different field teams [1,56].
For Cx. quinquefasciatus, in general, CDC GTs baited with odorous infusions exploiting oviposition behaviour are placed outside of houses and are the preferred method for collecting adult females. If the purpose of MX is to detect whether LF transmission is taking place within a community, then mosquito infection prevalence in females with undigested bloodmeals collected using CDC LTs placed inside houses may be useful, particularly since placing the traps indoors is more likely to select anthropophilic mosquitoes and W. bancrofti is anthroponotic. However, placing LTs in houses of individuals, who may or may not be infective to mosquitoes, may not provide representative estimates of LF prevalence in an entire community [23]. By contrast, an MCM comparison in the RMR of Brazil found that large battery-powered aspirators exploiting host-seeking behaviour collected significantly more Cx. quinquefasciatus females (38 times more blood-fed and 5 times more gravid females, as well as 2.6 times the total population of mosquitoes) than CDC LTs placed inside houses [25]. Moreover, residents preferred aspirators over fixed battery or AC-powered traps (e.g. CDC light or BGS) due to lower risk of battery theft, power cuts and the nuisance of light and noise in the bedroom at night.
For Ae. polynesiensis, methods exploiting host-seeking behaviour were preferred in Samoa for collecting outdoor biting adult female mosquitoes. A study comparing BGS traps, human-baited collections (HBCs) and UV LED CDC LTs found that baited BGS traps collected more mosquitoes than CDC LTs or HBCs, but there was no difference in the prevalent filarial infection rate between them. In addition, given that they are more ethically acceptable than HBCs, BGS traps were recommended [35]. For Anopheles mosquitoes, MCMs exploiting indoor resting behaviour such as PSCs inside houses are generally used in MX [ 22,24], but CDC LTs exploiting host-seeking behaviour (e.g. baited with CO2 or placed next to people sleeping under impregnated bed nets) have also been used in other studies [56].
It is crucial that the MX investigator or programme officer understands the likely targets of each MCM in order to capture the mosquito population that is most relevant to the work's objectives. For example, most post-MDA MX surveys for LF detect parasite DNA from any stage in the mosquito, but in order to verify when transmission has been interrupted, it is important to detect the L3 infective stage. MX sensitivity will be influenced by the MCM adopted: in the absence of transovarial transmission, nulliparous females that have never taken a bloodmeal will not contain any human pathogen DNA. If the MCM preferentially targets host-seeking females, it may catch a significant proportion of nulliparous females, as well as parous females, so may result in lower infection rates. However, an MCM that preferentially selects gravid females should result in a higher proportion of parous females and higher estimates of infection (containing any stage of parasite) and infectiveness (containing the infective stage to humans). Collecting mosquitoes resting indoors should result in a higher proportion of previously blood-fed females (possibly either infected or infective), but not all mosquito species are endophilic [23].
Similarly, for arbovirus surveillance, selection of an optimal trapping method is crucial in order to maximize the sensitivity of virus detection and improve cost-effectiveness [57]. A review focusing on MCMs specifically for arbovirus surveillance provided guidance on advantages and disadvantages of each method [58]. MCMs predominantly exploiting host-seeking behaviour (e.g. CDC light and BGS traps) may provide larger collections, particularly when CO2 is used as an attractant, than those exploiting resting or oviposition behaviour, but they require batteries or AC power [58]. Furthermore, obtaining supplies of baits such as CO2 is a constraint. Collecting resting mosquitoes using battery-powered aspirators provides proportionally more blood-fed females [59], but the total number of mosquitoes captured tends to be lower and the method also relies upon battery procurement. Ovitraps and GTs exploiting oviposition behaviour increase the likelihood of detecting positive pools, but collections tend to be smaller than the other MCMs [58].
(iii). Sample size
Establishing the appropriate sample size for a given MX system depends on the goal of the MX system. In an elimination context, the system must operate under strict criteria to establish if elimination is nearing, has been achieved, or has been sustained. However, the system may be more exploratory for many EID surveillance systems. Sample size calculations depend on estimates from previously existing literature on vector abundance and infection prevalence (ideally from the same, nearby or characteristically similar sites). Thus, they are also dependent on the vector–pathogen combinations to be monitored. Nevertheless, in general, some common assumptions apply. For areas where infection prevalence in the human population is high, it can be assumed that infection in the vector will be analogously high, thereby requiring fewer mosquitoes than if the human infection prevalences were low. By contrast, in areas where elimination activities are underway and human and mosquito infection prevalences have or can be assumed to have decreased, significantly more mosquitoes will be required to detect low infection levels than if infection levels were high.
A preliminary MX protocol for collecting resting, blood-fed Cx. quinquefasciatus mosquitoes recommended a sample size of greater than 3000 freshly blood-fed females [8]. This falls within the range for detecting low infection thresholds suggested through later modelling work [16]. However, the numbers of mosquitoes required to obtain accurate filarial prevalence rates in different geographical settings post-MDA depend on vector efficiency and may be as high as 5000–7500 Cx. quinquefasciatus, 2500 Anopheles and 22 000 Aedes females [31]. The sample size required to estimate an infection rate of 1% with a power of 0.80 was calculated as 2000 Anopheles females per implementation unit in Togo [24]. Other studies have set higher targets, but collecting and analysing over 10 000 mosquito females has proved challenging [33].
If, however, an MX system is being designed for EIDs, especially where this is little pre-existing data apart from perhaps vector abundance, then sample size considerations are entirely different and in such cases may not even apply in the traditional sense of needing a particular sample size to detect a difference in means. However, some important considerations may still apply related to adequate samples of mosquitoes needed to detect infection; these may relate to the likelihood of a given pathogen to be detected in a given vector species. For example, studies have estimated that it is extremely difficult to detect arboviruses in mosquitoes, even if greater than 1600 mosquitoes are collected [60,61]. It is not as simple as collecting more mosquitoes, MX systems must also consider how these mosquitoes are pooled for screening; in general, the larger the mosquito collection and more mosquitoes tested (including potentially varying the pool sizes), the greater the likelihood of detecting infection [62,63].
It should be noted that the effort required to obtain the desired sample size depends on several factors such as the season and time of day, e.g. it may be easier to collect high numbers after rainy season when mosquitoes are most abundant. Furthermore, some locations are more productive than others, and this may relate to factors such as sanitation. However, collecting higher numbers does not guarantee the ability to detect infection for diseases such as arboviruses or parasitic diseases in areas of low prevalence, and a cost–benefit evaluation should always be considered.
(iv). Timing: seasonality and time of collection
It is crucial that MX systems consider logistical and practical factors about the best time to optimize collections. This includes taking into account the seasonality of vector human biting for optimum yield, and the best strategy for trap placement in order to account for the large variation in household densities between clusters/villages in EUs [33] as well as the time of day when collections should occur.
Densities of a wide range of vectors and pathogens vary by season and time of year. Rainy and dry seasons may fluctuate not only by month but also geographically within the same country. Furthermore, vector densities often peak just after the rainy season, while pathogen densities may vary based on other factors more closely related to human characteristics (e.g. immunity) and behaviours. For parasitic diseases such as LF, there does not appear to be any discordance between detecting pathogen in the mosquito and human as the parasite may theoretically be detected at any point. For arboviruses, however, there appear to be patterns of arbovirus circulation in the human population (as evidenced by reported clinical disease and human laboratory-confirmed infection) and these do not always correspond to periods of peak vector abundance. For example, in the RMR, Brazil, peak mosquito abundance is usually just after the rainy season in the months of July–September; however, peak arboviral infection in humans has typically been detected November–March over the past five years (ZIKV, CHIKV) to decades (DENV) [25].
In addition to seasonality, optimal ‘time’ of the day for collections is an extremely important consideration since it relates closely to the behaviours of each mosquito species being targeted. For example, Cx. quinquefasciatus tend to bite at night, and blood-fed engorged females tend to rest on the walls so are ideally collected in the mornings. By contrast, Ae. aegypti bite during the day and are notoriously difficult to catch. Finally, many Anopheles spp. seek hosts at roughly dawn and dusk. Thus, an integrated MX system targeting more than one vector may need to be flexible and shift ideal collection periods per day towards a compromise between those time periods that may overlap and capture a greater variety of species (e.g. later in the morning than ideal for Cx. quinquefasciatus, and earlier in the day than ideal for Ae. aegypti).
(v). Scale and method of deployment
Accurate information concerning the spatial and temporal distribution of infected vectors could permit stakeholders to roll out pre-emptive public health interventions [64]. Maximizing trap number and placement to obtain accurate spatial information are paramount, with the former more related to vector and pathogen characteristics such as likely infection prevalence and the latter ideally guided by unique vector and environmental factors such as flight range and oviposition site availability. The scale and method of deployment depend on the purpose of the MX system.
For elimination contexts, particularly related to detecting elimination or recrudescence, it is best to design a system that covers an area as geographically wide and heterogeneous as possible. In such cases, the next consideration becomes the method of deployment; whereas rural sites depended on large-scale approaches such as random village sampling across districts or similar, urban sites have employed more granular approaches, leveraging city blocks or developing fixed geospatial grids.
In an MX study in two Sri Lankan EUs to investigate whether the number of trap sites where CDC GTs were placed affected the mosquito infection prevalence estimates obtained, mosquito infection rates did not significantly differ according to whether 75, 150 or 300 trap sites were used [28]. On mainland India, CDC GTs were placed at different densities using a sampling interval proportional to the number of households in PHCs versus a hotspot site within one PHC to determine optimum household-based MX sampling strategy for post-MDA surveillance [31]. Considering the spatial scale and transmission dynamics is also crucial when designing grid-based MX systems [25]. A recent systematic review on the use of mapping and monitoring for LF elimination concluded that MX could play a major role in monitoring and evaluation for LF, and that MX surveys could be of great value for integrated mapping of several VBDs with overlapping distributions [65].
For EID-focused MX systems, the approaches may vary widely, particularly when unidentified reservoir hosts are involved in transmission. Similar to MX for elimination, and if unconstrained by resources, such systems would ideally cover as wide a geographic area as possible and incorporate a wide variety of different types of sampling points (e.g. based on land-use, altitude, etc.). This should enable the detection of zoonotic as well as anthroponotic transmission [ 47,48]. However, in resource-limited situations, other options exist such as using index case methods and human hotspots (as detected by clinical disease or laboratory-confirmed infections) to guide where MX sampling points should be placed. Of course, an ideal MX system for EIDs would incorporate both a wide geographic area as well as hotspots into a complete MX platform offering basic (e.g. based on a grid) as well as enhanced (e.g. based on hotspots) VBD/EID surveillance.
(vi). Molecular tools and analysis
Molecular tools have improved significantly since the development of a simple DNA extraction method for high-throughput detection of W. bancrofti in mosquitoes [17]. For example, a backpack PCR system was developed in an attempt to provide a novel, simple field-based diagnostic platform for the detection of B. malayi DNA in pools of mosquitoes for MX [66]. It coupled a rapid and inexpensive DNA extraction methodology with an amplification platform and test-strip-based detection assay to replace the need for more expensive instrumentation and laboratory-based infrastructure increasing the feasibility of MX for LF surveillance, particularly in resource-constrained settings. The system may be adapted to allow for a parallel detection of W. bancrofti [66].
The high-throughput method for collecting excreta from a large numbers of mosquitoes then screening samples for arboviral, filarial and malarial DNA is promising even if it requires the development of new strategies for sample collection [50,67]. Techniques for testing mosquito saliva using honey-baited nucleic acid preservation cards or sugar bait stations for arboviruses provide evidence of pathogen transmission, not possible by processing pools, and are an alternate and sensitive method to mitigate against the problems of low prevalence of infection in mosquitoes, expensive labour costs and the need for specialized equipment [64,68]. A similar approach was used to detect malaria sporozoites in Anopheles [69]. An alternative cost-effective high-throughput method to replace ELISA and PCR for detecting Plasmodium in Anopheles used near-infrared spectroscopy [70]. Validation of these tools is ongoing, but the prospect of next-generation sequencing to analyse the virome of mosquito excreta for virus discovery as well as for arbovirus surveillance shows great promise [71].
(c). Context-driven factors
(i). Setting
The setting and geographic characteristics of where the MX system will be deployed is one of the most important factors to consider, including type of setting (urban, rural or mixed setting), continuous area or focal, human population density, residential layout, household structure (low, medium or high rise), elevation (flat, elevated, or mixed elevation), land-use and water (e.g. flooding risk, mosquito seasonality in relation to water availability for oviposition). One factor that cannot be underestimated is the issue of security, including if the study area is suitably safe for field teams to access and deploy tools, such as mosquito traps and geo-referencing devices, that use precious resources such as batteries (a target for theft for repurposing in many settings).
(ii). Local human population and health system
The local population must be considered when it comes to designing, deploying and successfully running MX systems. Local preferences and acceptability of MX systems are largely driven by experience with and education on VBDs, including the tremendous physical toll and dangers posed. Willingness to participate and acceptability of various MX tools and methods (included repeated visits and sustained contact with health officials conducting MX) often depend on buy-in from local health authorities, community leaders and cultural norms. For instance, many Latin American countries tend to favour a ‘bottom-up’ approach with participatory methods for vector control. However, the majority of Southeast Asian countries tend to operate with a ‘top-down’ approach where governments direct and manage vector control and surveillance without participation from the local populations (indeed, some countries accomplish this via social pressure and fines). It is crucial to understand the local population, cultural norms, acceptability of various MX tools and methods, and how local public health officials operate and exist with the local population in order to design appropriate MX systems for a given context. Other issues to consider include population migration, integrated vector management practices (e.g. water storage, use of bed nets, use of biological or chemical control) and socio-economic factors (e.g. seasonal workers or those who are never home during MX team work hours are not ideal targets for longitudinal MX systems that must use domestic space).
(iii). Cost
Since the realization that MX can serve as an effective non-invasive, sensitive monitoring and evaluation tool of VBDs in low prevalence settings [31], a few studies have looked at its cost-effectiveness in providing additional supplemental data for disease surveillance, but data are limited. In general, the most cost-effective sampling strategy will be site-specific, and will differ between countries [24]. In Togo, the financial resource factors taken into account when designing an MX sampling strategy were (i) budget, (ii) transport costs, (iii) daily allowances for personnel (entomologists, community volunteers and supervisors), and (iv) procurement of consumables and materials. The total estimated cost of the survey was $35 910 USD. Most of the outlay involved costs for training personnel, field mosquito collections and sample processing, but other costs included administration and vehicle maintenance. Taking into account additional donated reagents (∼$3000.00 USD), the mean approximated cost spent per village was $1051 USD (including personnel and sample processing) [24]. In Ghana, where the feasibility of using trained community vector collectors with minimal supervision to provide a low-cost, scaled-up MX was assessed, the total estimated annual cost in four districts was $30 042 USD. The majority (79.8%) was spent on personnel costs. Of the remaining capital costs, 88.7% was spent on transport and 11.3% on equipment [72]. In India, the cost components of MX surveys and TAS per EU were estimated and compared: $14 104 USD and $14 259 USD, respectively [40]. Similar to the above studies, the major cost component was personnel (63.6%), followed by transportation (17.1%). By contrast, the major cost component for TAS was supplies (60.5%, mostly for ICT), followed by personnel (37.5%) and transportation (2%) [40].
Costs for MX vary greatly, depending on factors such as local infrastructure and human capacity (e.g. the need for reference laboratories with trained staff) and the ability to procure tools and materials at concessionary prices. They also vary depending on specific laboratory costs associated with some types of MX (e.g. detecting RNA arboviruses via RT-PCR, requiring −80°C freezers) versus others (e.g. detecting W. bancrofti DNA via PCR, requiring −20°C freezers).
There is little information on the potential cost-effectiveness or cost benefit of MX as a supplementary tool to human infection surveillance. This is an area that needs much consideration and dialogue. For instance, it is possible that if research demonstrates the relative impact of MX for a much lower relative cost than human-focused surveillance, such evidence would be extremely influential in the policy space to further recommend MX (including, perhaps, the direct provision for its funding). This is especially important for diseases under elimination, including those heading towards the ‘endgame’ where human surveillance alone will become unfeasible at some point in the not too distant future.
(iv). Infrastructure
Local infrastructure (including continuity of funding for maintenance and improvement) is extremely important for considering long-term MX systems, especially those dependent on sample processing and storage. Field collection of mosquitoes may not vary by site, but the ability to conduct molecular surveillance is entirely dependent on adequate laboratories. For example, while a marker for infection, detection of Plasmodium spp. by dissection of Anopheles spp. is not technically MX if DNA or RNA is not being detected. Adequate laboratory facilities with appropriate equipment are crucial for long-term, standardized MX systems, and these include everything from freezers for sample storage to processing tools for maceration to PCR and sequencing machines; generators are essential. If insufficient laboratory capacity exists, including due to lack of a consistent electrical source, MX can still be conducted by using reference laboratories provided there are means to store and send samples in appropriate conditions to conserve genetic material (e.g. dry ice, FTA cards or solvents such as RNA later).
(v). Human capacity
Sufficient numbers of trained staff are required in order to carry out MX. This includes experienced entomologists, insect collectors and community volunteers who, when required for fieldwork, would need training on the best practices for mosquito collection and sample handing as well as supervision during the actual collections. Logistical considerations also need to be taken into account such as security and safety and venues for training workshops, meals and transportation allowances [24]. In addition to technical expertise in mosquito identification (especially for integrated disease surveillance and MX systems focused on EIDs), trained molecular biologists and laboratory facilities are required for processing the samples in the health system. Capacity building of human personnel, particularly for laboratory strengthening, is also essential [40].
5. Implications, future prospects and recommendations for molecular xenomonitoring
MX as a field has come a long way from its theoretical beginnings to the real-life applications employed for a variety of vectors and pathogens today. The implications of using MX for VBD surveillance are enormous. On the one hand, it can and is being used in elimination programmes to monitor progress and infection resurgence, thereby averting recrudescence. On the other, it can and is being used (albeit not by the name ‘MX’) in EID programmes focused on VBDs. In some sites, it is being developed for use in integrated programmes, thereby saving costs by leveraging tools, methods, staff, and laboratory space and capacity across multiple diseases at the same time. Currently, zoonoses are being monitored in various animals such as birds, bats and primates with significant investment by governments that have been particularly impacted (e.g. Latin America after multiple YFV outbreaks). Similarly, there is great potential for mosquito surveillance via MX systems to become standard, necessary investments to safeguard public health across the world, at once helping guarantee elimination of debilitating and fatal diseases (e.g. LF, malaria) as well as averting explosive epidemics (e.g. DENV, YFV) and EIDs (e.g. CHIKV, ZIKV).
Ideally, the programmatic community could move away from thinking of MX simply as a supplement to considering it as a possible replacement for human surveillance. However, for that to happen, a better understanding of the relationship between pathogen-infection rates in human and mosquito populations is required. Those data may no longer be obtainable due to the success of disease interventions for which MX is being used. For example, for both LF and malaria, large-scale and consistent elimination programmes deploying highly effective tools have effectively pushed human infection prevalence below thresholds to where it is infeasible to conduct large-scale, longitudinal human studies simply to establish corresponding relationships with mosquito infection prevalence. Nevertheless, there is an opportunity for these relationships to be established for certain EIDs (e.g. CHIKV, ZIKV).
Additionally, the research community still needs consensus on consistent terminology, as well as more information sharing to expand our knowledge, to continue to push towards new frontiers of tool and methodological development and deployment. Two broader concepts should be considered: (1) while several malaria and arboviral researchers have conducted MX in its basic forms, few have used terms related to MX, XS, or even included the words ‘monitoring’ or ‘surveillance’ when describing their overall pathogen screening approaches that incorporate mosquitoes as a proxy for human infection or disease; and (2) although not the focus of this article, it is important to recognize that many other non-mosquito arthropods, e.g. triatomine bugs, tsetse flies and sandflies, have been used for pathogen screening and have contributed greatly to a better understanding of VBDs such as Chagas disease, human African trypanosomiasis and leishmaniasis. Thus, there is great potential not only for mosquito-focused researchers to share knowledge and have some common consensus on MX (from tools and methods to terminology) but also to consider consultation on MX with the wider VBD community.
In conclusion, mosquito infection surveillance as a proxy for human disease has been occurring for nearly half a century via dissection methods to visualize the parasites of malaria and LF. Dramatic advances in molecular methods over the past two decades have provided researchers and programmers working on vector-borne parasitic diseases, as well as their arboviral disease counterparts, access to highly sensitive, high-throughput resources. Indeed, the increasing focus on arboviruses and emerging pathogens, often translated into massive infusions of rapid funding that has more recently eluded classical parasitological diseases, has allowed some researchers and programmatic officers focusing on MX to leverage the resources from one class of diseases towards another. Whether in relation to diseases under elimination or those that are emerging, there are multiple indications that the vector-borne disease community is entering a new phase of MX. In that case, it is hoped that some of the concepts and recommendations presented above may help researchers and programme managers alike to develop or optimize MX systems for the larger vector-borne disease community and, as always, for the benefit of public health.
Acknowledgements
The authors are grateful to their Faculties at the London School of Hygiene & Tropical Medicine for salary support and to Dr Miguella Mark-Carew for her constructive comments on the draft manuscript. We also appreciate the time invested by two independent reviewers and thank them for their valuable suggestions to improve the final submission.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors have made substantial contributions to the conception and writing of this opinion article, including analysis and interpretation of literature, and have given their final approval of the version to be published.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.WHO. 2013. Lymphatic filariasis: a handbook of practical entomology for national lymphatic filariasis elimination programmes, p. 14 Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Fauver JR, Gendernalik A, Weger-Lucarelli J, Grubaugh ND, Brackney DE, Foy BD. 2017. The use of xenosurveillance to detect human bacteria, parasites, and viruses in mosquito bloodmeals. Am. J. Trop. Med. Hyg. 97, 324–329. ( 10.4269/ajtmh.17-0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubaugh ND, et al. 2015. Xenosurveillance: a novel mosquito-based approach for examining the human-pathogen landscape. PLoS Negl. Trop. Dis. 9, e0003628 ( 10.1371/journal.pntd.0003628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molyneux DH, Taylor MJ. 2001. Current status and future prospects of the Global Lymphatic Filariasis Programme. Curr. Opin Infect. Dis. 14, 155–159. ( 10.1097/00001432-200104000-00008) [DOI] [PubMed] [Google Scholar]
- 5.Zeldenryk LM, Gray M, Speare R, Gordon S, Melrose W. 2011. The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl. Trop. Dis. 5, e1366 ( 10.1371/journal.pntd.0001366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams SA, et al. 2002. Development and standardization of a rapid, PCR-based method for the detection of Wuchereria bancrofti in mosquitoes, for xenomonitoring the human prevalence of bancroftian filariasis. Ann. Trop. Med. Parasitol. 96(Suppl. 2), S41–S46. ( 10.1179/000349802125002356) [DOI] [PubMed] [Google Scholar]
- 7.WHO. 2002. Defining the roles of vector control and xenomonitoring in the Global Programme to Eliminate Lymphatic Filariasis. Report no. 2002.3. Geneva, Switzerland: WHO/CDS/CPE/PVC.
- 8.Chadee DD, Williams SA, Ottesen EA. 2002. Xenomonitoring of Culex quinquefasciatus mosquitoes as a guide for detecting the presence or absence of lymphatic filariasis: a preliminary protocol for mosquito sampling. Ann. Trop. Med. Parasitol. 96, S47-S53. ( 10.1179/000349802125002365) [DOI] [PubMed] [Google Scholar]
- 9.Farid HA, Morsy ZS, Helmy H, Ramzy RMR, El Setouhy M, Weil GJ. 2007. A critical appraisal of molecular xenomonitoring as a tool for assessing progress toward elimination of lymphatic filariasis. Am. J. Trop. Med. Hyg. 77, 593–600. ( 10.4269/ajtmh.2007.77.593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gad AM, et al. 2007. Gravid trap collections of Culex pipiens for molecular xenomonitoring national programs for elimination of lymphatic filariasis. Am. J. Trop. Med. Hyg. 77, 104. [Google Scholar]
- 11.Ramzy RM, El Setouhy M, Helmy H, Farid HA, Gad AM, Weil GJ. 2007. Has Egypt eliminated lymphatic filariasis. Am. J. Trop. Med. Hyg. 77, 104. [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers EW, et al. 2009. Xenomonitoring of Wuchereria bancrofti and Dirofilaria immitis infections in mosquitoes from American Samoa: trapping considerations and a comparison of polymerase chain reaction assays with dissection. Am. J. Trop. Med. Hyg. 80, 774–781. ( 10.4269/ajtmh.2009.80.774) [DOI] [PubMed] [Google Scholar]
- 13.Erickson SM, Fischer K, Weil GJ, Christensen BM, Fischer PU. 2009. Distribution of Brugia malayi larvae and DNA in vector and non-vector mosquitoes: implications for molecular diagnostics. Parasit. Vectors 2, 56 ( 10.1186/1756-3305-2-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intapan PM, Thanchomnang T, Lulitanond V, Maleewong W. 2009. Rapid detection of Wuchereria bancrofti and Brugia malayi in mosquito vectors (Diptera: Culicidae) using a real-time fluorescence resonance energy transfer multiplex PCR and melting curve analysis. J. Med. Entomol. 46, 158–164. ( 10.1603/033.046.0119) [DOI] [PubMed] [Google Scholar]
- 15.Rao RU, Huang YF, Bockarie MJ, Susapu M, Laney SJ, Weil GJ. 2009. A qPCR-based multiplex assay for the detection of Wuchereria bancrofti, Plasmodium falciparum and Plasmodium vivax DNA. Trans. R Soc. Trop. Med. Hyg. 103, 365–370. ( 10.1016/j.trstmh.2008.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen EM, Stolk WA, Laney SJ, Michael E. 2009. The role of monitoring mosquito infection in the global programme to eliminate lymphatic filariasis. Trends Parasitol. 25, 319–327. ( 10.1016/j.pt.2009.03.013) [DOI] [PubMed] [Google Scholar]
- 17.Vasuki V, Subramanian S, Hoti SL, Jambulingam P. 2012. Use of a simple DNA extraction method for high-throughput detection of filarial parasite Wuchereria bancrofti in the vector mosquitoes. Parasitol. Res. 111, 2479–2481. ( 10.1007/s00436-012-3026-3) [DOI] [PubMed] [Google Scholar]
- 18.Thanchomnang T, et al. 2013. Rapid detection and identification of Wuchereria bancrofti, Brugia malayi, B. pahangi, and Dirofilaria immitis in mosquito vectors and blood samples by high resolution melting real-time PCR.. Korean J. Parasitol. 51, 645–650. ( 10.3347/kjp.2013.51.6.645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyab AK, Galal LA, Mahmoud AE, Mokhtar Y. 2015. Xenomonitoring of different filarial nematodes using single and multiplex PCR in mosquitoes from Assiut Governorate, Egypt. Korean J. Parasitol. 53, 77–83. ( 10.3347/kjp.2015.53.1.77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu BK, et al. 2013. Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl. Trop. Dis. 7, e2584 ( 10.1371/journal.pntd.0002584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilotte N, Unnasch TR, Williams SA. 2017. The current status of molecular xenomonitoring for lymphatic filariasis and onchocerciasis. Trends Parasitol. 33, 788–798. ( 10.1016/j.pt.2017.06.008) [DOI] [PubMed] [Google Scholar]
- 22.Owusu IO, de Souza DK, Anto F, Wilson MD, Boakye DA, Bockarie MJ, Gyapong JO. 2015. Evaluation of human and mosquito based diagnostic tools for defining endpoints for elimination of Anopheles transmitted lymphatic filariasis in Ghana. Trans. R Soc. Trop. Med. Hyg. 109, 628–635. ( 10.1093/trstmh/trv070) [DOI] [PubMed] [Google Scholar]
- 23.Irish SR, Stevens WMB, Derua YA, Walker T, Cameron MM. 2015. Comparison of methods for xenomonitoring in vectors of lymphatic filariasis in Northeastern Tanzania. Am. J. Trop. Med. Hyg. 93, 983–989. ( 10.4269/ajtmh.15-0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorkenoo MA, et al. 2018. Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo: no evidence for active transmission. Parasit. Vectors 11, 52 ( 10.1186/s13071-017-2611-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh A, et al. 2018. Development of an urban molecular xenomonitoring system for lymphatic filariasis in the Recife Metropolitan Region, Brazil. PLoS Negl. Trop. Dis. 12, e0006816 ( 10.1371/journal.pntd.0006816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moustafa MA, Salamah MMI, Thabet HS, Tawfik RA, Mehrez MM, Hamdy DM. 2017. Molecular xenomonitoring (MX) and transmission assessment survey (TAS) of lymphatic filariasis elimination in two villages, Menoufyia Governorate, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1143–1150. ( 10.1007/s10096-017-2901-3) [DOI] [PubMed] [Google Scholar]
- 27.Rao RU, Nagodavithana KC, Samarasekera SD, Wijegunawardana AD, Premakumara WDY, Perera SN, Settinayake S, Miller JP, Weil GJ. 2014. A comprehensive assessment of lymphatic filariasis in Sri Lanka six years after cessation of mass drug administration. PLoS Negl. Trop. Dis. 8, e3281 ( 10.1371/journal.pntd.0003281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao RU, et al. 2016. Programmatic use of molecular xenomonitoring at the level of evaluation units to assess persistence of lymphatic filariasis in Sri Lanka. PLoS Negl. Trop. Dis. 10, e0004722 ( 10.1371/journal.pntd.0004722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RU, et al. 2017. Reassessment of areas with persistent lymphatic filariasis nine years after cessation of mass drug administration in Sri Lanka. PLoS Negl. Trop. Dis. 11, e0006066 ( 10.1371/journal.pntd.0006066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao RU, Samarasekera SD, Nagodavithana KC, Goss CW, Punchihewa MW, Dassanayaka TDM, Ranasinghe USB, Mendis D, Weil GJ. 2018. Comprehensive assessment of a hotspot with persistent bancroftian filariasis in coastal Sri Lanka. Am. J. Trop. Med. Hyg. 99, 735–742. ( 10.4269/ajtmh.18-0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian S, et al. 2017. Application of a household-based molecular xenomonitoring strategy to evaluate the lymphatic filariasis elimination program in Tamil Nadu, India. PLoS Negl. Trop. Dis. 11, e0005519 ( 10.1371/journal.pntd.0005519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatri V, Amdare N, Chauhan N, Togre N, Reddy MV, Hoti SL, Kalyanasundaram R. 2019. Epidemiological screening and xenomonitoring for human lymphatic filariasis infection in select districts in the states of Maharashtra and Karnataka, India. Parasitol. Res. 118, 1045–1050. ( 10.1007/s00436-019-06205-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irish SR, et al. 2018. Molecular xenomonitoring for Wuchereria bancrofti in Culex quinquefasciatus in two districts in Bangladesh supports transmission assessment survey findings. PLoS Negl. Trop. Dis. 12, e0006574 ( 10.1371/journal.pntd.0006574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmaedick MA, Koppel AL, Pilotte N, Torres M, Williams SA, Dobson SL, Lammie PJ, Won KY. 2014. Molecular xenomonitoring using mosquitoes to map lymphatic filariasis after mass drug administration in American Samoa. PLoS Negl. Trop. Dis. 8, e3087 ( 10.1371/journal.pntd.0003087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hapairai LK, et al. 2015. Evaluation of traps and lures for mosquito vectors and xenomonitoring of Wuchereria bancrofti infection in a high prevalence Samoan village. Parasit. Vectors 8, 287 ( 10.1186/s13071-015-0886-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beng TS, et al. 2016. Molecular xenomonitoring of filarial infection in Malaysian mosquitoes under the national program for elimination of lymphatic filariasis. Southeast Asian J. Trop. Med. Public Health. 47, 617–624. [Google Scholar]
- 37.Vincent NJ, et al. 2017. Xenomonitoring of Wuchereria bancroftii infection before and after mass drug administration in Drekikier, Papua New Guinea. Am. J. Trop. Med. Hyg. 95, 57. [Google Scholar]
- 38.Irish SR, Moore SJ, Derua YA, Bruce J, Cameron MM. 2013. Evaluation of gravid traps for the collection of Culex quinquefasciatus, a vector of lymphatic filariasis in Tanzania. Trans. R Soc. Trop. Med. Hyg. 107, 15–22. ( 10.1093/trstmh/trs001) [DOI] [PubMed] [Google Scholar]
- 39.Rao RU, Samarasekera SD, Nagodavithana KC, Punchihewa MW, Ranasinghe USB, Weil GJ. 2019. Systematic sampling of adults as a sensitive means of detecting persistence of lymphatic filariasis following mass drug administration in Sri Lanka. PLoS Negl. Trop. Dis. 13, e0007365 ( 10.1371/journal.pntd.0007365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian S, et al. 2020. Molecular xenomonitoring as a post-MDA surveillance tool for global programme to eliminate lymphatic filariasis: field validation in an evaluation unit in India. PLoS Negl. Trop. Dis. 14, e0007862 ( 10.1371/journal.pntd.0007862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gass K, et al. 2012. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate bancroftian filariasis. PLoS Negl. Trop. Dis. 6, e1479 ( 10.1371/journal.pntd.0001479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau CL, Won KY, Lammie PJ, Graves PM. 2016. Lymphatic filariasis elimination in American Samoa: evaluation of molecular xenomonitoring as a surveillance tool in the endgame. PLoS Negl. Trop. Dis. 10, e0005108 ( 10.1371/journal.pntd.0005108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau CL, Won KY, Lammie PJ, Graves PM. 2017. Lymphatic filariasis elimination in American Samoa: evaluation of molecular xenomonitoring as a surveillance tool in the endgame. Am. J. Trop. Med. Hyg. 95, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Berg H, Kelly-Hope LA, Lindsay SW. 2013. Malaria and lymphatic filariasis: the case for integrated vector management. Lancet Infect. Dis. 13, 89–94. ( 10.1016/S1473-3099(12)70148-2) [DOI] [PubMed] [Google Scholar]
- 45.Ramesh A, Jeffries CL, Castanha P, Oliveira PAS, Alexander N, Cameron M, Braga C, Walker T. 2019. No evidence of Zika, dengue, or chikungunya virus infection in field-caught mosquitoes from the Recife Metropolitan Region, Brazil, 2015. Wellcome Open Res. 4, 93 ( 10.12688/wellcomeopenres.15295.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kading RC, Biggerstaff BJ, Young G, Komar N. 2014. Mosquitoes used to draw blood for arbovirus viremia determinations in small vertebrates. PLoS ONE 9, e99342 ( 10.1371/journal.pone.0099342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann A, Nitsche A, Kohl C. 2016. Viral metagenomics on blood-feeding arthropods as a tool for human disease surveillance. Int. J. Mol. Sci. 17, 1743 ( 10.3390/ijms17101743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bitome-Essono PY, et al. 2017. Tracking zoonotic pathogens using blood-sucking flies as ‘flying syringes’. eLife 6, e22069 ( 10.7554/eLife.22069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nascimento J, et al. 2018. Use of anthropophilic culicid-based xenosurveillance as a proxy for Plasmodium vivax malaria burden and transmission hotspots identification. PLoS Negl. Trop. Dis. 12, e0006909 ( 10.1371/journal.pntd.0006909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilotte N, Zaky WI, Abrams BP, Chadee DD, Williams SA. 2016. A novel xenomonitoring technique using mosquito excreta/feces for the detection of filarial parasites and malaria. PLoS Negl. Trop. Dis. 10, e0004641 ( 10.1371/journal.pntd.0004641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minetti C, et al. 2019. Field evaluation of DNA amplification of human filarial and malaria parasites using mosquito excreta/feces. bioRxiv ( 10.1101/2019.12.23.880740) [DOI]
- 52.WHO. 2017. Global vector control response 2017–2030. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 53.Burakoff A, Lehman J, Fischer M, Staples JE, Lindsey NP. 2018. West Nile virus and other nationally notifiable arboviral diseases—United States, 2016. MMWR Morb. Mortal. Wkly Rep. 67, 13–17. ( 10.15585/mmwr.mm6701a3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.da Costa CF, Dos Passos RA, Lima JBP, Roque RA, de Souza Sampaio V, Campolina TB, Secundino N, Pimenta PFP. 2017. Transovarial transmission of DENV in Aedes aegypti in the Amazon basin: a local model of xenomonitoring. Parasit. Vectors 10, 249 ( 10.1186/s13071-017-2194-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maniero VC, Rangel PSC, Coelho LMC, Silva CSB, Aguiar RS, Lamas CC, Cardozo SV. 2019. Identification of Zika virus in immature phases of Aedes aegypti and Aedes albopictus: a surveillance strategy for outbreak anticipation. Braz. J. Med. Biol. Res. 52, e8339 ( 10.1590/1414-43120198339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silver JB. 2008. Mosquito ecology: field sampling methods, 3rd edn Dordrecht, The Netherlands: Springer. [Google Scholar]
- 57.Pezzin A, Sy V, Puggioli A, Veronesi R, Carrieri M, Maccagnani B, Bellini R. 2016. Comparative study on the effectiveness of different mosquito traps in arbovirus surveillance with a focus on WNV detection. Acta Trop. 153, 93–100. ( 10.1016/j.actatropica.2015.10.002) [DOI] [PubMed] [Google Scholar]
- 58.Ramirez AL, van den Hurk AF, Meyer DB, Ritchie SA. 2018. Searching for the proverbial needle in a haystack: advances in mosquito-borne arbovirus surveillance. Parasit. Vectors 11, 320 ( 10.1186/s13071-018-2901-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. 2009. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J. Med. Entomol. 46, 1256–1259. ( 10.1603/033.046.0602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu W, Lampman R, Novak RJ. 2003. Problems in estimating mosquito infection rates using minimum infection rate. J. Med. Entomol. 40, 595–596. ( 10.1603/0022-2585-40.5.595) [DOI] [PubMed] [Google Scholar]
- 61.Gu W, Novak RJ. 2004. Short report: detection probability of arbovirus infection in mosquito populations. Am. J. Trop. Med. Hyg. 71, 636–638. ( 10.4269/ajtmh.2004.71.636) [DOI] [PubMed] [Google Scholar]
- 62.Gu W, Lampman R, Novak RJ. 2004. Assessment of arbovirus vector infection rates using variable size pooling. Med. Vet. Entomol. 18, 200–204. ( 10.1111/j.0269-283X.2004.00482.x) [DOI] [PubMed] [Google Scholar]
- 63.Katholi CR, Unnasch TR. 2006. Important experimental parameters for determining infection rates in arthropod vectors using pool screening approaches. Am. J. Trop. Med. Hyg. 74, 779–785. ( 10.4269/ajtmh.2006.74.779) [DOI] [PubMed] [Google Scholar]
- 64.Flies EJ, Toi C, Weinstein P, Doggett SL, Williams CR. 2015. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector Borne Zoonotic Dis. 15, 397–403. ( 10.1089/vbz.2014.1759) [DOI] [PubMed] [Google Scholar]
- 65.Srividya A, Subramanian S, Jambulingam P, Vijayakumar B, Raja JD. 2019. Mapping and monitoring for a lymphatic filariasis elimination program: a systematic review. Res. Rep. Trop. Med. 10, 43–90. ( 10.2147/RRTM.S134186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaky WI, Tomaino FR, Pilotte N, Laney SJ, Williams SA. 2018. Backpack PCR: a point-of-collection diagnostic platform for the rapid detection of Brugia parasites in mosquitoes. PLoS Negl. Trop. Dis. 12, e0006962 ( 10.1371/journal.pntd.0006962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girod R, et al. 2016. Detection of Chikungunya virus circulation using sugar-baited traps during a major outbreak in French Guiana. PLoS Negl. Trop. Dis. 10, e0004876 ( 10.1371/journal.pntd.0004876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fontaine A, Jiolle D, Moltini-Conclois I, Lequime S, Lambrechts L. 2016. Excretion of dengue virus RNA by Aedes aegypti allows non-destructive monitoring of viral dissemination in individual mosquitoes. Sci. Rep. 6, 24885 ( 10.1038/srep24885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brugman VA, Kristan M, Gibbins MP, Angrisano F, Sala KA, Dessens JT, Blagborough AM, Walker T. 2018. Detection of malaria sporozoites expelled during mosquito sugar feeding. Sci. Rep. 8, 7545 ( 10.1038/s41598-018-26010-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maia MF, Kapulu M, Muthui M, Wagah MG, Ferguson HM, Dowell FE, Baldini F, Ranford-Cartwright L. 2019. Detection of Plasmodium falciparum infected Anopheles gambiae using near-infrared spectroscopy. Malar. J. 18, 85 ( 10.1186/s12936-019-2719-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramírez AL, et al. 2020. Metagenomic analysis of the virome of mosquito excreta. mSphere. 5, e00587-20 ( 10.1128/mSphere.00587-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pi-Bansa S, et al. 2018. Implementing a community vector collection strategy using xenomonitoring for the endgame of lymphatic filariasis elimination. Parasit. Vectors 11, 672 ( 10.1186/s13071-018-3260-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.