Abstract
Gene drives are selfish genetic elements that can be re-designed to invade a population and they hold tremendous potential for the control of mosquitoes that transmit disease. Much progress has been made recently in demonstrating proof of principle for gene drives able to suppress populations of malarial mosquitoes, or to make them refractory to the Plasmodium parasites they transmit. This has been achieved using CRISPR-based gene drives. In this article, I will discuss the relative merits of this type of gene drive, as well as barriers to its technical development and to its deployment in the field as malaria control.
This article is part of the theme issue ‘Novel control strategies for mosquito-borne diseases'.
Keywords: gene drive, mosquito, anopheles, CRISPR, transgenic, vector control
1. Introduction
Malaria is a disease transmitted exclusively through the bite of an infected female mosquito. As she takes a bloodmeal, an infected mosquito can release a few Plasmodium parasites into the peripheral circulation of the human host and these are sufficient to establish infection, allowing other mosquitoes to take up parasites in subsequent bites and complete the cycle. Of the 3500 or so mosquito species that exist though, only those within the Anopheles genus are actually capable of transmitting human malaria. Among these, about 40 species are capable of transmitting malaria at a level of major concern to public health [1,2]. Looking in more detail at sub-Saharan Africa, where the majority of the malaria burden falls, an even more restricted set of Anopheles species are responsible for vast proportion of disease transmission. This means that vector control–reducing the numbers of mosquito vectors in a given area—can focus on a small number of species yet still achieve large gains in reducing malaria transmission.
The use of vector control in reducing malaria transmission is best illustrated by the demonstration that the halving of malaria-related deaths, from close to a million per year in 2000 to approximately 450 000 in 2015, is largely attributable to the use of insecticide-treated bednets and indoor residual spraying of insecticide, which kill mosquitoes as they look to bite or when they rest on treated walls after biting, respectively [3]. Despite these large gains, progress in this area is stalling, largely owing to the widespread emergence of insecticide resistance [4]. In addition to the search for novel insecticide classes and formulations, as well as improvements to their deployment, there is a need for additional innovation in vector control. One such innovation is the idea of genetic control—the deliberate introduction of genetic traits into a population that will affect its ability to transmit disease, either by suppressing the population or by affecting the intrinsic capacity of the insect to host the disease agent.
2. Genetic control
Genetic control of insects involves the deliberate release of individuals containing some desirable (for the purposes of control) genetic trait in order to introduce this trait into the extant population via mating. Because mating is a very species-specific process and since the process relies on released individuals seeking out the target population this type of approach can be very targeted in its effect. The most widely used form of genetic control is the sterile insect technique, first proposed in the 1950s [5]. The technique relies on the mass rearing of large numbers of sterile males which outnumber the wild population locally and compete with wild-type males for female mates. It has been used successfully to control a wide range of agricultural pests as well as urban populations of Aedes aegypti mosquitoes that serve as vectors of the Dengue and yellow fever viruses [6–8]. Because the technique generally entails mass rearing on an industrial scale and constant release, it requires sustained investment and is best suited to the localized control of relatively contained populations, where problematic immigration of mated females from outside the release area is less likely.
The possibility of making transgenic mosquitoes has opened up new possibilities for genetic control. Indeed there are already examples of modified versions of the sterile insect technique, which rely on sterility being conferred by a transgene, having been deployed in the field successfully to suppress urban mosquito populations [9–12]. What is more, the availability of an assembled genome for many of the major malaria vectors, coupled with an ability to manipulate these genomes through modern genome editing tools, offers the possibility of identifying the genetic determinants of key traits such as reproduction, host-seeking behaviour and parasite susceptibility. This, in turn, offers the possibility to modify these traits in wild populations, but one crucial question remains: how do you spread these traits in a population? The solution to this is not immediately trivial—any of these traits are unlikely to confer a significant fitness benefit and therefore will not be selected, meaning that their frequency in a given population will not increase over time. In fact, many of these engineered traits may even incur a negative fitness effect, upon the mosquito at least.
In the case of sub-Saharan Africa, where the malaria burden is highest, malaria vectors are rurally distributed across large geographical ranges and it may not be feasible to release sufficient numbers of mosquitoes in order to reach an appreciable frequency in the target control area, at scale [13]—even for an introduced trait that were neutral to the mosquito one would have to release, at minimum, a quantity of mosquitoes far in excess of the wild-type into every village population. This means that without some other force to push these traits through a population they would be rapidly lost, either owing to selection or genetic drift, or both. Such a force can be provided by a ‘gene drive', which describes any genetic element that is able to bias its inheritance among offspring. Often, the strength of the bias in transmission is sufficiently extreme that the gene drive element can increase in frequency even if each individual element imposes a fitness cost on the host. For this reason, gene drives have been proposed as a form of genetic control that could introduce traits into a population over a rapid timeframe, even starting from a very low release frequency [14].
3. Gene drives in Anopheles mosquitoes
In looking to build synthetic versions of gene drives, inspiration has been taken from naturally occurring examples of this phenomenon. These can include sex distorter genes or chromosomes, genetic underdominance, toxin : antitoxin systems and other selfish genetic elements that make extra copies of themselves in the germline [14–21].
In malaria-carrying mosquitoes, one such example that has proved the most fruitful to date is that of the homing endonuclease genes (HEGs) [14], that will form the bulk of the discussion here, although many of the considerations arising can be applied more broadly to gene drives in general.
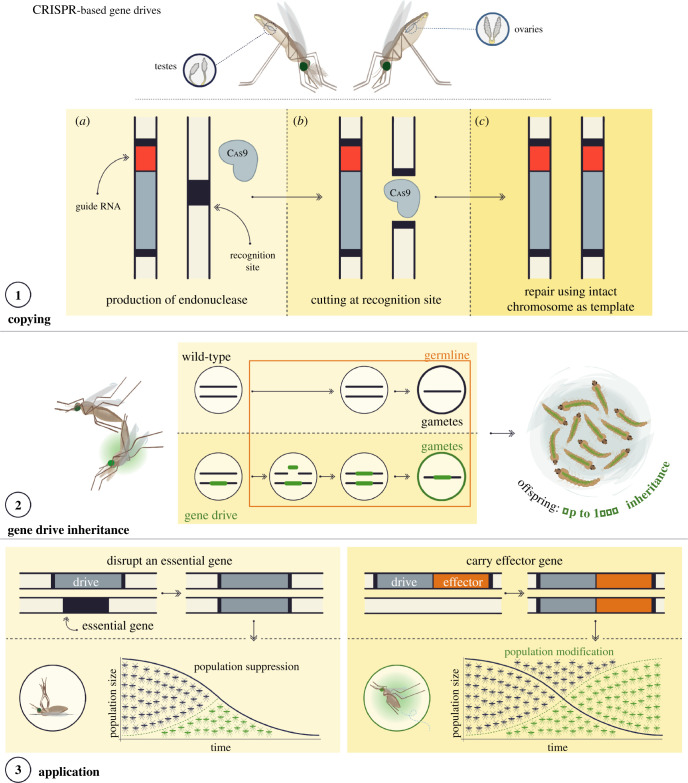
HEGs encode only a DNA endonuclease, which recognizes and cuts a unique DNA sequence. New endonuclease technologies such as CRISPR-Cas9, which are easier to re-programme to recognize a sequence of choice, have been repurposed to mimic the action of an HEG. Ordinarily an HEG resides on a chromosome within its own recognition sequence but, in a diploid organism, when this chromosome is paired with a chromosome that is void of the HEG and contains only the intact recognition sequence, that chromosome is cut, causing a double-stranded break in the DNA (figure 1, top). In repair of the broken strand, the loose DNA strands are resected slightly and anneal to the intact chromosome at regions of homology. The host cell DNA repair machinery uses this chromosome as a template on which to synthesize new DNA to ‘fill' the gap, in a process called homology-directed repair. In so doing it leads to ‘homing' of the HEG and a small amount of surrounding sequence so that it is now duplicated on both chromosomes. For homing to lead to a biased inheritance of the HEG it must occur in the cells that contribute to the next generation (the ‘germline' in multicellular organisms)—if it happens efficiently in the cells that become gametes, then an individual heterozygous for the HEG will produce a majority of gametes with a copy of the HEG and its associated trait, compared to the 50% that would be expected to receive it through normal Mendelian segregation (figure 1, middle). Therefore, the HEG can rapidly increase in frequency each generation.
Figure 1.
Mode of inheritance of CRISPR-based gene drives and their potential applications. Panel 1: the gene drive cassette consists of a source of Cas9 under a germline-specific promoter and a guide RNA designed to target the Cas9 to recognize and cut the target site on a wild-type chromosome. Because the gene drive is initially inserted within its own recognition site the chromosome containing the gene drive is immune from cleavage. Thus in an individual heterozygous for the gene drive, the wild-type chromosome is cut. The homology-directed repair pathway, invoked by the host cell, causes the broken ends of the cleaved chromosome to invade the intact homologous chromosome and pair at regions of homology before then using templated DNA synthesis along the intervening sequence, followed by resolution of the two chromosomes once this is complete. This ‘homing' process leads to a duplication of the gene drive so that it is now on both chromosomes. Panel 2: if the homing process happens in the germline prior to gamete formation then a higher proportion of the gametes from heterozygous individual will contain a copy of the gene drive than the 50% that would be expected if normal Mendelian inheritance applied. In Anopheles mosquitoes, the homing process can be very efficient and in some cases the rate of inheritance of a synthetic homing gene drive can approach 100%. Homing can occur in the germline of both sexes. Panel 3: left panel: population-wide knockout of some essential gene that produces a desirable phenotype (from a malaria control perspective) in individuals homozygous for the gene drive, such as female infertility, by virtue of the gene drive disrupting the coding sequence of the target gene. Importantly for this approach to work most effectively, ‘carrier' individuals heterozygous for the drive should be fully viable. right panel: population-wide spread of an effector gene as ‘cargo’. Gene drives that interfere with mosquito reproduction or viability aim to the eliminate or suppress the population to levels that do not support disease transmission (left panel). Alternatively, gene drives engineered to directly impair vector competence can invade the vector population, transforming it over time to become one that is refractory to malaria transmission (right panel).
In both Anopheles gambiae and Anopheles stephensi mosquitoes, CRISPR-based HEGs have been shown to show biased inheritance rates of close to 100% [22–24]. Broadly, in order to couple a trait of interest to the gene drive, two main approaches are available: the gene drive knocks out some essential gene in the mosquito; the gene drive is tightly linked to some desirable effector gene (a cargo) (figure 1, bottom panel). In the former case, the presence of the gene drive should cause a recessive phenotype, effectively acting as a sort of genomic parasite—as the gene drive spreads through the population heterozygous individuals are fully viable, meaning they continue to carry and transmit the gene drive, whereas individuals homozygous for the gene drive are non-viable, leading to a reduction in the reproductive capacity of the population. Examples of this type of drive have targeted genes essential for female viability and have been shown in the laboratory to have a strong suppressive effect on populations [24–26]. Examples of the latter type of drive with a cargo focus on incorporating anti-Plasmodium effectors have similarly been shown capable of spread in laboratory populations of mosquitoes [23,27]. The type of cargo has so far taken the form of single-chain antibody fragments but could feasibly incorporate other broad-acting anti-microbial peptides that show strong levels of inhibition of parasite strains in the laboratory [28,29].
One interesting possibility, yet to be realized, is the option of a hybrid of the two approaches whereby, rather than carrying an anti-Plasmodium gene as cargo, the gene drive instead disrupts a gene encoding for a mosquito ligand that is essential for the parasite to complete its life cycle in the mosquito.
4. Limitations and challenges of gene drive technology for malaria control
Gene drives that are designed to spread and impose a suppressive effect on either the mosquito or the parasite it transmits are expected eventually, like any intervention, to select for resistance in each organism, respectively. In order to mitigate against resistance, the usual rules apply: target sites and processes that are strongly constrained and target them at multiple points. This is perhaps best illustrated by the selection of resistant target site variants that occurred in the face of the first generation of homing-based gene drives that did not prioritize functional constraint in the choice of target site [27,30]. The resistant alleles that were selected both prevented cutting by the nuclease in the gene drive and conferred a positive fitness effect relative to the gene drive allele. By contrast, a gene drive designed to incapacitate a gene essential for female sexual development, targeting a highly constrained site, led to suppression of a laboratory population and failed to select for resistance [26]. The best indicator of functional constraint is sequence conservation over evolutionary time and in this aspect the availability of genomes from across the Anopheles genus and the resequencing of wild-caught A. gambiae and Anopheles coluzzii are an invaluable resource, allowing the identification of ultraconserved sites that are targetable by CRISPR-based gene drives [31–33]. As further contingency several guide RNAs can be included in the same gene drive construct in order to target multiple sites in the same target gene, where only one need be active in order for the gene drive to function [20,22,34].
If targeting the parasite directly, effector genes that form part of the gene drive cargo must be equally robust to parasite resistance. Again, contingency planning is available here by targeting the parasite at different stages throughout its life cycle in the mosquito, and choosing to interrupt parasite-specific pathways, or epitopes (if using single-chain antibodies) that are highly constrained [35,36].
5. Containment of gene drives
There are many flavours of gene drive potentially available but for the CRISPR-based homing gene drives so far developed in Anopheles mosquitoes, one of the most attractive and potent features of gene drives—their ability to spread from a very low initial frequency—is also one that causes concern. For example: accidental releases are hard to contain; a phased pathway of local test trials is challenging for a technology so invasive; issues of governance arise where the intervention spreads outwith the original release area, or across new territories. It is beyond the scope of this technical article to address these in detail but these concerns have prompted interest in alternative gene drive systems that are less invasive or require a threshold of release frequency to be superseded in order for the drive to invade the population [20,37,38]. As an intermediate, ‘split' gene drive systems, where the source of Cas9 is uncoupled from the element that is copied through homing, have also been proposed [39–41] and may allow useful optimization of the relevant components of a fully invasive gene drive prior to deployment.
An alternative strategy to deliberately limit the spread of a gene drive is to target it to an allele that is private and locally fixed in a particular sub-population [42,43]. Such private alleles might exist owing to a recent selection that acted directly on the allele itself, or owing to a selective sweep that happened to encompass the allele, or owing to a founder effect that fixed the allele by chance. It is important to distinguish between these scenarios because each would imply a differing level of functional constraint at the target site, and therefore a different propensity to tolerate drive-resistant alleles. For example, an allele that is fixed in population ‘by accident' is likely to be much more tolerant of mutations that render it resistant to a gene drive than a private allele that was the direct object of selection and therefore likely under some level of functional constraint.
6. Transferring gene drives to other Anopheles mosquitoes
Assuming its target site is conserved, a gene drive built in A. gambiae would be expected to introgress into A. coluzzii, and vice versa, since the level of reproductive isolation between these two species of the wider A. gambiae species complex is incomplete [44,45]. Indeed a precedent for a similar type of event is the recent adaptive introgression of insecticide-resistant alleles between these two species [46,47]. Even if hybridization between the two genomes occurs only rarely, as long as it happens at some appreciable frequency, CRISPR-based gene drives would be expected to complete their introgression into the new genome very rapidly, since in the act of homing from one chromosome to the other only the gene drive and a tiny amount of its original neighbouring genomic sequence are copied (figure 1, top).
The extent of reproductive isolation between Anopheles arabiensis, another significant malaria vector in the species complex, and A. gambiae is probably more extreme and although introgression can be forced in the laboratory, the extent to which it occurs in the wild is unclear.
Supposing one were to build a gene drive de novo, for example to target a different Anopheles species that was the dominant vector species locally, the following would be pre-requisites: an ability to edit the genome initially in order to introduce the gene drive cassette; promoter sequences that can direct Cas9 expression in the germline; a propensity for Cas9-induced cleavage to lead to homing of the gene drive allele (rather than repair through pathways that do not depend on homology); and high-quality genomic data in order to allow optimal choice of conserved target sites. For the prospects of making a transgenic organism, the universality of Cas9 genome editing as a tool is a boon and has been used to introduce germline modifications in a diverse range of malaria vectors including Anopheles funestus and Anopheles albimanus [48], meaning that it should be possible to build and test gene drives across a wider range of Anopheles species. Moreover, a recently developed technique that obviates the need to develop an embryo injection protocol for each species could also widen the scope of vectors to be targeted, providing it can be adapted to introduce genetic constructs rather than just induce small deletions, as is currently the case [49].
Assuming technical barriers to creating a gene drive in a mosquito species can be overcome, the optimal parameters for its performance, and the feasibility of its success in malaria control, will be exquisitely dependent on the particular ecology and life-history traits of the species at hand [50].
7. Summary
Targeting the mosquito vector in order to interrupt transmission has been the mainstay of successful malaria control programs over the years. Gene drives represent a powerful tool to achieve this in a targeted way that is species-specific, requires minimal infrastructure and is self-sustaining. Moreover, if successful, the benefits of this type of intervention would be available to all, regardless of differential access to healthcare.
Gene drives able to crash mosquito populations or to spread anti-parasite effectors to fixation have been shown to work in the laboratory. Following on from this promise, future steps will need to consider how to test these at scale and test their robustness and efficacy in larger population sizes under more realistic ecological settings. Because gene drives still represent a relatively novel technology, with unique characteristics, the pathway to regulating their use and obtaining consensus on whether and how to use them is challenging.
There has been a concerted effort by scientists working on gene drives to develop both a pathway to their development [35,51–53] and to establish minimum efficacy and safety criteria [54]. It is encouraging also to see a similar weight of attention given to the process of public engagement and knowledge transfer around gene drives [55–57]. This is especially important for gene drive mosquitoes, given that they are designed to be invasive and that they are genetically modified, both features that generate a certain sensitivity among the public.
Notwithstanding the transformative potential of gene drive technology it is unlikely that it, or any other novel malaria control tool for that matter, will be a silver bullet, given the proven resilience of both the parasite and the vector in the past. It is therefore essential that there is a combination of approaches, applied in a coordinated fashion to achieve maximal synergy between them, in order to achieve the goal of malaria elimination.
Acknowledgements
Manuela Bernardini kindly helped in the preparation of the figure.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. 2004. A global index representing the stability of malaria transmission. Am. J. Trop. Med. Hyg. 70, 486–498. ( 10.4269/ajtmh.2004.70.486) [DOI] [PubMed] [Google Scholar]
- 2.Sinka ME, et al. 2012. A global map of dominant malaria vectors. Parasit. Vectors 5, 69 ( 10.1186/1756-3305-5-69) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. ( 10.1038/nature15535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation. 2019. Guidelines for malaria vector control. Licence: CC BY-NC-SA 3.0 IGO. Geneva, Switzerland: WHO. [Google Scholar]
- 5.Knipling EF. 1959. Sterile-male method of population control. Science 130, 902–904. ( 10.1126/science.130.3380.902) [DOI] [PubMed] [Google Scholar]
- 6.Dyck VA, Hendrichs J, Robinson AS. 2005. Sterile insect technique: principles and practice in area-wide integrated pest management, xiv, 787p Dordrecht, The Netherlands: Springer. [Google Scholar]
- 7.Lindquist DA, Abusowa M, Hall MJ. 1992. The New World screwworm fly in Libya: a review of its introduction and eradication. Med. Vet. Entomol. 6, 2–8. ( 10.1111/j.1365-2915.1992.tb00027.x) [DOI] [PubMed] [Google Scholar]
- 8.Verysen MJB, Saleh K, Mramba F, Parker A, Feldmann U, Dyck VA, Msangi A, Bouyer J. 2014. Sterile insects to enhance agricultural development: the case of sustainable tsetse eradication on Unguja Island, Zanzibar, using an area-wide integrated pest management approach. PLoS Negl. Trop. Dis. 8, e2857 ( 10.1371/journal.pntd.0002857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, Malavasi A, Capurro ML. 2015. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 9, e0003864 ( 10.1371/journal.pntd.0003864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris AF, et al. 2012. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828–830. ( 10.1038/nbt.2350) [DOI] [PubMed] [Google Scholar]
- 11.Thomas DD, Donnelly CA, Wood RJ, Alphey LS. 2000. Insect population control using a dominant, repressible, lethal genetic system. Science 287, 2474–2476. ( 10.1126/science.287.5462.2474) [DOI] [PubMed] [Google Scholar]
- 12.Phuc HK, et al. 2007. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 5, 11 ( 10.1186/1741-7007-5-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 10, 295–311. ( 10.1089/vbz.2009.0014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burt A. 2003. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. R. Soc. B 270, 921–928. ( 10.1098/rspb.2002.2319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, Hay BA. 2007. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science 316, 597–600. ( 10.1126/science.1138595) [DOI] [PubMed] [Google Scholar]
- 16.Edgington MP, Alphey LS. 2017. Conditions for success of engineered underdominance gene drive systems. J. Theor. Biol. 430, 128–140. ( 10.1016/j.jtbi.2017.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galizi R, Doyle LA, Menichelli M, Bernardini F, Deredec A, Burt A, Stoddard BL, Windbichler N, Crisanti A. 2014. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 5, 3977 ( 10.1038/ncomms4977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton WD. 1967. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 19.Kidwell MG, Ribeiro JM. 1992. Can transposable elements be used to drive disease refractoriness genes into vector populations? Parasitol. Today 8, 325–329. ( 10.1016/0169-4758(92)90065-a) [DOI] [PubMed] [Google Scholar]
- 20.Oberhofer G, Ivy T, Hay BA. 2019. Cleave and rescue, a novel selfish genetic element and general strategy for gene drive. Proc. Natl Acad. Sci. USA 116, 6250–6259. ( 10.1073/pnas.1816928116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Melderen L, De Bast MS. 2009. Bacterial toxin-antitoxin systems: more than selfish entities? PLoS Genet. 5, e1000437 ( 10.1371/journal.pgen.1000437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champer J, Liu J, Oh SY, Reeves R, Luthra A, Oakes N, Clark AG, Messer PW. 2018. Reducing resistance allele formation in CRISPR gene drive. Proc. Natl Acad. Sci. USA 115, 5522–5527. ( 10.1073/pnas.1720354115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA. 2015. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl Acad. Sci USA 112, E6736–E6743. ( 10.1073/pnas.1521077112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond A, et al. 2016. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78–83. ( 10.1038/nbt.3439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond A, Kyrou K, Gribble M, Karlsson X, Morianou I, Galizi R, Beaghton A, Crisanti A, Nolan T. 2018. Improved CRISPR-based suppression gene drives mitigate resistance and impose a large reproductive load on laboratory-contained mosquito populations. bioRxiv, 360339 ( 10.1101/360339) [DOI] [PMC free article] [PubMed]
- 26.Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A. 2018. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 36, 1062–1066. ( 10.1038/nbt.4245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham TB, et al. 2019. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet. 15, e1008440 ( 10.1371/journal.pgen.1008440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaacs AT, Jasinskiene N, Tretiakov M, Thiery I, Zettor A, Bourgouin C, James AA. 2012. Transgenic Anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. Proc. Natl Acad. Sci. USA 109, E1922–E1930. ( 10.1073/pnas.1207738109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. 2012. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl Acad. Sci. USA 109, 12 734–12 739. ( 10.1073/pnas.1204158109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond AM, et al. 2017. The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLoS Genet. 13, e1007039 ( 10.1371/journal.pgen.1007039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Anopheles gambiae 1000 Genomes Consortium. 2017. Genetic diversity of the African malaria vector Anopheles gambiae. Nature 552, 96–100. ( 10.1038/nature24995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neafsey DE, et al. 2015. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347, 1258522 ( 10.1126/science.1258522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt H, Collier TC, Hanemaaijer MJ, Houston PD, Lee Y, Lanzaro GC. 2020. Abundance of conserved CRISPR-Cas9 target sites within the highly polymorphic genomes of Anopheles and Aedes mosquitoes. Nat. Commun. 11, 1425 ( 10.1038/s41467-020-15204-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandul NP, Liu J, Buchman A, Gantz VM, Bier E, Akbari OS. 2020. Assessment of a split homing based gene drive for efficient knockout of multiple genes. G3: Genes|Genomes|Genetics 10, 827–837. ( 10.1534/g3.119.400985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carballar-Lejarazu R, James AA. 2017. Population modification of Anopheline species to control malaria transmission. Pathog. Glob Health 111, 424–435. ( 10.1080/20477724.2018.1427192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall JM, Raban RR, Kandul NP, Edula JR, Leon TM, Akbari OS. 2019. Winning the tug-of-war between effector gene design and pathogen evolution in vector population replacement strategies. Front. Genet. 10, 1072 ( 10.3389/fgene.2019.01072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbari OS, Matzen KD, Marshall JM, Huang H, Ward CM, Hay BA. 2013. A synthetic gene drive system for local, reversible modification and suppression of insect populations. Curr. Biol. 23, 671–677. ( 10.1016/j.cub.2013.02.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leftwich PT, Edgington MP, Harvey-Samuel T, Carabajal Paladino LZ, Norman VC, Alphey L. 2018. Recent advances in threshold-dependent gene drives for mosquitoes. Biochem. Soc. Trans. 46, 1203–1212. ( 10.1042/BST20180076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, et al. 2020. Development of a confinable gene drive system in the human disease vector Aedes aegypti. Elife 9, e51701 ( 10.7554/eLife.51701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez Del Amo V, Bishop AL, Sanchez CH, Bennett JB, Feng X, Marshall JM, Bier E, Gantz VM. 2020. A transcomplementing gene drive provides a flexible platform for laboratory investigation and potential field deployment. Nat. Commun. 11, 352 ( 10.1038/s41467-019-13977-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble C, et al. 2019. Daisy-chain gene drives for the alteration of local populations. Proc. Natl Acad. Sci. USA 116, 8275–8282. ( 10.1073/pnas.1716358116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gantz VM, Bier E. 2016. The dawn of active genetics. Bioessays 38, 50–63. ( 10.1002/bies.201500102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudweeks J, et al. 2019. Locally fixed alleles: a method to localize gene drive to island populations. Sci. Rep. 9, 15821 ( 10.1038/s41598-019-51994-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pombi M, et al. 2017. Dissecting functional components of reproductive isolation among closely related sympatric species of the Anopheles gambiae complex. Evol. Appl. 10, 1102–1120. ( 10.1111/eva.12517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner TL, Hahn MW, Nuzhdin SV. 2005. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 3, e285 ( 10.1371/journal.pbio.0030285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarkson CS, et al. 2014. Adaptive introgression between Anopheles sibling species eliminates a major genomic island but not reproductive isolation. Nat. Commun. 5, 4248 ( 10.1038/ncomms5248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norris LC, Main BJ, Lee Y, Collier TC, Fofana A, Cornel AJ, Lanzaro GC. 2015. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc. Natl Acad. Sci. USA 112, 815–820. ( 10.1073/pnas.1418892112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Akbari OS, White BJ. 2018. Highly efficient site-specific mutagenesis in malaria mosquitoes using CRISPR. G3 (Bethesda) 8, 653–658. ( 10.1534/g3.117.1134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macias VM, McKeand S, Chaverra-Rodriguez D, Hughes GL, Fazekas A, Pujhari S, Jasinskiene N, James AA, Rasgon JL. 2020. Cas9-mediated gene-editing in the malaria mosquito Anopheles stephensi by ReMOT control. G3 (Bethesda) 10, 1353–1360. ( 10.1534/g3.120.401133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eckhoff PA, Wenger EA, Godfray HC, Burt A. 2017. Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proc. Natl Acad. Sci. USA 114, E255–E264. ( 10.1073/pnas.1611064114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adelman Z, et al. 2017. Rules of the road for insect gene drive research and testing. Nat. Biotechnol. 35, 716–718. ( 10.1038/nbt.3926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emerson C, James S, Littler K, Randazzo FF. 2017. Principles for gene drive research. Science 358, 1135–1136. ( 10.1126/science.aap9026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.James S, et al. 2018. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of Malaria in sub-Saharan Africa: recommendations of a scientific working group(dagger). Am. J. Trop. Med. Hyg. 98(6_Suppl), 1–49. ( 10.4269/ajtmh.18-0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James SL, Marshall JM, Christophides GK, Okumu FO, Nolan T. 2020. Toward the definition of efficacy and safety criteria for advancing gene drive-modified mosquitoes to field testing. Vector Borne Zoonotic Dis. 20, 237–251. ( 10.1089/vbz.2019.2606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Committee on Gene Drive Research in Non-Human Organisms. 2016. Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 56.Hartley S, et al. 2019. Knowledge engagement in gene drive research for malaria control. PLoS Negl. Trop. Dis. 13, e0007233 ( 10.1371/journal.pntd.0007233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thizy D, et al. 2019. Guidance on stakeholder engagement practices to inform the development of area-wide vector control methods. PLoS Negl. Trop. Dis. 13, e0007286 ( 10.1371/journal.pntd.0007286) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.