Abstract
Vector-borne diseases threaten the health of populations around the world. While key interventions continue to provide protection from vectors, there remains a need to develop and test new vector control tools. Cluster-randomized trials, in which the intervention or control is randomly allocated to clusters, are commonly selected for such evaluations, but their design must carefully consider cluster size and cluster separation, as well as the movement of people and vectors, to ensure sufficient statistical power and avoid contamination of results. Island settings present an opportunity to conduct these studies. Here, we explore the benefits and challenges of conducting intervention studies on islands and introduce the Bijagós archipelago of Guinea-Bissau as a potential study site for interventions intended to control vector-borne diseases.
This article is part of the theme issue ‘Novel control strategies for mosquito-borne diseases'.
Keywords: cluster-randomized trials, vector control, malaria, islands, Bijagós
1. Introduction
Vector-borne diseases (VBDs) contribute significantly to the global burden of disease and remain a threat to over 80% of the world's population [1,2]. The pathogens responsible for these infections are transmitted by mosquitoes, black flies, sand flies, ticks and other arthropods, the control of which is the principal method available for controlling many VBDs.
Vector control aims to limit the transmission of pathogens by reducing or eliminating human contact with the vector. The tools available for vector control include both chemical and non-chemical approaches that target either larval or adult stages [3]. Insecticide-treated nets (ITNs) and indoor residual spraying (IRS) have been the mainstay of malaria control [4]. However, recent decades have witnessed the development of novel interventions that approach the challenge of eliminating vector contact through the use of novel compounds for existing interventions, mass drug administration (MDA) of ivermectin, genetic modification and the introduction of bacteria that reduce or modify the vector population [5–9].
The importance of developing new tools has been emphasized since 2017 as evidence emerged that the global response to malaria had stalled. Malaria cases were found to have increased in several countries [10], and this was coupled with an increasing threat of Aedes-borne diseases. The global spread of dengue and chikungunya viruses, as well as outbreaks of Zika virus disease and yellow fever, clearly highlight the challenges faced with VBDs worldwide [11–13].
The World Health Organization's Global Vector Control Response strategy aims at strengthening vector control as a fundamental approach to preventing disease and responding to outbreaks and has two foundational elements—to enhance vector control capacity and capability, and to increase basic applied research and innovation [1]. Included in these foundations is a requirement to improve the evidence base of the impact of vector control interventions on infection and human disease, which is generally weak beyond the core interventions used for the control of malaria [14–16]. Relatively few field studies have been performed with some interventions, and these have often been poorly designed and conducted [17]. As a consequence, their outcomes are difficult to interpret and may give misleading results. There remains an urgent need to understand the efficacy of current interventions [18], and to measure the field suitability and performance of new interventions.
Randomized controlled trials (RCTs) provide the least biased and most robust estimate of an intervention's efficacy [19,20]. It is important to note that not all questions in vector control can be answered with RCTs, and other study designs, including observational studies, should be considered by investigators [17,21]. For example, Kleinschmidt et al. [22] used a prospective, observational cohort study design to investigate whether insecticide resistance was associated with a loss of effectiveness of long-lasting insecticidal nets. Other study designs may be more suitable where there is already strong evidence that an intervention works; it would be unethical to use a control arm that required study populations to use no ITNs or IRS.
Where RCTs are appropriate and can be conducted well, the allocation to treatment and control groups in a random fashion is expected to provide no systematic differences between groups that could be caused by confounding variables [23]. Mosquito trials typically involve area-wide, rather than individual, interventions, so their effects must be measured at community, or cluster, level [24]. However, in order to be able to provide evidence of efficacy, the study must carefully consider cluster size and cluster separation. The movement of people also needs to be considered, since it can contaminate the study results, generally giving bias in the direction of the null result [20].
2. Benefits of using islands for cluster-randomized trials
Avoidance of contamination is one of the primary reasons for using a cluster randomization design for field trials. One of the main strategies for reducing contamination is to choose clusters for the trial that are well separated [23]. Rural communities, which are often selected for such studies [25,26], have the benefit of being geographically dispersed, with less mixture of populations expected than in urban areas. Natural barriers, such as rivers or swamps, can also effectively increase the separation of clusters and prevent the mixture of populations. An extension of this concept is to base intervention studies, such as cluster-randomized trials (CRTs), on islands.
(a). Movement of human populations
Population movement is a major driver for infectious disease transmission and is known to have a significant role in the spatial spread of some diseases. The outbreak of Ebola in West Africa from 2014 to 2016 is an important recent example [27], but others include VBDs [28]. Dengue, transmitted by Aedes mosquitoes, is recognized as the most rapidly spreading mosquito-borne disease worldwide and is so intimately linked with population movement that mobile phone-based mobility estimates have been shown to predict the geographic spread and timing of epidemics in some settings [29].
In addition to affecting disease transmission [30–32], the movement of populations can impact disease control and elimination strategies. First, where healthcare provision is based around MDA, high population coverage is crucial to success [33]. Elimination strategies, in particular, demand that high levels of coverage are attained [34]. The movement of people may mean that some individuals are missed, coverage targets are not met, or there is re-introduction of disease [35].
Despite islands increasingly becoming part of global systems of migration and flows of resources, the movement of people to and from isolated islands is expected to be moderate compared with movement in mainland settings and, therefore, benefit the study of VBDs and other infectious diseases. Where studies are being conducted to assess the efficacy of an intervention, such as through CRTs, the movement of individuals between clusters could lead to dilution of the intervention's effects. Careful study design is needed to counter these issues [17]. In the case of mobile interventions such as insecticide-treated clothing or MDA of an endectocide, a type of systemic insecticide that has activity against both endo-parasites and ecto-parasites, substantial movement of treated individuals into control-group clusters could result in a reduction in the local mosquito population and an underestimate of the impact of the intervention. Conversely, in the trial of a repellent, those wearing repellents may divert mosquitoes to unprotected individuals [36] and in this situation inflate the apparent effect of the intervention. By conducting intervention studies on islands, these effects may be minimized.
(b). Movement of vector populations
The movement of vector populations is a further complication for intervention studies and is another motive for the use of islands. The dispersal range of Anopheles gambiae mosquitoes has been estimated through mark–release–recapture experiments to be less than 7 km, and more commonly in the range of 0.5–1 km [37–39]. This means that, if intervention clusters are considerably closer together, there is more opportunity for contamination. Experiments in which labelled An. gambiae were released from a Gambian village have shown that, while the majority of re-caught mosquitoes remained in the same village, there was also the movement of mosquitoes to neighbouring villages situated 1–1.4 km away. This suggested that movement could seriously affect the entomological evaluation of vector control programmes, such as studies with ITNs, in areas where treated and untreated villages were interspersed [40].
The restriction of movement of mosquito populations is particularly important for studies with genetically modified mosquitoes. The concern here is not just with assessing the impact of the intervention, which might be to use transgenes to suppress a local mosquito population or replace it with a population that is refractory to the development of pathogens [41,42], but one of mitigating risks. Genetically modified strains are designed to be competitive against wild populations, and it would be difficult to halt the spread of transgenes from a self-propagating, genetically modified mosquito population if some unanticipated negative side effects were identified following their release. As a result, it was recommended that the first field trials of genetically modified mosquitoes be carried out with individuals that are genetically sterile, and that the release site is geographically isolated, so that there is a negligible risk of spread of mosquitoes from any accidental release [43,44]. Oceanic or lacustrine islands are natural choices for such field sites and have been considered as release sites that would allow the success and risks associated with genetic modification of An. gambiae to be assessed.
Isolated riverine islands were selected for a field trial aimed at the suppression of insular Aedes albopictus populations through releases of irradiated male mosquitoes that also carried a transinfected Wolbachia strain incompatible with local females [45]. Several million factory-reared adult males were released in residential areas of islands in Nansha and Panyu Districts in Guangzhou, the city with the highest dengue transmission rate in China, and resulted in near elimination of field populations [45]. Before release, the mosquitoes were irradiated with an X-ray dose known to effectively sterilize females but to not negatively affect male competitiveness. Wolbachia is maternally inherited; accidental release of fertile females may, therefore, result in the unintended invasion of the novel Wolbachia strain in the local population and would render any future male releases carrying the strain ineffectual. The Nansha and Panyu District islands are relatively isolated. Although there is evidence of passive mosquito dispersal along with human transportation networks, nearby islands provided control sites for means of comparison [45]. Here, as elsewhere [46,47], islands have allowed for the precise monitoring of vector population suppression with limited interference from outside populations.
Rusinga Island in Lake Victoria, western Kenya, provides another example of the use of islands for the evaluation of vector control interventions. The island was used in a stepped wedge CRT to investigate the use of mass mosquito trapping for malaria control [48]. The island is connected to the mainland via a causeway, which is used for transport to the larger town of Mbita, where there is one of the two medical clinics that serve Rusinga Island. The island is a contained area, and, therefore, considered ideal for the use of a stepped wedge trial design. However, the island's proximity to the mainland, and its dependency on mainland infrastructure, may result in contamination from mainland vector populations. There are more remote islands in Lake Victoria, including Mageta, Magare and Ngodhe islands, which may be less likely to experience vector movements from the mainland [49].
3. Challenges of island cluster-randomized trials
While there are clear benefits of working on islands, such environments also present challenges to intervention studies. Island groups in remote settings have obvious logistical limitations, making it difficult for study teams and equipment to travel to and between study locations, and this can add study costs, which are often considerable for CRTs.
(a). Study clustering
To adequately establish the effect of intervention through CRTs, it is crucial to ensure adequate numbers of clusters to satisfy the study design. However, the number of islands in an archipelago may restrict options for clustering, and indeed, where a study requires a certain number of individuals, the population sizes on some islands be a limiting factor.
Sample size estimates must consider the possibility of within-cluster correlation as a result of between-cluster variability. For example, the availability of breeding sites for vectors, or differences in access to healthcare, may mean that there are differences in rates of infectious disease between clusters [23]. To ensure that study outcomes can be accurately observed, any heterogeneity between clusters must be taken into account when designing CRTs. Where there is a high degree of correlation in the outcome, whereby some clusters report low levels of disease prevalence and others much higher, it is more difficult to demonstrate an intervention effect without a very large number of clusters [21]. Studies of malaria prevalence suggest that for CRTs it may be necessary to assume high between-cluster variability when estimating realistic sample sizes [50].
(b). Translation of results to other settings
A further consideration for conducting research studies in an island setting is the ability to translate findings to other settings. The question of external validity asks whether the results obtained in a trial may be generalized to other settings, which may differ from the study setting with respect to characteristics that influence the outcome, and whether the results have been affected by particular geographical, temporal, socio-economic and ethnical factors that may not be present at other study sites [51]. Island populations may have different cultures and traditions, and can even differ genetically from those in other areas owing to genetic drift or differences in ancestral populations, potentially resulting in an effect modification [52,53]. It is conceivable that an intervention involving an island population would have different outcomes when implemented elsewhere. This holds for both human and vector populations. For instance, a glycine–serine mutation in the acetylcholinesterase (ace) gene that confers high levels of resistance to carbamates and organophosphates was not found to be present in An. gambiae from Bioko Island, 30 miles off the coast of Cameroon where bendiocarb resistance has been documented [54]. This indicates that there is little gene flow between Bioko Island and the nearest mainland populations. Insecticide treatments that are effective in controlling mosquito populations on Bioko might have limited effect in other areas. It is, therefore, important that the results of any efficacy studies are considered in the context of their settings. For these reasons, the World Health Organization Vector Control Advisory Group requires data from at least two well-conducted RCTs in different and complementary entomological settings, ideally covering two transmission seasons, to accept new first-in-class products for vector control [55]. While this can help ensure that products reaching the marketplace have public health value in more than one setting, it requires the use of more than one island group for intervention studies.
Islands were selected for the first interventions using RIDL (Release of Insects carrying a Dominant Lethal gene) mosquitoes. Approximately 3.3 million engineered male Aedes aegypti were released in a 23-week period in 2010 in a field site on Grand Cayman, an island in the Caribbean. Monitoring of ovitraps at the release sites and a control site indicated strong population suppression in the treated area during the last seven weeks of the release period [46]. This positive outcome and successful demonstration of population suppression provided encouragement for RIDL mosquitoes and genetic control strategies in general for population suppression. The island study was followed by sustained field releases in a suburb of Juazeiro, Bahia, Brazil. While reductions seen in ovitrap indices, compared with the adjacent no-release control area, were similar to those estimated in the Cayman trials [56], results from a large-scale release in Piracicaba have not been published in academic journals. Challenges in producing sufficient numbers of transgenic mosquitoes can limit the number released per hectare [46] and thus make it difficult to replicate successes in larger areas.
The control of arboviral diseases through the use of Wolbachia also requires the mass-rearing and release of mosquitoes. Wolbachia can reduce mosquito competence for a variety of RNA viruses, including dengue and chikungunya [57]. Many Wolbachia strains induce a sperm–egg inviability known as cytoplasmic incompatibility (CI) that provides female carriers with relative reproductive advantage, allowing the Wolbachia strain to invade naive insect populations [58]. The reproductive advantage conferred by CI increases with Wolbachia population frequency, although Wolbachia infection is also often accompanied by deleterious effects on some life-history traits. This combination of frequency-dependent fitness advantages and frequency-independent costs results in an invasion threshold—below the threshold the Wolbachia strain will tend to be lost from a population, but if the threshold is surpassed, the Wolbachia strain will tend to spread. It is, therefore, important that mosquito releases are sufficient to exceed the threshold frequency. In the case of the wMel Wolbachia infection in Ae. aegypti, it is estimated that the frequency of wMel must reach 20–30% in the population for successful invasion [59]. The rate at which Wolbachia spreads through a population depends on the distance mosquitoes disperse and the cost of Wolbachia infection, which are likely to be affected by environmental conditions [60]. Indeed, different rates of spreading have been observed at different release sites in Cairns, Australia [61]. Importantly, the ability of Wolbachia to become established can be affected by the isolation of the population. If an Aedes population is not isolated, a relatively small population of infected mosquitoes can be swamped by immigrants from surrounding Wolbachia-free populations, and the influx is expected to push the prevalence of Wolbachia below the invasion threshold [60]. There is, therefore, the assumption that an intervention will perform differently on an island from in a mainland setting, and theoretical models have been developed to guide programmes that deploy Wolbachia for the purposes of vector control [59].
4. The Bijagós archipelago
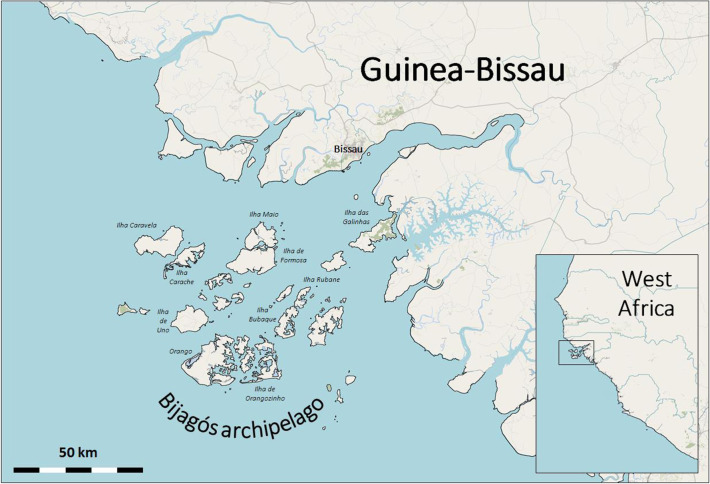
A unique setting for investigations of infectious disease epidemiology and the efficacy of control interventions is offered by the Bijagós archipelago of West Africa. The archipelago, made up of 88 islands, is situated within an area of approximately 13 000 km2 in the Atlantic Ocean, off the coast of Guinea-Bissau (figure 1). Collectively, the islands cover 900 km2, with the farthest being less than 100 km from the mainland.
Figure 1.
Bijagós archipelago, situated off the Atlantic coast of Guinea-Bissau. Inset shows position of Guinea-Bissau on the coast of West Africa. Adapted from OpenStreetMap. Credit: © OpenStreetMap contributors.
Eighteen of the islands are permanently inhabited, with an estimated population of around 24 000 people. Many of the others are reserved for seasonal agricultural use. The islands host a diversity of ecosystems from mangroves to forests, and provide habitats for a range of arthropod vectors of disease.
(a). Vector-borne diseases and current interventions on the Bijagós archipelago
The Bijagós archipelago is endemic for Plasmodium falciparum malaria and records the highest prevalence (more than 30 confirmed cases per 100 population) in Guinea-Bissau [62]. Standard malaria control interventions implemented on the Bijagós archipelago by the National Malaria Control Programme include ITNs, intermittent preventive treatment in pregnancy, and case diagnosis and treatment with artemisinin-based combination therapy (artemether–lumefantrine). There are no current plans for scale-up of additional malaria control measures on the islands, so there is an excellent opportunity to conduct interventional studies to reduce malaria transmission in these communities using novel cost-effective approaches.
(b). Previous studies on the Bijagós archipelago
Previous studies of the archipelago have monitored human activity and surveyed mosquito populations [63,64]. Using indoor adult light traps and larval dipping at potential breeding sites, our group characterized vectors likely to be responsible for the majority of malaria transmission. The island of Bubaque was found to maintain both wet- and dry-season An. gambiae s.l. populations, with An. gambiae s.s. being the primary wet season vector, and the salt water-tolerant species Anopheles melas likely to be responsible for the majority of dry-season transmission [64]. Anopheles melas is relatively rare [65], but can breed year-round in the island's abundant littoral habitats and mangrove swamps, and thus, as in other coastal areas [66,67], maintain a relatively constant dry-season population. By contrast, An. gambiae s.s. and Anopheles coluzzii populations on Bubaque decline as the freshwater habitats that support their breeding become scarce [64].
The entomological surveys on the archipelago report that An. gambiae s.s. and An. coluzzii reside in sympatry and exist as hybrid forms [64,68]. These hybrids are found at much higher frequencies than are observed elsewhere in Africa [69]. A moderate degree of resistance to α-cypermethrin, but full susceptibility to permethrin, has been recorded. Both kdr and metabolic resistance mechanisms were detected, which could compromise current control methods that rely on insecticide-treated bed nets [64].
The movement of people within and between a subset of the Bijagós islands was the subject of a study by Durrans et al. [63], which showed that, although there was likely less migration than on the mainland owing to their geographical remoteness, the movement was a common feature of island life for men and women alike. While this was a relatively small study, it showed that typical reasons for travel included subsistence activities, family events, income-generating activities, cultural festivities and healthcare. These movements often occurred erratically all-year-round, with the exception of seasonal travel within and between islands for agricultural purposes. Understanding the patterns of movement is important for tailoring and increasing the reach of public health interventions, and this analysis of the Bijogo population will facilitate future studies on the islands. Temporal movement and migration within the island communities can be mapped and monitored during trials, but there are also opportunities to measure epidemiological outcomes through active detection of disease cases in sentinel cohort populations, as has been used for vector control studies in other settings [70,71].
Marsden et al. [65] evaluated the suitability of the Bijagós archipelago as a potential field site for the release of genetically modified An. gambiae. Given the broad geographical distribution of An. gambiae species, there are surprisingly few islands where these mosquitoes are active in malaria transmission and where there is a sufficient geographical distance between clusters to ensure isolation. The study collected mosquitoes from a single site on three of the 88 islands, analysing 208 mosquitoes, in comparison with 998 mosquitoes collected and analysed from the Comoro Islands. However, despite the distance of the Bijagós islands from mainland Guinea-Bissau, there was no evidence of genetic sub-division between island and mainland sites, which suggests that there is some degree of ‘island hopping' on the archipelago. As a result of this evidence, the authors concluded that the Bijagós archipelago was not sufficiently isolated for the release of transgenic strains and found the island of Grande Comore in the Indian Ocean to be a more suitable site for trials with genetically modified strains [65]. Further, the presence of both An. coluzzii and An. gambiae s.s. on the Bijagós islands, and evidence of hybridization between them [68], would complicate the implementation of an isolated genetically modified mosquito trial on the archipelago.
A feature that was not relevant to the release of engineered mosquitoes, but is highly beneficial for other studies, is the presence of more than one island. The Comoro archipelago constitutes just four islands, while the Bijagós archipelago counts 18 inhabited islands, opening considerable opportunities for clustering of intervention and control arms in a CRT.
(c). Future vector control studies on the Bijagós archipelago
The Bijagós archipelago offers a setting for the evaluation of other vector control tools. However, it remains important to take potential contamination between clusters into consideration. If different islands of the archipelago were to serve as different clusters, mosquitoes originating on a control-group island that disperse to a treatment island, either through the inadvertent movement of mosquitoes with local boat traffic or by wind-assisted flight, could, as with human population movement, interfere with the interpretation of the impact of the intervention. Such impacts are expected to be smaller in island settings than in areas without such physical barriers, but they can be anticipated and minimized through study design.
To help minimize contamination, buffer zones can be designated within clusters. If buffer zones are used, each island is split into an inner core area and an outer buffer zone. The intervention is delivered to the whole island, but measurements are only taken in the core area. The advantage is that contamination can be reduced because it is more likely to occur in the buffer zone where no measurements are taken. A balance must be struck when deciding on the size of the buffer: the bigger the buffer the less contamination will occur in the core area, but the core area must be big enough to ensure a sufficiently large sample can be taken to maintain statistical power. The use of buffer zones, and their size, should consider the average distance travelled by mosquitoes and the amount of migration of local human populations. Such zones were used in the first randomized trial to provide evidence that IRS, when used in combination with ITNs, can give significant added protection against malarial infection compared with ITN use alone [72].
It is essential that any randomized trial is sufficiently powered to detect a meaningful difference between the two arms. For a CRT, the principal factors that determine power are: the number of clusters, the number of individuals measured in each cluster, the underlying prevalence/incidence of the endpoint, the difference between the two arms in the endpoint, and the variation between the clusters in the endpoint. A previous CRT that investigated the effect of ivermectin MDA on malaria only had eight clusters and was probably very underpowered [73]. A less appreciated consequence of using too few clusters is that some statistical models commonly used for the analysis of CRTs will not perform well and may lead to increased type 1 errors [73]. The Bijagós archipelago's 18 available inhabited islands would allow a medium-sized, two-armed, CRT to take place there and the sample size should be sufficient for many disease outcomes. For example, for a trial of the use of ivermectin on the archipelago, using data collected from previous studies, we estimate there will be over 90% power to detect a difference of 5 versus 10% malaria prevalence in the intervention and control arms, respectively.
Stratified randomization is often used in CRTs to increase power [23]. Clusters that are similar in some way are grouped into strata. Analysis is carried out within strata, and the similarity of clusters within each stratum reduces between-cluster variation and, hence, increases power. Care must be taken when deciding which variable(s) are used for stratification. If clusters are stratified on the basis of variables that are not associated with the study endpoint, then power may in fact be reduced. Which variables are strong predictors of endpoints will be specific to the disease in question; for VBDs, ecological variables related to the mosquito's life cycle may be appropriate. A common stratification variable that is appropriate in a wide variety of circumstances is a cluster-level baseline measure of disease prevalence of incidence.
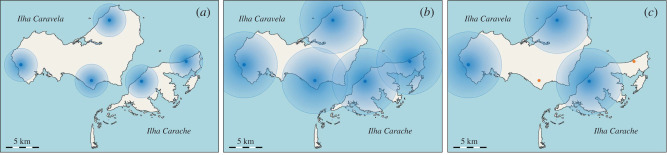
In the Bijagós archipelago, should more than 18 clusters, representing the number of inhabited islands, be needed in a CRT, islands must be split wherever possible following the above considerations. For example, the island of Caravela has a population numbering more than 4200 people. There are population centres on the north, south and western parts of the island, separated by at least 5 km of sea or land. The island could be split into two or more clusters. The clusters on an island could form a stratum for randomization, with one population centre being selected, at random, for assignment to the treatment arm and the other assigned to the control arm. This would allow for more clusters to be created beyond the natural limit of 18, but clearly each would have a smaller population size. The choice of buffer zone size might also have an impact on the power of the study. Large buffer zones will reduce the possibility of population mixing, but might demand that some sites are excluded from the study altogether (figure 2).
Figure 2.
Cluster selection and the use of buffer zones. Hypothetical buffer zones of 2.5 km radius (shown as blue discs) around villages (shown as blue dots) potentially allow for the establishment of multiple clusters on a single island (a). Buffer zones of 5 km radius, requiring villages to be at least 10 km apart, would prevent nearby villages from being in different intervention clusters (b). As a result, some villages (shown in orange) could be excluded from the study (c). Adapted from OpenStreetMap. Credit: © OpenStreetMap contributors.
A cluster-randomized control trial investigating the use of adjunctive ivermectin MDA to control malaria will be conducted in the Bijagós islands. Through interruption of transmission of malaria by reducing the human reservoir of P. falciparum and vector survival, dihydroartemisinin–piperaquine and adjunctive ivermectin MDA is expected to reduce the prevalence of the disease. Ivermectin has transformed the treatment of parasitic diseases, having been used effectively in MDA campaigns against onchocerciasis and lymphatic filariasis (LF) [74,75]. The drug has also been administered for head lice [76] and has had effects on scabies and intestinal helminths [77,78]. Ivermectin MDA for malaria control would provide an opportunity for collaboration between various disease control programmes, such as the Global Programme to Eliminate Lymphatic Filariasis (GPELF) and the Onchocerciasis Elimination Program of the Americas (OEPA).
Ivermectin is an attractive option for the control of malaria, as it kills mosquitoes that feed on individuals who have been administered an appropriate dose, and so targets mosquitoes regardless of whether they feed indoors or outdoors, during the day or at night [79]. Therefore, it can be used as a tool to complement ITNs and IRS. Sub-lethal effects of ivermectin on mosquitoes include reductions in fecundity and egg hatch rate [80,81], and it has also been shown to have a sporontocidal effect against Plasmodium vivax in host Anopheles [82]. This means that there can be community-wide effects of MDA campaigns, and modelling predictions suggest that ivermectin could be a valuable addition to malaria control in areas with seasonally persistent high malaria transmission, where existing interventions have failed to provide sufficient protection, or in areas that are approaching elimination [83].
The Bijagós archipelago has been targeted for an ivermectin campaign owing to its highly seasonal and stable malaria transmission. It also is co-endemic for malaria and neglected tropical diseases such as LF, soil-transmitted helminths and scabies. The control and elimination of these diseases has been prioritized by the Guinea Bissau Ministry of Public Health. Previous trachoma research on the islands has shown that MDA can be an effective and feasible strategy to eliminate infectious diseases in these communities [84]. MDA studies implemented to assess the efficacy of ivermectin for malaria control should employ both clinical and entomological primary outcomes [70]. Assessors should be blinded to the intervention received by participants to reduce detection biases, and entomological data should be collected through standardized methods, such as indoor traps for mosquito counts and an ovarian tracheation technique for determining mosquito age through parity [85,86]. Given that reductions in malaria prevalence may not necessarily correspond to reductions in clinical incidence [87], clinical outcomes should ideally include both measures of prevalence in the study population and measures of clinical incidence, determined through active case detection in cohorts.
The proposed ivermectin trial potentially places restrictions on the further use of the Bijagós archipelago for intervention studies. The number of islands, and size of their populations, only allow for a single trial of this type to be conducted at any one time. It may be expected that the ‘treated' clusters would have considerably different epidemics to take into consideration for follow-on trials, and it would be necessary to treat the ‘control’ clusters before subsequent trials can begin. It would also be necessary to allow a suitable period of time to pass before these clusters reach equivalence.
5. Conclusion
The evaluation of vector control tools requires well-designed studies that generate evidence of efficacy while minimizing potential biases. Although islands can have limitations, including restrictions on eligible populations and geography, they have unique features that align them well with CRT with novel vector control tools. The Bijagós islands are endemic for a range of vector-borne and neglected tropical diseases and have already been the subject of entomological and demographic studies that will facilitate future intervention and surveillance studies on the islands.
Caution should be taken in regarding islands as small-scale models of the wider world, but it is also important to avoid the danger of exceptionalism, regarding islands as being unique [88]. Many of the interventions that are currently in use or are in development could benefit from studies of efficacy and suitability, and conducting such trials on the Bijagós archipelago or other island groups will demonstrate their field suitability and potential value to public health.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the ideas and writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Medical Research Council (grant no. MR/S005013/1).
References
- 1.WHO. 2017. Global vector control response 2017–2030. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Golding N, et al. 2015. Integrating vector control across diseases. BMC Med. 13, 249 ( 10.1186/s12916-015-0491-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, Lindsay SW. 2020. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831 ( 10.1371/journal.pntd.0007831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okumu FO, Moore SJ. 2011. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar. J. 10, 208 ( 10.1186/1475-2875-10-208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores HA, O'Neill SL. 2018. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 16, 508–518. ( 10.1038/s41579-018-0025-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaccour C, Lines J, Whitty CJM. 2010. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J. Infect. Dis. 202, 113–116. ( 10.1086/653208) [DOI] [PubMed] [Google Scholar]
- 7.Protopopoff N, et al. 2018. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet 391, 1577–1588. ( 10.1016/S0140-6736(18)30427-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niang EHA, Bassene H, Fenollar F, Mediannikov O. 2018. Biological control of mosquito-borne diseases: the potential of Wolbachia-based interventions in an IVM framework. J. Trop. Med. 2018, 1470459 ( 10.1155/2018/1470459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paton DG, Childs LM, Itoe MA, Holmdahl IE, Buckee CO, Catteruccia F. 2019. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 567, 239–243. ( 10.1038/s41586-019-0973-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. 2018. World malaria report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 11.Pang T, Mak TK, Gubler DJ. 2017. Prevention and control of dengue—the light at the end of the tunnel. Lancet Infect. Dis. 17, e79–e87. ( 10.1016/S1473-3099(16)30471-6) [DOI] [PubMed] [Google Scholar]
- 12.Wilder-Smith A, Lee V, Gubler DJ. 2019. Yellow fever: is Asia prepared for an epidemic?. Lancet Infect. Dis. 19, 241–242. ( 10.1016/S1473-3099(19)30050-7) [DOI] [PubMed] [Google Scholar]
- 13.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi P-Y, Vasilakis N. 2016. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 130, 69–80. ( 10.1016/j.antiviral.2016.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HW, Breman JG, Teutsch SM, Liu S, Hightower AW, Sexton JD. 1995. The effectiveness of insecticide-impregnated bed nets in reducing cases of malaria infection: a meta-analysis of published results. Am. J. Trop. Med. Hyg. 52, 377–382. ( 10.4269/ajtmh.1995.52.377) [DOI] [PubMed] [Google Scholar]
- 15.Bhatt S, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. ( 10.1038/nature15535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherrard-Smith E, et al. 2018. Systematic review of indoor residual spray efficacy and effectiveness against Plasmodium falciparum in Africa. Nat. Commun. 9, 4982 ( 10.1038/s41467-018-07357-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. 2017. How to design vector control efficacy trials. Guidance on phase III vector control field trial design provided by the Vector Control Advisory Group. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 18.Wilson AL, Dhiman RC, Kitron U, Scott TW, van den Berg H, Lindsay SW. 2014. Benefit of insecticide-treated nets, curtains and screening on vector borne diseases, excluding malaria: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 8, e3228 ( 10.1371/journal.pntd.0003228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan YH. 2003. Randomised controlled trials (RCTs) - essentials. Singapore Med J. See http://www.smj.org.sg/article/randomised-controlled-trials-rcts-essentials (accessed: 22 July 2020).
- 20.Wolbers M, Kleinschmidt I, Simmons CP, Donnelly CA. 2012. Considerations in the design of clinical trials to test novel entomological approaches to dengue control. PLoS Negl. Trop. Dis. 6, e1937 ( 10.1371/journal.pntd.0001937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, Lindsay SW. 2015. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 31, 380–390. ( 10.1016/j.pt.2015.04.015) [DOI] [PubMed] [Google Scholar]
- 22.Kleinschmidt I, et al. 2018. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect. Dis. 18, 640–649. ( 10.1016/S1473-3099(18)30172-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes RJ, Moulton LH. 2017. Cluster randomized trials, 2nd edn Boca Raton, FL: Chapman & Hall. [Google Scholar]
- 24.James S, Simmons CP, James AA. 2011. Mosquito trials. Science 334, 771–772. ( 10.1126/science.1213798) [DOI] [PubMed] [Google Scholar]
- 25.Habluetzel A, Diallo DA, Esposito F, Lamizana L, Pagnoni F, Lengeler C, Traore C, Cousens SN. 1997. Do insecticide-treated curtains reduce all-cause child mortality in Burkina Faso? Trop. Med. Int. Health 2, 855–862. ( 10.1046/j.1365-3156.1997.d01-413.x) [DOI] [PubMed] [Google Scholar]
- 26.Mtove G, et al. 2016. The effectiveness of non-pyrethroid insecticide-treated durable wall lining to control malaria in rural Tanzania: study protocol for a two-armed cluster randomized trial. BMC Public Health 16, 633 ( 10.1186/s12889-016-3287-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander KA, et al. 2015. What factors might have led to the emergence of Ebola in West Africa?. PLoS Negl. Trop. Dis. 9, e0003652 ( 10.1371/journal.pntd.0003652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martens P, Hall L. 2000. Malaria on the move: human population movement and malaria transmission. Emerg. Infect. Dis. 6, 103–109. ( 10.3201/eid0602.000202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesolowski A, et al. 2015. Impact of human mobility on the emergence of dengue epidemics in Pakistan Trust Major Overseas Programme. Proc. Natl Acad. Sci. USA 112, 11 887–11 892. ( 10.1073/pnas.1504964112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerra CA, et al. 2019. Human mobility patterns and malaria importation on Bioko Island. Nat. Commun. 10, 2332 ( 10.1038/s41467-019-10339-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Menach A, Tatem AJ, Cohen JM, Hay SI, Randell H, Patil AP, Smith DL. 2011. Travel risk, malaria importation and malaria transmission in Zanzibar. Scient. Rep. 1, 93 ( 10.1038/srep00093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, Paz Soldan V, Kochel TJ, Kitron U, Elder JP, Scott TW. 2009. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl. Trop. Dis. 3, e481 ( 10.1371/journal.pntd.0000481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. 2017. Mass drug administration for falciparum malaria: a practical field manual. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 34.Stolk WA, Swaminathan S, van Oortmarssen GJ, Das K, Habbema JDF. 2003. Prospects for elimination of Bancroftian filariasis by mass drug treatment in Pondicherry, India: a simulation study. J. Infect. Dis. 188, 1371–1381. ( 10.1086/378354) [DOI] [PubMed] [Google Scholar]
- 35.Vegvari C, Truscott JE, Kura K, Anderson RM. 2019. Human population movement can impede the elimination of soil-transmitted helminth transmission in regions with heterogeneity in mass drug administration coverage and transmission potential between villages: a metapopulation analysis. Parasit. Vectors 12, 438 ( 10.1186/s13071-019-3612-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maia MF, Onyango SP, Thele M, Simfukwe ET, Turner EL, Moore SJ. 2013. Do topical repellents divert mosquitoes within a community? – Health equity implications of topical repellents as a mosquito bite prevention tool. PLoS ONE 8, e84875 ( 10.1371/journal.pone.0084875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Meillon B. 1937. Studies on insects of medical importance from southern Africa and adjacent territories. (Part IV.) Culicidae. 2. A note on Anopheles gambiae and Anopheles funestus in Northern Rhodesia. Publ. S. Afr. Inst. Med. Res. 40, 306–313. [Google Scholar]
- 38.Gillies MT. 1961. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bull. Entomol. Res. 52, 99–127. ( 10.1017/S0007485300055309) [DOI] [Google Scholar]
- 39.Epopa PS, Millogo AA, Collins CM, North A, Tripet F, Benedict MQ, Diabate A. 2017. The use of sequential mark-release-recapture experiments to estimate population size, survival and dispersal of male mosquitoes of the Anopheles gambiae complex in Bana, a west African humid savannah village. Parasit. Vectors 10, 376 ( 10.1186/s13071-017-2310-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson MC, Connor SJ, Quiñones ML, Jawara M, Todd J, Greenwood BM. 1995. Movement of Anopheles gambiae s.l. malaria vectors between villages in The Gambia. Med. Vet. Entomol. 9, 413–419. ( 10.1111/j.1365-2915.1995.tb00015.x) [DOI] [PubMed] [Google Scholar]
- 41.Meredith SEO, James AA. 1990. Biotechnology as applied to vectors and vector control. Ann. Parasitol. Hum. Comp. 65, 113–118. ( 10.1051/parasite/1990651113) [DOI] [PubMed] [Google Scholar]
- 42.Coleman PG, Alphey L. 2004. Editorial: genetic control of vector populations: an imminent prospect. Trop. Med. Int. Health 9, 433–437. ( 10.1111/j.1365-3156.2004.01225.x) [DOI] [PubMed] [Google Scholar]
- 43.Benedict MQ, Robinson AS. 2003. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 19, 349–355. ( 10.1016/S1471-4922(03)00144-2) [DOI] [PubMed] [Google Scholar]
- 44.James AA. 2005. Gene drive systems in mosquitoes: rules of the road. Trends Parasitol. 21, 64–67. ( 10.1016/j.pt.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 45.Zheng X, et al. 2019. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61. ( 10.1038/s41586-019-1407-9) [DOI] [PubMed] [Google Scholar]
- 46.Harris AF, et al. 2012. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828–830. ( 10.1038/nbt.2350) [DOI] [PubMed] [Google Scholar]
- 47.Homan T, et al. 2016. The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): a stepped-wedge cluster-randomised trial. Lancet 388, 1193–1201. ( 10.1016/S0140-6736(16)30445-7) [DOI] [PubMed] [Google Scholar]
- 48.Hiscox A, et al. 2016. Mass mosquito trapping for malaria control in western Kenya: study protocol for a stepped wedge cluster-randomised trial. Trials 17, 356 ( 10.1186/s13063-016-1469-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogola E, Villinger J, Mabuka D, Omondi D, Orindi B, Mutunga J, Owino V, Masiga DK. 2017. Composition of Anopheles mosquitoes, their blood-meal hosts, and Plasmodium falciparum infection rates in three islands with disparate bed net coverage in Lake Victoria, Kenya. Malar. J. 16, 1–12. ( 10.1186/s12936-017-2015-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peerawaranun P, et al. 2019. Intracluster correlation coefficients in the Greater Mekong Subregion for sample size calculations of cluster randomized malaria trials. Malar. J. 18, 428 ( 10.1186/s12936-019-3062-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dekkers OM, Elm EV, Algra A, Romijn JA, Vandenbroucke JP. 2010. How to assess the external validity of therapeutic trials: a conceptual approach. Int. J. Epidemiol. 39, 89–94. ( 10.1093/ije/dyp174) [DOI] [PubMed] [Google Scholar]
- 52.Osei-Atweneboana MY, Awadzi K, Attah SK, Boakye DA, Gyapong JO, Prichard RK. 2011. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 5, 998 ( 10.1371/journal.pntd.0000998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frankham R. 1997. Do island populations have less genetic variation than mainland populations? Heredity 78, 311–327. [DOI] [PubMed] [Google Scholar]
- 54.Hemingway J, Vontas J, Poupardin R, Raman J, Lines J, Schwabe C, Matias A, Kleinschmidt I. 2013. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc. Natl Acad. Sci. USA 110, 9397–9402. ( 10.1073/pnas.1307656110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO. 2017. Malaria Policy Advisory Committee Meeting, Geneva, Switzerland. Overview of WHO policy recommendations for malaria vector control interventions. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 56.Carvalho DO, Mckemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, Malavasi A, Capurro ML. 2015. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 9, e3864 ( 10.1371/journal.pntd.0003864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira LA, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139, 1268–1278. ( 10.1016/j.cell.2009.11.042) [DOI] [PubMed] [Google Scholar]
- 58.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 59.Turelli M, Barton NH. 2017. Deploying dengue-suppressing Wolbachia: robust models predict slow but effective spatial spread in Aedes aegypti. Theor. Popul. Biol. 115, 45–60. ( 10.1016/j.tpb.2017.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiggins FM. 2017. The spread of Wolbachia through mosquito populations. PLoS Biol. 15, e2002780 ( 10.1371/journal.pbio.2002780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt TL, et al. 2017. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 15, e2001894 ( 10.1371/journal.pbio.2001894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.WHO. 2018. Guinea-Bissau African region. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 63.Durrans S, Last A, Boiro H, Goncalves A, Mabey D, Greenland K. 2019. ‘Moving like birds’: a qualitative study of population mobility and health implications in the Bijagós Islands, Guinea Bissau. Soc. Sci. Med. 230, 204–213. ( 10.1016/j.socscimed.2019.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ant T, et al. 2020. A survey of Anopheles species composition and insecticide resistance on the island of Bubaque, Bijagos Archipelago, Guinea-Bissau.. Malar. J. 19, 27 ( 10.1186/s12936-020-3115-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marsden CD, et al. 2013. An analysis of two island groups as potential sites for trials of transgenic mosquitoes for malaria control. Evol. Appl. 6, 706–720. ( 10.1111/eva.12056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Awolola TS, Okwa O, Hunt RH, Ogunrinade AF, Coetzee M. 2002. Dynamics of the malaria-vector populations in coastal Lagos, south-western Nigeria. Ann. Trop. Med. Parasitol. 96, 75–82. ( 10.1179/000349802125000538) [DOI] [PubMed] [Google Scholar]
- 67.Jawara M, Pinder M, Drakeley CJ, Nwakanma DC, Jallow E, Bogh C, Lindsay SW, Conway DJ. 2008. Dry season ecology of Anopheles gambiae complex mosquitoes in The Gambia. Malar. J. 7, 156 ( 10.1186/1475-2875-7-156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marsden CD, et al. 2011. Asymmetric introgression between the M and S forms of the malaria vector, Anopheles gambiae, maintains divergence despite extensive hybridisation. Mol. Ecol. 20, 4983–4994. ( 10.1111/j.1365-294X.2011.05339.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Della Torre A, Tu Z, Petrarca V.. 2005. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect. Biochem. Mol. Biol. 35, 755–769. ( 10.1016/j.ibmb.2005.02.006) [DOI] [PubMed] [Google Scholar]
- 70.Foy BD, et al. 2019. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet 393, 1517–1526. ( 10.1016/S0140-6736(18)32321-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleinschmidt I, et al. 2015. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar. J. 14, 282 ( 10.1186/s12936-015-0782-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, Mosha FW, Kisinza W, Rowland M, Kleinschmidt I. 2014. Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med. 11, e1001630 ( 10.1371/journal.pmed.1001630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bradley J, Moulton LH, Hayes R. 2019. Analysis of the RIMDAMAL trial. Lancet 394, 1005–1006. ( 10.1016/S0140-6736(19)31663-0) [DOI] [PubMed] [Google Scholar]
- 74.Koroma JB, et al. 2018. Impact of five annual rounds of mass drug administration with ivermectin on onchocerciasis in Sierra Leone. Infect. Dis. Poverty 7, 30 ( 10.1186/s40249-018-0410-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dorkenoo A, Sodahlon Y, Mathieu E, Dubray C. 2011. Progress toward elimination of lymphatic filariasis — Togo, 2000–2009. Morbid. Mortal. Wkly Rep. 60, 989–991. [PubMed] [Google Scholar]
- 76.Coscione S, Esau T, Kekeubata E, Diau J, Asugeni R, Maclaren D, Steer AC, Kositz C, Marks M. 2018. Impact of ivermectin administered for scabies treatment on the prevalence of head lice in Atoifi, Solomon Islands. PLoS Negl. Trop. Dis. 12, e0006825 ( 10.1371/journal.pntd.0006825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin D, Wiegand R, Goodhew B, Mkocha H, Kasubi M. 2018. Impact of ivermectin mass drug administration for lymphatic filariasis on scabies in eight villages in Kongwa District, Tanzania. Am. J. Trop. Med. Hyg. 99, 937–939. ( 10.4269/ajtmh.18-0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohammed KA, Deb RM, Stanton MC, Molyneux DH. 2012. Soil transmitted helminths and scabies in Zanzibar, Tanzania following mass drug administration for lymphatic filariasis - a rapid assessment methodology to assess impact. Parasit. Vectors 5, 299 ( 10.1186/1756-3305-5-299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The Ivermectin Roadmappers. 2020. A roadmap for the development of ivermectin as a complementary malaria vector control tool. Am. J. Trop. Med. Hyg. 102, 3–24. ( 10.4269/ajtmh.19-0620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mekuriaw W, Balkew M, Messenger LA, Yewhalaw D, Woyessa A, Massebo F. 2019. The effect of ivermectin® on fertility, fecundity and mortality of Anopheles arabiensis fed on treated men in Ethiopia. Malar. J. 18, 357 ( 10.1186/s12936-019-2988-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sampaio VS, et al. 2016. Filling gaps on ivermectin knowledge: effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar. J. 15, 491 ( 10.1186/s12936-016-1540-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobylinski KC, et al. 2017. Ivermectin susceptibility and sporontocidal effect in Greater Mekong Subregion Anopheles. Malar. J. 16, 280 ( 10.1186/s12936-017-1923-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slater HC, et al. 2020. Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: a modelling study. Lancet. Infect. Dis. 20, P498–P508. ( 10.1016/S1473-3099(19)30633-4) [DOI] [PubMed] [Google Scholar]
- 84.Last AR, Burr SE, Harding-Esch E, Cassama E, Nabicassa M, Roberts C, Mabey DCW, Holland MJ, Bailey RL. 2017. The impact of a single round of community mass treatment with azithromycin on disease severity and ocular Chlamydia trachomatis load in treatment-naïve trachoma-endemic island communities in West Africa. Parasit. Vectors 10, 624 ( 10.1186/s13071-017-2566-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hugo LE, Quick-Miles S, Kay BH, Ryan PA. 2008. Evaluations of mosquito age grading techniques based on morphological changes. J. Med. Entomol. 45, 353–369. ( 10.1603/0022-2585(2008)45353:eomagt]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 86.Kenea O, Balkew M, Tekie H, Deressa W, Loha E, Overgaard HJ. 2019. Impact of combining indoor residual spraying and long-lasting insecticidal nets on Anopheles arabiensis in Ethiopia: results from a cluster randomized controlled trial. Malar. J. 18, 182 ( 10.1186/s12936-019-2811-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cameron E, et al. 2015. Defining the relationship between infection prevalence and clinical incidence of Plasmodium falciparum malaria. Nat. Commun. 6, 8170 ( 10.1038/ncomms9170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.King R. 2009. Geography, islands and migration in an era of global mobility. Isl. Stud. J. 4, 53–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.