Significance
Patients with small infarcts often demonstrate a poststroke acute dysexecutive syndrome resulting in failure to successfully re-integrate into society. The mechanism is poorly understood given that lesions are small and do not typically involve areas classically associated with cognitive decline. This knowledge gap makes designing treatment strategies difficult. We used MEG to evaluate changes in cerebral processing in individuals with small strokes. MEG showed a decrease in the amplitude of activation and temporal dispersion of activation peaks in areas responsible for visual word processing, along with an inability to modulate activity in response to task difficulty. These changes suggest disruption of network dynamics resulting in inefficient processing. Functional connectivity studies to isolate affected networks are the critical next step.
Keywords: stroke, recovery, magnetoencephalography
Abstract
Stroke patients with small central nervous system infarcts often demonstrate an acute dysexecutive syndrome characterized by difficulty with attention, concentration, and processing speed, independent of lesion size or location. We use magnetoencephalography (MEG) to show that disruption of network dynamics may be responsible. Nine patients with recent minor strokes and eight age-similar controls underwent cognitive screening using the Montreal cognitive assessment (MoCA) and MEG to evaluate differences in cerebral activation patterns. During MEG, subjects participated in a visual picture–word matching task. Task complexity was increased as testing progressed. Cluster-based permutation tests determined differences in activation patterns within the visual cortex, fusiform gyrus, and lateral temporal lobe. At visit 1, MoCA scores were significantly lower for patients than controls (median [interquartile range] = 26.0 [4] versus 29.5 [3], P = 0.005), and patient reaction times were increased. The amplitude of activation was significantly lower after infarct and demonstrated a pattern of temporal dispersion independent of stroke location. Differences were prominent in the fusiform gyrus and lateral temporal lobe. The pattern suggests that distributed network dysfunction may be responsible. Additionally, controls were able to modulate their cerebral activity based on task difficulty. In contrast, stroke patients exhibited the same low-amplitude response to all stimuli. Group differences remained, to a lesser degree, 6 mo later; while MoCA scores and reaction times improved for patients. This study suggests that function is a globally distributed property beyond area-specific functionality and illustrates the need for longer-term follow-up studies to determine whether abnormal activation patterns ultimately resolve or another mechanism underlies continued recovery.
Advances in acute stroke treatment have significantly reduced motor and language deficits, converting highly morbid large hemispheric lesions into smaller infarcts with better overall long-term outcomes (1, 2). Prior work has shown that the majority of individuals presenting for follow-up 4- to 6-wk postinfarct now exhibit what would be classified as “minor symptoms,” (3) with low stroke severity measured by the NIH Stroke Scale (NIHSS) (4) and modified Rankin Scale (mRS) (5) scores. Although these individuals lack a dense hemiparesis or aphasia, over half endorse some degree of cognitive impairment that significantly impacts their recovery. Interestingly, these symptoms are typically found to be independent of stroke size, location, or coexisting depression (6, 7).
Poststroke cognitive decline has a substantial presence in the literature (8–13). However, we find that rather than memory impairment or confusion, patients without prior cognitive disability report immediate difficulty with executive function, focus, concentration, and attention after a minor stroke, hereafter referred to as poststroke acute dysexecutive syndrome (PSADES) (3). Dysexecutive syndrome has been previously described in individuals with anatomic lesions (14) as well as disorders, such as schizophrenia (15) and Alzheimer’s disease (14), affecting the frontal lobes. When mild, the syndrome can be hard for others to appreciate, particularly, in previously high-functioning individuals, but poststroke, these deficits are detectable on screening tests, such as the Montreal cognitive assessment (MoCA) (16) and other scales of activities of daily living compared to age-matched controls (3). Despite the fact that following stroke, symptoms typically improve over the first 3–6 mo of recovery, PSADES impedes many successful well-educated individuals from returning to cognitively driven professions given the uncertainty of their prognosis. These decisions affect lifestyle and quality of life, resulting in lasting long-term consequences.
The pathophysiology underlying PSADES is poorly understood, as many times the inciting infarct is small and does not involve an area of the brain classically thought to be important for cognitive processing. Cognitive change due to deep white matter lesions (in multiplicity) has been well described (17), but there is no clear unifying physiological explanation regarding how a single small cortical or subcortical lesion may cause significant generalized cortical dysfunction. Some posit a “network” hypothesis suggesting that an individual requires an extensive system of neuronal connectivity, involving numerous cortical and subcortical regions, in order to complete a task (18). We propose that the cognitive dysfunction of PSADES may be the result of a disruption of general network dynamics due to lesions of the subcortical white matter tracts, which would, in turn, interfere with basic network function.
This study was designed as a first step in evaluating the role of network dynamics during tasks requiring attention, concentration, speed, and accuracy; all skills difficult for patients poststroke. We used magnetoencephalography (MEG) to determine the differences in cerebral activation patterns in nine individuals with small strokes versus a group of eight age-similar controls by measuring the amplitude and latency of cerebral responses during a visual comprehension task at two time points: ∼1- and 6-mo postinfarct. Our analysis focused on the early visual, M170, and M400 components of the event-related potential from the occipital lobe, fusiform gyrus, and lateral temporal lobe given their importance in visual recognition and language processing (19–22).
Materials and Methods
Study Population.
This study was approved by the Johns Hopkins University institutional review board. All participants provided written informed consent. Adult patients (age 18–70 y) recently hospitalized with MRI evidence of acute ischemic stroke at Johns Hopkins Bayview Medical Center, a large, urban Comprehensive Stroke Center, presented to the outpatient clinic for follow-up 4–6 wk following their admission. To avoid the confounding effects of severe hemiplegia or aphasia, only patients with minor stroke (NIHSS score on admission of <8) were recruited for participation. Individuals with large vessel occlusions (M1 and M2 branches) were also excluded. All patients were right handed with English as their native language. Those with prior history of dementia or incompletely treated psychiatric disease, uncorrected vision, hearing loss, or contraindication to MRI or MEG (claustrophobia, presence of metal implants, and severe obesity) were also excluded. Individuals underwent a comprehensive neurological examination and demonstrated no evidence of difficulty with reading, writing, naming, or comprehending written or spoken stimuli on baseline screening for aphasia, although formal testing of reading accuracy and response times was not performed. An age-similar population of individuals without history of prior stroke was recruited as a control group for comparison. Cognitive and functional status were assessed using: the MoCA, NIHSS, mRS, Barthel Index (23), and F-A-S verbal fluency test (24). Student’s t tests were used to compare means between stroke patients and controls for continuous variables, and χ2 analysis was used for categorical variables at each visit. However, over concern for the relatively small sample size and potentially skewed distribution, MoCA scores were reported using the median and interquartile range (IQR). Medians were compared across groups (strokes versus controls) using the nonparametric Mann–Whitney U Test and across time points (visit 1 versus visit 2) using the Wilcoxon Signed Rank Test. Similarly, given the relatively good functional status of both populations, mRSs were compared as a binary variable (0 versus 1) using χ2 analysis.
MEG Recording.
Magnetic fields were recorded using a 157-channel whole-head MEG system (KIT-Eagle Technology, Kanazawa Japan) with an online 200-Hz low-pass filter and a 60-Hz notch filter at a sampling rate of 1 kHz. Before the recording event, each participant’s head shape was digitized using three fiducials and five marker positions. Five coils attached to the participant’s head at the marker points were used to localize their head position relative to the MEG sensors. Head position was determined before and after the task to account for possible movement. During the recording session, participants were positioned lying down in a dimly lit magnetically shielded room (VAC, Hanau, Germany). Magnetic sensors were arranged in a uniform helmet-shaped array at the bottom of the recording dewar with ∼25 mm between the centers of each 15.5-mm-diameter coil. In this device, sensors are configured as first-order axial gradiometers with a baseline of 50 mm and field sensitivity of 5 fT/√Hz or better in the white noise region (>1 kHz). For more detailed information regarding MEG image acquisition, quality measures, data processing, and analysis, please see our SI Appendix.
Visual Comprehension Task.
Magnetic fields were recorded while the participant completed a visual picture–word matching task. The task was composed of four parts: familiarization, match mismatch, pairs naming, and pairs description. Participants began with familiarization, during which they were presented with a series of 30 images taken from the Boston Naming Test (25). Each image was projected onto a screen ∼2 ft in front of them followed after a brief pause by the object’s name: [image (2 s) → pause (2 s) → word (2 s)]. After familiarization, participants began the matching paradigms. During match mismatch, they were presented with the same sequence, but the word matched the object’s name for only half the trials. For the other half of the trials, the word was the name of another object seen during familiarization. Participants had to decide as quickly and accurately as possible whether the word matched the image and push the button corresponding to “yes” or “no” (n = 60). All images and words had been previously seen during the familiarization phase. Pairs naming and pairs description were purposefully designed to be more cognitively challenging. For pairs naming, a pair of images (either semantically related [n = 24] or with the same first sound [n = 24]) were presented together; one above the other on the screen. After 4 s, a fixation cross appeared in between the images, to guide attention to the center of the screen. After another 2 s, the fixation cross was replaced by a word (the name of one of the objects). The participant was asked to decide which image the name corresponded to by pushing button “A” or “B.” Pairs description was identical to pairs naming, except that rather than a name, the presented word was a one-word description of one of the images. Participants had not seen the descriptions before, but they were composed of familiar words of significantly higher frequency (26) than the object names (mean logarithm word frequency 3.2 versus 2.1, P < 0.001). For each task, reaction times were measured from the time the word first appeared on the screen to the time the participant pushed the response button. Responses were classified as appropriate versus inappropriate based on whether they were correct in their match and were recorded along with the MEG signal for further processing.
Data Processing.
Continuous MEG raw data were analyzed using Eelbrain 0.31.7 and MNE-Python 0.19.0 software packages (27–29). Flat and noisy channels were excluded from analysis. Extraneous artifacts were removed with temporal signal space separation (30). Then, data were band-pass filtered between 1 and 40 Hz using a zero-phase finite impulse response filter (MNE-Python default settings). For each recording, independent component analysis was used to remove biological artifacts, such as eye blinks and heartbeat. Data epochs related to presentation of images or words were then extracted from −100–1,000 ms relative to stimulus onset and down-sampled to 200 Hz. Epochs containing recording artifacts were detected by visual examination and excluded. Trials with incorrect responses were also removed along with trials with reaction times greater than 2 SDs above the subject’s mean reaction time for the subtask (mean number removed = 16.2 trials for patients and 9.5 for controls). In total, 87% of epochs were included for patients and 94% for controls. These epochs were averaged to estimate the response to images and words for each participant.
Sensor rms Analysis.
For an initial estimate of response magnitudes, epochs from all tasks were combined, and averaged responses were analyzed using the rms across sensors. The rms was computed for each time point in the response, and the resulting time series were analyzed using cluster-based permutation tests (31). For each time point between 50 and 600 ms a t value was computed comparing activation between individuals with minor stroke and controls. This time window was chosen to include our predetermined peaks of interest (visual, M170, and M400). Clusters were formed based on contiguous time points where the t value exceeded a value equivalent to uncorrected P = 0.05. Each cluster was evaluated by comparing its cluster mass (sum of all t values in the cluster) with a distribution determined from 10,000 random permutations of the data. When the P value was between 0.040–0.060, a 10-fold increase in the number of permutations was used to verify the precision of the result. The rms analysis was performed separately to compare stroke patients and controls for visit 1 and visit 2. Independent t tests were used to compare activation patterns between groups at each visit. Paired t tests were used to compare differences for each group between visits.
Source Localization Analysis.
The digitized head shapes were used to coregister the “fsaverage” template brain provided with FreeSurfer (32) to each participant’s head using uniform scaling, translation, and rotation. A source space was defined based on fourfold icosahedral subdivision of the white matter surface with virtual current dipoles having a perpendicular orientation relative to the surface. Activity, time locked to the events of interest, was source localized using distributed minimum norm estimates with dynamic statistical parametric mapping (dSPM) normalization (33, 34). Spatiotemporal maps of brain activity were extracted from three predetermined areas of interest. These areas were defined by combining multiple labeled areas from the “aparc” parcellation: occipital lobe (combining aparc labels: pericalcarine fissure, cuneus, and lateral occipital lobe), fusiform gyrus (single aparc label), and lateral temporal lobe (combining aparc labels: superior, middle, inferior, transverse temporal gyri, superior temporal sulcus, and temporal pole) (31, 35, 36). These areas were chosen because of our interest in the early visual, word form (M170), and semantic processing (M400) responses. The anatomical regions are displayed in Fig. 1. Preliminary review confirmed expected responses in these areas (Fig. 1). Spatiotemporal cluster-based permutation tests based on group (2) × task (4) ANOVA F values were used to detect significant effects in each area. Activity time courses were then visualized in regions of interest (ROIs) based on significant clusters. Large clusters (based on positive F values) often combined regions with positive and negative (signed) current directions due to varying cortical folding. In the fusiform gyrus, we accounted for this by selecting clusters with constant orientation (anterior versus posterior fusiform gyrus). This was not possible for the lateral temporal lobe because all clusters spanned several gyri. In order to visualize temporal activity, which is dominated by currents in the vertical direction, we flipped the sign of the signal at all downward-pointing current dipoles before averaging. To decide whether to collapse the data points across groups or across tasks when visualizing main effect clusters, we tested for an interaction with a less conservative univariate ANOVA on the average value per participant/condition in each cluster. Cluster-based tests using independent t tests were used to evaluate for differences in activation between groups. Paired t tests were used to compare changes within groups between visits.
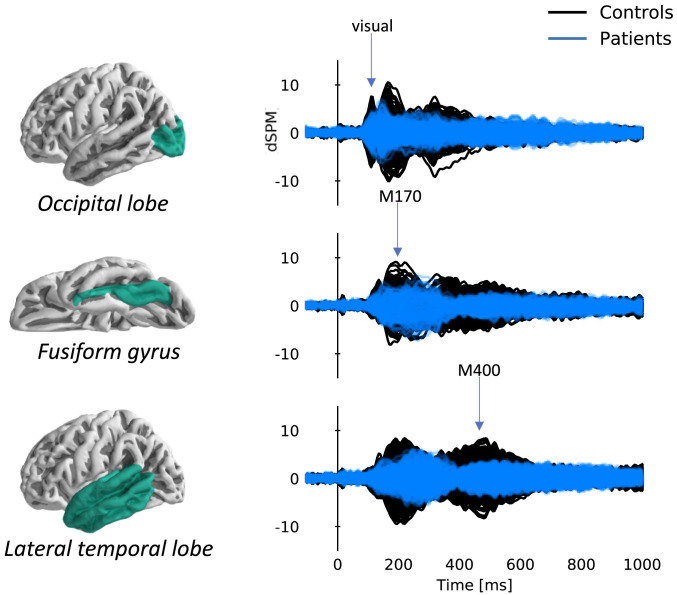
Fig. 1.
Overlay plots of cerebral activation time courses corresponding to each of the predefined areas of interest highlighted in green (created by combining aparc labels as described in our Methods section): the occipital lobe (visual response), fusiform gyrus (M170 word form response) and lateral temporal lobe (M400 semantic processing response) for stroke patients and controls at visit 1 (n = 9 patients and 8 controls). Responses shown correspond to the average response to all words included in the analysis. Each trace corresponds to one virtual current dipole in the source model. dSPM, related to a standardized Z score, represents the current estimate normalized by the variance of the noise estimate (34). Note the decreased amplitude and lack of clear peaks for stroke patients compared to controls.
Results
Comparison of Behavioral Outcomes.
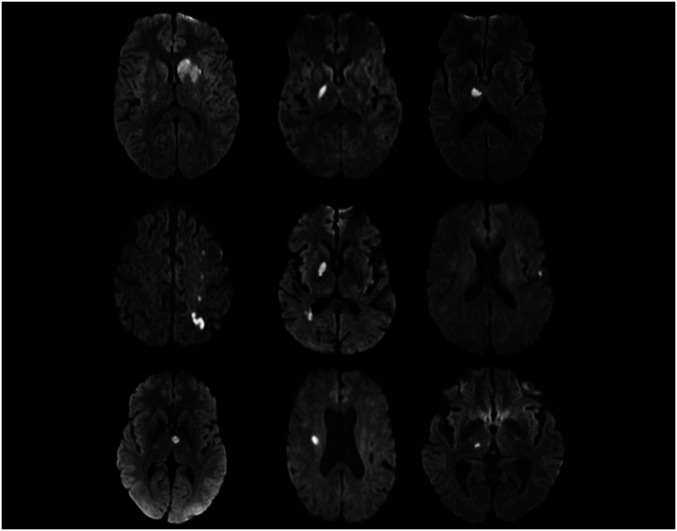
Nine patients with minor strokes were recruited over the study period along with eight age-similar controls. Six in each group were able to return for visit 2. Due to the COVID-19 pandemic recruitment was halted and the others were unable to return for visit 2. All patients were classified as “minor stroke” based on our predefined inclusion criteria. The average time from stroke onset to first MEG assessment was 51 d; 239 d to visit 2. The average stroke volume was 2.8 cc. Four of the nine lesions were located in the left hemisphere, and the majority were subcortical lacunar strokes involving white matter pathways. MRIs illustrating the size and location of the infarcts are displayed in Fig. 2.
Fig. 2.
Clinical MRI scans of the nine stroke patients (diffusion weighted imaging sequences) obtained during their hospital admission showing small acute infarcts without involvement of areas typically associated with cognitive dysfunction.
Patients with stroke were similar to controls (Table 1) except for a lower level of baseline education and a higher medical comorbidity index (driven predominantly by the stroke itself). Cognitive scores on the MoCA were significantly lower for stroke patients at visit 1 compared to controls (median score [IQR] = 26.0 [4] versus 29.5 [3], P = 0.005) despite other similarities. Reaction times for all parts of the Visual Comprehension Task tended to be longer for stroke patients with the greatest clinical effect seen during the pairs naming portion of the task, and stroke patients made a larger number of errors compared to controls (Table 1). Results remained consistent when only those participants (n = 6 patients and 6 controls) who completed both visits were compared. At visit 2, cognitive performance was no longer significantly different between groups, although scores were still lower for stroke patients. Reaction times improved for both patients and controls. Even though tests comparing performance at visit 1 to visit 2 for each group were not significant, a numerically greater improvement in stroke patients both with respect to the MoCA and their reaction times on the visual task resulted in a reduction of the overall difference between groups. Individual subject scores are reported in the SI Appendix.
Table 1.
Clinical characteristics and behavioral outcomes
| Visit 1 | Visit 2 | |||||
| Controls (n = 8) | Strokes (n = 9) | P | Controls (n = 6) | Strokes (n = 6) | P | |
| Age (mean years, SD) | 58.0 (13.1) | 59.8 (15.7) | 0.805 | |||
| Sex (n male, %) | 4 (50) | 4 (44) | 0.819 | |||
| Race (n black, %) | 2 (25) | 4 (44) | 0.402 | |||
| Handedness (n right, %) | 7 (88) | 9 (100) | 0.274 | |||
| Level of education (mean years, SD) | 18.6 (3.6) | 14.1 (4.4) | 0.036 | |||
| Occupation (level) | 0.266 | |||||
| History of dementia (n, %) | 0 (0) | 0 (0) | 1.000 | |||
| Family support (n, %) | 8 (100) | 7 (78) | 0.156 | |||
| Ambulation status (n walking, %) | 8 (100) | 9 (100) | 1.000 | |||
| Comorbidity index (mean points, SD) | 0.5 (1.4) | 2.2 (1.7) | 0.041 | |||
| Driving (n, %) | 8 (100) | 6 (67) | 0.072 | |||
| Hemisphere (n left, %) | 4 (44) | |||||
| Volume (mean cc, SD) | 2.8 (3.5) | |||||
| Time to the MEG (mean days, SD) | 51.4 (20.5) | 239.2 (12.5) | ||||
| Hours of sleep preimaging (mean hours, SD) | 7.1 (1.6) | 6.9 (2.0) | 0.841 | 6.6 (0.8) | 6.8 (2.2) | 0.801 |
| Clinical performance | ||||||
| NIHSS (mean points, SD) | 0 (0) | 0.7 (1.1) | 0.114 | 0 (0) | 0.2 (0.4) | 0.341 |
| mRS (median, IQR) | 0 (0) | 1.0 (0) | <0.001* | 0 (0) | 0.5 (1) | 0.046* |
| MoCA (median, IQR) | 29.5 (3) | 26.0 (4) | 0.005† | 29.5 (1) | 27.0 (3) | 0.221† |
| Barthel Index (mean points, SD) | 100 (0) | 100 (0) | 1.000 | 100 (0) | 100 (0) | 1.000 |
| F letters (mean n, SD) | 14.6 (4.1) | 14.1 (4.9) | 0.829 | 16.5 (4.0) | 15.6 (8.5) | 0.822 |
| S letters (mean n, SD) | 15.6 (3.2) | 15.8 (7.7) | 0.967 | 18.3 (5.5) | 15.4 (6.9) | 0.45 |
| Boys names (mean n, SD) | 19.9 (2.9) | 18.5 (7.5) | 0.636 | 22.5 (4.2) | 21.2 (8.5) | 0.748 |
| MEG visual comprehension task | ||||||
| Reaction time total (mean s, SD) | 0.8818 (0.2398) | 1.5343 (0.9247) | 0.0724 | 0.7615 (0.1590) | 1.1423 (0.5565) | 0.138 |
| Number incorrect total (mean n, SD) | 3.0 (1.2) | 9.7 (7.9) | 0.032 | 2.0 (1.3) | 13.8 (19.0) | 0.152 |
| Match mismatch | ||||||
| Reaction time total (mean s, SD) | 0.7155 (0.2349) | 1.3212 (1.0075) | 0.144 | 0.7127 (0.2088) | 0.9618 (0.6260) | 0.377 |
| Reaction time correct (mean s, SD) | 0.7132 (0.2339) | 1.2221 (0.9465) | 0.189 | 0.7129 (0.2085) | 0.9627 (0.6203) | 0.372 |
| Reaction time incorrect (mean s, SD) | 0.8651 (0.5379) | 1.9160 (1.7927) | 0.2915 | 0.5848 (0.1779) | 0.9606 (0.7679) | 0.449 |
| Number incorrect (mean n, SD) | 0.9 (0.7) | 3.6 (4.0) | 0.102 | 0.5 (0.0548) | 2.2 (2.1) | 0.094 |
| Pairs naming | ||||||
| Reaction time total (mean s, SD) | 0.8136 (0.1899) | 1.5333 (0.9062) | 0.044 | 0.7009 (0.1418) | 1.0225 (0.4157) | 0.103 |
| Reaction time correct (mean s, SD) | 0.8098 (0.1857) | 1.4536 (0.7162) | 0.026 | 0.7000 (0.1415) | 1.0501 (0.4783) | 0.116 |
| Reaction time incorrect (mean s, SD) | 1.2410 (0.2557) | 2.5409 (2.8480) | 0.471 | 0.8887 (0.0255) | 1.0144 (0.4146) | 0.702 |
| Number incorrect (mean n, SD) | 0.6 (0.9) | 2.6 (2.8) | 0.086 | 0.3 (0.5) | 5.7 (8.6) | 0.159 |
| Pairs description | ||||||
| Reaction time total (mean s, SD) | 1.1155 (0.3492) | 1.8096 (0.9939) | 0.081 | 0.8810 (0.1530) | 1.4776 (0.6289) | 0.048 |
| Reaction time correct (mean s, SD) | 1.1086 (0.3390) | 1.7149 (0.8194) | 0.071 | 0.8783 (0.1567) | 1.5103 (0.7136) | 0.06 |
| Reaction time incorrect (mean s, SD) | 1.3669 (0.8357) | 2.7742 (2.6739) | 0.175 | 0.9369 (0.2865) | 1.6812 (0.5598) | 0.0294 |
| Number incorrect (mean n, SD) | 1.6 (0.9) | 3.6 (2.9) | 0.094 | 1.2 (1.0) | 6 (8.5) | 0.195 |
Means compared using Student’s t test; percentages compared using χ2 analyses.
mRS 0 versus 1 compared using χ2 analysis.
MOCA scores compared using the Mann–Whitney U Test.
MEG rms Differences: Decreased Amplitude and Temporal Dispersion.
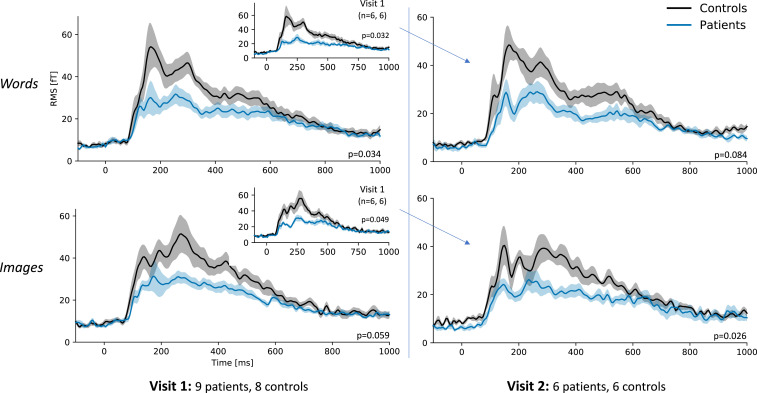
The overall magnitude of brain responses to images and words was assessed by taking the rms over all sensors (Fig. 3). Compared to controls, stroke patients exhibited a significantly different pattern of activation: reduced response to words and lack of clear activation peaks (temporal dispersion) between 50 and 600 ms, P = 0.034). A similar pattern was seen following image presentation (P = 0.059). When restricted to the participants who underwent imaging at both visits, groups were significantly different at visit 1 for both words and images (P = 0.032 and P = 0.049, respectively). At visit 2, the responses of patients and controls appeared to be more similar, especially when directly comparing to the group of 12 subjects with complete data for both visits (see Fig. 3 inlay). In addition, there was no longer a significant difference between groups in the response to words. However, paired t tests comparing patterns of activation between visit 1 and visit 2 did not demonstrate a statistically significant change in activation pattern between visits for either group.
Fig. 3.
MEG rms analysis demonstrating a significant difference in activation patterns between 50 and 600 ms for both words and images at visit 1 for stroke patients versus controls. This difference was no longer significant for words at visit 2. Activation patterns appear more visually similar between groups at visit 2, especially when compared to the visit 1 activation patterns of only the participants (n = 6 patients and 6 controls) who had repeat imaging performed (Inlays). Error bars represent the within-subject SEM (48).
Source Localization: The Effect of Task Difficulty and Modulation of Cerebral Activation.
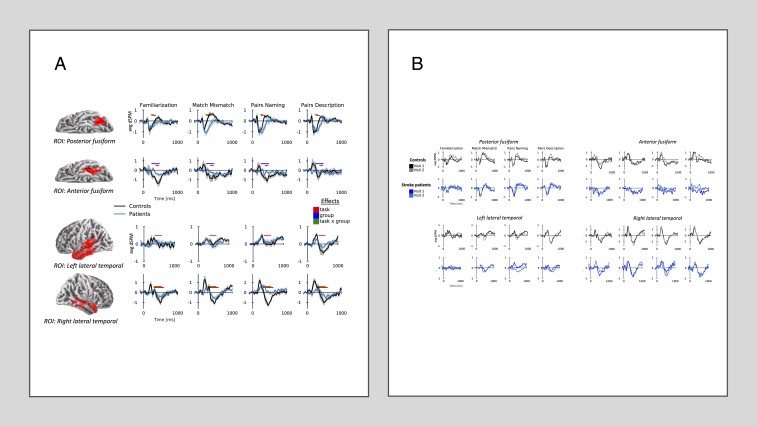
Source analysis was used to individually evaluate the response to words within the three predefined areas of interest, chosen to evaluate early visual, word form, and semantic processing responses. At visit 1, within the occipital lobe (50–150 ms), there were no significant differences seen between groups or as a function of task difficulty. Analysis of the bilateral fusiform gyri (150–600 ms) yielded several overlapping significant clusters (Table 2). Two clusters demonstrated a significant group by task interaction because all clusters exhibited a similar interaction when less conservatively evaluated data were not collapsed across tasks or groups. Fig. 4A shows the time course of activation based on representative clusters in the left anterior and posterior fusiform gyri (20). In the posterior fusiform gyrus, both groups exhibited an early response characterized by negative deflection. Significantly, in controls only, this response was followed by a positive deflection which varied in amplitude depending on the task. In the anterior fusiform gyrus, the later response was more pronounced than in the posterior fusiform gyrus. In general, controls exhibited the ability to modulate the amplitude of their cerebral activation in response to task difficulty, while stroke patients displayed activation of consistently low amplitude for all stimuli. For subjects who underwent complete testing (both visit 1 and visit 2; n = 6 stroke patients and 6 controls), the results for both patients and controls remained relatively consistent across visits within each ROI (Fig. 4B).
Table 2.
ANOVA evaluating group and task differences within the fusiform gyrus and lateral temporal lobe at visits 1 and 2 with significant clusters
| Sources (n) | Hemisphere | tstart (ms) | tstop (ms) | v | p | Effect |
| Fusiform gyrus | ||||||
| Visit 1 | ||||||
| 44 | Left | 0.360 | 0.460 | 1316.7 | 0.023 | Task |
| 33 | Left | 0.245 | 0.375 | 1033.3 | 0.044 | Task |
| 71 | Right | 0.205 | 0.560 | 7345.9 | <0.001 | Task |
| 47 | Left | 0.240 | 0.480 | 5778 | 0.041 | Group |
| 49 | Left | 0.220 | 0.315 | 1175.3 | 0.032 | Task × group |
| 51 | Right | 0.285 | 0.410 | 1055.8 | 0.042 | Task × group |
| Visit 2 | ||||||
| 34 | Left | 0.170 | 0.300 | 1122.6 | 0.048 | Task |
| 57 | Left | 0.315 | 0.555 | 3847 | <0.001 | Task |
| 66 | Right | 0.190 | 0.525 | 6452.3 | <0.001 | Task |
| Lateral temporal lobe | ||||||
| Visit 1 | ||||||
| 233 | Left (pole) | 0.355 | 0.585 | 7757.4 | 0.001 | Task |
| 243 | Right (superior) | 0.300 | 0.600 | 15501 | <0.001 | Task |
| 120 | Right (superior) | 0.300 | 0.535 | 3499.9 | 0.025 | Task × group |
| Visit 2 | ||||||
| 202 | Left (superior) | 0.330 | 0.495 | 6416.6 | 0.004 | Task |
| 247 | Right (superior) | 0.300 | 0.595 | 13578 | <0.001 | Task |
Task by group interactions are present at visit 1 but not visit 2.
Fig. 4.
(A) At visit 1 there are task effects by the group (n = 9 patients and 8 controls) present within the fusiform gyrus and lateral temporal lobe. Bars indicate the temporal extent of significant clusters found in the spatiotemporal cluster-based analysis. Time courses are shown within ROIs defined from significant clusters, outlined in red. Times series demonstrate that for controls, the shape of the waveform varies as a function of task while remaining relatively flat and typically around zero for stroke patients. dSPM, related to a Z score, represents the current estimate normalized by the variance of the noise estimate but is averaged across sources in the ROI, which accounts for the lower amplitude compared to Fig. 1. Error bars indicate the within-subject SEM. (B) Waveforms remain consistent for both stroke patients and controls across visits. Only subjects with complete data from visits 1 and 2 (n = 6 patients and 6 controls) are displayed for comparison.
A similar pattern was seen bilaterally in the lateral temporal lobes (300–600 ms), where three significant clusters were found. One cluster conservatively demonstrated the task by group interaction; however, all three were found, on less conservative testing, to display a similar pattern. This task by group interaction disappeared at visit 2 in both the fusiform gyrus and the lateral temporal lobe (Table 2) as stroke patients began to demonstrate increased variability of activation across tasks, although there was not enough improvement to be considered significantly different between visits.
Discussion
Poststroke dementia is well documented in the literature, particularly, after large cortical strokes affecting areas known to be important in cognitive function (37–40). In contrast, the acute dysexecutive dysfunction observed in individuals with small cortical or subcortical infarcts is a distinct syndrome, presenting as impairment of attention, concentration, and processing speed. Given the typically small infarct size and varying locations of the lesions, the physiology of this dysfunction is not immediately clear. Our results confirm that clinically there is a difference between groups both in overall cognition (MoCA scores) and reaction times. While the differences in scores may at first appear small (3.5 points on the MoCA at visit 1), it is important to point out that this was a “cognitively normal,” relatively high-functioning cohort prestroke with an average age of only 60 y. In this population, MoCA scores would be expected to be well above 26. Furthermore, the difference in cognition for an individual with a MoCA score of 26 versus 30 is great with a large decrement represented per point decrease. Therefore, a difference in 3.5 points would be seen by physicians as large and clinically important. We also saw an improvement over time within the stroke group. Again, although small, this change is also clinically meaningful and illustrates that their initial visit 1 MoCA score did not represent their cognitive baseline. Notably, we saw that while stroke patients performed worse than controls at both visits but improved over time, controls displayed similar scores at visits 1 and 2, arguing against improvement being due solely to a practice effect.
Comparable to cognitive decline, longer reaction times have been reported in older individuals and those with lower levels of education (41); however, our findings correspond to patient-reported outcomes of feeling “slow and fuzzy,” not at their baseline. Additionally, with each patient serving as their own control and demonstrating improvement between visit 1 and visit 2, the educational difference alone is unlikely to explain the results. These differences in reaction time are small but consistent across tasks that require attention, concentration, and are time sensitive. Importantly, they are also functionally meaningful to patients. Slowed responses are accompanied by alterations in cerebral activation patterns on MEG, indicating that MEG may be a useful biomarker to gauge both severity and improvement in individuals who report such symptoms following their infarct.
The pattern of reduced amplitude and temporal dispersion observed on MEG during task completion may provide some insight into the underlying neurophysiology of PSADES. We suggest that the dysfunction results from disruption of the timing coherence of the signal between the thalamus and the cortex or between separate cortical regions, similar to that responsible for the temporal dispersion seen on the EMG within the peripheral nervous system with incomplete nerve lesions involving axon bundles (42). The overall functional picture relates to the issue of altered isochronicity, a fundamental property of brain function (43). Stimuli that require significant processing (images and words that need to be matched) display a significant difference in evoked response between groups as the necessary information is delayed in reaching its target. Likewise, areas responsible for higher degrees of processing during a comprehension task (e.g., fusiform gyrus and lateral temporal lobe) show a larger effect size difference in response than other areas, such as visual cortex. This suggests that the more cognitive processing required, the larger the difference between groups, consistent with a disconnect in the processing loop.
More direct indications that the observed temporal dispersion arises from thalamocortical or corticocortical disruption could be obtained from MEG studies evaluating connectivity in individuals with minor strokes. However, even when confirmed, the question remains, how does such disruption result in symptomatic cognitive dysfunction? The issue of processing speed may be one of latency. It is well established that a single task does not occur within the brain in isolation. The importance of thalamocortical projections has been described (44). During completion of even simple tasks information must pass back and forth between the cortex and the thalamus multiple times in order to be fully processed. In order for a visual image to be perceived it must appear first in the visual cortex within the occipital lobe. The signal then travels from the primary visual cortex to association cortices within the frontal, temporal, and parietal regions before moving through the limbic system and ultimately to areas responsible for action. The thalamus integrates this information between processing points. By placing a lesion along the path, each pass between the cortex and the thalamus results in an “accumulating latency.” We suggest that for functions requiring multiple relays between the thalamus and the cortical areas signal disruption is magnified. As a result, even small strokes disrupting the network result in significantly slowed response times and noticeable difficulty performing activities of daily living quickly and accurately. Corticocortical signals would suffer analogous degradation. These results are consistent with other studies showing injury to white matter tracts results in decreased processing speed for brain injured individuals (45).
We also observed a difference in the ability to modulate cerebral activity in response to task difficulty. At visit 1, controls were able to vary their amplitude of activation between tasks. Stroke patients demonstrated little variability, displaying uniform responses for all task components. Results were consistent at visit 2; however, there was no longer a significant task by group interaction as stroke patients began to display some variation in activation. This ability to modulate activity based on the difficulty of the task allows for cognitive efficiency. An inability to modulate may indicate an inefficient system, resulting in the need for significant effort to be put forth for all tasks, ultimately leading to cognitive exhaustion, and may explain some of the cognitive fatigue endorsed by patients early in their recovery course (46). Whether the inability to modulate activity is due to increased psychological stressors in patients after stroke or an alternative etiology, patients began to show improved modulation of neural activity along with their improved cognitive performance and reaction times at visit 2.
In this study, we describe an acute dysexecutive syndrome due to network disruption rather than dysfunction of a particular brain region. While determining the underlying changes in cerebral activation in those with PSADES is a critical first step, it leaves many unanswered questions. There is some improvement in activity modulation for tasks for stroke patients, which may account for some of the reported improvement in cognitive fatigue 3- to 6-mo poststroke; however, there is no significant change in the pattern of temporal dispersion, despite improved clinical scores at visit 2. It is unclear if over time the differences will resolve as there is rerouting of white matter tracts in the thalamocortical or corticocortical circuits. Formal tests of functional connectivity may better elucidate the changes and potential reorganization of networks leading to improvement.
This study has limitations. It is a relatively small sample of patients due to the need to halt recruitment in the setting of a pandemic. This may have contributed to less robust statistical results with respect to the cognitive differences, activation patterns, and changes over time between groups, particularly, when the same tools were used to reevaluate performance only 6 mo later. However, the greater than 3-point difference in cognition between stroke patients and controls is a difference that would be viewed as clinically important by physicians, particularly, since this was a group without previous cognitive complaints. While there is the possibility of practice effect impacting results, neuropsychological tests such as the MoCA are routinely readministered for longitudinal follow-up at the 6-mo time point, and prior studies have reported relatively good sensitivity and specificity with readministration of the MoCA, even within the first 3 mo (47). Most importantly, no such practice effect was seen in the control group, arguing against it being the sole cause of clinical improvement.
Despite any limitations, this study represents an important advancement in understanding the mechanisms underlying PSADES and describing network disruption as a potential cause of a dysexecutive syndrome rather than purely anatomical dysfunction. We have demonstrated that acutely, patients with even minor strokes can have impaired overall cognitive performance compared to age-similar controls with specific difficulties with attention, concentration, and processing speed; and that patterns of activation on MEG demonstrate decreased amplitude and temporal dispersion. Further studies evaluating thalamocortical and corticocortical connectivity to confirm the underlying pathophysiology of PSADES and explore the potential to modify the impaired circuits through treatment interventions are needed.
Supplementary Material
Acknowledgments
We thank The Stroke Center at Johns Hopkins Bayview Medical Center along with Erin Lawrence and Dawn Merbach for their tireless work in caring for patients and recruiting for the study. This study was funded, in part, through an American Heart Association Innovative Project Award (AHA 18IPA34170313) and through the generous support of the Iorizzo family.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2013231117/-/DCSupplemental.
Data Availability.
Anonymized MEG fif files have been deposited in the University of Maryland Data Repositories (https://doi.org/10.13016/n9j1-mwne). All other study data are included in the article and SI Appendix.
References
- 1.Demchuk A. M. et al.; ESCAPE Trial Investigators , Endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) trial: Methodology. Int. J. Stroke 10, 429–438 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Campbell B. C. et al.; EXTEND-IA Investigators , Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 372, 1009–1018 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Marsh E. B., et al. , Pre-stroke employment results in better patient-reported outcomes after minor stroke: Short title: Functional outcomes after minor stroke. Clin. Neurol. Neurosurg. 165, 38–42 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Brott T., et al. , Measurements of acute cerebral infarction: A clinical examination scale. Stroke 20, 864–870 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Rankin J., Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott. Med. J. 2, 200–215 (1957). [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., et al. , Depression after minor stroke: Prevalence and predictors. J. Psychosom. Res. 79, 143–147 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Winward C., Sackley C., Metha Z., Rothwell P. M., A population-based study of the prevalence of fatigue after transient ischemic attack and minor stroke. Stroke 40, 757–761 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Pendlebury S. T., Rothwell P. M., Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 8, 1006–1018 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Pendlebury S. T., Cuthbertson F. C., Welch S. J., Mehta Z., Rothwell P. M., Underestimation of cognitive impairment by mini-mental state examination versus the montreal cognitive assessment in patients with transient ischemic attack and stroke: A population-based study. Stroke 41, 1290–1293 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Pendlebury S. T., Wadling S., Silver L. E., Mehta Z., Rothwell P. M., Transient cognitive impairment in TIA and minor stroke. Stroke 42, 3116–3121 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Leys D., Hénon H., Mackowiak-Cordoliani M. A., Pasquier F., Poststroke dementia. Lancet Neurol. 4, 752–759 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Hénon H., et al. , Poststroke dementia: Incidence and relationship to prestroke cognitive decline. Neurology 57, 1216–1222 (2001). [DOI] [PubMed] [Google Scholar]
- 13.del Ser T., et al. , Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke 36, 2670–2675 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Wilson B. A., Evans J. J., Emslie H., Alderman N., Burgess P., The development of an ecologically valid test for assessing patients with a dysexecutive syndrome. Neuropsychol. Rehabil. 8, 213–228 (1998). [Google Scholar]
- 15.Katz N., Tadmor I., Felzen B., Hartman-Maeir A., The Behavioural Assessment of the Dysexecutive Syndrome (BADS) in schizophrenia and its relation to functional outcomes. Neuropsychol. Rehabil. 17, 192–205 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine Z. S., et al. , The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Skrobot O. A. et al.; VICCCS group , The vascular impairment of cognition classification consensus study. Alzheimers Dement. 13, 624–633 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Sporns O., Tononi G., Kötter R., The human connectome: A structural description of the human brain. PLOS Comput. Biol. 1, e42 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J., Harris A., Kanwisher N., Stages of processing in face perception: An MEG study. Nat. Neurosci. 5, 910–916 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Thesen T., et al. , Sequential then interactive processing of letters and words in the left fusiform gyrus. Nat. Commun. 3, 1284 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutas M., Federmeier K. D., Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annu. Rev. Psychol. 62, 621–647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCandliss B. D., Cohen L., Dehaene S., The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 7, 293–299 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Wade D. T., Collin C., The Barthel ADL index: A standard measure of physical disability? Int. Disabil. Stud. 10, 64–67 (1988). [DOI] [PubMed] [Google Scholar]
- 24.Spreen O., Benton A. L., Neurosensory Center Comprehensive Examination for Aphasia (University of Victoria, Victoria, BC, 1977). [Google Scholar]
- 25.Kaplan E., Googlass H., Weintraub S., Boston naming test (Lea & febiger, philadelphia, ed. 2, 1983. [Google Scholar]
- 26.Brysbaert M., New B., Moving beyond Kucera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav. Res. Methods 41, 977–990 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Gramfort A., et al. , MNE software for processing MEG and EEG data. Neuroimage 86, 446–460 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gramfort A., et al. , MEG and EEG data analysis with MNE-Python. Front. Neurosci. 7, 267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodbeck C., Das P., Brooks T. L., Reddigari S., Christianbrodbeck/eelbrain: 0.31, Version v0.31. https://github.com/christianbrodbeck/Eelbrain. Accessed 4 December 2020.
- 30.Taulu S., Simola J., Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 51, 1759–1768 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Maris E., Oostenveld R., Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Fischl B., FreeSurfer. Neuroimage 62, 774–781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale A. M., Sereno M. I., Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J. Cogn. Neurosci. 5, 162–176 (1993). [DOI] [PubMed] [Google Scholar]
- 34.Dale A. M., et al. , Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 26, 55–67 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Desikan R. S., et al. , An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Brodbeck C., Pylkkänen L., Language in context: Characterizing the comprehension of referential expressions with MEG. Neuroimage 147, 447–460 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Milner B., Effects of different brain lesions on card sorting: The role of the frontal lobes. Arch. Neurol. 9, 90–100 (1963). [Google Scholar]
- 38.Burgess P. W., Strategy application disorder: The role of the frontal lobes in human multitasking. Psychol. Res. 63, 279–288 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Blaxton T. A., Theodore W. H., The role of the temporal lobes in recognizing visuospatial materials: Remembering versus knowing. Brain Cogn. 35, 5–25 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Moscovitch M., Cognitive resources and dual-task interference effects at retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology 8, 524 (1994). [Google Scholar]
- 41.Tun P. A., Lachman M. E., Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Dev. Psychol. 44, 1421–1429 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung T., Prasad K., Lloyd T. E., Peripheral neuropathy: Clinical and electrophysiological considerations. Neuroimaging Clin. N. Am. 24, 49–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda M., Yamamoto T., Llinás R., The isochronic band hypothesis and climbing fibre regulation of motricity: An experimental study. Eur. J. Neurosci. 13, 315–326 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Yeh F. C., et al. , Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage 178, 57–68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turken A., et al. , Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42, 1032–1044 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen K., Marsh E. B., Chronic post-stroke fatigue: It may no longer be about the stroke itself. Clin. Neurol. Neurosurg. 174, 192–197 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Julayanont P., et al. , The montreal cognitive assessment—basic: A screening tool for mild cognitive impairment in illiterate and low‐educated elderly adults. J. Am. Geriatr. Soc. 63, 2550–2554 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Loftus G. R., Masson M. E. J., Using confidence intervals in within-subject designs. Psychon. Bull. Rev. 1, 476–490 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized MEG fif files have been deposited in the University of Maryland Data Repositories (https://doi.org/10.13016/n9j1-mwne). All other study data are included in the article and SI Appendix.