Abstract
Objective
Time-of-flight magnetic resonance angiography (MRA) is limited by clip-induced artifacts after cerebral aneurysmal clipping. Recently, ultrashort echo time was shown to reduce metal artifacts. We assessed the pointwise encoding time reduction with radial acquisition (PETRA) sequence in subtraction-based MRA as an ultrashort echo time method during follow-up for clipping surgery.
Methods
We retrospectively evaluated 114 branches of 63 aneurysms in 56 patients treated with titanium clips using MRA and 3-dimensional computed tomography angiography. The appearance using each method was compared, and the associations between visibility on PETRA-MRA, clip number and shape, and amount of hematoma were examined. Furthermore, the visibility of the aneurysm remnants and 2 clipping cases with cobalt-chromium-nickel-molybdenum clips were evaluated.
Results
No branches were visible using time-of-flight-MRA, but 79 of 114 branches (69.3%) were visible on PETRA-MRA. PETRA-MRA was effective for follow-up imaging in 33 of 63 aneurysms (52.4%). The median vessel diameters were 1.67 mm (interquartile range, 1.24–2.62 mm) and 0.96 mm (interquartile range, 0.59–1.53 mm) in the visible and invisible groups, respectively. Only the vessel diameter correlated significantly (P < 0.001) with the visibility on PETRA-MRA. A receiver operating characteristic curve for the association between the vessel diameter and visibility on PETRA-MRA showed a cutoff value of 1.26 mm for vessel diameter. Cobalt-chromium-nickel-molybdenum clips produced a strong artifact, even on PETRA-MRA. All 4 residual aneurysms were visible on PETRA-MRA.
Conclusions
PETRA-MRA can be useful for follow-up aneurysm imaging when the diameter of vessels adjacent to the clip exceeds 1.26 mm. However, its usefulness is limited to titanium clips.
Key words: Cerebral aneurysm, Clipping, PETRA, Ultrashort echo time
Abbreviations and Acronyms: 3DCTA, 3-Dimensional computed tomography angiography; ACA, Anterior cerebral artery; CCNM, Cobalt-chromium-nickel-molybdenum; CE TR-MRA, Contrast-enhanced time-resolved MRA; MRA, Magnetic resonance angiography; PETRA, Pointwise encoding time reduction with radial acquisition; SAH, Subarachnoid hemorrhage; TOF, Time-of-flight; UTE, Ultrashort echo time
Introduction
Aneurysmal clipping has been widely used for cases of unruptured aneurysms or aneurysmal subarachnoid hemorrhage (SAH). Considering the possibility of remnant aneurysms or de novo aneurysms, follow-up imaging is important. Three-dimensional computed tomography angiography (3DCTA) is an established technique used for follow-up after clipping. However, there are risks associated with contrast media and radiation exposure.1 Nonenhanced time-of-flight (TOF)-magnetic resonance angiography (MRA) is another useful noninvasive technique; however, the arteries around the clip will usually be invisible owing to clip-induced artifacts. MRA with ultrashort echo time (UTE) reduces the metal artifact but is inadequate for evaluating regions adjacent to the clipped aneurysms.2 Recently, a few studies reported the usefulness of pointwise encoding time reduction with radial acquisition (PETRA), another MRA technique using UTE, for the assessment of arteries and the aneurysmal sac in the vicinity of a titanium clip.3,4 PETRA is a quiet sequence using UTE technology characterized by quiet imaging noise due to the low variability of the tilting magnetic field. Inversion recovery pulses are used as a prepulse to improve contrast, and it produces 3-dimensional T1-weighted images.5 This technique can be very useful, because it allows for postoperative evaluation without contrast media, which is especially important for patients who are allergic to contrast agents or have renal dysfunction. However, with UTE-MRA, it is difficult to improve the resolution. In contrast, TOF-MRA is more advantageous for fine vessel delineation.
Therefore, we investigated the extent to which patients could be evaluated using PETRA-MRA after cerebral aneurysm clipping.
Methods
Patients
The present retrospective study included 56 patients who had undergone surgical clipping using titanium clips for ruptured or unruptured aneurysms and TOF-MRA, PETRA-MRA, and 3DCTA for follow-up imaging from March 2018 to April 2020 in our hospital. Six patients were excluded because of severe motion artifacts. Thus, 50 patients (40 women and 10 men; age range, 35–86 years; median age, 70 years) with 63 aneurysms were enrolled in the present study. In addition to these patients, we also evaluated 2 cases of clipping using cobalt-chromium-nickel-molybdenum (CCNM) alloy clips. The Suwa Red Cross Hospital research ethics committee approved the present study. All personal patient information was removed from the database to protect patient privacy.
Imaging Acquisition
For the patients with titanium clip use, imaging studies were performed in the same chronological order after surgical clipping of ruptured aneurysms, whenever possible. 3DCTA examinations were performed immediately or 1 day after the surgery. TOF-MRA and PETRA-MRA were performed 7 days postoperatively in unruptured cases and 14 days postoperatively in ruptured cases, when the effects of vasospasm had decreased. In the 2 cases that used CCNM clips, the patients had undergone clipping surgery >10 years ago and PETRA-MRA was performed in an outpatient clinic in May 2018.
TOF-MRA and PETRA-MRA were performed using a 1.5T clinical scanner (MAGNETOM Area [Siemens, Erlangen, Germany]) using a 20-channnel head-neck coil. The detailed scan parameters for TOF-MRA were as follows: repetition time/echo time, 25 ms/7.0 ms; flip angle, 20°; field of view, 180 × 180 mm; matrix, 256 × 256; thickness, 0.6 mm; voxel size, 0.9 × 0.7 × 0.6 mm; and acquisition time, 5 minutes, 21 seconds. The detailed scan parameters for PETRA-MRA were as follows: repetition time/echo time, 3.32 ms/0.07 ms; flip angle, 6°; field of view, 280 × 280 mm; matrix, 288 × 288; thickness, 0.97 mm; voxel size, 0.97 × 0.97 × 0.97 mm; acquisition time with and without a presaturation pulse, 2 minutes, 53 seconds and 4 minutes, 47 seconds; and total acquisition time, 7 minutes, 40 seconds. To clearly visualize the cerebral arteries, a presaturation pulse was added. 3DCTA examinations were performed using a 320-row computed tomography scanner (Aquillion ONE; Canon Medical Systems, Tochigi, Japan). Scan parameters comprised: rotation period, 1.0 sec; tube voltage, 100 kV; tube current, 420 mA; field of view, 200 mm; matrix, 512 × 512; slice thickness, 0.5 mm; and voxel size, 0.9 × 0.7 × 0.6 mm.
Image Analysis
Diameters of the proximal and branched arteries of the aneurysm were measured on reconstructed 3DCTA images using a picture archiving and communication system (Synapse software, version 4.1, FUJIFILM Medical Systems USA, Stamford, Connecticut, USA). Three neurosurgeons (A.N., Y.K., and N.W.) subjectively and independently assessed the visibility of proximal and branched vessel continuity at the clipping site and remnant component in both MRA findings. All vessel diameters were measured on the 3DCTA, but not on the MRAs. Vascular diameters were measured at the narrowest point within 5 mm of the clipping site (Figure 1). In the case of branched arteries, the diameter of 2 branched arteries was measured. In the case of ≥3 arteries, such as trifurcation, the 2 larger arteries were measured. In the case of anterior communicating artery aneurysms, the diameter was measured with the anterior cerebral artery (ACA) A1 on the developed side as the proximal artery and anterior communicating artery and distal ACA on the ipsilateral side as the branched artery. Small vessels such as the anterior choroidal artery, which could not be confirmed by 3DCTA, were excluded.
Figure 1.
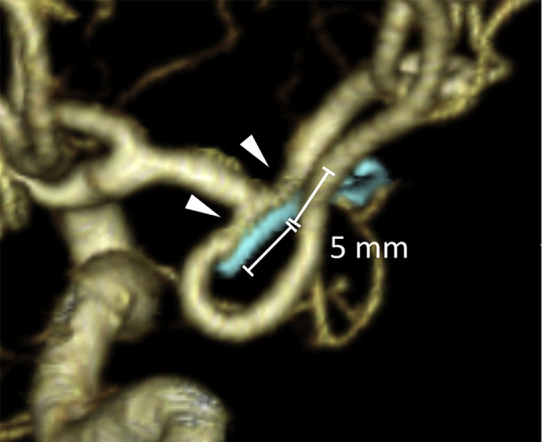
Diagram showing the measurements of vessel diameters on 3-dimensional computed tomography angiography. Vessel diameters were measured at the narrowest point (arrowheads) within 5 mm of the clipping site.
In contrast to PETRA-MRA using subtraction methods, interpretation of TOF-MRA can be difficult or impossible in the presence of a hematoma.6 As such, the amount of SAH around the clipping site was assessed using a fluid-attenuated inversion recovery sequence and classified into 3 groups: no SAH (no hematoma), small SAH (hematoma thickness <10 mm), and large SAH (hematoma thickness ≥10 mm).
Because the amount of metal tends to create artifacts,1,7,8 the number and shape of the clips used for clipping were examined. The shape of the clips was classified as fenestrated, bayonet, and other. In cases in which a remnant of aneurysm was present after clipping, the height and width of that remnant were measured on 3DCTA.
Statistical Analysis
For the patients with titanium clips, statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan),9 a graphic user interface for R (R Foundation for Statistical Computing, Vienna, Austria).10 More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. The Mann-Whitney U test was performed to compare the visibility for TOF-MRA and PETRA-MRA. The vessel diameter, number of clips, shape of the clips, and the amount of SAH were selected as investigatory factors. To assess the association between the MRA findings and these factors, a multivariate analysis was performed using a binominal logistic regression model. The 2 patients with CCNM clips were evaluated individually because the CCNM alloy is known to produce large artifacts7,11 and is rarely used at present. The interrater agreement for each of the MRA techniques was evaluated using Fleiss's fixed marginal multirater kappa using the Online Kappa Calculator (available at: http://justusrandolph.net/kappa/).12,13 The results were interpreted as poor (<0.40), intermediate to good (0.40–0.75), or excellent (>0.75). Continuous variables are summarized as the median and interquartile range. A P value <0.05 was considered statistically significant.
Results
Patient and Aneurysm Characteristics
The characteristics of the patients and aneurysms treated with titanium clips are shown in Table 1. We analyzed the data from 50 patients, of whom 7 had multiple aneurysms. Of the 7 patients, 5 had had 2 aneurysms and 2 had had 5 aneurysms. Thus, a total of 63 aneurysms were included, of which 41 were unruptured and 22 were ruptured. The distribution of the 63 aneurysm locations was as follows: middle cerebral artery aneurysm, 24 (38.1%); internal carotid artery aneurysm, 20 (31.7%); and ACA aneurysm, 19 (30.2%). Of the 63 aneurysms, 3 were nonbranching aneurysms (ACA A1, paraclinoid internal carotid artery, and middle cerebral artery M1 aneurysms), and 9 branched vessels were excluded owing to a lack of visibility on 3DCTA. Therefore, 114 branched vessels were analyzed. The patients were treated using Sugita titanium aneurysm clips II (Mizuho Medical, Tokyo, Japan) made of titanium. The number of clips used was as follows: 1 clip was required in 49, 2 in 13, and 3 in 1 of the 63 aneurysms. Fenestrated clips were applied in 5, bayonet clips in 14, and other shapes in 44 of the 63 aneurysms. Of the 63 aneurysms, no SAH was confirmed in 42, a small SAH in 18, and a large SAH in 3 aneurysms.
Table 1.
Patient Characteristics (Titanium Clips)
| Parameter | Value |
|---|---|
| Age (years) | |
| Median | 70 |
| Range | 35–86 |
| Sex (n) | |
| Female | 40 |
| Male | 10 |
| Aneurysmal location | |
| MCA (n; %) | 24 (38.1) |
| MCA bifurcation | 22 |
| MCA M1 | 2 |
| ICA (n; %) | 20 (31.7) |
| ICA PCoA | 14 |
| ICA anterior choroidal artery | 4 |
| Paraclinoid ICA | 1 |
| ICA bifurcation | 1 |
| ACA (n; %) | 19 (30.2) |
| ACoA | 11 |
| Distal ACA | 7 |
| ACA A1 | 1 |
| No. of clips used (n; %) | |
| 1 | 49 (77.8) |
| 2 | 13 (20.6) |
| 3 | 1 (1.6) |
| Clip shape (n; %) | |
| Fenestrated type | 5 (7.9) |
| Bayonet type | 14 (22.2) |
| Other | 44 (69.9) |
| Amount of SAH (n; %) | |
| No SAH | 42 (66.7) |
| Small SAH | 18 (28.6) |
| Large SAH | 3 (4.7) |
ACA, anterior cerebral artery; ACoA, anterior communicating artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCoA, posterior communicating artery; SAH, subarachnoid hemorrhage.
Visibility of Vessel Continuity
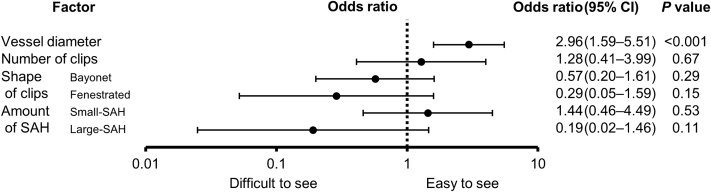
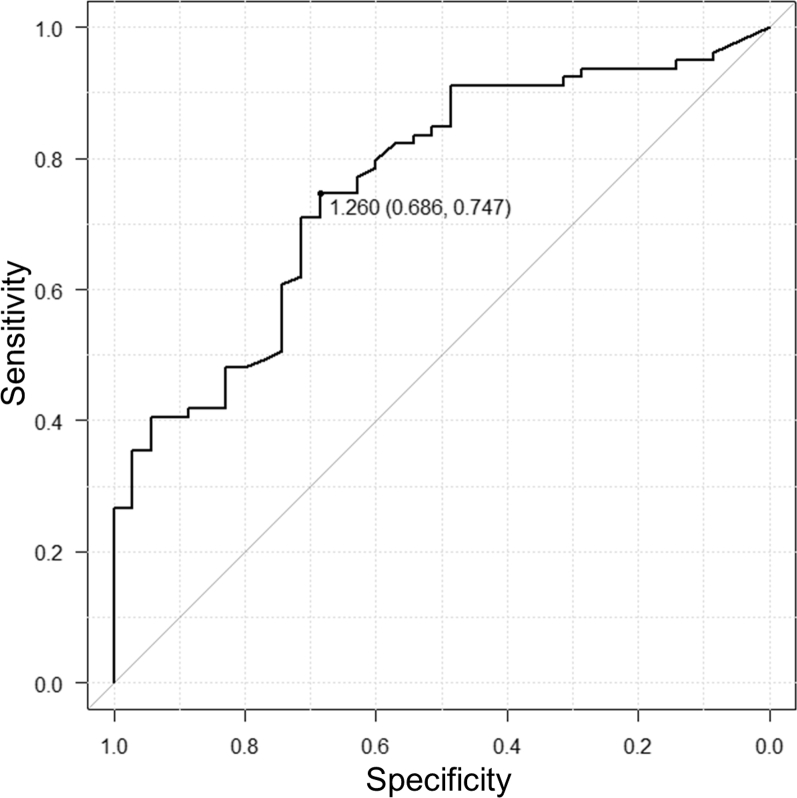
3DCTA, PETRA-MRA, and TOF-MRA images for the representative cases are shown in Figure 2. Of all 114 branches, 79 (69.3%) were visible on PETRA-MRA, with excellent interrater agreement (κ = 0.81; 95% confidence interval [CI], 0.73–0.90) and no branches (0%) were visible on TOF-MRA. Of the 63 aneurysms, the continuity of all vessels was visible in 33 aneurysms (52.4%) and ≥1 vessel in 55 aneurysms (87.3%) on PETRA-MRA. The visibility of vessel continuity was significantly better for PETRA-MRA than for TOF-MRA (P < 0.001). The median vessel diameters were 1.67 mm (interquartile range, 1.24–2.62 mm) and 0.96 mm (interquartile range, 0.59–1.53 mm) in the visible and invisible groups, respectively (P < 0.001; Figure 3). Multivariate analysis revealed that the vessel diameter was the only factor that correlated significantly (odds ratio, 2.96; 95% CI, 1.59–5.51; P < 0.001) with the visibility on PETRA-MRA (Figure 4). The number of clips, the shape of the clips, and the amount of SAH were poorly associated with visibility. A receiver operating characteristic curve for the association between the vessel diameter and visibility on PETRA-MRA showed that the cutoff value for the vessel diameter was 1.26 mm (Figure 5). In the 2 cases with CCNM clips, neither the parent vessels nor the branches were visible (Figure 6).
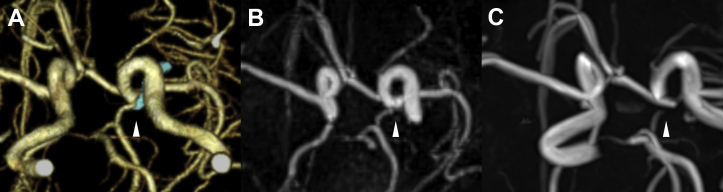
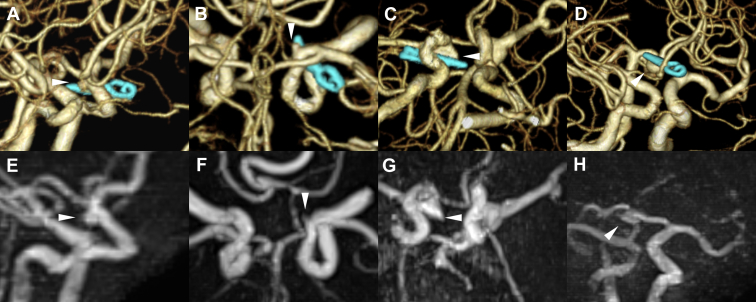
Figure 2.
Postoperative examination of the unruptured left internal carotid artery (ICA) posterior communicating artery (PCoA) aneurysmal clipping observed from the caudal side. (A) Three-dimensional computed tomography angiogram showing the PCoA, ICA, and Sugita Titanium Aneurysm Clips II (Mizuho Medical, Tokyo, Japan; arrowhead). (B) The PCoA and ICA are visible on pointwise encoding time reduction with radial acquisition (arrowhead). (C) The PCoA and ICA around the clip are not visible owing to clip-induced artifact on time-of-flight magnetic resonance angiography (arrowhead).
Figure 3.
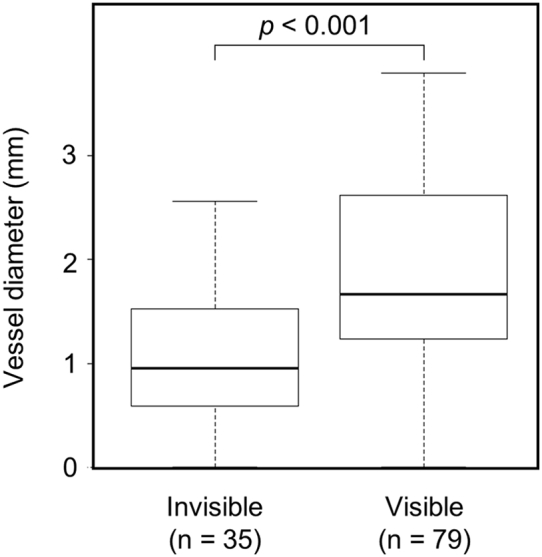
Comparison of branched vessel diameters with and without vessel continuity on pointwise encoding time reduction with radial acquisition magnetic resonance angiography. The vessel diameters were 1.67 mm (range, 1.24–2.62 mm) and 0.96 mm (range, 0.59–1.53 mm) in the visible and invisible groups, respectively (P < 0.001; Mann-Whitney U test).
Figure 4.
Binomial logistic regression analysis of the association between factors and visibility on pointwise encoding time reduction with radial acquisition magnetic resonance angiography. Only a larger vessel diameter correlated significantly with visibility. CI, confidence interval; SAH, subarachnoid hemorrhage.
Figure 5.
Receiver operating characteristic curve showing the association between vessel diameter and visibility on pointwise encoding time reduction with radial acquisition magnetic resonance angiography. The cutoff value for the vessel diameter was 1.26 mm, and the sum of the sensitivity (0.747) and specificity (0.686) was the maximum. The area under the curve was 0.759, with a 95% confidence interval of 0.666–0.852.
Figure 6.
Images of 2 cases of clipping surgery with clips made of cobalt-chromium-nickel-molybdenum alloy. (A) Three-dimensional computed tomography angiogram showing a fenestrated type clip at the anterior communicating artery site. (B) Clip-induced artifact (arrowheads) limited assessment of the artery in the vicinity of the clip on pointwise encoding time reduction with radial acquisition magnetic resonance angiography. (C) Three-dimensional computed tomography angiogram showing a curved type clip at the right middle cerebral artery bifurcation. (D) Pointwise encoding time reduction with radial acquisition magnetic resonance angiogram showing strong artifacts (arrowheads) around the clip, making the vicinity around the clip not visible.
Visibility of Remnant Aneurysm
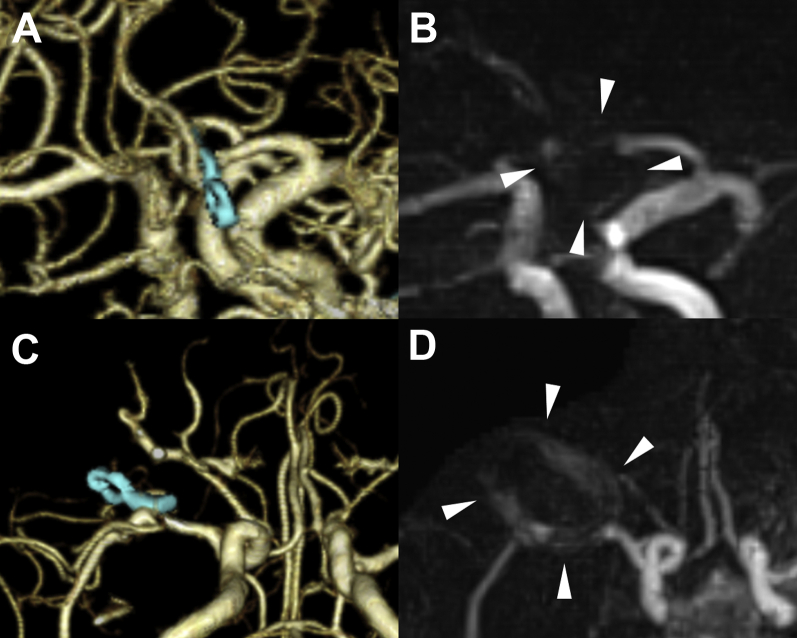
A remnant aneurysm was observed in 4 patients, and the details are presented in Table 2. All 4 remnant components were visible on PETRA-MRA, with excellent interrater agreement (κ = 1.00; Figure 7).
Table 2.
Characteristics of Patients With Incomplete Clipping
| Pt. No. | Age (years) | Sex | Aneurysm |
Amount of SAH | Clips |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Remnant Component Size (mm) |
Rupture | ||||||||
| Width | Height | n | Shape | Material | ||||||
| 3 | 84 | F | ICA PCoA | 2.4 | 2.7 | Yes | Large SAH | 1 | Bayonet | Titanium |
| 10 | 76 | F | ICA PCoA | 1.6 | 4.2 | No | No SAH | 1 | Other | Titanium |
| 31 | 82 | F | ICA PCoA | 3.9 | 4.4 | Yes | Small SAH | 2 | Other | Titanium |
| 33 | 86 | F | ACoA | 5.4 | 4.3 | Yes | Small SAH | 1 | Other | Titanium |
ACoA, anterior communicating artery; F, female; ICA, internal carotid artery; PCoA, posterior communicating artery; SAH, subarachnoid hemorrhage.
Figure 7.
Images of 4 cases with incomplete aneurysmal clipping. Both 3-dimensional computed tomography angiograms (Upper Row) and pointwise encoding time reduction with radial acquisition magnetic resonance angiograms (Lower Row) revealed remnant components of the aneurysms (arrowheads). (A) Patient 3, an 84-year-old woman with a ruptured left internal carotid artery (ICA) posterior communicating artery (PCoA) aneurysm clipping. The massive hematoma caused no confirmation other than that the neck of the aneurysm and a part of the aneurysm was left. (B) Patient 10, a 76-year-old woman with an unruptured left ICA PCoA aneurysm clipping. A part of the aneurysm was left intentionally to preserve the perforator branch of the PCoA that had adhered to the aneurysm. (C) Patient 31, an 82-year-old woman with a ruptured right ICA PCoA aneurysm clipping. Dome clipping with 2 clips was performed to preserve the PCoA. (D) Patient 33, an 86-year-old woman with a ruptured anterior communication artery aneurysm clipping. Dome clipping was performed owing to severe atherosclerosis.
Discussion
The objective of the present study was to assess the efficacy of PETRA-MRA as a follow-up imaging procedure after aneurysm clipping. We found that PETRA-MRA is effective for follow-up imaging of aneurysms after treatment with titanium alloy clips and for detecting remnant aneurysms compared with TOF-MRA. However, PETRA-MRA is not useful for aneurysms treated with CCNM alloy clips. The results of the present study also showed excellent interrater agreement for the image analysis among the 3 neurosurgeons (A.N., Y.K., and N.W.), who had assessed the data.
Visible Vessel Diameter
The vessel diameter was the only factor with significant differences when stratified by the visibility on PETRA-MRA. The visibility was improved with larger vessel diameters using PETRA-MRA. Compared with the 3DCTA and TOF-MRA images, smaller vessels were difficult to see using PETRA-MRA owing to the wide field of view and the low resolution. If the vessel diameter were larger than the cutoff value of 1.26 mm, it was more likely to be visible on PETRA-MRA. The cutoff value is greater than the 0.9-mm slice thickness of PETRA-MRA, which we assumed resulted from the factors discussed in the next sections.
Clip Artifacts
We found no association between the number of clips and the degree of visibility. The visibility could possibly be worsened by the use of fenestrated or bayonet clips compared with other types. We speculated that when using fenestrated or bayonet clips, the clip heads with greater metal volumes will be in closer proximity to the vessels and more susceptible to metal artifact. The clips tended to be delineated as a signal void area fully surrounded by high signal on the original PETRA-MRA images.4 The surrounding high signal can lead to underestimation or, possibly, overestimation of the visibility on the reconstructed images, affecting the interrater agreement. In the 2 cases using CCNM clips, large artifacts appeared that covered the entire clip (Figure 6). The CCNM alloy, which was examined in the present study, is a material of Sugita Aneurysm Clips (Mizuho Medical), and is also known as Elgiloy (Elgiloy Specialty Metals, Elgin, Illinois, USA). The CCNM alloy causes a very strong metal artifact compared with titanium.7,8,11 Shortening of the echo time reduces clip-induced artifacts.2,14,15 However, the effect on the CCNM alloy was insufficient using PETRA-MRA with a UTE of 0.07 ms. Thus, the use of titanium clips is recommended to ensure adequate visibility on magnetic resonance imaging.
SAH Artifacts
The visibility of vessels was slightly, but not significantly, worse in the presence of a large SAH. However, it was not worsened with small SAHs. Because PETRA-MRA uses subtraction techniques, a small amount of hematoma probably did not interfere with delineation in the presence of a small SAH. However, with a large amount of hematoma enclosing the clip, the image processing sequence would not have been able to eliminate the effect of the hematoma artifacts in the presence of a large SAH. Using TOF-MRA, high hematoma signals were incorporated into the maximal intensity projection reconstruction, and the vessels around the hematoma and the vessels in the vicinity of the clip were not recognized.
Motion Artifacts
Of the 56 patients, 6 had apparent motion artifacts found during PETRA-MRA but not during TOF-MRA or 3DCTA. The subtraction technique was added to improve the image quality; however, PETRA-MRA required a longer time than TOF-MRA (PETRA-MRA, 7 minutes, 40 seconds; TOF-MRA, 5 minutes, 21 seconds). The longer time required for PETRA-MRA allows for more time for movement of the patient's body and misregistration.
1.5T or 3T Magnetic Field
We previously reported the dynamics of cerebral aneurysm clips in the 3T magnetic field.16,17 Despite increasing the resolution, the 3T magnetic field also increases the amount of torque, translational force, metal temperature, and metal artifact compared with 1.5T. Recently, Katsuki et al.18 reported the usefulness of 3T MRA with UTE, which reduced the metal artifacts and increased resolution. Higher magnetic fields with UTE might improve the weakness of PETRA-MRA's low resolution. In a 1.5T magnetic resonance imaging environment, vessel continuity was observed in 69.3% of cases in the present study. If the credibility of the PETRA-MRA findings was considered reasonable compared with 3DCTA, a subsequent outpatient follow-up examination might be possible using PETRA-MRA without contrast media and radiation exposure. In cases involving medical devices in the body, such as pacemakers, the examination could be performed in a 1.5T environment but not in a 3T environment.
Other Studies and Techniques
TOF-MRA is a widely used MRA technique that relies on the intrinsic magnetic properties of the flowing blood to produce a signal.19 TOF-MRA depends on the steady inflow of the magnetized blood and is affected by various artifacts, such as metal artifacts. As in previous studies, none of the 114 branches were visible on TOF-MRA in the present study. Various techniques have been developed to overcome the disadvantages of TOF-MRA. Recently, Ryu et al.20 reported a study of 119 patients with 126 treated aneurysms using silent MRA, which is also a sequence using UTE (echo time, 0.016 ms), similar to PETRA-MRA. The study included 16 clipped aneurysms, demonstrating that silent MRA improved visualization of the treated site and detection of the remnant aneurysm sac compared with TOF-MRA. Their results are consistent with those from the present study. Among other noninvasive techniques is contrast-enhanced time-resolved MRA (CE TR-MRA).21 CE TR-MRA is a multiphasic MRA examination that generates multiphasic angiographic images with a temporal resolution of 2–6 seconds at a submillimeter isotropic spatial resolution.22 CE TR-MRA has been reported to be particularly effective for shunt disease23,24 and aneurysms.25 Grossberg et al.21 reported a study of 37 flow diverter-treated aneurysms and found both positive and negative predictive values of 92% comparing CE TR-MRA and digital subtraction angiography in terms of aneurysm occlusion. However, because the clips were described as a susceptibility artifact, CE TR-MRA might not be effective for aneurysms treated with clipping. In that respect, PETRA-MRA is superior to CE TR-MRA because it does not require contrast agents and it attenuates metal artifacts.
In contrast to previous studies, we included a relatively large number of aneurysms treated by surgical clipping instead of endovascular treatment. Moreover, we assessed the relationship between the treated site visualization, shape and number of clips, amount of SAH, and, in particular, the vessel diameter. To the best of our knowledge, the present study is the first numerical report showing the cutoff value of the vessel diameter visible on PETRA-MRA.
Study Limitations
Because of the inadequate image resolution, 1.5T PETRA-MRA is not suitable for the assessment of small vessels. All 4 remnant components were visible on PETRA-MRA in our study; however, the minimum size that can be confirmed using PETRA-MRA is still unknown. The smallest residual component among the 4 cases was 1.6 mm. Thus, smaller sizes might not be visible on PETRA-MRA. Furthermore, owing to the inadequate resolution, it might not be possible to detect small, slow-growing aneurysms and remnant components. Therefore, PETRA-MRA is not an absolute alternative modality to 3DCTA and should only be used for specific cases. 3DCTA might be preferred to PETRA-MRA in cases with a remnant component after SAH owing to the high rebleeding risk. As stated, the appearance of high signal artifacts around the clips could lead to an over- or underestimation of the visibility. Attention is required for proper image evaluation. Additionally, patients with SAH might have large amounts of hematoma present or it might not be possible to keep the patient resting, which can result in poor images not useful for assessment and analysis. The vessel diameter was the only factor with significant differences, although the findings with fenestrated clips (P = 0.15) and large SAH (P = 0.11) were almost statistically significant. These differences might have been significant with an increasing number of cases. Our study was a single-center, retrospective review, limited to the evaluation of a relatively small number of aneurysms treated with only one brand name of aneurysm clips (i.e., Sugita titanium aneurysm clips II [Mizuho Medical]). A prospective, multicenter study evaluating titanium clips from other manufacturers is required to fully assess the usefulness of PETRA-MRA for this indication.
Conclusions
PETRA-MRA is a useful method for follow-up imaging after cerebral aneurysm clipping, especially when the vessel diameter adjacent to the clip is ≥1.26 mm. This method could be valuable for the follow-up examination of aneurysm remnants. However, the clip material is limited to titanium with PETRA-MRA because the CCNM alloy causes a strong metal artifact.
CRediT authorship contribution statement
Akihiro Nishikawa: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Visualization. Yukinari Kakizawa: Conceptualization, Investigation, Resources, Data curation, Writing - review & editing, Supervision, Project administration. Naomichi Wada: Investigation, Resources. Yasunaga Yamamoto: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Visualization. Masahito Katsuki: Formal analysis, Resources. Toshiya Uchiyama: Resources.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.van Loon J.J.L., Yousry T.A., Fink U., Seelos K.C., Reulen H.-J., Steiger H.J. Postoperative spiral computed tomography and magnetic resonance angiography after aneurysm clipping with titanium clips. Neurosurgery. 1997;41:851–857. doi: 10.1097/00006123-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Gönner F., Lövblad K.O., Heid O. Magnetic resonance angiography with ultrashort echo times reduces the artefact of aneurysm clips. Neuroradiology. 2002;44:755–758. doi: 10.1007/s00234-002-0825-8. [DOI] [PubMed] [Google Scholar]
- 3.Katsuki M., Kakizawa Y., Yamamoto Y., Nishikawa A., Wada N., Uchiyama T. Magnetic resonance angiography with ultrashort echo time evaluates cerebral aneurysm with clip. Surg Neurol Int. 2020;11:65. doi: 10.25259/SNI_59_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takubo S., Kawasaki K., Nagatari T., Matsumoto M., Kageyama T. Clinical usefulness of ultra-short TE MRA for follow-up imaging after cerebral aneurysm clipping. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2020;76:177–184. doi: 10.6009/jjrt.2020_JSRT_76.2.177. [DOI] [PubMed] [Google Scholar]
- 5.Grodzki D.M., Jakob P.M., Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA) Magn Reson Med. 2012;67:510–518. doi: 10.1002/mrm.23017. [DOI] [PubMed] [Google Scholar]
- 6.Wilcock D.J., Jaspan T., Worthington B.S. Problems and pitfalls of 3-D TOF magnetic resonance angiography of the intracranial circulation. Clin Radiol. 1995;50:526–532. doi: 10.1016/s0009-9260(05)83186-1. [DOI] [PubMed] [Google Scholar]
- 7.Lawton M.T., Heiserman J.E., Prendergast V.C., Zabramski J.M., Spetzler R.F. Titanium aneurysm clips: part III—clinical application in 16 patients with subarachnoid hemorrhage. Neurosurgery. 1996;38:1170–1175. doi: 10.1097/00006123-199606000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Heindel W., Friedmann G., Bunke J., Thomas B., Firsching R., Ernestus R.I. Artifacts in MR imaging after surgical intervention. J Comput Assist Tomogr. 1986;10:596–599. doi: 10.1097/00004728-198607000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Comprehensive R Archive Network. https://cran.r-project.org/ Available at:
- 11.Takikawa S., Houkin K., Itosaka H., Saitoh H., Abe H. Usefulness and limitation of cerebral aneurysm clipping using titanium clip. Surg Cereb Stroke. 1997;25:189–194. [Google Scholar]
- 12.Fleiss J.L. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–382. [Google Scholar]
- 13.Randolph J.J. 2008. Online Kappa Calculator.http://justus.randolph.name/kappa Available at: [Google Scholar]
- 14.Robson M.D., Gatehouse P.D., Bydder M., Bydder G.M. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27:825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Schmalbrock P., Yuan C., Chakeres D.W., Kohli J., Pelc N.J. Volume MR angiography: methods to achieve very short echo times. Radiology. 1990;175:861–865. doi: 10.1148/radiology.175.3.2343137. [DOI] [PubMed] [Google Scholar]
- 16.Kakizawa Y., Seguchi T., Horiuchi T., Hongo K. Cerebral aneurysm clips in the 3-Tesla magnetic field. J Neurosurg. 2010;113:859–869. doi: 10.3171/2010.3.JNS091346. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe A., Seguchi T., Koyama J. Investigation of radiofrequency-induced temperature elevation of aneurysm clips in a 3.0-Tesla magnetic resonance environment. Neurosurgery. 2007;61:1062–1066. doi: 10.1227/01.neu.0000303202.69747.d2. [DOI] [PubMed] [Google Scholar]
- 18.Katsuki M., Narita N., Ozaki D., Sato Y., Iwata S., Tominaga T. Three Tesla magnetic resonance angiography with ultrashort echo time describes the arteries near the cerebral aneurysm with clip and the peripheral cerebral arteries. Surg Neurol Int. 2020;11:224. doi: 10.25259/SNI_329_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard B.M., Hu R., Barrow J.W., Barrow D.L. Comprehensive review of imaging of intracranial aneurysms and angiographically negative subarachnoid hemorrhage. Neurosurg Focus. 2019;47:1–13. doi: 10.3171/2019.9.FOCUS19653. [DOI] [PubMed] [Google Scholar]
- 20.Ryu K.H., Baek H.J., Moon J.I. Usefulness of noncontrast-enhanced silent magnetic resonance angiography (MRA) for treated intracranial aneurysm follow-up in comparison with time-of-flight MRA. Neurosurgery. 2020;87:220–228. doi: 10.1093/neuros/nyz421. [DOI] [PubMed] [Google Scholar]
- 21.Grossberg J.A., Howard B.M., Saindane A.M. The use of contrast-enhanced, time-resolved magnetic resonance angiography in cerebrovascular pathology. Neurosurg Focus. 2019;47:1–6. doi: 10.3171/2019.9.FOCUS19627. [DOI] [PubMed] [Google Scholar]
- 22.Grist T.M., Mistretta C.A., Strother C.M., Turski P.A. Time-resolved angiography: past, present, and future. J Magn Reson Imaging. 2012;36:1273–1286. doi: 10.1002/jmri.23646. [DOI] [PubMed] [Google Scholar]
- 23.Razek A.A.K.A., Gaballa G., Megahed A.S., Elmogy E. Time resolved imaging of contrast kinetics (TRICKS) MR angiography of arteriovenous malformations of head and neck. Eur J Radiol. 2013;82:1885–1891. doi: 10.1016/j.ejrad.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Machet A., Portefaix C., Kadziolka K. Brain arteriovenous malformation diagnosis: value of time-resolved contrast-enhanced MR angiography at 3.0T compared to DSA. Neuroradiology. 2012;54:1099–1108. doi: 10.1007/s00234-012-1024-x. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.W., Roh H.G., Moon W.J. Time-resolved 3D contrast-enhanced MRA on 3.0T: a non-invasive follow-up technique after stent-assisted coil embolization of the intracranial aneurysm. Korean J Radiol. 2011;12:662–670. doi: 10.3348/kjr.2011.12.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]