Abstract
Objective
The present work was aimed to evaluate the effect of valproic acid (VPA),Parathyroid hormone (1–34) (PTH)+VPA on Ti rods osseointegration in ovariectomized rats and further investigation of the possible mechanism.
Methods
The MC3T3-E1 cells were co-cultured with VPA,PTH + VPA and induced to osteogenesis, and the cell viability,mineralization ability were observed by MTT and ALP staining,Alizarin Red staining and Western blotting. Twelve weeks after bilateral ovariectomy, all animals were randomly divided into four groups: group OVX and VPA,PTH + VPA, and all the rats received Ti implants and animals belong to group VPA,PTH + VPA received valproic acid (300 mg/day), valproic acid (300 mg/day) plus Parathyroid hormone (1–34) every 3 days (60 μg/kg), respectively, treatment until death at 12 weeks. Micro-CT, histology, biomechanical testing, bone metabolism index and Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis were used to observe the therapeutic effect and explore the possible mechanism.
Results
Results shown that VPA decreased new bone formation around the surface of titanium rods and push-out force other than group OVX. Histology, Micro-CT and biochemical analysis results showed combined application of systemic VPA showed harmful effects than OVX group on bone formation in osteopenia rats, with the worse effects on CTX-1, P1NP and microarchitecture as well as biomechanical parameters by down-regulated gene expression of Runx2, OCN, Smad1, BMP-2 and OPG, while up-regulated RANKL. However, after PTH treatment, the above indicators were significantly improved.
Conclusions
The present study suggests that systemic use of VPA may bring harm to the stability of titanium implants in osteoporosis, PTH can reverse the negative effect of VPA on the osseointegration of titanium rods in ovariectomized rats.
Translational potential of this article
According to our research, when patients with epilepsy have osteoporotic fractures, after joint replacement or internal fixation, continue to use sodium valproate for anti-epileptic therapy, the possibility of postoperative loosening increases, again on the basis of It can be reversed with the anti-osteoporosis drug parathyroid hormone (1-34).
Keywords: Osteoporosis, Osseointegration, Titanium implants, Parathyroid hormone (1–34), Valproic acid
Introduction
With the increase of elderly people, the development of osteoporosis and the concomitant fragility fractures are a worldwide health problem, affecting approximately 200 million people, which leads to heavy burden on the health care system and society [1]. Bone quality, which is a significant determinant of the survival rate of an implant, depends on the bone mineral density as well as the spatial structure of trabecular bone [2]. For orthopedic implant placement, the presence of sufficient bone volume is the most important of prerequisites [3]. Metabolic disorders have an important impact on bone structure and bone repair, as osteoporosis and osteoporosis-related diseases are associated with a decreased BMD and increased risk of nonunion of fracture [4,5] (Table 1).
Table 1.
Nucleotide sequences for real-time RT-PCR primers.
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Runx-2 OC Smad1 BMP-2 RANKL OPG GAPDH |
TCCCAGTATGAGAGTAGGTGTCC GAGGGCAGTAAGGTGGTGAA GTGGAAACAGGGCGACGAAG ACGACGGTAAAGGACATC AGCCGAGACTACGGCAAGTA ACACACCAACTGCAGCTCAC ACATTGTTGCCATCAACGAC |
GGCTCAGATAAGAGGGGTAAGAC CCTAAACGGTGGTGCCATAG AGGGAGCGAGGAATGGTGAC ATGGTTGGTGGAGTTCAG AGACCACCTGACCCAGTCC TGTCCACCAGAACACTCAGC TACTCAGCACCAGCATCACC |
It has been recently recognized that seizure-related falls of epilepsy is associated increased morbidity, including neurocognitive deficits, gait disturbances, falls, bone fractures, and osteoporosis [6,7]. There is consistent evidence that patients with long-term treatment with antiepileptic drugs have an increased risk of osteoporosis. Patients with epilepsy have significant impairments to bone health, with a 2- to 3-fold increased risk of fractures [8]. Valproic acid (2-propylpentanoic acid) (VPA), a short-chain fatty acid class of histone deacetylase inhibitor (HDACI),which is a widely used antiepileptic drug with a broad range of effects and broad clinical efficacy. Recent clinical study have confirmed VPA is associated with decreased BMD of trochanter and Ward’s triangle after administered for 24 months [9]. Although the molecular mechanism underlying this event is not fully understood, a number of theories have been proposed to explain why anti-epileptic drugs affect bone; this includes reduced levels of vitamin D metabolites, reduced calcium absorption, inhibition of the cellular response to PTH, hyperparathyroidism, vitamin K deficiency and calcitonin deficiency [10,11].
Parathyroid hormone(1–34)(PTH), human recombinant parathyroid hormone 1–34 fragment, which was first approved by FDA to increase bone formation for use in the United States, is one of the most widely used anti-osteoporosis drugs and has previously been shown to effectively treat osteoporosis [12]. PTH, a key regulator of calcium and phosphate homeostasis, which is produced by the parathyroid grand and essential for calcium homeostasis and bone remodeling. Previous animal and clinical studies showed that intermittent parathyroid hormone (PTH) could increase the BMD of Hip and spine bone by regulation the delicate balance between bone resorption and bone formation [13,14]. Our previous studies have demonstrated that the effects of intermittent PTH is associated with significant improvement titanium rods osseointegration [15,16].
Despite data demonstrating increased osseointegration of implants with intermittent PTH administration, there are limited data about the association of long-term maintenance therapy with VPA and PTH. We hypothesize that intermittent PTH injection enhances titanium rods osseointegration in VPA-treated OVX rats. Therefore, the purpose of this study was to observe whether intermittent subcutaneous administration of PTH can reverse the efficacy of VPA on inhibition of osseointegration in osteoporotic bone.
Materials and methods
Experimental animals
Health adult female Sprague–Dawley rats (n = 40), weighing (200–250 g, Central Laboratory of Yijishan Hospital) were used in this study. All rats were maintained in a temperature-controlled environment (22 ± 3 °C), 55 ± 5% relative humidity under a 12-h light/dark cycle and humidity and fed standard laboratory food and water. Animal care and all experimental procedures were carried out in accordance with guidelines under the Animal Experimentation of Wannan Medical College.
Cell culture, treatment and evaluation
MC3T3-E1 cells, an osteoblast precursor cell line derived from mouse, were purchased from the Institute of Biochemistry and Cell Biology, CAS (Shanghai, China). MC3T3-E1 cells were cultured at 37 °C and 5% CO2 atmosphere in DMEM containing 10% FBS. Thereafter, β-glycerophosphate (10 mM), l-ascorbic acid (50 μg/mL) and dexamethasone (10 nM) were added into DMEM to induce osteogenic differentiation. After reaching confluence, the DMEM were added with VPA(10 μM), or PTH(10 μM) treatment or no drug treatment (Control group, Con).
MTT detection
After 3 day treatment, 0.02 ml of MTT solution (5 mg/ml) was added to each well and the plates were incubated at 37 °C for 4 h to estimate cell viability. After that, the DMSO was added to dissolve formazan crystals. The absorbance value was measured at 570 nm using a plate reader (Bio-Rad Inc, Hercules, CA, USA).
Alizarin red and ALP staining
To detect mineralization (calcium deposits) and ALP activity, 2 or 3 weeks after induction cells were fixed with 4% paraformaldehyde, and stained with Alizarin Red S (2%; Sigma–Aldrich) or ALP staining kit (Beyotime Institute of Biotechnology, Shanghai, China) and the samples were observed by inverted phase contrast microscopy (Eclipse TE2000-U; Nikon, Japan).
Western blot analyses
To detect the expression levels of osteogenesis-related proteins, we performed western blot analyses. After 7 days treatment, the treated cells were harvested and the RIPA lysis buffer and BCA protein Assay (Beyotime, Shanghai, China) was used to extract and determine the total proteins. SDS–polyacrylamide gel electrophoresis (10%) was performed to separate the sample protein (20 μg/lane).Then, the proteins were transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin (Solarbio, Beijing, China) at room temperature for 2 h in Tris-buffered saline and Tween 20 (TBST), the membranes were probed with the primary antibodies such as with antibodies against the following proteins: alkaline phosphatase (ALP) (1:1000, ab33923), bone morphogenetic protein 2(BMP-2) (1:1000, ab135434), runt-related transcription factor 2 (RUNX-2), osteocalcin (OC) (1:1000, ab139412),type I collagen (COL-1) (1:1000, ab78034) (all from Abcam, Cambridge, UK); and GAPDH (1:1000, TA-08; ZSGB-Bio,Beijing, China)and incubated at 4 °C overnight. The appropriate secondary antibody was applied (1:2000; horseradish peroxidase anti-mouse and horseradish peroxidase anti-rabbit) at room temperature for 1 h. Quantitative analysis of protein expression was accomplished by scanning autoradiogram and densitometry (Image J, NIH).
Surgery and treatment
A bilateral ovariectomized (OVX) or sham operation was performed using standard methods, the rats were allowed for 12 weeks to conduct an osteoporosis as previous reports described [17,18]. The femurs of each five randomly selected OVX rats and the five sham-operated ones were harvested for bone mineral density (BMD) evaluation by micro computed tomography (Micro-CT) and hematoxylin-eosin (HE) staining to confirm the establishment of osteoporosis. Afterwards all OVX animals were randomly divided into three experimental groups: OVX group, VPA and PTH + VPA group. There were 10 rats in each groups. Afterwards, the titanium rods [1 mm external diameter and 20 mm length (Zhejiang Guangci Medical Appliance Co., Ltd., Ningbo, China) ] were inserted bilaterally in all animals. The rats in OVX group, VPA and PTH + VPA group were treated with 1 ml of isotonic saline; valproic acid (VPA) 300 mg/kg/day, valproic acid (VPA) 300 mg/kg/day plus PTH every 3 days (60 μg/kg), respectively. All drugs were purchased from SIGMA. The doses of SVP and PTH were determined according to previous experiments where they showed protective effects in OVX rodent models [19,20].At 12 weeks postoperatively, all of the rats were euthanized by the over-dose administration of anesthetic agents. Subsequently, femur with implants and blood were harvested and evaluated by biochemical analysis, histological, Micro-CT, and push-out tests and Real-time quantitative RT-PCR (RT-qPCR).
Micro-CT scanning
For microarchitecture, trabecular bone architecture of defected area was determined by Micro-CT (Micro-CT μm 100, SCANCO Medical, Switzerland).The.
The entire trabecular compartment around implant from slice 2 mm below the growth plate to distal 100 slices was defined of volume of interest (VOI) to analysis the bone Osseointegration. The bone volume per total volume (BV/TV); trabecular number (Tb.N), thickness (Tb.Th), and spacing (Tb.Sp), the mean connective density (Conn.D), and the mean trabecular separation (Tb.Sp) within VOI were measured as previously described [21,22].
Histological examination
Following Micro-CT scanning, the femur specimens were dehydrated by alcohols and embedded in PMMA. The specimens were cut into 200-μm thick sections using a microtome (Lecia Microsystems Ltd, Wetzlar, Germany) and were subsequently ground and polished to a thickness of 40–50 μm as previously described [23,24]. The femur specimens were stained in Von-Gieson for histopathological examination.
Mechanical test
The right femora were cut at the level of both the proximal end of the rod and the distal end of it. After that, the distal ends of the femora were resected 2 mm in length, and then, the distal ends of the implants were exposed 2 mm in length. Each femur was fixed on a metal base with resin. The shear strength at the bone-implant interface was measured using a universal testing machine (Instron 4302; Instron, Norwood, MA, USA) operated in stroke-control mode at a constant displacement of 0.5 mm/min. A vector of the push-out force was applied parallel to the long axis of the rod. The peak load was obtained before failure to measure the bone-implant shear strength [25,26].
Biochemical analysis
At the end of the experiment, blood was collected from the animals fasted overnight. Serum was separated and the aliquots were employed for the estimation of collagen type 1 cross-linked C-telopeptide (CTX-1), procollagen I N-terminal propeptide (PINP). Rat antibody specific ELISA kits for CTX and PINP were procured from R&D System, UK. The intra-assay coefficient of variance (CV) of CTX-1 is 10% and inter-assay CV: 11%. The intra-assay CV of PINP is 7.4% and inter-assay CV is 8.0%.
Reverse transcription-quantitative polymerase chain reaction(RT-qPCR) analysis
Total RNA was prepared and isolated using Triol reagent (Invitrogen Corp., Carlsbad, CA, USA) as previously described [26,24]. Briefly, tissue samples around implant were homogenized using a mortar and pestle with liquid nitrogen, using 5 ml of TRIzol per 50–100 mg of tissue. Insoluble material was removed from the homogenate by centrifugation for 10 min at 10,000 rpm at 4 °C. RNA was reverse transcribed into cDNA using Primer Script RT reagent kit (Takara, Dalian, China) following standard procedures of the manufacturer’s instructions. The quantitative real-time PCR was performed using the Applied Biosystems 7500 Sequence Detection System (ABI, Vernon, CA, USA). The expressions of the Runt-related transcription factor 2 (Runx-2), OC, osteocalcin; bone morphogenetic protein 2(BMP-2), small mothers against deca-pentaplegic homologs-1 (Smad1),osteoprotegerin (OPG), and receptor activator of nuclear factor kappa-B ligand (RANKL) were normalized to a housekeeping gene, GAPDH.
Statistical analysis
Measurement data were described as mean ± standard deviation (mean ± SD). Analysis was were performed with commercially available statistical software (SPSS 19·0; SPSS Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) and post-hoc Tukey test were carried out for comparisons between different groups. For all analyses, statistical significance was defined as P < 0.05.
Results
Animals
Due to anesthetic accident and infection, 6 rats died following the establishment ovariectomy or bone implant model. The survived 34 rats were included from further analysis.
Confirmation of osteoporosis model
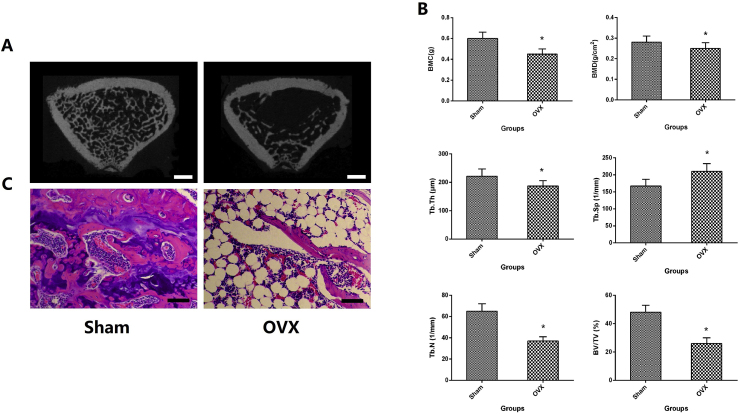
The results of histological and Micro-CT performed on the distal metaphysis of femur in Fig. 1. Compared with Sham group, the quantity and quality of trabecular bone were significantly lower than that in OVX group. In quantitative analysis of BMD, BMC, BV/TV, Tb.N, Tb. Sp and Tb.Th, statistically, there were obviously significant difference between OVX and the Sham group (P < 0.05).
Figure 1.
A. The Micro-CT 3D scanning of trabecular bone at femoral metaphyseal; B. Quantitative results of Micro-CT 3D scanning of trabecular bone expressed as BMD, BMC, BV/TV, Tb.N, Tb.Sp and Tb.Th ; C. histological analysis of femoral metaphysis. ∗p < 0.05 vs. Sham group, the scale bar represents 1 mm.
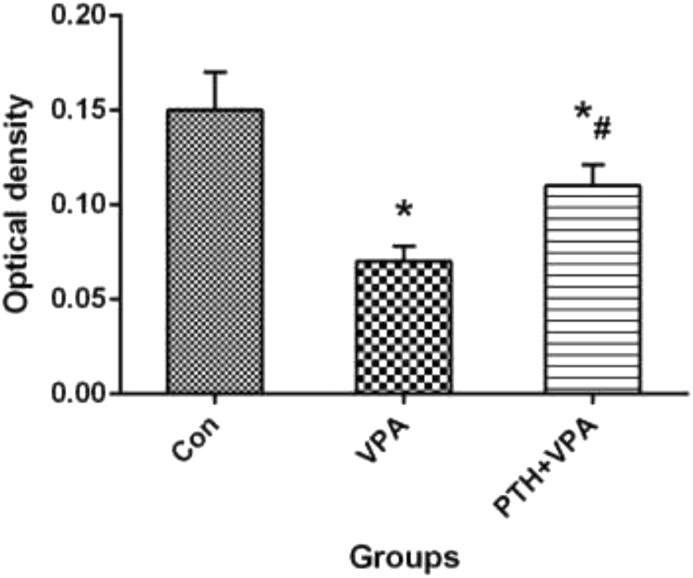
Osteoblastic viability
The cell viability by MTT assessment of three groups were shown in Fig. 2. After VPA treatment, the cell activity of VPA group was significantly lower than that of Con group (P<0.05), but the cell activity of PTH + VPA group was significantly higher than that of VPA group (P<0.05).
Figure 2.
MTT assay of cell viability with different treatment. ∗p < 0.05 vs. Con group, #p < 0.05 vs. VPA group.
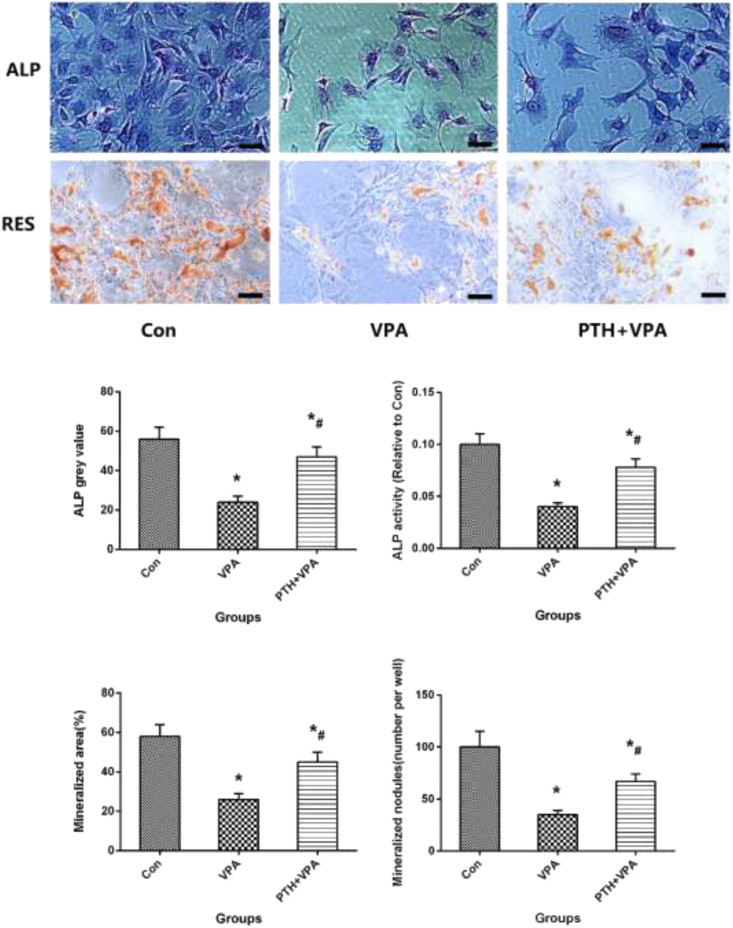
Alizarin red and ALP staining
Alizarin red and ALP staining with quantification of area in osteogenic differentiation of MC3T3-E1 cells, as shown in Fig. 3. The Mineralized nodules (number per well), Mineralized area (%),ALP grey value and ALP activity of VPA group is significantly higher than Con group (p < 0.05), but the values of osteogenic differentiation of MC3T3-E1 cells of PTH + VPA group was significantly higher than that of VPA group (P<0.05).
Figure 3.
Representative images of alizarin red staining and ALP staining, ALP grey value of staining, ALP activity, Mineralized nodules (number per well) and Mineralized area (%)of different groups. ∗p < 0.05 vs. Con group, #p < 0.05 vs. VPA group, the scale bar represents 100 μm.
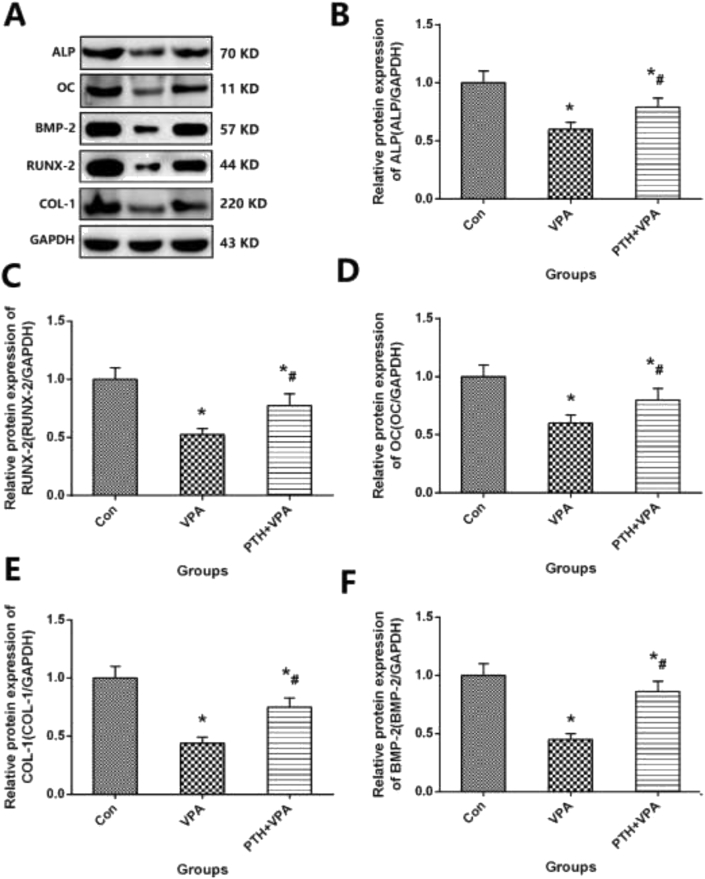
Osteogenic protein expression of MC3T3-E1 cells
Protein expression in osteogenic differentiation of MC3T3-E1 cells with different meddle, as shown in Fig. 4. The values of osteogenic protein expression such as ALP、BMP-2、RUNX-2、OC and COL-1 in VPA group is significantly higher than Con group (p < 0.05), but the values of ALP、BMP-2、RUNX-2、OC and COL-1 in PTH + VPA group was significantly higher than that of VPA group (P<0.05).
Figure 4.
A Representative images of Osteogenic Protein Expression and Osteogenic Protein Expression for BMP-2、ALP、RUNX-2、OC and COL-1 (A–F) of MC3T3-E1 cells; ∗p < 0.05 vs. Con group, #p < 0.05 vs.VPA group.
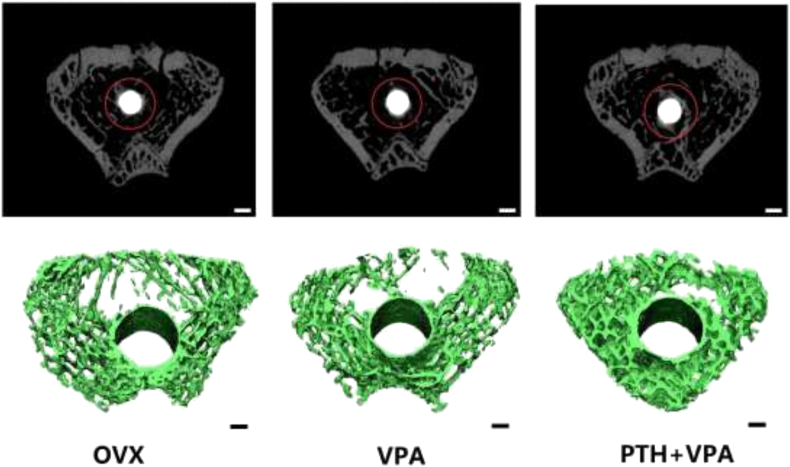
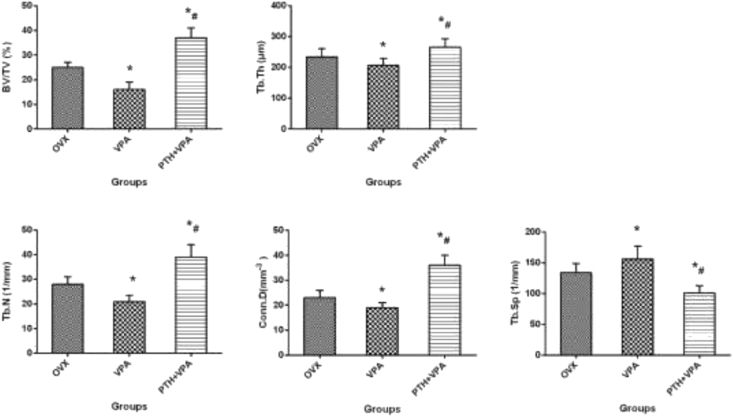
Micro-CT evaluation
The 3D reconstruction of the VOI area clearly depicted the effects of different treatments on bone osseointegration qualitatively (Fig. 5). The quantitative results were expressed as BV/TV, Tb.N, Tb.Sp,Conn.D and Tb.Th (Fig. 6). Compared with OVX group, VPA group revealed the poor values of BV/TV, Tb.N, Tb.Sp,Conn.D and Tb.Th (p < 0.05). But the values of BV/TV, Conn.D, Tb.N, Tb.Sp and Tb.Th in PTH + VPA group were significantly better than that of VPA group (P < 0.05).
Figure 5.
Representative images of the 3D reconstruction of Bone osseointegration around implants (bar 1 nm).
Figure 6.
Quantitative results expressed as BV/TV, Tb.N, Tb.Sp,Conn.D and Tb.Th of VOI area. ∗p < 0.05 vs. Con group, #p < 0.05 vs. VPA group.
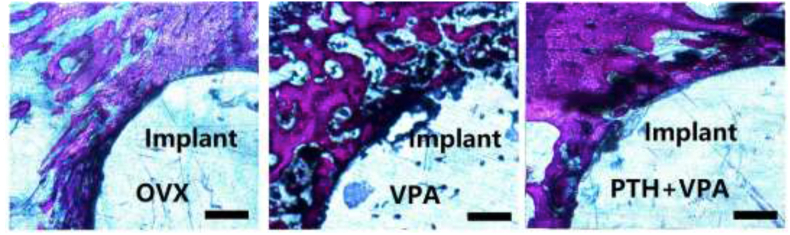
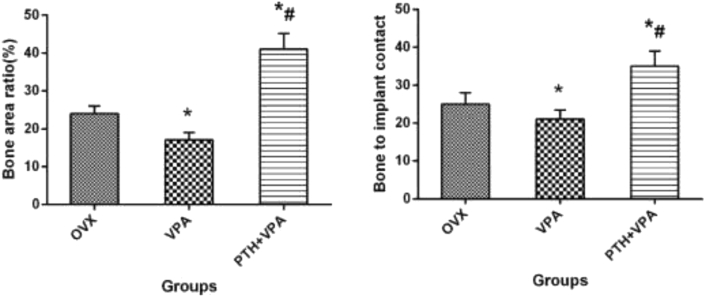
Histological analysis
Histological images showing bone osseointegration for different treatment, as shown in Fig. 7. Compared to group OVX, System administration VPA significantly decreased Bone area ratio (BAR) and Bone to implant contact (BIC) in histomorphometric analysis (P < 0.05; Fig. 8). However, PTH + VPA treatment revealed the highest values of BAR and BIC when compared with VPA group and OVX group (p < 0.05).
Figure 7.
Histological analysis through toluidine blue Von-Gieson (bar 200 μm).
Figure 8.
BIC and BAR of implants after 12 weeks treatment. ∗p < 0.05 vs. Con group, #p < 0.05 vs. VPA group.
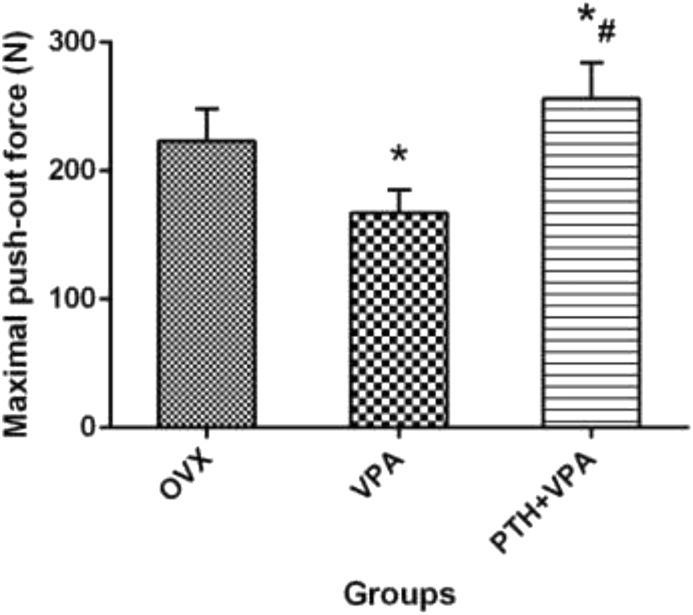
Biomechanical test
The maximum push-out force of different treatment were test as shown in Fig. 9. Compared to group OVX, System administration VPA significantly decreased the maximum push-out force (P < 0.05; Fig. 9). However, PTH + VPA treatment revealed the highest values of the maximum push-out force when compared with VPA group and OVX group (p < 0.05).
Figure 9.
The maximum push-out force of different treatment, ∗p < 0.05 vs. Con group, #p < 0.05 vs. VPA group.
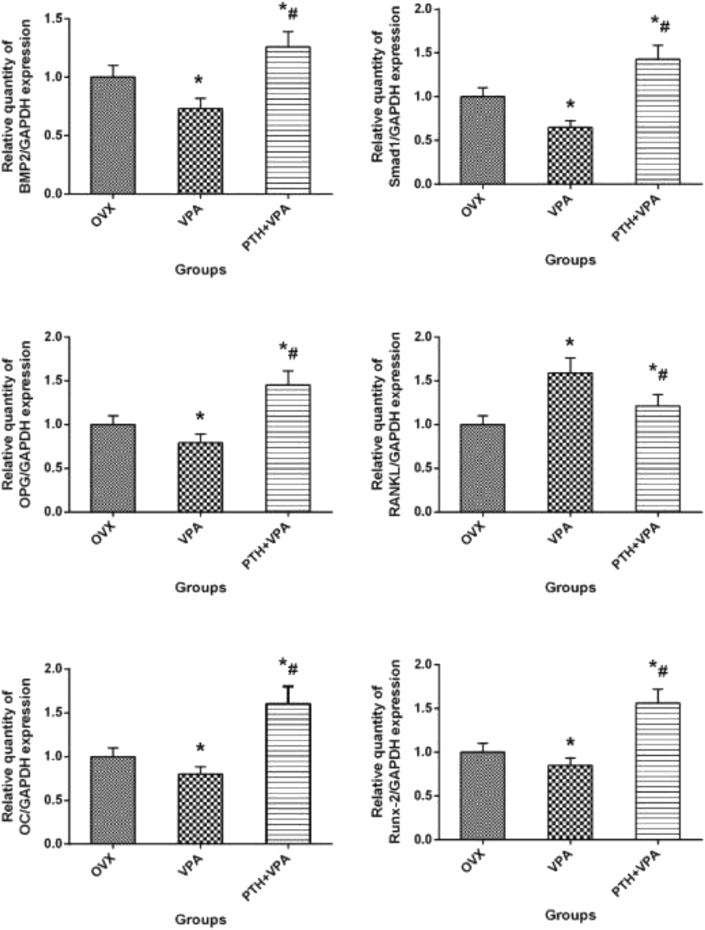
RT-qPCR analysis
Gene expression of bone tissue around implants after different treatment, as shown in Fig. 10. At 12 weeks, VPA group showed decreased BMP-2, OC, RUNX-2, Smad1, OPG than the OVX group (p < 0.05), while VPA group exhibited increased RANKL than the OVX group (p < 0.05). However, PTH + VPA treatment presented the strongest effect on BMP-2, OC, RUNX-2, Smad1, OPG and RANKL when compared with VPA group and OVX group (p < 0.05).
Figure 10.
Gene expression of VIO area bone tissue after different treatment. ∗p < 0.05 vs. OVX group, #p < 0.05 vs. VPA group.
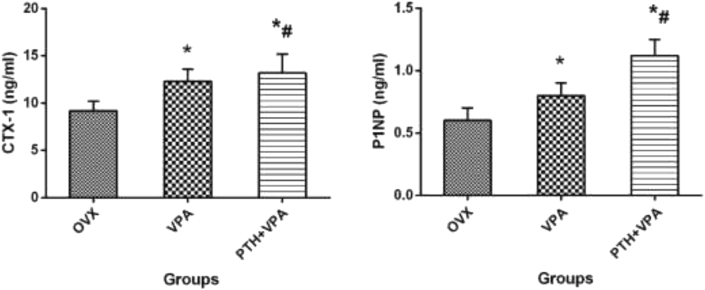
Bone turnover markers
The bone turnover markers clearly depicted the differences between OVX and treated groups (Fig. 11). Compared with group OVX, group VPA showed a higher CTX-1 and P1NP (P < 0.05). However, PTH + VPA treatment presented the strongest effect on CTX-1 and P1NP when compared with VPA group and OVX group (p < 0.05).
Figure 11.
Bone turnover markers expressed as CTX-1 and P1NP for each group. ∗p < 0.05 vs. OVX group, #p < 0.05 vs. VPA group.
Discussions
Hard tissue repair and regeneration cost hundreds of billions of dollars annually worldwide, and the need has substantially increased as the population has aged. However, the treatment of osteoporotic fractures remains an important area to target, yet as clinicians, we are still faced with an osteoporotic population that sustains the lower regeneration capacity of imbalanced bone remodeling as compared with the normal bone [26], [27], [28]. A large body of evidence indicates that antiepileptic drugs (AEDs) impact bone metabolism negatively leading to impaired bone quality and increased risk of fractures [19], [29], [30], [31]. In view of the side effects of valproic acid on affect bone metabolism and cause osteoporosis, this study selected clinical use of the converted dose for valproic acid, using an ovariectomized rat model to observe the effects of the drug on Ti osseointegration. These results support our initial hypothesis that VPA maintenance therapy has a negative effect on osseointegration in OVX rats. In the present study, PTH was used to intervene Ti osseointegration of ovariectomized rats treated with VPA, and the results confirmed the hypothesis that PTH significantly enhanced bone formation in ovariectomized rats even with VPA treatment.
In vitro, the effects of valproic acid alone or valproic acid with Parathyroid hormone(1-34) on cell proliferation and osteogenic differentiation of MC3T3-E1 cells were systematically evaluated. In the in vitro differentiation assay, mineralization of MC3T3-E1 osteoblasts and primary cultured osteoblasts was demonstrated with negative effects by Alizarin red staining assay and ALP activity assay after valproic acid treatment. In vitro tests indicated that valproic acid significantly decreases proliferation and osteoblastic differentiation in MC3T3-E1 osteoblasts and primary cultured osteoblasts. As we all know, during bone formation, cytokines synthesized and secreted by osteoblasts regulate the differentiation and maturation of osteoclasts such as ALP, BMP-2, COL-1, RUNX-2 and OC [32]. In the process of valproic acid-mediated osteoblast differentiation, VPA significantly reduces ALP activity and the expressions of key bone formation markers, such as BMP-2, COL-1, RUNX-2 and OC. When cultured with PTH, mouse osteoblastic MC3T3-E1 cells showed increased differentiation and mineralization by by MTT, ALP and Alizarin Red staining (ARS). PTH has also been shown to stimulate the proliferation of osteoblastic MC3T3-E1 cells, although VPA inhibited the differentiation.
In vivo study, osseointegration of a femoral implant in rats was retarded by OVX, and valproic acid further aggravated the OVX-induced poor osseointegration, likely promoting bone healing by preventing imbalanced bone turnover. We also found a tendency for greater variation in suppression tendency for Ti osseointegration in the VPA group when compared with the OVX group. Micro-CT and histometric analysis showed that the valproic acid treatment group showed the lowest new bone area percentage, and somewhat surprisingly the VPA group had a lower new bone area percentage than the OVX group. In terms of mechanical stability experiments, the valproic acid treatment was shown to have a detrimental effect on the mechanical stability of Ti when subjected to a mechanical experiments. In vivo and in vitro experiments indicate that clinically commonly used antiepileptic drug valproic acid have an inhibitory effect on bone formation around Ti. Micro-CT scanning and histological examination showed that bone formation in the PTH+VPA treatment group significantly increased, and mechanical experiments showed that the stability of titanium rod was also significantly improved.
Why can PTH antagonize the inhibitory effect of VPA on Ti osseointegration in ovariectomized rats? To further investigate the possible mechanism of VPA and PTH on Ti osseointegration, we detected the expression of bone remodeling-related genes and specific bone metabolism-related indicators. As is known to all, bone marrow mesenchymal stem cells (BMSCs) are the multipotential stem cells with the capacity to differentiate into osteoblasts in bone tissue under the control of several transcription factors. An adequate supply of osteoblasts from their precursors, BMSCs, is critical to bone formation. The BMP/SMAD signal pathway and OPG/RANKL signal pathway play an important role in bone remodeling [33]. BMP-2 can promote chondrocyte differentiation from BMSCs and exert anabolic effects by stimulating synthesis of extracellular matrix proteins osteoblasts from their precursors by activating SMAD1/5/8 [34]. Runt-related transcription factor 2 (RUNX2), a mammalian homologue of Drosophila runt, is an osteoblast master transcription factor. In addition, RUNX-2 is required for differentiation of precursor cells into mature osteoblasts [1]. Pre-osteoblasts differentiate into mature osteoblasts that deposit the necessary components to form bone matrix, followed by mineralization. Eventually, mature, mineralizing osteoblasts become embedded in the newly secreted bone matrix and undergo terminal differentiation to form osteocytes through activating terminal osteogenic marker genes such as osteocalcin (OC) [35]. RANKL (receptor activator of NF-κB ligand), its receptor RANK, and the natural inhibitor osteoprotegerin (OPG) are essential for the development and activation of osteoclasts [36]. The relative levels of RANKL and OPG expression in the bone microenvironment are the key to determining osteoclast formation and activity.
In this study, VPA treatment likely stimulate expression of RANKL and suppress expression of Runx-2, OC, Smad1, BMP-2 and OPG by osteoblasts or their progenitors. Increased serum levels of RANKL and decreased levels of Runx-2, OC, Smad1, BMP-2 and OPG have been associated with a poor osseointegration in VPA group. Restoring the RANKL/OPG imbalance by treatment with PTH not only reduces VPA-associated bone lesions but also halts disease progression in animal models. To test whether the PTH had an effect on markers of bone metabolism under VPA treatment, we selected and compared the change of P1NP and CTX as the biochemical markers of bone formation and resorption, respectively. Furthermore, this finding was also supported by some previous investigations [19], [37]. PTH moderately increased osteogenic gene (Runx-2, OC, Smad1, BMP-2 and OPG) expression and decreased osteoclastogenic gene (RANKL/OPG) expression, which indicated that the OPG/RANKL and BMP-2/Smad1 signal pathway were activation, and finally enhance the ability of bone repair and osseointegration, even in the case of VPA treatment.
There are several shortcomings associated with the present study. In this study, only one anti-epileptic drug VPA was selected for the experiment, and the drug dose was obtained by referencing the clinically used drug dose conversion. Whether it is suitable for rats needs to be further clarified. This study only observed the effect of VPA on osseointegration in ovariectomized rats at 12 weeks. It is not known whether early treatment and long-term treatment also have harmful effects on osseointegration. This study did not select the effects of different anti-epileptic drugs and different doses of drugs on the osseointegration of titanium osteoporosis, so the following research needs to further observe the effects of different drugs on this situation.
In conclusion, this pilot study suggests a possible association between the poor osseointegration of titanium implants and osteoporotic patient treatment with VPA that may be of considerable clinical importance. Our currently study further demonstrated that PTH can antagonize the negative effects of VPA on osteoblasts and osteoporotic rats, and improve the osseointegration ability of Ti in osteoporotic state.
Declaration of competing interest
There is none of the authors having any potential conflict of interest for our manuscript entitled “Parathyroid hormone (1–34) can reverse the negative effect of valproic acid on the osseointegration of titanium rods in ovariectomized rats” to be considered for publication in Journal of Orthopaedic Translation.
Acknowledgments
This study was supported by a grant from the University Natural Science Research Project of Anhui Province (CN)(grant no. KJ2017A266), Funding of “Peak”Training Program and “Panfeng”Innovation Team Project for Scientific Research of Yijishan Hospital, Wannan Medical College (grant no. GF2019G04, PF2019005, GF2019T02 and PF2019007), National Natural Science Foundation of China(82002322), Talented Scholars of Wannan Medical College (YR201917) and Young and Middle-aged Key Project of Wannan Medical College(WK2020ZF16).
References
- 1.Y L., H W., Xz Z., N L., Yc G., Tp C. Pentraxin 3 promotes the osteoblastic differentiation of MC3T3-E1 cells through the PI3K/Akt signaling pathway. Biosci Rep. 2020;40(6) doi: 10.1042/bsr20201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi E., Kaneuji A., Tsuda R., Numata Y., Ichiseki T., Fukui K. The influence of cement thickness on stem subsidence and cement creep in a collarless polished tapered stem:When are thick cement mantles detrimental? Bone & joint research. 2017;6(5):351–357. doi: 10.1302/2046-3758.65.BJR-2017-0028.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oki Y., Doi K., Makihara Y., Kobatake R., Kubo T., Tsuga K. Effects of continual intermittent administration of parathyroid hormone on implant stability in the presence of osteoporosis: an in vivo study using resonance frequency analysis in a rabbit model. J Appl Oral Sci. 2017;25(5):498–505. doi: 10.1590/1678-7757-2016-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolaou V.S., Efstathopoulos N., Kontakis G., Kanakaris N.K., Giannoudis P.V. The influence of osteoporosis in femoral fracture healing time. Inj Extra. 2009;40(10):202. doi: 10.1016/j.injury.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Debby H., Hom-Lay W. Medical contraindications to implant therapy: Part II: relative contraindications. Implant Dent. 2007;16(1):13–23. doi: 10.1097/ID.0b013e31803276c8. [DOI] [PubMed] [Google Scholar]
- 6.M F., Ml W., By W. Survey of risk factors for osteoporosis and osteoprotective behaviors among patients with epilepsy. Epilepsy Behav : E&B. 2015;45:217–222. doi: 10.1016/j.yebeh.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 7.N J., Lm L., Cj M., Hj P., J M., Wd L. Association of antiepileptic drugs with nontraumatic fractures: a population-based analysis. Arch Neurol. 2011;68(1):107–112. doi: 10.1001/archneurol.2010.341. [DOI] [PubMed] [Google Scholar]
- 8.Pc S., Dj W., Jg W., Tp V.S., Ac E. Use of antiepileptic drugs and risk of fractures: case-control study among patients with epilepsy. Neurology. 2006;66(9):1318–1324. doi: 10.1212/01.wnl.0000210503.89488.88. [DOI] [PubMed] [Google Scholar]
- 9.Ecevit C., Aydogan A., Kavakli T., Altinoz S. Effect of carbamazepine and valproate on bone mineral density. Pediatr Neurol. 2004;31(4):279–282. doi: 10.1016/j.pediatrneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 10.S K., P K., Kk P., D V. Homocysteine in neurological disease: a marker or a cause? CNS Neurol Disord - Drug Targets. 2011;10(3):361–369. doi: 10.2174/187152711794653797. [DOI] [PubMed] [Google Scholar]
- 11.S K., Kk P., D V. Insights into liaison between antiepileptic drugs and bone. Drug Discov Today. 2009;14:428–435. doi: 10.1016/j.drudis.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ll D., F W., H W., Dh W., Q L., Yx M. Osteoporosis drugs for prevention of clinical fracture in white postmenopausal women: a network meta-analysis of survival data. Osteoporos Int : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2020;31(5):961–971. doi: 10.1007/s00198-019-05183-4. [DOI] [PubMed] [Google Scholar]
- 13.F C., Ef E., C R., Pd M., N G., C K. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res : the official journal of the American Society for Bone and Mineral Research. 2011;26(3):503–511. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 14.Ml B., P C., Ev G., Df K., Pd D., Bh M. Teriparatide and raloxifene reduce the risk of new adjacent vertebral fractures in postmenopausal women with osteoporosis. Results from two randomized controlled trials. J Bone Jt Surg Am Vol. 2009;91(6):1329–1338. doi: 10.2106/jbjs.H.01030. [DOI] [PubMed] [Google Scholar]
- 15.Zs T., Ws Z., Bl B., W C., Yx L., Xb Y. The effects of combined human parathyroid hormone (1-34) and simvastatin treatment on the interface of hydroxyapatite-coated titanium rods implanted into osteopenia rats femurs. J Mater Sci Mater Med. 2016;27(3):43. doi: 10.1007/s10856-015-5650-9. [DOI] [PubMed] [Google Scholar]
- 16.Zs T., Ws Z., Z Q, Kk T., Zl H., Hm X. Intermittent administration of human parathyroid hormone (1-34) increases fixation of strontium-doped hydroxyapatite coating titanium implants via electrochemical deposition in ovariectomized rat femur. J Biomater Appl. 2016;30(7):952–960. doi: 10.1177/0885328215610898. [DOI] [PubMed] [Google Scholar]
- 17.Zs T., Ws Z., Xj W., X Z., L W., Jb X. Prevention of ovariectomy-induced osteoporosis in rats : comparative study of zoledronic acid, parathyroid hormone (1-34) and strontium ranelate. Zeitschrift fur Gerontologie und Geriatrie. 2019;52(2):139–147. doi: 10.1007/s00391-018-1376-x. [DOI] [PubMed] [Google Scholar]
- 18.Zs T., Yx L., W C., Zl H., Kk T., Q Z. Effect of teriparatide on repair of femoral metaphyseal defect in ovariectomized rats. Zeitschrift fur Gerontologie und Geriatrie. 2016;49(5):423–428. doi: 10.1007/s00391-015-0949-1. [DOI] [PubMed] [Google Scholar]
- 19.Parveen B., Tiwari A., Jain M., Pal S., Chattopadhyay N., Tripathi M. The anti-epileptic drugs valproate, carbamazepine and levetiracetam cause bone loss and modulate Want inhibitors in normal and ovariectomised rats. Bone. 2018;113:57–67. doi: 10.1016/j.bone.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Tao Z., Zhou W., Qiang Z., Tu K., Huang Z., Xu H. Intermittent administration of human parathyroid hormone (1-34) increases fixation of strontium-doped hydroxyapatite coating titanium implants via electrochemical deposition in ovariectomized rat femur. J Biomater Appl. 2016;30(7):952–960. doi: 10.1177/0885328215610898. [DOI] [PubMed] [Google Scholar]
- 21.Li Y.F., Li X.D., Bao C.Y., Chen Q.M., Zhang H., Hu J. Promotion of peri-implant bone healing by systemically administered parathyroid hormone (1-34) and zoledronic acid adsorbed onto the implant surface. Osteoporos Int : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(3):1063–1071. doi: 10.1007/s00198-012-2258-5. [DOI] [PubMed] [Google Scholar]
- 22.Gabet Y., Kohavi D., Kohler T., Baras M., Müller R., Bab I. Trabecular bone gradient in rat long bone metaphyses: mathematical modeling and application to morphometric measurements and correction of implant positioning. J Bone Miner Res. 2008;23(1):48–57. doi: 10.1359/jbmr.070901. [DOI] [PubMed] [Google Scholar]
- 23.Zs T., Xj W., Ws Z., Xj W., W L., M Y. Local administration of aspirin with β-tricalcium phosphate/poly-lactic-co-glycolic acid (β-TCP/PLGA) could enhance osteoporotic bone regeneration. J Bone Miner Metabol. 2019;37(6):1026–1035. doi: 10.1007/s00774-019-01008-w. [DOI] [PubMed] [Google Scholar]
- 24.Z T., W Z., X W., H L., N M., Y L. Local administration of aspirin improves osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats through activation of the Notch signaling pathway. J Biomater Appl. 2020;34(7):1009–1018. doi: 10.1177/0885328219889630. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Li Q., Zhu S., Luo E., Li J., Feng G. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31(34):9006–9014. doi: 10.1016/j.biomaterials.2010.07.112. [DOI] [PubMed] [Google Scholar]
- 26.Zs T., Xj W., M Y., Hg X. Local administration with silymarin could increase osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats. J Biomater Appl. 2019;34(5):664–672. doi: 10.1177/0885328219863290. [DOI] [PubMed] [Google Scholar]
- 27.Uezono M., Takakuda K., Kikuchi M., Suzuki S., Moriyama K. Hydroxyapatite/collagen nanocomposite-coated titanium rod for achieving rapid osseointegration onto bone surface †. J Biomed Mater Res B Appl Biomater. 2013;101B(6):1031–1038. doi: 10.1002/jbm.b.32913. [DOI] [PubMed] [Google Scholar]
- 28.L-V N., F-F J., Jm R., Bm S., H-H S., L-V A. Bioactive surfaces vs. Conventional surfaces in titanium dental implants: a comparative systematic review. J Clin Med. 2020;9(7) doi: 10.3390/jcm9072047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.X D., W Y., L Z., W C., Z Q., D C. Tailorable hierarchical structures of biomimetic hydroxyapatite micro/nano particles promoting endocytosis and osteogenic differentiation of stem cells. Biomaterials science. 2020;8(12):3286–3300. doi: 10.1039/d0bm00443j. [DOI] [PubMed] [Google Scholar]
- 30.B P., Ak T., M J., S P., N C., M T. The anti-epileptic drugs valproate, carbamazepine and levetiracetam cause bone loss and modulate Want inhibitors in normal and ovariectomised rats. Bone. 2018;113:57–67. doi: 10.1016/j.bone.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 31.A S., S M., E C., L P., G S., L G. Antiepileptic drugs and bone health: current concepts. Psychopharmacol Bull. 2020;50(2):36–44. [PMC free article] [PubMed] [Google Scholar]
- 32.Aa P., S A., M S., K J., C E., J H. Bone health screening practices among neurologists in patients on antiepileptic drugs: a quality improvement Project. Pediatr Neurol. 2020;102:49–55. doi: 10.1016/j.pediatrneurol.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 33.J F., L P., J L., T T., Y C. Effects of Second-Generation Antiepileptic Drugs Compared to First-Generation Antiepileptic Drugs on Bone Metabolism in Patients with Epilepsy: a Meta-Analysis. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2019;51(8):511–521. doi: 10.1055/a-0963-0054. [DOI] [PubMed] [Google Scholar]
- 34.dSdO Jc, Er L., Ck S., R O., Ir G.-J. Immunohistochemistry evaluation of BMP-2 with β-tricalcium phosphate matrix, polylactic and polyglycolic acid gel, and calcium phosphate cement in rats. Oral Maxillofac Surg. 2017;21(2):247–258. doi: 10.1007/s10006-017-0624-3. [DOI] [PubMed] [Google Scholar]
- 35.D H., X H., D Z., Q Z., C Y. Two novel polysaccharides from rhizomes of Cibotium barometz promote bone formation via activating the BMP2/SMAD1 signaling pathway in MC3T3-E1 cells. Carbohydr Polym. 2020;231:115732. doi: 10.1016/j.carbpol.2019.115732. [DOI] [PubMed] [Google Scholar]
- 36.H L., B N., Z D., S Z., T L., B Y. Bacitracin promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by stimulating the bone morphogenetic protein-2/Smad axis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;103:588–597. doi: 10.1016/j.biopha.2018.04.084. [DOI] [PubMed] [Google Scholar]
- 37.Cy T., W C., Y L., J W., Y Z., A M. Runx1 up-regulates chondrocyte to osteoblast lineage commitment and promotes bone formation by enhancing both chondrogenesis and osteogenesis. Biochem J. 2020;477(13):2421–2438. doi: 10.1042/bcj20200036. [DOI] [PMC free article] [PubMed] [Google Scholar]