Significance
Hopanoids are bacterial membrane lipids that are abundant in the geologic record. Hopanoids methylated at the C-2 position (2-methylhopanoids) are particularly abundant during Mesozoic episodes of ocean anoxia, yet the sources and factors that contribute to accumulation of these biomarkers remain poorly understood. Here, we report 2-methylhopanoid production in Nitrobacter, a ubiquitous group of nitrite-oxidizing bacteria that are favored under nutrient-rich growth environments, and we demonstrate that 2-methylhopanoid biosynthesis depends on cobalamin (vitamin B12). We propose that Nitrobacter spp. acquire their cobalamin from ammonia-oxidizing archaea in a mutualistic relationship that promotes nitrification. Therefore, accumulation of 2-methylhopanoids during the Mesozoic could have resulted from intensification of the oxidative steps of the marine nitrogen cycle in a nutrient-rich ocean.
Keywords: nitrification, hopanoids, biomarker, cobalamin, oceanic anoxic event
Abstract
Bacterial hopanoid lipids are ubiquitous in the geologic record and serve as biomarkers for reconstructing Earth’s climatic and biogeochemical evolution. Specifically, the abundance of 2-methylhopanoids deposited during Mesozoic ocean anoxic events (OAEs) and other intervals has been interpreted to reflect proliferation of nitrogen-fixing marine cyanobacteria. However, there currently is no conclusive evidence for 2-methylhopanoid production by extant marine cyanobacteria. As an alternative explanation, here we report 2-methylhopanoid production by bacteria of the genus Nitrobacter, cosmopolitan nitrite oxidizers that inhabit nutrient-rich freshwater, brackish, and marine environments. The model organism Nitrobacter vulgaris produced only trace amounts of 2-methylhopanoids when grown in minimal medium or with added methionine, the presumed biosynthetic methyl donor. Supplementation of cultures with cobalamin (vitamin B12) increased nitrite oxidation rates and stimulated a 33-fold increase of 2-methylhopanoid abundance, indicating that the biosynthetic reaction mechanism is cobalamin dependent. Because Nitrobacter spp. cannot synthesize cobalamin, we postulate that they acquire it from organisms inhabiting a shared ecological niche—for example, ammonia-oxidizing archaea. We propose that during nutrient-rich conditions, cobalamin-based mutualism intensifies upper water column nitrification, thus promoting 2-methylhopanoid deposition. In contrast, anoxia underlying oligotrophic surface ocean conditions in restricted basins would prompt shoaling of anaerobic ammonium oxidation, leading to low observed 2-methylhopanoid abundances. The first scenario is consistent with hypotheses of enhanced nutrient loading during OAEs, while the second is consistent with the sedimentary record of Pliocene–Pleistocene Mediterranean sapropel events. We thus hypothesize that nitrogen cycling in the Pliocene–Pleistocene Mediterranean resembled modern, highly stratified basins, whereas no modern analog exists for OAEs.
Hopanoids are a structurally diverse class of isoprenoid lipids that are involved in bacterial membrane homeostasis by mediating membrane organization and stress response (1–4). As chemical fossils, hopanoids and their diagenetic products are ubiquitous in the geologic record where they serve as important biomarkers for our planet’s biogeochemical and microbial evolution from the Proterozoic onward (5–8). Specifically, a subgroup of hopanoids methylated at the C-2 position (2-methylhopanoids) has been used as biomarkers for cyanobacteria (9) and invoked as evidence for the proliferation of nitrogen-fixing cyanobacteria during intervals such as Mesozoic ocean anoxic events (OAEs) (10, 11) and the Paleocene–Eocene Thermal Maximum (12).
The importance of hopanoids as biomarkers has sustained interest in understanding their sources and their role in bacterial physiology, leading to multiple recent studies that challenge prior assumptions (2, 3, 13–15). Specifically, the occurrence of 2-methylhopanoids in diverse alphaproteobacteria, including the anoxygenic phototroph Rhodopseudomonas palustris (16) and other freshwater and soil bacteria (17–21), illustrates that 2-methylhopanoids are not exclusive to cyanobacteria. Although 2-methylhopanoids predominantly originate from cyanobacteria in environments such as freshwater and lagoonal microbial mats (22, 23), it is plausible that other bacteria could have contributed to the geologic record of 2-methylhopanoids. However, it is unlikely that freshwater cyanobacteria were primary contributors to the accumulation of 2-methylhopanoids in offshore marine environments during Mesozoic OAEs. Thus, numerous uncertainties surround the origin of 2-methylhopanoids in the geological record and the reasons behind their prevalence during episodes of ocean anoxia.
Screening of genomes and metagenomes for the hpnP gene encoding a hopanoid C-2 methyltransferase (24) has led to the identification of a small subset of freshwater and soil bacteria as putative 2-methylhopanoid producers but has not revealed instances in marine cyanobacteria (24, 25). A more recent gene homology analysis suggested the presence of the hpnP gene in diverse marine cyanobacteria (26). However, 2-methylhopanoids have not been detected in any of these cyanobacteria (15), suggesting this recent study (26) may have detected related genes of different function.
Identification of common source organisms and elucidation of the underlying biochemistry of 2-methylhopanoid biosynthesis could help constrain the factors controlling their geologic record. Based on previous detection of the hpnP gene in a species of the alphaproteobacterial genus Nitrobacter (24), we hypothesized that Nitrobacter spp. could be an important but previously overlooked source of 2-methylhopanoids. Nitrobacter spp. are nitrite-oxidizing bacteria (NOB) that are abundant in soil, fresh water, and the oceans where they share an ecological niche with other NOB (Nitrospina, Nitrospira, Nitrococcus spp.), ammonia-oxidizing archaea (AOA), and ammonia-oxidizing bacteria (27–29).
Here, we use a combination of genomic analyses and culture experiments with the model organism Nitrobacter vulgaris AB1 to elucidate the factors driving 2-methylhopanoid biosynthesis in this taxon. Our results suggest that the reaction mechanism is not only dependent on a radical S-adenosylmethionine (SAM) enzyme (23) but also on the enzymatic cofactor cobalamin (vitamin B12). Because Nitrobacter spp. are cobalamin auxotrophs, we hypothesize that synergistic interaction between Nitrobacter and cobalamin-producing nitrifying archaea could be an important control on 2-methylhopanoid production. This hypothesis is consistent with enhanced production of 2-methylhopanoids under conditions of intensified oxidative nitrogen cycling in past environments.
Results and Discussion
NOB Produce 2-Methylhopanoids.
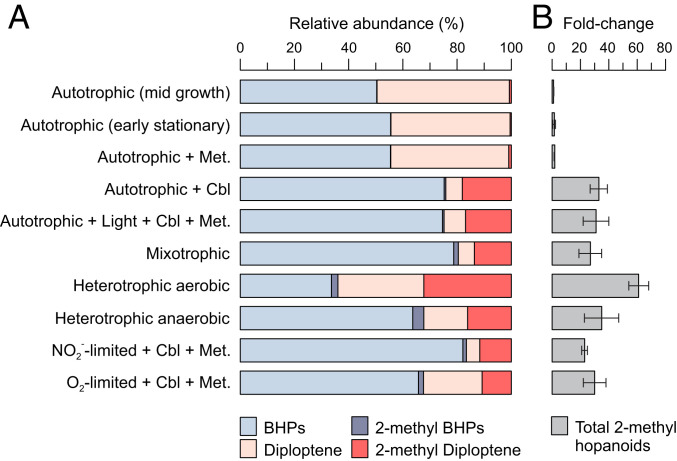
Identification of the shc gene, responsible for the initial step of hopanoid biosynthesis, and of hpnP (24) and hpnR (30) as the methyltransferases responsible for hopanoid methylation at C-2 and C-3, respectively, facilitates detection of potential methylhopanoid producers in cultures and environmental samples (25). Here, we identified shc and hpnP gene homologs in chemoautotrophic NOB of the genus Nitrobacter. All available Nitrobacter genomes (n = 6) contain shc and hpnP homologs (SI Appendix, Table S1), confirming the notion that 2-methylhopanoid synthesis is common among alphaproteobacteria (24). Similarly, Nitrobacter-like hpnP sequences are found in marine metagenomes (SI Appendix, Fig. S1), although short read length limits the number of confidently identified homologs. The Nitrobacter hpnP homologs are most closely related to alphaproteobacterial hpnP (SI Appendix, Fig. S1) and are located in a cluster of hopanoid biosynthesis genes with a similar ordering to that observed in R. palustris TIE-1 (SI Appendix, Fig. S2). Despite the presence of the hpnP gene, we detected only trace amounts of 2-methylhopanoids (2-methyl diploptene, 2-methyl bacteriohopanetetrol, and 2-methyl bacterioaminohopanetriol; 0.6% of all hopanoids) in N. vulgaris grown chemoautotrophically in a defined minimal medium containing only inorganic salts (Fig. 1).
Fig. 1.
Hopanoid composition of N. vulgaris at different growth conditions (average of triplicate cultures). (A) Relative abundances of polyfunctionalized (BHPs) and nonfunctionalized (diploptene) hopanoids and their C-2–methylated derivatives (2-methyl). (B) Fold change in total 2-methylhopanoid abundance (2-methyl BHPs + 2-methyl diploptene) relative to autotrophic early stationary-phase growth. Fold change values are given as averages of triplicate cultures with errors bars representing propagated uncertainties (one SD). SI Appendix, Table S2 has detailed information of fold change of individual hopanoid classes. Tested growth conditions: autotrophic (NO2− as electron donor), autotrophic + methionine (Met.), autotrophic + cobalamin (Cbl), autotrophic + Met. + Cbl + 6-/18-h light/dark cycles, mixotrophic (NO2− + complex organics), heterotrophic aerobic (complex organics but no NO2−), heterotrophic anaerobic (complex organics but N2 headspace), autotrophic NO2-limited chemostat, and autotrophic O2-limited chemostat.
The paucity of 2-methylhopanoids in autotrophic, axenic N. vulgaris cultures suggested that 2-methylhopanoid production depended upon a critical substrate that was not available or a condition that was not met. Previous studies on R. palustris and cyanobacteria indicated accumulation of 2-methylhopanoids only under specific growth conditions, such as in resting stages of the heterocystous cyanobacterium Nostoc punctiforme (31) or depending on the light regime in Antarctic lacustrine microbial mats (22). However, we observed no difference in 2-methylhopanoid abundance in N. vulgaris harvested during midlogarithmic growth phase or in stationary phase or when grown in light/dark cycles (Fig. 1 and SI Appendix, Table S2). We thus hypothesized that the need for a specific enzymatic cofactor was limiting 2-methylhopanoid production in N. vulgaris. Isotope labeling experiments indicate that the methyl groups in 2-methylhopanoids of Methylobacterium organophilum (32) and R. palustris (16), as well as in the homologous 3-methylhopanoids of Acetobacter pasteurianus (32), derive from methionine. Identification of a radical SAM domain in hpnP and hpnR (24, 30) is consistent with a mechanism in which the methyl groups of methylhopanoids are derived from methionine via SAM. Methionine may thus be a prerequisite and potentially a limiting factor for 2-methylhopanoid production.
Bacteria synthesize the essential amino acid methionine using either the cobalamin-dependent methionine synthase metH or the cobalamin-independent synthase metE (33). All sequenced Nitrobacter genomes contain homologs of both metH and metE (SI Appendix, Table S3). In the presence of cobalamin, metE is likely down-regulated in favor of metH by a cobalamin-binding riboswitch directly upstream of metE in the N. vulgaris genome (SI Appendix, Fig. S3). However, in the absence of both cobalamin and methionine in the medium, Nitrobacter spp. must use metE to sustain growth. The metE pathway is less efficient and has an ∼50-fold slower turnover rate than metH (34), suggesting that methionine supplementation could increase both growth rate and 2-methylhopanoid production when cobalamin is absent. To address this question, N. vulgaris cultures were grown with and without 100 µM methionine in the absence of cobalamin. Nitrite oxidation rates of methionine-supplemented N. vulgaris cultures were higher compared with methionine-free cultures (Fig. 2), and abundance of 2-methylhopanoids was increased approximately twofold relative to the control (Fig. 1B and SI Appendix, Table S2). Nevertheless, relative abundances of 2-methylhopanoids (predominantly 2-methyl diploptene) remained low at 1.0 ± 0.14% of total hopanoids (control: 0.6 ± 0.03%), suggesting that other factors must control hopanoid methylation in N. vulgaris.
Fig. 2.
(A) Maximum nitrite oxidation rates of N. vulgaris during chemolithoautotrophic growth on methionine-free and cobalamin-free medium (control) or with 0.5 μM methionine (Met) or 0.5 µM cobalamin (Cbl) added to the medium. The error bars represent one SD of average nitrite oxidation rates calculated from quadruplicate cultures (three-point linear approximation). (B) Biomass-normalized concentrations of hopanoids in N. vulgaris. The total amount of NO2− oxidized in each experiment and replicate was identical at 7.5 mM. The error bars represent one SD of triplicate cultures.
Experimentation with different growth conditions subsequently showed that 2-methylhopanoid relative abundance was higher in mixotrophic (15 ± 4%), heterotrophic aerobic (35 ± 3%), and heterotrophic anaerobic cultures (20 ± 7%) (Fig. 1B). During mixotrophic and heterotrophic growth, the medium contained the complex organic substrates yeast extract and peptone, which are rich in amino acids and vitamins including cobalamin, suggesting that these organic substrates provided an essential cofactor for 2-methylhopanoid biosynthesis.
Biosynthesis of 2-Methylhopanoids Is Cobalamin-Dependent.
Subsequent experiments suggested that the missing cofactor in 2-methylhopanoid biosynthesis was cobalamin. Supplementation of chemoautotrophic cultures with cobalamin resulted in an ∼33-fold increase in 2-methylhopanoid abundance, which is similar to the 27- to 61-fold increase in cultures grown on complex medium (Fig. 1B and SI Appendix, Table S2). Chemostat experiments with chemoautotrophic cultures in which the supply of either O2 (the electron acceptor in nitrite oxidation) or NO2− (electron donor) was limited showed similar 2-methylhopanoid enrichment compared with batch cultures when both were supplemented with cobalamin (Fig. 1). These experiments further support the view that 2-methylhopanoid biosynthesis is primarily controlled by cobalamin availability and not by growth stage or growth rate.
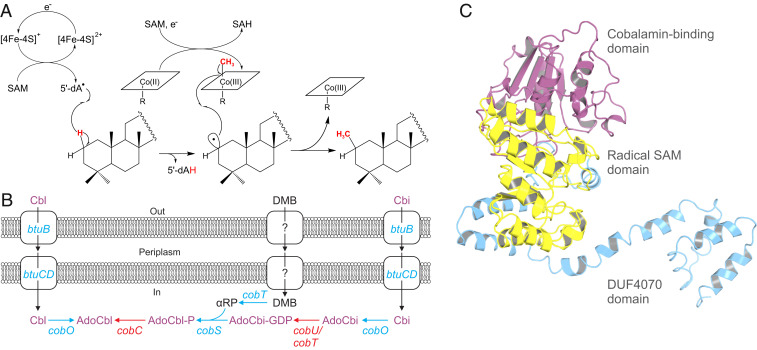
Although cobalamin has long been known to be involved in radical reactions (35, 36), it has not yet been shown how cobalamin is involved in 2-methylhopanoid biosynthesis. Our inspection of the genomes of Nitrobacter and other bacteria reveals that all confirmed hpnP and hpnR sequences contain a cobalamin-binding N-terminal domain (Pfam PF02310) in addition to a radical SAM domain (CxxxCxxCxC motif) (24, 30, 37). However, the hpnP and hpnR sequences differ significantly from canonical cobalamin radical enzymes. The specific DxHxxG cobalamin-binding motif (36) of adenosylcobalamin (AdoCbl)-dependent enzymes is not conserved among extant hpnP and hpnR protein sequences, suggesting a different binding mechanism. Further, the existence of both a radical SAM domain and a cobalamin-binding domain suggests a reaction mechanism distinct from the AdoCbl-mediated methylation found, for instance, in metH, which contains the DxHxxG motif but lacks a radical SAM domain (38). Based on these characteristics, the hpnP and hpnR gene products likely belong to the class B radical SAM methylase family. Enzymes of this family use methylcobalamin rather than AdoCbl as cofactor (39). Although this family contains ∼7,000 annotated proteins, involvement of cobalamin has been demonstrated only for a few candidates (36).
The genetic and experimental evidence for involvement of both methionine and cobalamin in 2-methylhopanoid formation indicates a reaction mechanism congruent with other class B radical SAM enzymes (39–41) that is based on the capacity of cobalamins to act as alkyl donors (42). Here, we propose that the reaction mechanism uses two SAM moieties as well as cobalamin to form 2-methylhopanoids and by analogy, 3-methylhopanoids (Fig. 3A). The first SAM would be reductively cleaved by a [4Fe-4S]+ cluster to form a 5′deoxyadenosyl radical and methionine. The second SAM would transfer a methyl group to a cobalamin lacking an upper ligand, forming methylcobalamin. Two sequential radical reactions then lead to the formation of methylhopanoids: the 5′deoxyadenosyl radical would abstract a hydrogen atom from the hopanoid at the C-2 position forming 5′deoxyadenosine and a hopanoid radical intermediate. The hopanoid radical is then available to attack the methyl group of methylcobalamin, forming the 2- or 3-methylhopanoid. This proposed reaction mechanism is consistent with previously observed incorporation of 13C- and 2H-labeled methyl groups from methionine into 2-methylhopanoids (16, 32). Based on this evidence, Zundel and Rohmer (32) hypothesized that an unsaturation at C-2 would be required for 2-methylhopanoid biosynthesis. However, if HpnP is a bona fide class B methylase, it may be able to methylate sp3 carbons without a need for prior unsaturation. Given that no hopanoid bearing an unsaturation at the C-2 position was found in N. vulgaris or reported for other bacteria containing 2-methylhopanoids, we suggest that the precursor to 2-methylhopanoids is a saturated hopanoid. The reaction mechanism proposed here could also apply to HpnR, which catalyzes biosynthesis of 3-methylhopanoids and similarly contains both radical SAM and cobalamin-binding domains.
Fig. 3.
(A) Proposed reaction scheme for the formation of 2-methylhopanoids by a cobalamin (Cbl)-dependent radical SAM methylase. The 5′-deoxyadenosyl (5′dA•) radical is generated by reductive cleavage of SAM by a [4Fe-4S]+ cluster. Methylcobalamin and S-adenosylhomocysteine (SAH) are formed by transfer of a methyl group from SAM to Cbl (corrin ring represented by square, R = lower ligand). The 5′dA• radical abstracts hydrogen from the hopanoid substrate followed by reaction of the hopanoid radical intermediate with the methyl group of methylcobalamin. (B) Proposed Cbl salvage pathway reconstructed from Nitrobacter spp. genomes (blue, protein homolog present; red, absent). Cbls (missing upper ligand) or Cbl biosynthetic intermediates such as cobinamides (Cbis; missing upper and lower ligands) are transported into the cell via the btuCD transporter (note missing Cbl/Cbi-binding protein btuF) (SI Appendix, Table S4). The upper ligand adenosine (Ado) is added to form AdoCbl. Scavenging of Cbi additionally requires synthesis of the lower ligand from 5,6-dimethylbenzimidazole (DMB) via α-ribazole phosphate (αRP). Question marks indicate a putative unknown transporter. (C) Structure of N. vulgaris HpnP modeled using Phyre2 (11). Functional domains obtained via InterPro7 (10) are highlighted in purple (Cbl binding), yellow (radical SAM), and blue (domain of unknown function 4070 [DUF4070]).
The specificity of HpnP for C-2 and HpnR for the C-3 positions could be related to conformational differences between the three methylene carbon atoms on the hopanoid A ring, warranting further structural elucidation of these enzymes. Protein modeling indicates that the secondary and tertiary structures of HpnP (Fig. 3C) resemble the cobalamin-dependent SAM enzyme OxsB (SI Appendix, Fig. S4). Although OxsB is not a methylase (43), it allows binding of SAM in two distinct modes, with one mode orienting SAM close to the Fe-S cluster to allow radical generation and a second mode orienting SAM close to cobalamin (35). By analogy, the capability of hopanoid methylases to bind SAM in both modes could allow sequential radical generation and methyl donation from SAM to cobalamin.
The reaction mechanism proposed here could be tested using genetic manipulation experiments. Although no genetic system is currently available for Nitrobacter spp., the genetically tractable alphaproteobacterium R. palustris TIE-1 may be a promising target as it contains both a complete cobalamin biosynthesis pathway and the hpnP gene (SI Appendix, Table S4) (3, 24, 44). Deletion of the corrinoid adenosyltransferase gene cobO could help exclude a role of AdoCbl (instead of methylcobalamin) in 2-methylhopanoid formation. Finally, deletion of genes required for biosynthesis of the lower ligand could help elucidate cobalamin-binding mechanisms of HpnP.
Potential Function of 2-Methylhopanoids in Nitrobacter.
During autotrophic growth, cobalamin addition had a greater effect than methionine addition on the rate of nitrite oxidation (Fig. 2). This indicates that cobalamin has an additional growth-enhancing effect beyond its role in methionine biosynthesis via MetH. The growth rate-enhancing effect of cobalamin may be attributable to a direct phenotype effect conferred by 2-methylhopanoid production. In R. palustris, 2-methylhopanoids increase membrane rigidity (13), and analogously to sterols, hopanoids increase membrane ordering and integrity (2, 3, 13, 45). This results in reduced leakage of protons and other ions across the membrane (45, 46), thus improving cellular energetic efficiency and growth rates.
Conversely, the observed enhancement of nitrite oxidation rates is not easily attributable to other cobalamin-dependent processes. Other than MetH and HpnP, Nitrobacter genomes contain only two additional cobalamin-dependent proteins: cobalamin-dependent class II ribonucleotide reductase (RNR; B2M20_08295) and methylmalonyl-Coenzyme A mutase (B2M20_12005, B2M20_11995). Nitrobacter spp. likely utilize methylmalonyl-Coenzyme A mutase for heterotrophic catabolism similar to other bacteria (47, 48), and thus, this enzyme would not be necessary for chemoautotrophic growth. On the other hand, RNR is a required enzyme under all growth conditions, but Nitrobacter spp. have two RNRs: cobalamin-dependent class II and the cobalamin-independent class I. The use of class II instead of class I RNR by Nitrobacter spp. should primarily reflect cobalamin availability because the class I RNR genes (B2M20_16355, B2M20_16350) are located directly downstream of a cobalamin riboswitch in Nitrobacter genomes (SI Appendix, Fig. S5). As in other bacteria, binding of cobalamin to this riboswitch would result in down-regulation of class I RNR gene transcription and necessitate use of class II RNR (49). However, a switch between RNRs would likely not affect growth rates as both RNRs are equally efficient: deletion of either class I or class II RNR had no effect on growth of Streptomyces coelicolor (49, 50). It is thus likely that the cobalamin-induced increase in nitrite oxidation rate is solely attributable to the enrichment of 2-methylhopanoids.
Availability of cobalamin results in further changes in hopanoid composition of N. vulgaris that may be significant for adaptation to environmental stress. N. vulgaris grown with cobalamin produces significantly less diploptene than cobalamin-limited cultures (P < 0.05, two-tailed t test), while total hopanoid content does not change significantly (P > 0.05) (Fig. 2B). Because 2-methyl diploptene does not replace diploptene quantitatively, it appears unlikely that their contents are simply controlled by a product–precursor relationship. Instead, 2-methyl diploptene may be more efficient than diploptene, providing beneficial membrane rigidification at significantly lower concentrations. Remodeling of hopanoid composition and specifically, accumulation of 2-methylhopanoids have been shown to be essential for high-temperature acclimatization in N. punctiforme (4). The availability of cobalamin may, therefore, be essential to membrane homeostasis and thus, the adaptability of Nitrobacter spp. and other NOB to environmental stress.
Nitrobacter spp. Are Cobalamin Auxotrophs.
Cobalamin-stimulated 2-methylhopanoid production in N. vulgaris contrasts with a previous study reporting high levels of 2-methylhopanoids but no further stimulation by cobalamin in R. palustris (51). This contrasting outcome is explained by key biosynthetic differences between these organisms. R. palustris has a complete aerobic cobalamin biosynthetic pathway and thus, does not require cobalamin supplementation under aerobic conditions (SI Appendix, Table S4). In addition, R. palustris was grown in medium containing yeast extract (51), a rich source of cobalamin. By contrast, all sequenced Nitrobacter species lack complete cobalamin biosynthetic pathways and specifically cannot synthesize the central cobinamide moiety (SI Appendix, Table S4). Absence of cobalamin biosynthesis also could explain the dependence of Nitrobacter spp. on complex organics for anaerobic growth, which requires use of the cobalamin-dependent class II RNR for DNA synthesis. Nitrobacter spp. may take up extracellular cobalamin or cobinamides through a transmembrane transporter system (btuBCD) (SI Appendix, Table S4) and attach the upper (adenosine) and lower axial ligands (5,6-dimethylbenzimidazole) to the cobinamide moiety (Fig. 3C). We, therefore, postulate that Nitrobacter spp. are cobalamin auxotrophs that take up cobalamin or cobinamides.
The apparent absence of cobalamin biosynthetic capacity in Nitrobacter spp. compared with closely related bacteria (SI Appendix, Table S4) can be parsimoniously explained by loss of the cobalamin pathway in Nitrobacter spp. Ancestral-state reconstructions using the marker genes cobF and cbiD support this view, suggesting that the last common ancestor of the class Rhizobiales and the family Bradyrhizobiaceae was capable of cobalamin biosynthesis (SI Appendix, Fig. S6). The biosynthesis of cobalamin requires more than 20 steps and thus, poses a high metabolic and genetic burden (52), which may have promoted gene loss in Nitrobacter. The use of cobalamin-independent isozymes and a ready supply of cobalamin in their habitat could have further promoted gene loss. Aquatic Nitrobacter spp. inhabit remineralization zones alongside a multitude of cobalamin-producing microbes, including diverse heterotrophic bacteria (53–55). Additionally, while ammonia-oxidizing bacteria cannot synthesize cobalamin, AOA are significant sources of cobalamin (SI Appendix, Table S4) (53, 56, 57). The heterogeneous distribution of cobalamin biosynthetic capacity underlies mutualistic relationships between cobalamin producers and cobalamin auxotrophs: for example, between marine heterotrophic bacteria and eukaryotic algae (54, 58). A mutualistic relationship between Nitrobacter and AOA is plausible, as ammonia oxidizers would benefit from removal of the waste product nitrite as well as from potential reciprocal feeding on urea or other substrates (59). Cobalamin-based mutualism could thus influence environmental nitrification rates, with implications for nitrogen cycling in natural and artificial systems.
Implications for 2-Methylhopanoid Paleorecords.
Reevaluating the sources of marine 2-methylhopanoids.
The accumulation of 2-methylhopanoids during episodes of ocean anoxia, most prominently during Mesozoic OAEs, commonly has been interpreted to indicate the proliferation of nitrogen-fixing cyanobacteria (10, 11, 60–62). Here, we reevaluate this idea and introduce an alternative hypothesis that pelagic (distal open ocean) deposits of 2-methylhopanoids are largely derived from NOB. A dominant NOB source maintains the connection between 2-methylhopanoid abundances and changes in the marine nitrogen cycle and importantly, does not preclude a concomitant major expansion of marine nitrogen-fixing cyanobacteria.
Historically, the attribution of 2-methylhopanoids to cyanobacteria was based primarily on 1) the occurrence of 2-methylhopanoids in some nitrogen-fixing freshwater cyanobacteria, particularly of the order Nostocales (15, 63, 64), and 2) accumulation of 2-methylhopanoids during periods of enhanced nitrogen fixation in the geologic past (10, 11, 60–62). In addition, the stable carbon isotopic composition (δ13C values of −28 to −32‰) of 2-methylhopanoids deposited during OAE1a (Aptian) is consistent with carbon fixation via the Calvin–Benson–Bassham (CBB) cycle (10, 62). These δ13C signatures cannot be considered diagnostic, however, as the CBB cycle is broadly distributed among bacteria. Similarly, 2-methylhopanoid biosynthesis is not exclusive to cyanobacteria but is also widely distributed among alphaproteobacteria. Based on these ambiguities, the use of 2-methylhopanoids as biomarkers for cyanobacteria has been questioned (25, 65).
To comprehensively assess the occurrence of hpnP in cyanobacteria and its correspondence to habitat type and nitrogen fixation, we surveyed all publicly available cyanobacterial genomes (n = 1,184) (SI Appendix, Fig. S7). These analyses reveal that none of the 72 finished cyanobacterial genomes (=40 species) from marine or brackish habitats contain hpnP copies (SI Appendix, Fig. S8), in agreement with earlier reports based on fewer genomes (25, 66). After also incorporating unfinished genomes, only 1 of 739 marine cyanobacterial species contains an hpnP homolog: a filamentous cyanobacterium (ESFC-1) originally isolated from an intertidal mat (67). We detected and removed false positives in marine cyanobacteria described by an earlier study (26) that were caused by high sequence similarity of orthologous sequences (SI Appendix, Supplementary Methods). By contrast, hpnP is prevalent (∼30% of all species) (SI Appendix, Fig. S8) in terrestrial and freshwater cyanobacteria, particularly within the order Nostocales (SI Appendix, Fig. S7). Based on this contrast between freshwater/terrestrial and marine species, we propose that the occurrence of hpnP in cyanobacteria is not related to taxonomy but to habitat, in agreement with previous ecological interpretations proposed for the hpnP distribution in alphaproteobacteria (25, 65, 66). This hypothesis is supported by the observation that hpnP is absent in genomes of marine and brackish planktonic ecotypes even in clades where all or most terrestrial ecotypes contain hpnP (e.g., Nodularia spp. in the order Nostocales) (SI Appendix, Fig. S9). Together with observations from modern, marginally marine environments (23), our findings indicate that occurrence of hpnP in marine cyanobacteria is limited to nonplanktonic, mat-forming ecotypes. Finally, analysis of the nitrogen fixation marker gene nifH shows that the link between 2-methylhopanoids and nitrogen fixation is tenuous. Although some cyanobacteria that produce 2-methylhopanoids can fix nitrogen, the majority of nitrogen-fixing cyanobacteria do not produce 2-methylhopanoids (SI Appendix, Figs. S7–S9) (25). Conversely, as previously stated (64), this also would imply that an absence of 2-methylhopanoids cannot be interpreted as an absence or decrease of cyanobacterial nitrogen fixation.
A contribution of marine cyanobacteria to the 2-methylhopanoid signal in pelagic OAE environments would require either 1) cyanobacterial populations that are not extant in the modern ocean (i.e., they either became extinct or are so rare as to remain undetected) and/or 2) invasion of freshwater/coastal ecotypes into pelagic environments. The latter might be prompted by opening of new environmental niches, such as decreased nitrogen/phosphorous (N/P) ratios that promote nitrogen fixation and/or reduced salinity due to enhanced freshwater runoff. While the extinction hypothesis is not falsifiable and therefore, must remain a possibility, studies of modern and recent analog environments do not support the invasion hypothesis. Free-living filamentous or heterocystous cyanobacteria are not common in the (sub-)tropical open ocean due to ecological constraints, including high sea surface temperature (68), which would have been globally exacerbated under Mesozoic greenhouse conditions. Even in modern marine environments where large blooms of nitrogen-fixing filamentous/heterocystous cyanobacteria do occur, 2-methylhopanoids are not abundant. In the temperate Baltic Sea, cyanobacterial blooms induced by low N/P ratios are dominated by hpnP-lacking Nostocales species (69), and consequently, the Pleistocene–Holocene paleorecord contains no 2-methylhopanoids (70). Similarly, enhanced nitrogen fixation in the stratified anoxic Arctic Ocean during the Eocene was associated with hpnP-lacking, nitrogen-fixing cyanobacterial symbionts of freshwater ferns (65, 71) (Trichormus azollae) (SI Appendix, Fig. S9). Finally, Neogene sapropel events in the subtropical Mediterranean Sea that featured conditions superficially similar to OAEs (low N/P, water column stratification, anoxia, enhanced nitrogen fixation) together with an expansion of heterocystous, nitrogen-fixing cyanobacteria (Nostocales) (72) resulted in the deposition of only trace amounts of methylhopanoids (73) relative to large amounts of nonmethylated hopanoids (74, 75).
An alternative explanation for the apparent connection between marine nitrogen cycle perturbations and 2-methylhopanoid accumulation is their production by NOB. The δ13C values of 2-methylhopanoids deposited during OAE1a, as described above, would be consistent with an origin from Nitrobacter spp., as these organisms also are autotrophs that use the CBB cycle for carbon fixation. Accordingly, N. vulgaris yields biomass and hopanoids that are fractionated by 23.2 ± 0.3 and 31.9 ± 0.4‰, respectively, relative to CO2 when grown in chemoautotrophic batch cultures (SI Appendix, Table S5). These fractionations are close to the maximum theoretical fractionation of the CBB cycle and are indistinguishable from culture data of other organisms using this cycle, including cyanobacteria (76, 77).
Although the model organism N. vulgaris prefers brackish and freshwater environments, marine ecotypes are common throughout the oceans (28) and share major traits with other Nitrobacter spp., including cobalamin auxotrophy and presence of an hpnP homolog (e.g., Nitrobacter sp. Nb-311) (SI Appendix, Fig. S1 and Table S4). High relative abundances of C30 2-methylhopanoids (i.e., 2-methyl diploptene/2-methyl diplopterol) observed during OAEs (∼10 to 45% of hopanoids) (10, 11, 60–62) are congruent with the distribution in N. vulgaris (33 to 75%) (SI Appendix, Table S6), when considering dilution of the signal through production of desmethyl-C30 hopanoids by other bacteria. In addition to C30 2-methylhopanoids, OAE records often contain high abundances (up to 20%) of the C31–C35 2-methylhopanoid degradation products of diagenetically less stable 2-methyl bacteriohopanepolyols (BHPs) (10, 78). These 2-methyl BHPs are produced in lower amounts by N. vulgaris (up to 6% relative to nonmethyl BHPs under laboratory culture conditions) (SI Appendix, Table S6) compared with OAE records. This discrepancy could indicate additional sources of 2-methylhopanoids during OAEs, from other as yet unidentified bacterial groups. However, our results also demonstrate that BHP biosynthesis and relative abundances depend strongly on growth conditions (SI Appendix, Table S6), and therefore, it also remains possible that further changes in gene expression (such as the hpnG and hpnH genes for BHP biosynthesis from diploptene) (79) as well as phenotypic variation could lead to increased 2-methyl BHP production in Nitrobacter spp. Environmental stressors such as high temperatures may cause increased 2-methylhopanoid production, something that remains to be assessed in culture. Congruence between our culture experiments and the geologic record implicates nitrite-oxidizing Nitrobacter spp. as a source of 2-methylhopanoids but leaves open the question of the biogeochemical and geological significance of this association.
Two distinct modes of nitrogen cycling during ocean anoxia.
Despite their relative abundance in OAE sediments, 2-methylhopanoids are rare in modern anoxic environments and recent anoxic events such as Pliocene–Pleistocene Mediterranean sapropels (73), which like OAEs, are associated with marine nitrogen cycle perturbations (80, 81). This contrasting behavior raises questions about oceanographic influences on 2-methylhopanoid production.
If 2-methylhopanoids indicate the proliferation of Nitrobacter spp., they must reflect unusually elevated nitrite concentrations in the oxic zone. In the modern oxic ocean, ammonium released from organic matter remineralization is oxidized to nitrite by AOA, which is in turn oxidized to nitrate by NOB. Efficient cycling of ammonia and nitrite results in consistently low concentrations of these substrates and the proliferation of energy-efficient NOB (Nitrospina) and AOA (Thaumarchaeota) with high substrate affinity (K strategists) (82–87). Similarly, in the redox transition zones of modern oxygen minimum zones and anoxic basins, nitrite is maintained at low concentrations not only by NOB but also, by competing anaerobic ammonia-oxidizing (anammox) bacteria that use nitrite as an electron acceptor. Since anammox bacteria are inhibited by oxygen and have a lower affinity for nitrite than many NOB except for Nitrobacter, the balance between aerobic and anaerobic nitrite cycling in the ocean ultimately depends on the intensity of deoxygenation, the total nutrient load, and the dynamics of local water column structure (82, 86–89). In both modern scenarios, K-strategist NOB and anammox bacteria outcompete the r-strategist Nitrobacter (lower substrate affinity, lower energy efficiency, and higher growth rate) for nitrite. We, therefore, postulate that ocean oxygenation and circulation play a major role via their control on the balance between aerobic and anaerobic nitrogen cycling processes.
Maintenance of sufficiently high concentrations and fluxes of nitrite would require both elevated total ocean nutrient concentrations and overturning circulation vigorous enough to promote oxidative nitrogen cycling (i.e., OAE-like conditions) (90). Upwelling of ammonium-rich deep water into the oxic zone, as suggested previously for the Cretaceous Atlantic Ocean (90–93), could have fueled nitrification and consequently, the proliferation of Nitrobacter spp. resulting in the observed deposition of 2-methylhopanoids (10). Due to the mobilization of cobalt under anoxic conditions (94, 95), upwelling could have supplied the cobalt necessary for production of cobalamin by Thaumarchaeota. Although deep waters in strongly density-stratified water bodies such as the Mediterranean Sea during sapropel deposition or the modern Black Sea also accumulate ammonium, slower vertical mixing yields a sharper and stable chemocline dominated by slow, diffusive nutrient supply and low nitrite and oxygen concentrations. We propose that such physical regimes lead instead to prevalence of anammox bacteria and K-strategist NOB and a consequent paucity of 2-methylhopanoids.
The geologic record provides evidence for the existence of these two distinct modes of nitrite cycling and allows developing testable hypotheses about biomarker distributions (Fig. 4). Increased accumulation of AOA lipid biomarkers (crenarchaeol) relative to bulk organic matter is common to both OAEs and Mediterranean sapropels (96, 97), consistent with intensified nitrogen cycling in both scenarios. In contrast, anammox biomarkers such as stereoisomers of bacteriohopanetetrol including bacteriohopanetetrol-II (98) are prevalent in Mediterranean sapropels (74, 75) but have not been detected in OAEs, possibly due to their thermodynamic instability. Although their absence from OAEs may be related to low preservation potential, characteristically 13C-depleted (75, 99) homohopanoid degradation products should have accumulated if anammox bacteria were prevalent, given that bacteriohopanetetrol-II is the most abundant hopanoid in some sapropels (74). Although further research is needed, there is currently no evidence that homohopanoids in OAE deposits are 13C depleted (62), suggesting low abundance of anammox bacteria. In summary, biomarker records suggest the existence of 1) aerobically dominated nitrite cycling during OAEs and 2) combined aerobic–anaerobic nitrite cycling during Mediterranean sapropel events (Fig. 4).
Fig. 4.
Conceptual sketch of microbial community composition (size of circles indicating hypothetical abundance), relative fluxes of nitrogen species (thickness of arrows; blue, aerobic oxidation of ammonia; purple, aerobic oxidation of nitrite; red, anammox; gray, denitrification), water column structure (curves for nitrogen species: estimated trends in concentration relative to each other and positions relative to oxic/anoxic and photic/aphotic zones), and the resulting sedimentary biomarker record (peak height indicating arbitrary abundance) for two end-member states of nitrite cycling. BHT-II, bacteriohopanetetrol-II (anammox bacteria); Cren, crenarchaeol (AOA); 2-methyl, 2-methylhopanoids (Nitrobacter-type NOB).
Importantly, biomarker evidence and nitrogen cycle modeling suggest intensification of multiple nitrogen cycle processes (nitrogen fixation, nitrification, denitrification) during anoxic events (10, 74, 90, 97, 100). However, proliferation of Nitrobacter and other NOB during OAEs may have exerted a feedback on the nitrogen cycle by limiting the intensification of fixed nitrogen loss via anammox. This process may have helped cyanobacterial nitrogen fixation keep pace with reductive nitrogen loss, thereby sustaining primary productivity and the consequent formation of black shales.
Further studies are required to test the predicted covariation of crenarchaeol and 2-methylhopanoids as well as the anticorrelation of 2-methylhopanoids and bacteriohopanetetrol-II (Fig. 4). Global transects of biomarker distributions could further be used to investigate the spatial and temporal decoupling of aerobic and anaerobic nitrogen cycling processes predicted from nitrogen cycle modeling (90). Regardless, nitrifier ecotypes coexist in a continuum, implying that nitrifiers such as Nitrospina, Nitrospira, Nitrococcus, Nitrosomonas, and Nitrosococcus spp. contribute to the deposition of non–2-methylated hopanoids, as indicated by the presence of the shc gene and absence of the hpnP gene (SI Appendix, Table S1) (101, 102). We, therefore, expect spatial variation in biomarker records that results from variability in nitrification rates and ecotypes in response to contrasting water column structure. Similarly, any sedimentary contribution of 2-methylhopanoids from cyanobacteria would most likely be associated with coastal and shallow shelf environments that harbor mat-forming, filamentous, and heterocystous cyanobacteria.
Conclusions
We suggest that cobalamin-dependent nitrifier mutualism mechanistically links 2-methylhopanoid deposition by Nitrobacter spp. to intensification of the marine nitrogen cycle. Combined analysis of biomarkers from Nitrobacter, AOA, and anammox bacteria could be used to quantify the relative importance of a circulation-driven, high-nutrient loading nitrogen cycle in which considerable nitrite reaches the upper water column (nominally an “aerobic-dominated” cycle) vs. a relatively more stratified, lower to moderate nutrient concentration nitrogen cycle in which anammox contributes significantly to denitrification and surface waters remain more oligotrophic (nominally an “anaerobic-dominated” cycle). These relative states of the nitrogen cycle must be related to primary productivity in the geologic past: the record of 2-methylhopanoids implies that nitrogen cycling during Mediterranean sapropel deposition was largely similar to modern anoxic basins, whereas OAE-type nitrogen cycling has no apparent analog in the modern ocean.
Materials and Methods
Detailed methods can be found in SI Appendix. N. vulgaris AB1 was grown at 28 °C in pH 7.5 artificial freshwater medium modified from Bock et al. (103). Cultures were harvested in stationary phase after nitrite was completely consumed or optical density stopped increasing (SI Appendix, Fig. S10). Biomass was collected using filtration. Lipids were extracted using a Bligh and Dyer extraction (104) following the modifications of Sturt et al. (105) and Saénz (106). Stable carbon isotopic composition of dissolved inorganic carbon, lipids, and biomass was analyzed using mass spectrometry. BHPs and hopanoids were quantified on acetylated aliquots using coupled high-performance liquid chromatography–mass spectrometry as described previously (107).
Supplementary Material
Acknowledgments
This work was funded through the Gordon and Betty Moore Foundation (A.P.) and NSF Grants 1843285 and 1702262. T.W.E. acknowledges funding from the Alexander von Humboldt-Gesellschaft through the Feodor Lynen Fellowship. Research at the Massachusetts Institute of Technology was otherwise supported by NASA Exobiology Program Grant 18-EXXO18-0039. We thank B. David Naafs and an anonymous reviewer for their constructive comments that helped improve an earlier version of this manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012357117/-/DCSupplemental.
Data Availability.
All lipid data, growth parameters, and results of genomic analyses are included in the article, the SI Appendix, and in the Harvard Dataverse (DOI: 10.7910/DVN/IZY3JA).
References
- 1.Ourisson G., Rohmer M., Poralla K., Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu. Rev. Microbiol. 41, 301–333 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Sáenz J. P., et al. , Hopanoids as functional analogues of cholesterol in bacterial membranes. Proc. Natl. Acad. Sci. U.S.A. 112, 11971–11976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welander P. V., et al. , Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 191, 6145–6156 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci J. N., Morton R., Kulkarni G., Summers M. L., Newman D. K., Hopanoids play a role in stress tolerance and nutrient storage in the cyanobacterium Nostoc punctiforme. Geobiology 15, 173–183 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Brocks J. J., Banfield J., Unravelling ancient microbial history with community proteogenomics and lipid geochemistry. Nat. Rev. Microbiol. 7, 601–609 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Brocks J. J., Pearson A., Building the biomarker tree of life. Rev. Mineral. Geochem. 59, 233–258 (2005). [Google Scholar]
- 7.Briggs D. E. G., Summons R. E., Ancient biomolecules: Their origins, fossilization, and role in revealing the history of life. Bioessays 36, 482–490 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Ourisson G., Albrecht P., Hopanoids. 1. Geohopanoids: The most abundant natural products on Earth? Acc. Chem. Res. 25, 398–402 (1992). [Google Scholar]
- 9.Summons R. E., Jahnke L. L., Hope J. M., Logan G. A., 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 400, 554–557 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Kuypers M. M. M., van Breugel Y., Schouten S., Erba E., Sinninghe Damsté J. S., N2-fixing cyanobacteria supplied nutrient N for Cretaceous oceanic anoxic events. Geology 32, 853–856 (2004). [Google Scholar]
- 11.Blumenberg M., Wiese F., Imbalanced nutrients as triggers for black shale formation in a shallow shelf setting during the OAE 2 (Wunstorf, Germany). Biogeosciences 9, 4139–4153 (2012). [Google Scholar]
- 12.Lyons S. L., et al. , Palaeocene–Eocene thermal maximum prolonged by fossil carbon oxidation. Nat. Geosci. 12, 54 (2019). [Google Scholar]
- 13.Wu C.-H., Bialecka-Fornal M., Newman D. K., Methylation at the C-2 position of hopanoids increases rigidity in native bacterial membranes. eLife 4, e05663 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni G., Wu C.-H., Newman D. K., The general stress response factor EcfG regulates expression of the C-2 hopanoid methylase HpnP in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 195, 2490–2498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sáenz J. P., Waterbury J. B., Eglinton T. I., Summons R. E., Hopanoids in marine cyanobacteria: Probing their phylogenetic distribution and biological role. Geobiology 10, 311–319 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Rashby S. E., Sessions A. L., Summons R. E., Newman D. K., Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proc. Natl. Acad. Sci. U.S.A. 104, 15099–15104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot H. M., Rohmer M., Farrimond P., Rapid structural elucidation of composite bacterial hopanoids by atmospheric pressure chemical ionisation liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 21, 880–892 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Sahm H., Rohmer M., Bringer-Meyer S., Sprenger G. A., Welle R., “Biochemistry and physiology of hopanoids in bacteria” in Advances in Microbial Physiology, Rose A. H., Ed. (Academic Press, 1993), pp. 247–273. [DOI] [PubMed] [Google Scholar]
- 19.Bisseret P., Zundel M., Rohmer M., Prokaryotic triterpenoids. 2. 2 beta-Methylhopanoids from Methylobacterium organophilum and Nostoc muscorum, a new series of prokaryotic triterpenoids. Eur. J. Biochem. 150, 29–34 (1985). [DOI] [PubMed] [Google Scholar]
- 20.Renoux J.-M., Rohmer M., Prokaryotic triterpenoids. New bacteriohopanetetrol cyclitol ethers from the methylotrophic bacterium Methylobacterium organophilum. Eur. J. Biochem. 151, 405–410 (1985). [DOI] [PubMed] [Google Scholar]
- 21.Bravo J.-M., Perzl M., Härtner T., Kannenberg E. L., Rohmer M., Novel methylated triterpenoids of the gammacerane series from the nitrogen-fixing bacterium Bradyrhizobium japonicum USDA 110. Eur. J. Biochem. 268, 1323–1331 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Matys E. D., et al. , Bacteriohopanepolyols across environmental gradients in Lake Vanda, Antarctica. Geobiology 17, 308–319 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Garby T. J., Walter M. R., Larkum A. W. D., Neilan B. A., Diversity of cyanobacterial biomarker genes from the stromatolites of Shark Bay, Western Australia. Environ. Microbiol. 15, 1464–1475 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Welander P. V., Coleman M. L., Sessions A. L., Summons R. E., Newman D. K., Identification of a methylase required for 2-methylhopanoid production and implications for the interpretation of sedimentary hopanes. Proc. Natl. Acad. Sci. U.S.A. 107, 8537–8542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci J. N., et al. , Diverse capacity for 2-methylhopanoid production correlates with a specific ecological niche. ISME J. 8, 675–684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornejo-Castillo F. M., Zehr J. P., Hopanoid lipids may facilitate aerobic nitrogen fixation in the ocean. Proc. Natl. Acad. Sci. U.S.A. 116, 18269–18271 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward B. B., Glover H. E., Lipschultz F., Chemoautotrophic activity and nitrification in the oxygen minimum zone off Peru. Deep-Sea Res. A Oceanogr. Res. Pap. 36, 1031–1051 (1989). [Google Scholar]
- 28.Ward B. B., Carlucci A. F., Marine ammonia- and nitrite-oxidizing bacteria: Serological diversity determined by immunofluorescence in culture and in the environment. Appl. Environ. Microbiol. 50, 194–201 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santoro A. E., Richter R. A., Dupont C. L., Planktonic marine archaea. Annu. Rev. Mar. Sci. 11, 131–158 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Welander P. V., Summons R. E., Discovery, taxonomic distribution, and phenotypic characterization of a gene required for 3-methylhopanoid production. Proc. Natl. Acad. Sci. U.S.A. 109, 12905–12910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doughty D. M., Hunter R. C., Summons R. E., Newman D. K., 2-Methylhopanoids are maximally produced in akinetes of Nostoc punctiforme: Geobiological implications. Geobiology 7, 524–532 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zundel M., Rohmer M., Prokaryotic triterpenoids. 3. The biosynthesis of 2 β-methylhopanoids and 3 β-methylhopanoids of Methylobacterium organophilum and Acetobacter pasteurianus ssp. pasteurianus. Eur. J. Biochem. 150, 35–39 (1985). [DOI] [PubMed] [Google Scholar]
- 33.Ferla M. P., Patrick W. M., Bacterial methionine biosynthesis. Microbiology (Reading) 160, 1571–1584 (2014). [DOI] [PubMed] [Google Scholar]
- 34.González J. C., Banerjee R. V., Huang S., Sumner J. S., Matthews R. G., Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli: Two solutions to the same chemical problem. Biochemistry 31, 6045–6056 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Bridwell-Rabb J., Grell T. A. J., Drennan C. L., A rich man, poor man story of S-adenosylmethionine and cobalamin revisited. Annu. Rev. Biochem. 87, 555–584 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Bridwell-Rabb J., Drennan C. L., Vitamin B., Vitamin B12 in the spotlight again. Curr. Opin. Chem. Biol. 37, 63–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sofia H. J., Chen G., Hetzler B. G., Reyes-Spindola J. F., Miller N. E., Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: Functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 29, 1097–1106 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drennan C. L., Matthews R. G., Ludwig M. L., Cobalamin-dependent methionine synthase: The structure of a methylcobalamin-binding fragment and implications for other B12-dependent enzymes. Curr. Opin. Struct. Biol. 4, 919–929 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Bauerle M. R., Schwalm E. L., Booker S. J., Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation. J. Biol. Chem. 290, 3995–4002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato S., Kudo F., Kuzuyama T., Hammerschmidt F., Eguchi T., C-methylation catalyzed by Fom3, a cobalamin-dependent radical S-adenosyl-l-methionine enzyme in fosfomycin biosynthesis, proceeds with inversion of configuration. Biochemistry 57, 4963–4966 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Wang B., et al. , Stereochemical and mechanistic investigation of the reaction catalyzed by Fom3 from Streptomyces fradiae, a cobalamin-dependent radical S-adenosylmethionine methylase. Biochemistry 57, 4972–4984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosimann H., Kräutler B., Methylcorrinoids methylate radicals-their second biological mode of action? Angew. Chem. Int. Ed. Engl. 39, 393–395 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Bridwell-Rabb J., Zhong A., Sun H. G., Drennan C. L., Liu H. W., A B12-dependent radical SAM enzyme involved in oxetanocin A biosynthesis. Nature 544, 322–326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao Y., Kappler A., Croal L. R., Newman D. K., Isolation and characterization of a genetically tractable photoautotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl. Environ. Microbiol. 71, 4487–4496 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poger D., Mark A. E., The relative effect of sterols and hopanoids on lipid bilayers: When comparable is not identical. J. Phys. Chem. B 117, 16129–16140 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Haines T. H., Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 40, 299–324 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Haller T., Buckel T., Rétey J., Gerlt J. A., Discovering new enzymes and metabolic pathways: Conversion of succinate to propionate by Escherichia coli. Biochemistry 39, 4622–4629 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Savvi S., et al. , Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: Implications for propionate metabolism during growth on fatty acids. J. Bacteriol. 190, 3886–3895 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borovok I., Gorovitz B., Schreiber R., Aharonowitz Y., Cohen G., Coenzyme B12 controls transcription of the Streptomyces class Ia ribonucleotide reductase nrdABS operon via a riboswitch mechanism. J. Bacteriol. 188, 2512–2520 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borovok I., et al. , Alternative oxygen-dependent and oxygen-independent ribonucleotide reductases in Streptomyces: Cross-regulation and physiological role in response to oxygen limitation. Mol. Microbiol. 54, 1022–1035 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Wu C.-H., et al. , Quantitative hopanoid analysis enables robust pattern detection and comparison between laboratories. Geobiology 13, 391–407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danchin A., Braham S., Coenzyme B12 synthesis as a baseline to study metabolite contribution of animal microbiota. Microb. Biotechnol. 10, 688–701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heal K. R., et al. , Two distinct pools of B12 analogs reveal community interdependencies in the ocean. Proc. Natl. Acad. Sci. U.S.A. 114, 364–369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croft M. T., Lawrence A. D., Raux-Deery E., Warren M. J., Smith A. G., Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Lu X., Heal K. R., Ingalls A. E., Doxey A. C., Neufeld J. D., Metagenomic and chemical characterization of soil cobalamin production. ISME J. 14, 53–66 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doxey A. C., Kurtz D. A., Lynch M. D., Sauder L. A., Neufeld J. D., Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. ISME J. 9, 461–471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallam S. J., et al. , Genomic analysis of the uncultivated marine crenarchaeote Cenarchaeum symbiosum. Proc. Natl. Acad. Sci. U.S.A. 103, 18296–18301 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazamia E., et al. , Mutualistic interactions between vitamin B12 -dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 14, 1466–1476 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Koch H., et al. , Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. U.S.A. 112, 11371–11376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao C., et al. , Biogeochemical evidence for euxinic oceans and ecological disturbance presaging the end-Permian mass extinction event. Earth Planet. Sci. Lett. 281, 188–201 (2009). [Google Scholar]
- 61.Kasprak A. H., et al. , Episodic photic zone euxinia in the northeastern Panthalassic Ocean during the end-Triassic extinction. Geology 43, 307–310 (2015). [Google Scholar]
- 62.Dumitrescu M., Brassell S. C., Biogeochemical assessment of sources of organic matter and paleoproductivity during the early Aptian oceanic anoxic event at shatsky rise, ODP Leg 198. Org. Geochem. 36, 1002–1022 (2005). [Google Scholar]
- 63.Garby T. J., et al. , Lack of methylated hopanoids renders the cyanobacterium Nostoc punctiforme sensitive to osmotic and pH stress. Appl. Environ. Microbiol. 83, e00777-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talbot H. M., et al. , Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Org. Geochem. 39, 232–263 (2008). [Google Scholar]
- 65.Newman D. K., Neubauer C., Ricci J. N., Wu C.-H., Pearson A., Cellular and molecular biological approaches to interpreting ancient biomarkers. Annu. Rev. Earth Planet. Sci. 44, 493–522 (2016). [Google Scholar]
- 66.Ricci J. N., Michel A. J., Newman D. K., Phylogenetic analysis of HpnP reveals the origin of 2-methylhopanoid production in Alphaproteobacteria. Geobiology 13, 267–277 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Everroad R. C., et al. , Permanent draft genome of strain ESFC-1: Ecological genomics of a newly discovered lineage of filamentous diazotrophic cyanobacteria. Stand. Genomic Sci. 11, 53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staal M., Meysman F. J. R., Stal L. J., Temperature excludes N2-fixing heterocystous cyanobacteria in the tropical oceans. Nature 425, 504–507 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Bauersachs T., Talbot H. M., Sidgwick F., Sivonen K., Schwark L., Lipid biomarker signatures as tracers for harmful cyanobacterial blooms in the Baltic Sea. PLoS One 12, e0186360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blumenberg M., et al. , Bacteriohopanepolyols record stratification, nitrogen fixation and other biogeochemical perturbations in Holocene sediments of the central Baltic Sea. Biogeosciences 10, 2725–2735 (2013). [Google Scholar]
- 71.Bauersachs T., et al. , Fossilized glycolipids reveal past oceanic N2 fixation by heterocystous cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 107, 19190–19194 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bale N. J., et al. , Biomarker evidence for nitrogen-fixing cyanobacterial blooms in a brackish surface layer in the Nile River plume during sapropel deposition. Geology 47, 1088–1092 (2019). [Google Scholar]
- 73.Löhr S. C., Kennedy M. J., George S. C., Williamson R. J., Xu H., Sediment microfabric records mass sedimentation of colonial cyanobacteria and extensive syndepositional metazoan reworking in Pliocene sapropels. Depos. Rec. 4, 293–317 (2018). [Google Scholar]
- 74.Rush D., et al. , Biomarker evidence for the occurrence of anaerobic ammonium oxidation in the eastern Mediterranean Sea during Quaternary and Pliocene sapropel formation. Biogeosciences 16, 2467–2479 (2019). [Google Scholar]
- 75.Hemingway J. D., et al. , A novel method to measure the 13C composition of intact bacteriohopanepolyols. Org. Geochem. 123, 144–147 (2018). [Google Scholar]
- 76.Sakata S., et al. , Carbon isotopic fractionation associated with lipid biosynthesis by a cyanobacterium: Relevance for interpretation of biomarker records. Geochim. Cosmochim. Acta 61, 5379–5389 (1997). [DOI] [PubMed] [Google Scholar]
- 77.Wilkes E. B., Carter S. J., Pearson A., CO2-dependent carbon isotope fractionation in the dinoflagellate Alexandrium tamarense. Geochim. Cosmochim. Acta 212, 48–61 (2017). [Google Scholar]
- 78.French K. L., Tosca N. J., Cao C., Summons R. E., Diagenetic and detrital origin of moretane anomalies through the Permian–Triassic boundary. Geochim. Cosmochim. Acta 84, 104–125 (2012). [Google Scholar]
- 79.Schmerk C. L., et al. , Elucidation of the Burkholderia cenocepacia hopanoid biosynthesis pathway uncovers functions for conserved proteins in hopanoid-producing bacteria. Environ. Microbiol. 17, 735–750 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Calvert S. E., Nielsen B., Fontugne M. R., Evidence from nitrogen isotope ratios for enhanced productivity during formation of eastern Mediterranean sapropels. Nature 359, 223–225 (1992). [Google Scholar]
- 81.Sachs J. P., Repeta D. J., Oligotrophy and nitrogen fixation during eastern mediterranean sapropel events. Science 286, 2485–2488 (1999). [DOI] [PubMed] [Google Scholar]
- 82.Bristow L. A., et al. , Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters. Proc. Natl. Acad. Sci. U.S.A. 113, 10601–10606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nowka B., Daims H., Spieck E., Comparison of oxidation kinetics of nitrite-oxidizing bacteria: Nitrite availability as a key factor in niche differentiation. Appl. Environ. Microbiol. 81, 745–753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacob J., et al. , Oxidation kinetics and inverse isotope effect of marine nitrite-oxidizing isolates. Aquat. Microb. Ecol. 80, 289–300 (2017). [Google Scholar]
- 85.Martens-Habbena W., Berube P. M., Urakawa H., de la Torre J. R., Stahl D. A., Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., et al. , Nitrifier adaptation to low energy flux controls inventory of reduced nitrogen in the dark ocean. Proc. Natl. Acad. Sci. U.S.A. 117, 4823–4830 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Straka L. L., Meinhardt K. A., Bollmann A., Stahl D. A., Winkler M. H., Affinity informs environmental cooperation between ammonia-oxidizing archaea (AOA) and anaerobic ammonia-oxidizing (Anammox) bacteria. ISME J. 13, 1997–2004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bristow L. A., et al. , N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nat. Geosci. 10, 24–29 (2017). [Google Scholar]
- 89.Lam P., et al. , Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc. Natl. Acad. Sci. U.S.A. 104, 7104–7109 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naafs B. D. A., et al. , Fundamentally different global marine nitrogen cycling in response to severe ocean deoxygenation. Proc. Natl. Acad. Sci. U.S.A. 116, 24979–24984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Higgins M. B., Robinson R. S., Husson J. M., Carter S. J., Pearson A., Dominant eukaryotic export production during ocean anoxic events reflects the importance of recycled NH4+. Proc. Natl. Acad. Sci. U.S.A. 109, 2269–2274 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Junium C. K., Arthur M. A., Nitrogen cycling during the cretaceous, cenomanian-turonian oceanic anoxic event II: Cretaceous nitrogen cycling. Geochem. Geophys. Geosyst. 8, Q03002 (2007). [Google Scholar]
- 93.Jenkyns H. C., Geochemistry of oceanic anoxic events. Geochem. Geophys. Geosyst. 11, Q03004 (2010). [Google Scholar]
- 94.Swanner E. D., et al. , Cobalt and marine redox evolution. Earth Planet. Sci. Lett. 390, 253–263 (2014). [Google Scholar]
- 95.Saito M. A., Sigman D. M., Morel F. M. M., The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean–Proterozoic boundary? Inorg. Chim. Acta 356, 308–318 (2003). [Google Scholar]
- 96.Kuypers M. M. M., et al. , Archaeal remains dominate marine organic matter from the early Albian oceanic anoxic event 1b. Palaeogeogr. Palaeoclimatol. Palaeoecol. 185, 211–234 (2002). [Google Scholar]
- 97.Polik C. A., Elling F. J., Pearson A., Impacts of paleoecology on the TEX86 Sea Surface temperature proxy in the Pliocene-Pleistocene Mediterranean Sea. Paleoceanogr. Paleoclimatol. 33, 1472–1489 (2018). [Google Scholar]
- 98.Schwartz-Narbonne R., et al. , A unique bacteriohopanetetrol stereoisomer of marine anammox. Org. Geochem. 143, 103994 (2020). [Google Scholar]
- 99.Schouten S., et al. , Stable carbon isotopic fractionations associated with inorganic carbon fixation by anaerobic ammonium-oxidizing bacteria. Appl. Environ. Microbiol. 70, 3785–3788 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuypers M. M. M., et al. , Massive expansion of marine archaea during a mid-Cretaceous oceanic anoxic event. Science 293, 92–95 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Kharbush J. J., Thompson L. R., Haroon M. F., Knight R., Aluwihare L. I., Hopanoid-producing bacteria in the Red Sea include the major marine nitrite oxidizers. FEMS Microbiol. Ecol. 94, 10.1093/femsec/fiy063 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Seemann M., Bisseret P., Tritz J.-P., Hooper A. B., Rohmer M., Novel bacterial triterpenoids of the hopane series from Nitrosomonas europaea and their significance for the formation of the C35 bacteriohopane skeleton. Tetrahedron Lett. 40, 1681–1684 (1999). [Google Scholar]
- 103.Bock E., Sundermeyer-Klinger H., Stackebrandt E., New facultative lithoautotrophic nitrite-oxidizing bacteria. Arch. Microbiol. 136, 281–284 (1983). [Google Scholar]
- 104.Bligh E. G., Dyer W. J., A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 105.Sturt H. F., Summons R. E., Smith K., Elvert M., Hinrichs K.-U., Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry'–new biomarkers for biogeochemistry and microbial ecology. Rapid Commun. Mass Spectrom. 18, 617–628 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Sáenz J. P., “Exploring the distribution and physiological roles of bacterial membrane lipids in the marine environment,” PhD thesis, Massachusetts Institute of Technology and Woods Hole Oceanographic Institution, Cambridge, MA (2010).
- 107.Matys E. D., et al. , Bacteriohopanepolyols along redox gradients in the Humboldt current system off northern Chile. Geobiology 15, 844–857 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All lipid data, growth parameters, and results of genomic analyses are included in the article, the SI Appendix, and in the Harvard Dataverse (DOI: 10.7910/DVN/IZY3JA).