Highlight
-
•
Apatinib showed promising efficacy and an acceptable safety profile in patients with advanced AFPGC.
-
•
Based on the higher expression of VEGF-C of AFPGC compared with common GC, application of antiangiogenic therapy may be a profitable strategy.
-
•
The results of this prospective real-world study provides first-hand data of apatinib for AFPGC as a rare disease with poorer prognosis.
Keywords: Alpha-fetoprotein, AFP-producing gastric cancer, Apatinib, Target therapy
Abbreviations: AFPGC, alpha-fetoprotein-producing gastric cancer; PFS, progression-free survival; OS, median overall survival; AEs, adverse events; CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; GC, gastric cancer; HACs, hepatoid adenocarcinoma of the stomach; VEGFR, vascular endothelial growth factor; cFDA, China Food and Drug Administration; ELISA, enzyme-linked immunosorbent assay; ECOG, Eastern Cooperative Oncology Group; CT, computed tomography; ORR, objective response rate; DCR, disease control rate; RECIST, Response Evaluation Criteria in Solid Tumors; CTCAE, Common Terminology Criteria Adverse Events; CI, confidence interval; PR, partial response; SD, stable disease; PD, progression disease
Abstract
Background
Alpha-fetoprotein-producing gastric cancer (AFPGC) poses a therapeutic challenge worldwide because of its poor prognosis. This study aimed to evaluate the efficacy and safety of antiangiogenic drug apatinib in advanced AFPGC in a real-world setting.
Methods
From September 2015 to December 2017, twenty-one patients identified with AFPGC from the clinical trial AHEAD-G202, an open-label, prospective, multicenter, non-interventional study of apatinib for advanced metastatic gastric cancer, were enrolled to perform this analysis. Patients received oral apatinib as monotherapy or combination therapy. A treatment cycle was defined as 28 days. The primary outcome was progression-free survival (PFS) and overall survival (OS), and the secondary outcomes included safety, objective response rate (ORR), and disease control rate (DCR).
Results
Twenty patients were evaluated for the apatinib efficacy analysis. The ORR of apatinib was 10%, whereas the DCR was 70%. The median PFS was 3.5 months [95%confidence interval (CI): 2.34–4.66]. The median OS was 4.5 months (95%CI: 3.49–5.51). Median OS of AFPGC patients without carcinoembryonic antigen (CEA) elevation achieved 30.8 months. CEA elevation was considered to be a potential independent predictive factor for OS (P = 0.030) and PFS (P = 0.047) by the analysis of multivariate analysis. The most common grade 3 to 4 adverse events (AEs) were hypertension (4.8%), hand-foot syndrome (4.8%), anorexia (4.8%), and vomiting and nausea (4.8%).
Conclusion
Apatinib showed promising efficacy and an acceptable safety profile in patients with advanced AFPGC. Antiangiogenic therapy may be a good strategy for the treatment of AFPGC as a rare sub-type of gastric cancer.
Trial registration
AHEAD-G202 (NCT02668380).
Introduction
Alpha-fetoprotein-producing gastric cancer (AFPGC) is a special type of gastric cancer. Αlpha-fetoprotein (AFP) is a plasma glycoprotein that is mainly synthesized from the yolk sac and the fetal liver during fetal development [1]. Elevated serum AFP levels may be detected in solid tumors of other organs, including stomach, lung, pancreas, gallbladder, and colon [2], [3], [4], [5], [6], [7]. AFP elevation in gastric cancer (GC) is the most common situation in the extrahepatic tumors [2]. In 1970, Bourreille et al. [8] first reported a case of AFP-elevated gastric cancer with liver metastasis; at that point, AFPGC was named. AFPGC exhibits more aggressive biological behavior than common GC, which may result from a high rate of liver metastasis, vascular invasion, and lymph node metastasis [9,10].
Currently, there are some studies of AFPGC, most of which are cases or reviews concerning the clinicopathologic features. There are few data in the literature about the treatment. Common chemotherapeutic regimens, such as platinum, taxane, and fluropyrimidines for GC currently have had poor efficacy in AFPGC. Apatinib is an oral tyrosine kinase inhibitor that selectively targets vascular endothelial growth factor-2 (VEGFR-2). The agent was approved by China Food and Drug Administration (cFDA) in 2014 for the treatment of advanced GC as third line therapy in China [11]. AHEAD-G202 is a noninterventional study to investigated the safety and effectiveness of apatinib in advanced gastric cancer patients in a real-world setting. We analyzed 21 patients with AFPGC as a subgroup of the study AHEAD-G202 (NCT02668380).
Materials and methods
Ethics statement
This study (AHEAD-G202) was reviewed and approved by the ethic committees or institutions review boards of the institutions below (Approval code:HS-806) :
Peking Union Medical College Hospital
The Fourth Hospital of Hebei Medical University
Peking University Third Hospital
Tianjin Medical University Cancer Institute and Hospital
Shanxi Academy of Medical Sciences, Shanxi Dayi Hospital
First Hospital of Qinhuangdao
Written informed consent to participation in the study was obtained from each patient enrolled in this study.
Patients and treatment
From September 2015 to December 2017, a total of 337 patients with advanced metastatic GC were enrolled in the clinical trial AHEAD-G202. Serum AFP level was detected by enzyme-linked immunosorbent assay (ELISA). AFP cut-off value was defined as 20 ng/mL (normal range, 0–20 ng/ml), as determined from Roche Cobas 6000 Automatic Electrochemiluminescence Immunoassay Analyzer in the laboratory of our hospital. All patients with elevated serum AFP levels (>20 ng/ml) were enrolled.
Patients received apatinib orally at 250–850 mg once daily for 4 weeks per cycle initially. The dose was modified by the oncologists according to the patients’ physical status. The dosage adjustment was based on the AEs. The chemotherapy or other targeted therapy combined with apatinib was hinged on physicians’ determination. Continued treatment until disease progression or intolerable toxicity or withdrew consent from the study.
Data collection and patient evaluation
All the patients were regularly followed up from the date of enrollment in the AHEAD-G202 trial. We collected the data from the patients’ medical records. All the clinicopathological variables and AEs were evaluated in all the patients. We used the Eastern Cooperative Oncology Group (ECOG) performance scale to evaluate the performance status. Enhanced computed tomography (CT) and laboratory tests were performed every 8 weeks during apatinib treatment according to routine clinical practice. Evaluation of treatment response was based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Treatment-related toxicities were graded according to Common Terminology Criteria Adverse Events (CTCAE) version 4.0. Clinical assessment and follow up were undertaken during regular visits at 8–12 weeks according to routine clinical practice.
Statistical analysis
All the data were analyzed via SPSS 22.0 software (SPSS Inc., IL, US) . The primary outcome of the study were PFS and OS.The secondary outcomes included ORR, DCR and safety. Treatment responses and AEs were both aggregated in the form of frequency counts and percentages. The OS was measured from the date of initial administration with apatinib to the date of death due to any cause. The PFS was defined as a duration from the date of initiation of apatinib to disease progression. The ORR and DCR were analyzed on the basis of frequency counts. The ORR included complete response (CR) and partial response (PR), which were assessed using the RECIST v 1.1. DCR was defined as the percentage of patients with stable disease (SD), CR, or PR.
All demographic information, clinicopathological data, and treatment-related data were coded and resembles as a database. Survival analysis was performed using the Kaplan-Meier method, and the subgroup analysis was performed according to log-rank statistics. Univariate analysis and multivariate analysis were performed to evaluate the predictive factors. Multivariate analyses were carried out using the Cox proportional hazards model to analyze. All statistical analyses were two sided. P<0.05 was considered significant.
Results
Clinicopathologic characteristics
Three hundred thirty-seven patients were enrolled in the AHEAD-G202 study. A total of 21 patients (6.2%) confirmed as AFPGC were included in the present study. The median age of all patients with AFPGC was 64 years (range, 37–84 y), and 76.2% of the patients were men. Eleven patients were initially diagnosed with metastatic GC, whereas 10 patients had recurrent disease after radical gastrectomy. Sixteen patients (76.2%) had hepatic metastasis, and five patients (23.8%) were diagnosed with peritoneal metastasis. Two patients had both hepatic metastasis and peritoneal metastasis. Some patients also had lymph nodes (61.9%), lung (19.0%), leptomeningeal (4.8%), brain (4.8%) or adrenal (4.8%) metastasis. Compared with the non-AFPGC patients in the AHEAD-G202 study, 33.2% of the non-AFPGC patients had hepatic metastasis and 9.2% had peritoneal metastasis. Of the cases, 57.1% were identified as poorly differentiated adenocarcinoma, which was consistent with the non-AFPGC patients in the AHEAD-G202 study (57.6%). Two patients were diagnosed with immunohistochemical AFP-positive HAC. The AFP level of the patients ranged from 26.4 to 56,317 ng/mL, with a median value of 167.8 ng/mL. Fifty percent of cases in the AFP greater than 10,000 ng/mL group were diagnosed with early-occurrence of liver metastasis.
Treatment with apatinib
The apatinib treatment regimen was according to the individual physician's choice. Apatinib was used as monotherapy in 11 patients (52.4%). Eleven patients received apatinib combined with other drugs including S-1 (3 patients), capecitabine (3 patients), irinotecan (2 patients), paclitaxel-albumin (1 patient), trastuzumab (1 patient), and oxaliplatin (1 patient). One patient received apatinib as first-line therapy; seven patients received apatinib as second-line therapy; four received apatinib as third-line therapy; and eight received apatinib as ≥ fourth-line therapy. The initial dose of apatinib was 500 to 850 mg in 57.2% of the patients, and 250 mg in 42.8% of the patients.
Efficacy
Twenty patients were evaluated for the apatinib efficacy analysis (Table 1). Two patients achieved PR, 12 patients had SD, and 6 patients had progressive disease (PD). The overall ORR was 10%, and the DCR was 70%.
Table 1.
The ORR and DCR of apatinib treatment.
| Factors | No. Of patients(%) | Response (n) | Efficacy | |||
|---|---|---|---|---|---|---|
| PR | SD | PD | ORR | DCR | ||
| Total | 20 (100) | 2 | 12 | 6 | 10% | 70% |
All the patients were followed-up until December 19, 2018. The median follow-up duration was 27 months (range, 13–30 months). The Kaplan-Meier curves of PFS and OS are displayed in Figs 1A and 1B. The median PFS of all the patients given apatinib was 3.5 months (95% CI: 2.34–4.66). The median OS in the whole group was 4.5 months (95%CI: 3.49–5.51). The 1-year survival rate was 20%. Four patients (20%) survived for less than 3 months, nine (45%) for 3 to 6 months, and eight (40%) patients survived for more than 6 months. AFP decreases greater than 50% were more common in the OS of greater than 6 months group compared with the OS of less than 6 months group (62.5% vs. 33%); however, the difference was not significant (P = 0.081). CEA elevation was more common in OS less than 6 months group compared with the OS of greater than 6 months group (100% vs. 50%, P = 0.029).
Fig. 1.
Kaplan-Meier estimates of progression-free survival (PFS) (A) and overall survival (OS) (B) of 21 patients with AFPGC. (A) Median PFS was 3.5 months with apatinib. (B) Median OS was 4.5 months with apatinib.
Safety
By last follow-up, all the 21 patients had discontinued apatinib therapy due to disease progression or intolerable adverse effects. The median duration of apatinib treatment was 91 days (range, 5–755 d). Twenty-one patients were evaluated for the safety analysis. The most common AEs included hypertension (33.3%), fatigue (23.8%), and myelosuppression (19.0%). In total, the incidence of grades 3 to 4 AEs was 19.0% including hypertension (4.8%), hand-foot syndrome (4.8%), anorexia (4.8%), and vomiting and nausea (4.8%). No treatment-related death occurred during apatinib administration. Seven patients had reduced dosage because of toxicity. The main reasons for dose reduction were hypertension, oral ulcer, and diarrhea. One patient withdrew from the study because of hypertension. Hoarseness and bradycardia were observed respectively in two patients. The details of all AEs are summarized in Table 2.
Table 2.
Adverse events of 21 patients.
| Adverse event | No. Of patients(%) | ||
|---|---|---|---|
| Total | Grades 1–2 | Grades 3–4 | |
| Hypertension | 7 (33.3) | 6 (28.6) | 1 (4.8) |
| Fatigue | 5 (23.8) | 5 (23.8) | 0 |
| Myelosuppression | 4 (19.0) | 4 (19.0) | 0 |
| Hand-foot syndrome | 3 (14.3) | 2 (9.5) | 1 (4.8) |
| Anorexia | 3 (14.3) | 2 (9.5) | 1 (4.8) |
| Oral ulcer | 3 (14.3) | 3 (14.3) | 0 |
| Hoarseness | 2 (9.5) | 2 (9.5) | 0 |
| Nausea or Vomiting | 2 (9.5) | 1 (4.8) | 1 (4.8) |
| Bradycardia | 2 (9.5) | 2 (9.5) | 0 |
| Diarrhea | 1 (4.8) | 1 (4.8) | 0 |
| Proteinuria | 1 (4.8) | 1 (4.8) | 0 |
| Hematochezia | 1 (4.8) | 1 (4.8) | 0 |
Predictive factors
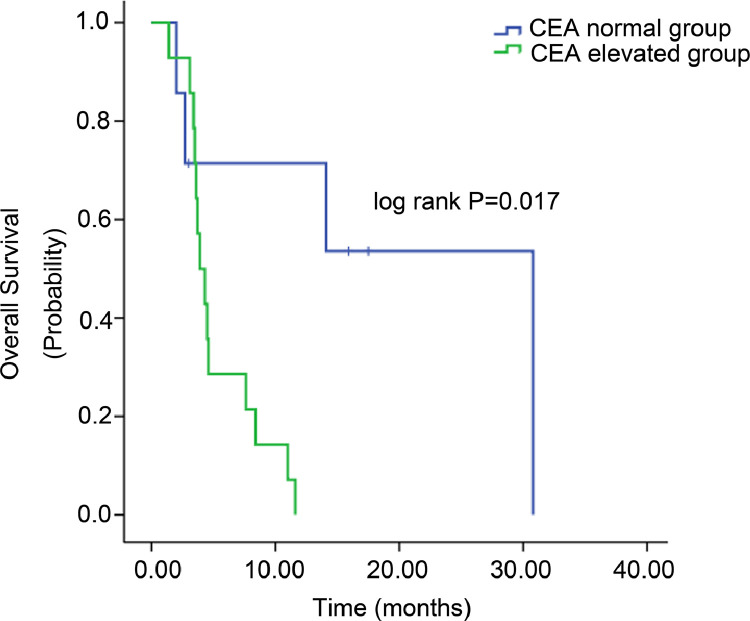
As shown in Supplemental Table 1, we brought other clinicopathologic factors into our analysis to investigate the predictive factors. Univariate analysis showed that CEA level (P = 0.029, Fig 2A) and grade 3 or 4 AEs (P = 0.013, Fig 2B) were associated with PFS, whereas an association with OS was found in CEA elevation (P = 0.017, Fig 3), number of metastatic organs (P = 0.02), and grade 3 or 4 AEs (P = 0.022). We performed multivariate analysis using gender, CEA elevation, number of metastatic organs, liver metastasis, lines of apatinib, and grade 3 or 4 AEs(Tables 3 and 4). CEA elevation (P = 0.047) and grade 3 or 4 AEs (P = 0.029) were considered to be independent predictive factors for PFS in multivariate analysis. Multivariate analysis for OS demonstrated that only CEA elevation was considered to be a potential independent predictive factor (P = 0.030). Further, median OS of AFPGC patients without carcinoembryonic antigen (CEA) elevation achieved 30.8 months(Fig 3).
Fig. 2.
Kaplan-Meier estimates of progression-free survival (PFS) for different factors. (A) PFS of patients with normal CEA level and those with elevated CEA level (P = 0.029). (B) PFS of patients with grades 3 or 4 AEs and those without grades 3 or 4 AEs (P = 0.013).
Fig. 3.
Overall survival (OS) of patients with normal CEA level and those with elevated CEA level (P = 0.017).
Table 3.
Multivariate analysis for PFS.
| Factors | HR | P |
|---|---|---|
| CEA level | 3.654 (1.016–13.136) | 0.047 |
| Grade 3/4 AE | 0.225 (0.059–0.855) | 0.029 |
Table 4.
Multivariate analysis for OS.
| Factors | HR | P |
|---|---|---|
| CEA level | 5.547 (1.184–25.997) | 0.030 |
Discussion
AFPGC is a rare tumor that has been reported in 15% of GC in western countries [12] and in 1.3% to 6.6% of GC in Asian countries including China [13,14]. Most AFPGC cases are reported by Japan. The incidence in the United States is much higher than that in Japan; however, the reason is unknown. In the current study, 6.2% of the patients from AHEAD-G202 were diagnosed with AFPGC. All the patients received an AFP test; thus, the incidence is reliable and is also consistent with the morbidity reported in China. Liver metastasis was reported to occur in 33% to 72% of AFPGC cases [15,16], which was similar to that in our study (76.2%). AFPGC was reported to have higher rates of peritoneal metastasis and lymph node metastasis [10,17]. Similar data were demonstrated in our study in lymph node metastasis (61.9%), and 23.8% of the patients had peritoneal implantation, which was lower than previously reported (71.2%) [10]. Peritoneal invasion was difficult to discover, which could be one explanation. The incidence of liver metastasis and peritoneal metastasis in our study were higher than the non-AFPGC cases in the AHEAD-G202 study, which showed that it was a characteristic of AFPGC. In our study, cases with higher AFP level (>10,000 ng/mL) seemed more likely to be associated with early occurrence of liver metastases during disease progression. AFP level was reported to be related to ulcerative type of AFPGC [18], but there are limited data about the association of AFP level and other characteristics.
In recent years, although several biological or targeted therapies are available for GC, the prognosis of advanced disease is still poor, with a 5-year survival rate that is less than 20%. The median OS of advanced AFPGC was reported to be 9.3 months [19], which was poorer. The 5-year survival rate was 8.3% to 11.9% for all stages of AFPGC [20,21]. There is currently no standard regimen. Some regimens have been reported including S-1, cisplatin, taxane, and fluorouracil [22], [23], [24]. The combination of platinum and fluropyrimidines are frequently used with an ORR of 25% to 43.9%, lower than that for triplet regimens (66.7%) in AFPGC [25]. In a report of 10 cases of metastatic AFPGC, the regimen of fluoropyrimidine and platinum, a combination of paclitaxel and cisplatin, or TS-1 single had an ORR as 8.3% and a DCR of 50% [26]. The combination of irinotecan and mitomycin C showed promising efficacy in an AFPGC case with a DFS of 36 months [27]. The general ORR of chemotherapy in AFPGC was reported to be 8.3% to 66.7% as a first-line therapy, which is quite diverse among different reports because of the small sample sizes. A report of 7 advanced AFPGC patients treated with immunotherapy plus chemotherapy compared with 14 patients as control treated with chemotherapy with or without herceptin/apatinib as first-line treatment was published [28]. The median PFS was 22.0 months in immunotherapy group (4.3 months in control group). The median OS of the control group was 16.0 months (14.0 months in chemotherapy alone subgroup, 20.0 months in chemotherapy plus herceptin/apatinib subgroup), while the mOS of patients receiving immunotherapy was not reached. This study suggests immunotherapy plus chemotherapy may be a treatment option for AFPGC patients. There were no adequate data on efficacy of therapy as multi-line therapy.
Kanei et al. [29] reported that vascular endothelial growth factor type C (VEGF-C) expressed higher in AFPGC cases compared with common GC. AFP was considered to have the ability of up-regulating the VEGF-C expression, which may lead to unfavorable prognosis [30]. Therefore, the approaches targeting the VEGF-C-VEGFR2 are promising for AFPGC. Ramucirumab targeting VEGFR2 was reported to be effective in an AFPGC patient, with an OS of 16 months [31]. Sorafenib is one of the anti-VEGF drugs that has been reported to be effective, with a PFS of 2 and 4 months, respectively, in two AFPGC cases [32,33].
Apatinib targeting VEGFR2 has been proven effective in the treatment of advanced GC. Compared with the data in several trials of apatinib in common GC (ORR 2.84%−13.04%, DCR 34.78–58.33%) [34], [35], [36], more patients reached disease control (70%), and the ORR (10%) seemed to be nearly efficient. In preceding studies of apatinib as multiline therapy in common GC, the PFS ranged from 2.6 to 3.7 months, which is similar to our results, whereas OS (6.5 mo) seemed better [34,35]. The front-line application of apatinib is not common in general GC. Considering the importance of VEGF expression in AFPGC, early medication of apatinib may be a good strategy. In our study, as a first- or second-line therapy, apatinib got a PFS of 10 months and an OS of 14 months, which were much better than that in the third or more line therapy (3.2, 6.4 months) in the current study (Supplemental Table). The efficacy of triplet-regimen chemotherapy (Docetaxel, cisplatin, 5-fluorouracil; DCF) showed an OS of 9.3 months as the first line in AFPGC [19]; however, the AEs of DCF were prominent. Compared with a DCF regimen as first line, the efficacy of apatinib is worth exploring. This front-line benefit provided us a promising idea for the strategy of apatinib in AFPGC. Early treatment with anti-VEGF therapy in AFPGC may be beneficial. As multiline therapy, the efficacy of apatinib is also acceptable compared with other regimens in AFPGC. The ORR of double-drug chemotherapy was 8.3% to 26.3% as a multiline therapy, which is comparable to our results [25,26]. The PFS of the double-drug regimen was 3.47 months, which is similar to our overall PFS of 3.5 months [26]. The efficacy of apatinib as fourth line showed a PFS of 5 months in an AFPGC case report, which is promising [37]. We got an OS of 14 months in first- or second-line therapy, which was even better than the high-intensity DCF regimen. The toxicity of apatinib was more tolerable than that of DCF in real-world application. Our results demonstrated the preliminary acceptable efficacy of apatinib in multiline and frontline treatment of AFPGC with low toxicity. The results suggested that the antiangiogenic drug apatinib may improve the prognosis of patients with AFPGC.
The incidence of grades 3 or 4 AEs in the current study was almost consistent with trials in common GC, except that the occurrence of anorexia (4.8% vs. 0%) and nausea/vomiting (4.8% vs. 0%) was notable [34]. The AEs were considered acceptable, which made apatinib a more tolerable option.
Some factors were reported to be associated with poor prognosis of AFPGC, including TNM stage [38], AFP level [39], and AFP decline [40]. The CEA elevation was an independent risk factor for poor prognosis in our study. The prognostic role of CEA in common GC has been reported [41]. Most articles found that AFP level was a risk factor [39]. There was no significant difference due to the small sample size in our study. With respect to the patients with an AFP decline after the treatment, we found a difference between subgroups both in PFS and OS analysis, whereas the p value was not significant. An decline in AFP usually occurs in the duration of effective treatment. Some reports considered AFP decline (≧50%) after chemotherapy as a good predictive factor of AFPGC [25]. In our study, 45% of the patients demonstrated a decrease in AFP level after apatinib treatment, and most of these cases had a better therapeutic effect. We deduced that the non-statistical result was limited by small sample sizes. Focusing on AFP fluctuation may still be helpful in predicting apatinib efficacy and monitoring recurrence.
Limited successful options in the treatment of AFPGC have existed in recent years. Our study provides first-hand efficacy and safety data of apatinib for AFPGC in the real world. The survival benefit was promising and the toxicity was consistent with the data in common GC.
We have limitations of our present study. First, the sample size is not sufficiently large because of the rare morbidity. Second, the data were evaluated from trials limited to the Asian population. Third, this study involved some heavily pre-treated patients, which may have lead to the poor tolerance and lower dosage of apatinib.
In conclusion, AFPGC is a rare type of gastric cancer with a worse prognosis. The optimal treatment remains a challenge. The current study provides more information about the treatment of apatinib in AFPGC in real-world setting. The heavily pretreated advanced gastric cancer patients may benefit from apatinib with acceptable toxicity after dosage adjustment. Anti-VEGFR therapy with apatinib for AFPGC may be a alternative approach. Further investigations with larger sample sizes should be undertaken.
Disclosure statement
An Open Label, Prospective, Multicenter, Non-interventional Study of Apatinib for Chemotherapy-Refractory Advanced Metastatic Gastric Cancer (AHEAD-G202). ClinicalTrials.gov Identifier: NCT02668380. The following represents disclosure information provided by authors of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Jiangsu HengRui Medcine Co., Ltd. This work was supported by grants from 2016 PUMCH Science Fund for Junior Faculty (Pumch-2016–1.13) and grants from Chinese Anti-cancer Association (CORP-143–09).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.101004.
Appendix. Supplementary materials
References
- 1.Bergstrand C.G., Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand. J. Clin. Lab. Invest. 1956;8:174–179. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- 2.Metzgeroth G., Strobel P., Baumbusch T., Reiter A., Hastka J. Hepatoid adenocarcinoma -review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33:263–269. doi: 10.1159/000305717. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata T., Yamagata Y., Nakanishi M., Matsunaga K., Minakata Y., Ichinose M. A case of primary lung cancer producing alpha-fetoprotein. Can. Respir. J. 2004;11:504–506. doi: 10.1155/2004/510350. [DOI] [PubMed] [Google Scholar]
- 4.Matsueda K., Yamamoto H., Yoshida Y., Notohara K. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP) J. Gastroenterol. 2006;41:1011–1019. doi: 10.1007/s00535-006-1889-8. [DOI] [PubMed] [Google Scholar]
- 5.Kinjo T., Taniguchi H., Kushima R., Sekine S., Oda I., Saka M., Gotoda T., Kinjo F., Fujita J., Shimoda T. Histologic and immunohistochemical analyses of α -fetoprotein-producing cancer of the stomach. Am. J. Surg. Pathol. 2012;36:56–65. doi: 10.1097/PAS.0b013e31823aafec. [DOI] [PubMed] [Google Scholar]
- 6.Cappetta A., Bergamo F., Mescoli C., Lonardi S., Rugge M., Zagonel V. Hepatoid adenocarcinoma of the colon: what should we target? Pathol. Oncol. Res. 2012;18:93–96. doi: 10.1007/s12253-011-9424-5. [DOI] [PubMed] [Google Scholar]
- 7.Isonishi S., Ogura A., Kiyokawa T., Suzuki M., Kunito S., Hirama M., Tachibana T., Ochiai K., Tanaka T. Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly woman. Int. J. Clin. Oncol. 2009;14:70–73. doi: 10.1007/s10147-008-0800-4. [DOI] [PubMed] [Google Scholar]
- 8.Bourreille J., Metayer P., Sauger F., Matray F., Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277–1278. (in French) [PubMed] [Google Scholar]
- 9.Ishikura H., Fukasawa Y., Ogasawara K., Natori T., Tsukada Y., Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840–848. doi: 10.1002/1097-0142(19850815)56:4<840::aid-cncr2820560423>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Cheng Y., Sheng W., Lu H., Xu Y., Long Z., Zhu H., Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J. Surg. Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 11.Roviello G., Ravelli A., Polom K. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016;372(2):187–191. doi: 10.1016/j.canlet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 12.McIntire K.R., Waldmann T.A., Moertel C.G., Go V.L. Serum alpha-fetoprotein in patients with neoplasms of the gastroin- testinal tract. Cancer Res. 1975;35:991–996. [PubMed] [Google Scholar]
- 13.Chang Y.C., Nagasue N., Abe S., Taniura H., Kumar D.D., Nakamura T. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am. J. Gastroenterol. 1992;87:321–325. [PubMed] [Google Scholar]
- 14.Li Xiao-Dong, Wu Chang-Ping, Ji Mei. Characteristic analysis of α-fetoprotein-producing gastric carcinoma in China. World J. Surg. Oncol. 2013;11:246. doi: 10.1186/1477-7819-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata Y., Sato K., Kodama M., Nanjyo H. Alpha-fetoprotein-producing early gastric cancer of the remnant stomach: report of a case. Surg. Today. 2007;37:995–999. doi: 10.1007/s00595-007-3501-0. [DOI] [PubMed] [Google Scholar]
- 16.Vivekanandarajah A., Atallah J.P., Gupta S. Alpha-fetoproteinproducing nonmetastatic gastric adenocarcinoma: a rare entity. J. Gastrointest. Cancer. 2014;45:225–227. doi: 10.1007/s12029-013-9498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talamonti M.S., Kim S.P., Yao K.A., Wayne J.D., Feinglass J., Bennett C.L., Rao S. Surgical outcomes of patients with gastric carcinoma: the importance of primary tumor location and microvessel invasion. Surgery. 2003;134:720–727. doi: 10.1016/s0039-6060(03)00337-4. discussion 727-729. [DOI] [PubMed] [Google Scholar]
- 18.Qu B.G., Bi W.M., Qu B.T., Qu T., Han X.H., Wang H., Liu Y.X., Jia Y.G. PRISMA-compliant article: clinical characteristics and factors influencing prognosis of patients with hepatoid adenocarcinoma of the stomach in China. Medicine (Baltimore). 2016;95:e3399. doi: 10.1097/MD.0000000000003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkaya Y., Demirci N.S., Kurtipek A. Clinicopathological and prognostic characteristics in patients with AFP-secreting gastric carcinoma. Mol. Clin. Oncol. 2017 Aug;7(2):267–274. doi: 10.3892/mco.2017.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata Y., Sato K., Kodama M., Nanjyo H. Alphafetoprotein-producing early gastric cancer of the remnant stomach: report of a case. Surg. Today. 2007;37:995–999. doi: 10.1007/s00595-007-3501-0. [DOI] [PubMed] [Google Scholar]
- 21.Nagai E., Ueyama T., Yao T., Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. CancerCancer. 1993;72:1827–1835. doi: 10.1002/1097-0142(19930915)72:6<1827::aid-cncr2820720606>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka M., Inoue N., Someda H., Noguchi M., Sawada M., Azuma K. [A case of AFP-producing gastric cancer resected after efficient S-1/CDDP combination chemotherapy] Gan To Kagaku Ryoho. 2011 Jan;38(1) 105e8. [PubMed] [Google Scholar]
- 23.Fukuda K., Ito S., Shimizu K., Mikami E., Sakuraba S., Shiroki T. [Retrospective analysis concerning AFP-producing gastric cancer] Gan To Kagaku Ryoho. 2013 Feb;40(2):191e5. [PubMed] [Google Scholar]
- 24.Kunoki N., Nishiyama R., Ryuzaki H., Oonishi M., Yamamoto T., Uno A. A case of alpha-fetoprotein-producing gastric cancer with hepatic metastasis successfully treated with combination chemotherapy. Nihon Shokakibyo Gakkai Zasshi. 2008 Oct;105(10):1489e95. [PubMed] [Google Scholar]
- 25.Wang Ya-Kun, Shen Lin, Jiao Xi. Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J. Gastroenterol. 2018;24(2):266–273. doi: 10.3748/wjg.v24.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek S.K., Han S.W., DY O., Im S.A., Kim T.Y., Bang Y.J. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11(1):56. doi: 10.1186/1471-230X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirao K., Hirasaki S., Tsuzuki T., Kajiwara T., Hyodo I. Unresectable alpha fetoprotein-producing gastric cancer successfully treated with irinotecan and mitomycin C after S-1 failure. Intern. Med. 2004;43(2):106e10. doi: 10.2169/internalmedicine.43.106. [DOI] [PubMed] [Google Scholar]
- 28.Li Wei, Li Qian, Yu Yiyi. Effect of immune checkpoint inhibitors plus chemotherapy on advanced gastric cancer patients with elevated serum AFP or hepatoid adenocarcinoma. Cancer Manag. Res. 2020;12:11113–11119. doi: 10.2147/CMAR.S276969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamei S., Kono K., Amemiya H. Evaluation of VEGF and VEGF-C expression in gastric cancer cells producing alpha-fetoprotein. J. Gastroenterol. 2003;38:540–547. doi: 10.1007/s00535-002-1099-y. [DOI] [PubMed] [Google Scholar]
- 30.Yonemura Y., Endo Y., Fujita H. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 31.Yasuhiro A., Miho T., keisuke A.I.B.A., al et. Significant response to ramucirumab monotherapy in chemotherapy-resistant recurrent alpha‑fetoprotein-producing gastric cancer: a case report. Oncol. Lett. 2017;14:3039–3042. doi: 10.3892/ol.2017.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koneri K., Hirono Y., Fujimoto D., Sawai K., Morikawa M., Murakami M., Goi T., Iida A., Katayama K., Yamaguchi A. Five-year survival of alpha-fetoprotein-producing gastric cancer with synchronous liver metastasis: a case report. J. Gastric Cancer. 2013;13:58–64. doi: 10.5230/jgc.2013.13.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Y.U., Wang L., Yang N., Gong X., Zhang Y.U., Qin S. Successful multimodal therapy for an α-fetoprotein-producing gastric cancer patient with simultaneous liver metastases. Oncol. Lett. 2015;10:3021–3025. doi: 10.3892/ol.2015.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Qin S., Xu J. Randomized, double-blind, placebo-controlled phase III trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 2016;34(13):1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Qin S., Xu J. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 2013;31(26):3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Yong, Han Chun, Li Juan. Efficacy and safety for Apatinib treatment in advanced gastric cancer: a real world study. Sci Rep. 2017;7(1):13208. doi: 10.1038/s41598-017-13192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X.R., Zhu M.L., Wang Q. A case report of targeted therapy with apatinib in a patient with advanced gastric cancer and high serum level of alpha-fetoprotein. Medicine (Baltimore) 2016;95(37) doi: 10.1097/MD.0000000000004610. article e4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Ruji, Yang Qinyi, Dong Xuqiang. Clinicopathologic and prognostic characteristics of alpha-fetoprotein–producing gastric cancer. Oncotarget. 2017;8(14):23817–23830. doi: 10.18632/oncotarget.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun W., Liu Y., Shou D., Sun Q., Shi J., Chen L., Liang T., Gong W. AFP (alpha fetoprotein): who are you in gastrology? Cancer Lett. 2015;357:43–46. doi: 10.1016/j.canlet.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Yakun Wang, Lin Shen, Xiaotian Zhang. The prognosis and clinicopathological characteristics of 70 gastric cancer patients with elevated serum AFP. Chin. J. Oncol. 2017;39(7) doi: 10.3760/cma.j.issn.0253-3766.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.H., Jun K.H., Jung H., Park I.S., Chin H.M. Prognostic value of preoperative serum levels of five tumor markers (carcinoembryonic antigen, CA19-9, alpha-fetoprotein, CA72-4, and CA125) in gastric cancer. Hepatogastroenterology. 2014;61:863–869. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.