Significance
The host environment in which infection occurs plays a crucial role in the interaction between Pseudomonas aeruginosa and antibiotic treatments. To make up for the lack of new antibiotics, alternative approaches, such as combination therapy and discovery of novel activity of known antibiotics in the host environment, are vital for treatment of pathogens such as P. aeruginosa. This research demonstrates how in vitro conditions that closely mimic human infection more accurately reflect the antimicrobial activity of antibiotics. We have shown that azithromycin (AZM) has increased activity and synergy with synthetic peptides in physiologically relevant in vitro conditions and in vivo. Furthermore, this research also provides potential alternative mechanisms for the activity of AZM against P. aeruginosa.
Keywords: azithromycin, host-mimicking media, RNA-Seq, Pseudomonas aeruginosa, antibiotic susceptibility
Abstract
Pseudomonas aeruginosa causes severe multidrug-resistant infections that often lead to bacteremia and sepsis. Physiologically relevant conditions can increase the susceptibility of pathogens to antibiotics, such as azithromycin (AZM). When compared to minimal-inhibitory concentrations (MICs) in laboratory media, AZM had a 16-fold lower MIC in tissue culture medium with 5% Mueller Hinton broth (MHB) and a 64-fold lower MIC in this tissue culture medium with 20% human serum. AZM also demonstrated increased synergy in combination with synthetic host-defense peptides DJK-5 and IDR-1018 under host-like conditions and in a murine abscess model. To mechanistically study the altered effects of AZM under physiologically relevant conditions, global transcriptional analysis was performed on P. aeruginosa with and without effective concentrations of AZM. This revealed that the arn operon, mediating arabinosaminylation of lipopolysaccharides and related regulatory systems, was down-regulated in host-like media when compared to MHB. Inactivation of genes within the arn operon led to increased susceptibility of P. aeruginosa to AZM and great increases in synergy between AZM and other antimicrobial agents, indicating that dysregulation of the arn operon might explain increased AZM uptake and synergy in host-like media. Furthermore, genes involved in central and energy metabolism and ribosome biogenesis were dysregulated more in physiologically relevant conditions treated with AZM, likely due to general changes in cell physiology as a result of the increased effectiveness of AZM in these conditions. These data suggest that, in addition to the arn operon, there are multiple factors in host-like environments that are responsible for observed changes in susceptibility.
The opportunistic pathogen Pseudomonas aeruginosa is found ubiquitously in the environment but is of interest due to its high intrinsic resistance and ability to rapidly develop acquired antibiotic resistance (1). P. aeruginosa is one of the major causes of infection in the lungs of individuals with cystic fibrosis (CF). CF is a genetic disease characterized by defective anion channels in specialized epithelial cells, leading to ineffective mucociliary clearance and excessive inflammation, enabling chronic bacterial colonization (2). P. aeruginosa is also a major cause of nosocomial bacteremia and sepsis, often transmitted from local infections such as pneumonia or burn wounds into systemic bloodstream infections for which the mortality rate is as high as 48% (3). Antibiotics used to treat P. aeruginosa infections include aminoglycosides, such as gentamicin and tobramycin; carbapenems, such as imipenem; cephalosporins, such as ceftazidime; fluoroquinolones, such as ciprofloxacin; penicillins in combination with β-lactamase inhibitors; monobactams, such as aztreonam; and fosfomycin; with polymyxins, such as colistin, as a last resort (4). However, increasing antibiotic resistance in pathogens such as P. aeruginosa greatly impacts clinical and surgical procedures, where resistant infections can seriously complicate even minor injuries (1). For this reason, the World Health Organization has declared P. aeruginosa as one of the most critical pathogens to target for drug research and development (5). Unfortunately, the rate of discovery of new antibiotics and new chemical classes for treatment is currently extremely low (6).
To address these issues, researchers are exploring alternatives or adjuvants to traditional antibiotic treatments, including host defense (antimicrobial) peptides (HDPs) and novel or overlooked activities of existing antimicrobial agents (7, 8). Synthetic derivatives of HDPs have shown some success as antiinfective, antibiofilm, and antiabscess treatments and can be used synergistically with conventional antibiotics (9–11). Additionally, although traditional antibiotic assays use nutrient-rich media such as Mueller Hinton broth (MHB), and therefore do not reflect the in vivo environment (carbon sources, iron availability, etc.), recent research has started to explore antimicrobial activity in assays that are more biologically relevant (12–15). These studies are finding unconventional activities of antibiotics and HDPs in conditions that mimic human blood, lung sputum, or bowel fluid that are not observed in nutrient-rich media used for conventional microbial growth and antimicrobial testing. Of particular interest are studies done with the macrolide antibiotic azithromycin (AZM). This antibiotic is a derivative of erythromycin, belonging to a class of antibiotics that inhibit bacterial growth by binding the 23S ribosomal RNA and stalling peptide elongation by preventing the addition of amino acids to the polypeptide chain (16). AZM has modest activity against P. aeruginosa in MHB and is not considered an active antibiotic against this pathogen (15). However, it has been shown to be effective in clinical trials to treat CF patients and has been proposed to alter quorum-sensing, virulence-factor production, and susceptibility through the decreased expression of efflux systems in P. aeruginosa (15, 17–19).
The success of an antimicrobial treatment depends on the interactions between the antimicrobial agent, the host, and the infecting bacteria (20). Pathogens being treated with antimicrobial therapies likely rely on different physiological processes during growth in different conditions, and recent studies are exploring transcriptional differences in bacteria related to virulence and antibiotic resistance when grown in media that are relevant to the type of infection studied (21–25). Although various studies have been done on the activity of AZM, only a few have focused on the effects of AZM specifically in the host environment, and none has looked at global transcriptomics of P. aeruginosa treated with AZM in the host environment.
In this study, RNA-sequencing (RNA-Seq) was used to examine global transcriptional changes that occurred in P. aeruginosa when grown under physiologically relevant conditions, both untreated and treated with low levels of AZM, comparing them to similar treatment in nutrient-rich laboratory media. Interestingly, differential gene expression in our physiologically relevant media, when compared to rich broth, demonstrated strong overlap with results for Pseudomonas isolated directly from patients (26). Thus, this research allowed us to study the impact of growth in a physiologically relevant host-like environment on acquired hypersusceptibility to AZM treatment and to explore how changing the growth conditions of an organism can alter how it is targeted by this antibiotic.
Results
P. aeruginosa Demonstrated Increased Susceptibility to Macrolides in Physiologically Relevant Conditions.
It was previously demonstrated that macrolide antibiotics have increased activity against gram-negative pathogens in conditions that are more physiologically relevant than MHB (12, 15, 27). Our first analysis was to verify these findings by measuring the minimal inhibitory concentrations (MICs) of the macrolide antibiotics erythromycin, clarithromycin, and AZM against wild-type (WT) P. aeruginosa PAO1. Assays were performed in MHB as a nutrient-rich laboratory control and enriched tissue culture medium, Roswell Park Memorial Institute (RPMI)-1640 + 5% MHB (RPMI), and RPMI-1640 + 5% MHB + 20% human serum (RPMI/serum) to mimic wound exudate or human blood. RPMI-1640, utilized in tissue culture, contains a mixture of inorganic salts optimized for the growth of mammalian cells, together with bicarbonate, vitamins, amino acids, glutathione, and glucose. Human serum contains a unique combination of different proteins, electrolytes, antibodies, antigens, and hormones that are important components of the blood. In these more biologically relevant media conditions, macrolide antibiotics showed significantly increased antimicrobial activity compared to MHB (Fig. 1A). The dibasic macrolide AZM had a 16-fold lower MIC in RPMI and a 64-fold lower MIC in RPMI/serum conditions, whereas the monobasic macrolides erythromycin and clarithromycin demonstrated only twofold changes in both media. This is compared to nine other antimicrobial agents from different classes that had decreased activity in physiologically relevant media (SI Appendix, Table S1). Time kill curves with AZM were also performed on P. aeruginosa grown in MHB, RPMI, and RPMI/serum conditions treated with 8 µg/mL AZM (Fig. 1B). At this concentration, AZM, usually considered a bacteriostatic antibiotic, had little or no inhibitory effect on P. aeruginosa grown in MHB but exhibited significant killing of P. aeruginosa grown in RPMI and RPMI/serum conditions (P < 0.001).
Fig. 1.
P. aeruginosa PAO1 demonstrated increased susceptibility to macrolide antibiotics under growth conditions that were physiologically relevant to human infection. (A) MICs of macrolides, expressed as micrograms per milliliter, vs. WT P. aeruginosa grown in MHB, RPMI + 5% MHB (RPMI), and RPMI + 5% MHB + 20% human serum (RPMI/serum). Values are the mode of a minimum of n = 3. (B) Time kill curves were performed against WT P. aeruginosa with 8 µg/mL AZM treatment in all three media. ***P < 0.0001 using an unpaired t test (n = 3).
Titration of human serum into MHB and RPMI +5% MHB was performed to measure if the presence of serum had a dose-dependent effect on the MIC of AZM (Table 1). In both MHB and RPMI, increasing the concentration of serum decreased the MIC of AZM. In MHB, human serum concentrations between 5 and 50% led to stepwise decreases in the MIC, with values from 64 µg/mL to below 1 µg/mL, respectively. In RPMI, the addition of 5% serum decreased the MIC by only 2-fold, while the addition of 30% human serum decreased the MIC by more than 16-fold, to <0.25 µg/mL. The addition of H2O did not have the same effect on the MIC of AZM in either media, having no effect up to 30% and only reducing the MIC by up to fourfold in MHB and by up to twofold in RPMI when 50% H2O was added.
Table 1.
Medium-dependent activity of AZM against P. aeruginosa PAO1
| Added serum or H2O, % | MIC, µg/mL | |||
| MHB + serum | MHB + H2O | RPMI/serum | RPMI + H2O | |
| 5 | 128 | 128 | 4 | 8 |
| 10 | 64 | 128 | 2 | 8 |
| 20 | 8 | 128 | 1 | 8 |
| 30 | 4 | 128 | <0.25 | 8 |
| 40 | 4 | 64 | <0.25 | 8 |
| 50 | <1 | 32 | <0.25 | 4 |
MICs of AZM against P. aeruginosa PAO1 grown in MHB or tissue culture media RPMI + 5% MHB (RPMI) with increasing concentrations of human serum or H2O.
AZM Synergized with Synthetic Peptides in Vitro and in Vivo.
Although AZM is not usually considered clinically as an antibiotic treatment for P. aeruginosa infections, it has been shown to improve morbidity and mortality outcomes when used in combination with typical clinical treatments (such as tobramycin and DNase) to treat CF patients chronically infected with P. aeruginosa (17). In this study, the ability of AZM to synergize with synthetic cationic HDPs, IDR-1018 and DJK-5, was examined using checkerboard titration assays. These peptides have antibiofilm and antiinfective activity against a broad range of gram-negative and gram-positive pathogens in vitro and in vivo and are a promising alternative treatment to traditional antibiotics (10, 11, 28–30). Synergy in vitro was determined based on the fractional inhibitory concentration indices (FICIs) of the combination, where values below 0.5 demonstrated high synergy, 0.5 to 1 demonstrated weak synergy or an additive effect, and values greater than 1 indicated antagonism or no synergy. FICIs of AZM + DJK-5 and AZM + IDR-1018 combinations are shown in Fig. 2A, with corresponding fold changes in each individual MIC of each agent. Strong synergy with DJK-5 was only evident under physiologically relevant conditions, with MICs of AZM decreasing by four- or eightfold and the MIC of DJK-5 decreasing by fourfold. This was more synergistic than in MHB, where there were fold changes in MICs of four- and twofold for AZM and peptide, respectively, resulting in an FICI of 0.75. For IDR-1018, synergistic combinations between peptide and AZM decreased the AZM MIC by fourfold in host-mimicking conditions, whereas the IDR-1018 MIC was only decreased by twofold in both MHB and host-mimicking conditions.
Fig. 2.
AZM strongly synergized with antimicrobial peptides under physiologically relevant conditions and in a cutaneous P. aeruginosa infection model. (A) In vitro, AZM was combined with DJK-5 and IDR-1018 in checkerboard titration assays in MHB and under physiologically relevant conditions: RPMI + 5% MHB (RPMI) and RPMI + 5% MHB + 20% human serum (RPMI/serum). FICI values below 0.5 indicate high synergistic activity, 0.5 to 1 indicate weak synergy or an additive effect, values greater than 1 indicate antagonism or no synergy. (B and C) In vivo, synergy was observed in a murine abscess model. Mice were infected with bacteria and treated with 1 mg/kg AZM, 0.1 mg/kg DJK-5, 14 mg/kg IDR-1018, or a combination of AZM and peptide. Synergy occurred when the effect of the combination of drugs on abscess size (B) and bacterial CFU (C) recovered was significantly (P < 0.01) more pronounced than the sum of the effects of each agent alone in comparison to the saline-treated controls after 18 h. Statistical analysis was performed using one-way ANOVA, Kruskal–Wallis test with Dunn’s correction (two-sided). The asterisks indicate significant differences to the WT: *P < 0.05; **P < 0.01; ***P < 0.001. The open boxes indicate significant differences of the combination therapy over the sum of the effects of each agent alone: *P < 0.05; **P < 0.01.
The combination of AZM with peptides was also tested for synergy in a murine cutaneous abscess model. Synergy in vivo is considered to have occurred when the effect of the combination of drugs is significantly more pronounced than the sum of the effects of each agent alone, when referenced to the saline-treated controls. Each of these treatments had a significantly enhanced effect in decreasing abscess size (Fig. 2B), and the combination of 0.1 mg/kg DJK-5 with 1 mg/kg AZM had a significant decrease in colony-forming units (CFUs) recovered from the abscess (Fig. 2C) 18 h after infection when compared to any of the single treatments. This indicated a synergistic effect of AZM and peptides in vivo against high-density infections, being especially pronounced in the case of the AZM/DJK-5 combination. In these studies, saline-treated mice had a mortality rate of 45%, while DJK-5–treated mice and AZM-treated mice demonstrated 22 and 20% mortality, respectively. No mortality was observed in the IDR-1018 treatment group or in the combined-treatment synergy groups.
Tissue Culture Media with Human Serum Mimicked Physiologically Relevant Conditions for Growth of P. aeruginosa.
To assess whether RPMI and RPMI/serum conditions are physiologically relevant, and to probe mechanisms, global transcriptional patterns of P. aeruginosa PAO1 grown in RPMI and RPMI/serum conditions were compared to gene expression of P. aeruginosa grown in MHB. Subsequently, the differences in gene expression were compared to previously published data on P. aeruginosa from human samples (26, 31). RNA-Seq was performed on P. aeruginosa PAO1 grown to an optical density at 600 nm of 0.5 in MHB, RPMI, and RPMI/serum conditions, untreated or treated with effective concentrations of AZM that reduced but did not inhibit growth (SI Appendix, Fig. S1 A–C). Comparing P. aeruginosa grown in RPMI and RPMI/serum to those grown in MHB, there were, respectively, 1,165 up-regulated and 827 down-regulated genes in RPMI and 1,218 up-regulated and 988 down-regulated genes in RPMI/serum; of these, 971 up-regulated genes and 701 down-regulated genes were in common (SI Appendix, Fig. S2).
To examine how the host-like conditions utilized here reflected human patient conditions, these results were compared to the Pseudomonas transcriptome analyses of Cornforth and coworkers in human samples (26, 31). Transcriptomes of P. aeruginosa from expectorated CF patient sputum and wound tissue samples were used as templates to compare our untreated in vitro transcriptomes, using a method developed by Cornforth et al. (31). Comparisons were done by calculating z scores for the mean read counts of each gene under each “model” condition cf. the mean read counts of each gene in the in vivo “templates.” The percentages of genes in the models within each SD of the mean of the template were then plotted (SI Appendix, Fig. S3). The percentage of genes within 2 SDs of the mean of the in vivo template is referred to as the accuracy score (AS2) (31) (Tables 2 and 3). In vitro datasets from Synthetic Cystic Fibrosis sputum Medium2 (SCFM2), lysogeny broth (LB), morpholinepropanesulfonicacid (MOPS) succinate, uninfected CF airway epithelial cells (CFECs), respiratory syncytial virus (RSV)-coinfected CFECs (RSV-CFECs), and a murine chronic wound model, analyzed previously by Cornforth et al. (31), were also included in this analysis to allow us to gauge how our in vitro models performed relative to other models (Table 2 and SI Appendix, Fig. S3). When calculating accuracy against the combined in vivo human samples, RPMI and RPMI/serum transcriptomes had greater similarity (AS2 values) than both nutrient-rich laboratory media LB and MHB and minimal medium Mops succinate, with AS2 values of 85 and 84% for RPMI and RPMI/serum, respectively. MHB had a lower AS2 of 72%, LB an AS2 of 77%, and Mops succinate an AS2 of 80%. Airway epithelial models performed similarly to our media, and SCFM2 and the mouse wound model were somewhat better. The values for RPMI and RPMI/serum (74 and 72%) were somewhat lower when compared to human sputum but still outperformed the other in vitro models, with the exception of SCFM2 (AS2 of 82%). Other, more complex models had AS2 values of 79, 75, and 84% for CFECs, RSV-infected epithelial cells, and murine wound transcriptomes, respectively. Similarly, when compared to just the chronic wound and burn samples as the in vivo target, RPMI and RPMI/serum outperformed most synthetic media, with AS2 of 82 and 81% for RPMI and RPMI/serum, respectively. This was better than LB, MHB, and Mops succinate (AS2 = 76, 70, and 76%, respectively) and on par with RSV-infected and uninfected CFECs that had AS2 values of 81 and 82%, respectively.
Table 2.
AS2 for gene expression in P. aeruginosa PAO1
| Model | AS2, % | ||
| Combined in vivo | Sputum | Chronic wound/burn | |
| Resampled in vivo | 100 | 100 | 100 |
| Mouse wound model | 92 | 84 | 88 |
| SCFM2 | 91 | 82 | 88 |
| CFECs | 86 | 79 | 82 |
| RPMI | 85 | 74 | 82 |
| RPMI/serum | 84 | 72 | 81 |
| RSV-CFECs | 83 | 75 | 81 |
| Mops succinate | 80 | 72 | 76 |
| LB | 77 | 69 | 76 |
| MHB | 72 | 65 | 70 |
AS2 for gene expression in P. aeruginosa PAO1 grown in MHB, RPMI + 5% MHB (RPMI), and RPMI + 5% MHB + 20% human serum (RPMI/serum) and previously published in vitro models, when compared to in vivo transcriptomes in CF sputum and chronic wound (31), showed greater similarity between the host-mimicking conditions used in this study and in vivo samples cf. nutrient-rich laboratory media. The percentage of genes within 2 SDs (AS2) of the mean expression for each gene in CF sputum (total, 2,437 genes) and chronic wound samples (957 genes) or a combined in vivo dataset (835 genes) (31) was determined with z-score calculations using the mean of normalized read counts.
Table 3.
AS2 for the expression of genes in PseudCap (32) functional classes
| Functional category | AS2, % | ||
| RPMI | RPMI/serum | LB | |
| Adaptation and protection | 75 | 75 | 73 |
| Amino acid metabolism and biosynthesis | 84 | 78 | 80 |
| Antibiotic resistance and susceptibility | 79 | 79 | 64 |
| Biosynthesis of cofactor prosthetic groups and carriers | 86 | 84 | 84 |
| Cell division | 94 | 94 | 88 |
| Cell wall LPS capsule | 87 | 91 | 89 |
| Central intermediary metabolism | 80 | 80 | 76 |
| Chaperone and heat shock | 100 | 100 | 94 |
| Chemotaxis | 89 | 67 | 78 |
| Energy metabolism | 75 | 73 | 72 |
| Fatty acid and phospholipid metabolism | 90 | 86 | 86 |
| Membrane | 83 | 80 | 73 |
| Motility | 90 | 81 | 81 |
| Nucleotide biosynthesis and metabolism | 93 | 93 | 75 |
| Protein secretion and export | 93 | 100 | 87 |
| Putative enzyme | 90 | 88 | 86 |
| Secreted factors | 100 | 100 | 67 |
| Transcription, RNA processing and degradation | 91 | 91 | 78 |
| Transcriptional regulator | 73 | 64 | 59 |
| Translation and posttranslational modification | 90 | 88 | 71 |
| Transport small molecules | 84 | 81 | 69 |
| Two-component regulators | 95 | 89 | 68 |
AS2 for the expression of genes in PseudCap (32) functional classes where transcriptomes from RPMI + 5% MHB (RPMI) and RPMI + 5% MHB + 20% human serum (RPMI/serum) outperformed transcriptomes in LB compared to in vivo transcriptomic data from CF sputum samples and chronic wound. The AS2 represents the percentage of genes within 2 SDs of the mean expression levels in CF sputum and chronic wound samples as determined with z-score calculations using the mean of normalized read counts. Functional categories that performed as well or better than the other in vitro models Mops succinate and SCFM2 are bolded.
A similar analysis was performed to measure the similarity in dysregulated genes between combined in vivo transcriptomes and in vitro conditions for each of the PseudoCap (32) functional classes individually (Table 3 and SI Appendix, Table S2). Compared to the synthetic nutrient-rich medium LB, RPMI and/or RPMI/serum had higher AS2 values against combined in vivo datasets in 22 functional classes (Table 3), with, e.g., for RPMI, 50% of functional classes having AS2 values >90% and 82% of classes having AS2 values >80%. Additionally, transcriptomes from RPMI and/or RPMI/serum performed as well or better than transcriptomes from SCFM2 in 12 (55%) of these functional classes and all 22 classes for Mops succinate (SI Appendix, Table S2). Interestingly, genes involved in functional classes that make up the majority of antibiotic targets had higher AS2 values in RPMI and RPMI/serum than other in vitro media (Table 3). Genes belonging to the functional classes “Antibiotic resistance and susceptibility” (AS2 of 79% in RPMI and RPMI/serum), “Two-component regulators” (AS2 of 95 and 89% for RPMI and RPMI/serum, respectively), “Biosynthesis of cofactors, prosthetic groups, and carriers” (AS2 of 86 and 84%), “Transcription, RNA processing and degradation” (AS2 of 91% for both host-mimicking media), “Cell division” (AS2 of 94% for both host-mimicking media), “Translation and posttranslational modification” (AS2 of 90 and 88% for RPMI and RPMI/serum, respectively), and “Transcriptional regulators” (AS2 of 73 and 64%) all outperformed the transcriptomes from nutrient-rich laboratory media. The first four of these also performed as well or better than SCFM2, the highest-performing model for this in vivo dataset comparison (Table 3 and SI Appendix, Table S2). Additional high-performing classes under both host-mimicking media conditions used in this study included “Chaperone and heat shock” (AS2 = 100%), “Fatty acid and phospholipid metabolism” (AS2 = 86 to 90%), “Protein secretion and export” (AS2 = 93 to 100%), “Membrane” (AS2 = 80 to 83%), “Transport small molecules” (AS2 = 81 to 84%), and “Motility” (AS2 = 81 to 90%) (Table 3).
Growth in Physiologically Relevant Media Led to Altered Regulation of Cell Surface Modification in P. aeruginosa.
To elucidate potential reasons for the altered effects of AZM on P. aeruginosa, untreated (Fig. 3) and treated (Fig. 4) transcriptomes were compared between physiologically relevant media and MHB. Given that P. aeruginosa grown in physiologically relevant growth conditions has such a distinct transcriptome from growth in MHB, and because it appears to be more relevant to human infection, we looked within dysregulated genes in host-like conditions for a subset of 465 known resistome genes in P. aeruginosa collected from previous studies (33–37) (Fig. 3A). The resistome genes could be categorized into three groups: genes for which mutants had increased resistance to certain antibiotics (resistant mutants), genes for which mutants had decreased resistance to certain antibiotics (hypersusceptible mutants), and genes for which mutants had mixed effects depending on the antibiotics that were tested in resistome studies (mixed mutants). In physiologically relevant media conditions, 96 of these resistome genes were dysregulated relative to their expression when P. aeruginosa was grown in MHB. Of these, in RPMI, we found 53 up-regulated genes that conferred resistance when mutated and 30 down-regulated genes that conferred hypersusceptibility when mutated. In RPMI/serum, we found 56 up-regulated genes that conferred resistance when mutated and 34 down-regulated genes that conferred hypersusceptibility when mutated. These genes were largely involved in membrane integrity and modification, iron acquisition and utilization, energy metabolism, small-molecule transport, and regulation. The most significant difference in expression was seen in the arn operon (arnBCADTEF), for which genes were down-regulated between 17- and 40-fold in physiologically relevant media compared to MHB (Fig. 3B). The arn operon mediates the addition of 4-aminoarabinose to lipid A of lipopolysaccharide (LPS), which blocks self-promoted uptake, leading to resistance in P. aeruginosa to cationic antimicrobials (33). Regulation of this operon is partially mediated by PhoPQ in Pseudomonas, which was also significantly down-regulated by 12- and 7-fold in RPMI and 15- and 9-fold in RPMI/serum for phoP and phoQ, respectively, when compared to MHB (Fig. 3C). Dysregulation of the arn and phoPQ operons were also examined using qRT-PCR, and the same trends in regulation were observed (SI Appendix, Table S3). Analysis of the transcriptome under host-mimicking conditions revealed other candidate genes that might explain the increased susceptibility under host-like conditions, cf. MHB, including two component regulators affecting arn operon expression and other susceptibility determinants (38–40) such as phoPQ (7.35- to 14.7-fold down-regulated), parRS (1.5- to 1.7-fold down-regulated), pmrAB (1.6- to 2.2-fold down-regulated), galU, pyrD, lptC, and tpiA (Fig. 3 and Dataset S1). All of these genes are involved in induction of resistance and/or when deleted lead to increased susceptibility to the cationic antibiotic polymyxin B (PMB) and were down-regulated under host-mimicking conditions. Additionally, other genes involved in membrane permeability were also modestly dysregulated (SI Appendix, Table S4). These include genes involved in LPS production and export that were up-regulated in RPMI and RPMI/serum.
Fig. 3.
P. aeruginosa PAO1 grown in RPMI + 5% MHB (RPMI) and RPMI + 5% MHB + 20% human serum (RPMI/serum) displayed distinct transcriptional changes in genes previously proposed to be involved in resistance and susceptibility to antibiotics when compared to P. aeruginosa grown in MHB. (A) Heatmap shows those resistome genes with a log2 fold change ≥ ±2. Genes are grouped by PseudoCap functional class and mutant type, where mutant type indicates whether a mutant in that gene was predicted to be resistant, susceptible, or to have mixed effects to antibiotics. The arn operon (B) and two-component systems (C) were highly dysregulated in host conditions compared to MHB.
Fig. 4.
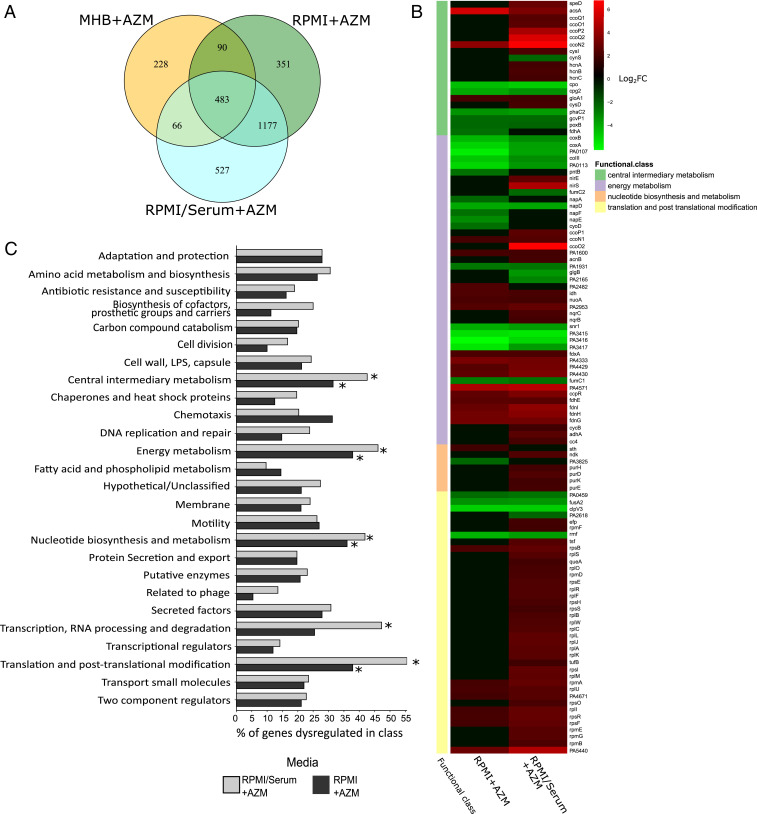
P. aeruginosa PAO1 grown in RPMI + 5% MHB (RPMI) and RPMI + 5% MHB + 20% human serum (RPMI/serum) had more genes differentially expressed after AZM treatment cf. cells grown in MHB. (A) Venn diagram of numbers of genes and overlap. (B) Functional classes significantly enriched by AZM treatment under physiologically relevant conditions but not in MHB, as determined using a hypergeometric distribution calculation with a false-discovery rate-corrected P value of <0.05. * indicates functional classes enriched after AZM treatment in either RPMI or RPMI/serum but not MHB. (C) Heatmap showing log2 fold change (Log2FC) of genes in some functional classes that were significantly enriched after AZM treatment in host-like media vs. MHB (Log2FC ≥ ±2 to enable capture of the most significantly dysregulated genes).
Increased Effects of AZM in Physiologically Relevant Media Led to Increased Dysregulation of Known AZM Targets and Previously Undescribed Patterns of Dysregulated Genes.
To elucidate the effects of AZM on known and previously undescribed targets of P. aeruginosa under conditions relevant to the human host, the global transcriptome of AZM-treated cultures was compared to that of untreated cultures in all three media conditions. AZM is an amphipathic, cationic macrolide that is thought to enter bacterial cells through interaction with surface molecules and self-promoted uptake and has multiple proposed targets (18, 19, 27, 41, 42). In our analysis, we found protease-encoding lasA and lasB as well as the hypothetical gene cluster PA0585–PA0587 were all down-regulated by more than 20-fold in P. aeruginosa treated with AZM under RPMI and RPMI/serum conditions but only slightly down-regulated in MHB (Table 4). Polysaccharide genes and quorum-sensing genes were also down-regulated in RPMI/serum by up to eightfold but were not significantly down-regulated in MHB conditions (Table 4). Unlike previous research, we did not observe down-regulation of oprM after AZM treatment, and it was actually up-regulated by about twofold under our physiologically relevant conditions (Table 4). Further analysis of the genes encoding major antibiotic efflux systems of P. aeruginosa revealed no down-regulation of most of these genes in any growth conditions after AZM treatment (SI Appendix, Table S5). Furthermore, the genes in the MexXY efflux system were significantly up-regulated in response to AZM treatment, which is known to occur in response to ribosome-targeting antibiotics (43) and PMB (26). The effects of AZM on dysregulation of quorum-sensing genes and the mexXY and oprM genes were verified with qRT-PCR (SI Appendix, Table S6).
Table 4.
| Locus tag | Name | Fold change due to AZM treatment | ||
| MHB | RPMI | RPMI/serum | ||
| PA3477 | rhlR | −1.8 | −3.6 | −8.5 |
| PA3476 | rhlI | −1.9 | — | −2.3 |
| PA1430 | lasR | — | −3.5 | −4.3 |
| PA1432 | lasI | — | — | −2.0 |
| PA2587 | pqsH | — | −2.3 | −6.3 |
| PA1003 | mvfR (pqsR) | — | 2.2 | −2.6 |
| PA0996 | pqsA | — | 1.9 | −3.0 |
| PA0997 | pqsB | — | — | −4.9 |
| PA0998 | pqsC | — | — | −4.8 |
| PA0999 | pqsD | — | — | −2.6 |
| PA1000 | pqsE | — | — | −2.0 |
| PA2586 | gacA | — | — | −1.6 |
| PA2395 | pvdO | −3.1 | −2.3 | — |
| PA2396 | pvdF | — | −3.1 | −2.6 |
| PA2397 | pvdE | — | −2.5 | −3.5 |
| PA2398 | fpvA | −3.9 | — | — |
| PA2399 | pvdD | — | −2.1 | — |
| PA1148 | toxA | — | −2.6 | −5.5 |
| PA2663 | ppyR | −3.5 | −4.1 | −2.1 |
| PA1871 | lasA | −4.0 | −27.0 | −45.4 |
| PA3724 | lasB | −5.0 | −25.1 | −27.8 |
| PA2231 | pslA | 1.9 | 2.4 | — |
| PA2232 | pslB | — | — | −2.7 |
| PA2233 | pslC | — | — | −3.2 |
| PA2234 | pslD | — | — | −2.5 |
| PA2235 | pslE | — | — | −2.7 |
| PA2236 | pslF | — | −2.4 | −4.8 |
| PA2237 | pslG | — | — | −3.4 |
| PA2238 | pslH | — | −3.4 | −4.8 |
| PA2239 | pslI | — | −2.6 | −5.0 |
| PA0427 | oprM | — | 1.9 | 2.1 |
| PA3297 | — | 1.7 | 2.5 | |
| PA0585 | — | −2.6 | −4.2 | |
| PA0586 | −3.1 | −69.0 | −59.2 | |
| PA0587 | −2.7 | −47.2 | −44.5 | |
| PA0588 | −2.7 | −27.7 | −29.4 | |
Values are shown as fold change, obtained from RNA-Seq experiments (with adjusted P ≤ 0.05) for P. aeruginosa PAO1 treated with effective concentrations of AZM vs. untreated samples after growth in MHB, RPMI + 5% MHB (RPMI), and RPMI + 5% MHB + 20% human-serum (RPMI/serum). Samples with a dash signify no change. Not shown are rsmZ, rsmY, pvdN, mexA, or mexB, for which no changes were observed in any medium.
Exploring the complete transcriptome for AZM treatment effects between MHB and host-like conditions, more genes were found to be dysregulated by AZM treatment under physiologically relevant conditions than in MHB (Fig. 4A). Thus, there were 975 genes up-regulated and 1,126 genes down-regulated in RPMI by AZM treatment and 1,074 genes up-regulated and 1,179 genes down-regulated in RPMI/serum conditions by AZM treatment. This is compared to only 394 genes up-regulated and 473 genes down-regulated in MHB upon AZM treatment. Specifically, the subset of genes that were dysregulated by AZM treatment in biologically relevant conditions was significantly higher than with AZM treatment in MHB. This highlights genes that were uniquely important for resistance or susceptibility to AZM under physiologically relevant conditions. To examine this, we compared the dysregulation of genes after AZM treatment in RPMI or RPMI/serum to the effects of AZM treatment in MHB. There were 1,814 genes that were significantly dysregulated by treatment in the physiologically relevant conditions when compared to treatment in MHB. Enrichment of PseudoCap functional classes of genes indicated that pathways involved in central and energy metabolism, nucleotide biosynthesis and metabolism, transcription, RNA processing and degradation, and translation and posttranslational modification were significantly dysregulated with AZM treatment in physiologically relevant conditions compared to AZM treatment in MHB (Fig. 4B). The most dysregulated genes within these functional classes (log2 fold change ≥ ±2) are shown in Fig. 4C. Of these genes, many involved in central and energy metabolism tended to be down-regulated, while nucleotide biosynthesis and translation genes were up-regulated with AZM treatment in physiologically relevant conditions compared to treatment in MHB. Interestingly, many of these genes were involved in nitrogen metabolism. Genes related to translation and posttranslational modification were dysregulated in RPMI/serum with AZM treatment but not significantly dysregulated in RPMI with AZM treatment, these being mainly genes involved in ribosomal biogenesis.
Deletion of the arn Operon in P. aeruginosa Increased Susceptibility to AZM and the Ability of AZM to Synergize with Other Antimicrobials.
Previous studies proposed that AZM increases membrane permeability of P. aeruginosa in host conditions (12, 15), and our results showed that the phoPQ and arn operons were significantly down-regulated under our physiologically relevant media conditions. Therefore, we hypothesized that the deletion of the genes belonging to the arn operon would increase susceptibility of P. aeruginosa to AZM and increase synergy of AZM with other antimicrobial agents. To test the effect of mutations in genes in the phoPQ and arn operons in P. aeruginosa, mutants from an ordered transposon library (44) containing insertions within genes in these operons were tested for susceptibility in MIC assays (SI Appendix, Table S7). Interestingly, phoP::lacZ and arnD::phoA, arnT::lacZ, and arnE::phoA had greater than twofold decreases in MIC but not phoQ::phoA or the other transposon mutants tested. PhoQ is proposed to negatively regulate PhoP-dependent activation of genes involved in cell surface modification and resistance, and the observation of decreased MICs in the phoP but not the phoQ mutant was in agreement with this (39, 45). A deletion mutant was constructed for the entire arn operon in the PAO1 background and was also tested for susceptibility to AZM in the different media (Table 5). In these assays, PAO1 ∆arn had a twofold decreased MIC in MHB and RPMI/serum conditions. A complemented mutant showed MICs equal to WT values.
Table 5.
Altered susceptibility of P. aeruginosa PAO1 ∆arn to AZM
| Mutant name | MIC, µg/mL | ||
| MHB | RPMI | RPMI/serum | |
| PAO1 | 128 | 8 | 2 |
| ∆arn | 64 | 8 | 1 |
| ∆arn+arn | 128 | 8 | 2 |
Bacteria were grown in MHB, RPMI+5%MHB (RPMI), and RPMI + 5% MHB + 20% human serum (RPMI/serum). MICs for deletion mutant (∆arn in which PA3552 to PA3558 were deleted) and the complemented mutant (∆arn+arn) compared to WT PAO1 are presented.
To evaluate whether the deletion of the arn operon, and resulting disruption of cell surface modifications, could be responsible for the increased synergy of AZM with other antimicrobials under host-like conditions, the arn operon mutant was tested for synergy with DJK-5, PMB, and colistin (Table 6). These peptides were chosen because they have synergy with AZM against PAO1 in host-mimicking conditions but minimal to no synergy in laboratory media. In MHB, PAO1 ∆arn had synergy with DJK-5 and PMB that resulted in FICIs of 0.5 and 0.38, which aligns with FICI values observed in the WT strain under physiologically relevant conditions. For the mutant strain, the combined MICs of AZM and/or peptide were altered by only twofold in MHB compared to WT. Regarding colistin, ∆arn demonstrated increased synergy under all three media conditions with FICI values of 0.38 in MHB and RPMI/serum and 0.63 in RPMI. This compared to values of 0.5, 1, and 0.5 in the WT strain.
Table 6.
Synergy of AZM with DJK-5, PMB, and colistin (COL) against P. aeruginosa PAO1 WT, ∆arn, and ∆arn+arn-complemented strain
| Strain | Peptide | Media | FICI | Reduction (fold change) in AZM MIC | Reduction (fold change) in peptide MIC |
| PAO1 WT | DJK-5 | MHB | 0.75 | 4 | 2 |
| RPMI | 0.50 | 4 | 4 | ||
| RPMI/serum | 0.38 | 8 | 4 | ||
| PMB | MHB | 0.75 | 4 | 2 | |
| RPMI | 1.00 | 2 | 2 | ||
| RPMI/serum | 0.50 | 4 | 4 | ||
| COL | MHB | 0.50 | 4 | 4 | |
| RPMI | 1.00 | 2 | 2 | ||
| RPMI/serum | 0.50 | 4 | 4 | ||
| PAO1 ∆arn | DJK-5 | MHB | 0.50 | 4 | 4 |
| RPMI | 0.50 | 4 | 4 | ||
| RPMI/serum | 0.38 | 8 | 4 | ||
| PMB | MHB | 0.38 | 8 | 4 | |
| RPMI | 2.00 | 4 | 2 | ||
| RPMI/serum | 0.50 | 4 | 4 | ||
| COL | MHB | 0.38 | 8 | 4 | |
| RPMI | 0.63 | 8 | 2 | ||
| RPMI/serum | 0.38 | 8 | 4 | ||
| PAO1 ∆arn+arn | DJK-5 | MHB | 0.50 | 4 | 4 |
| RPMI | 0.50 | 4 | 4 | ||
| RPMI/serum | 0.63 | 8 | 2 | ||
| PMB | MHB | 0.63 | 8 | 2 | |
| RPMI | 2.00 | 1 | 1 | ||
| RPMI/serum | 0.56 | 16 | 2 | ||
| COL | MHB | 0.63 | 8 | 2 | |
| RPMI | 0.75 | 4 | 2 | ||
| RPMI/serum | 0.75 | 4 | 2 |
Synergy was measured using checkerboard titration assays in MHB, RPMI + 5% MHB (RPMI), and RPMI + 5% MHB + 20% human serum (RPMI/serum). FICI values (rounded to two decimal places) below 0.5 are highly synergistic (shown by bolded typeface), 0.5 to 1 indicate weak synergy or additive nature, and higher than 1 indicate no synergy or antagonism.
Discussion
This study confirmed that AZM is significantly more effective against P. aeruginosa in RPMI media and RPMI media containing 20% human serum and demonstrates significant killing in physiologically relevant media, although it is normally considered bacteriostatic (Fig. 1). The effect of serum on the MIC of AZM against P. aeruginosa occurred in a dose-dependent manner, but the same effect was not found for the addition of other antimicrobials, including some that are used clinically for treatment of P. aeruginosa infections (SI Appendix, Table S1). These results suggested that components of tissue culture media and human serum are important factors affecting susceptibility of P. aeruginosa in host-like conditions, rather than simply limiting nutrient availability, that alters bacterial susceptibility to multiple antibiotics (46). Furthermore, previous research demonstrated that AZM synergizes with the polycationic lipopeptide antibiotic colistin, a last resort polymyxin used to treat multidrug resistant gram-negative infections (12). In this study, two synthetic antimicrobial peptides, IDR-1018 and DJK-5, were utilized because they have the ability to eradicate bacterial growth and biofilm formation in a broad-spectrum manner (10, 28), although it has been proposed that their antimicrobial effectiveness is limited by the host environment (47). We found that AZM was highly synergistic with these peptides both in vitro, in physiologically relevant media, and in vivo, in a cutaneous murine abscess model (Fig. 2) that is highly refractory to conventional antibiotic treatment (31). This synergistic ability allowed for both antimicrobial peptides and AZM to be effective at substantially lower concentrations, which, in clinical settings, could greatly improve efficacy, reduce cost of treatment and potential toxicity, and decrease the chances of resistance development.
Transcriptional changes of P. aeruginosa grown in RPMI and RPMI/serum in the absence of AZM treatment were found to demonstrate greater similarity to previously published transcriptomic data (26, 31) for P. aeruginosa isolated from chronic infections in CF sputum and wounds (Table 2 and SI Appendix, Fig. S3) than those observed in MHB and LB. When compared against in vivo transcriptomes from wound and burn alone, our models also outperformed Mops succinate and performed as well as uninfected or RSV-infected CF epithelial cell models. Other models, such as murine wounds and SCFM2 medium, somewhat outperformed RPMI and RPMI/serum when compared to in vivo transcriptomes comprised of mainly human sputum samples; however, RPMI and RPMI/serum were still found to be good mimics for a variety of important functional classes, particularly those covering transcription and its regulation. These results indicate that our in vitro host-mimicking media are more relevant conditions to test antibiotics than traditional laboratory media commonly employed for MIC studies (48) and that they might represent a better mimic of the wound exudate or blood than human sputum. Importantly, expression of many functions and pathways dysregulated in bacteria taken directly from patients were similarly dysregulated in host-like media (Table 3 and SI Appendix, Table S2). Indeed, this analysis indicated that the expression of genes belonging to functional classes representing the majority of antibiotic targets were similarly altered both during growth in a human host and in our host-like conditions. Overall, these data support the use of RPMI and RPMI with human serum as in vitro conditions that somewhat mirror conditions in the patient and suggest that their use in antimicrobial-susceptibility testing might be more relevant to infection conditions than traditional assays in MHB (48).
AZM treatment in the host environment had a greater impact on gene expression in RPMI and RPMI/serum conditions, with more than twice the number of genes dysregulated under each condition when compared to the number in MHB (Fig. 4). Altered expression of many central metabolism and ribosome-related genes in physiologically relevant media is likely due to general changes in cell physiology due to the increased effectiveness of AZM in these conditions. This might suggest that components in RPMI and serum, e.g., different nitrogen and carbon sources and/or concentrations, could play a role in increased AZM susceptibility. Future analysis of susceptibility in host-mimicking conditions should explore the effects of specific components in RPMI and serum, to elucidate how they impact susceptibility.
AZM treatment down-regulates the expression of QS genes and genes involved in virulence factors, such as exotoxin A, proteases, and Psl polysaccharides (18). The hypothetical gene cluster PA0585–PA0587, proposed to influence pyocyanin production and swarming, shows decreased expression in P. aeruginosa treated with AZM (49). In our study, many of these genes were more dysregulated in the presence of human serum, than in MHB or RPMI + 5% MHB, supporting the increased potency of AZM observed in this condition. Interestingly, the Las and Rhl QS systems were down-regulated in all three media conditions, while dysregulation of the PQS system was more specific to the serum-containing condition, which might have influenced AZM susceptibility. It was previously proposed that down-regulation of major intrinsic outer membrane efflux channel OprM explained increased AZM activity against P. aeruginosa in serum-containing conditions (15). In contrast, we found that genes involved in major antibiotic efflux systems of P. aeruginosa were not significantly down-regulated in host conditions (15).
Among the genes dysregulated in P. aeruginosa grown under physiologically relevant conditions were genes implicated in the resistome of P. aeruginosa in response to various antibiotics (33, 37, 38). Such resistome genes could contribute to the altered susceptibility of P. aeruginosa to antibiotics when grown in host-like conditions. Thus, important regulatory genes, those involved in resistance to cationic antibiotics and genes involved in metabolism, iron uptake, and membrane production and modification were among those dysregulated in physiologically relevant conditions. Importantly, genes in the arn and phoPQ operons were greatly dysregulated in RPMI and RPMI/serum, relative to MHB (Fig. 3). Down-regulation of the arn operon through phoPQ, pmrAB, and/or other regulatory mechanisms, when P. aeruginosa was grown in physiologically relevant media, would likely increase self-promoted uptake (27) of AZM, thus increasing its effectiveness and ability to synergize with cationic peptides, as observed here. Inactivation of genes in the arn operon increased the activity of AZM and the ability of AZM to synergize with the peptide DJK-5 and the cationic antimicrobials PMB and colistin (Tables 1 and 5). This is relevant to the use of combined therapies in the clinic and suggests that cell membrane permeabilization plays a very important role in the activity of AZM in host infections and synergy with other antimicrobials. The synergy between antimicrobial peptides and AZM observed in the murine abscess model could be determined in part by basal arn operon expression (and/or other resistance determinants) as well as its reduced induction by AZM and/or peptides in this model. Based on the results in Table 6, we speculate that disruption of the arn operon might further increase synergy observed in this model. However, since knocking out this operon did not increase susceptibility in MHB to levels observed under host conditions, there must be other targets of AZM in host conditions that specifically allow it to become more effective. Given the altered expression of genes involved in regulation, cationic antibiotic susceptibility, metabolism, and iron uptake in P. aeruginosa grown on physiologically relevant media, and the further altered expression of carbon and nitrogen metabolism genes after AZM treatment, we propose that one of these features might be a crucial component of human serum that alters susceptibility of P. aeruginosa in clinical infections.
This research provides an exploration of the effects of AZM in a host-like environment, validates feasible in vitro growth conditions for testing of antimicrobial activities, and provides evidence for specific pathways dysregulated in the host environment that contribute to antibiotic uptake and synergy. We propose that analyses like those provided here are crucial to understand the effects of antibiotics in the host.
Materials and Methods
Detailed methods are included in SI Appendix, Supplementary Materials and Methods.
Bacterial Strains and Growth Conditions.
P. aeruginosa PAO1 H103 (50) and transposon insertion mutants (44) were used here. Plasmids and primers are summarized in SI Appendix, Table S8. Media included laboratory control MHB, RPMI-1640 supplemented with 5% MHB (referred to as RPMI), and RPMI-1640 supplemented with 5% MHB and 20% human serum pooled from anonymous donors (RPMI/serum).
Antimicrobial-Susceptibility Assays.
MIC assays were performed as previously described (48). Time kill curves were performed in three biological replicates by treating with 0 or 8 µg/mL AZM. Synergy assays exposed P. aeruginosa to combination treatments using a checkerboard titration approach (51). The FICI was calculated as described previously (51).
Synergy between AZM and Peptides in a Cutaneous Mouse Infection Model.
Animal experiments were performed following the Canadian Council on Animal Care guidelines and were approved by The University of British Columbia Animal Care Committee (certificate no. A14-0363). Synergy between AZM and peptides DJK-5 and IDR-1018 was tested in a murine abscess model, as previously described (11, 52).
Global Transcriptome Analysis in Physiologically Relevant Conditions.
RNA-Seq was performed on planktonic cultures, untreated or treated with concentrations of AZM reduced growth to ∼70% of untreated samples after 1 h (namely 16, 8, and 6 µg/mL for MHB, RPMI, and RPMI/serum, respectively; SI Appendix, Fig. S1). RNA, extracted as previously described, was sequenced on Illumina HiSEq. 2500. Experimental setup and data analysis are described in detail in SI Appendix; see also SI Appendix, Fig. S1.
Construction of PAO1 Deletion Mutant and Complementation.
The arn operon deletion mutant was constructed using double-homologous recombination as previously described (30, 53). Complementation was done by amplifying the full open reading frame using appropriate primers and cloning with the shuttle vector pBBR1MCS-5 (54).
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Travis Blimkie in RNA-Seq analysis and Zack Dang for MICs and clone screening. Research funding came from Canadian Institutes for Health Research Foundation Grant FDN-154287. C.R.B. received a Doctoral Studentship Award from Cystic Fibrosis Canada. D.P. received a Cystic Fibrosis Postdoctoral Fellowship (Canada) and a Research Trainee Award from the Michael Smith Foundation for Health Research. R.E.W.H. holds a Canada Research Chair in Health and Genomics and a University of British Columbia Killam Professorship.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2007626117/-/DCSupplemental.
Data Availability.
RNA-Seq data on differentially expressed genes have been deposited in the Gene Expression Omnibus (GEO) database under Sequence Read Archive accession no. SRP264926 (GEO series accession no. GSE151259).
References
- 1.Diggle S. P., Whiteley M., Microbe profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology (Reading) 166, 30–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flume P. A., Van Devanter D. R., State of progress in treating cystic fibrosis respiratory disease. BMC Med. 10, 88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattemer A., et al. , Bacterial and clinical characteristics of health care- and community-acquired bloodstream infections due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57, 3969–3975 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti M., Vena A., Croxatto A., Righi E., Guery B., How to manage Pseudomonas aeruginosa infections. Drugs Context 7, 212527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO , Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics (WHO, 2017). https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed 9 April 2017.
- 6.Singh S. B., Confronting the challenges of discovery of novel antibacterial agents. Bioorg. Med. Chem. Lett. 24, 3683–3689 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Hancock R. E. W., Rethinking the antibiotic discovery paradigm. EBioMedicine 2, 629–630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun E., Belanger C. R., Haney E. F., Hancock E. W., “Host defense (antimicrobial) peptides” in Peptide Applications in Biomedicine, Biotechnology and Bioengineering, Koutsopoulos S., Ed. (Elsevier, 2018), pp. 253–285. [Google Scholar]
- 9.Cherkasov A., et al. , Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem. Biol. 4, 65–74 (2009). [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente-Núñez C., et al. , D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 22, 196–205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour S. C., et al. , Bacterial abscess formation is controlled by the stringent stress response and can be targeted therapeutically. EBioMedicine 12, 219–226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L., et al. , Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine 2, 690–698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeaman M. R., Gank K. D., Bayer A. S., Brass E. P., Synthetic peptides that exert antimicrobial activities in whole blood and blood-derived matrices. Antimicrob. Agents Chemother. 46, 3883–3891 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colquhoun J. M., Wozniak R. A. F., Dunman P. M., Clinically relevant growth conditions alter Acinetobacter baumannii antibiotic susceptibility and promote identification of novel antibacterial agents. PLoS One 10, e0143033 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buyck J. M., et al. , Increased susceptibility of Pseudomonas aeruginosa to macrolides and ketolides in eukaryotic cell culture media and biological fluids due to decreased expression of oprM and increased outer-membrane permeability. Clin. Infect. Dis. 55, 534–542 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Poehlsgaard J., Douthwaite S., The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 3, 870–881 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Saiman L. et al.; Macrolide Study Group , Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: A randomized controlled trial. JAMA 290, 1749–1756 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Imperi F., Leoni L., Visca P., Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 5, 178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swatton J. E., et al. , Impact of azithromycin on the quorum sensing-controlled proteome of Pseudomonas aeruginosa. PLoS One 11, e0147698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller M., de la Peña A., Derendorf H., Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: Kill curves versus MIC. Antimicrob. Agents Chemother. 48, 369–377 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colmer-Hamood J. A., Dzvova N., Kruczek C., Hamood A. N., “In vitro analysis of Pseudomonas aeruginosa virulence using conditions that mimic the environment at specific infection sites” in Host-Microbe Interactions, Francisco M. S., Francisco B. S., Eds. (Progress in Molecular Biology and Translational Science, Academic Press, 2016), pp. 151–191, chap. 6. [DOI] [PubMed] [Google Scholar]
- 22.Crabbé A., et al. , Antimicrobial efficacy against Pseudomonas aeruginosa biofilm formation in a three-dimensional lung epithelial model and the influence of fetal bovine serum. Sci. Rep. 7, 43321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruczek C., Qaisar U., Colmer-Hamood J. A., Hamood A. N., Serum influences the expression of Pseudomonas aeruginosa quorum-sensing genes and QS-controlled virulence genes during early and late stages of growth. MicrobiologyOpen 3, 64–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruczek C., et al. , Major transcriptome changes accompany the growth of Pseudomonas aeruginosa in blood from patients with severe thermal injuries. PLoS One 11, e0149229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tata M., et al. , RNASeq based transcriptional profiling of Pseudomonas aeruginosa PA14 after short- and long-term anoxic cultivation in synthetic cystic fibrosis sputum medium. PLoS One 11, e0147811 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornforth D. M., et al. , Pseudomonas aeruginosa transcriptome during human infection. Proc. Natl. Acad. Sci. U.S.A. 115, E5125–E5134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmer S., Li Z. S., Hancock R. E. W., Influence of outer membrane mutations on susceptibility of Escherichia coli to the dibasic macrolide azithromycin. J. Antimicrob. Chemother. 29, 27–33 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Reffuveille F., de la Fuente-Núñez C., Mansour S., Hancock R. E. W., A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 58, 5363–5371 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Fuente-Núñez C., Reffuveille F., Haney E. F., Straus S. K., Hancock R. E. W., Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10, e1004152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pletzer D., Wolfmeier H., Bains M., Hancock R. E. W., Synthetic peptides to target stringent response-controlled virulence in a Pseudomonas aeruginosa murine cutaneous infection model. Front. Microbiol. 8, 1867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornforth D. M., Diggle F. L., Melvin J. A., Bomberger J. M., Whiteley M., Quantitative framework for model evaluation in microbiology research using Pseudomonas aeruginosa and cystic fibrosis infection as a test case. mBio 11, e03042-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winsor G. L., et al. , Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 44, D646–D653 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breidenstein E. B. M., Khaira B. K., Wiegand I., Overhage J., Hancock R. E. W., Complex ciprofloxacin resistome revealed by screening a Pseudomonas aeruginosa mutant library for altered susceptibility. Antimicrob. Agents Chemother. 52, 4486–4491 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez-Ortega C., Wiegand I., Olivares J., Hancock R. E. W., Martínez J. L., Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to beta-lactam antibiotics. Antimicrob. Agents Chemother. 54, 4159–4167 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dötsch A., et al. , Genomewide identification of genetic determinants of antimicrobial drug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53, 2522–2531 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schurek K. N., et al. , Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52, 4213–4219 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fajardo A., et al. , The neglected intrinsic resistome of bacterial pathogens. PLoS One 3, e1619 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schurek K. N., et al. , Involvement of pmrAB and phoPQ in polymyxin B adaptation and inducible resistance in non-cystic fibrosis clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53, 4345–4351 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutu A. D., Rodgers N. S., Park J., Moskowitz S. M., Pseudomonas aeruginosa high-level resistance to polymyxins and other antimicrobial peptides requires cprA, a gene that is disrupted in the PAO1 strain. Antimicrob. Agents Chemother. 59, 5377–5387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández L., et al. , Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob. Agents Chemother. 54, 3372–3382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kai T., et al. , A low concentration of azithromycin inhibits the mRNA expression of N-acyl homoserine lactone synthesis enzymes, upstream of lasI or rhlI, in Pseudomonas aeruginosa. Pulm. Pharmacol. Ther. 22, 483–486 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Tan H., et al. , PA3297 counteracts antimicrobial effects of azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 7, 317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeannot K., Sobel M. L., El Garch F., Poole K., Plésiat P., Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 187, 5341–5346 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs M. A., et al. , Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 100, 14339–14344 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macfarlane E. L., Kwasnicka A., Ochs M. M., Hancock R. E., PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 34, 305–316 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Radlinski L., Conlon B. P., Antibiotic efficacy in the complex infection environment. Curr. Opin. Microbiol. 42, 19–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dostert M., Belanger C. R., Hancock R. E. W., Design and assessment of anti-biofilm peptides: Steps toward clinical application. J. Innate Immun. 11, 193–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiegand I., Hilpert K., Hancock R. E. W., Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Pérez-Martínez I., Haas D., Azithromycin inhibits expression of the GacA-dependent small RNAs RsmY and RsmZ in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 55, 3399–3405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L., Dhillon P., Yan H., Farmer S., Hancock R. E., Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 3317–3321 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan H., Hancock R. E. W., Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45, 1558–1560 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pletzer D., Mansour S. C., Wuerth K., Rahanjam N., Hancock R. E. W., New mouse model for chronic infections by Gram-negative bacteria enabling the study of anti-infective efficacy and host-microbe interactions. MBio 8, e00140-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zumaquero A., Macho A. P., Rufián J. S., Beuzón C. R., Analysis of the role of the type III effector inventory of Pseudomonas syringae pv. phaseolicola 1448a in interaction with the plant. J. Bacteriol. 192, 4474–4488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pletzer D., Braun Y., Weingart H., Swarming motility is modulated by expression of the putative xenosiderophore transporter SppR-SppABCD in Pseudomonas aeruginosa PA14. Antonie van Leeuwenhoek 109, 737–753 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq data on differentially expressed genes have been deposited in the Gene Expression Omnibus (GEO) database under Sequence Read Archive accession no. SRP264926 (GEO series accession no. GSE151259).