Introduction
Acidosis and hypoxia in the tumor microenvironment are immunosuppressive and therapeutic approaches are emerging to mitigate their effects and improve immune therapy. While immunotherapy has shown great promise, it is limited by numerous immunosuppressive mechanisms in the microenvironment, such as checkpoints, inhibitory cytokines, and suppressor cells. A less well understood, but emerging, suppressive mechanism is the physiological microenvironment of tumors, specifically hypoxia and acidosis. Due to imbalances between vascularity and cellular growth patterns, sub-regions of tumors (habitats) contain microenvironments that harbor these metabolic stressors. Hypoxia and acidity have an influence on almost all immune cell components, and negatively affect responses to current immunotherapies. There is emerging evidence that these stressors can be directly or indirectly targeted to improve immune therapy response, and that such interventions will undoubtedly provide benefit, especially if applied in a biomarker-driven adaptive manner.
Hypoxia, Acidosis and Immune Function
Hypoxia is a characteristic of many solid tumors, which is caused by the imbalance between the vascular oxygen supply and cellular consumption due to the fast growth of tumor cells combined with poor perfusion. In general, hypoxia limits/inhibits immune cell functions by negatively affecting production of cytokines, receptors and gene expression. Mechanisms of hypoxia induction are fairly well known and include hypoxia-inducible factor alpha 1-α (HIF1-α) and Nrf2 in target cells and release of reactive nitrogen species, and adenosine into the microenvironment. In general, these effects result in decreased function of natural killer cells, upregulation of checkpoint ligands, increased infiltration and activity of myeloid derived suppressor cells and regulatory T cells. A recent and thorough review has summarized the effects of hypoxia on resistance to immune therapy (1). Hypoxia provides several advantages for tumor cells to help them escape from immune surveillance by the induction of immune suppression and the development of tumor variants (Figure 1). For example, a number of studies have clearly shown that the programmed death checkpoint ligand 1 (PD-L1) is induced by hypoxia via HIF1-α in myeloid derived suppressor cells, macrophages, dendritic cells, and tumor cells. It was shown that PD-L1 blockade in hypoxia resulted with reduced T cell suppression by myeloid derived suppressor cells. This suggests that HIF1-α inhibition can be combined with PD-L1 blockade to improve immunotherapy responses in patients.(2)
Figure. 1. Effect of hypoxia and acidosis on immune function.
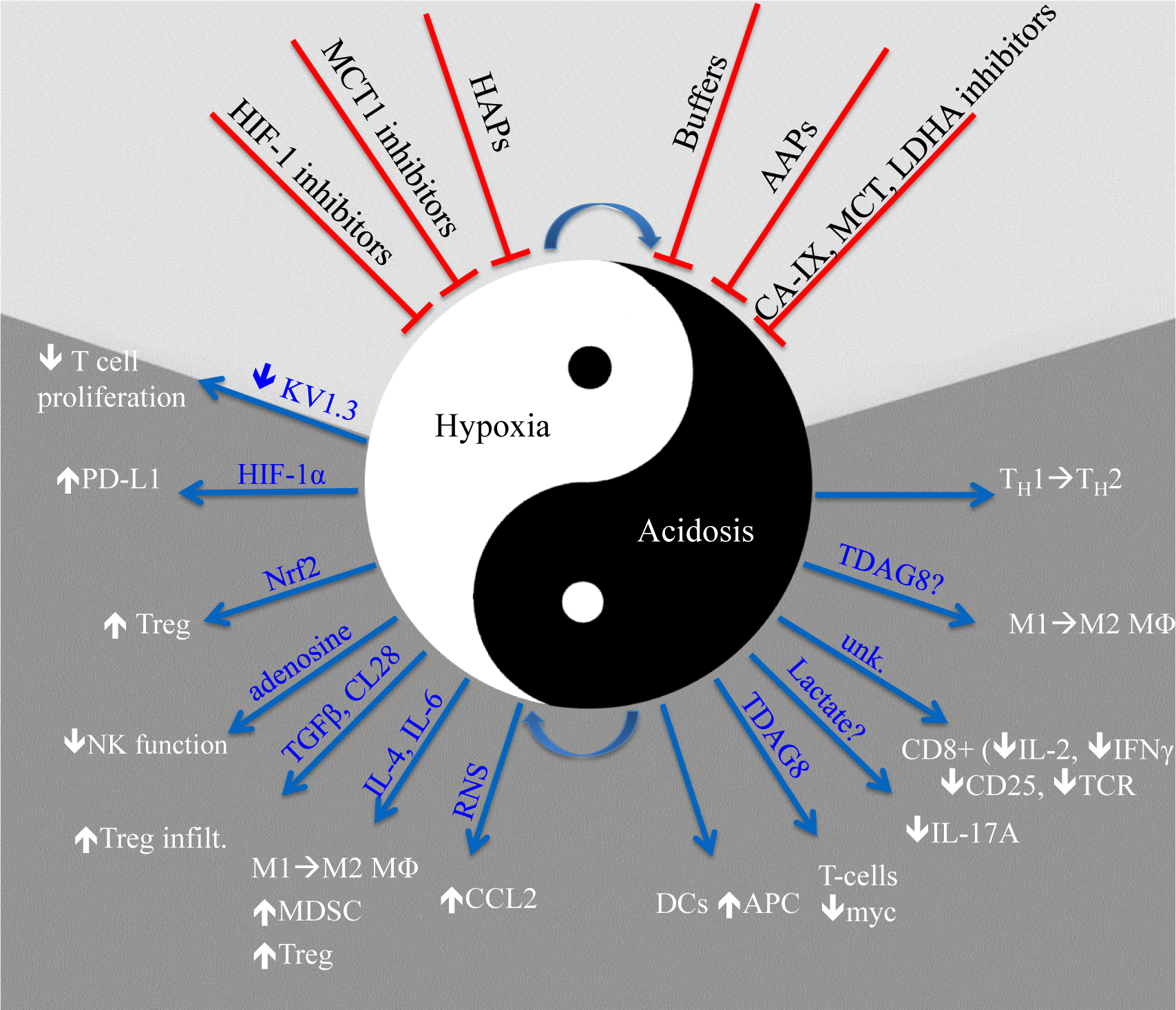
Hypoxia and acidosis are often associated with each other, and indeed hypoxia can lead to acidosis by upregulation of fermentation and acidosis can exacerbate hypoxia by inducing oxidative metabolism in otherwise non-hypoxic tissues. Under the light of accumulating evidence about the association of hypoxia and low pH with therapy resistance, different strategies have been developed to alleviate/ inhibit their effects (demonstrated on the top of the figure with red arrows). Blue arrows used to show the effects of hypoxia and acidosis on immune cells.
Acidosis is one of the most frequent characteristics of solid tumors as a result of metabolic switch to glycolysis and rapid growth with poor vascular support.(3). Less is known regarding the effect of acidic microenvironment on immune cells when compared to hypoxia despite its observation in many cancer types (4). In isolated cases, TDAG8 has been implicated, but this is not general (personal observations). It is known that lactate can potently exacerbate the effects of low pH and, in some cases, can mediate effects without changes in pH. In general the effects of acidity on immune function include changes in macrophage and helper T cell polarities towards pro-inflammatory phenotypes, induction of anergy in CD8+ Teff cells, and increased antigen presentation capacity of DCs.
In vitro, acidity profoundly suppresses CD8+ effector T-cell functions, albeit by unknown mechanisms. Accumulating evidence also indicates that low pH affects other immune cells, including dendritic cells, myeloid derived suppressor cells, and induces macrophages polarization from anti-inflammatory (M1) and pro-inflammatory (M2) phenotype (5). Despite a number of consistent observations, there remain many vacancies in our knowledge regarding the effect of pH on immune surveillance.
Monitoring of tumor hypoxia and pH
In order to consider treatment paradigms to target hypoxia and acidosis to improve immune surveillance and therapy, it is critical to be able to image these physiological parameters longitudinally in patients, which could be used to guide therapeutic choices, inclusion criteria on trials, and/or to adapt therapy during response. Although multiple pre-clinical approaches are available, to date there are no approved methods with which to accomplish this clinically. Several trials have shown promise that clinical hypoxia and acidosis monitoring will be available in the near future, discussed in (6) for hypoxia and (7) for pH. Approaches for both pH and oxygen monitoring include radioisotope tracer methods, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT); or magnetic resonance methods, such as magnetic resonance imaging (MRI) and electron paramagnetic resonance imaging (EPRI). Each of these has their own strengths and limitations. While nuclear medicine techniques have the advantage of being able to interrogate the entire patient, the hypoxia and acidity specific tracers developed to date generally suffer from a small dynamic range and lack of commercial production. MRI techniques, such as oxygen-enhanced MRI (OEMRI) are advantageous in that they are label-free and widely available, yet they are challenging to quantify. EPRI, while superbly quantitative, is not generally available in the clinic. Additionally, there is emerging evidence that deep analyses of combined FDG-PET and MRI on standard clinical images can be used to infer hypoxic and acidic habitats in tumors, but this research is in its infancy and PET-MRI systems are not widely available. Despite this current deficit, there is a growing need to image both tumor oxygen and acid-base status and hence, it is expected that techniques will soon emerge and be available in clinical practice.
Targeting Hypoxia to Improve Immunotherapy
As a common characteristic of tumors, hypoxia is not only a negative prognostic factor but also is a promising therapeutic target. There are different strategies for targeting hypoxia. For instance, HIF-1 overexpression by hypoxic tumors is related to treatment resistance and poor prognostic performance, which makes this transcription factor a promising target. Novel small molecules targeting HIF-1 are in early clinical development, and these could alleviate the HIF-associated suppressive mechanisms, such as elaboration of PD-L1.
An alternative approach is to use hypoxia-activated prodrugs, HAPs, that contain therapeutic moieties that are released in low oxygen environments (8). Evofosfamide (TH-302) is a representative HAP that has been through phase III clinical trials in pancreatic cancer and sarcoma. However, these trials did not meet their clinical endpoints, possibly due to trial design, which was not biomarker driven (9). Despite this setback, there remains continued enthusiasm for the use of HAPs, and it emphasizes the need to include a positive imaging test as part of inclusion criteria. In short, HAPs will not be effective if tumors are not hypoxic, and reduced effectiveness of conventional therapies would also be expected in a hypoxic control arm. Notably, a new clinical trial (NCT 03098160) has been initiated with the aim of combining hypoxia-activated prodrug (evofosfamide) with ipilimumab. This includes patients with confirmed metastatic or locally advanced prostate cancer, metastatic pancreatic cancer, melanoma or HPV-negative squamous cell carcinoma of head and neck that have progressed on standard therapy.(10)
Targeting metabolic vulnerabilities of hypoxic cells by, e.g. manipulating lactate metabolism is another approach to kill hypoxic cells. It has been shown that there is a metabolic symbiosis between oxidative and fermentative cancer cells as oxidative cells use the waste product of fermentation, lactate, as an energy source by using MCT1 as a lactate shuttle. This makes MCT1 inhibition a strategy to kill hypoxic cells with glucose starvation (8).
Targeting Acidosis to improve Immunotherapy
Therapies based on reversal of tumor acidosis utilizing systemic oral buffers have shown striking results as monotherapy in a variety of pre-clinical models. Further, buffers in combination with immunotherapies can lead to improved durable outcomes. Oral bicarbonate buffer (200 mM ad lib) is commonly used to neutralize tumor acidity in animals, and in combination with anti-CTLA-4, anti PD-1, or adoptive T cell therapy, led to improved durable responses and cures in syngeneic models of melanoma and pancreatic cancer (11). However, buffer therapy has been challenging to translate to the clinic. Three trials (NCT01350583, NCT01198821, and NCT01846429) failed to expand due to poor taste or GI discomfort after ongoing oral bicarbonate buffer. Hence, although targeting tumor acidity with buffers appears to have benefit in combination with immune therapy, alternatives are needed for clinical translation. Another approach to directly target pH uses CEACAM6-targeted Jack bean urease (L-DOS47), which raises pH through the breakdown of endogenous urea into 2 NH4+ and 1 HCO3− (12).
An alternative to directly targeting pH is to use acid-activated pro-drugs, AAPs. Among these are clinically-approved proton pump inhibitors, such as Omeprazole, which have been shown to raise tumor pH and improve efficacy of checkpoint blockade in pre-clinical studies. These tetracyclic sulfonamides covalently target cysteine residues of proteins in an acid-catalyzed reaction. While the canonical target is the gastric H+K ATPase, in extragastric tissues it is not known whether the acid pH of tumors is sufficient to activate these agents or whether they are only active in the endosomal/lysosomal compartment. Further alternative AAPs include a large number of nanoparticles that have been developed that disassociate in acid media. Acid-activated nanoparticles have not yet been extensively used in combination with immune therapies.
A further alternative is to target the metabolism of cancer cells that is responsible for producing acidosis in the first place (3). Carbonic Anhydrase IX (CA-IX) controls intra- and extracellular acid-base balance to maintain survival and promote invasion. It is a key regulator of extracellular acidity and inhibition of CA-IX modulates its role in pH regulation. Monocarboxylate transporters (MCTs) that facilitate the export of lactate and H+ have role on extracellular acidosis, promoting survival and invasive characteristic of cancer cells. There is strong effect for targeting MCTs and lactate production order to reduce microenvironment acidification to remove its suppressive effects on immune functions (13, 14).
Targeting the microenvironment to improve immune therapy
While it is accepted that the physiological microenvironment is a barrier to chemo- and radio-therapies, an emerging concept is that it also inhibits effectiveness of immunotherapies. Tumor hypoxia and acidosis are major players in the tumor microenvironment that generate therapy resistance. In vitro studies have shown that hypoxia and acidosis have both direct and indirect effects on all facets of the innate and adaptive immune systems and are, in general, suppressors of immune surveillance. The effects of hypoxia appear to be mediated by HIF1-α. In contrast, the mediator(s) of acid inhibition are not known, but are suspected to be one (or more) of G-protein coupled acid receptors (TDAG8, OGR1) or acid stimulated ion channels (ASIC1–4). In animal models, there is strong evidence that hypoxia and acidosis are responsible for therapy resistance and suppressed immune responses. Further, there is increasing evidence that manipulation of hypoxia and acidity in animal tumor models can improve outcomes to immune therapies. In the clinic, trials that aim to target hypoxia or acidity mostly lack biomarker-driven inclusion criteria and this remains a major challenge going forward. Further, tumor hypoxia and acidity should be monitored in patients during treatment in order to adapt therapies based on the evolution and response of hypoxic and acidic habitats.
In summary, manipulating tumor hypoxia and acidosis is a promising approach to improve immunotherapy; however, an obvious barrier is the lack of effective drugs, hence makes the area of developing new drugs to target them highly demanded.
Funding Information:
This article is published as part of a supplement sponsored by NuOmix-Research k.s. The conference was financially supported by Protina Pharmazeutische GmbH, Germany and Sirius Pharma, Germany, and organized by NuOmix-Research k.s. Neither company had any role in writing of the manuscript.
Footnotes
Conflicts of Interest: SD and AI-H received grant support from Helix Biopharma. RJG received grant support from Helix Biopharma & Healthmyne, and NIH Small Business Innovation Research (SBIR) with Healthmyne, Inc; RJG also holds stock HealthMyne, Inc (H-M), which is a commercial quantitative PACS system for reading and analysis of radiographic scans, and held stock in Helix until 2017. SP-T received grant support from the American Cancer Society.
REFERENCES
- 1.Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA, Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 36, 439–445 (2017); published online EpubJan 26 ( 10.1038/onc.2016.225). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarus D, Peters C, Stockmann A, Eliasof S, Jayaraman L, Abstract 3209: CRLX101, an investigational nanoparticle-drug conjugate of camptothecin, demonstrates synergy with immunotherapy agents in preclinical models. Cancer Research 76, 3209–3209 (2016) 10.1158/1538-7445.am2016-3209). [DOI] [Google Scholar]
- 3.McDonald PC, Chafe SC, Dedhar S, Overcoming Hypoxia-Mediated Tumor Progression: Combinatorial Approaches Targeting pH Regulation, Angiogenesis and Immune Dysfunction. Frontiers in cell and developmental biology 4, 27 (2016) 10.3389/fcell.2016.00027). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lardner A, The effects of extracellular pH on immune function. J Leukoc Biol 69, 522–530 (2001); published online EpubApr ( [PubMed] [Google Scholar]
- 5.El-Kenawi A, Gatenbee C, Robertson-Tessi M, Bravo R, Dhillon J, Balagurunathan Y, Berglund A, Visvakarma N, Ibrahim-Hashim A, Choi J, Luddy K, Gatenby R, Pilon-Thomas S, Anderson A, Ruffell B, Gillies R, Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. British journal of cancer 121, 556–566 (2019) 10.1038/s41416-019-0542-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J, Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 9, 674–687 (2012); published online EpubDec ( 10.1038/nrclinonc.2012.171). [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Lin Y, Gillies RJ, Tumor pH and its measurement. J Nucl Med 51, 1167–1170 (2010); published online EpubAug ( 10.2967/jnumed.109.068981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson WR, Hay MP, Targeting hypoxia in cancer therapy. Nat Rev Cancer 11, 393–410 (2011); published online EpubJun ( 10.1038/nrc3064). [DOI] [PubMed] [Google Scholar]
- 9.Lindner LH, Hypoxia-activated prodrug: an appealing preclinical concept yet lost in clinical translation. Lancet Oncol 18, 991–993 (2017); published online EpubAug ( 10.1016/S1470-2045(17)30401-1). [DOI] [PubMed] [Google Scholar]
- 10.Jindal V, Immunotherapy: a glimmer of hope for metastatic prostate cancer. Chinese Clinical Oncology 7, (2018). [DOI] [PubMed] [Google Scholar]
- 11.Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mule JJ, Ibrahim-Hashim A, Gillies RJ, Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res 76, 1381–1390 (2016); published online EpubMar 15 ( 10.1158/0008-5472.CAN-15-1743). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong WY, DeLuca CI, Tian B, Wilson I, Molund S, Warriar N, Govindan MV, Segal D, Chao H, Urease-induced alkalinization of extracellular pH and its antitumor activity in human breast and lung cancers. J Exp Ther Oncol 5, 93–99 (2005). [PubMed] [Google Scholar]
- 13.Damgaci S, Ibrahim-Hashim A, Enriquez-Navas PM, Pilon-Thomas S, Guvenis A, Gillies RJ, Hypoxia and acidosis: immune suppressors and therapeutic targets. Immunology 154, 354–362 (2018) 10.1111/imm.12917). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty JR, Cleveland JL, Targeting lactate metabolism for cancer therapeutics. The Journal of clinical investigation 123, 3685–3692 (2013) 10.1172/JCI69741). [DOI] [PMC free article] [PubMed] [Google Scholar]


