Abstract
Background
Following a meropenem shortage, we implemented a post-prescription review with feedback (PPRF) in November 2015 with mandatory infectious disease (ID) consultation for all meropenem and imipenem courses > 72 hours. Providers were made aware of the policy via an electronic alert at the time of ordering.
Methods
A retrospective study was conducted at the University of Washington Medical Center (UWMC) and Harborview Medical Center (HMC) to evaluate the impact of the policy on antimicrobial consumption and clinical outcomes pre- and post-intervention during a 6-year period. Antimicrobial use was tracked using days of therapy (DOT) per 1,000 patient-days, and data were analyzed by an interrupted time series.
Results
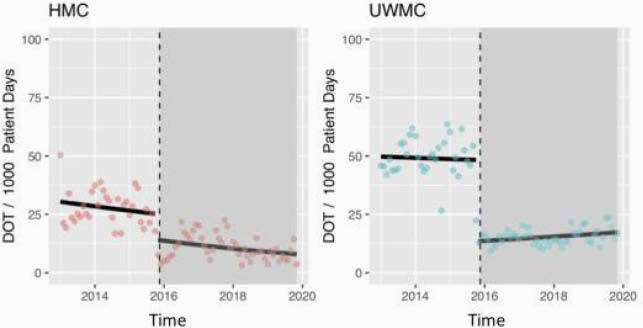
There were 4,066 and 2,552 patients in the pre- and post-intervention periods, respectively. Meropenem and imipenem use remained steady until the intervention, when a marked reduction in DOT/1,000 patient-days occurred at both hospitals (UWMC: percentage change -72.1%, (95% CI -76.6, -66.9), P < 0.001; HMC: percentage change -43.6%, (95% CI -59.9, -20.7), P = 0.001). Notably, although the intervention did not address antibiotic use until 72 hours after initiation, there was a significant decline in meropenem and imipenem initiation (“first starts”) in the post-intervention period, with a 64.9% reduction (95% CI 58.7, 70.2; P < 0.001) at UWMC and 44.7% reduction (95% CI 28.1, 57.4; P < 0.001) at HMC.
Meropenem and Imipenem DOT (January 2013 – November 2019)
Conclusion
Mandatory ID consultation and PPRF for meropenem and imipenem beyond 72 hours resulted in a significant and sustained reduction in the use of these antibiotics and notably impacted their up-front usage.
Disclosures
All Authors: No reported disclosures


