Introduction
Angiomyomatous hamartoma (AMH) of the lymph node (LN) is an uncommon, benign vascular proliferation of unknown etiology. Histologically, it is characterized by the replacement of nodal architecture with blood vessels, smooth muscle cells, and variable amounts of fibrous and adipose tissue in a collagenous stroma.1,2 First described in 1992, there are now 30 cases reported in the literature, primarily involving the inguinal and femoral LNs; rarely, cervical and submandibular nodal involvement have been described.1,3, 4, 5, 6, 7 Herein we present the first case, to our knowledge, of AMH arising within a postauricular node and clinically masquerading as an epidermal inclusion cyst.
Case report
A 33-year-old healthy man presented for evaluation of two subcutaneous nodules on his vertex scalp and right side of the postauricular neck. He reported that both lesions arose in adolescence and grew over time. The lesion on the vertex scalp had been excised several years previously but had recently recurred. The postauricular lesion had no prior treatment. Both lesions were asymptomatic without a history of inflammation or fluid drainage. Review of systems was negative. On physical examination, there were a 7-mm, firm, nontender, mobile subcutaneous nodule on the vertex scalp and a 9-mm, firm, nontender, partially mobile subcutaneous nodule on the right postauricular neck, both without overlying punctum or skin discoloration (Fig 1).
Fig 1.
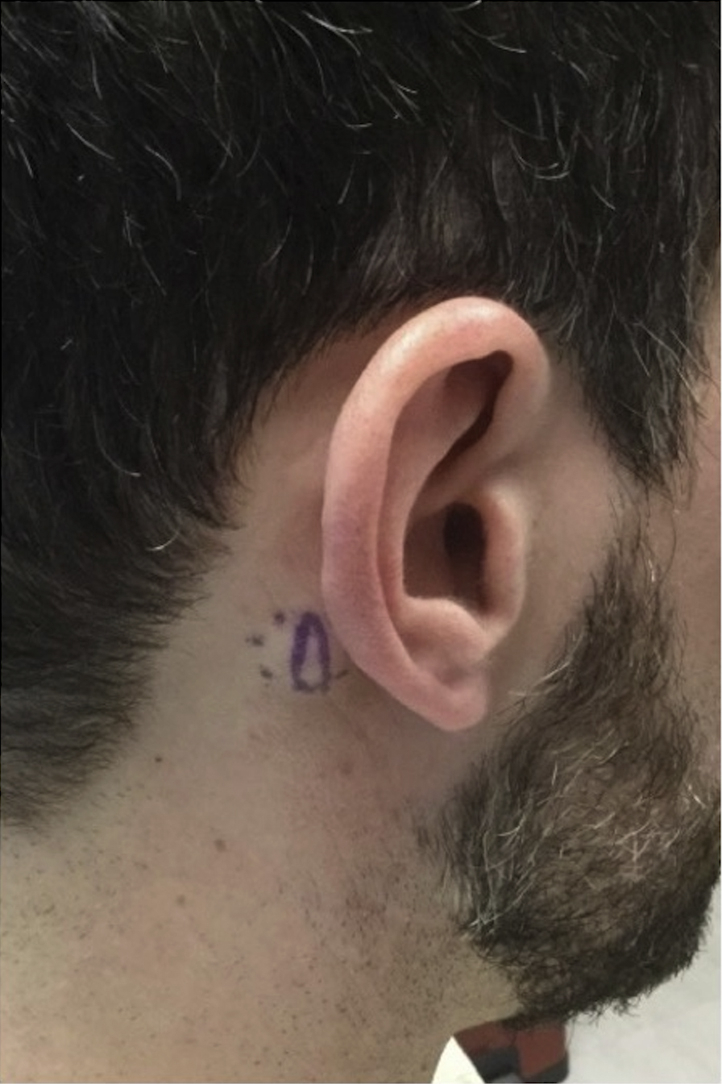
AMH presenting as a subcutaneous nodule on the right side of the postauricular neck, without overlying punctum or skin discoloration. AMH, Angiomyomatous hamartoma.
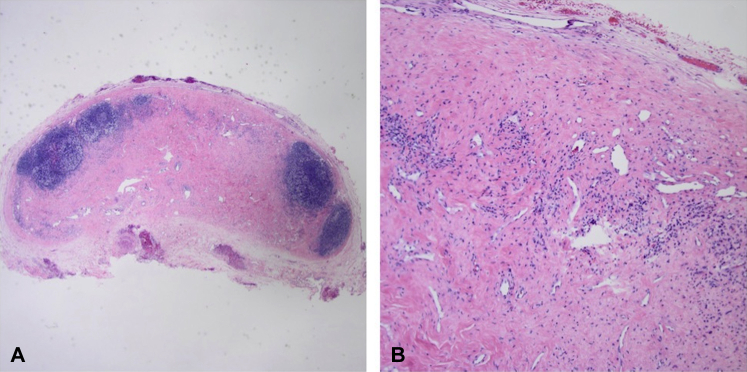
The patient underwent excision of both lesions with local anesthesia. Grossly, the excised specimen from the scalp was firm, round, and pearly in color, consistent with a cyst, and histologic evaluation confirmed a diagnosis of pilar cyst. The postauricular neck specimen, however, was irregularly shaped, inelastic, and yellow to violaceous in color. Histopathologic evaluation of this specimen revealed a circumscribed collection of lymphoid tissue surrounding a prominent proliferation of irregularly distributed thick-walled blood vessels and smooth muscle cells within a dense collagenous stroma (Fig 2).
Fig 2.
Hematoxylin-eosin staining of AMH. A, Resected LN magnification with a periphery of remaining lymphoid tissue and central replacement of LN architecture with AMH. (Original magnification: ×4.) B, Irregularly distributed, thick-walled blood vessels and smooth muscle cells within a dense collagenous stroma. (Original magnification: ×10). AMH, Angiomyomatous hamartoma; LN, lymph node.
There were no features suggestive of malignancy. The lesion was subsequently diagnosed as AMH of a postauricular LN. Postoperative 7-month follow-up found no evidence of recurrence.
Discussion
Chan et al first described AMH as part of a study characterizing primary vascular tumors of LNs.3 The term “angiomyomatous hamartoma” was used to reflect the extensive replacement of nodal parenchyma with blood vessels, smooth muscle cells, and adipose tissue seen on histology. It appears to be a rare nodal condition, with only 30 cases reported in the literature, with a predilection for inguinal and femoral LNs and only isolated cases reported in cervical and submandibular LNs.4, 5, 6, 7 Although the rarity of AMH may be due to under-reporting, our case represents novel involvement of the postauricular node and supports previous reports that AMH may present outside the inguinal or femoral nodal basins.
The epidemiology and pathophysiology of AMH have not been well elucidated. There is no apparent predilection for sex or age, with the age at presentation ranging from 8 months to 83 years.1,4 There has been some controversy regarding whether AMH of LN is its own histologic entity or a variant of previously described conditions, such as lymphangioleiomyomatosis (LAM); however, a recent review of 21 cases demonstrated its immunohistochemical distinction.1 The condition appears to be idiopathic and has been theorized to arise from a reparative reaction against nodal inflammation.3
Clinically, AMH of LN presents as an indolent subcutaneous nodule or mass within the inguinal or femoral LNs, but as our case demonstrates, it may occur in the head and neck region. The differential diagnosis includes common benign conditions, such as cyst or lipoma, and malignant conditions, including lymphoma. Unlike an epidermal cyst, lesions of AMH of LN lack an overlying punctum or history of inflammation and drainage.6 Rarely, AMH may present with pain, which is thought to be due to nerve compression, and thus, AMH may clinically mimic an angiolipoma.5 Ultrasound may be helpful to differentiate between these entities and suggests that the lesion is within an LN; however, ultrasound is neither sensitive nor specific.4 Ultimately, the diagnosis of AMH of LN is made by histologic evaluation.
Relevant histologic differential diagnoses include leiomyomatosis, angiomyolipoma of the LN, and LAM, all of which affect the deep nodes of the abdomen and pelvis.2,8,9 In comparison with AMH of LN, nodal leiomyomatosis lacks a significant vascular component.9 AMH of LN may be distinguished from angiomyolipoma of the retroperitoneal LNs with the use of immunohistochemistry, as the latter is human melanoma black-45 positive and is also associated with multifocal angiomyolipoma of the kidney.8,9 LAM of the pelvic and para-aortic LNs is also human melanoma black-45 positive as well as cathepsin K-positive. AMH of LN does not stain with these markers.2,9 Interestingly, variations of LAM can lack a key characteristic of obvious lymphatic channels and may present as benign fascicular spindle proliferations that highly resemble AMH without immunohistochemical staining.1 This raises the question of whether earlier diagnoses of AMH predating such immunologic stains may have actually been variants of the aforementioned pathologies. Additional immunohistochemical stains that may confirm the diagnosis of AMH from other vascular tumors include actin, desmin, and human herpesvirus-8.
Surgical excision is often performed for both diagnosis and treatment of AMH of LN and is often curative.1,5,8 However, an exception to this standard treatment became evident in a case of AMH that recurred 14 years after initial surgical excision and presented with symptoms of pain and burning secondary to compressive neuralgia. The mass was subsequently treated with intralesional steroid injections, rather than repeat excision, and it demonstrated no further signs of growth.5
To the best of our knowledge, this is the first case of AMH of LN within the postauricular chain, and our findings corroborate previous reports demonstrating that AMH of LN can affect LNs outside the inguinal and femoral regions. The purpose of this report is to increase awareness of this vascular proliferation and highlight its benign clinical course and cure by surgical resection. Our case underscores the importance of considering a broad differential diagnosis and maintaining a high index of suspicion when evaluating nondescript subcutaneous nodules or masses near nodal basins.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Moh M., Sangoi A.R., Rabban J.T. Angiomyomatous hamartoma of lymph nodes, revisited: clinicopathologic study of 21 cases, emphasizing its distinction from lymphangioleiomyomatosis of lymph nodes. Hum Pathol. 2017;68:175–183. doi: 10.1016/j.humpath.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Mridha A.R., Ranjan R., Kinra P., Ray R., Khan S.A., Shivanand G. Angiomyomatous hamartoma of popliteal lymph node: an unusual entity. J Pathol Transl Med. 2015;49(2):156–158. doi: 10.4132/jptm.2013.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J.K., Frizzera G., Fletcher C.D., Rosai J. Primary vascular tumors of lymph nodes other than Kaposi's sarcoma: analysis of 39 cases and delineation of two new entities. Am J Surg Pathol. 1992;16(4):335–350. doi: 10.1097/00000478-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Catania V.D., Manzoni C., Novello M., Lauriola L., Coli A. Unusual presentation of angiomyomatous hamartoma in an eight-month-old infant: case report and literature review. BMC Pediatr. 2012;12(1):172. doi: 10.1186/1471-2431-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolley C.A., Oswald J., Chen J. Painful inguinal angiomyomatous hamartoma responsive to conservative pain management: a case report. A&A Pract. 2019;13(10):373–375. doi: 10.1213/XAA.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 6.Barzilai G., Schindler Y., Cohen-Kerem R. Angiomyomatous hamartoma in a submandibular lymph node: a case report. Ear Nose Throat J. 2009;88(3):831–832. [PubMed] [Google Scholar]
- 7.Laeng R.H., Hotz M.A., Borisch B. Angiomyomatous hamartoma of a cervical lymph node combined with haemangiomatoids and vascular transformation of sinuses. Histopathology. 1996;29(1):80–84. [PubMed] [Google Scholar]
- 8.Arava S., Gahlot G.P., Deepak R., Sharma M.C., Nath D., Ashok S. Angiomyomatous hamartoma of lymph nodes: clinicopathological study of 6 cases with review of literature. Indian J Pathol Microbiol. 2016;59(2):206. doi: 10.4103/0377-4929.182039. [DOI] [PubMed] [Google Scholar]
- 9.Ram M., Alsanjari N., Ansari N. Angiomyomatous hamartoma: a rare case report with review of the literature. Rare Tumors. 2009;1(2):75–78. doi: 10.4081/rt.2009.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]