Introduction
Sarcoidosis is an inflammatory disease characterized by the formation of granulomas in affected tissues. Sarcoidosis can occur in nearly any organ; approximately one third of patients have skin involvement, which is occasionally the only site of disease.1 Cutaneous sarcoidosis commonly affects the face and can lead to significant morbidity. Angiolupoid sarcoidosis is a clinical variant, which favors the central face, especially the nasal bridge, and is characterized by prominent telangiectasia formation overlying inflammatory lesions.2
The only FDA-approved treatment for sarcoidosis is corticosteroids, which are approved for pulmonary involvement. No approved treatments for cutaneous sarcoidosis exist. For cutaneous sarcoidosis, topical and/or intralesional corticosteroids are often used; however, their utility is limited by local adverse effects, including atrophy and hypopigmentation. Other topical therapies, such as calcineurin inhibitors, are often ineffective. Systemic therapies including hydroxychloroquine, methotrexate, antibiotics, and tumor necrosis factor alfa (TNF-α) inhibitors are not reproducibly effective,3 and their use may be difficult to justify, when disease is limited.
The pathogenesis of sarcoidosis has yet to be fully elucidated, but a chronic T cell response appears to play an important role in driving granuloma formation.4 We and others have found that, in particular, CD4+ T-cell–derived interferon gamma (IFN-γ) may be a primary driver of disease through macrophage activation. Other cytokines such as interleukin 6 (IL-6) may also play a role.5,6 Both IFN-γ and IL-6 (and other cytokines) signal via the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway, and the activity of these cytokines leads to constitutive activation of JAK-STAT signaling in sarcoidosis.5,6 Indeed, we have shown that oral tofacitinib (a JAK1/3 inhibitor) can be effective in patients with sarcoidosis,5,6 and we are currently performing a clinical trial of oral tofacitinib for patients with severe sarcoidosis (NCT03910543). Others have reported the efficacy oral ruxolitinib (JAK1/2 inhibitor) for sarcoidosis, also with promising results.7,8
Despite these observations, topical JAK inhibition has not yet been evaluated in cutaneous sarcoidosis. Here, we describe the combined use of tofacitinib ointment 2% and pulsed dye laser (PDL) to successfully treat a patient with angiolupoid sarcoidosis.
Case report
A 50-year-old woman with a 5-year history of sarcoidosis presented for evaluation and discussion of treatment options. She had a history of a T1a melanoma 5 years previously, treated with surgery alone. Her sarcoidosis was discovered incidentally during her melanoma workup. An abnormal chest X-ray led to computed tomography of the chest, which showed hilar and mediastinal lymphadenopathy with peribronchovascular and subpleural opacities. These findings were deemed consistent with sarcoidosis and have been followed and remained stable. Two years later, plaques developed on the patient's face and arms. A biopsy from the upper portion of the arm showed sarcoidal granulomas (Fig 1).
Fig 1.
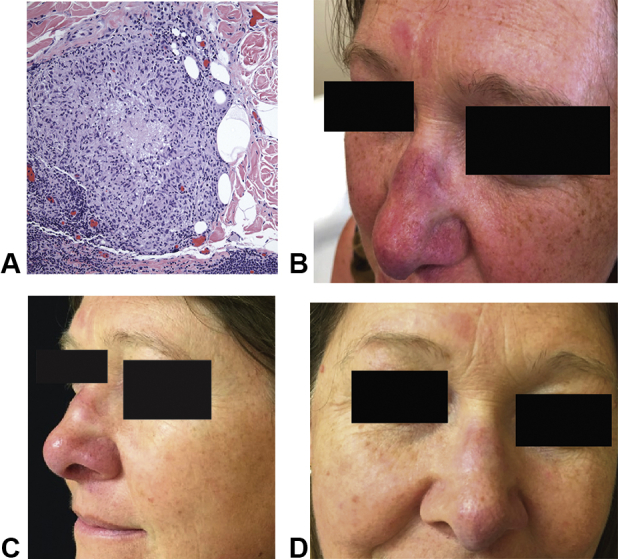
Histologic and clinical features of sarcoidosis and response to tofacitinib ointment 2% and PDL. A, A biopsy from the upper portion of the left arm showed well-formed granulomas composed of epithelioid histiocytes and a sparse lymphocytic infiltrate. Stains for acid-fast bacilli and fungal organisms were negative. Polarization was negative. B, Clinical image at baseline (while the patient was taking methotrexate 15 mg weekly). C, Clinical image after 5 months of tofacitinib ointment 2% and before the second session of PDL. D, Clinical image after 1 year of tofacitinib 2% ointment treatment and 3 sessions of PDL. PDL, Pulsed dye laser.
The lung involvement was felt not to require treatment by her pulmonologist. For her cutaneous disease, the patient had previously been treated with triamcinolone cream 0.1% for up to 2 weeks at a time, which was ineffective. The use of topical tacrolimus ointment 0.1% was also ineffective. A trial of doxycycline 50 mg twice daily and later methotrexate 15 mg weekly were both ineffective after 3-month trials of each. She was intolerant of hydroxychloroquine (dizziness). Given her history of melanoma and personal preference, the use of TNF-α inhibition was not pursued.
Physical examination (while the patient was taking methotrexate) showed indurated, slightly infiltrated pink-red plaques on the nasal dorsum, tip, alae, and glabella. Prominent telangiectasias overlying the lesions on the nose were observed (Fig 1). With regard to facial involvement, the cutaneous sarcoidosis activity and morphology instrument9 activity score was 15. Given the emerging experience with oral JAK inhibition in sarcoidosis at our institution and elsewhere, we decided to proceed with compounded tofacitinib 2% ointment twice daily.
After 10 weeks of topical tofacitinib, improvement in erythema and induration was noted; however, the telangiectasia failed to improve. At this time, PDL was initiated for a total of 3 treatments over 5 months with improvement in the telangiectasia. Tofacitinib was continued through the PDL and has been used continuously for a total of 18 months. The patient has experienced ongoing improvement, with no development of new telangiectasias. At her most recent follow-up, her cutaneous sarcoidosis activity and morphology instrument activity score was 8; the lesions on the face were judged to be minimally active, and their distribution had not changed. She has experienced no adverse effects from therapy.
Discussion
Oral JAK inhibition is being actively pursued as a new approach for sarcoidosis (NCT03793439 and NCT03910543), given the promise of this approach observed in case reports and small series.5, 6, 7, 8 However, topical JAK inhibition for cutaneous sarcoidosis has not yet been evaluated or reported. Topical JAK inhibition is being widely pursued in dermatology in inflammatory dermatoses, such as vitiligo and atopic dermatitis, among many others.
Here, in a patient with sarcoidosis, tofacitinib ointment 2% was effective in reducing erythema and induration of sarcoidal lesions and resulted in durable improvement. However, the telangiectasias already present in this angiolupoid presentation persisted. PDL was effective in reducing existing telangiectasias once the inflammation was controlled with tofacitinib. The use of PDL in sarcoidosis has been reported previously in case reports but is not thought to affect the underlying inflammation.10 We hypothesize that ongoing tofacitinib treatment, by controlling underlying inflammation, has prevented the formation of additional, new telangiectasias. The mechanism by which telangiectasias develop in angiolupoid sarcoidosis is unclear but may involve production of vascular endothelial growth factor and other angiogenic factors by activated macrophages. Although JAK inhibition would not inhibit these factors directly, it would prevent upstream activation of the macrophages by interferon gamma, indirectly preventing formation of new telangiectasias. Additional work is required to determine the reproducibility of this approach. We expect ongoing treatment will be needed to maintain the effect in this patient.6
This report suggests that topical JAK inhibition may be a viable approach for localized cutaneous sarcoidosis. Cases where granulomatous inflammation is more superficial may be most amendable to such approaches; deep dermal and/or subcutaneous involvement may be less responsive. Further evaluation is warranted.
Conflicts of interest
Dr King is an investigator for Concert Pharmaceuticals Inc, Eli Lilly and Company, and Pfizer Inc; is a consultant to and/or investigator for and/or has served on advisory boards for Aclaris Therapeutics, Arena Pharmaceuticals, Bristol-Meyers Squibb, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, Pfizer Inc, and Viela Bio; and is on the speaker's bureau for Regeneron and Sanofi Genzyme. Dr Damsky has research funding from Pfizer, is a consultant for Eli Lilly and Twi Biotechnology, and receives licensing fees from EMD/Sigma/Millipore in unrelated work. Authors Singh and Wang and Drs Heald, McNiff, Suozzi, and Leventhal have no conflicts of interest to declare.
Footnotes
Funding sources: Dr Damsky is supported by a Career Development Award from the Dermatology Foundation. Dr King is supported by the Ajay and Ranjini Poddar Fund for Dermatologic Diseases Research.
IRB approval status: Not applicable.
References
- 1.Noe M.H., Rosenbach M. Cutaneous sarcoidosis. Curr Opin Pulm Med. 2017;23(5):482–486. doi: 10.1097/MCP.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 2.Wu M.C., Lee J.Y. Cutaneous sarcoidosis in southern Taiwan: clinicopathologic study of a series with high proportions of lesions confined to the face and angiolupoid variant. J Eur Acad Dermatol Venereol. 2013;27(4):499–505. doi: 10.1111/j.1468-3083.2012.04473.x. [DOI] [PubMed] [Google Scholar]
- 3.Wanat K.A., Rosenbach M. A practical approach to cutaneous sarcoidosis. Am J Clin Dermatol. 2014;15(4):283–297. doi: 10.1007/s40257-014-0079-3. [DOI] [PubMed] [Google Scholar]
- 4.Broos C.E., Hendriks R.W., Kool M. T-cell immunology in sarcoidosis: disruption of a delicate balance between helper and regulatory T-cells. Curr Opin Pulm Med. 2016;22(5):476–483. doi: 10.1097/MCP.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 5.Damsky W., Thakral D., McGeary M.K., Leventhal J., Galan A., King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82(3):612–621. doi: 10.1016/j.jaad.2019.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damsky W., Thakral D., Emeagwali N., Galan A., King B. Tofacitinib treatment and molecular analysis of cutaneoussarcoidosis. N Engl J Med. 2018;379(26):2540–2546. doi: 10.1056/NEJMoa1805958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotenberg C., Besnard V., Brillet P.Y., Giraudier S., Nunes H., Valeyre D. Dramatic response of refractory sarcoidosis under ruxolitinib in a patient with associated JAK2-mutated polycythemia. Eur Respir J. 2018;52(6):1801482. doi: 10.1183/13993003.01482-2018. [DOI] [PubMed] [Google Scholar]
- 8.Wei J.J., Kallenbach L.R., Kreider M., Leung T.H., Rosenbach M. Resolution of cutaneous sarcoidosis after Janus kinase inhibitor therapy for concomitant polycythemia vera. JAAD Case Rep. 2019;5(4):360–361. doi: 10.1016/j.jdcr.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbach M., Yeung H., Chu E.Y. Reliability and convergent validity of the cutaneous sarcoidosis activity and morphology instrument for assessing cutaneous sarcoidosis. JAMA Dermatol. 2013;149(5):550–556. doi: 10.1001/jamadermatol.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cliff S., Felix R.H., Singh L., Harland C.C. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1(1):49–52. doi: 10.1080/14628839950517101. [DOI] [PubMed] [Google Scholar]


