Abstract
Coronavirus disease 2019 (COVID-19) pandemic has affected more than 40 million people worldwide. Some patients had episodes of symptom recurrence after the first episode of infection with variable intervals. There are multiple issues and hypotheses about re-infection or re-activation of the virus, especially in immunocompromised patients. In this paper, we present details of an individual with a recent history of COVID-19 who proceeded to acute myeloid leukaemia M3 and immunosuppression by chemotherapy, then we review some recently published articles about possible re-infection or re-activation.
Keywords: Acute myeloid leukaemia, coronavirus disease-19, re-infection
Case history
A 15-year-old boy was referred to our haematology clinic because of pancytopenia (white blood cell count (WBC) 3200/μL, haemoglobin 10.5 mg/dL, platelets 88 000/μL). His mother complained of her son's sudden icteric sclera. He had no past medical, surgical or medication history. One month previously, he had had an episode of coronavirus disease 2019 (COVID-19) with signs and symptoms of cough, dyspnoea and patchy infiltration in the left lung. He had two negative PCR tests. The symptoms then gradually resolved, and only some residual patchy infiltration was visible on lung CT scan. In the physical examination, there were no signs of lymphadenopathies nor of splenomegaly. The PCR test for severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) was negative, but the antibody test was positive for IgG (33 IU/mL; typically <5 IU/mL) but negative for IgM (3.5 IU/mL; typically <5 IU/mL).
He became a candidate for bone marrow biopsy and aspiration. Results for bone marrow biopsy, aspiration (Fig. 1) and flow cytometry were compatible with acute myeloid leukaemia M3 with positive PML-RARα. After admission, he had no signs or symptoms of active infection, so the chemotherapy regimen started with all-trans retinoic acid (tretinoin; ATRA) (45 mg/m2) and arsenic trioxide (0.15 mg/kg) daily. After about 10 days of treatment, his weight increased by about 10 kg and the WBC count reached 29 000/μL. As with differentiation syndrome, we started dexamethasone 8 mg twice daily and a single dose of idarubicin 12 mg/m2, although he had no sign of respiratory distress. After 3 days, the WBC count decreased to 13 000/μL but then rose again to about 23 000/μL 3 days later, so another single dose of idarubicin 12 mg/m2 was administered. With this second dose, the WBC count dropped to 800/μL, haemoglobin to 7.5 mg/dL and platelets to 15 000/μL. By continuing arsenic trioxide and withholding the ATRA, the WBC count started to decrease. We restarted ATRA 45 mg/m2 and with this decrement, his weight returned to normal.
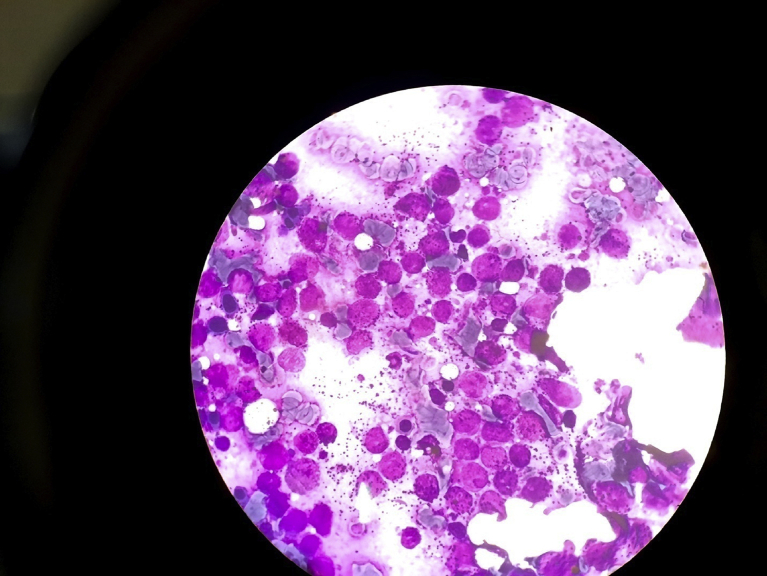
FIG. 1.
Bone marrow aspiration shown diffuse infiltration of promyelocytes.
In the admission course, as he was neutropenic, he became febrile with a temperature of about 39.0°C. He had a cough, shivering and myalgia. We continued ATRA and arsenic trioxide but evaluated him for febrile neutropenia aetiologies. On lung CT scan, a new patchy infiltration was seen in his right lung. Empirical antibiotics (meropenem, vancomycin and levofloxacin) were administered. As the patient's general condition worsened we added the antifungal agent liposomal amphotericin 3 mg/kg to the empirical antibiotics.
After 24 hours, he became severely dyspnoeic, and O2 saturation dropped to 75%. The CT scan showed severe bilateral ground-glass patchy infiltrations compatible with COVID-19 lung involvement (Fig. 2). The patient was intubated and concomitantly a pulmonologist performed a bronchoscopy and took a mini-bronchoalveolar lavage; this was sent for galactomannan and Gram smear, culture and COVID-19 PCR test. After 48 hours, the bronchoalveolar lavage galactomannan was negative, but viral load measured by COVID RT-PCR cycle threshold (CT levels) was 521 868 217 copies/mL.
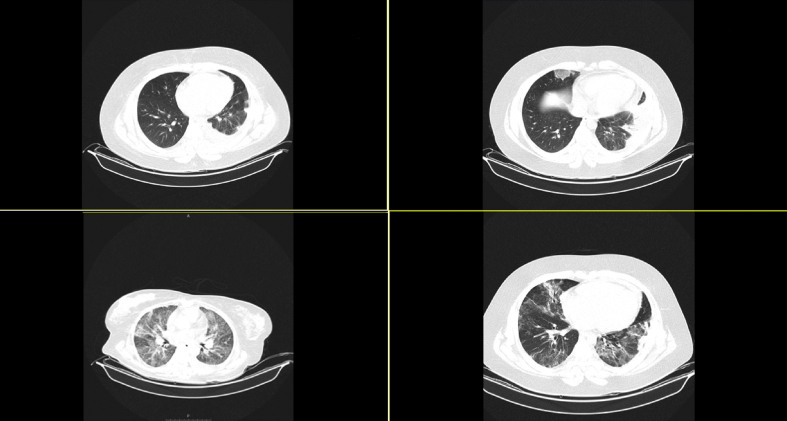
FIG. 2.
Upper left: CT scan at first day of admission to hospital; upper right: CT scan showing a new infiltration in the right lower lobe after patient became febrile with neutropenia; lower left: CT scan showing bilateral infiltration suggesting new episode of coronavirus disease 2019; lower right: CT scan on the day of discharge consistent with partial healing.
We started interferon-β (1.2 million units subcutaneously every other day) and remdesivir (200 mg on day 1 and then 100 mg for 4 days after that) with 3 days of methylprednisolone 500 mg followed by dexamethasone 8 mg twice daily continued. Although ATRA and arsenic trioxide continued during the antiviral treatment. He was also evaluated for pulmonary thromboembolism with pulmonary CT-angiography, which was consistent with thromboembolism. We transfused platelets and maintained the platelet count above 50 000/μL, and started enoxaparin (60 units subcutaneously twice daily). After 5 days on mechanical ventilation, O2 saturation began to rise and some evidence of respiratory recovery was seen. On day 8, the patient was extubated. Antibiotics and antifungal treatments were continued for 14 days, then stopped. Dexamethasone was tapered gradually and continuously. By continuing ATRA and arsenic trioxide administration the platelet count increased gradually and the patient became transfusion independent. Six weeks after admission, the patient was discharged with good general condition and without any dependency on oxygen. WBC was 5600/μL, haemoglobin was 10.5 mg/dL and platelets were 145 000/μL at the time of discharge. Anti-coagulant continued after discharge.
Discussion
Up to October 2020, COVID-19 had affected more than 40 million people with more than one million deaths worldwide. Two significant concerns about SARS-CoV-2, the virus that causes COVID-19, are re-infection and prolonged viral shedding [1,2]. Some patients have positive SARS-CoV-2 PCR tests early after the recovery from infection despite antibody production [3]. Some studies have shown that antibody titres begin to drop about 2 months later [4]. The virus may persist in the body in respiratory secretions while the patient has no symptoms; it can spread throughout the body into the different organs such as the spleen or lymph nodes, which cannot be detected by nasopharyngeal swab [5]. It has been suggested that antibodies are produced against virus spike proteins, which can mutate and lead to reduced neutralization [6,7]. During the second infection described here, IgG antibodies were undetectable after the diagnosis, which would be justifiable considering the low burden of disease in the first episode of infection in some patients [8,9]. T-cell immunity may have a pivotal role in long-term protection against the virus by providing targets against the spike protein with helper and cytotoxic T cells [10,11]. During the convalescence period, viral shedding is still ongoing. There is some evidence that patients with immunodeficiency, such as glucocorticoid use, have prolonged viral shedding [12]. In a report from the COCOREC study group in France, they reported on 11 patients with confirmed viral re-infection at least 3 weeks after the first episode. Four of them had a mild relapse and seven of them had severe relapses and were admitted to intensive care. It was suggested that in patients with mild relapse, prolonged exposure and reduced immunity made them susceptible to re-infection. However, in severe relapses, suboptimal control of infection leads to second infection [13,14]. There are several reports of SARS-CoV-2 re-infection with mild clinical pictures or without any symptoms. The latter may be diagnosed with a positive PCR test in which it could be sample contamination or misdiagnosis due to detecting non-infectious RNA. The PCR test cannot differentiate between infectious and non-infectious RNA, so not all test positives will be a clinical relapse [[15], [16], [17], [18]]. Lancman et al. described a 55-year-old woman with acute lymphoblastic leukaemia who was positive for SARS-CoV-2 infection after induction chemotherapy with severe respiratory signs and symptoms. After receiving remdesivir and showing clinical improvement, she became infected again with positive PCR 1 month later after consolidation therapy. Results of the antibody test were negative despite previous positive results. These supported the SARS-CoV-2 re-activation issue because of a short interval between consolidation therapy and PCR positivity [19]. In this paper, we report on a patient with acute myeloid leukaemia who had previously been infected with SARS-CoV-2 with positive IgG serology and negative PCR at the time of admission. However, as he became leukopenic and lymphopenic in the course of treatment, he became infected again with SARS-CoV-2 and became severely symptomatic, which was confirmed by lung imaging and a positive PCR test result. There are some issues concerning re-infection. First, it may be possible that after a first infection, the virus is not fully removed from bodily secretions, the lymphatic system or pulmonary infiltration as in our patient, So it may be quiescent until an immunosuppression event occurs, which it becomes active again. Chemotherapeutic agents that interact with B-cell function, such as anti-CD20 agents, may impact antibody production against SARS-CoV-2. Phillips et al. reported on an individual with acute lymphoblastic leukaemia who had severe COVID-19 before starting induction chemotherapy. He received only steroids and non-myeloablative chemotherapeutic agents until the critical period of infection had passed, then he received a full course of chemotherapy. In their paper Phillips et al. recommended that after passing the critical phase of infection, chemotherapeutic agents could be introduced [20]. However, this needs more attention, as our report and that of Lancman et al. [19] describe new episodes COVID-19 after myeloablative chemotherapy, so starting chemotherapy would not be completely safe. A recently published report from the Memorial Sloan Kettering Cancer Center in New York, demonstrated severe COVID-19 in 20% of patients with cancer and a 12% Case fatality rate. Treatment with immune checkpoint inhibitors predicted both hospitalization and severe disease [21]. A recent report by Choi et al. [22] described a 40-year-old man with antiphospholipid syndrome, who had received immunosuppressive agents because of alveolar haemorrhage. In the course of his first infection with SARS-CoV-2 until his death, he had four episodes of COVID-19, one new infection and three recurrences.
Second, it may be possible that the virus can transform into a new mutational status, which is more virulent [23], or there may be secondary infection with a new viral strain. However, it is necessary to define the exact genome in each course of infection. Nevertheless, because the previous PCR result was negative, it would not possible to compare the genomic study results in the two episodes of COVID-19. In the Case of new mutational status, it could be possible that a new mutation interacts with a different lymphocyte colony and makes them replicative, which would lead to another phase of cytokine release. So, it may be possible that significantly immunocompromised patients can acquire this infection several times. This issue needs further investigations to confirm these observations.
In summary, we reported on a boy with acute myeloid leukaemia M3, who had a previous history of COVID-19. After administration of chemotherapeutic agents he became infected again with positive findings on CT scan and also PCR test. This may be due to re-activation or re-infection, and needs further investigation.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Helsinki declaration. Informed consent was obtained from all individuals.
Conflict of interest
The authors declare that they have no conflict of interest.
Fundings
The authors received no financial support for the research, author-ship and/or publication of the article.
References
- 1.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.To K.K.W., Cheng V.C.C., Cai J.P., Chan K.H., Chen L.L., Wong L.H. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020;1:e111–e118. doi: 10.1016/S2666-5247(20)30053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 7.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.C. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. bioRxiv. 2020 doi: 10.7554/eLife.61312. [Preprint]. 2020.07.21.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., To K.K., Chan K.H., Wong Y.C., Zhou R., Kwan K.Y. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect. 2020;9:1664–1670. doi: 10.1080/22221751.2020.1791738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 10.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 11.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020 Jul 29 doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 12.Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 Mar 28:ciaa344. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gousseff M., Penot P., Gallay L., Batisse D., Benech N., Bouiller K., in behalf of the COCOREC study group Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020 doi: 10.1016/j.jinf.2020.06.073. S0163-4453(20)30454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sah R., Rodriguez-Morales A.J., Jha R., Chu D.K.W., Gu H., Peiris M. Complete genome sequence of a 2019 novel coronavirus (SARS-CoV-2) strain isolated in Nepal. Microbiol Resour Announc. 2020;9 doi: 10.1128/MRA.00169-20. e00169–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L.D., Li H., Ye Y.M., Wu Z., Huang Y.P., Zhang W.L. A COVID-19 patient with multiple negative results for PCR assays outside Wuhan, China: a Case report. BMC Infect Dis. 2020;20:517. doi: 10.1186/s12879-020-05245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi R., Naidu R., Sheth S., Chakravarthy K. Efficacy of serology testing in predicting reinfection in patients with SARS-CoV-2. Disaster Med Public Health Prep. 2020 Jun 24:1–3. doi: 10.1017/dmp.2020.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancman G., Mascarenhas J., Bar-Natan M. Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:131. doi: 10.1186/s13045-020-00968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips L., Pavisic J., Kaur D., Dorrello N.V., Broglie L., Hijiya N. Successful management of SARS-CoV-2 acute respiratory distress syndrome and newly diagnosed acute lymphoblastic leukemia. Blood Adv. 2020;4:4358–4361. doi: 10.1182/bloodadvances.2020002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020 Nov 11 doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A. Genomic evidence for reinfection with SARS-CoV-2: a Case study. Lancet Infect Dis. 2020 Oct 12 doi: 10.1016/S1473-3099(20)30764-7. S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]